Abstract
Vestibular sensation is essential for gaze stabilization, balance, and perception of gravity. The vestibular receptors in mammals, Type I and Type II hair cells, are located in five small organs in the inner ear. Damage to hair cells and their innervating neurons can cause crippling symptoms such as vertigo, visual field oscillation, and imbalance. In adult rodents, some Type II hair cells are regenerated and become re-innervated after damage, presenting opportunities for restoring vestibular function after hair cell damage. This article reviews features of vestibular sensory cells in mammals, including their basic properties, how they develop, and how they are replaced after damage. We discuss molecules that control vestibular hair cell regeneration and highlight areas in which our understanding of development and regeneration needs to be deepened.
Keywords: Vestibular, hair cell, supporting cell, development, regeneration
1. Introduction
Vestibular hair cells are sensory receptors in the inner ear that detect head motion and thereby enable animals to orient their bodies and coordinate movements. In mammals, vestibular hair cells and their innervating neurons degenerate with age [1–3], and they can be destroyed by therapeutic drugs such as aminoglycoside antibiotics [4, 5]. Extensive loss of vestibular sensory cells is highly debilitating and can elicit nauseating bouts of dizziness, imbalance, and incapacitation. Vestibular deficits are prevalent in the human population. They are estimated to affect 35% of the U.S. population >40 years old, and they increase significantly with age [6]. Although mammals compensate after vestibular hair cell loss by invoking visual and proprioceptive senses, functional deficits can persist and affect balance throughout life.
The pathology of vestibular aging and toxicity is complex, affecting various cell types and structures in the sensory organs, neurons of the vestibular ganglion, and the central pathways to which the neurons project [1–4, 7]. Indeed, the degree to which losses of vestibular hair cells and neurons contribute to vestibular dysfunction in humans is not well understood. Regeneration of vestibular hair cells is one treatment strategy being explored for some forms of vestibular dysfunction. The majority of hair cells in mammalian vestibular organs are formed during embryogenesis. However, adult mammals can regenerate a subpopulation of these cells after damage, increasing the likelihood that cellular repair or replacement could potentially benefit millions of people suffering from vestibular deficits. This review summarizes the current state of knowledge on development, damage, and regeneration of sensory cell types in the mammalian vestibular system and highlights critical information gaps that must be addressed before new therapies for vestibular dysfunction can be defined.
2. The mammalian vestibular organs contain a diverse array of cell types
The sense of balance is achieved by integrating vestibular, visual, and somatosensory inputs. Five vestibular organs located in the inner ear sense head position and movements in different directions (Fig. 1). Mechanosensitive hair cells are receptor cells located in the sensory epithelium of each vestibular organ. Hair cells and their innervating neurons detect head velocity and acceleration when a specialized bundle of stiff finger-like projections (stereocilia) located at their apical surface is deflected in response to head movement. There are two types of vestibular sensory epithelia. Maculae are found in the utricle and the saccule. The stereocilia of macular hair cells are weighted by small stones (otoconia), enabling the cells to sense linear head acceleration and gravity. Cristae (lateral, anterior, and posterior) lie at the end of the three semicircular canals and sense head rotations.
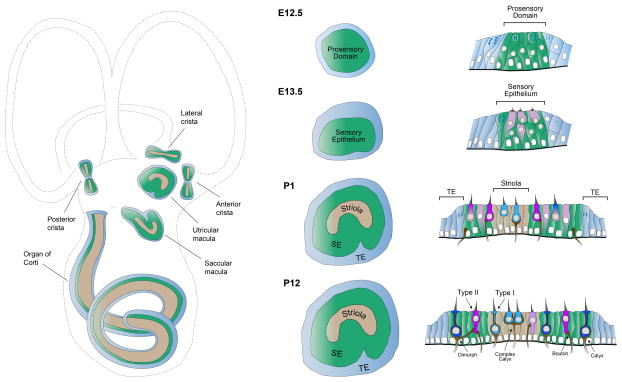
Figure 1. The sensory organs of the mouse inner ear.
The structure of the inner ear sensory organs is shown (left column), as well as the development of the utricular macula in surface (middle column) and cross-sectional (right column) views. The most mature epithelia are shown at the bottom. Left column, Detection of sound or acceleration occurs in the sensory epithelia (green), which are ordered patches comprised of mechanosensitive hair cells and supporting cells. The lateral, posterior, and anterior cristae detect rotational acceleration, the utricle and saccule detect linear acceleration, and the cochlea detects sound. In mammals, each sensory epithelium (green) contains a specialized set of hair cells (tan) that enhance range or sensitivity. In the vestibular organs, these specialized cells are located centrally within the epithelium. Middle and right columns, Surface views and cross-sections depicting development of the mouse utricular macula. By E12.5, a pseudostratified layer of neuroepithelial cells within the otocyst differentiates to form a prosensory domain (green), the precursor to the utricular macula. Neuroepithelial cells surrounding the prosensory domain form the non-sensory transitional epithelium (TE, blue). Prosensory cells exit the cell cycle and begin to differentiate into the first hair cells at E13.5. By birth (P1), progenitors are completing final rounds of cell division. The crescent-shaped striola (tan) has distinguished itself from the surrounding extrastriolar zones (green). Many hair cells display the morphological and electrophysiological characteristics of Type I and II hair cells and have formed connections with vestibular nerve endings. By P12, maturation of the sensory epithelium is nearly complete.
Each vestibular sensory epithelium is composed of hair cells and supporting cells (Fig. 1, bottom right), which share similarities with epithelial and glial cells. Each macula has two anatomical zones: a central striola in which specialized afferent terminals are located and a surrounding extrastriola. In or around the striola, hair cells are divided along a line of polarity reversal in which the vector of maximum sensitivity of their stereocilia reverses direction. Polarity reversal allows for detection of linear acceleration in opposing directions. Each crista is also divided into central and peripheral zones, but in contrast to the maculae, all hair bundles in the crista share a common orientation.
Vestibular hair cells are divided into two subtypes, Type I and Type II [8–14]. Type I and Type II hair cells are found in both central and peripheral zones of all five vestibular organs, usually in almost equal ratios, of all mammals that have been examined, including humans. This has sometimes been confused with findings from birds and reptiles, in which Type I hair cells are only found in central zones [15, 16]. The functional differences between hair cell subtypes and regions are still being elucidated, although accumulating evidence indicates that Type I hair cells, particularly those located centrally within the sensory epithelium, may be better suited for detecting acceleration during high frequency head movements compared to Type II hair cells [17, 18].
Type I hair cells are unique to amniotes. They are classically defined by the presence of cup-shaped, calyceal afferent innervation, whereas Type II hair cells synapse upon discrete bouton afferent terminals [10]. Distinct morphological differences such as cell shape and stereocilia width and length have also been linked to each subtype [11, 19–21]. Relatively little is known about what separates vestibular hair cell subtypes at the molecular level, but differences in Sox2 transcription factor expression [22] and calcium binding protein expression [9, 23] can be reliable indicators. Rapid progress on this front will likely soon be made as high throughput profiling methods like single-cell RNA-sequencing should allow for characterization of differences at the whole transcriptome level [24]. A growing list of electrophysiological differences also distinguish Type I and Type II hair cells [25–34], and regional differences in physiological recordings confirm that Type I and Type II hair cells may be further subdivided based on central versus peripheral location [25, 26, 35–41].
Diversity amongst the afferent vestibular ganglion neurons that innervate hair cells is somewhat more complex. Morphologically, terminals of the neurons can be broken into two types: calyces and boutons. Each neuron usually branches multiple times and innervates several hair cells [42, 43]. Neurons whose arbors exclusively form bouton endings are least common and only found in peripheral zones, whereas neurons whose arbors exclusively form calyceal endings are only found in central zones. The vast majority of afferent neurons are dimorphic; they branch to form both calyceal and bouton endings. Dimorphs are found in both central and peripheral zones, but individual neurons do not cross zones. Finally, some central calyces are complex, meaning that one calyx from the same branch can extend to innervate multiple neighboring hair cells, whereas the peripheral calyces that originate from dimorphs only innervate a single hair cell.
In mature mice, peripheral afferents that innervate the maculae project centrally to distinct regions of the brain, depending on the zone from which they arise. Afferent nerves from the medial half of the utricle project almost exclusively to vestibular nuclei, while afferents in the lateral half travel primarily to the cerebellum [44]. The converse is true in the saccule. These zones have been termed “cerebellar macula” and “vestibular macula”. The functional significance of this segregation of projections is not known. However, since a given stimulus activates one macular zone and not the other, it has been proposed that this would result in facilitation of one nucleus and defacilitation of the other. Given the central circuitry, this could result in enhanced vestibular tuning [44].
Another major feature distinguishing individual vestibular ganglion neurons is their background firing pattern [45–47]. The calyx-only and dimorphic neurons in the central zones exhibit irregular discharge of action potentials, whereas the bouton-only afferents and dimorphs in peripheral zones are more regular. Irregular afferents show larger gain at higher frequency stimuli than do regular afferents, suggesting central afferents are better at detecting the onset of rapid movements [48, 49]. A clear connection between hair cell subtype, afferent terminal type, and discharge regularity is obscured, however, by the fact that Type I/Type II hair cells and calyces/boutons are found in both central and peripheral zones. Therefore, mechanisms by which each cell type in each region encodes head movements must be elucidated before we learn how to reconstruct injured vestibular organs such that function is restored. Experiments showing that low and mid frequency components of the vestibulo-ocular reflex remain intact after silencing irregular afferents suggest that these inputs to the central nervous system may be dispensable for some forms of vestibular function [50]. However, the full impact of removing and replacing distinct neural and hair cell subtypes on vestibular function is still actively under investigation.
While sensory hair cells and neurons might be the most essential cell types for vestibular function, there are other cell types present within vestibular organs that have key roles. Supporting cells anchor hair cells into the sensory epithelium, generate material for the overlying structures that are essential for stereocilia displacement, clear dead hair cells and debris, and help maintain ion homeostasis (supporting cell features and functions are reviewed in [51, 52]). Supporting cells also play the key role of serving as precursors to new hair cells in adulthood (discussed in detail below). Clear evidence exists that, at least during development, there are two subtypes of vestibular supporting-cell-like progenitors separated by the central and peripheral regions, particularly within the maculae [24, 53–55]. Whether these differences persist in mature supporting cells at adulthood remains to be determined.
Surrounding the sensory epithelium is a transitional region, followed by a thinner epithelium that arches up to form a roof and enclose the lumen. Dark cells in this thinner epithelium, and to a lesser extent cells from the transitional region, are essential for secreting potassium (K) and maintaining the high K concentration of the endolymph in utricles and cristae. In addition, macrophages send processes or migrate periodically into the sensory epithelium [56]. Finally, the sensory epithelium sits on a bed of stromal mesenchyme, which contains poorly characterized cell types including, but likely not limited to, Schwann cells, fibroblasts, immune cells, and capillaries. Since less is known about many of these cell types and their importance in regeneration, they will not be covered further here.
3. Development
The sensory epithelia of the vestibular organs derive from the otic placode, a patch of head ectoderm that forms during early development. The placode grows in size and invaginates into the mesoderm, forming a fluid-filled ball called the otocyst.
The first signs of sensorineural cell fate decisions become apparent when neuroblasts delaminate from the ventral region of the otocyst (E9.5 in mouse) and migrate to the ventro-medial location, where they will eventually differentiate into neurons of the vestibular ganglion. Shortly thereafter (E11.5–E12.5 in mouse), the hair cells within the maculae and cristae begin to emerge from separate patches of neuroepithelial progenitors located more dorsally in the ventral otocyst. Interestingly, many of the non-sensory epithelial cells that separate the vestibular organs appear to derive from the same lineage as their sensory counterparts [57]. Two pouches of cells evaginate from the otocyst near the equator of the ventral and dorsal hemispheres to form the anterior/posterior and lateral canals, respectively (E12.5–E13.5 in mouse). During this process, these pouches increase in size, and then form fusion plates that resorb to produce the tube-like canals. For a more in-depth discussion of these early stages of inner ear development and the molecular cues that control them, the reader is referred to a chapter by Alsina and Whitfield in this issue and to two other reviews [58, 59].
In mice and rats, mitotic tracing experiments have shown that cell cycle exit in the sensory epithelia of the utricle and cristae begins around E13.5/E14.5 and occurs in a pronounced central-to-peripheral pattern [60–62]. Because these studies collected specimens at adult ages after labeling during embryogenesis, they did not determine whether cell cycle exit is temporally coupled to hair cell differentiation in vestibular organs. However, one study correlated the locations of terminal mitoses and the onset of hair cell markers in cristae, suggesting that unlike the cochlea, hair cell differentiation might occur in conjunction or soon after cell cycle exit in those organs [62].
Production of hair cells and supporting cells in the mouse utricle is a protracted process, extending into the postnatal period [60, 63] (Fig. 1). At E13.5, the utricular macula is comprised mostly of the region that will lie medial to the line of polarity reversal, and it is not until late in embryonic development that the lateral region expands. During the 2-week period following birth, approximately half of the hair cell population is formed [63]. Although studies suggest hair cells differentiate in a central-to-peripheral pattern, mirroring cell cycle exit [61, 62], definitive evidence remains to be presented. During embryonic and postnatal development, hair cells in any particular region display various states of morphological and electrophysiological maturation, suggesting that maturation might not be regionally synchronized [26, 30, 64]. It should be noted that hair cell formation appears to be restricted to embryogenesis in humans [65], in contrast to rats and mice.
In mice, newly formed vestibular hair cells are first identified by expression of cell-specific molecular markers such as Atoh1 mRNA as early as E11.5 [57] and Pou4f3 protein shortly thereafter at E12.5 [66]. Hair bundles are first detected around E13.5 in utricles [64, 67] and E15 in cristae [68]. Physiological properties of hair cells, including mechanotransduction-gated and voltage-gated ion currents, develop during the late embryonic and neonatal periods [25, 26, 30, 41].
The lineages of hair cell subtypes within developing vestibular epithelia have not been elucidated. Morphological and electrophysiological evidence indicates that the two subtypes differentiate in parallel, beginning around E18 in mice [25, 26, 69]. While these data suggest the two subtypes diverge from a common immature hair cell, it has also been posited that Type I hair cells transit through a Type II stage on the way to becoming a “super-differentiated” hair cell [70–72]. For example, Kirkegaard and Jørgensen [71] noted in normal adult bat utricles that some hair cells resembled Type II hair cells in morphology and possessed partial calyceal afferent contacts, suggesting Type I hair cells derive from Type II hair cells. Further, during hair cell regeneration in chickens, Weisleder et al. [70] observed that in the striola, Type II hair cell replacement precedes that of Type I hair cell replacement, suggesting Type I’s pass through a Type II stage. Alternatively, mitotic tracing experiments in developing rats show that the progenitors that give rise to Type I’s exit the cell cycle prior to Type II progenitors, suggesting that Type I’s might differentiate first [61]. Since these questions have not been resolved, it remains possible that each hair cell subtype develops independently, even perhaps through different progenitor populations. Elucidation of markers that distinguish different cell types, as well as cell fate-mapping studies, will help to clarify the mechanisms and dynamics of hair cell genesis.
Synaptogenesis occurs concurrent with hair cell differentiation (Fig. 1). In the mouse utricle, sporadic bouton synaptic contacts and partial calyces are detected by E15 and E18, respectively; however, boutons do not become widespread until E18 and full calyces are not apparent until several days after birth [25, 73–75]. In mouse cristae, the first synapses are seen at E20, and innervation appears mature by P10 [76]. Vestibular neural processing, as assessed by the vestibulo-ocular reflex, is also mature around this time [77]. Importantly, some cell-type-specific morphological and physiological features of hair cells develop when innervation is interrupted [25, 78, 79], which indicates hair cell specification does not necessarily rely on continual input from neurons.
When hair cells first differentiate, the actin-rich stereocilia of the hair bundle extend around a centrally positioned cilium. As the stereocilia elongate, the cilium moves to one side of the apical surface, marking the first signs of planar cell polarity (PCP) within the epithelium [80]. In the utricle and saccule, PCP is essential for the establishment of the line of polarity reversal. PCP appears to be independent of subtype identity, since Type I and Type II hair cells are evenly distributed on both sides of this line. An in-depth review of the molecules that establish and maintain PCP is provided by Perrin in this issue.
4. Vestibular cell types and subtypes might exhibit differential susceptibility to damage
Age-related degeneration of Type I and Type II hair cells has been observed in surface preparations and sections of human maculae and cristae [1, 2, 13, 81–84]. Similar declines have been observed in mouse and guinea pig [85, 86]. In several of the aforementioned studies, aged maculae showed less hair cell loss than the cristae, suggesting the utricle and saccule might be less susceptible. Concomitant with loss of sensory hair cells, numbers of vestibular ganglion neurons appear to decrease with age as well [13, 84, 87–90], and losses of both sensory hair cells and neurons correlate with deterioration of vestibular organ function, as measured by multiple tests [6, 91–94]. It remains to be determined whether different regions of the peripheral organs show varying rates of age-associated hair cell loss, or whether there are subtler forms of degeneration that could impact function, such as the synaptopathy that has been carefully documented within the cochlea [95].
In addition to age-related degeneration, more acute traumatic events such as exposure to ototoxins can also harm vestibular cells and function. Hair cells appear to be one of the more susceptible vestibular cell types; however, loss of vestibular ganglion neurons and damage to supporting cells has also been observed [52, 96, 97]. For example, chronic exposure to 3,3′-iminodipropionitrile can lead to transient disassembly of calyceal synapses in the absence of any overt hair cell or ganglion damage [98]. Finally, several studies have found differential susceptibility of hair cell subtypes – Type I versus Type II, macular versus ampullary, and central versus peripheral – to various ototoxins [4, 49, 99–104].
5. Spontaneous regeneration of vestibular hair cells after damage declines with age but persists into adulthood
Remarkably, many adult non-mammalian vertebrates respond to vestibular hair cell death by generating replacements that become innervated and restore sensory function within days or weeks (for a review on cross-species comparisons, see [105]). In birds, which have been most well studied, many replacement hair cells arise as a result of neighboring supporting cells reentering the cell cycle and dividing [106–111]. Post-mitotic cells then differentiate into new hair cells or supporting cells. Studies in amphibians and birds suggest new hair cells may arise via a non-mitotic process, called direct transdifferentiation, during which supporting cells phenotypically convert into hair cells [112–117]. In birds, regenerated Type I and Type II hair cells acquire many normal physiological properties [72, 118], reconnect with afferent nerve fibers [119, 120], and substantially restore vestibular function [121, 122]. However, some limitations exist. For instance, in pigeons, some features of innervation, such as terminal field structure, are not fully restored in the crista after hair cell damage, and deficiencies in gaze stability persist. In order for full functional recovery to occur, organs will likely have to reestablish appropriate numbers and patterning of Type I and II hair cells, as well as precise innervation of the new sensory cells.
Supporting cells in vestibular organs of embryonic and newborn mice are also naturally capable of proliferative hair cell regeneration [54, 123] (Fig. 2). However, most vestibular supporting cells lose the ability to divide during the first week after birth [60, 63, 123–126]. Very few vestibular supporting cells in adult rodents undergo mitosis after complete or near-complete destruction of hair cells (Fig. 2) [127–136]. Nonetheless, adult rodents regenerate some vestibular hair cells, the vast majority of which presumably arise via phenotypic conversion of supporting cells into hair cells [100, 101, 131–134] (Fig. 3). Golub et al. [133] estimated that 17% of hair cells were replaced in utricles of adult mice. Further, there is morphological evidence that humans, even those who are very old, have the capacity to form new utricular hair cells [137]. This is in stark contrast to the nearby cochlea, in which no hair cells are replaced after damage in adulthood [101] (Fig. 3).
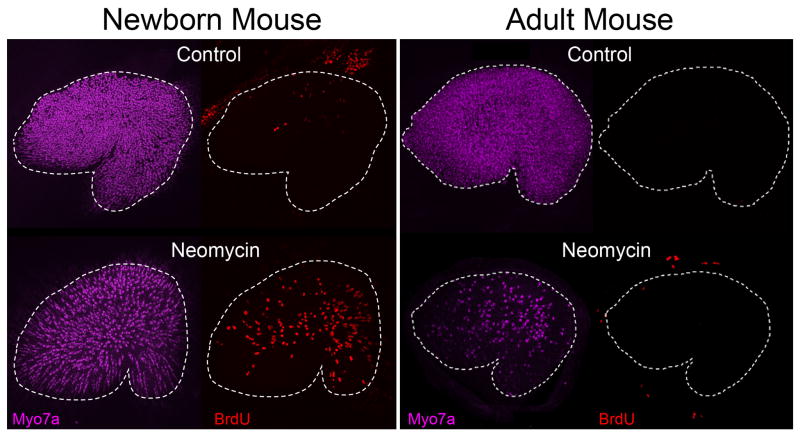
Figure 2. The capacity for mitotic regeneration in the utricle becomes limited during the first postnatal week in mice.
Confocal images of utricles from newborn (P0) or adult (P80) mice that were explanted and cultured for 24 h with or without 3mM Neomycin. After Neomycin treatment, utricles were cultured in the presence of BrdU for an additional 72 h, at which point the organs were fixed an immunolabeled for BrdU and the hair cell marker Myo7a. A significant number of supporting cells in P0 mice reentered the cell cycle in response to damage, whereas few if any supporting cells incorporated BrdU in adults. Note that equivalent Neomycin treatment kills many more hair cells in adults than in neonates. One hypothesis for this difference is that many hair cells in the neonatal epithelium are immature and lack mechanotransduction, the route by which aminoglycosides preferentially enter hair cells [195]. Neonatal mouse images reproduced from [123].
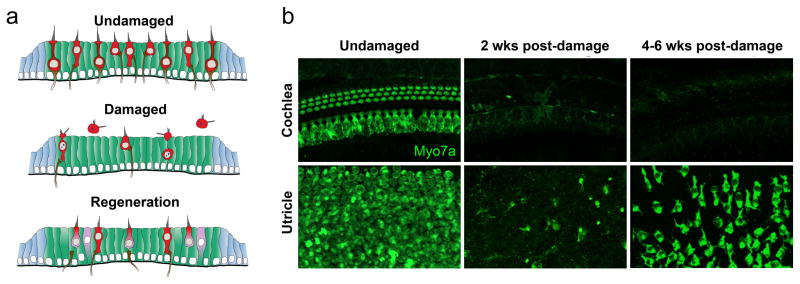
Figure 3. A subpopulation of vestibular hair cells is regenerated after near-complete ablation.
a. Schematic showing the utricular macula under normal conditions, after damage, and after regeneration. b. Confocal images of the mid-apical region of the organ of Corti in the cochlea (top) and the extrastriolar region of the utricle (bottom) in normal conditions, at 2 weeks post-damage (induced by diphtheria toxin in Pou4f3DTR mice, see [133], and at 4–6 weeks post-damage. In both sensory organs, hair cells (green) were killed by diphtheria toxin, and replacement hair cells were detected in the utricle but not the cochlea.
Cell fate-mapping studies have demonstrated new hair cells in adult rodents arise from supporting cells [132]. Oddly, however, morphological analysis indicates that all new hair cells possess short, thin stereocilia and basolateral processes, and they lack calyceal afferent endings, indicating only Type II hair cells are replaced, even after long recovery periods [100, 101, 131, 133]. It is not known at this time why Type I hair cells are not regenerated in mammals or if this partial replacement of new Type II hair cells results in significant functional improvement. In birds, for comparison, the full complement of Type I and Type II hair cells is regenerated after damage [70, 138].
As discussed above, very little supporting cell division accompanies vestibular hair cell replacement in adult mammals. This indicates a non-mitotic form of regeneration must occur. In this case, supporting cells act as post-mitotic hair cell precursors. As expected, in the absence of sufficient supporting cell renewal, supporting cell numbers are reduced during hair cell regeneration in rodents [101, 133]. These observations raise the question of whether stem-like cells exist to replace supporting cells once they convert into hair cells. One hallmark of stem cells is self-renewal, which enables clonal growth of dissociated cells in culture and repeated regeneration of specialized cells in vivo. Cells with the capacity for self-renewal and formation of hair cell-like cells have been isolated from utricles of young mice [139], but numbers of these cells appear to wane significantly after the first postnatal week [140]. Consistent with the findings in neonates, striolar supporting cells in P3 mice selectively upregulate the well-characterized stem cell marker Lgr5 in response to hair cell damage and show enhanced capacity for proliferative hair cell regeneration [54]. However, the finding that extrastriolar supporting cells also regenerate hair cells in neonates and adults indicates that Lgr5-expressing cells are not the only cells with regenerative capacity in the vestibular epithelium.
Mature birds and fish regenerate hair cells in auditory or lateral line organs after repeated injuries separated by recovery periods [141, 142], which is consistent with persistence of stem cells in mature hair-cell epithelia of non-mammals. Furthermore, under normal physiological conditions, supporting cells in birds divide and replace vestibular hair cells that have undergone programmed cell death [143–146]. A recent unpublished study from the laboratories of Jennifer Stone and Brandon Cox used fate-mapping to demonstrate that small numbers of utricular hair cells die and are replaced in adult mice under normal physiological conditions, confirming anatomical observations from earlier studies [100, 127, 147]. While these observations do not confirm the presence of a stem-like cell in vestibular epithelia of adult mammals, they demonstrate that hair cell replacement is a natural innate process in mammals and provide opportunity for investigators to identify signals that restrict this process to Type II hair cells.
6. Lessons from development: molecular level control of proliferation and differentiation in adult vestibular epithelia
A possible explanation for distinct regenerative capacities across animals is that mammalian supporting cells execute a more specialized differentiation program than non-mammalian supporting cells, creating more barriers to de-differentiation after damage. Consistent with this hypothesis, mammalian supporting cells develop extensive cytoskeletal and junctional specializations around the same time their capacity for proliferative regeneration wanes, whereas such specializations are absent in many species of regenerating non-mammals [125, 148, 149]. However, these differences are not fully understood, which poses an obstacle to scientists trying to stimulate hair cell regeneration in humans as a treatment for balance disorders. To circumvent this, one strategy under investigation is to overcome restrictions on proliferation and differentiation in adult vestibular epithelia by awakening molecular programs that promote hair cell and supporting cell formation during development.
Enhancing the number of supporting cells that convert into hair cells could be the simplest and safest route, since it only involves one step and poses less oncogenic risk. The basic helix-loop-helix transcription factor Atoh1 is the earliest known determinant of a hair cell fate [150–152]. Atoh1 expression is re-activated after hair cell loss in adult mouse utricles as new hair cells form and differentiate [132, 153]. Forced expression of Atoh1 in supporting cells using viral vectors appears to augment hair cell regeneration in damaged vestibular organs from adult rodents [154–158]. However, functional recovery was small or absent, and electrophysiological properties of new hair cells were not carefully examined. In contrast with results from adenoviral vectors, evidence from transgenic mice with inducible Atoh1 expression indicates utricular supporting cells become refractory to Atoh1-mediated hair cell conversion within a month after birth [159]. One aspect that has yet to be addressed is whether the means of Atoh1 overexpression (i.e., viral delivery to the ear or activation in transgenic mice) is a factor that contributes to different outcomes.
The Notch pathway is critical for both hair cell and supporting cell differentiation during development (reviewed in [160, 161]). Ligands expressed on the surfaces of differentiating hair cells bind the Notch receptor on adjacent undifferentiated cells. As a result, the cleaved Notch receptor represses Atoh1 transcription, blocking new cells from acquiring the hair cell fate. In neonatal mice, blockade of Notch receptor cleavage results in massive supporting-cell-to-hair-cell conversion [55]. A smaller but significant effect of Notch receptor inactivation upon hair cell regeneration is observed in adult vestibular organs after killing hair cells [132, 134].
Another potent regulator of vestibular hair cell regeneration is canonical Wnt signaling. In neonatal mice, forced expression of the Wnt signaling effector, β-catenin, promotes mitotic hair cell replacement, particularly in the striola [54]. However, Wu et al. [162] found that pharmacological inhibition of Notch or activation of β-catenin alone failed to trigger supporting cell division in adult mouse utricles. Nevertheless, simultaneous inactivation of Notch and activation of β-catenin led to a small but significant increase in supporting cell division.
Many of the aforementioned studies measured hair cell regeneration using markers of young hair cells. Accordingly, it remains unclear whether initiating the earliest stages of hair cell differentiation is sufficient for creating a functional hair cell or if additional factors will be necessary to induce replacement of both Type I and II hair cells in adults, with proper hair bundle maturation, PCP, and innervation. Most likely, multiple manipulations will be required to achieve significant recovery of vestibular function after hair cell damage has occurred.
By its very nature, conversion of supporting cells into hair cells will result in supporting cell depletion, unless supporting cells are replaced by cell division. Extracellular stimulation of proliferative regeneration with exogenous factors like growth factors would be ideal, and a number of studies have shown that select fibroblast growth factors, transforming growth factors, insulin-like growth factors, epidermal growth factors, and Wnt agonists can elicit cell cycle reentry of vestibular supporting cells in vitro and in vivo [162–167]. Unfortunately, responsiveness to growth factors and Wnt agonists declines sharply with age despite continued expression of these ligands’ receptors, which has led researchers to seek alternative means of stimulating proliferation in adults.
Cyclin dependent kinase inhibitors (CDKIs) such as p19Ink4d, p21Cip1, p27Kip1 and pocket proteins such as Rb1, Rbl1/p107, and Rbl2/p130 have vital roles in turning off the proliferation machinery as the cells in eukaryotes stop DNA replication and cell division. Deletion experiments have shown that p19Ink4d, p21Cip1, p27Kip1, Rb1, and Rbl2/p130 contribute to cell cycle exit in cochlear progenitor cells [168–176], and some of these findings have been extended to the vestibular organs. Rb1 deletion in embryonic and neonatal mice can lead to cell cycle entry of pre-existing, differentiated hair cells and supporting cells in the utricle [169, 170], and it appears that some dividing hair cells may survive [171]. Interestingly, unlike in the cochlea, co-deletion of p19Ink4d and p21Cip1 has no effect on proliferation in the vestibular organs, indicating that the postmitotic state is at least partly regulated by distinct CDKIs in different regions of the inner ear [168]. The influence of p27Kip1 and Rbl2/p130 is not well characterized in the vestibular system.
Overexpressing positive regulators of the cell cycle might bypass repressive signals like pocket proteins and CDKIs. CyclinD1 expression declines as hair cells and supporting cells exit the cell cycle, and forced expression of cyclin D1 in neonatal and adult mouse utricles triggers cell cycle reentry of hair cells and/or supporting cells [177, 178]. Conditional deletion of N-Myc in the embryonic ear reduces growth of the sensory epithelium by inhibiting proliferation [179, 180], and overexpression of N-Myc (J. Burns, unpublished results) or c-Myc, a closely related family member, results in robust proliferation of supporting cells in the adult mouse utricle in vitro [181]. Neither cyclin D1 nor c-Myc overexpression is able to induce re-growth of the sensory epithelium, as proliferating cells either become arrested in G2 or undergo apoptosis after passing through M phase, apparently due to accumulation of DNA damage [182]. Alternatively, reprogramming mature supporting cells to a progenitor-like state might make supporting cells more amenable to cell cycle reentry. The pioneer transcription factors Sox4 and Sox11 are downregulated in utricular supporting cells in the neonatal period, and overexpression of these genes in juvenile mice restores sufficient plasticity to induce supporting cell proliferation and the formation of a limited number of new hair cells in vitro [183].
7. Future outlook
In contrast to the cochlea, the cellular plasticity that persists into adulthood within mammalian vestibular organs could make restoration of vestibular function more feasible in the near-term than overcoming the many challenges associated with hearing loss. Morphology, patterning, and physiology of vestibular cells also appear to be less complex than their cochlear counterparts. However, the vestibular organs are still relatively intricate structures that contain a diverse array of cell types and subtypes. Important outstanding work in the field includes elucidating the molecular mechanisms that control cell type diversity, understanding how cellular maturation limits plasticity in mammals but not non-mammals, physiological testing of the extent to which spontaneous regeneration restores function, and further characterization of the cell types and structures that are most susceptible to damage and/or most necessary for proper vestibular sensation. Studies on regeneration of vestibular ganglion neurites are particularly lacking.
Emerging technological breakthroughs position the field to move ahead at a quicker pace. A number of high throughput methods are being used to profile the transcriptome and proteome during processes like hair cell differentiation [24, 184–186], growth of the hair bundle [187, 188], the cellular response to ototoxic insult [189], and hair cell regeneration in non-mammals [190–192]. As applied to differentiation and regeneration, single-cell transcriptional profiling is particularly powerful, since it characterizes these processes for distinct cell types and subtypes in an unbiased manner. For example, single-cell RNA-Seq was recently used to reconstruct the early stages of hair cell differentiation in the neonatal mouse utricle [24], and work is underway to extend this trajectory to include branching into Type I and Type II subtypes as well as maturation of supporting cells. Molecular characterization of cells appears to be less of a hurdle now more than ever; thus the biggest bottleneck moving forward is the throughput of the perturbation methods used to validate the function of candidate genes and proteins. This bottleneck may be opened by in vitro systems like organoids and improvements in genetic manipulation like CRISPR/Cas9. Thousands of miniature vestibular sensory epithelia can now be generated in a dish from embryonic stem cells, and the epithelia contain mechanotransducing Type I and Type II hair cells [193, 194].
In summary, there are many outstanding questions that remain to be explored in vestibular development and regeneration. More attention appears to be focused on the vestibular organs in recent years as the debilitating effects of balance disorders become increasingly apparent. Equipped with higher throughput tools, researchers are set to solve the riddle of how to regenerate different vestibular cell types and restore sensory function.
Acknowledgments
We would like to thank Mark Warchol (Washington University, St Louis) for critical comments on the manuscript, as well as the efforts of reviewers and editors who helped build this special journal issue. The Stone lab receives research support from National Institutes of Health (DC013695, DC013358, DC013771) and the Hearing Health Foundation’s Hearing Restoration Project.
Abbreviations
- K
potassium
- CDKIs
cyclin dependent kinase inhibitors
- PCP
planar cell polarity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Merchant SN, Velazquez-Villasenor L, Tsuji K, Glynn RJ, Wall C, 3rd, Rauch SD. Temporal bone studies of the human peripheral vestibular system. Normative vestibular hair cell data. Ann Otol Rhinol Laryngol Suppl. 2000;181:3–13. doi: 10.1177/00034894001090s502. [DOI] [PubMed] [Google Scholar]
- 2.Rauch SD, Velazquez-Villaseñor L, Dimitri PS, Merchant SN. Decreasing hair cell counts in aging humans. Ann N Y Acad Sci. 2001;942:220–7. doi: 10.1111/j.1749-6632.2001.tb03748.x. [DOI] [PubMed] [Google Scholar]
- 3.Velazquez-Villasenor L, Merchant SN, Tsuji K, Glynn RJ, Wall C, 3rd, Rauch SD. Temporal bone studies of the human peripheral vestibular system. Normative Scarpa’s ganglion cell data. Ann Otol Rhinol Laryngol Suppl. 2000;181:14–9. doi: 10.1177/00034894001090s503. [DOI] [PubMed] [Google Scholar]
- 4.Tsuji K, Velazquez-Villasenor L, Rauch SD, Glynn RJ, Wall C, 3rd, Merchant SN. Temporal bone studies of the human peripheral vestibular system. Aminoglycoside ototoxicity. Ann Otol Rhinol Laryngol Suppl. 2000;181:20–5. doi: 10.1177/00034894001090s504. [DOI] [PubMed] [Google Scholar]
- 5.Ishiyama G, Finn M, Lopez I, Tang Y, Baloh RW, Ishiyama A. Unbiased quantification of Scarpa’s ganglion neurons in aminoglycoside ototoxicity. J Vestib Res. 2005;15:197–202. [PubMed] [Google Scholar]
- 6.Agrawal Y, Carey JP, Della Santina CC, Schubert MC, Minor LB. Disorders of balance and vestibular function in US adults: data from the National Health and Nutrition Examination Survey, 2001–2004. Arch Intern Med. 2009;169:938–44. doi: 10.1001/archinternmed.2009.66. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez JC, Diaz C, Suarez C, Fernandez JA, Gonzalez del Rey C, Navarro A, et al. Neuronal loss in human medial vestibular nucleus. Anat Rec. 1998;251:431–8. doi: 10.1002/(SICI)1097-0185(199808)251:4<431::AID-AR2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 8.Desai SS, Ali H, Lysakowski A. Comparative morphology of rodent vestibular periphery. II. Cristae ampullares. J Neurophysiol. 2005;93:267–80. doi: 10.1152/jn.00747.2003. [DOI] [PubMed] [Google Scholar]
- 9.Desai SS, Zeh C, Lysakowski A. Comparative morphology of rodent vestibular periphery. I. Saccular and utricular maculae. J Neurophysiol. 2005;93:251–66. doi: 10.1152/jn.00746.2003. [DOI] [PubMed] [Google Scholar]
- 10.Wersall J. Studies on the structure and innervation of the sensory epithelium of the cristae ampulares in the guinea pig; a light and electron microscopic investigation. Acta Otolaryngol Suppl. 1956;126:1–85. [PubMed] [Google Scholar]
- 11.Li A, Xue J, Peterson EH. Architecture of the mouse utricle: macular organization and hair bundle heights. J Neurophysiol. 2008;99:718–33. doi: 10.1152/jn.00831.2007. [DOI] [PubMed] [Google Scholar]
- 12.Gopen Q, Lopez I, Ishiyama G, Baloh RW, Ishiyama A. Unbiased stereologic type I and type II hair cell counts in human utricular macula. Laryngoscope. 2003;113:1132–8. doi: 10.1097/00005537-200307000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Lopez I, Ishiyama G, Tang Y, Tokita J, Baloh RW, Ishiyama A. Regional estimates of hair cells and supporting cells in the human crista ampullaris. J Neurosci Res. 2005;82:421–31. doi: 10.1002/jnr.20652. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez C, Lysakowski A, Goldberg JM. Hair-cell counts and afferent innervation patterns in the cristae ampullares of the squirrel monkey with a comparison to the chinchilla. J Neurophysiol. 1995;73:1253–69. doi: 10.1152/jn.1995.73.3.1253. [DOI] [PubMed] [Google Scholar]
- 15.Si X, Zakir MM, Dickman JD. Afferent innervation of the utricular macula in pigeons. J Neurophysiol. 2003;89:1660–77. doi: 10.1152/jn.00690.2002. [DOI] [PubMed] [Google Scholar]
- 16.Xue J, Peterson EH. Hair bundle heights in the utricle: differences between macular locations and hair cell types. J Neurophysiol. 2006;95:171–86. doi: 10.1152/jn.00800.2005. [DOI] [PubMed] [Google Scholar]
- 17.Eatock RA, Songer JE. Vestibular hair cells and afferents: two channels for head motion signals. Annu Rev Neurosci. 2011;34:501–34. doi: 10.1146/annurev-neuro-061010-113710. [DOI] [PubMed] [Google Scholar]
- 18.Contini D, Zampini V, Tavazzani E, Magistretti J, Russo G, Prigioni I, et al. Intercellular K(+) accumulation depolarizes Type I vestibular hair cells and their associated afferent nerve calyx. Neuroscience. 2012;227:232–46. doi: 10.1016/j.neuroscience.2012.09.051. [DOI] [PubMed] [Google Scholar]
- 19.Ricci AJ, Rennie KJ, Cochran SL, Kevetter GA, Correia MJ. Vestibular type I and type II hair cells. 1: Morphometric identification in the pigeon and gerbil. J Vestib Res. 1997;7:393–406. [PubMed] [Google Scholar]
- 20.Lysakowski A, Goldberg JM. A regional ultrastructural analysis of the cellular and synaptic architecture in the chinchilla cristae ampullares. J Comp Neurol. 1997;389:419–43. doi: 10.1002/(sici)1096-9861(19971222)389:3<419::aid-cne5>3.0.co;2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pujol R, Pickett SB, Nguyen TB, Stone JS. Large basolateral processes on type II hair cells are novel processing units in mammalian vestibular organs. J Comp Neurol. 2014;522:3141–59. doi: 10.1002/cne.23625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oesterle EC, Campbell S, Taylor RR, Forge A, Hume CR. Sox2 and JAGGED1 expression in normal and drug-damaged adult mouse inner ear. J Assoc Res Otolaryngol. 2008;9:65–89. doi: 10.1007/s10162-007-0106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simmons DD, Tong B, Schrader AD, Hornak AJ. Oncomodulin identifies different hair cell types in the mammalian inner ear. J Comp Neurol. 2010;518:3785–802. doi: 10.1002/cne.22424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burns JC, Kelly MC, Hoa M, Morell RJ, Kelley MW. Single-cell RNA-Seq resolves cellular complexity in sensory organs from the neonatal inner ear. Nature communications. 2015;6:8557. doi: 10.1038/ncomms9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rusch A, Lysakowski A, Eatock RA. Postnatal development of type I and type II hair cells in the mouse utricle: acquisition of voltage-gated conductances and differentiated morphology. J Neurosci. 1998;18:7487–501. doi: 10.1523/JNEUROSCI.18-18-07487.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geleoc GS, Risner JR, Holt JR. Developmental acquisition of voltage-dependent conductances and sensory signaling in hair cells of the embryonic mouse inner ear. J Neurosci. 2004;24:11148–59. doi: 10.1523/JNEUROSCI.2662-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holt JR, Vollrath MA, Eatock RA. Stimulus processing by type II hair cells in the mouse utricle. Ann N Y Acad Sci. 1999;871:15–26. doi: 10.1111/j.1749-6632.1999.tb09172.x. [DOI] [PubMed] [Google Scholar]
- 28.Songer JE, Eatock RA. Tuning and timing in mammalian type I hair cells and calyceal synapses. J Neurosci. 2013;33:3706–24. doi: 10.1523/JNEUROSCI.4067-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bao H, Wong WH, Goldberg JM, Eatock RA. Voltage-gated calcium channel currents in type I and type II hair cells isolated from the rat crista. J Neurophysiol. 2003;90:155–64. doi: 10.1152/jn.00244.2003. [DOI] [PubMed] [Google Scholar]
- 30.Wooltorton JR, Gaboyard S, Hurley KM, Price SD, Garcia JL, Zhong M, et al. Developmental changes in two voltage-dependent sodium currents in utricular hair cells. J Neurophysiol. 2007;97:1684–704. doi: 10.1152/jn.00649.2006. [DOI] [PubMed] [Google Scholar]
- 31.Rusch A, Eatock RA. A delayed rectifier conductance in type I hair cells of the mouse utricle. J Neurophysiol. 1996;76:995–1004. doi: 10.1152/jn.1996.76.2.995. [DOI] [PubMed] [Google Scholar]
- 32.Rennie KJ, Correia MJ. Potassium currents in mammalian and avian isolated type I semicircular canal hair cells. J Neurophysiol. 1994;71:317–29. doi: 10.1152/jn.1994.71.1.317. [DOI] [PubMed] [Google Scholar]
- 33.Rennie KJ, Ricci AJ, Correia MJ. Electrical filtering in gerbil isolated type I semicircular canal hair cells. J Neurophysiol. 1996;75:2117–23. doi: 10.1152/jn.1996.75.5.2117. [DOI] [PubMed] [Google Scholar]
- 34.Correia MJ, Lang DG. An electrophysiological comparison of solitary type I and type II vestibular hair cells. Neurosci Lett. 1990;116:106–11. doi: 10.1016/0304-3940(90)90394-o. [DOI] [PubMed] [Google Scholar]
- 35.Holt JC, Chatlani S, Lysakowski A, Goldberg JM. Quantal and nonquantal transmission in calyx-bearing fibers of the turtle posterior crista. J Neurophysiol. 2007;98:1083–101. doi: 10.1152/jn.00332.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hurley KM, Gaboyard S, Zhong M, Price SD, Wooltorton JR, Lysakowski A, et al. M-like K+ currents in type I hair cells and calyx afferent endings of the developing rat utricle. J Neurosci. 2006;26:10253–69. doi: 10.1523/JNEUROSCI.2596-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kharkovets T, Hardelin JP, Safieddine S, Schweizer M, El-Amraoui A, Petit C, et al. KCNQ4, a K+ channel mutated in a form of dominant deafness, is expressed in the inner ear and the central auditory pathway. Proc Natl Acad Sci U S A. 2000;97:4333–8. doi: 10.1073/pnas.97.8.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rennie KJ, Weng T, Correia MJ. Effects of KCNQ channel blockers on K(+) currents in vestibular hair cells. Am J Physiol Cell Physiol. 2001;280:C473–80. doi: 10.1152/ajpcell.2001.280.3.C473. [DOI] [PubMed] [Google Scholar]
- 39.Rocha-Sanchez SM, Morris KA, Kachar B, Nichols D, Fritzsch B, Beisel KW. Developmental expression of Kcnq4 in vestibular neurons and neurosensory epithelia. Brain Res. 2007;1139:117–25. doi: 10.1016/j.brainres.2006.12.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schweizer FE, Savin D, Luu C, Sultemeier DR, Hoffman LF. Distribution of high-conductance calcium-activated potassium channels in rat vestibular epithelia. J Comp Neurol. 2009;517:134–45. doi: 10.1002/cne.22148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li GQ, Meredith FL, Rennie KJ. Development of K(+) and Na(+) conductances in rodent postnatal semicircular canal type I hair cells. American journal of physiology Regulatory, integrative and comparative physiology. 2010;298:R351–8. doi: 10.1152/ajpregu.00460.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernandez C, Baird RA, Goldberg JM. The vestibular nerve of the chinchilla. I. Peripheral innervation patterns in the horizontal and superior semicircular canals. J Neurophysiol. 1988;60:167–81. doi: 10.1152/jn.1988.60.1.167. [DOI] [PubMed] [Google Scholar]
- 43.Fernandez C, Goldberg JM, Baird RA. The vestibular nerve of the chinchilla. III. Peripheral innervation patterns in the utricular macula. J Neurophysiol. 1990;63:767–80. doi: 10.1152/jn.1990.63.4.767. [DOI] [PubMed] [Google Scholar]
- 44.Maklad A, Kamel S, Wong E, Fritzsch B. Development and organization of polarity-specific segregation of primary vestibular afferent fibers in mice. Cell Tissue Res. 2010;340:303–21. doi: 10.1007/s00441-010-0944-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baird RA, Desmadryl G, Fernandez C, Goldberg JM. The vestibular nerve of the chinchilla. II. Relation between afferent response properties and peripheral innervation patterns in the semicircular canals. J Neurophysiol. 1988;60:182–203. doi: 10.1152/jn.1988.60.1.182. [DOI] [PubMed] [Google Scholar]
- 46.Goldberg JM, Desmadryl G, Baird RA, Fernandez C. The vestibular nerve of the chinchilla. V. Relation between afferent discharge properties and peripheral innervation patterns in the utricular macula. J Neurophysiol. 1990;63:791–804. doi: 10.1152/jn.1990.63.4.791. [DOI] [PubMed] [Google Scholar]
- 47.Goldberg JM, Smith CE, Fernandez C. Relation between discharge regularity and responses to externally applied galvanic currents in vestibular nerve afferents of the squirrel monkey. J Neurophysiol. 1984;51:1236–56. doi: 10.1152/jn.1984.51.6.1236. [DOI] [PubMed] [Google Scholar]
- 48.Sadeghi SG, Chacron MJ, Taylor MC, Cullen KE. Neural variability, detection thresholds, and information transmission in the vestibular system. J Neurosci. 2007;27:771–81. doi: 10.1523/JNEUROSCI.4690-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hullar TE, Della Santina CC, Hirvonen T, Lasker DM, Carey JP, Minor LB. Responses of irregularly discharging chinchilla semicircular canal vestibular-nerve afferents during high-frequency head rotations. J Neurophysiol. 2005;93:2777–86. doi: 10.1152/jn.01002.2004. [DOI] [PubMed] [Google Scholar]
- 50.Minor LB, Goldberg JM. Vestibular-nerve inputs to the vestibulo-ocular reflex: a functional-ablation study in the squirrel monkey. J Neurosci. 1991;11:1636–48. doi: 10.1523/JNEUROSCI.11-06-01636.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wan G, Corfas G, Stone JS. Inner ear supporting cells: rethinking the silent majority. Semin Cell Dev Biol. 2013;24:448–59. doi: 10.1016/j.semcdb.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Monzack EL, Cunningham LL. Lead roles for supporting actors: critical functions of inner ear supporting cells. Hear Res. 2013;303:20–9. doi: 10.1016/j.heares.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rau A, Legan PK, Richardson GP. Tectorin mRNA expression is spatially and temporally restricted during mouse inner ear development. J Comp Neurol. 1999;405:271–80. [PubMed] [Google Scholar]
- 54.Wang T, Chai R, Kim GS, Pham N, Jansson L, Nguyen DH, et al. Lgr5+ cells regenerate hair cells via proliferation and direct transdifferentiation in damaged neonatal mouse utricle. Nature communications. 2015;6:6613. doi: 10.1038/ncomms7613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Collado MS, Thiede BR, Baker W, Askew C, Igbani LM, Corwin JT. The postnatal accumulation of junctional E-cadherin is inversely correlated with the capacity for supporting cells to convert directly into sensory hair cells in mammalian balance organs. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:11855–66. doi: 10.1523/JNEUROSCI.2525-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaur T, Hirose K, Rubel EW, Warchol ME. Macrophage recruitment and epithelial repair following hair cell injury in the mouse utricle. Front Cell Neurosci. 2015;9:150. doi: 10.3389/fncel.2015.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raft S, Koundakjian EJ, Quinones H, Jayasena CS, Goodrich LV, Johnson JE, et al. Cross-regulation of Ngn1 and Math1 coordinates the production of neurons and sensory hair cells during inner ear development. Development. 2007;134:4405–15. doi: 10.1242/dev.009118. [DOI] [PubMed] [Google Scholar]
- 58.Wu DK, Kelley MW. Molecular mechanisms of inner ear development. Cold Spring Harb Perspect Biol. 2012;4:a008409. doi: 10.1101/cshperspect.a008409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Groves AK, Fekete DM. Shaping sound in space: the regulation of inner ear patterning. Development. 2012;139:245–57. doi: 10.1242/dev.067074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ruben RJ. Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta Otolaryngol. 1967;(Suppl 220):1–44. [PubMed] [Google Scholar]
- 61.Sans A, Chat M. Analysis of temporal and spatial patterns of rat vestibular hair cell differentiation by tritiated thymidine radioautography. J Comp Neurol. 1982;206:1–8. doi: 10.1002/cne.902060102. [DOI] [PubMed] [Google Scholar]
- 62.Slowik AD, Bermingham-McDonogh O. A central to peripheral progression of cell cycle exit and hair cell differentiation in the developing mouse cristae. Dev Biol. 2016;411:1–14. doi: 10.1016/j.ydbio.2016.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burns JC, On D, Baker W, Collado MS, Corwin JT. Over Half the Hair Cells in the Mouse Utricle First Appear After Birth, with Significant Numbers Originating from Early Postnatal Mitotic Production in Peripheral and Striolar Growth Zones. J Assoc Res Otolaryngol. 2012 doi: 10.1007/s10162-012-0337-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Denman-Johnson K, Forge A. Establishment of hair bundle polarity and orientation in the developing vestibular system of the mouse. J Neurocytol. 1999;28:821–35. doi: 10.1023/a:1007061819934. [DOI] [PubMed] [Google Scholar]
- 65.Severinsen SA, Sorensen MS, Kirkegaard M, Nyengaard JR. Stereological estimation of total cell numbers in the young human utricular macula. Acta Otolaryngol. 2010;130:773–9. doi: 10.3109/00016480903397694. [DOI] [PubMed] [Google Scholar]
- 66.Xiang M, Gao WQ, Hasson T, Shin JJ. Requirement for Brn-3c in maturation and survival, but not in fate determination of inner ear hair cells. Development. 1998;125:3935–46. doi: 10.1242/dev.125.20.3935. [DOI] [PubMed] [Google Scholar]
- 67.Mbiene JP, Favre D, Sans A. The pattern of ciliary development in fetal mouse vestibular receptors. A qualitative and quantitative SEM study. Anat Embryol (Berl) 1984;170:229–38. doi: 10.1007/BF00318726. [DOI] [PubMed] [Google Scholar]
- 68.Mbiene JP, Sans A. Differentiation and maturation of the sensory hair bundles in the fetal and postnatal vestibular receptors of the mouse: a scanning electron microscopy study. J Comp Neurol. 1986;254:271–8. doi: 10.1002/cne.902540210. [DOI] [PubMed] [Google Scholar]
- 69.Nordemar H. Postnatal development of the vestibular sensory epithelium in the mouse. Acta Otolaryngol. 1983;96:447–56. doi: 10.3109/00016488309132731. [DOI] [PubMed] [Google Scholar]
- 70.Weisleder P, Tsue TT, Rubel EW. Hair cell replacement in avian vestibular epithelium: supporting cell to type I hair cell. Hear Res. 1995;82:125–33. doi: 10.1016/0378-5955(94)00169-q. [DOI] [PubMed] [Google Scholar]
- 71.Kirkegaard M, Jorgensen JM. Continuous hair cell turnover in the inner ear vestibular organs of a mammal, the Daubenton’s bat (Myotis daubentonii) Die Naturwissenschaften. 2000;87:83–6. doi: 10.1007/s001140050015. [DOI] [PubMed] [Google Scholar]
- 72.Masetto S, Correia MJ. Electrophysiological properties of vestibular sensory and supporting cells in the labyrinth slice before and during regeneration. J Neurophysiol. 1997;78:1913–27. doi: 10.1152/jn.1997.78.4.1913. [DOI] [PubMed] [Google Scholar]
- 73.Anniko M, Nordemar H, Sobin A. Principles in embryonic development and differentiation of vestibular hair cells. Otolaryngol Head Neck Surg. 1983;91:540–9. doi: 10.1177/019459988309100513. [DOI] [PubMed] [Google Scholar]
- 74.Anniko M. Formation and maturation of the vestibular ganglion. ORL J Otorhinolaryngol Relat Spec. 1985;47:57–65. doi: 10.1159/000275746. [DOI] [PubMed] [Google Scholar]
- 75.Mbiene JP, Favre D, Sans A. Early innervation and differentiation of hair cells in the vestibular epithelia of mouse embryos: SEM and TEM study. Anat Embryol (Berl) 1988;177:331–40. doi: 10.1007/BF00315841. [DOI] [PubMed] [Google Scholar]
- 76.Desmadryl G, Sans A. Afferent innervation patterns in crista ampullaris of the mouse during ontogenesis. Brain research Developmental brain research. 1990;52:183–9. doi: 10.1016/0165-3806(90)90234-p. [DOI] [PubMed] [Google Scholar]
- 77.Faulstich BM, Onori KA, du Lac S. Comparison of plasticity and development of mouse optokinetic and vestibulo-ocular reflexes suggests differential gain control mechanisms. Vision Res. 2004;44:3419–27. doi: 10.1016/j.visres.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 78.Anniko M, Nordemar H, Wersäll J. Genesis and maturation of vestibular hair cells. Adv Otorhinolaryngol. 1979;25:7–11. doi: 10.1159/000402909. [DOI] [PubMed] [Google Scholar]
- 79.Kim WY, Fritzsch B, Serls A, Bakel LA, Huang EJ, Reichardt LF, et al. NeuroD-null mice are deaf due to a severe loss of the inner ear sensory neurons during development. Development. 2001;128:417–26. doi: 10.1242/dev.128.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tilney LG, Tilney MS, DeRosier DJ. Actin filaments, stereocilia, and hair cells: how cells count and measure. Annu Rev Cell Biol. 1992;8:257–74. doi: 10.1146/annurev.cb.08.110192.001353. [DOI] [PubMed] [Google Scholar]
- 81.Rosenhall U. Mapping of the cristae ampullares in man. Ann Otol Rhinol Laryngol. 1972;81:882–9. doi: 10.1177/000348947208100622. [DOI] [PubMed] [Google Scholar]
- 82.Rosenhall U. Vestibular macular mapping in man. Ann Otol Rhinol Laryngol. 1972;81:339–51. doi: 10.1177/000348947208100305. [DOI] [PubMed] [Google Scholar]
- 83.Rosenhall U. Degenerative patterns in the aging human vestibular neuro-epithelia. Acta Otolaryngol. 1973;76:208–20. doi: 10.3109/00016487309121501. [DOI] [PubMed] [Google Scholar]
- 84.Richter E. Quantitative study of human Scarpa’s ganglion and vestibular sensory epithelia. Acta Otolaryngol. 1980;90:199–208. doi: 10.3109/00016488009131716. [DOI] [PubMed] [Google Scholar]
- 85.Severinsen SA, Raarup MK, Ulfendahl M, Wogensen L, Nyengaard JR, Kirkegaard M. Type I hair cell degeneration in the utricular macula of the waltzing guinea pig. Hear Res. 2008;236:33–41. doi: 10.1016/j.heares.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 86.Park JC, Hubel SB, Woods AD. Morphometric analysis and fine structure of the vestibular epithelium of aged C57BL/6NNia mice. Hear Res. 1987;28:87–96. doi: 10.1016/0378-5955(87)90156-0. [DOI] [PubMed] [Google Scholar]
- 87.Alidina A, Lyon MJ. Aging rat vestibular ganglion: I. Quantitative light microscopic evaluation. Am J Otolaryngol. 1990;11:174–81. doi: 10.1016/0196-0709(90)90034-s. [DOI] [PubMed] [Google Scholar]
- 88.Lyon MJ, King JM. Aging rat vestibular ganglion: II. Quantitative electron microscopic evaluation. Ann Otol Rhinol Laryngol. 1997;106:753–8. doi: 10.1177/000348949710600908. [DOI] [PubMed] [Google Scholar]
- 89.Park JJ, Tang Y, Lopez I, Ishiyama A. Unbiased estimation of human vestibular ganglion neurons. Ann N Y Acad Sci. 2001;942:475–8. doi: 10.1111/j.1749-6632.2001.tb03773.x. [DOI] [PubMed] [Google Scholar]
- 90.Ishiyama G, Geiger C, Lopez IA, Ishiyama A. Spiral and vestibular ganglion estimates in archival temporal bones obtained by design based stereology and Abercrombie methods. J Neurosci Methods. 2011;196:76–80. doi: 10.1016/j.jneumeth.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 91.Mock B, Jones TA, Jones SM. Gravity receptor aging in the CBA/CaJ strain: a comparison to auditory aging. J Assoc Res Otolaryngol. 2011;12:173–83. doi: 10.1007/s10162-010-0247-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mock BE, Vijayakumar S, Pierce J, Jones TA, Jones SM. Differential effects of Cdh23(753A) on auditory and vestibular functional aging in C57BL/6J mice. Neurobiol Aging. 2016;43:13–22. doi: 10.1016/j.neurobiolaging.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Agrawal Y, Zuniga MG, Davalos-Bichara M, Schubert MC, Walston JD, Hughes J, et al. Decline in semicircular canal and otolith function with age. Otol Neurotol. 2012;33:832–9. doi: 10.1097/MAO.0b013e3182545061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee SK, Cha CI, Jung TS, Park DC, Yeo SG. Age-related differences in parameters of vestibular evoked myogenic potentials. Acta Otolaryngol. 2008;128:66–72. doi: 10.1080/00016480701387108. [DOI] [PubMed] [Google Scholar]
- 95.Sergeyenko Y, Lall K, Liberman MC, Kujawa SG. Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J Neurosci. 2013;33:13686–94. doi: 10.1523/JNEUROSCI.1783-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sedó-Cabezón L, Boadas-Vaello P, Soler-Martín C, Llorens J. Vestibular damage in chronic ototoxicity: a mini-review. Neurotoxicology. 2014;43:21–7. doi: 10.1016/j.neuro.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 97.Slattery EL, Oshima K, Heller S, Warchol ME. Cisplatin exposure damages resident stem cells of the mammalian inner ear. Dev Dyn. 2014;243:1328–37. doi: 10.1002/dvdy.24150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sedó-Cabezón L, Jedynak P, Boadas-Vaello P, Llorens J. Transient alteration of the vestibular calyceal junction and synapse in response to chronic ototoxic insult in rats. Dis Model Mech. 2015;8:1323–37. doi: 10.1242/dmm.021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lindeman HH. Regional differences in sensitivity of the vestibular sensory epithelia to ototoxic antibiotics. Acta oto-laryngologica. 1969;67:177–89. doi: 10.3109/00016486909125441. [DOI] [PubMed] [Google Scholar]
- 100.Forge A, Li L, Corwin JT, Nevill G. Ultrastructural evidence for hair cell regeneration in the mammalian inner ear. Science. 1993;259:1616–9. doi: 10.1126/science.8456284. [DOI] [PubMed] [Google Scholar]
- 101.Forge A, Li L, Nevill G. Hair cell recovery in the vestibular sensory epithelia of mature guinea pigs. J Comp Neurol. 1998;397:69–88. [PubMed] [Google Scholar]
- 102.Lyford-Pike S, Vogelheim C, Chu E, Della Santina CC, Carey JP. Gentamicin is primarily localized in vestibular type I hair cells after intratympanic administration. J Assoc Res Otolaryngol. 2007;8:497–508. doi: 10.1007/s10162-007-0093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sun DQ, Lehar M, Dai C, Swarthout L, Lauer AM, Carey JP, et al. Histopathologic Changes of the Inner ear in Rhesus Monkeys After Intratympanic Gentamicin Injection and Vestibular Prosthesis Electrode Array Implantation. J Assoc Res Otolaryngol. 2015;16:373–87. doi: 10.1007/s10162-015-0515-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wersall J, Hawkins JE., Jr The vestibular sensory epithelia in the cat labyrinth and their reactions in chronic streptomycin intoxication. Acta oto-laryngologica. 1962;54:1–23. doi: 10.3109/00016486209126917. [DOI] [PubMed] [Google Scholar]
- 105.Warchol ME. Sensory regeneration in the vertebrate inner ear: differences at the levels of cells and species. Hear Res. 2011;273:72–9. doi: 10.1016/j.heares.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 106.Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240:1772–4. doi: 10.1126/science.3381100. [DOI] [PubMed] [Google Scholar]
- 107.Jones JE, Corwin JT. Replacement of lateral line sensory organs during tail regeneration in salamanders: identification of progenitor cells and analysis of leukocyte activity. J Neurosci. 1993;13:1022–34. doi: 10.1523/JNEUROSCI.13-03-01022.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jones JE, Corwin JT. Regeneration of sensory cells after laser ablation in the lateral line system: hair cell lineage and macrophage behavior revealed by time-lapse video microscopy. J Neurosci. 1996;16:649–62. doi: 10.1523/JNEUROSCI.16-02-00649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science. 1988;240:1774–6. doi: 10.1126/science.3381101. [DOI] [PubMed] [Google Scholar]
- 110.Balak KJ, Corwin JT, Jones JE. Regenerated hair cells can originate from supporting cell progeny: evidence from phototoxicity and laser ablation experiments in the lateral line system. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1990;10:2502–12. doi: 10.1523/JNEUROSCI.10-08-02502.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Weisleder P, Rubel EW. Hair cell regeneration after streptomycin toxicity in the avian vestibular epithelium. J Comp Neurol. 1993;331:97–110. doi: 10.1002/cne.903310106. [DOI] [PubMed] [Google Scholar]
- 112.Baird RA, Steyger PS, Schuff NR. Mitotic and nonmitotic hair cell regeneration in the bullfrog vestibular otolith organs. Ann N Y Acad Sci. 1996;781:59–70. doi: 10.1111/j.1749-6632.1996.tb15693.x. [DOI] [PubMed] [Google Scholar]
- 113.Baird RA, Burton MD, Lysakowski A, Fashena DS, Naeger RA. Hair cell recovery in mitotically blocked cultures of the bullfrog saccule. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11722–9. doi: 10.1073/pnas.97.22.11722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Taylor RR, Forge A. Hair cell regeneration in sensory epithelia from the inner ear of a urodele amphibian. The Journal of Comparative Neurology. 2005;484:105–20. doi: 10.1002/cne.20450. [DOI] [PubMed] [Google Scholar]
- 115.Shang J, Cafaro J, Nehmer R, Stone J. Supporting cell division is not required for regeneration of auditory hair cells after ototoxic injury in vitro. J Assoc Res Otolaryngol. 2010;11:203–22. doi: 10.1007/s10162-009-0206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Roberson DW, Kreig CS, Rubel EW. Light microscopic evidence that direct transdifferentiation gives rise to new hair cells in regenerating avian auditory epithelium. Aud Neurosci. 1996;2:195–205. doi: 10.1002/jnr.20271. [DOI] [PubMed] [Google Scholar]
- 117.Roberson DW, Alosi JA, Cotanche DA. Direct transdifferentiation gives rise to the earliest new hair cells in regenerating avian auditory epithelium. J Neurosci Res. 2004;78:461–71. doi: 10.1002/jnr.20271. [DOI] [PubMed] [Google Scholar]
- 118.Masetto S, Correia MJ. Ionic currents in regenerating avian vestibular hair cells. Int J Dev Neurosci. 1997;15:387–99. doi: 10.1016/s0736-5748(96)00099-8. [DOI] [PubMed] [Google Scholar]
- 119.Zakir M, Dickman JD. Regeneration of vestibular otolith afferents after ototoxic damage. J Neurosci. 2006;26:2881–93. doi: 10.1523/JNEUROSCI.3903-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Haque A, Zakir M, Dickman JD. Regeneration of vestibular horizontal semicircular canal afferents in pigeons. J Neurophysiol. 2009;102:1274–86. doi: 10.1152/jn.91000.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dickman JD, Lim I. Posture, head stability, and orientation recovery during vestibular regeneration in pigeons. J Assoc Res Otolaryngol. 2004;5:323–36. doi: 10.1007/s10162-004-4047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Carey JP, Fuchs AF, Rubel EW. Hair cell regeneration and recovery of the vestibuloocular reflex in the avian vestibular system. J Neurophysiol. 1996;76:3301–12. doi: 10.1152/jn.1996.76.5.3301. [DOI] [PubMed] [Google Scholar]
- 123.Burns JC, Cox BC, Thiede BR, Zuo J, Corwin JT. In vivo proliferative regeneration of balance hair cells in newborn mice. J Neurosci. 2012;32:6570–7. doi: 10.1523/JNEUROSCI.6274-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Collado MS, Burns JC, Meyers JR, Corwin JT. Variations in shape-sensitive restriction points mirror differences in the regeneration capacities of avian and mammalian ears. PloS One. 2011;6:e23861. doi: 10.1371/journal.pone.0023861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Burns JC, Corwin JT. Responses to cell loss become restricted as the supporting cells in mammalian vestibular organs grow thick junctional actin bands that develop high stability. J Neurosci. 2014;34:1998–2011. doi: 10.1523/JNEUROSCI.4355-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Davies D, Magnus C, Corwin JT. Developmental changes in cell-extracellular matrix interactions limit proliferation in the mammalian inner ear. Eur J Neurosci. 2007;25:985–98. doi: 10.1111/j.1460-9568.2007.05355.x. [DOI] [PubMed] [Google Scholar]
- 127.Rubel EW, Dew LA, Roberson DW, Warchol ME, Corwin JT, Forge A, et al. Mammalian Vestibular Hair Cell Regeneration. Science. 1995;267:701–7. doi: 10.1126/science.7839150. [DOI] [PubMed] [Google Scholar]
- 128.Yamashita H, Oesterle EC. Induction of cell proliferation in mammalian inner-ear sensory epithelia by transforming growth factor alpha and epidermal growth factor. Proc Natl Acad Sci U S A. 1995;92:3152–5. doi: 10.1073/pnas.92.8.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li L, Forge A. Morphological evidence for supporting cell to hair cell conversion in the mammalian utricular macula. Int J Dev Neurosci. 1997;15:433–46. doi: 10.1016/s0736-5748(96)00102-5. [DOI] [PubMed] [Google Scholar]
- 130.Kuntz AL, Oesterle EC. Transforming growth factor alpha with insulin stimulates cell proliferation in vivo in adult rat vestibular sensory epithelium. J Comp Neurol. 1998;399:413–23. [PubMed] [Google Scholar]
- 131.Kawamoto K, Izumikawa M, Beyer LA, Atkin GM, Raphael Y. Spontaneous hair cell regeneration in the mouse utricle following gentamicin ototoxicity. Hear Res. 2009;247:17–26. doi: 10.1016/j.heares.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lin V, Golub JS, Nguyen TB, Hume CR, Oesterle EC, Stone JS. Inhibition of notch activity promotes nonmitotic regeneration of hair cells in the adult mouse utricles. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:15329–39. doi: 10.1523/JNEUROSCI.2057-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Golub JS, Tong L, Ngyuen TB, Hume CR, Palmiter RD, Rubel EW, et al. Hair cell replacement in adult mouse utricles after targeted ablation of hair cells with diphtheria toxin. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:15093–105. doi: 10.1523/JNEUROSCI.1709-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Slowik AD, Bermingham-McDonogh O. Hair cell generation by notch inhibition in the adult mammalian cristae. J Assoc Res Otolaryngol. 2013;14:813–28. doi: 10.1007/s10162-013-0414-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tanyeri H, Lopez I, Honrubia V. Histological evidence for hair cell regeneration after ototoxic cell destruction with local application of gentamicin in the chinchilla crista ampullaris. Hear Res. 1995;89:194–202. doi: 10.1016/0378-5955(95)00137-7. [DOI] [PubMed] [Google Scholar]
- 136.Warchol ME, Lambert PR, Goldstein BJ, Forge A, Corwin JT. Regenerative proliferation in inner ear sensory epithelia from adult guinea pigs and humans. Science. 1993;259:1619–22. doi: 10.1126/science.8456285. [DOI] [PubMed] [Google Scholar]
- 137.Taylor RR, Jagger DJ, Saeed SR, Axon P, Donnelly N, Tysome J, et al. Characterizing human vestibular sensory epithelia for experimental studies: new hair bundles on old tissue and implications for therapeutic interventions in ageing. Neurobiol Aging. 2015;36:2068–84. doi: 10.1016/j.neurobiolaging.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dye BJ, Frank TC, Newlands SD, Dickman JD. Distribution and time course of hair cell regeneration in the pigeon utricle. Hear Res. 1999;133:17–26. doi: 10.1016/s0378-5955(99)00046-5. [DOI] [PubMed] [Google Scholar]
- 139.Li H, Liu H, Heller S. Pluripotent stem cells from the adult mouse inner ear. Nat Med. 2003;9:1293–9. doi: 10.1038/nm925. [DOI] [PubMed] [Google Scholar]
- 140.Oshima K, Grimm CM, Corrales CE, Senn P, Martinez Monedero R, Geleoc GS, et al. Differential distribution of stem cells in the auditory and vestibular organs of the inner ear. J Assoc Res Otolaryngol. 2007;8:18–31. doi: 10.1007/s10162-006-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cruz IA, Kappedal R, Mackenzie SM, Hailey DW, Hoffman TL, Schilling TF, et al. Robust regeneration of adult zebrafish lateral line hair cells reflects continued precursor pool maintenance. Dev Biol. 2015;402:229–38. doi: 10.1016/j.ydbio.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Niemiec AJ, Raphael Y, Moody DB. Return of auditory function following structural regeneration after acoustic trauma: behavioral measures from quail. Hear Res. 1994;79:1–16. doi: 10.1016/0378-5955(94)90122-8. [DOI] [PubMed] [Google Scholar]
- 143.Jorgensen JM, Mathiesen C. The avian inner ear. Continuous production of hair cells in vestibular sensory organs, but not in the auditory papilla. Naturwissenschaften. 1988;75:319–20. doi: 10.1007/BF00367330. [DOI] [PubMed] [Google Scholar]
- 144.Roberson DF, Weisleder P, Bohrer PS, Rubel EW. Ongoing production of sensory cells in the vestibular epithelium of the chick. Hear Res. 1992;57:166–74. doi: 10.1016/0378-5955(92)90149-h. [DOI] [PubMed] [Google Scholar]
- 145.Kil J, Warchol ME, Corwin JT. Cell death, cell proliferation, and estimates of hair cell life spans in the vestibular organs of chicks. Hear Res. 1997;114:117–26. doi: 10.1016/s0378-5955(97)00166-4. [DOI] [PubMed] [Google Scholar]
- 146.Goodyear RJ, Gates R, Lukashkin AN, Richardson GP. Hair-cell numbers continue to increase in the utricular macula of the early posthatch chick. J Neurocytol. 1999;28:851–61. doi: 10.1023/a:1007070121751. [DOI] [PubMed] [Google Scholar]
- 147.Lambert PR, Gu R, Corwin JT. Analysis of small hair bundles in the utricles of mature guinea pigs. Am J Otol. 1997;18:637–43. [PubMed] [Google Scholar]
- 148.Burns JC, Christophel JJ, Collado MS, Magnus C, Carfrae M, Corwin JT. Reinforcement of cell junctions correlates with the absence of hair cell regeneration in mammals and its occurrence in birds. The Journal of comparative neurology. 2008;511:396–414. doi: 10.1002/cne.21849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Burns JC, Collado MS, Oliver ER, Corwin JT. Specializations of intercellular junctions are associated with the presence and absence of hair cell regeneration in ears from six vertebrate classes. J Comp Neurol. 2013;521:1430–48. doi: 10.1002/cne.23250. [DOI] [PubMed] [Google Scholar]
- 150.Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, et al. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–41. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- 151.Woods C, Montcouquiol M, Kelley MW. Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nat Neurosci. 2004;7:1310–8. doi: 10.1038/nn1349. [DOI] [PubMed] [Google Scholar]
- 152.Driver EC, Sillers L, Coate TM, Rose MF, Kelley MW. The Atoh1-lineage gives rise to hair cells and supporting cells within the mammalian cochlea. Dev Biol. 2013;376:86–98. doi: 10.1016/j.ydbio.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang GP, Chatterjee I, Batts SA, Wong HT, Gong TW, Gong SS, et al. Notch signaling and Atoh1 expression during hair cell regeneration in the mouse utricle. Hear Res. 2010;267:61–70. doi: 10.1016/j.heares.2010.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kraft S, Hsu C, Brough DE, Staecker H. Atoh1 induces auditory hair cell recovery in mice after ototoxic injury. Laryngoscope. 2013;123:992–9. doi: 10.1002/lary.22171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Schlecker C, Praetorius M, Brough DE, Presler RG, Jr, Hsu C, Plinkert PK, et al. Selective atonal gene delivery improves balance function in a mouse model of vestibular disease. Gene therapy. 2011 doi: 10.1038/gt.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Staecker H, Praetorius M, Baker K, Brough DE. Vestibular hair cell regeneration and restoration of balance function induced by math1 gene transfer. Otol Neurotol. 2007;28:223–31. doi: 10.1097/MAO.0b013e31802b3225. [DOI] [PubMed] [Google Scholar]
- 157.Xu JC, Huang DL, Hou ZH, Guo WW, Sun JH, Zhao LD, et al. Type I hair cell regeneration induced by Math1 gene transfer following neomycin ototoxicity in rat vestibular sensory epithelium. Acta Otolaryngol. 2012;132:819–28. doi: 10.3109/00016489.2012.673233. [DOI] [PubMed] [Google Scholar]
- 158.Shou J, Zheng JL, Gao WQ. Robust generation of new hair cells in the mature mammalian inner ear by adenoviral expression of Hath1. Mol Cell Neurosci. 2003;23:169–79. doi: 10.1016/s1044-7431(03)00066-6. [DOI] [PubMed] [Google Scholar]
- 159.Gao Z, Kelly MC, Yu D, Wu H, Lin X, Chi FL, et al. Spatial and Age-Dependent Hair Cell Generation in the Postnatal Mammalian Utricle. Molecular neurobiology. 2015 doi: 10.1007/s12035-015-9119-0. [DOI] [PubMed] [Google Scholar]
- 160.Kelley MW. Regulation of cell fate in the sensory epithelia of the inner ear. Nat Rev Neurosci. 2006;7:837–49. doi: 10.1038/nrn1987. [DOI] [PubMed] [Google Scholar]
- 161.Campbell DP, Chrysostomou E, Doetzlhofer A. Canonical Notch signaling plays an instructive role in auditory supporting cell development. Sci Rep. 2016;6:19484. doi: 10.1038/srep19484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wu J, Li W, Lin C, Chen Y, Cheng C, Sun S, et al. Co-regulation of the Notch and Wnt signaling pathways promotes supporting cell proliferation and hair cell regeneration in mouse utricles. Sci Rep. 2016;6:29418. doi: 10.1038/srep29418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gu R, Montcouquiol M, Marchionni M, Corwin JT. Proliferative responses to growth factors decline rapidly during postnatal maturation of mammalian hair cell epithelia. Eur J Neurosci. 2007;25:1363–72. doi: 10.1111/j.1460-9568.2007.05414.x. [DOI] [PubMed] [Google Scholar]
- 164.Zheng JL, Helbig C, Gao WQ. Induction of cell proliferation by fibroblast and insulin-like growth factors in pure rat inner ear epithelial cell cultures. J Neurosci. 1997;17:216–26. doi: 10.1523/JNEUROSCI.17-01-00216.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zheng JL, Frantz G, Lewis AK, Sliwkowski M, Gao WQ. Heregulin enhances regenerative proliferation in postnatal rat utricular sensory epithelium after ototoxic damage. J Neurocytol. 1999;28:901–12. doi: 10.1023/a:1007078307638. [DOI] [PubMed] [Google Scholar]
- 166.Oesterle EC, Cunningham DE, Westrum LE, Rubel EW. Ultrastructural analysis of [3H]thymidine-labeled cells in the rat utricular macula. The Journal of Comparative Neurology. 2003;463:177–95. doi: 10.1002/cne.10756. [DOI] [PubMed] [Google Scholar]
- 167.Hume CR, Kirkegaard M, Oesterle EC. ErbB expression: the mouse inner ear and maturation of the mitogenic response to heregulin. J Assoc Res Otolaryngol. 2003;4:422–43. doi: 10.1007/s10162-002-3008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Laine H, Doetzlhofer A, Mantela J, Ylikoski J, Laiho M, Roussel MF, et al. p19(Ink4d) and p21(Cip1) collaborate to maintain the postmitotic state of auditory hair cells, their codeletion leading to DNA damage and p53-mediated apoptosis. J Neurosci. 2007;27:1434–44. doi: 10.1523/JNEUROSCI.4956-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mantela J, Jiang Z, Ylikoski J, Fritzsch B, Zacksenhaus E, Pirvola U. The retinoblastoma gene pathway regulates the postmitotic state of hair cells of the mouse inner ear. Development. 2005;132:2377–88. doi: 10.1242/dev.01834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sage C, Huang M, Karimi K, Gutierrez G, Vollrath MA, Zhang DS, et al. Proliferation of functional hair cells in vivo in the absence of the retinoblastoma protein. Science. 2005;307:1114–8. doi: 10.1126/science.1106642. [DOI] [PubMed] [Google Scholar]
- 171.Sage C, Huang M, Vollrath MA, Brown MC, Hinds PW, Corey DP, et al. Essential role of retinoblastoma protein in mammalian hair cell development and hearing. Proc Natl Acad Sci U S A. 2006;103:7345–50. doi: 10.1073/pnas.0510631103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126:1581–90. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- 173.Rocha-Sanchez SM, Scheetz LR, Contreras M, Weston MD, Korte M, McGee J, et al. Mature mice lacking Rbl2/p130 gene have supernumerary inner ear hair cells and supporting cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:8883–93. doi: 10.1523/JNEUROSCI.5821-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Walters BJ, Liu Z, Crabtree M, Coak E, Cox BC, Zuo J. Auditory hair cell-specific deletion of p27Kip1 in postnatal mice promotes cell-autonomous generation of new hair cells and normal hearing. J Neurosci. 2014;34:15751–63. doi: 10.1523/JNEUROSCI.3200-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Weber T, Corbett MK, Chow LM, Valentine MB, Baker SJ, Zuo J. Rapid cell-cycle reentry and cell death after acute inactivation of the retinoblastoma gene product in postnatal cochlear hair cells. Proc Natl Acad Sci U S A. 2008;105:781–5. doi: 10.1073/pnas.0708061105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yu Y, Weber T, Yamashita T, Liu Z, Valentine MB, Cox BC, et al. In vivo proliferation of postmitotic cochlear supporting cells by acute ablation of the retinoblastoma protein in neonatal mice. J Neurosci. 2010;30:5927–36. doi: 10.1523/JNEUROSCI.5989-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Laine H, Sulg M, Kirjavainen A, Pirvola U. Cell cycle regulation in the inner ear sensory epithelia: role of cyclin D1 and cyclin-dependent kinase inhibitors. Dev Biol. 2010;337:134–46. doi: 10.1016/j.ydbio.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 178.Loponen H, Ylikoski J, Albrecht JH, Pirvola U. Restrictions in cell cycle progression of adult vestibular supporting cells in response to ectopic cyclin d1 expression. PloS One. 2011;6:e27360. doi: 10.1371/journal.pone.0027360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dominguez-Frutos E, Lopez-Hernandez I, Vendrell V, Neves J, Gallozzi M, Gutsche K, et al. N-myc controls proliferation, morphogenesis, and patterning of the inner ear. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:7178–89. doi: 10.1523/JNEUROSCI.0785-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kopecky B, Santi P, Johnson S, Schmitz H, Fritzsch B. Conditional deletion of N-Myc disrupts neurosensory and non-sensory development of the ear. Developmental dynamics : an official publication of the American Association of Anatomists. 2011;240:1373–90. doi: 10.1002/dvdy.22620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Burns JC, Yoo JJ, Atala A, Jackson JD. MYC Gene Delivery to Adult Mouse Utricles Stimulates Proliferation of Postmitotic Supporting Cells In Vitro. PloS one. 2012;7:e48704. doi: 10.1371/journal.pone.0048704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Laos M, Anttonen T, Kirjavainen A, Af Hallstrom T, Laiho M, Pirvola U. DNA damage signaling regulates age-dependent proliferative capacity of quiescent inner ear supporting cells. Aging. 2014;6:496–510. doi: 10.18632/aging.100668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gnedeva K, Hudspeth AJ. SoxC transcription factors are essential for the development of the inner ear. Proc Natl Acad Sci U S A. 2015;112:14066–71. doi: 10.1073/pnas.1517371112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Scheffer DI, Shen J, Corey DP, Chen ZY. Gene Expression by Mouse Inner Ear Hair Cells during Development. J Neurosci. 2015;35:6366–80. doi: 10.1523/JNEUROSCI.5126-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cai T, Jen HI, Kang H, Klisch TJ, Zoghbi HY, Groves AK. Characterization of the transcriptome of nascent hair cells and identification of direct targets of the atoh1 transcription factor. J Neurosci. 2015;35:5870–83. doi: 10.1523/JNEUROSCI.5083-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Elkon R, Milon B, Morrison L, Shah M, Vijayakumar S, Racherla M, et al. RFX transcription factors are essential for hearing in mice. Nature communications. 2015;6:8549. doi: 10.1038/ncomms9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Shin JB, Streijger F, Beynon A, Peters T, Gadzala L, McMillen D, et al. Hair bundles are specialized for ATP delivery via creatine kinase. Neuron. 2007;53:371–86. doi: 10.1016/j.neuron.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Shin JB, Longo-Guess CM, Gagnon LH, Saylor KW, Dumont RA, Spinelli KJ, et al. The R109H variant of fascin-2, a developmentally regulated actin crosslinker in hair-cell stereocilia, underlies early-onset hearing loss of DBA/2J mice. J Neurosci. 2010;30:9683–94. doi: 10.1523/JNEUROSCI.1541-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tao L, Segil N. Early transcriptional response to aminoglycoside antibiotic suggests alternate pathways leading to apoptosis in sensory hair cells in the mouse inner ear. Front Cell Neurosci. 2015;9:190. doi: 10.3389/fncel.2015.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ku YC, Renaud NA, Veile RA, Helms C, Voelker CC, Warchol ME, et al. The transcriptome of utricle hair cell regeneration in the avian inner ear. J Neurosci. 2014;34:3523–35. doi: 10.1523/JNEUROSCI.2606-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Jiang L, Romero-Carvajal A, Haug JS, Seidel CW, Piotrowski T. Gene-expression analysis of hair cell regeneration in the zebrafish lateral line. Proc Natl Acad Sci U S A. 2014;111:E1383–92. doi: 10.1073/pnas.1402898111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Steiner AB, Kim T, Cabot V, Hudspeth AJ. Dynamic gene expression by putative hair-cell progenitors during regeneration in the zebrafish lateral line. Proc Natl Acad Sci U S A. 2014;111:E1393–401. doi: 10.1073/pnas.1318692111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Koehler KR, Mikosz AM, Molosh AI, Patel D, Hashino E. Generation of inner ear sensory epithelia from pluripotent stem cells in 3D culture. Nature. 2013;500:217–21. doi: 10.1038/nature12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Liu XP, Koehler KR, Mikosz AM, Hashino E, Holt JR. Functional development of mechanosensitive hair cells in stem cell-derived organoids parallels native vestibular hair cells. Nature communications. 2016;7:11508. doi: 10.1038/ncomms11508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Marcotti W, van Netten SM, Kros CJ. The aminoglycoside antibiotic dihydrostreptomycin rapidly enters mouse outer hair cells through the mechano-electrical transducer channels. J Physiol. 2005;567:505–21. doi: 10.1113/jphysiol.2005.085951. [DOI] [PMC free article] [PubMed] [Google Scholar]