Abstract
Substance use disorders are diagnosed as a manifestation of inappropriate behavioral allocation towards abused drugs and away from other behaviors maintained by more adaptive nondrug reinforcers (e.g., work and social relationships). Substance use disorder treatment goals include not only decreasing drug-maintained behavior but also promoting behavioral reallocation toward these socially adaptive alternative reinforcers. Preclinical drug self-administration procedures that offer concurrent access to both drug and nondrug reinforcers provide a translationally relevant dependent measure of behavioral allocation that may be useful for candidate medication evaluation. In contrast to other abused drugs, such as heroin or cocaine, preclinical methamphetamine versus food choice procedures have been a more recent development. We hypothesize that preclinical to clinical translatability would be improved by the evaluation of repeated pharmacological treatment effects on methamphetamine self-administration under a methamphetamine versus food choice procedure. In support of this hypothesis, a literature review suggests strong concordance between preclinical pharmacological treatment effects on methamphetamine versus food choice in nonhuman primates and clinical medication treatment effects on methamphetamine self-administration in human laboratory studies or methamphetamine abuse metrics in clinical trials. In conclusion, this literature suggests preclinical methamphetamine versus food choice procedures may be useful in developing innovative pharmacotherapies for methamphetamine use disorder.
Keywords: methamphetamine, choice, addiction, dopamine, nonhuman primate
Introduction
Methamphetamine is an N-methyl analog of amphetamine and a clinically available monoamine transporter substrate that is approved by the U.S. Food and Drug Administration (FDA) for the treatment of attention-deficit hyperactivity disorder and obesity (http://www.accessdata.fda.gov/drugsatfda_docs/label/2007/005378s026lbl.pdf). Similar to amphetamine, methamphetamine has a higher affinity for the dopamine (DA) and norepinephrine (NE) transporters (DAT, NET) compared to the serotonin (5-HT) transporter (SERT).1–3 This in vitro selectivity for DAT versus SERT is also consistent with methamphetamine being more potent in vivo to increase extracellular dopamine versus serotonin levels in brain regions involved in drug reinforcement and abuse, such as the nucleus accumbens and striatum.4–6 Furthermore, laboratory studies in mice,7 rats,8 cats,9 nonhuman primates,10,11 and humans12 have demonstrated that methamphetamine functions as a reinforcer under multiple drug self-administration procedures. Overall, this body of literature is consistent with the U.S. Drug Enforcement Administration (DEA) classification of methamphetamine as a schedule II controlled substance with high abuse liability.
Methamphetamine use disorder remains a significant and global public health issue.13,14 Furthermore, methamphetamine use disorder remains one of the few substance use disorders for which there are zero candidate medications that have showed consistent therapeutic efficacy in clinical trials.15 Moreover, a high priority for all drug abuse research is the development of both safer and more efficacious medications to treat all classes of substance use disorders. Toward this goal, preclinical drug self-administration procedures have improved our understanding of the environmental and biological determinants of drug reinforcement. In addition, preclinical candidate medication treatment evaluation on drug self-administration has provided good, although not perfect, concordance with candidate medication treatment evaluation in both human laboratory drug self-administration studies and abused drug use metrics in clinical trials.16–18 Two experimental attributes that appear to promote concordant preclinical to clinical results with other abused drugs, such as heroin19 and cocaine,20,21 are (1) repeated candidate medication–treatment regimens to model treatment regimens that are employed in both human laboratory drug self-administration studies and clinical trials and (2) candidate medication–treatment assessment in drug self-administration procedures that assess choice between concurrent availability of an abused drug and an alternative nondrug reinforce, such as food (in preclinical studies) or money (in human laboratory studies). Whether these two experimental attributes promote concordant preclinical to clinical results for methamphetamine remains unexplored. Accordingly, this review has two main aims. First, we discuss the major pharmacological manipulations on intravenous methamphetamine versus food choice in preclinical studies with the goal of assessing the translational validity of candidate medication treatment results from preclinical methamphetamine versus food choice studies to human laboratory drug self-administration studies and clinical trials. For comparison, we also discuss the results of these same pharmacological manipulations following either acute or subchronic treatments on intravenous methamphetamine self-administration under schedules of reinforcement other than a concurrent schedule of food and methamphetamine availability. The goal here is to assess concordance between different preclinical drug self-administration procedures. Second, we discuss the implications of these findings and potential future directions for candidate methamphetamine use disorder medication development.
Rationale for preclinical choice procedures
Preclinical drug self-administration procedures are the most commonly used procedure to assess candidate substance use disorder treatments.16,17,22 Although there are numerous drug self-administration procedural variants, this review will focus on preclinical drug versus food choice procedures where behavior is maintained on two different response manipulanda by two different consequent stimuli.23,24 For example, completing the response requirement on one response manipulandum results in intravenous methamphetamine delivery, whereas completing the response requirement on a different but concurrently available manipulandum results in food pellet delivery. Furthermore, the use of preclinical drug versus food choice procedures for substance use disorder treatment evaluation is founded on three main principles. First, substance use disorders can be defined as “a disorder of behavioral allocation” that manifests as maladaptive behavioral allocation toward drug use and away from behavior maintained by alternative nondrug reinforcers, such as a job or social relationships.25,26 Moreover, the diagnosis of substance use disorders is based on both explicit and implicit measures of behavioral allocation between the abused drug and alternative nondrug reinforcers.27 On the basis of this conceptualization, two critical substance use disorder treatment goals are not only to decrease drug-maintained behavior but also to increase adaptive behavior maintained by alternative nondrug reinforcers.23,28 Preclinical choice procedures allow for an explicit, albeit simplified, assessment of behavioral allocation between a concurrently available drug and nondrug reinforcer. Second, human laboratory drug self-administration studies almost exclusively utilize drug versus nondrug choice procedures to determine candidate medication treatment efficacy,16,17 and increased homology between preclinical and human laboratory drug self-administration parameters has been hypothesized to enhance translational concordance.29–31 Finally, preclinical drug versus nondrug choice procedures provide dependent measures that may facilitate pharmacological treatment interpretation. For example, a decrease in methamphetamine self-administration is achievable by either a selective reduction in methamphetamine reinforcement (the desired outcome) or a nonselective reduction in rates of operant behavior (undesired outcome suggestive of adverse behavioral or physiological effects). Because drug versus nondrug choice procedures provide distinct dependent measures of drug reinforcement32,33 (measured by behavioral allocation (Fig. 1A)) and general behavioral competence (measured by rate of operant behavior (Fig. 1B)), a desirable candidate medication treatment effect would be a decrease in methamphetamine choice and a complementary increase in nondrug choice with minimal effects on rates of operant responding. In contrast, an undesirable candidate medication treatment effect would be no change or increase in methamphetamine choice (Fig. 1B) and a significant decrease in rates of operant responding (Fig. 1D and 1F).
Figure 1.
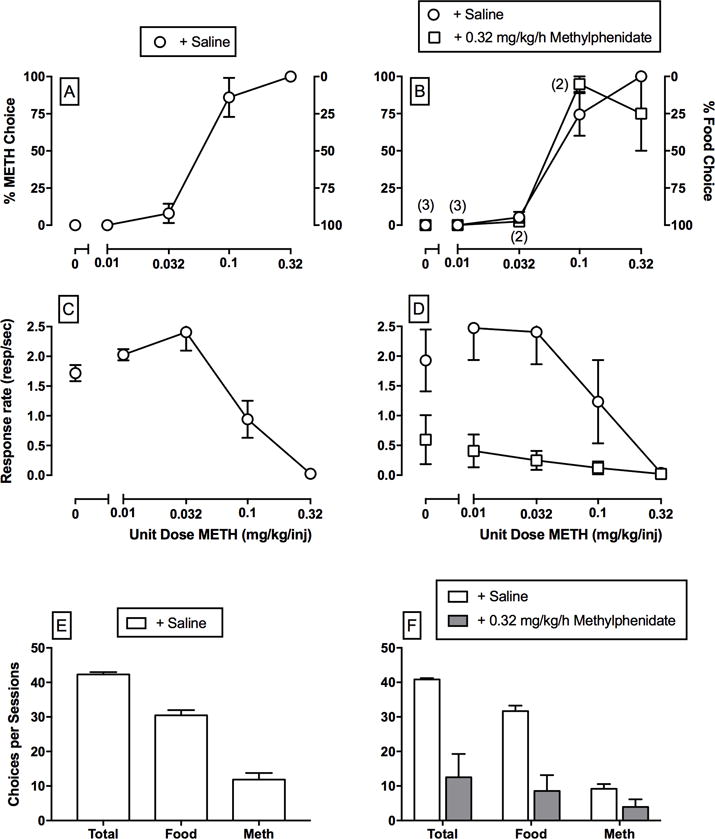
Choice between different unit methamphetamine doses (0–0.32 mg/kg/injection) and 1-g food pellets in rhesus monkeys (n = 4) under a concurrent FR10:FR100 schedule of methamphetamine injections and food availability under conditions of either subchronic saline or 0.32 mg/kg/h methylphenidate treatment. Methylphenidate treatment results have been previously published.46 Panels (A–D) abscissae: unit methamphetamine dose in mg/kg/injection. Top left ordinates: percent methamphetamine choice. Top right ordinates: percent food choice. Middle ordinates: rates of operant responding in responses per second. Bottom abscissae: dependent measure. Bottom ordinates: number of choices completed during the entire methamphetamine choice session. All dashed lines represent the mean (± standard error of the mean.) of three consecutive saline treatment days. Data points represent the mean of last 3 treatment days of each 7-day treatment period. Numbers in parentheses indicate the number of subjects contributing to that data point if less than the total number of subjects (four) tested and denote a component where the subject failed to complete at least one ratio requirement. These results demonstrate three main findings from drug versus food choice procedures. First, baseline (+ saline) methamphetamine versus food choice increases in a monotonic function as the unit methamphetamine dose increases. Second, rates of operant responding are not predictive of methamphetamine versus food choice. Lastly, preclinical methamphetamine versus food choice procedures provide dissociable measures of methamphetamine reinforcement (percent drug choice; panels A and B) and measures of operant rates of responding (C–F) that are differentially sensitive to pharmacological treatment effects.
Evaluation of candidate medications in preclinical drug versus food choice procedures
Preclinical drug versus food choice procedures have been used for more than 30 years to evaluate candidate medications for other abused drugs, such as cocaine34 and heroin.35,36 However, the establishment of methamphetamine versus food choice and its subsequent use in the candidate medication development process has only occurred within the past several years. A PubMed literature search revealed a total of 5 published preclinical studies that have determined subchronic (≥ 3 consecutive treatment days) pharmacological treatment effects with 9 different compounds on methamphetamine versus food choice in nonhuman primates. Methamphetamine versus food choice has also been recently established in rats;37 however, there are no published reports of subchronic pharmacological treatments on methamphetamine versus food choice in rats. The body of this preclinical literature is summarized in Table 1. Based on the results of this literature search, a second literature search was conducted to identify whether similar pharmacological treatments, either acute (1-day) or subchronic, had been examined on methamphetamine self-administration under other schedules of reinforcement to allow for translational assessment across different preclinical methamphetamine self-administration procedures. The body of this preclinical literature is summarized in Table 2.
Table 1.
Summary of published manuscripts reporting subchronic (≥ 3 consecutive days) pharmacological treatment effects on intravenous (+)-methamphetamine self-administration under a concurrent-choice schedule.
| # | (Dose in mg/kg/injection) | Alternative reinforcer | Species | Main treatment examined | Effect | Ref. |
|---|---|---|---|---|---|---|
| Monoamine transporter substrate treatments | ||||||
| 1 | METH (0.01–0.32) | Food pellet | Rhesus | Effect of D-amphetamine | ─/↓ | 46 |
| Monoamine transporter inhibitor treatments | ||||||
| 2 | METH (0.01–0.32) | Food pellet | Rhesus monkey | Effect of bupropion | ─ | 68 |
| 3 | METH (0.01–0.32) | Food pellet | Rhesus | Effect of methylphenidate and cocaine | ─ | 46 |
| Dopamine receptor antagonist and partial agonist treatments | ||||||
| 4 | METH (0.01–0.3) | Food pellet | Rhesus | Effect of buspirone and PG619 | ─ | 101 |
| 5 | METH (0.01–0.3) | Food pellet | Rhesus | Effect of PG01037 | ─ | 93 |
| 6 | METH (0.01–0.32) | Food pellet | Rhesus | Effect of risperidone | ↑ | 68 |
| Serotonin receptor antagonist treatments | ||||||
| 7 | METH (0.01–0.32) | Food pellet | Rhesus | Effect of pimavanserin | ─ | 113 |
Note: Columns show methamphetamine (METH) doses, the alternative reinforcer, the species in which studies were conducted, the primary treatment examined and outcome, and the reference. Studies are categorized as classes of treatment compounds discussed in the manuscript. A horizontal dashed line (─) represents a pharmacological treatment that did not significantly alter methamphetamine choice up to doses that produced other behavioral effects, such as suppression of rates of operant behavior. A downward arrow (↓) represents a pharmacological treatment that decreased methamphetamine choice. An upward arrow (↑) represents a pharmacological treatment that increased methamphetamine choice.
Table 2.
Summary of published manuscripts reporting acute (1 day) or subchronic (≥ 3 consecutive days) pharmacological treatment effects on intravenous (+)-methamphetamine self-administration.
| # | (Dose in mg/kg/injection) | Schedule | Species | Treatment regimen | Main treatment examined | Effect | Ref. |
|---|---|---|---|---|---|---|---|
| Monoamine transporter substrate treatments | |||||||
| 1 | METH (0.001–0.056) | FR10 | Rhesus | Acute | Effect of methamphetamine | ↓ | 51 |
| 2 | METH (0.06) | FR5 | Rat | Acute | Effect of methamphetamine | ↓ | 52 |
| 3 | METH (0.01–0.32) | FR5 | Rat | Acute | Effect of d-amphetamine | ↓/↑ | 50 |
| 4 | METH (0.06) | FR5 | Rat | Acute /subchronic | Effect of phentermine | ↓ (Acute) ↓ (Subchronic) |
52 |
| 5 | METH (0.06) | FR5 | Rat | Acute /subchronic | Effect of fenfluramine | ↓ (Acute) ─ (Subchronic) |
52 |
| 6 | METH (0.06) | FR5 | Rat | Acute | Effect of phentermine + fenfluramine | ─ | 52 |
| Monoamine transporter inhibitor treatments | |||||||
| 7 | METH (0.001–0.056) | FR10 | Rhesus monkey | Acute | Effect of bupropion | ↓ | 51 |
| 8 | METH (0.025) | FR1, FR3 | Rat | Subchronic | Effect of bupropion | ↓ | 69 |
| 9 | METH (0.025–0.1) | FR5 | Rat | Acute /subchronic | Effect of bupropion | ↓ (Acute) ↓ (Subchronic) |
70 |
| 10 | METH (0.001–0.056) | FR10 | Rhesus | Acute | Effect of methylphenidate | ─ | 51 |
| 11 | METH (0.02) | FR1 | Rat | Acute | Effect of modafanil | ↓ | 71 |
| 12 | METH (0.01–0.32) | FR5 | Rat | Acute | Effect of WIN35,428 | ↓/↑ | 50 |
| 13 | METH (0.01–0.32) | FR5 | Rat | Acute | Effect of JHW007, AHN 2-005, and AHN 1-055 | ↓ | 50 |
| Dopamine receptor antagonist and partial agonist treatments | |||||||
| 14 | METH (0.05) | FR1, PR | Rat | Acute | Effect of aripiprazole | ↓ | 100 |
| 15 | METH (0.05) | FR1, PR | Rat | Acute | Effect of CJB090 | ↓ | 94 |
| 16 | METH (0.05) | FR2, PR | Rat | Acute | Effect of PG01037 | ─ (FR) ↓ (PR) | 94 |
| 17 | METH (0.05) | FR2, PR | Rat | Acute | Effect of PG01037 | ─ (FR) ↓ (PR) | 95 |
| 18 | METH (0.05) | FR2, PR | Rat | Acute | Effect of SB-277011A | ─ (FR) ↓ (PR) | 96 |
Note: Columns show methamphetamine (METH) dose, the schedule of reinforcement (FR fixed ratio; PR, progressive ratio), the species in which studies were conducted, the treatment regimen, the primary treatment examined and outcome, and the reference. Studies are categorized as classes of treatment compounds discussed in the manuscript. A horizontal dashed line (─) represents a pharmacological treatment that did not significantly alter methamphetamine self-administration. A downward arrow (↓) represents a pharmacological treatment that decreased methamphetamine self-administration. An upward arrow (↑) represents a pharmacological treatment that increased methamphetamine self-administration.
Effects of monoamine transporter substrates
One pharmacotherapy approach for methamphetamine addiction might be other monoamine transporter substrates, because these compounds would share the same mechanism of action as methamphetamine and thus function as potential agonist-based substitution pharmacotherapies. The therapeutic goal with this pharmacotherapeutic approach would be to replace illicit methamphetamine use with a pharmacologically similar compound that could be administered orally. Two examples of this approach that have been approved by the FDA and are clinically used are methadone maintenance for opioid addiction38,39 and the nicotine patch for tobacco addiction.40
Monoamine transporter substrates, such as amphetamine, represent a class of drugs that function as substrates for DAT, NET, and SERT and produce pharmacological effects by promoting neurotransmitter release from the presynaptic neuron.1,3,41 Furthermore, monoamine transporter substrates are currently approved by the FDA and clinically used as pharmacotherapies for a wide range of mental health disorders, including attention deficit hyperactivity disorder, narcolepsy, and binge eating. To date, only treatment with the DAT- and NET-selective substrate D-amphetamine has been examined on methamphetamine-maintained behaviors in both clinical trials and human laboratory studies. D-Amphetamine treatment has failed to significantly decrease methamphetamine use in clinical trials.42–44 D-Amphetamine treatment also did not significantly attenuate methamphetamine self-administration under a progressive-ratio procedure in a single human laboratory study,45 although there was a trend for a decrease, and the D-amphetamine doses examined were small compared to the clinical trials. Consistent with these clinical trial and human laboratory results, a single preclinical study46 in nonhuman primates has also reported that subchronic D-amphetamine treatment failed to decrease methamphetamine versus food choice at the group analysis level. However, individual subject analysis revealed methamphetamine choice was decreased in two of the four monkeys.46 Altogether, this literature does not provide robust support for the utility of monoamine transporter substrates as potential agonist-based substitution pharmacotherapies for methamphetamine use disorder. Moreover, this literature also suggests that monoamine transporter substrates may have utility in some methamphetamine users under an individualized medicine” approach,47 an approach that is also supported by several case reports.48,49
In contrast to the clinical, human laboratory, and preclinical methamphetamine choice studies described above, three other monoamine transporter substrates, besides D-amphetamine, have been evaluated as treatments under simple FR schedules of methamphetamine reinforcement. Acute D-amphetamine treatment increased low rates of methamphetamine-maintained behavior maintained by small methamphetamine doses and decreased high rates of methamphetamine-maintained behavior maintained by intermediate methamphetamine doses.50 These results were interpreted as an undesirable pharmacological treatment profile because acute D-amphetamine pretreatment shifted the methamphetamine dose-effect function to the left. Acute (+)-methamphetamine pretreatment has also been shown to decrease rates of methamphetamine self-administration under an FR schedule in both monkeys51 and rats.52 Furthermore, methamphetamine pretreatment effects were also determined on food-maintained responding to assess behavioral selectivity and facilitate dissociations between a selective decrease in methamphetamine self-administration compared with a more general decrease in motor competence. Although methamphetamine pretreatment did not significantly decrease food-maintained responding in monkeys,51 there was a trend for decreased rates of operant responding, suggesting modest behavioral selectivity. Consistent with the methamphetamine results, treatment with DA and NE versus the 5-HT-selective monoamine transporter substrate phentermine also attenuated rates of methamphetamine self-administration under both acute and subchronic treatment regimens.52 Behavioral selectivity was also assessed in this study, and phentermine was equally potent to decrease rates of responding in the methamphetamine discrimination and methamphetamine self-administration procedures. Thus, these results do not suggest phentermine selectively decreases methamphetamine self-administration. The final monoamine transporter substrate that has been evaluated was 5-HT versus the DA and NE-selective substrate fenfluramine. The rationale for fenfluramine evaluation as a pharmacological treatment was based on preclinical evidence implicating serotonergic regulation of dopamine neurotransmission (for review, see Refs. 53 and 54). Acute fenfluramine treatment decreased rates of methamphetamine self-administration, but tolerance developed to fenfluramine treatment effects during repeated treatments such that the fenfluramine effect was absent after 4 treatment days.52 Overall, these results highlight the importance of experimental designs that include repeated, subchronic pharmacological dosing regimens and determining behavioral selectivity on nondrug-maintained responding.
Effects of monoamine transporter inhibitors
Another agonist-like pharmacotherapy approach for methamphetamine addiction might be other drugs that interact with monoamine transporters by inhibiting neurotransmitter uptake back into the presynaptic neuron. The functional pharmacological consequence of transporter inhibition is similar to monoamine transporter substrates in that synaptic neurotransmitter levels are increased. Monoamine transporter inhibitors are also currently FDA approved and clinically used as pharmacotherapies for a wide range of mental health disorders, including attention deficit hyperactivity disorder and depression. To date, treatments with seven different monoamine transporter inhibitors on methamphetamine-maintained behaviors have been examined in both clinical trials and human laboratory studies, and these can be broadly categorized into selective and non-selective monoamine transporter inhibitors.
First, three different DAT-selective inhibitors have been the most extensively examined: bupropion, modafanil, and methylphenidate. Both bupropion55–59 and modafanil60–62 have failed to significantly decrease methamphetamine use in clinical trials compared with placebo. Upon reanalysis of these clinical trial results, a bupropion treatment effect in light methamphetamine users, defined as self-reported methamphetamine use for 1–18 of the last 30 days, was unmasked.61 However, when a clinical trial was specifically designed to determine bupropion treatment efficacy in light methamphetamine users, bupropion treatment was not significantly different than placebo.55 Furthermore, both bupropion63 and modafanil64 treatments failed to attenuate methamphetamine self-administration in human laboratory studies. For methylphenidate, two clinical trials have reported negative results with methylphenidate65,66 and one trial67 reported a significant decrease in amphetamine-positive urines that enrolled both amphetamine and methamphetamine users. Consistent with the generally poor treatment effectiveness of DAT-selective monoamine transporter inhibitors to decrease methamphetamine use in humans, both subchronic bupropion and methylphenidate treatment also failed to attenuate methamphetamine versus food choice in monkeys.18,68 Furthermore, individual subject analysis did not reveal a therapeutic-like bupropion or methylphenidate treatment effect on methamphetamine choice.
These same three DAT-selective inhibitors have also been evaluated under other schedules of methamphetamine reinforcement. Both acute and subchronic bupropion treatments have decreased rates of methamphetamine self-administration in both rhesus monkeys51 and rats,69,70 but these treatment effects were not selective for methamphetamine- versus food-maintained responding. Acute modafanil pretreatment has also been reported to decrease rates of methamphetamine self-administration in rats,71 although behavioral selectivity was not determined. In contrast to the bupropion and modafanil results, acute methylphenidate pretreatment failed to alter methamphetamine self-administration in monkeys at doses that decreased food-maintained responding.51 In addition, two other DAT inhibitors have been examined. Similar to the D-amphetamine pretreatment results described above under a simple FR schedule of methamphetamine reinforcement, pretreatment with the DAT-selective inhibitor WIN35,428 also increased rates of methamphetamine self-administration maintained by small methamphetamine doses and decreased rates of methamphetamine self-administration maintained by intermediate methamphetamine doses.50 However, pretreatment with atypical DAT inhibitors JHW007, AHN 2-005, and AHN 1-05550 produced a downward shift in the methamphetamine self-administration dose–effect function, although behavioral selectivity was not determined. The clinical implications of these atypical inhibitors treatment effects remains to be empirically determined under other schedules of methamphetamine reinforcement, under experimental conditions that determine behavioral selectivity, and under subchronic treatment regimens.
In addition to DAT- and NET-selective inhibitors, SERT-selective inhibitors have also been examined in clinical trials. Similar to fenfluramine, the rationale for evaluation of SERT inhibitors as pharmacological treatments is founded on preclinical evidence implicating serotonergic regulation of dopaminergic neurotransmission (for review, see Refs. 53 and 54). Fluoxetine,72 paroxetine,73 and sertraline74 have all failed to attenuate methamphetamine use in clinical trials. No selective SERT inhibitor treatments in either human laboratory studies or preclinical methamphetamine versus food choice procedures have been published. However, unpublished observations from our laboratory suggest that subchronic treatment with the SERT-selective inhibitor citalopram has failed to decrease methamphetamine versus food choice in rhesus monkeys. Second, treatment with the non-selective NET and SERT inhibitor imipramine has also failed to attenuate methamphetamine use in clinical trials.75,76 There are no published studies evaluating imipramine treatment effects in either human laboratory studies or preclinical methamphetamine versus food choice procedures. However, treatment with the non-selective monoamine transporter inhibitor cocaine failed to attenuate methamphetamine versus food choice in nonhuman primates.46 These preclinical results were somewhat surprising given previous evidence that methamphetamine treatment attenuated cocaine versus food choice in monkeys77 and attenuated cocaine use in a clinical trial.78 Altogether, these human and preclinical results highlight that different pharmacological treatment approaches are necessary for treating methamphetamine versus cocaine use disorder, and comingling both disorders into a general stimulant category, despite their differences in mechanism of action, may confound interpretation.
One potential explanation for the clinical failure of monoamine transporter inhibitors to attenuate methamphetamine reinforcement may be related to chronic methamphetamine-induced neurobiological alterations. For example, human imaging studies have reported significantly lower DAT availability in methamphetamine users compared with controls.79–82 Consequently, there may be less DAT protein available for DAT inhibitors, such as bupropion, methylphenidate, or modafanil, to bind and exert their pharmacological effects. Thus, the ability of DAT inhibitors to function as an agonist-like medication may be compromised owing to chronic methamphetamine-induced neurobiological changes.
Effects of dopamine receptor antagonists and partial agonists
Pharmacological antagonists are available for the receptors that are specifically targeted by certain drugs of abuse, and in these cases, antagonists have been evaluated for their efficacy to reduce drug-maintained behavior. For example, the µ-opioid agonist heroin produces its abuse-related reinforcing effects by binding to and activating µ-opioid receptors. Although patient compliance remains a significant obstacle, the opioid antagonist naltrexone is an FDA-approved pharmacotherapy for opioid use disorders.83 Furthermore, consistent with the therapeutic efficacy of µ-opioid antagonists in detoxified heroin-dependent humans,84 subchronic treatment with another µ-opioid antagonist, naloxone, decreases heroin choice and increases food choice in non-opioid–dependent nonhuman primates.85 Methamphetamine is hypothesized to produce its abuse-related behavioral effects primarily by binding to and traversing DA transporters to induce DA release from presynaptic neurons into the synaptic cleft.1,86 Thus, methamphetamine functions as an indirect DA receptor agonist because it does not directly bind to and activate DA receptors. On the basis of the hypothesis that DA receptors mediate the abuse-related effects of methamphetamine,87,88 DA receptor antagonists have been evaluated as candidate pharmacotherapies for treating methamphetamine use disorder.
In clinical trials, DA D2 receptor antagonist–based pharmacotherapies with risperidone89–91 have failed to reduce methamphetamine use. Furthermore, a recent meta-analysis examining antipsychotic treatment efficacy for cocaine- or amphetamine-type use disorders concluded that antipsychotic compounds that function primarily as DA receptor antagonists have no treatment efficacy and were associated with significantly more adverse effects.92 In agreement with these clinical results, subchronic risperidone treatment failed to attenuate methamphetamine versus food choice in nonhuman primates.68 Moreover, targeting specific DA receptor subtypes, such as D3 receptors, has not improved the therapeutic efficacy. Although selective DA D3 receptor antagonists have not been examined in either clinical trials or human laboratory studies, both subchronic buspirone and PG01037 treatment did not attenuate methamphetamine versus food choice in nonhuman primates.93 Altogether, this literature does not support DA D2 or D3 antagonists as effective pharmacotherapies for methamphetamine use disorder.
DA receptor antagonists have also been evaluated under other schedules of methamphetamine reinforce. In contrast to the methamphetamine versus food choice study referenced above, acute PG01037 or SB-277011A pretreatment attenuated methamphetamine self-administration under a PR schedule, but not an FR, schedule of methamphetamine reinforcement94–96. However, the acute effects of PG01037 treatment were not selective for methamphetamine, as the potency of PG01037 to decrease methamphetamine- versus sucrose-maintained behavior was similar95. Overall, the lack of PG01037 treatment behavioral selectivity suggests a generalized decrease in rates of operant behavior and not a selective decrease in methamphetamine reinforcement. This interpretation is also consistent with the reported PG01037 treatment effects on methamphetamine versus food choice.
Another strategy to reduce the undesirable effects of DA receptor antagonists has been the development of DA receptor partial agonists. Treatment with the DA D2 partial agonist aripiprazole failed to decrease methamphetamine use in clinical trials67, 97, 98 and methamphetamine self-administration in a human laboratory setting under a PR procedure99. Although aripiprazole treatment has not been examined in preclinical methamphetamine versus food choice procedures, it has been examined under other schedules of reinforcement. Acute aripiprazole pretreatment decreased methamphetamine self-administration under both PR and FR schedules of reinforcement100, although behavioral selectivity was not determined. Recently, subchronic treatment with the DA D3-selective partial agonist PG619 failed to decrease methamphetamine versus food choice in nonhuman primates101. No human laboratory or clinical trials examining DA D3-selective partial agonists as treatments for methamphetamine use disorder have been published. Overall, these results do not support the clinical effectiveness of DA D2 or D3 receptor partial agonists for treating methamphetamine use disorder.
One potential explanation for the failure of DA receptor antagonists and DA receptor partial agonists to attenuate methamphetamine reinforcement may also be related to chronic methamphetamine-induced neurobiological alterations. For example, human imaging studies have reported significantly lower DA D2 receptor availability in methamphetamine addicts compared with controls,102 and the working hypothesis is that decrease in DA D2 receptors reflects mostly postsynaptic D2 receptors.103 In contrast to DA D2 receptors, nucleus accumbens DA D1 receptors were reported to be increased in methamphetamine users postmortem.104 Because all DA antagonists examined as candidate anti–methamphetamine addiction pharmacotherapies are relatively selective for DA D2 versus D1 family subtypes,105 these treatments could potentially leave DA D1 receptors unopposed for methamphetamine-induced DA to bind and activate subsequent second-messenger systems, resulting in enhanced methamphetamine reinforcement.
Effects of 5-HT antagonists
Accumulating scientific evidence has implicated the serotonergic system as one potential modulator of both mesolimbic and nigrostrial dopaminergic activity (for review, see Refs. 53 and 54). Targeting specific serotonin receptor subtypes directly implicated in mesolimbic dopaminergic activity may decrease methamphetamine reinforcement more effectively than the selective SERT inhibitor or releaser treatment results reported above. For example, subchronic treatment with a 5-HT3 antagonist decreased nucleus accumbens extracellular DA levels.106 However, treatment with the 5-HT3 antagonist ondansetron failed to decrease methamphetamine use in a clinical trial.107 Another 5-HT receptor that has been implicated in modulating dopaminergic activity is the 5-HT2A receptor. Pretreatment with a 5-HT2A antagonist has attenuated amphetamine-induced increases in extracellular DA levels in rat striatum and nucleus accumbens108,109 and the caudate nucleus in nonhuman primates.110 However, subchronic treatment with the 5-HT2A inverse agonist/antagonist pimavanserin, which was just approved by the FDA for the treatment of Parkinson-induced psychosis,111,112 failed to decrease methamphetamine versus food choice in nonhuman primates.113 Altogether, this clinical trial and methamphetamine versus food choice study does not provide compelling evidence for the clinical effectiveness of selectively targeting 5-HT3 or 5-HT2A receptors as candidate medications for methamphetamine use disorder.
Implications and future directions
Preclinical drug versus food choice procedures are an emerging methodology for evaluating subchronic candidate medication treatment effects for methamphetamine and other substance use disorders. In particular, these procedures (1) provide a simplified model of the clinical methamphetamine use disorder context with multiple concurrently available reinforcers, (2) model choice procedures utilized to assess candidate medication treatment effects in human laboratory studies, and (3) provide a dependent measure of methamphetamine-reinforcing efficacy that is less sensitive to reinforcement-independent rate-altering drug effects. In summary, this literature review suggests that preclinical drug versus food choice procedures examining subchronic pharmacological treatment effects have produced concordant results with both human laboratory drug self-administration studies and clinical trials. In contrast, more traditional drug self-administration procedures that provide rate-based measures of methamphetamine reinforcement have produced less concordant results with both human laboratory drug self-administration studies and clinical trials. Given the “growing therapeutic graveyard”114 for methamphetamine use disorder pharmacotherapy, this review highlights the rationale for utilizing preclinical methamphetamine versus food choice procedures in the development of a translational anti–methamphetamine use disorder pharmacotherapy pipeline similar to a pipeline approach that was recently described for cocaine use disorder.21
One potential future direction would be to improve our understanding of the basic behavioral pharmacology principles that determine methamphetamine versus food choice. For example, a recent methamphetamine versus food choice study in rats suggested that escalation of methamphetamine self-administration under an FR schedule of reinforcement was not predictive of methamphetamine versus food choice.37 In particular, the role of biological variables, such as sex or genotype, and environmental variables, such as social housing conditions or nondrug reinforcers other than food/sweetened liquid, as determinants of methamphetamine versus food choice are unknown. If these biological and environmental variables affected methamphetamine choice, these results would provide new and innovative research avenues to improve our understanding of methamphetamine use disorder. Moreover, the use of nondrug alternative reinforcers other than food would also potentially minimize the potential anorectic effects of both the self-administered drug and pharmacological treatments being evaluated as candidate medications.
Although no class of compounds reviewed above demonstrated a consistent and robust pharmacotherapeutic profile for methamphetamine use disorders across preclinical and human laboratory studies and clinical trials, there was a small signal of potential treatment efficacy for indirect DA agonists. Because members of this drug class function as indirect DA agonists, another future direction might be the evaluation of direct DA receptor agonists as candidate anti–methamphetamine use disorder pharmacotherapies. For example, both direct DA D1 and D2 receptor agonists produce methamphetamine-like discriminative stimulus effects in monkeys.87 These results suggest that DA D1 and D2 receptor activation is sufficient to produce methamphetamine-like effects consistent with an agonist-like pharmacological profile. To date, there are no published studies examining treatment effects with DA D1 or D2 agonists on methamphetamine self-administration in either preclinical or human laboratory studies. Based on the scientific literature discussed in this review, preclinical experimental designs that incorporate both subchronic treatment regimens and metrics of methamphetamine reinforcement using a methamphetamine choice procedure would appear to produce the best translationally relevant results.
Acknowledgments
The author is supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Number R01 DA031718. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of interest
The author has no conflicts of interest to declare.
References
- 1.Rothman RB, et al. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 2.Simmler LD, et al. Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol. 2013;168:458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freyberg Z, et al. Mechanisms of amphetamine action illuminated through optical monitoring of dopamine synaptic vesicles in drosophila brain. Nat Commun. 2016;7:10652. doi: 10.1038/ncomms10652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baumann MH, et al. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37:1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gough B, et al. Comparative effects of substituted amphetamines (PMA, MDMA, and METH) on monoamines in rat caudate. Ann N Y Acad Sci. 2002;965:410–420. doi: 10.1111/j.1749-6632.2002.tb04182.x. [DOI] [PubMed] [Google Scholar]
- 7.Carney JM, et al. Establishment of chronic intravenous drug self-administration in the C57BL/6J mouse. Neuroreport. 1991;2:477–480. doi: 10.1097/00001756-199108000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Yokel RA, Pickens R. Self-administration of optical isomers of amphetamine and methylamphetamin by rats. J Pharmacol Exp Ther. 1973;187:27–33. [PubMed] [Google Scholar]
- 9.Balster RL, Kilbey MM, Ellinwood EH., Jr Methamphetamine self-administration in the cat. Psychopharmacologia. 1976;46:229–233. doi: 10.1007/BF00421107. [DOI] [PubMed] [Google Scholar]
- 10.Deneau G, Yanagita T, Seevers MH. Self-administration of psychoactive substances by the monkey. Psychopharmacologia. 1969;16:30–48. doi: 10.1007/BF00405254. [DOI] [PubMed] [Google Scholar]
- 11.Balster RL, Schuster CR. A comparison of d-aphetamine, l-amphetamine, and methamphetamine self-administration in rhesus monkeys. Pharmacol Biochem Behav. 1973;1:67–71. doi: 10.1016/0091-3057(73)90057-9. [DOI] [PubMed] [Google Scholar]
- 12.Hart CL, et al. Methamphetamine self-administration by humans. Psychopharmacology. 2001;157:75. doi: 10.1007/s002130100738. [DOI] [PubMed] [Google Scholar]
- 13.UNODC. World Drug Report 2015. United Nations Office on Drugs and Crime; Vienna: 2015. [Google Scholar]
- 14.DEA. National Foreensic Laboratory Information System: 2014 Annual Report. Department of Justice Drug Enforcement Administration Springfield; VA: 2015. [Google Scholar]
- 15.Brensilver M, Heinzerling KG, Shoptaw S. Pharmacotherapy of amphetamine-type stimulant dependence: An update. Drug and Alcohol Rev. 2013;32:449–460. doi: 10.1111/dar.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haney M, Spealman R. Controversies in translational research: drug self-administration. Psychopharmacology. 2008;199:403–419. doi: 10.1007/s00213-008-1079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Comer SD, et al. The role of human drug self-administration procedures in the development of medications. Drug Alcohol Depend. 2008;96:1–15. doi: 10.1016/j.drugalcdep.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banks ML, et al. Use of Preclinical Drug Vs. Food Choice Procedures to Evaluate Candidate Medications for Cocaine Addiction. Curr Treat Options Psych. 2015;2:136–150. doi: 10.1007/s40501-015-0042-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Negus SS, Banks ML. Medications Development for Opioid Abuse. Cold Spring Harb Perspect Med. 2013;3:a012104. doi: 10.1101/cshperspect.a012104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banks ML, et al. Preclinical assessment of lisdexamfetamine as an agonist medication candidate for cocaine addiction: Effects in rhesus monkeys trained to discriminate cocaine or to self-administer cocaine in a cocaine versus food choice procedure. Int J Neuropsychopharmacol. 2015;18 doi: 10.1093/ijnp/pyv009. pii: pyv009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Czoty PW, Stoops WW, Rush CR. Evaluation of the “Pipeline” for development of medications for cocaine use disorder: A review of translational preclinical, human laboratory, and clinical trial research. Pharmacol Rev. 2016;68:533–562. doi: 10.1124/pr.115.011668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mello NK, Negus SS. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology. 1996;14:375–424. doi: 10.1016/0893-133X(95)00274-H. [DOI] [PubMed] [Google Scholar]
- 23.Banks ML, Negus SS. Preclinical determinants of drug choice under concurrent schedules of drug self-administration. Adv Pharmacol Sci. 2012;2012:281768. doi: 10.1155/2012/281768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bergman J, Paronis CA. Measuring the reinforcing strength of abused drugs. Mol Interv. 2006;6:273–283. doi: 10.1124/mi.6.5.9. [DOI] [PubMed] [Google Scholar]
- 25.Heyman GH. Addiction: A disorder of choice. Harvard University Press; Cambridge: 2009. [Google Scholar]
- 26.Ahmed SH. Validation crisis in animal models of drug addiction: Beyond non-disordered drug use toward drug addiction. Neurosci Biobehav Rev. 2010;35:172–184. doi: 10.1016/j.neubiorev.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Association, American Pyschiatric. Diagnositc and Statistical Manual of Mental Disorders. Fifth. American Psychiatric Association; Arlington, VA: 2013. [Google Scholar]
- 28.Vocci FJ. Can replacement therapy work in the treatment of cocaine dependence? And what are we replacing anyway? Addiction. 2007;102:1888–1889. doi: 10.1111/j.1360-0443.2007.02014.x. [DOI] [PubMed] [Google Scholar]
- 29.Lile JA, et al. Development of a translational model to screen medications for cocaine use disorder II: Choice between intravenous cocaine and money in humans. Drug Alcohol Depend. 2016;165:111–119. doi: 10.1016/j.drugalcdep.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson AR, et al. Development of a translational model to screen medications for cocaine use disorder I: Choice between cocaine and food in rhesus monkeys. Drug Alcohol Depend. 2016;165:103–110. doi: 10.1016/j.drugalcdep.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foltin RW, et al. Development of translational preclinical models in substance abuse: Effects of cocaine administration on cocaine choice in humans and non-human primates. Pharmacol Biochem Behav. 2015;134:12–21. doi: 10.1016/j.pbb.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woolverton WL, Johanson CE. Preference in rhesus monkeys given a choice between cocaine and d,l-cathinone. J Exp Anal Behav. 1984;41:35–43. doi: 10.1901/jeab.1984.41-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banks ML, et al. Relationship between response rates and measures of reinforcing strength using a choice procedure in monkeys. Behav Pharmacol. 2008;19:365–369. doi: 10.1097/FBP.0b013e32830990bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woolverton WL, Balster RL. Effects of antipsychotic compounds in rhesus monkeys given a choice between cocaine and food. Drug Alcohol Depend. 1981;8:69–78. doi: 10.1016/0376-8716(81)90088-0. [DOI] [PubMed] [Google Scholar]
- 35.Griffiths RR, Wurster RM, Brady JV. Discrete-trial choice procedure: Effects of naloxone and methadone on choice between food and heroin. Pharmacol Rev. 1975;27:357–365. [PubMed] [Google Scholar]
- 36.Wurster RM, et al. Reduction of heroin self-administration in baboons by manipulation of behavioral and pharmacological conditions. Pharmacol Biochem Behav. 1977;7:519–528. doi: 10.1016/0091-3057(77)90248-9. [DOI] [PubMed] [Google Scholar]
- 37.Caprioli D, et al. Persistent palatable food preference in rats with a history of limited and extended access to methamphetamine self-administration. Addiction Biol. 2015;20:913–926. doi: 10.1111/adb.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dole VP, Nyswander ME, Kreek M. Narcotic blockade. Arch Intern Med. 1966;118:304–309. [PubMed] [Google Scholar]
- 39.Bell J. Pharmacological maintenance treatments of opiate addiction. Br J Clin Pharmacol. 2014;77:253–263. doi: 10.1111/bcp.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elrashidi MY, Ebbert JO. Emerging drugs for the treatment of tobacco dependence: 2014 update. Expert Opin Emerg Drugs. 2014;19:243–260. doi: 10.1517/14728214.2014.899580. [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez-Menchaca AA, et al. S(+)amphetamine induces a persistent leak in the human dopamine transporter: molecular stent hypothesis. Br J Pharmacol. 2012;165:2749–2757. doi: 10.1111/j.1476-5381.2011.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galloway GP, et al. A randomized, placebo-controlled trial of sustained-release dextroamphetamine for treatment of methamphetamine addiction. Clin Pharmacol Ther. 2011;89:276–282. doi: 10.1038/clpt.2010.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Longo M, et al. Randomized controlled trial of dexamphetamine maintenance for the treatment of methamphetamine dependence. Addiction. 2010;105:146–154. doi: 10.1111/j.1360-0443.2009.02717.x. [DOI] [PubMed] [Google Scholar]
- 44.Shearer J, et al. Pilot randomized controlled study of dexamphetamine substitution for amphetamine dependence. Addiction. 2001;96:1289–1296. doi: 10.1046/j.1360-0443.2001.96912898.x. [DOI] [PubMed] [Google Scholar]
- 45.Pike E, et al. Methamphetamine self-administration in humans during D-amphetamine maintenance. J Clin Psychopharmacol. 2014;34:675–681. doi: 10.1097/JCP.0000000000000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwienteck KL, Banks ML. Effects of 7-day continuous d-amphetamine, methylphenidate, and cocaine treatment on choice between methamphetamine and food in male rhesus monkeys. Drug Alcohol Depend. 2015;155:16–23. doi: 10.1016/j.drugalcdep.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nader MA. Chapter 1 - Animal models for addiction medicine: From vulnerable phenotypes to addicted individuals. In: Hamed E, Martin PP, editors. Prog Brain Res. Vol. 224. Elsevier; 2016. pp. 3–24. [DOI] [PubMed] [Google Scholar]
- 48.Sherman JP. Dexamphetamine for “speed” addiction. Med J Australia. 1990;153:306. doi: 10.5694/j.1326-5377.1990.tb136930.x. [DOI] [PubMed] [Google Scholar]
- 49.Charnaud B, Griffiths V. Levels of intravenous drug misuse among clients prescribed oral dexamphetamine or oral methadone: a comparison. Drug Alcohol Depend. 1998;52:79–84. doi: 10.1016/s0376-8716(98)00052-0. [DOI] [PubMed] [Google Scholar]
- 50.Hiranita T, et al. Preclinical efficacy of N-substituted benztropine analogs as antagonists of methamphetamine self-administration in rats. J Pharmacol Exp Ther. 2014;348:174–191. doi: 10.1124/jpet.113.208264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schindler CW, et al. Comparison of the effects of methamphetamine, bupropion, and methylphenidate on the self-administration of methamphetamine by rhesus monkeys. Exp Clin Psychopharmacol. 2011;19:1–10. doi: 10.1037/a0022432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Munzar P, et al. Effects of dopamine and serotonin-releasing agents on methamphetamine discrimination and self-administration in rats. Psychopharmacology. 1999;141:287. doi: 10.1007/s002130050836. [DOI] [PubMed] [Google Scholar]
- 53.Alex KD, Pehek EA. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol Ther. 2007;113:296–320. doi: 10.1016/j.pharmthera.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Howell LL, Cunningham KA. Serotonin 5-HT2 receptor interactions with dopamine function: Implications for therapeutics in cocaine use disorder. Pharmacol Rev. 2015;67:176–197. doi: 10.1124/pr.114.009514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anderson AL, et al. Bupropion for the treatment of methamphetamine dependence in non-daily users: A randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2015;150:170–174. doi: 10.1016/j.drugalcdep.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heinzerling KG, et al. Pilot randomized trial of bupropion for adolescent methamphetamine abuse/dependence. Journal of Adolesc Health. 2013;52:502–505. doi: 10.1016/j.jadohealth.2012.10.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heinzerling KG, et al. Randomized, placebo-controlled trial of bupropion in methamphetamine-dependent participants with less than daily methamphetamine use. Addiction. 2014;109:1878–1886. doi: 10.1111/add.12636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elkashef AM, et al. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33:1162–1170. doi: 10.1038/sj.npp.1301481. [DOI] [PubMed] [Google Scholar]
- 59.Shoptaw S, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2008;96:222–232. doi: 10.1016/j.drugalcdep.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heinzerling KG, et al. Randomized, double-blind, placebo-controlled trial of modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2010;109:20–29. doi: 10.1016/j.drugalcdep.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anderson AL, et al. Modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2012;120:135–141. doi: 10.1016/j.drugalcdep.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shearer J, et al. A double-blind, placebo-controlled trial of modafinil (200 mg/day) for methamphetamine dependence. Addiction. 2009;104:224–233. doi: 10.1111/j.1360-0443.2008.02437.x. [DOI] [PubMed] [Google Scholar]
- 63.Stoops WW, et al. Naltrexone and bupropion, alone or combined, do not alter the reinforcing effects of intranasal methamphetamine. Pharmacol Biochem Behav. 2015;129:45–50. doi: 10.1016/j.pbb.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De La Garza R, et al. Evaluation of modafinil effects on cardiovascular, subjective, and reinforcing effects of methamphetamine in methamphetamine-dependent volunteers. Drug Alcohol Depend. 2010;106:173–180. doi: 10.1016/j.drugalcdep.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miles SW, et al. Extended-release methylphenidate for treatment of amphetamine/methamphetamine dependence: a randomized, double-blind, placebo-controlled trial. Addiction. 2013;108:1279–1286. doi: 10.1111/add.12109. [DOI] [PubMed] [Google Scholar]
- 66.Ling W, et al. Sustained-release methylphenidate in a randomized trial of treatment of methamphetamine use disorder. Addiction. 2014;109:1489–1500. doi: 10.1111/add.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tiihonen J, et al. A comparison of aripiprazole, methylphenidate, and placebo for amphetamine dependence. Am J Psychiatry. 2007;164:160–162. doi: 10.1176/ajp.2007.164.1.160. [DOI] [PubMed] [Google Scholar]
- 68.Banks ML, Blough BE. Effects of environmental maniuplations and bupropion and risperidone treatments on choice between methamphetamine and food in rhesus monkeys. Neuropsychoharmacology. 2015;40:2198–2206. doi: 10.1038/npp.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reichel CM, Linkugel JD, Bevins RA. Bupropion differentially impacts acquisition of methamphetamine self-administration and sucrose-maintained behavior. Pharmacol Biochem Behav. 2008;89:463–472. doi: 10.1016/j.pbb.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reichel CM, et al. Bupropion attenuates methamphetamine self-administration in adult male rats. Drug Alcohol Depend. 2009;100:54–62. doi: 10.1016/j.drugalcdep.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reichel CM, See RE. Chronic modafinil effects on drug-seeking following methamphetamine self-administration in rats. Int J Neuropsychopharmacol. 2012;15:919–929. doi: 10.1017/S1461145711000988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Batki SL, et al. Methamphetamine quantitative urine concentrations during a controlled trial of fluoxetine treatment: Preliminary analysis. Ann N Y Acad Sci. 2000;909:260–263. doi: 10.1111/j.1749-6632.2000.tb06688.x. [DOI] [PubMed] [Google Scholar]
- 73.Piasecki MP, et al. An exploratory study: The use of paroxetine for methamphetamine craving. J Psychoactive Drugs. 2002;34:301–304. doi: 10.1080/02791072.2002.10399967. [DOI] [PubMed] [Google Scholar]
- 74.Heinzerling KG, et al. Randomized, placebo-controlled trial of baclofen and gabapentin for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2006;85:177–184. doi: 10.1016/j.drugalcdep.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 75.Galloway GP, et al. A controlled trial of imipramine for the treatment of methamphetamine dependence. J Subst Abuse Treat. 1996;13:493–497. doi: 10.1016/s0740-5472(96)00154-7. [DOI] [PubMed] [Google Scholar]
- 76.Galloway GP, et al. Imipramine for the treatment of cocaine and methamphetamine dependence. J Addict Dis. 1994;13:201–216. doi: 10.1300/j069v13n04_08. [DOI] [PubMed] [Google Scholar]
- 77.Banks ML, Blough BE, Negus SS. Effects of monoamine releasers with varying selectivity for releasing dopamine/norepinephrine versus serotonin on choice between cocaine and food in rhesus monkeys. Behav Pharmacol. 2011;22:824–836. doi: 10.1097/FBP.0b013e32834d63ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mooney ME, et al. Effects of oral methamphetamine on cocaine use: A randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2009;101:34–41. doi: 10.1016/j.drugalcdep.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Volkow ND, et al. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- 80.Volkow ND, et al. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Volkow ND, et al. Recovery of dopamine transporters with methamphetamine detoxification is not linked to changes in dopamine release. NeuroImage. 2015;121:20–28. doi: 10.1016/j.neuroimage.2015.07.035. [DOI] [PubMed] [Google Scholar]
- 82.Sekine Y, et al. Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. Am J Psychiatry. 2001;158:1206–1214. doi: 10.1176/appi.ajp.158.8.1206. [DOI] [PubMed] [Google Scholar]
- 83.Comer SD, Sullivan MA, Hulse GK. Sustained-release naltrexone: novel treatment for opioid dependence. Expert Opin Investig Drugs. 2007;16:1285–1294. doi: 10.1517/13543784.16.8.1285. [DOI] [PubMed] [Google Scholar]
- 84.Comer SD, et al. Injectable, sustained-release naltrexone for the treatment of opioid dependence: A randomized, placebo-controlled trial. Arch Gen Psychiatry. 2006;63:210–218. doi: 10.1001/archpsyc.63.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Negus SS. Choice between heroin and food in nondependent and heroin-dependent rhesus monkeys: Effects of naloxone, buprenorphine, and methadone. J Pharmacol Exp Ther. 2006;317:711–723. doi: 10.1124/jpet.105.095380. [DOI] [PubMed] [Google Scholar]
- 86.Goodwin JS, et al. Amphetamine and methamphetamine differentially affect dopamine transporters in vitro and in vivo. J Biol Chem. 2009;284:2978–2989. doi: 10.1074/jbc.M805298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tidey JW, Bergman J. Drug discrimination in methamphetamine-trained monkeys: Agonist and antagonist effects of dopaminergic drugs. J Pharmacol Exp Ther. 1998;285:1163–1174. [PubMed] [Google Scholar]
- 88.Wise RA. Roles for nigrostriatal—not just mesocorticolimbic—dopamine in reward and addiction. Trends Neurosci. 2009;32:517–524. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meredith CW, et al. An open-label pilot study of risperidone in the treatment of methamphetamine dependence. J Psychoactive Drugs. 2007;39:167–172. doi: 10.1080/02791072.2007.10399875. [DOI] [PubMed] [Google Scholar]
- 90.Meredith CW, et al. Open trial of injectable risperidone for methamphetamine dependence. J Addict Med. 2009;3:55–65. doi: 10.1097/ADM.0b013e31818e2185. [DOI] [PubMed] [Google Scholar]
- 91.Nejtek VA, et al. Do atypical antipsychotics effectively treat co-occurring bipolar disorder and stimulant dependence? A randomized, double-blind trial. J Clin Psychiatry. 2008;69:1257–1266. doi: 10.4088/jcp.v69n0808. [DOI] [PubMed] [Google Scholar]
- 92.Kishi T, et al. Antipsychotics for cocaine or psychostimulant dependence: systematic review and meta-analysis of randomized, placebo-controlled trials. J Clin Psychiatry. 2013;74:e1169–1180. doi: 10.4088/JCP.13r08525. [DOI] [PubMed] [Google Scholar]
- 93.John WS, Newman AH, Nader MA. Differential effects of the dopamine D3 receptor antagonist PG01037 on cocaine and methamphetamine self-administration in rhesus monkeys. Neuropharmacology. 2015;92:34–43. doi: 10.1016/j.neuropharm.2014.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Orio L, et al. The dopamine D3 receptor partial agonist CJB090 and antagonist PG01037 decrease progressive ratio responding for methamphetamine in rats with extended-access. Addiction Biol. 2010;15:312–323. doi: 10.1111/j.1369-1600.2010.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Higley AE, et al. PG01037, a novel dopamine D3 receptor antagonist, inhibits the effects of methamphetamine in rats. J Psychopharmacology. 2011;25:263–273. doi: 10.1177/0269881109358201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Higley AE, et al. Dopamine D3 receptor antagonist SB-277011A inhibits methamphetamine self-administration and methamphetamine-induced reinstatement of drug-seeking in rats. Eur J Pharmacol. 2011;659:187–192. doi: 10.1016/j.ejphar.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Coffin PO, et al. Aripiprazole for the treatment of methamphetamine dependence: a randomized, double-blind, placebo-controlled trial. Addiction. 2013;108:751–761. doi: 10.1111/add.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sulaiman AH, et al. A randomized, placebo-controlled trial of aripiprazole for the treatment of methamphetamine dependence and associated psychosis. Int J Psychiatry Clin Pract. 2013;17:131–138. doi: 10.3109/13651501.2012.667116. [DOI] [PubMed] [Google Scholar]
- 99.Stoops WW, et al. Influence of aripiprazole pretreatment on the reinforcing effects of methamphetamine in humans. Prog Neuropsychopharmacol Biol Psychiatry. 2013;47:111–117. doi: 10.1016/j.pnpbp.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wee S, et al. Effect of aripiprazole, a partial dopamine D2 receptor agonist, on increased rate of methamphetamine self-administration in rats with prolonged session duration. Neuropsychopharmacology. 2007;32:2238–2247. doi: 10.1038/sj.npp.1301353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.John WS, et al. Effects of buspirone and the dopamine D3 receptor compound PG619 on cocaine and methamphetamine self-administration in rhesus monkeys using a food-drug choice paradigm. Psychopharmacology. 2015;232:1279–1289. doi: 10.1007/s00213-014-3760-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Volkow ND, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: Association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- 103.Hume SP, et al. The potential of high-resolution positron emission tomography to monitor striatal dopaminergic function in rat models of disease. J Neurosci Methods. 1996;67:103–112. [PubMed] [Google Scholar]
- 104.Worsley JN, et al. Dopamine D1 receptor protein is elevated in nucleus accumbens of human, chronic methamphetamine users. Mol Psychiatry. 2000;5:664–672. doi: 10.1038/sj.mp.4000760. [DOI] [PubMed] [Google Scholar]
- 105.Lako IM, et al. Estimating dopamine D(2) receptor occupancy for doses of 8 antipsychotics: a meta-analysis. J Clin Psychopharmacology. 2013;33:675–681. doi: 10.1097/JCP.0b013e3182983ffa. [DOI] [PubMed] [Google Scholar]
- 106.Liu W, Thielen RJ, McBride WJ. Effects of repeated daily treatments with a 5-HT3 receptor antagonist on dopamine neurotransmission and functional activity of 5-HT3 receptors within the nucleus accumbens of Wistar rats. Pharmacol Biochem Behav. 2006;84:370–377. doi: 10.1016/j.pbb.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 107.Johnson BA, et al. A preliminary randomized, double-blind, placebo-controlled study of the safety and efficacy of ondansetron in the treatment of methamphetamine dependence. Int J Neuropsychopharmacol. 2008;11:1–14. doi: 10.1017/S1461145707007778. [DOI] [PubMed] [Google Scholar]
- 108.Auclair A, et al. Role of serotonin2A receptors in the d-amphetamine-induced release of dopamine: comparison with previous data on α1b-adrenergic receptors. J Neurochem. 2004;91:318–326. doi: 10.1111/j.1471-4159.2004.02714.x. [DOI] [PubMed] [Google Scholar]
- 109.Porras G, et al. 5-HT2A and 5-HT2C/2B receptor subtypes modulate dopamine release induced in vivo by amphetamine and morphine in both the rat nucleus accumbens and striatum. Neuropsychopharmacology. 2002;26:311–324. doi: 10.1016/S0893-133X(01)00333-5. [DOI] [PubMed] [Google Scholar]
- 110.Murnane KS, et al. Selective serotonin 2A receptor antagonism attenuates the effects of amphetamine on arousal and dopamine overflow in non-human primates. J Sleep Res. 2013;22:581–588. doi: 10.1111/jsr.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cummings J, et al. Pimavanserin for patients with parkinson’s disease psychosis: A randomised, placebo-controlled phase 3 trial. Lancet. 2014;383:533–540. doi: 10.1016/S0140-6736(13)62106-6. [DOI] [PubMed] [Google Scholar]
- 112.Walsh S. FDA approves first drug to treat hallucinations and delusions associated with Parkinson’s disease. 2016;2016 [Google Scholar]
- 113.Banks ML. Effects of 7-day repeated treatment with the 5-HT2A inverse agonist/antagonist pimavanserin on methamphetamine vs. food choice in male rhesus monkeys. Drug Alcohol Depend. 2016;165:260–264. doi: 10.1016/j.drugalcdep.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Carson DS, Taylor ER. Commentary on heinzerling et al. (2014): A growing methamphetamine dependence therapeutics graveyard. Addiction. 2014;109:1887–1888. doi: 10.1111/add.12709. [DOI] [PubMed] [Google Scholar]


