Abstract
Background
Anemia and vitamin D deficiency are highly prevalent in critical illness, and vitamin D status has been associated with hemoglobin concentrations in epidemiologic studies. We examined the effect of high-dose vitamin D therapy on hemoglobin and hepcidin concentrations in critically ill adults.
Materials and Methods
Mechanically ventilated critically ill adults (N=30) enrolled in a pilot double-blind, randomized, placebo-controlled trial of high-dose vitamin D3 were included in this analysis. Participants were randomized to receive placebo, 50,000 IU D3, or 100,000 IU D3 daily for 5 days (totaling 250,000 IU D3, and 500,000 IU D3, respectively). Blood was drawn weekly throughout hospitalization for up to 4 weeks. Linear mixed-effects models were used to assess change in hemoglobin and hepcidin concentrations by treatment group over time.
Results
At enrollment, >75% of participants in all groups had plasma 25-hydroxyvitamin D [25(OH)D] concentrations <30 ng/mL and >85% of participants across groups were anemic. In the 500,000 IU D3 group, hemoglobin concentrations increased significantly over time (Pgroup*time=0.01) compared to placebo, but did not change in the 250,000 IU D3 group (Pgroup*time=0.59). Hepcidin concentrations decreased acutely in the 500,000 IU D3 group relative to placebo after 1 week (P=0.007). Hepcidin did not change significantly in the 250,000 IU D3 group.
Conclusion
In these critically ill adults, treatment with 500,000 IU D3 was associated with increased hemoglobin concentrations over time and acutely reduced serum hepcidin concentrations. These findings suggest that high-dose vitamin D may improve iron metabolism in critical illness, and should be confirmed in larger studies.
Keywords: vitamin D, hemoglobin, hepcidin, anemia, critical illness
Introduction
Anemia is highly prevalent in critical illness. Nearly two thirds of adults have anemia on admission to the intensive care unit (ICU) and an even greater proportion develops anemia within the first week of admission.1, 2 The presence of anemia is associated with increased risk of mortality, cardiovascular morbidity, and decreased oxygen-carrying capacity, potentially prolonging the requirement for mechanical ventilation.3 This may be particularly concerning in critically ill patients with pre-existing cardiopulmonary disease. Traditional therapies for anemia in critical illness include blood transfusions, erythropoiesis stimulating agents, and iron repletion, however, these are not without risks and there is controversy regarding their efficacy in improving patient survival.4–7 In light of the high burden of anemia and associated adverse outcomes, investigation into safe and efficacious alternative or complementary therapies to improve hemoglobin concentrations in critically ill adults is warranted.
Anemia in critical illness may develop due to repeated blood sampling, hemorrhage, renal disease, inflammation, and nutrient deficiencies including iron, folate, and vitamin B12.3 Recently, vitamin D deficiency has also been identified as a risk factor for anemia, particularly anemia of inflammation.8, 9 Where iron deficiency anemia occurs due to absolute iron deficiency - depletion of iron stores, most often measured via ferritin concentrations in the blood, and reduced circulating iron - anemia of inflammation is characterized by reduced circulating iron in the context of normal or elevated ferritin concentrations due to sequestration of iron within cells of the reticuloendothelial system10. In critical illness, inflammation may contribute to anemia due to elevations in pro-inflammatory cytokines and hepcidin, the major iron-regulatory hormone.11 Increases in cytokines and hepcidin result in shortened red blood cell lifespan, decreased iron absorption, and iron sequestration within macrophages, limiting the amount of iron in circulation to support erythropoiesis and hemoglobin synthesis.12 Vitamin D has been shown to reduce pro-inflammatory cytokines and suppress hepcidin transcription, thereby potentially improving iron egress from cells and increasing the amount of iron in circulation to support erythropoiesis. Furthermore, the hepcidin antimicrobial peptide gene (HAMP) has been found to contain a vitamin D response element, thus lending biological plausibility to the observed association between vitamin D deficiency and anemia.13–15
We have previously reported a high prevalence of vitamin D insufficiency and anemia in ICUs at our centers,16, 17 and the VITdAL-ICU study found that serum hemoglobin concentrations were higher in the vitamin D-treated group than placebo 28 days after intervention18 However, few studies, particularly in the ICU population, have explored the therapeutic effect of vitamin D with hemoglobin as the primary outcome of interest. Therefore, we aimed to 1) examine the impact of high-dose vitamin D3 supplementation on hemoglobin concentrations in critically ill adults and 2) evaluate the effect of vitamin D on serum hepcidin concentrations to better understand the role of vitamin D in iron metabolism in this population. We hypothesized that treatment with high-dose vitamin D3 would increase hemoglobin concentrations and reduce hepcidin concentrations in this population of critically ill adults.
Methods
Study design and participants
The study population for this analysis was derived from participants enrolled in a pilot (N=30) double-blinded, randomized, placebo-controlled trial of high-dose vitamin D3 regimens in mechanically ventilated critically ill adults (NCT01372995).19 The parent study was designed to test the efficacy of high-dose vitamin D3 regimens in improving plasma 25-hydroxyvitamin D [25(OH)D] concentrations to levels ≥ 30 ng/mL. Briefly, upon enrollment participants were stratified on Acute Physiology and Chronic Health Evaluation II (APACHE II) score (≤ 15 or > 15) and randomized to receive placebo, a total enteral dose of 250,000 IU (6,250 μg) of cholecalciferol (D3), or a total enteral dose of 500,000 IU (12,500 μg) D3. Participants were not included/excluded from the study on the basis of their 25(OH)D status at enrollment. The study drug was administered in 5 equal doses over 5 days (i.e. the 500,000 IU group received a dose of 100,000 IU daily for 5 days after enrollment). Pills were dissolved in sterile water and administered through an enteral feeding tube. The cholecalciferol was manufactured from Tischon Corp. (Westbury, NY) and BioTech Pharmacal (Fayetteville, AR). Inclusion criteria were age ≥ 18 years, receiving care in ICUs at Emory University Hospital, Emory University Hospital Midtown, or Grady Memorial Hospital, anticipated mechanical ventilation of ≥ 72 hours after study enrollment, anticipated survival and ICU stay of ≥ 96 hours, and enteral access and ability to tolerate enteral study drug administration. Potential participants were excluded due to pregnancy, shock, hypercalcemia, receipt of high-dose vitamin D therapy in the preceding 6 months, chronic renal dysfunction requiring dialysis, AIDS, cirrhosis, and receipt of any investigational drug in the 60 days prior to study entry. This study was approved by the Emory University Institutional Review Board and written informed consent was obtained from the patient or legally authorized representative prior to enrollment.
Data collection
Data on demographics, medical history, and admitting diagnosis were collected on study entry.19 Blood was drawn at study enrollment, and weekly throughout the hospitalization for up to 4 weeks for assessment of plasma 25(OH)D and serum hepcidin concentrations. Plasma 25(OH)D concentrations were measured using an automated chemiluminescent technique (IDS-iSYS automated machine, Immunodiagnostic Systems, Inc., Fountain Hills, AZ) in a laboratory which participates in the Vitamin D External Quality Assessment Scheme (DEQAS, site #606) and the National Institute of Standards and Technology/NIH Vitamin D Metabolites Quality Assurance Program to ensure the accuracy of 25(OH)D measurements. Hepcidin concentrations were measured using an electrochemiluminescence immunoassay (Eli Lilly and Company, Indianapolis, IN, USA) as previously described.14, 15, 20, 21 Hemoglobin measurements within 24 hours of the 25(OH)D measurement were abstracted from the electronic medical record, starting when the patient was enrolled in the study. Hemoglobin concentrations were determined via automated cell counting using standard hospital methods. Anemia was defined based on World Health Organization criteria as hemoglobin concentrations < 13 g/dL in men and < 12 g/dL in non-pregnant women.22 Data on receipt and volume of red blood cell transfusion were extracted from participant electronic medical records. The transfusion trigger used at our sites is typically a hemoglobin concentration of 7 g/dL.
Statistical analysis
Descriptive statistics were performed for all variables and presented as mean ± standard deviation (SD) for normally distributed continuous variables or percentages for categorical variables. Non-normally distributed variables (hemoglobin and hepcidin) were transformed to the natural logarithmic scale for analysis. Such variables were subsequently back-transformed so as to be expressed in their original unit of measurement as geometric means (95% confidence interval [CI]). Comparisons of characteristics between treatment groups at enrollment were performed using one-way ANOVA for continuous variables and Fisher’s exact tests for categorical variables.
Analysis of repeated measures was performed using linear mixed-effects models to ascertain mean differences in hemoglobin and hepcidin concentrations by time, treatment group, and time*treatment group interaction. Any differences between groups in outcomes over time are reflected in the time*treatment group term. Beta-coefficients from linear mixed-effects models with log-transformed outcome variables (hemoglobin and hepcidin) were back-transformed and expressed as geometric mean ratios (GMR) with their corresponding 95% CI. A GMR of 1 indicates no treatment effect. A one-way ANOVA with Tukey’s multiple testing correction was used to determine differences in treatment groups at specific time points, and paired t-tests were used to examine within group changes in outcomes.
Sensitivity analyses were performed in which potentially confounding variables (age, sex, race, hemoglobin concentration at enrollment, hepcidin concentration at enrollment, transfusion volume, and admission ICU) were added to the model one at a time to determine the influence of each of these variables on the hemoglobin and hepcidin outcomes. Additional sensitivity analyses were performed with the linear mixed-effects models in which models were restricted to those with 25(OH)D concentrations < 30 ng/mL at enrollment to determine if enrollment 25(OH)D status affected our outcomes. All analyses were two-sided with an alpha of 0.05, and performed using SAS v. 9.4 (SAS Institute Inc., Cary, NC).
Results
Demographic, biochemical, and health status characteristics at study enrollment were similar across treatment groups (Table 1). The median time from ICU admission to study enrollment was 4 days (IQR: 5), and did not differ between groups (P=0.53). The study population was largely male, overweight or obese, and approximately half were African American. The vast majority of participants had 25(OH)D concentrations < 30 ng/mL at study enrollment. Nearly all of the participants were anemic at study enrollment, and the mean APACHE II score indicated a high degree of illness severity among the study population. Several participants had underlying comorbidities at enrollment including diabetes and heart disease. In the 500,000 IU D3 group, there was a higher proportion of a prior diagnosis of coronary artery disease compared to the other two groups. The groups did not differ in the proportion of participants who received blood transfusions, or in the total volume of blood received throughout the course of the study.
Table 1.
Characteristics by Treatment Group at Time of Study Enrollment.
| Characteristic | Placebo (n = 10) |
250,000 IU D3 (n = 9) |
500,000 IU D3 (n = 11) |
Pa |
|---|---|---|---|---|
| Age (yrs)b | 64.8 ± 17.5 | 56.4 ± 15.4 | 68.1 ± 18.6 | 0.33 |
| Men [n (%)] | 6 (60.0) | 5 (55.6) | 8 (72.7) | 0.72 |
| Race | 0.09 | |||
| African American [n (%)] | 4 (40.0) | 7 (77.8) | 3 (27.3) | |
| Caucasian [n (%)] | 5 (50.0) | 2 (22.2) | 8 (72.7) | |
| American Indian/Alaskan [n (%)] | 1 (10.0) | 0 (0.0) | 0 (0.0) | |
| BMI (kg/m2)b,c | 28.2 ± 9.9 | 33.4 ± 6.3 | 30.2 ± 6.1 | 0.36 |
| Plasma 25(OH)D (ng/mL)b | 21.5 ± 12.2 | 23.2 ± 7.8 | 20.0 ± 7.3 | 0.75 |
| 25(OH)D < 20 ng/mL [n (%)] | 5 (50.0) | 3 (33.3) | 5 (45.5) | 0.81 |
| 25(OH)D < 30 ng/mL [n (%)] | 8 (80.0) | 7 (77.8) | 10 (90.9) | 0.70 |
| Hemoglobin (g/dL)d | 8.7 (7.7, 9.8) | 10.3 (9.1, 11.7) | 9.6 (8.5, 10.7) | 0.14 |
| Anemia [n (%)]e | 10 (100.0) | 8 (88.9) | 10 (90.9) | 0.75 |
| Hepcidin (ng/mL)d | 40.9 (15.4, 108.8) | 31.7 (11.3, 88.9) | 14.0 (5.5, 35.7) | 0.25 |
| APACHE II Scoreb | 23.2 ± 8.8 | 20 ± 10.1 | 19.0 ± 7.5 | 0.53 |
| Admission ICU | 0.16 | |||
| Medical | 7 (70.0) | 4 (44.4) | 3 (27.3) | |
| Surgical | 3 (30.0) | 5 (55.6) | 8 (72.7) | |
| Infection on admission [n (%)] | 6 (60.0) | 4 (44.4) | 3 (27.3) | 0.38 |
| Coronary artery disease [n (%)] | 1 (10) | 2 (22.2) | 7 (63.6) | 0.03 |
| Congestive heart failure [n (%)] | 1 (10) | 2 (22.2) | 5 (45.5) | 0.20 |
| COPD [n (%)] | 2 (20) | 1 (11.1) | 4 (36.4) | 0.49 |
| Asthma [n (%)] | 1 (10) | 1 (11.1) | 0 (0) | 0.52 |
| Diabetes [n (%)] | 4 (40.0) | 1 (11.1) | 2 (18.2) | 0.32 |
| Received blood transfusion [n(%)]f | 4 (40.0) | 2 (22.2) | 3 (27.3) | 0.78 |
| Transfusion volume (mL)b,g | 775 ± 505.8 | 700 ± 495.0 | 916.7 ± 700.6 | 0.91 |
25(OH)D, 25-hydroxyvitamin D; APACHE II, Acute Physiology and Chronic Health Evaluation II; BMI, body mass index; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; IU, international unit
ANOVA for continuous variables, Fisher’s exact test for categorical variables
mean ± SD
n=8 for 250,000 IU D3 group
Geometric mean (95% confidence interval)
Hemoglobin <13 g/dL for men, <12 g/dL for women
Receipt of any blood transfusion during the course of the study
Total volume of packed red cells received during the course of the study, among those who received a transfusion
As previously reported19, plasma 25(OH)D concentrations increased significantly after 1 week in the groups that received 250,000 IU D3 and 500,000 IU D3, (to 45 ± 20 ng/mL and 55 ± 14 ng/mL, respectively) , compared to no change in the placebo group (P<0.001). These effects were sustained through week 4.
Hemoglobin concentrations increased significantly over time in the group that received 500,000 IU D3 (Figure 1). Compared to the placebo group, those who received 500,000 IU D3 demonstrated a significant 8% increase in hemoglobin concentrations per week [GMR: 1.08 (95% CI: 1.02, 1.15), Pgroup*time=0.01]. Hemoglobin concentrations in the 250,000 IU D3 group did not change significantly over time relative to placebo [GMR: 0.99 (95% CI: 0.94, 1.04), P=0.59]. By week 3, hemoglobin concentrations were significantly higher in the 500,000 IU D3 group compared to placebo [11.30 g/dL (95% CI: 9.34, 13.68) vs. 8.19 g/dL (95% CI: 7.26, 9.24), P=0.03]. The prevalence of anemia remained high throughout the course of the study and did not differ significantly between groups at any time point (P=1.00 at week 1, and P=0.72 at week 2; all remaining participants were anemic at weeks 3 and 4).
Figure 1.
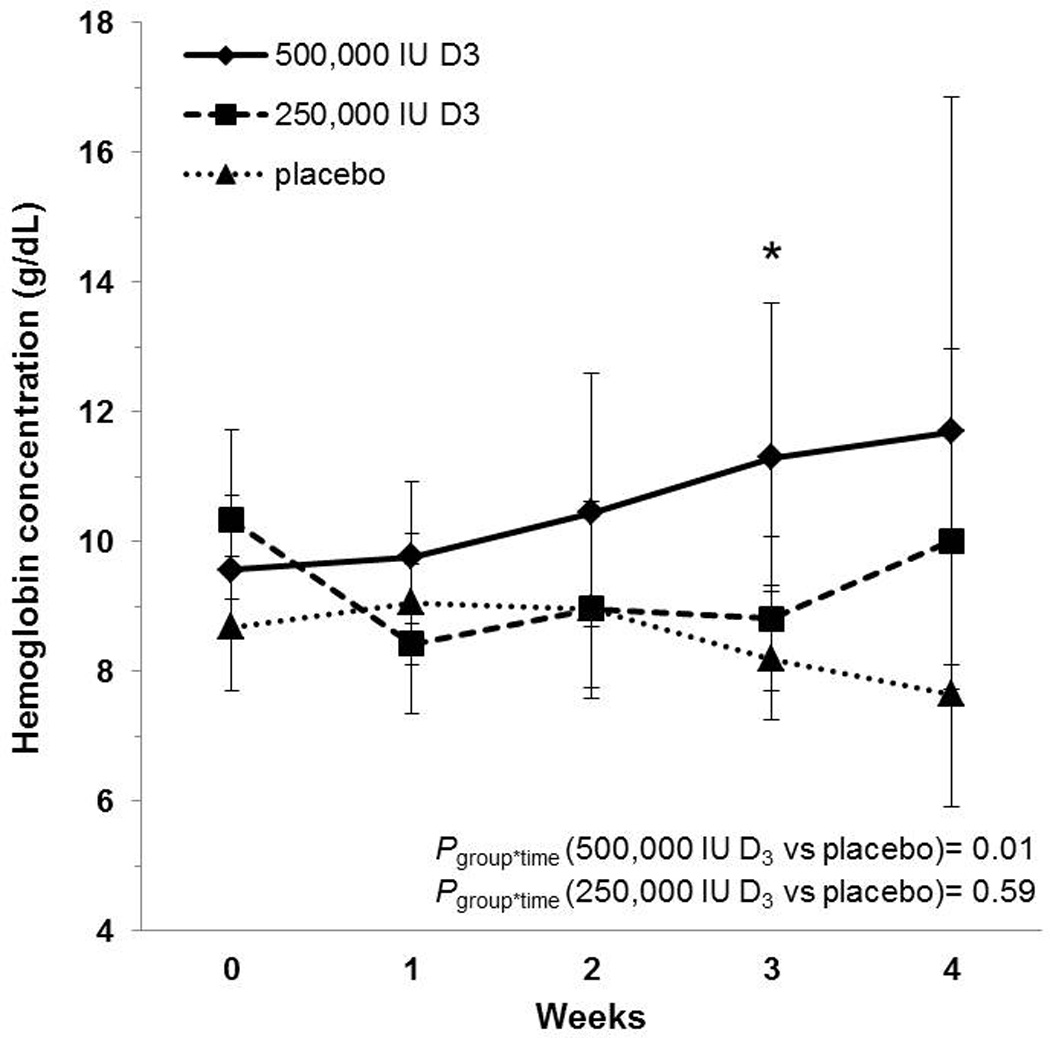
Geometric mean hemoglobin concentrations with corresponding 95% confidence intervals in critically ill adults. Hemoglobin concentrations are reported across time and by treatment group. Hemoglobin concentrations increased significantly over time in the group that received 500,000 IU D3 compared to the placebo group; there was no significant change in the 250,000 IU D3 group. By three weeks, hemoglobin concentrations in the 500,000 IU D3 group differed significantly from the placebo group; there were no statistically significant differences between groups at other time points. *P<0.05; group*time, group-by-time interaction. Sample sizes in the placebo, 250,000 IU D3, and 500,000 IU D3 groups, respectively: enrollment n = 10, 9, 11; 1 week n = 9, 9, 9; 2 weeks n = 8, 6, 5; 3 weeks n = 5, 4, 2; 4 weeks n = 2, 2, 1.
Hepcidin concentrations were only obtained for up to 3 weeks (Figure 2). During that period, there was not a statistically significant group-by-time interaction with hepcidin concentrations in either vitamin D-treated group relative to the placebo group [Pgroup*time (500,000 IU D3 vs placebo) = 0.44; Pgroup*time (250,000 IU D3 vs placebo) = 0.60]. However, by 1 week, hepcidin concentrations did differ significantly between the 500,000 IU D3 and placebo groups (P=0.007). The 250,000 IU D3 group did not differ from either the 500,000 IU D3 group (P=0.22) or the placebo group (P=0.38) at 1 week. Within group analyses showed that hepcidin concentrations decreased acutely by 66% after 1 week in the 500,000 IU D3 group, relative to study enrollment [GMR: 0.34 (95% CI: 0.20, 0.59), P=0.002]. No significant changes were observed within the 250,000 IU D3 or placebo groups from enrollment to1 week (P=0.08 and P=0.48, respectively).
Figure 2.
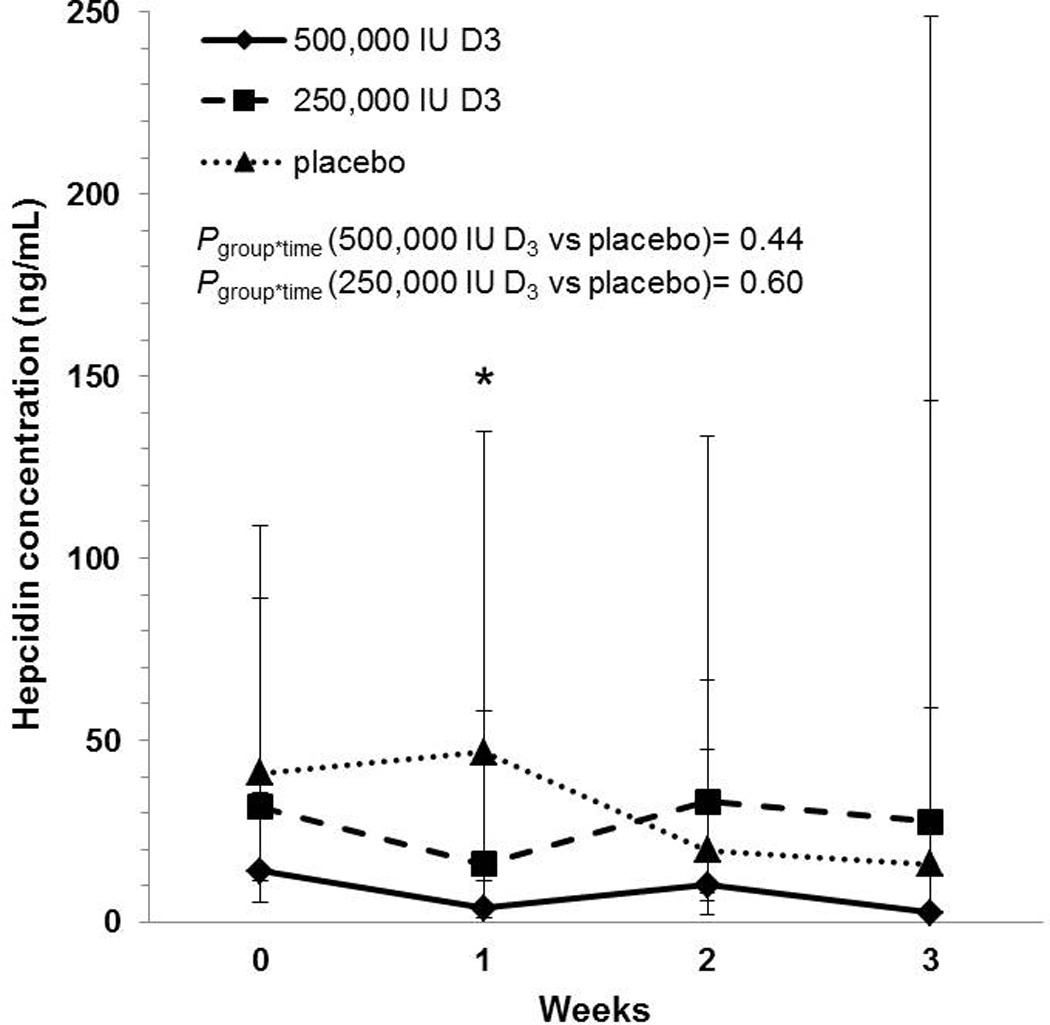
Geometric mean hepcidin concentrations with corresponding 95% confidence intervals in critically ill adults. Hepcidin concentrations are reported across time and by treatment group. There were no significant differences over time in hepcidin concentrations in either vitamin D-treated group relative to placebo. Hepcidin concentrations in the 500,000 IU D3 group differed significantly from the placebo group one week after dosing; there were no statistically significant differences between groups at other time points. *P<0.05; group*time, group-by-time interaction. Sample sizes in the placebo, 250,000 IU D3, and 500,000 IU D3 groups, respectively: enrollment n = 10, 9, 11; 1 week n = 9, 6, 9; 2 weeks n = 8, 6, 5; 3 weeks n = 4, 4, 2.
Sensitivity analyses
Controlling separately for potentially confounding variables age, sex, race, hemoglobin concentration at enrollment, hepcidin concentration at enrollment, transfusion volume, and admission ICU did not affect the results of the linear mixed-effects models. The group-by-time interaction for hemoglobin remained significant in the 500,000 IU D3 group (P<0.03 for all) and non-significant in the 250,000 IU D3 (P>0.45 for all). The group-by-time interaction for hepcidin remained non-significant for both the 500,000 IU D3 (P>0.35 for all) and 250,000 IU D3 (P>0.45 for all) relative to placebo with the addition of each control variable.
When linear mixed-effects models were restricted to those with 25(OH)D < 30 ng/mL at the time of study enrollment, hemoglobin and hepcidin results were largely unchanged. Compared to the placebo group, those who received 500,000 IU D3 demonstrated a significant 10% increase in hemoglobin concentrations per week [GMR: 1.10 (95% CI: 1.04, 1.18), Pgroup*time=0.003]. Hemoglobin concentrations in the 250,000 IU D3 group remained unchanged relative to placebo [GMR: 0.99 (95% CI: 0.93, 1.05), P=0.67]. There was not a statistically significant group-by-time interaction with hepcidin concentrations in either vitamin D-treated group relative to the placebo group [Pgroup*time (500,000 IU D3 vs placebo) = 0.34; Pgroup*time (250,000 IU D3 vs placebo) = 0.47].
Discussion
In this population of mechanically ventilated critically ill adults, we found that treatment with a total enteral dose of 500,000 IU D3 was associated with a significant increase in hemoglobin concentrations over time. Although not sustained, there was an acute decrease in hepcidin concentrations by 1 week following treatment with 500,000 IU D3 relative to the placebo group. No significant changes in hemoglobin or hepcidin concentrations were observed in the 250,000 IU D3 group compared to the placebo group, suggesting a potential dose-related effect of vitamin D3 on these outcomes.
Our findings are consistent with studies of patients with chronic kidney disease (CKD) in which treatment with vitamin D or its analogues increased hemoglobin concentrations.23, 24 However, studies in other patient populations have been mixed, but this was likely due to differences in the dose and form of vitamin D administered, as well as the prevalence of vitamin D deficiency and type of anemia in these studies.25–27 Indeed, Sooragonda et al26 found that among generally healthy adults with iron deficiency anemia, treatment with 600,000 IU of cholecalciferol did not result in further increases in hemoglobin concentrations following correction of iron deficiency. This would suggest that the effects of vitamin D are likely specific to anemia of inflammation, which is consistent with the proposed mechanism of action outlined below.
The acute reduction in hepcidin that we observed is consistent with another study from our group in which healthy volunteers who received high-dose vitamin D3, experienced a significant reduction in hepcidin concentrations after one week, compared to the placebo group15.Given the physiology of iron recycling, the acute reduction in hepcidin observed in the 500,000 IU D3 group likely potentiated the increase in hemoglobin, even though the change in hepcidin was not sustained over time.28 During anemia of inflammation, which is a component of anemia in critical illness11, hepcidin is elevated, blocking iron egress from cells, and sequestering iron within cells of the reticuloendothelial system.12 This process limits the iron in circulation that can be used to support erythropoiesis and hemoglobin synthesis. Reductions in hepcidin may restore iron recycling by allowing iron to exit cells and be utilized in hemoglobin synthesis and erythropoiesis. Thus increases in hemoglobin concentrations are likely to be preceded by reductions in hepcidin in the context of anemia of inflammation. Our findings of an acute reduction in hepcidin concentration with an increase over time in hemoglobin concentrations in the 500,000 IU D3 group are in line with this process, and the gene coding for hepcidin has been demonstrated to contain a vitamin D response element13, lending strong biological plausibility to our findings. Other potential mechanisms underlying our findings may involve a role for vitamin D in supporting erythropoiesis through induction of erythroid progenitor cell proliferation29–31; however this was not evaluated in the present study.
Unexpectedly, we did not see significant changes in either hemoglobin or hepcidin in response to 250,000 IU D3, despite significant increases in 25(OH)D concentrations in this group. It is possible that there may be differences in characteristics of the individuals randomized to these two groups which were not measured in this study, or differences in unknown factors related to response to high-dose vitamin D3. Another possibility is that there is a threshold level of 25(OH)D that may need to be reached to provide adequate substrate for the local production of 1,25(OH)2D by the macrophage to regulate hepcidin concentrations. While the groups were generally balanced at study enrollment, they differed in the prevalence of coronary artery disease, with the 500,000 IU D3 group having a higher proportion with this condition. It is possible that this group was more responsive to vitamin D therapy in terms of hemoglobin and hepcidin concentrations. Indeed, in observational studies in patients scheduled for cardiac surgery and coronary angiography, higher 25(OH)D concentrations were associated with higher hemoglobin concentrations, and protective against anemia.32, 33 Another potential explanation may be that even though 25(OH)D concentrations increased with both dosing regimens, it is possible that there was a differential between free 25-hydroxyvitamin D in these groups. Recent evidence has indicated that free 25-hydroxyvitamin D may be a better marker of vitamin D bioactivity than total 25(OH)D concentrations.34 Thus if the higher dose of vitamin D resulted in greater concentrations of free 25-hydroxyvitamin D, this may explain the observed changes in hemoglobin and hepcidin.
Our findings have potential clinical implications for vitamin D repletion as a safe and efficacious adjunct therapy for anemia in critical illness. Even though anemia was not completely resolved in our study population, hemoglobin concentrations did improve to a clinically meaningful extent in the group which received 500,000 IU D3. The improvement in hemoglobin may reduce the frequency and necessity of blood transfusions. Transfusions are widely used in the ICU, but there is controversy regarding their use and threshold for initiation, as studies have found transfusions to be associated with increased length of hospital stay, hospital costs, and mortality.1, 2, 35, 36 Studies indicate that the hemoglobin threshold for initiation of transfusions typically falls between 7–9 g/dL and may vary by patient characteristics.1, 37, 38 If vitamin D can improve hemoglobin concentrations to a level above which transfusions may be initiated, as was demonstrated in our study, this may have positive effects on patient outcomes.
Strengths of our study included the rigorous clinical trial design, novel research question, and the well-characterized study population. However, there were important limitations. First, this trial was not specifically designed and powered with hemoglobin as the primary outcome, and the sample size of this pilot trial was relatively small. Nonetheless, the significant increase in hemoglobin that was observed, despite the small sample size, suggests that this was a fairly robust finding. We were also limited in that the randomization was unbalanced with respect to coronary artery disease. With only one person in the placebo group having the disease, we were unable to adjust for this variable in our analysis. We therefore cannot rule out the effect of this potential confounder on our outcomes, and our findings related to the 500,000 IU D3 group may lack generalizability to populations with a different prevalence of underlying comorbidities. Another potential limitation of this study was that the median time from ICU admission to study enrollment was 4 days. Therefore, the plasma 25(OH)D, serum hepcidin, and hemoglobin concentrations obtained at study enrollment may not reflect the participants’ true baseline levels as concentrations of these biochemical markers may decrease after ICU admission due to hemodilution or other unknown factors. Finally, we were unable to fully evaluate the effect of other anemia treatments that may have been given as part of the patients’ medical care. We did, however, find that the number of patients who received transfusions during their hospitalization did not differ by treatment group, nor did the volume of packed red cells received, suggesting that the increase in hemoglobin in the 500,000 IU D3 group was unlikely due to differences in receipt of transfusions in this group.
In conclusion, we found that treatment with 500,000 IU D3 was associated with a significant increase in hemoglobin concentrations over time and an acute reduction in hepcidin concentrations in critically ill adults. Larger clinical trials of high-dose vitamin D3 in critically ill adults with hemoglobin as the primary outcome are warranted to confirm these findings and further elucidate the therapeutic effect of vitamin D on anemia in critical illness.
Clinical Relevancy Statement.
Anemia is highly prevalent in critical illness and associated with adverse patient outcomes. Vitamin D deficiency, also common in critical illness, has been identified as a risk factor for anemia. Our finding that hemoglobin concentrations increased following treatment with high-dose vitamin D is clinically relevant for clinicians and researchers contemplating therapeutic options for anemia in critically ill adults. Pending confirmation in larger studies, vitamin D repletion may have implications as a safe and alternative adjunct therapy to traditional therapies for anemia in critical illness, such as transfusions which may carry risks for the patient.
Acknowledgments
Financial disclosure: This work was supported in part by grants from the National Institutes of Health T32 DK007734 (EMS), T32 DK007298 (JLJ), K01 DK102851 (JAA), R21 HL110044 (GSM, TRZ, VT), K24 DK096574 (TRZ), UL1 TR000454 (Atlanta Clinical & Translational Science Institute). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in the design, analysis or writing of this article.
Footnotes
Conflicts of interest: JHS and RJK are employed by Eli Lilly and Company and performed the hepcidin assay. Eli Lilly and Company played no role in the study design or the decision to publish.
Statement of authorship: Ellen M. Smith, Greg S. Martin, Thomas R. Ziegler, and Vin Tangpricha contributed to the conception and design of the work; Ellen M. Smith, Jennifer L. Jones, Jenny E. Han, Jessica A. Alvarez, Susu M. Zughaier, John H. Sloan, and Robert J. Konrad contributed to the acquisition and analysis of the data. Ellen M. Smith drafted the manuscript, and all authors contributed to the interpretation of the data and critically revised the manuscript. All authors read and approved the final manuscript and agree to be accountable for ensuring the accuracy and integrity of all aspects of the work.
References
- 1.Corwin HL, Gettinger A, Pearl RG, et al. The CRIT Study: Anemia and blood transfusion in the critically ill--current clinical practice in the United States. Crit Care Med. 2004 Jan;32(1):39–52. doi: 10.1097/01.CCM.0000104112.34142.79. [DOI] [PubMed] [Google Scholar]
- 2.Vincent JL, Baron JF, Reinhart K, et al. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288(12):1499–1507. doi: 10.1001/jama.288.12.1499. [DOI] [PubMed] [Google Scholar]
- 3.Hayden SJ, Albert TJ, Watkins TR, Swenson ER. Anemia in critical illness: insights into etiology, consequences, and management. Am J Respir Crit Care Med. 2012;185(10):1049–1057. doi: 10.1164/rccm.201110-1915CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Retter A, Wyncoll D, Pearse R, et al. Guidelines on the management of anaemia and red cell transfusion in adult critically ill patients. Br J Haematol. 2013;160(4):445–464. doi: 10.1111/bjh.12143. [DOI] [PubMed] [Google Scholar]
- 5.Pieracci FM, Stovall RT, Jaouen B, et al. A multicenter, randomized clinical trial of IV iron supplementation for anemia of traumatic critical illness. Crit Care Med. 2014;42(9):2048–2057. doi: 10.1097/CCM.0000000000000408. [DOI] [PubMed] [Google Scholar]
- 6.Zarychanski R, Turgeon AF, McIntyre L, Fergusson DA. Erythropoietin-receptor agonists in critically ill patients: a meta-analysis of randomized controlled trials. CMAJ. 2007;177(7):725–734. doi: 10.1503/cmaj.071055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dejam A, Malley BE, Feng M, et al. The effect of age and clinical circumstances on the outcome of red blood cell transfusion in critically ill patients. Crit Care. 2014;18(4):487. doi: 10.1186/s13054-014-0487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perlstein TS, Pande R, Berliner N, Vanasse GJ. Prevalence of 25-hydroxyvitamin D deficiency in subgroups of elderly persons with anemia: association with anemia of inflammation. Blood. 2011;117(10):2800–2806. doi: 10.1182/blood-2010-09-309708. [DOI] [PubMed] [Google Scholar]
- 9.Smith EM, Alvarez JA, Martin GS, Zughaier SM, Ziegler TR, Tangpricha V. Vitamin D deficiency is associated with anaemia among African Americans in a US cohort. Br J Nutr. 2015;113(11):1732–1740. doi: 10.1017/S0007114515000999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 11.Sihler KC, Napolitano LM. Anemia of inflammation in critically ill patients. J Intensive Care Med. 2008;23(5):295–302. doi: 10.1177/0885066608320836. [DOI] [PubMed] [Google Scholar]
- 12.Nemeth E, Ganz T. Anemia of inflammation. Hematol Oncol Clin North Am. 2014;28(4):671–681. vi. doi: 10.1016/j.hoc.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bacchetta J, Zaritsky JJ, Sea JL, et al. Suppression of iron-regulatory hepcidin by vitamin D. J Am Soc Nephrol. 2014;25(3):564–572. doi: 10.1681/ASN.2013040355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zughaier SM, Alvarez JA, Sloan JH, Konrad RJ, Tangpricha V. The role of vitamin D in regulating the iron-hepcidin-ferroportin axis in monocytes. J Clin Transl Endocrinol. 2014;1(1):19–25. doi: 10.1016/j.jcte.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith EM, Alvarez JA, Kearns MD, et al. High-dose vitamin D3 reduces circulating hepcidin concentrations: A pilot, randomized, double-blind, placebo-controlled trial in healthy adults. Clin Nutr. 2016 doi: 10.1016/j.clnu.2016.06.015. pii: S0261-5614(16)30148-0. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeng L, Yamshchikov AV, Judd SE, et al. Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. J Transl Med. 2009;7:28. doi: 10.1186/1479-5876-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kempker JA, West KG, Kempker RR, et al. Vitamin D status and the risk for hospital-acquired infections in critically ill adults: a prospective cohort study. PLoS One. 2015;10(4):e0122136. doi: 10.1371/journal.pone.0122136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amrein K, Schnedl C, Holl A, et al. Effect of high-dose votamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial. JAMA. 2014;312(15):1520–1530. doi: 10.1001/jama.2014.13204. [DOI] [PubMed] [Google Scholar]
- 19.Han JE, Jones JL, Tangpricha V, et al. High dose vitamin D administration in ventilated intensive care unit patients: a pilot double blind randomized placebo controlled trial. J Clin Transl Endocrinol. 2016;4:59–65. doi: 10.1016/j.jcte.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butterfield AM, Luan P, Witcher DR, et al. A dual-monoclonal sandwich ELISA specific for hepcidin-25. Clin Chem. 2010;56(11):1725–1732. doi: 10.1373/clinchem.2010.151522. [DOI] [PubMed] [Google Scholar]
- 21.Troutt JS, Rudling M, Persson L, et al. Circulating human hepcidin-25 concentrations display a diurnal rhythm, increase with prolonged fasting, and are reduced by growth hormone administration. Clin Chem. 2012;58(8):1225–1232. doi: 10.1373/clinchem.2012.186866. [DOI] [PubMed] [Google Scholar]
- 22.McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr. 2009;12(4):444–454. doi: 10.1017/S1368980008002401. [DOI] [PubMed] [Google Scholar]
- 23.Lin CL, Hung CC, Yang CT, Huang CC. Improved anemia and reduced erythropoietin need by medical or surgical intervention of secondary hyperparathyroidism in hemodialysis patients. Ren Fail. 2004;26(3):289–295. doi: 10.1081/jdi-120039528. [DOI] [PubMed] [Google Scholar]
- 24.Riccio E, Sabbatini M, Bruzzese D, et al. Effect of paricalcitol vs calcitriol on hemoglobin levels in chronic kidney disease patients: a randomized trial. PLoS One. 2015;10(3):e0118174. doi: 10.1371/journal.pone.0118174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ernst JB, Tomaschitz A, Grubler MR, et al. Vitamin D supplementation and hemoglobin levels in hypertensive patients: a randomized controlled trial. Int J Endocrinol. 2016;2016:6836402. doi: 10.1155/2016/6836402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sooragonda B, Bhadada SK, Shah VN, Malhotra P, Ahluwalia J, Sachdeva N. Effect of vitamin D replacement on hemoglobin concentration in subjects with concurrent iron-deficiency anemia and vitamin D deficiency: a randomized, single-blinded, placebo-controlled trial. Acta Haematol. 2015;133(1):31–35. doi: 10.1159/000357104. [DOI] [PubMed] [Google Scholar]
- 27.Madar AA, Stene LC, Meyer HE, Brekke M, Lagerløv P, Knutsen KV. Effect of vitamin D3 supplementation on iron status: a randomized, double-blind, placebo-controlled trial among ethnic minorities living in Norway. Nutr J. 2015;15(1):74. doi: 10.1186/s12937-016-0192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Drygalski A, Adamson JW. Iron metabolism in man. JPEN J Parenter Enteral Nutr. 2013 Sep;37(5):599–606. doi: 10.1177/0148607112459648. [DOI] [PubMed] [Google Scholar]
- 29.Smith EM, Tangpricha V. Vitamin D and anemia: insights into an emerging association. Curr Opin Endocrinol Diabetes Obes. 2015;22:432–438. doi: 10.1097/MED.0000000000000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alon DB, Chaimovitz C, Dvilansky A, et al. Novel role of 1,25(OH)2D3 in induction of erythroid progenitor cell proliferation. Exp Hematol. 2002;30:403–409. doi: 10.1016/s0301-472x(02)00789-0. [DOI] [PubMed] [Google Scholar]
- 31.Aucella F, Scalzulli RP, Gatta G, Vigilante M, Carella AM, Stallone C. Calcitriol increases burst-forming unit-erythroid proliferation in chronic renal failure. A synergistic effect with r-HuEpo. Nephron Clin Pract. 2003;95(4):c121–c127. doi: 10.1159/000074837. [DOI] [PubMed] [Google Scholar]
- 32.Ernst JB, Zittermann A, Pilz S, et al. Independent associations of vitamin D metabolites with anemia in patients referred to coronary angiography: the LURIC study. Eur J Nutr. 2016 Jan 8; doi: 10.1007/s00394-015-1149-x. [DOI] [PubMed] [Google Scholar]
- 33.Zittermann A, Kuhn J, Dreier J, et al. Association of 25-hydroxyvitamin D with anemia risk in patients scheduled for cardiac surgery. Int J Lab Hematol. 2014;36(1):29–36. doi: 10.1111/ijlh.12112. [DOI] [PubMed] [Google Scholar]
- 34.Shieh A, Chun RF, Ma C, et al. Effects of high-dose vitamin D2 versus vitamin D3 on total and free 25-hydroxyvitamin D and markers of calcium balance. J Clin Endocrinol Metab. 2016:jc20161871. doi: 10.1210/jc.2016-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zilberberg MD, Stern LS, Wiederkehr DP, Doyle JJ, Shorr AF. Anemia, transfusions and hospital outcomes among critically ill patients on prolonged acute mechanical ventilation: a retrospective cohort study. Crit Care. 2008;12(2):R60. doi: 10.1186/cc6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walsh TS, Boyd JA, Watson D, et al. Restrictive versus liberal transfusion strategies for older mechanically ventilated critically ill patients: a randomized pilot trial. Crit Care Med. 2013;41(10):2354–2363. doi: 10.1097/CCM.0b013e318291cce4. [DOI] [PubMed] [Google Scholar]
- 37.Thomas J, Jensen L, Nahirniak S, Gibney RT. Anemia and blood transfusion practices in the critically ill: a prospective cohort review. Heart Lung. 2010;39(3):217–225. doi: 10.1016/j.hrtlng.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]


