Abstract
Background
Vitamin D insufficiency (a serum 25(OH)D < 30 ng/ml) has been associated with asthma morbidity.
Objective
To examine vitamin D insufficiency, asthma and lung function among U.S. children and adults.
Methods
Using data from NHANES for 2001–2010, we examined vitamin D insufficiency and: 1) current asthma or wheeze in 10,860 children (6–17 years) and 24,115 adults (18–79 years), and 2) lung function in a subset of participants. Logistic or linear regression was used for the multivariable analysis, adjusting for age, gender, race/ethnicity, income, body mass index, smoking, and C-reactive protein level.
Results
Vitamin D insufficiency was associated with current asthma (odds ratio [OR]=1.35, 95% confidence interval [CI]=1.11–1.64) and current wheeze in children, as well as with current wheeze in adults (OR=1.17, 95%CI=1.04–1.31). After stratifying the analysis by race/ethnicity and (in adults) current smoking, vitamin D insufficiency was associated with current asthma and wheeze in non-Hispanic white children only; in adults, vitamin D insufficiency was associated with current wheeze in non-Hispanic whites and blacks. Vitamin D insufficiency was also associated with lower FEV1 and FVC in children and adults. When analyzing each NHANES wave separately, vitamin D insufficiency prevalence was 72%–76% from 2001 to 2006, and then decreased from 2007 to 2010 (64%–65%); interestingly, asthma prevalence decreased for the first time from 2007–2008 (8.2%) to 2009–2010 (7.4%).
Conclusions
We show racial/ethnic-specific associations between vitamin D insufficiency and current asthma or wheeze in children and adults. Moreover, we report parallel recent decrements in the prevalence of vitamin D insufficiency and asthma.
Keywords: Vitamin D insufficiency, Asthma, Wheezing, Lung function, NHANES
INTRODUCTION
Asthma is a common respiratory disease and a significant public health problem worldwide. In the United States (U.S.), the prevalence of asthma increased from 3.1% in 1980 to 8.4% in 20101. The causes of this “asthma epidemic” are unclear but likely to be due to changes in the environment or lifestyle. Vitamin D status, which is assessed by measurement of serum or plasms 25-hydroxyvitamin D (25[OH]D), is key to the metabolism of calcium and phosphorus. While the definition of vitamin D deficiency or insufficiency on the basis of 25(OH)D values is still under debate2, vitamin D insufficiency, defined as a serum 25-hydroxyvitamin D3 (25[OH]D) < 30 ng/ml, is now commonly found in children3 and adults4 in the U.S., likely due to reduced outdoor activity (and thus sun exposure) over the last few decades. Since vitamin D insufficiency is particularly frequent among members of demographic groups at high risk for asthma, such as African Americans, Puerto Ricans and obese individuals5,6, there has been considerable recent interest in exploring a potential role of vitamin D in the pathogenesis of asthma.
Experimental findings and results from epidemiologic studies have provided suggestive but inconclusive evidence for vitamin D insufficiency as a cause of asthma or morbidity from asthma, with generally more consistent findings of a possible link in children than in adults7–16. Such data provided support for two ongoing randomized clinical trials (RCTs) of prenatal vitamin D for the prevention of childhood asthma17,18. In the largest of those two trials (n=876), children born to women who received vitamin D at 4,400 IU/day during pregnancy had a lower incidence of asthma and recurrent wheeze at age 3 years than those born to women who received 400 IU/day of vitamin D during pregnancy (24.3% vs. 30.4%), but this difference (6.1%) was not statistically significant (P=0.05)17. However, the study lacked sufficient statistical power to detect an effect ≤20%17, and longer follow-up of the participating children is ongoing to determine whether the observed effect of vitamin D on asthma is persistent and clinically important. With regard to asthma morbidity, two RCTs have reported that vitamin D did not reduce the rate of first treatment failure or exacerbation among adults with persistent asthma and vitamin D insufficiency19,20.
However, the larger of those two trials reported that adults in the vitamin D arm received a significantly lower dose of inhaled corticosteroids than those in the placebo arm, suggesting steroid-enhancing effects of vitamin D. Moreover, an exploratory analysis in that trial showed that adults who achieved vitamin D sufficiency while in the intervention arm had a significantly lower risk of severe asthma exacerbations than those in the placebo group21. Small clinical trials in children have shown supportive but inconclusive evidence of beneficial effects of vitamin D in preventing asthma exacerbations21,22 and improving asthma control23,24, and a larger clinical trial in U.S. school-aged children is ongoing (NCT02687815, PI: Celedón JC).
Given continued controversy and a paucity of large-scale studies, we examined the relationship between vitamin D insufficiency and: current asthma, current wheeze, and lung function among children and adults who participated in the U.S. National Health and Nutrition Examination Survey (NHANES) from 2001 to 2010. Moreover, we examined temporal trends for vitamin D insufficiency and current asthma during the study period.
METHODS
Subject recruitment and study procedures
NHANES is a cross-sectional nationwide survey of a representative sample of the U.S. population. As part of the study design, ethnic minorities (African Americans, Asians and Mexican Americans) and individuals 60 years and older are over-sampled, to achieve adequate statistical power for data analysis in these groups.
Serum 25(OH)D level was measured in study participants at the National Center for Environmental Health, using the DiaSorin RIA kit (Stillwater MN) for NHANES 2001–2006, and liquid chromatography-tandem mass spectrometry (LC-MS/MS) for the NHANES 2007–2010 data. In order to allow comparison of standardized 25(OH)D levels across the two study periods, a variety of regression equations were developed to convert NHANES 2001–2006 RIA measurements to a standardized LC-MS/MS-equivalent 25(OH)D level25. Serum total 25(OH)D (in SI units of nmol/L) was defined as the sum of 25(OH)D3 and 25(OH)D2 and then converted to conventional units (ng/mL) using the suggested conversion factors: 1 nmol/L = 0.40066 ng/mL25.
Spirometry was available in NHANES 2007–2008 and 2009–2010. Participants were not eligible for spirometry if they were on supplemental oxygen or had painful ear infections, current chest pain or a physical problem with forceful expiration, surgery (of the eye, chest or the abdomen) in the prior 3 months, heart disease, history of an aneurysm or a detached retina, hemoptysis, or history of a collapsed lung or tuberculosis exposure. Eligible participants performed spirometry following American Thoracic Society (ATS) recommendations26. The best forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) were selected for analysis. Participants whose baseline FEV1/FVC ratio was below the lower limit of normal (LLN)27,28 and/or whose baseline FEV1 was below 70% of the predicted value for their demographic characteristics underwent a repeat spirometry, 15 minutes after inhalation of albuterol. Participants were excluded from bronchodilator administration if they had recently used a short-acting inhaled β2-agonist or had a previous adverse reaction to albuterol; had a history of congenital heart disease, hypertension, major arrhythmia or an implanted defibrillator; or were pregnant or breastfeeding. Age-, gender-, and race/ethnicity-specific percent predicted (%predicted) values of FEV1, FVC, and FEV1/FVC were calculated adopting normal equations for spirometric parameters of the general US population27.
Informed consent was obtained from all study participants. NHANES was approved by the institutional review board of the National Center for Health Statistics of the Center for Disease Control and Prevention (CDC). Further details on study measurements and procedures may be found in the NHANES website (http://www.cdc.gov/nchs/nhanes.htm); specific details on spirometry and bronchodilator administration can be found in the NHANES spirometry procedures manual29.
Statistical analysis
Vitamin D insufficiency was defined as a serum 25(OH)D <30 ng/ml. Current asthma was defined by a positive answer to both of the following questions: “Has a doctor or other health professional ever told you that you have asthma?”, and “Do you still have asthma?”. Participants who answered no to both questions were selected as controls. Current wheeze was defined by a positive answer to the question “In the past 12 months, have you had wheezing or whistling in your chest?”. Bronchodilator response (BDR) was defined as: ([post-bronchodilator FEV1 − pre-bronchodilator FEV1]/pre-bronchodilator FEV1) × 100.
Sampling weights, stratification, and clusters provided in the NHANES dataset were incorporated into the analysis, in order to account for the complex NHANES survey design and obtain proper estimates and their standard errors. Wald chi-square tests and t-tests were used for bivariate analyses of binary and continuous variables, respectively. In children, body mass index (BMI) z-scores were calculated based on the 2000 U.S. CDC growth charts30. Logistic regression was used for the multivariable analysis of vitamin D insufficiency and current asthma, current wheeze and BDR. Linear regression was used for the multivariable analysis of vitamin D insufficiency and lung function measures (FEV1, FVC, and FEV1/FVC). All multivariable models were adjusted for age, gender, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, or other), household income (<$20,000/year or ≥$20,000/year), BMI (for adults) or BMI z-score (for children), and serum levels of cotinine and C-reactive protein (CRP). Multivariable models of BDR were additionally adjusted for baseline (pre-bronchodilator) FEV1. All statistical analyses were conducted using the SAS SURVEY procedure and SAS 9.3 software (SAS Institute Inc., Cary, NC).
RESULTS
During the study period (2001 to 2010), a total of 10,860 children aged 6 to 17 years and 24,115 adults aged 18 to 79 years who had serum vitamin D measures and information on asthma status were included in the current analysis. Of these participants, 3,001 children and 8,347 adults from NHANES 2007–2010 also had baseline spirometry data; 234 (7.8%) of these children and 744 (8.9%) of these adults had a BDR measurement.
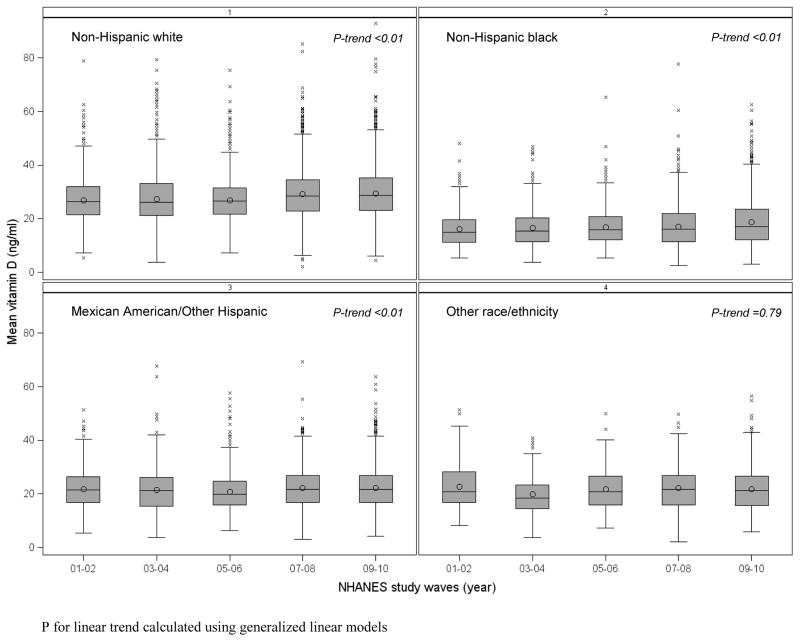
The overall prevalence of current asthma among study participants was 10.9% in children and 7.6% in adults. The main characteristics of participants are shown in Table 1. Compared to children without asthma (n=9,668), those with asthma (n=1,192) were more likely to be non-Hispanic Black and to have health insurance coverage, a higher BMI z-score, a lower serum vitamin D level and a higher prevalence of vitamin D insufficiency, lower FEV1/FVC, and higher BDR. Compared to adults without asthma (n=22,286), those with asthma (n=1,829) were more likely to be female and non-Hispanic white or black, and to have: health insurance coverage, an annual household income <$20,000, higher BMI, higher levels of serum cotinine and C-reactive protein, lower FEV1 and FVC, lower FEV1/FVC, and higher BDR. Vitamin D insufficiency was common in both children and adults (68% to 75%), and most common in non-Hispanic blacks (94% to 97%). Figure 1 shows vitamin D levels by race/ethnicity for each NHANES study wave (2001–2002 to 2009–2010). Overall, non-Hispanic blacks had lower vitamin D level (mean = 16.0–18.6 ng/ml) than other racial or ethnic groups. Moreover, an upward trend of increased vitamin D levels, specifically in the periods 2007–2008 and 2009–2010, was seen in the U.S. population.
Table 1.
Characteristics of participants in NHANES 2001–2010, by current asthma status
| Characteristics | Children 6–17 years | Adults 18–79 years | ||
|---|---|---|---|---|
|
| ||||
| Current asthma
| ||||
| No n=9,668 |
Yes n=1,192 |
No n=22,286 |
Yes n=1,829 |
|
| Age (years) | 11.7 ± 0.1 | 12.1 ± 0.1 | 44.1 ± 0.2 | 44.2 ± 0.5 |
| Male gender | 4833 (51.2) | 655 (53.9) | 11046 (49.8) | 662 (36.3)‡ |
| Race/ethnicity | ||||
| Non-Hispanic white | 2779 (60.1) | 316 (56.0)‡ | 10327 (70.1) | 967 (74.2)‡ |
| Non-Hispanic Black | 2737 (13.6) | 465 (20.8) | 4560 (10.8) | 433 (12.4) |
| Mexican American/other Hispanic | 3685 (19.6) | 352 (17.7) | 4619 (13.4) | 349 (8.4) |
| Other | 467 (6.6) | 59 (5.1) | 980 (5.7) | 80 (5.0) |
| Health insurance coverage (yes) | 8041 (88.0) | 1083 (94.0)‡ | 16672 (80.1) | 1507 (85.5)‡ |
| Annual household income < $20,000 | 2169 (16.3) | 288 (17.1) | 4680 (14.8) | 518 (21.1)‡ |
| Body mass index (kg/m2) | 20.8 (0.1) | 22.2 (0.2)‡ | 28.2 ± 0.1 | 30.5 ± 0.3‡ |
| BMI z-score | 0.58 (0.02) | 0.88 (0.04)‡ | - | - |
| Serum cotinine level (ng/ml) | 6.7 ± 0.5 | 9.5 ± 2.0 | 63.2 ± 2.1 | 72.0 ± 4.0‡ |
| Current smoker | - | - | 4723 (23.8) | 453 (26.8) |
| Serum C-reactive protein (mg/dl) | 0.14 ± 0.01 | 0.18 ± 0.02 | 0.39 ± 0.01 | 0.53 ± 0.02‡ |
| Serum vitamin D level (ng/ml) | 26.9 ± 0.1 | 25.7 ± 0.4† | 25.7 ± 0.2 | 25.0 ± 0.3 |
| Vitamin D insufficiency (<30 ng/ml) | 7685 (67.7) | 995 (75.9)‡ | 7052 (69.9) | 1046 (72.3) |
| Non-Hispanic White | 1527 (54.6) | 199 (64.6) | 6379 (61.8) | 621 (65.5) |
| Non-Hispanic Black | 2584 (93.8) | 440 (94.4) | 4287 (94.4) | 416 (96.5) |
| Mexican American/other Hispanic | 3183 (84.1) | 308 (88.0) | 5552 (86.3) | 299 (87.8) |
| Other | 391 (83.6) | 48 (82.4) | 834 (85.3) | 70 (86.5) |
| Pre-bronchodilator FEV1 (L)1 | 2.73 ± 0.03 | 2.76 ± 0.07 | 3.28 ± 0.02 | 2.82 ± 0.03‡ |
| Pre-bronchodilator FVC, (L)1 | 3.14 ± 0.03 | 3.28 ± 0.08 | 4.18 ± 0.02 | 3.77 ± 0.05‡ |
| Pre-bronchodilator FEV1/FVC (%)1 | 86.5 ± 0.2 | 83.4 ± 0.5‡ | 78.5 ± 0.2 | 74.5 ± 0.4‡ |
| Bronchodilator response as a percent of baseline FEV1 (BDR, %)1 | 9.0 ± 0.6 | 13.2 ± 1.5‡ | 5.5 ± 0.2 | 10.9 ± 1.1‡ |
Results are shown as mean ± standard error (SE) for continuous variables, and as N (%) for binary variables.
P<0.05 or
P<0.01 for current asthma vs no current asthma (within each age group).
Data are only available in NHANES 2007–2010.
Figure 1.
Vitamin D level (25[OH]D, ng/ml) by race/ethnicity and NHANES study waves (2001–2002 to 2009–2010)
The multivariable analysis of vitamin D insufficiency and current asthma or current wheeze is shown in Table 2. After adjusting for age, gender, race/ethnicity, household income, BMI, and serum cotinine and CRP levels, vitamin D insufficiency was significantly associated with increased odds of current asthma and current wheeze in children, as well as with current wheeze in adults. After stratification by race or ethnicity, vitamin D insufficiency remained significantly associated with current wheeze in non-Hispanic white children and adults, as well as with current asthma in non-Hispanic white adults. However, there was no significant association between vitamin D insufficiency and current wheeze or current asthma in children or adults in other racial or ethnic groups. We obtained similar results to those shown in Table 2 when the multivariable analyses were adjusted for health insurance coverage instead of household income (data not shown).
Table 2.
Multivariable analysis* of vitamin D insufficiency and current asthma or current wheeze by race/ethnicity, NHANES 2001–2010
| Children (total=10,860) | Adults (total=24,115) | |||
|---|---|---|---|---|
|
|
||||
| Outcomes | N1 | Odds ratio, (95% confidence interval), P-value | N1 | Odds ratio, (95% confidence interval), P-value |
| Current asthma | ||||
| All participants | 995 | 1.35 (1.11, 1.64), <0.01 | 1046 | 1.06 (0.93, 1.21), 0.39 |
| Non-Hispanic whites | 199 | 1.41 (1.12, 1.77), <0.01 | 621 | 1.07 (0.93, 1.24), 0.36 |
| Non-Hispanic blacks | 440 | 1.11 (0.69, 1.79), 0.68 | 416 | 1.53 (0.91, 2.58), 0.11 |
| Mexican American/other Hispanic | 308 | 1.35 (0.85, 2.14), 0.20 | 299 | 0.95 (0.61, 1.48), 0.83 |
| Current wheeze | ||||
| All participants | 978 | 1.24 (1.02, 1.52), 0.03 | 2597 | 1.17 (1.04, 1.31), <0.01 |
| Non-Hispanic whites | 228 | 1.40 (1.11, 1.75), <0.01 | 1264 | 1.22 (1.08, 1.38), <0.01 |
| Non-Hispanic blacks | 383 | 0.71 (0.47, 1.06), 0.10 | 660 | 1.29 (0.91, 1.81), 0.15 |
| Mexican American/other Hispanic | 316 | 0.91 (0.63, 1.32), 0.61 | 561 | 1.00 (0.72, 1.38), 0.98 |
All multivariable models were adjusted for age, gender, household income, cotinine, C-reactive protein, BMI (in adults) and BMIZ (in children). The model for all participants was additionally adjusted for race/ethnicity
N = participants with vitamin D insufficiency and current asthma (or current wheeze). See Table 1 for the number of participants with vitamin D insufficiency but no current asthma or current wheeze.
In a secondary analysis, we repeated the multivariable analysis in adults after stratification by current smoking, in order to minimize potential misclassification of chronic obstructive pulmonary disease (COPD) as current asthma or current wheeze (Supplementary Table 1). In this analysis, vitamin D insufficiency was significantly associated with current wheeze among non-smoking non-Hispanic white and black adults. We found no significant association between vitamin D insufficiency and current asthma (regardless of current smoking) or current wheeze in current smokers.
Table 3 shows the results of the multivariable analysis of vitamin D insufficiency and lung function measures among participants in NHANES 2007–2010. In children, vitamin D insufficiency was significantly associated with 110 ml to 129 ml decrements in FEV1 and FVC. In adults, vitamin D insufficiency was significantly and inversely associated with FEV1 (−70 mL) and FVC (−118 mL), but significantly and positively associated with FEV1/FVC (0.45%, 95% CI=0.05%–0.85%). Similar results were obtained when the analysis was repeated using %predicted values of FEV1, FVC and FEV1/FVC (supplemental Table 2) and after stratification by current asthma (Supplemental Table 3). Vitamin D insufficiency was not associated with BDR in either children or adults.
Table 3.
Multivariable analysis* of vitamin D insufficiency and lung function measures, NHANES 2007–2010
| Lung Function Measures | Children (n=3,001) | Adults (n=8,347) |
|---|---|---|
|
| ||
| β (95% confidence interval), P-value | ||
| Pre-bronchodilator FEV1 (mL)1 | −110.17 (−146.79, −75.54), <0.01 | −69.68 (−94.17, −45.18), <0.01 |
| Pre-bronchodilator FVC, (mL)1 | −129.13 (−176.09, −82.19), <0.01 | −118.11 (−150.43, −85.79), <0.01 |
| Pre-bronchodilator FEV1/FVC (%) | −0.06 (−0.84, 0.72), 0.88 | 0.45 (0.05, 0.85), 0.03 |
| Bronchodilator response (BDR,%)2,3 | 0.50 (0.20, 1.21), 0.12 | 0.69 (0.36, 1.36), 0.29 |
All models were adjusted for age, gender, race/ethnicity, household income, current asthma, cotinine, C-reactive protein, BMI (in adults) or BMIZ (in children).
Additionally adjusted for height and height square
The model for bronchodilator response were additionally adjusting for baseline (pre-bronchodilator) FEV1.
Bronchodilator response defined as ≥ 12% increment from baseline FEV1 in children, and as ≥12% and ≥ 200ml increment from baseline in adults P for linear trend calculated using generalized linear models
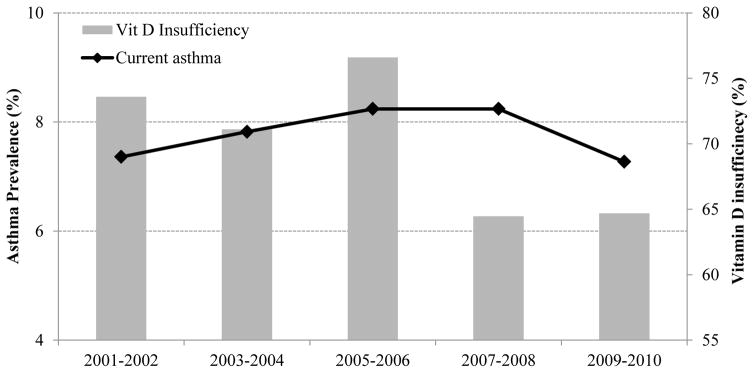
We next examined estimates of the prevalence of vitamin D insufficiency and current asthma for each NHANES study wave from 2001 to 2010 (Figure 2). In this analysis, the estimated prevalence of vitamin D insufficiency was 72% to 76% in 2001–2006, and then decreased to 64%–65% in 2007–2010. Of interest, the estimated prevalence of current asthma decreased from 8.2% in 2007–2008 to 7.4% in 2009–2010, in parallel with the reduction in the estimated prevalence of vitamin D insufficiency for the same period.
Figure 2.
Prevalence of current asthma and vitamin D insufficiency (25[OH]D <30 ng/ml) by NHANES study waves (2001–2002 to 2009–2010), in all participants
DISCUSSION
To our knowledge, this is the first study to examine the relation between vitamin D insufficiency and current asthma or current wheeze in a large sample of U.S. children and adults. Vitamin D insufficiency was common, being present in two thirds of study participants. Vitamin D insufficiency was associated with current asthma and current wheeze in non-Hispanic white children, as well as with current wheeze in non-Hispanic white and black adults without history of current smoking. Vitamin D insufficiency was not associated with current asthma or current wheeze in non-white (black or Mexican) children or Mexican adults. Temporal trends in NHANES showed that a decrement in the estimated prevalence of vitamin D insufficiency was followed by decreased prevalence of current asthma.
Consistent with our findings in the U.S. NHANES, vitamin D insufficiency or vitamin D deficiency (a serum 25[OH]D <20 ng/ml) has been associated with asthma or wheeze in population-based studies of Peruvian8, Australian31 and Canadian children32, but not significantly associated with asthma in a large study of Israeli adults12. Although two recent RCTs of vitamin D supplementation (with 2800 IU/day17 or 4000 ID/day18) during pregnancy failed to show a significant effect on asthma or wheeze (recurrent or persistent) in the first 3 years of life, the RCT using the larger dose of vitamin D reported a non-statistically significant trend (P=0.05) for a protective effect against asthma or recurrent wheeze, as well as a significant protective effect against degree of allergic sensitization (i.e. fewer positive IgE tests to allergens) in a secondary analysis18. Both trials had insufficient statistical power to detect small to moderate effects on asthma or wheeze, and continued follow up of participating children is needed to determine potential effects of vitamin D supplementation on asthma per se (as this disease cannot be confidently diagnosed in children younger than 6 years).
Growing evidence indicates that vitamin D affects innate and adaptive immune responses relevant to childhood asthma. In murine models, vitamin D deficiency is associated with airway hyper-responsiveness (AHR), as well as with increased pro-inflammatory cytokines and reduced T-regulatory (T-reg) cells in bronchoalveolar lavage fluid (BALF)33. Moreover, vitamin D supplementation attenuates allergen-induced inflammation and AHR in sensitized mice33,34. Among children, three common genetic variants in the class I MHC–restricted T cell–associated molecule gene (CRTAM) have been associated with asthma exacerbations in those with vitamin D deficiency35, and polymorphisms in the gene for the vitamin D receptor have been associated with asthma365. Whether gene-by-vitamin D interactions explain inconsistent results across observational studies of asthma is uncertain.
We found a significant association between vitamin D insufficiency and decreased FEV1 or FVC in children and adults, regardless of co-existing asthma. Our results for lung function are thus consistent with those of prior reports in children37,38,39 and adults40. In a study of adults followed for 11 years, those with asthma and low serum 25(OH)D (<20 ng/mL) at baseline had larger declines in FEV1, FVC and FEV1/FVC than those with a serum 25(OH)D ≥20 ng.ml). In general agreement with our results, that study showed that the inverse association between vitamin D deficiency and lung function was more pronounced among adults who were non-smokers and did not use inhaled corticosteroids14. In contrast to our results, serum or plasma 25(OH)D was not associated with lung function in a Danish population-based study of 4999 adults15 or in a Canadian Health Measure Survey that included 1,421 children41. Moreover, the VIDA (Vitamin D Add-on Therapy Enhances Corticosteroid Responsiveness in Asthma) trial found no significant effects of vitamin D on lung function (a secondary outcome) in adults with persistent asthma and vitamin D insufficiency19. The discrepant findings for vitamin D and lung function may be explained by differences in age, race/ethnicity, number of participants, or degree of residual confounding across studies.
In our study, vitamin D insufficiency was significantly associated with asthma or wheeze in non-Hispanic white children, but not in non-Hispanic black or Mexican children. We found similarly negative results for asthma or wheeze in non-Hispanic black children when lower cut points for vitamin D (e.g., <20 ng/ml or <25 ng/ml) were used (data not shown). Such negative findings may be explained by lack of variability in vitamin D status. Among non-Hispanic black children, >90% had a vitamin D level <30 ng/ml and >50% had a vitamin D level <20 ng/ml. In contrast to our results in children, vitamin D insufficiency was significantly associated with current wheeze among non-smoking white and black adults, suggesting that age may modify the effect of vitamin D on wheeze in non-Hispanic blacks. Similarly, Mexican ethnicity may modify the effects of vitamin D insufficiency on asthma, given null results among Mexican children and adults in our study.
A recent study reported that trends in overall childhood asthma prevalence plateaued in 2008, and then declined significantly in 201342. Although our finding of parallel decrements in asthma and vitamin D insufficiency must be cautiously interpreted due to inherent limitations of ecological results, they are both intriguing and worth exploring in future longitudinal studies.
Our study has considerable strengths, including large and ethnically diverse cohorts of both children and adults, and ability to adjust for potential confounders such as cigarette smoking (measured by serum cotinine), low-grade systematic inflammation (measured by CRP), and obesity. We also acknowledge several limitations of our findings. First, we cannot determine a temporal relationship between vitamin D insufficiency and asthma or lung function in a cross-sectional study. Second, we cannot exclude residual confounding by unmeasured variables, including atopy, use of asthma medications, outdoor activities, seasonal variation in vitamin D measurements, and dietary intake of other nutrients. Third, we cannot stratify the analysis of vitamin D insufficiency and lung function by race or ethnicity, due to small sample size. Finally, the cut points for serum vitamin D levels associated with optimal general (non-musculoskeletal) health are uncertain and under study2,43. The potential effects of vitamin D on asthma and lung health and what adequate levels should be recommended require further investigation.
In summary, vitamin insufficiency was highly prevalent among children and adults who participated in a large U.S. survey (NHANES). In NHANES, vitamin D insufficiency was associated with current asthma or current wheeze in non-Hispanic white children, as well as with current wheeze in non-smoking non-Hispanic white and black adults. Moreover, vitamin D insufficiency was associated with reduced FEV1 and FVC in children and adults. Ongoing RCTs should help determine whether vitamin D supplementation prevents asthma or severe asthma exacerbations during childhood.
Supplementary Material
Highlights.
What is already known about this topic?
Vitamin D deficiency or insufficiency may increase the risk of asthma or morbidity from asthma, particularly in children.
What does this article add to our knowledge?
We show racial/ethnic-specific effects of vitamin D insufficiency on current asthma or wheeze. Moreover, we show parallel recent decrements in the prevalence of vitamin D insufficiency and asthma among participants in different study waves.
How does this study impact current management guidelines?
At this time, vitamin D cannot be recommended for asthma treatment. Clinicians should consider screening for vitamin D deficiency (which causes musculoskeletal problems) in high-risk groups (e.g., Blacks, Puerto Ricans, institutionalized individuals and the obese).
Acknowledgments
Sources of funding: Dr. Celedón’s contribution was supported by grants HL079966, HL117191, and HL119952 from the U.S. National Institutes of Health, and by The Heinz Endowments.
Abbreviations
- 25(OH)D
25-hydroxy vitamin D
- BDR
bronchodilator response
- BMI
body mass index
- CRP
C-reactive protein
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- NHANES
National Health and Nutrition Examination Survey
- RCTs
randomized clinical trials
Footnotes
Author contributions: Conception and study design: Y-Y.H., E.F., and J.C.C.; Data analysis and interpretation: Y-Y.H. and E.F., drafting of the manuscript for intellectual content: Y-Y.H., E.F., and J.C.C. All authors approved the final version of the manuscript prior to submission
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moorman JE, Akinbami LJ, Bailey CM, et al. National Center for Health Statistics Centers for Disease Control and Prevention, editor. National Surveillance of Asthma: United States, 2001–2010. Hyattsville, Maryland: 2012. [Google Scholar]
- 2.Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clinic proceedings. 2011;86:50–60. doi: 10.4065/mcp.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mansbach JM, Ginde AA, Camargo CA., Jr Serum 25-hydroxyvitamin D levels among US children aged 1 to 11 years: do children need more vitamin D? Pediatrics. 2009;124:1404–10. doi: 10.1542/peds.2008-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr. 2008;88:558S–64S. doi: 10.1093/ajcn/88.2.558S. [DOI] [PubMed] [Google Scholar]
- 5.Pereira-Santos M, Costa PR, Assis AM, Santos CA, Santos DB. Obesity and vitamin D deficiency: a systematic review and meta-analysis. Obesity reviews: an official journal of the International Association for the Study of Obesity. 2015;16:341–9. doi: 10.1111/obr.12239. [DOI] [PubMed] [Google Scholar]
- 6.Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol. 2007;120:1031–5. doi: 10.1016/j.jaci.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 7.Paul G, Brehm JM, Alcorn JF, Holguin F, Aujla SJ, Celedon JC. Vitamin D and asthma. Am J Respir Crit Care Med. 2012;185:124–32. doi: 10.1164/rccm.201108-1502CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Checkley W, Robinson CL, Baumann LM, et al. 25-hydroxy vitamin D levels are associated with childhood asthma in a population-based study in Peru. Clin Exp Allergy. 2015;45:273–82. doi: 10.1111/cea.12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolokotroni O, Papadopoulou A, Middleton N, et al. Vitamin D levels and status amongst asthmatic and non-asthmatic adolescents in Cyprus: a comparative cross-sectional study. BMC Public Health. 2015;15:015–1385. doi: 10.1186/s12889-015-1385-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beigelman A, Zeiger RS, Mauger D, et al. The association between vitamin D status and the rate of exacerbations requiring oral corticosteroids in preschool children with recurrent wheezing. J Allergy Clin Immunol. 2014;133:1489–92. 92 e1–3. doi: 10.1016/j.jaci.2014.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brehm JM, Acosta-Perez E, Klei L, et al. Vitamin D insufficiency and severe asthma exacerbations in Puerto Rican children. Am J Respir Crit Care Med. 2012;186:140–6. doi: 10.1164/rccm.201203-0431OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Confino-Cohen R, Brufman I, Goldberg A, Feldman BS. Vitamin D, asthma prevalence and asthma exacerbations: a large adult population-based study. Allergy. 2014;69:1673–80. doi: 10.1111/all.12508. [DOI] [PubMed] [Google Scholar]
- 13.Wu AC, Tantisira K, Li L, Fuhlbrigge AL, Weiss ST, Litonjua A. Effect of vitamin D and inhaled corticosteroid treatment on lung function in children. Am J Respir Crit Care Med. 2012;186:508–13. doi: 10.1164/rccm.201202-0351OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brumpton BM, Langhammer A, Henriksen AH, et al. Vitamin D and Lung Function Decline in Adults With Asthma: The HUNT Study. Am J Epidemiol. 2016:18. doi: 10.1093/aje/kwv243. [DOI] [PubMed] [Google Scholar]
- 15.Thuesen BH, Skaaby T, Husemoen LL, Fenger M, Jorgensen T, Linneberg A. The association of serum 25-OH vitamin D with atopy, asthma, and lung function in a prospective study of Danish adults. Clin Exp Allergy. 2015;45:265–72. doi: 10.1111/cea.12299. [DOI] [PubMed] [Google Scholar]
- 16.Korn S, Hubner M, Jung M, Blettner M, Buhl R. Severe and uncontrolled adult asthma is associated with vitamin D insufficiency and deficiency. Respir Res. 2013;14:1465–9921. doi: 10.1186/1465-9921-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chawes BL, Bonnelykke K, Stokholm J, et al. Effect of Vitamin D3 Supplementation During Pregnancy on Risk of Persistent Wheeze in the Offspring: A Randomized Clinical Trial. Jama. 2016;315:353–61. doi: 10.1001/jama.2015.18318. [DOI] [PubMed] [Google Scholar]
- 18.Litonjua AA, Carey VJ, Laranjo N, et al. Effect of Prenatal Supplementation With Vitamin D on Asthma or Recurrent Wheezing in Offspring by Age 3 Years: The VDAART Randomized Clinical Trial. Jama. 2016;315:362–70. doi: 10.1001/jama.2015.18589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castro M, King TS, Kunselman SJ, et al. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: the VIDA randomized clinical trial. JAMA. 2014;311:2083–91. doi: 10.1001/jama.2014.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martineau AR, MacLaughlin BD, Hooper RL, et al. Double-blind randomised placebo-controlled trial of bolus-dose vitamin D3 supplementation in adults with asthma (ViDiAs) Thorax. 2015;70:451–7. doi: 10.1136/thoraxjnl-2014-206449. [DOI] [PubMed] [Google Scholar]
- 21.Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr. 2010;91:1255–60. doi: 10.3945/ajcn.2009.29094. [DOI] [PubMed] [Google Scholar]
- 22.Yadav M, Mittal K. Effect of vitamin D supplementation on moderate to severe bronchial asthma. Indian J Pediatr. 2014;81:650–4. doi: 10.1007/s12098-013-1268-4. [DOI] [PubMed] [Google Scholar]
- 23.Tachimoto H, Mezawa H, Segawa T, Akiyama N, Ida H, Urashima M. Improved Control of Childhood Asthma with Low-Dose, Short-Term Vitamin D Supplementation: A Randomized, Double-Blind, Placebo-Controlled Trial. Allergy. 2016;3:12856. doi: 10.1111/all.12856. [DOI] [PubMed] [Google Scholar]
- 24.Majak P, Olszowiec-Chlebna M, Smejda K, Stelmach I. Vitamin D supplementation in children may prevent asthma exacerbation triggered by acute respiratory infection. J Allergy Clin Immunol. 2011;127:1294–6. doi: 10.1016/j.jaci.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 25.Analytical Note for 25-Hydroxyvitamin D Data Analysis using NHANES III (1988–1994), NHANES 2001–2006, and NHANES 2007–2010. [Accessed April 21, 2016];2015 at http://wwwn.cdc.gov/Nchs/Nhanes/VitaminD/AnalyticalNote.aspx?h=https://wwwn.cdc.gov/Nchs/Nhanes/2009-2010/VID_F.htm&t=VID_F%20Doc.
- 26.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. The European respiratory journal. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 27.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. American journal of respiratory and critical care medicine. 1999;159:179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG., Jr Pulmonary function between 6 and 18 years of age. Pediatric pulmonology. 1993;15:75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 29.Center for Disease Control and Prevention. National Health and Nutrition Examination Survey (NHANES) Respiratory Health Spirometry Procedures Manual. 2008. [Google Scholar]
- 30. [Accessed April 20, 2016];A SAS Program for the 2000 CDC Growth Charts (ages 0 to <20 years) 2015 at http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm.
- 31.Hollams EM, Hart PH, Holt BJ, et al. Vitamin D and atopy and asthma phenotypes in children: a longitudinal cohort study. Eur Respir J. 2011;38:1320–7. doi: 10.1183/09031936.00029011. [DOI] [PubMed] [Google Scholar]
- 32.Niruban SJ, Alagiakrishnan K, Beach J, Senthilselvan A. Association of vitamin D with respiratory outcomes in Canadian children. Eur J Clin Nutr. 2014;68:1334–40. doi: 10.1038/ejcn.2014.121. [DOI] [PubMed] [Google Scholar]
- 33.Agrawal T, Gupta GK, Agrawal DK. Vitamin D supplementation reduces airway hyperresponsiveness and allergic airway inflammation in a murine model. Clin Exp Allergy. 2013;43:672–83. doi: 10.1111/cea.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fischer KD, Hall SC, Agrawal DK. Vitamin D Supplementation Reduces Induction of Epithelial-Mesenchymal Transition in Allergen Sensitized and Challenged Mice. PLoS One. 2016:11. doi: 10.1371/journal.pone.0149180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Du R, Litonjua AA, Tantisira KG, et al. Genome-wide association study reveals class I MHC-restricted T cell-associated molecule gene (CRTAM) variants interact with vitamin D levels to affect asthma exacerbations. J Allergy Clin Immunol. 2012;129:368–73. doi: 10.1016/j.jaci.2011.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tizaoui K, Berraies A, Hamdi B, Kaabachi W, Hamzaoui K, Hamzaoui A. Association of vitamin D receptor gene polymorphisms with asthma risk: systematic review and updated meta-analysis of case-control studies. Lung. 2014;192:955–65. doi: 10.1007/s00408-014-9648-8. [DOI] [PubMed] [Google Scholar]
- 37.Zosky GR, Hart PH, Whitehouse AJ, et al. Vitamin d deficiency at 16 to 20 weeks’ gestation is associated with impaired lung function and asthma at 6 years of age. Ann Am Thorac Soc. 2014;11:571–7. doi: 10.1513/AnnalsATS.201312-423OC. [DOI] [PubMed] [Google Scholar]
- 38.Gazibara T, den Dekker HT, de Jongste JC, et al. Associations of maternal and fetal 25-hydroxyvitamin D levels with childhood lung function and asthma: the Generation R Study. Clin Exp Allergy. 2016;46:337–46. doi: 10.1111/cea.12645. [DOI] [PubMed] [Google Scholar]
- 39.Gupta A, Sjoukes A, Richards D, et al. Relationship between serum vitamin D, disease severity, and airway remodeling in children with asthma. Am J Respir Crit Care Med. 2011;184:1342–9. doi: 10.1164/rccm.201107-1239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sutherland ER, Goleva E, Jackson LP, Stevens AD, Leung DY. Vitamin D levels, lung function, and steroid response in adult asthma. Am J Respir Crit Care Med. 2010;181:699–704. doi: 10.1164/rccm.200911-1710OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khan S, Mai XM, Chen Y. The link between plasma 25-Hydroxyvitamin D and lung function in general and asthmatic children. Pediatric Allergy, Immunology, and Pulmonology. 2014;27:87–91. doi: 10.1089/ped.2013.0312. [DOI] [PubMed] [Google Scholar]
- 42.Akinbami LJ, Simon AE, Rossen LM. Changing Trends in Asthma Prevalence Among Children. Pediatrics. 2016:137. doi: 10.1542/peds.2015-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.National Institute of Health. Vitamin D, Fact Sheet for Health Professionals. National Institute of Health; 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.