Abstract
Second anterior cruciate ligament rupture is a common and devastating injury among young women who return to sport after ACL reconstruction, but it is inadequately understood. The purpose of this study was to compare gait biomechanics and return-to-sport time frames in a matched cohort of young female athletes who, after primary ACLR, returned to sport without reinjury or sustained a second ACL injury. Approximately six months after primary reconstruction, fourteen young women (age 16 ± 2 years) involved in jumping, cutting, and pivoting sports underwent motion analysis testing after physical therapy and impairment resolution. Following objective return-to-sport clearance, seven athletes sustained a second ACL rupture within 20 months of surgery (13.4 ± 4.9 months). We matched them by age, sex, and sport-level to seven athletes who returned to sports without re-injury. Data were analyzed using a previously validated, EMG-informed, patient-specific musculoskeletal model. Compared to athletes without re-injury, athletes who sustained a second ACL injury received surgery sooner (p=0.023), had post-operative impairments resolved earlier (p=0.022), reached criterion-based return-to-sport benchmarks earlier (p=0.024), had higher body mass index (p=0.039), and walked with lower peak knee flexor muscle forces bilaterally (p=0.021). Athletes who sustained a second injury also tended to walk with larger (p=0.089) and more symmetrical peak knee flexion angles and less co-contraction, all indicative of a more normal gait pattern.
Keywords: ACL, re-injury, gait biomechanics, musculoskeletal modeling, return-to-sport
Introduction
Hundreds of thousands of anterior cruciate ligament (ACL) ruptures occur annually within the United States alone,1 with over 100,000 individuals undergoing ACL reconstruction (ACLR) each year.2,3 Even after reconstructive surgery, only 55% of athletes return to their pre-injury competitive level of sport.4 Moreover, compared to previously uninjured controls, athletes who do return to sport after ACLR are approximately 15 times more likely to sustain a second ACL injury.5 Younger female athletes may be at an especially high risk of second ACL injury,5,6 with approximately 30% sustaining a graft or contralateral ACL rupture within the first two years of returning to sport after ACLR.7,8 The impact and sequelae of second ACL injury are often devastating. A recent meta-analysis reported poorer knee function and a higher incidence of radiographic osteoarthritis in patients following revision versus primary ACLR.9 Therefore, understanding why second ACL injuries occur is a critically important research question.
The risk for second ACL injury is influenced by many factors, some of which are modifiable.10,11 Timing of return-to-sport (RTS) and biomechanical deficits are two potentially modifiable factors that are linked to second ACL injury risk.5,7,12–15 Early RTS following primary ACLR places athletes at a higher risk for graft rupture or contralateral ACL injury.5,7,12,15 Athletes who returned to competition within the first seven months were nearly three times more likely to sustain a second ACL injury than those who returned after seven months, with the majority of the injuries occurring within the first month of returning to sport.12 A recently published study by Grindem and colleagues found that the rate of knee re-injury could be reduced by 51% for each month RTS was delayed for up to nine months post-operatively.15 In addition to early RTS, altered movement patterns of the trunk and lower extremities are strongly associated with primary ACL injury risk, and thus likely play a role in second injury risk as well.13,14,16 Notably, young athletes who sustained a second ACL injury exhibited biomechanical deficits in knee, hip, and trunk control during a drop landing task assessed after RTS clearance following primary ACLR.13 Athletes with impaired hip-ankle coordination in the sagittal plane may also be at greater risk for second ACL injury.14
Prior studies demonstrate that athletes who perform poorly on objective performance measures six months after primary ACLR walk with larger kinematic and kinetic gait inter-limb asymmetries than those athletes who perform well.17 Poor functional performance is also associated with asymmetrical tibiofemoral joint loading.18 These studies point to a link between gait asymmetry and functional performance following ACLR; however the association between gait biomechanics and second ACL injury is unknown.
Previous studies have used musculoskeletal modeling to estimate and report muscle forces in healthy subjects19,20 as well as individuals with osteoarthritis,21 stroke,22 ACL deficiency,17,23,24 and ACL reconstruction.24–26 Altered tibiofemoral joint loading has implications for the development of osteoarthritis following ACL injury and reconstruction,24,25 but joint loading has not been assessed in relationship to second injury risk. The use of gait analysis, electromyography, and musculoskeletal modeling can uniquely examine gait biomechanics and second ACL injury risk by estimating muscle and joint contact forces.
Therefore, the purpose of this study was to compare gait biomechanics and return-to-sport time frames in a matched cohort of young female athletes who, following primary ACLR and impairment resolution, returned to sport without re-injury (ACLx1) or sustained a second ACL injury (ACLx2). We hypothesized that there would be differences in RTS time frames and knee gait mechanics, muscle forces, and joint contact forces between ACLx1 and ACLx2 subjects.
Methods
Subjects
This study is an individual case-control study (level of evidence: 3b). This study was approved by the University of Delaware IRB, and informed consent was obtained from all subjects as well as a parent/guardian when the subject was a minor. Fourteen young female athletes (age 16.1 ± 1.7 years; range: 13-19 years) were included in this study from part of an ongoing, prospective randomized control trial with 70 subjects (30 women) currently enrolled. All subjects participated in level I or II sports (i.e., sports involving jumping, cutting, and pivoting)27,28 prior to primary ACL rupture and subsequent ACLR, and planned to return to their pre-injury sporting level. Athletes were excluded from participation if they had grade 3 concomitant ligament injury, osteochondral defects >1cm2, or significant previous lower extremity injury. Following primary ACLR, all subjects underwent physical therapy and met the following criteria prior to study enrollment and motion analysis testing: minimal to no effusion,29 symmetric and full knee range of motion, ≥80% quadriceps strength limb symmetry index, ≥12 weeks post-operative, initiation of a running progression, and ability to hop pain-free on each leg.
Following ACLR, impairment resolution, motion analysis testing, and a progressive return-to-play training program,30 all athletes returned to sport within the first year after ACLR. Athletes received RTS clearance when they met the following objective criteria:31 ≥90% quadriceps strength limb symmetry index, ≥90% limb symmetry on four single-legged hops (i.e., single, crossover, triple, and timed 6 meter),32,33 and ≥90% on the Knee Outcome Survey Activities of Daily Living Scale.34 All athletes were followed two years post-operatively. Seven athletes sustained a noncontact (N=6) or partial contact (N=1; contact to body, contralateral injury) mechanism of second ACL injury to either their ipsilateral (N=3 graft ruptures) or contralateral (N=4) knee during sport activities within the first two years post-operatively (ACLx2: 13.4 ± 4.9 months post-ACLR). We matched these subjects by sex, age, sport-level, and graft type (for those whose second injuries were graft ruptures) to seven athletes who successfully returned to their pre-injury level of sport competition within the first year of ACLR without re-injury (ACLx1). Matching was done to create a homogenous comparison group and to control for several known large independent risk factors for second ACL injury, including age7,8,10,11,35–38 and activity level.8,11,36 By closely controlling for sex, age, and activity level, however, we were unable to match by autograft type all ACLx2 subjects who sustained contralateral second ACL injuries. During the matching process, we prioritized sex, age, sport-level, and graft type for those whose second injuries were graft ruptures.
Motion Analysis Testing
Motion analysis testing occurred following impairment resolution after primary ACLR (ACLx1: 7.3 ± 1.9 months; ACLx2: 4.9 ± 1.5 months post primary ACLR; Table 1), which was prior to athletes receiving clearance to return to sport. Thirty-nine retroreflective markers were placed on the bilateral lower extremities and pelvis. We collected motion data during gait at 120Hz using an eight camera motion analysis system (VICON, Oxford, UK). An embedded force platform (Bertec Corporation, Columbus, OH) was used to collect kinetic data at 1080Hz. Subjects walked at a self-selected gait speed maintained throughout testing to within ±5%. Kinematic and kinetic variables were calculated via inverse dynamics using commercial software (Visual3D, C-Motion, Germantown, MD). Variables of interest included gait velocity as well as peak values during the first half of stance for the following variables: knee flexion angle (pKFA), internal knee extension moment (pKEM), knee adduction angle (pKAA), and internal knee adduction moment (pKAM). Moments were normalized to mass*height (kg*m) to allow comparisons between subjects and groups.39
Table 1.
Demographic characteristics and timing variables in the ACLx1 and ACLx2 groups
| Demographic Variable | ACLx1 | ACLx2 | P-value |
|---|---|---|---|
| Age (years) | 16.0 ± 1.7 | 16.3 ± 1.9 | .741 |
| Body Mass Index (kg/m2) | 21.4 ± 1.8 | 24.5 ± 3.0 | .039* |
| Graft Type | 4 HS, 3 BPTB | 7 HS, 0 BPTB | .192 |
| Timing Variable | ACLx1 | ACLx2 | P-value |
|---|---|---|---|
| Injury to Surgery (weeks) | 6.8 ± 2.5 | 4.1 ± 1.0 | .023* |
| Surgery to Meeting Criteria for Enrollment (months) | 7.3 ± 1.9 | 4.9 ± 1.5 | .022* |
| Surgery to RTS Clearance (months) | 9.5 ± 1.9 | 6.8 ± 1.9 | .024* |
Abbreviation: HS: hamstring autograft; BPTB: bone patellar-tendon bone autograft; RTS: return-to-sport
p<0.05.
Electromyography
Surface electromyography (EMG) data were recorded bilaterally from seven lower extremity muscles per limb (rectus femoris, medial and lateral vastii, medial and lateral hamstrings, medial and lateral gastrocnemii) at 1080Hz (MA-300 EMG System, Motion Lab Systems, Baton Rouge, LA). We prepared the skin surface by shaving and abrading the skin prior to electrode placement, which was done in accordance with previous work.23 EMG data were high-pass filtered (2nd-order Butterworth at 30Hz), rectified, and low-pass-filtered (6Hz) to create a linear envelope. Maximal volitional isometric contractions were used to normalize EMG data in the following positions: seated with knees secured in approximately 60° of flexion for quadriceps; prone with knees secured in 30° of flexion for hamstrings; standing plantarflexion holding counter for resistance for gastrocnemii.23
EMG-Driven Model
All subjects’ gait data were analyzed using a validated,40 EMG-informed, musculoskeletal modeling approach previously described in detail.23,40,41 Briefly, this patient-specific modeling approach uses a Hill-type muscle fiber model. An iterative, simulated annealing process establishes optimal muscle parameters. Joint contact forces are estimated using a frontal plane moment algorithm that balances external knee adduction moments with internal knee moments (i.e., individual muscle forces × moment arms). Subsequently, three predicted walking trials per limb per subject were used for analysis. Variables of interest included knee extensor (i.e., quadriceps) muscle forces, knee flexor (i.e., combined hamstrings and gastrocnemii) muscle forces, and medial tibiofemoral joint contact force (which has larger magnitude and greater validity than lateral joint contact forces42). Specifically, we compared peak knee extensor muscle forces (pEXT) during the first half (i.e., loading phase) of stance; pEXT occurrence (stance phase normalized to 100%); knee flexor muscle forces at peak knee extension moment (FLEX @ pKEM);23 peak knee flexor muscle forces (pFLEX) during the second half of stance; pFLEX occurrence (stance phase normalized to 100%); and peak medial compartment contact force (pMCCF) during the first half of stance. Muscle and joint contact forces were normalized to each subject's body weight and reported in body weight (BW) units, hence allowing comparison across subjects.39
Model Tuning
Comparing the inverse dynamics sagittal moment curve to the calibrated EMG-driven model moment curve, the model tuning coefficient of determination (R2) was 0.836 ± 0.088 and the root mean square error (RMSE) was 6.9 ± 3.7%. These statistics validate the model predictions and are similar to a previous study analyzing ACL deficient subjects.23
Statistical Analysis
Statistical analyses were performed using SPSS Version 23.0 (IBM Corp, Armonk, NY) and Microsoft Excel. We utilized t-tests and Fisher's Exact Test to compare demographics (i.e., age at surgery, body mass index, graft type) as well as time from injury to surgery, surgery to enrollment/motion analysis testing (i.e., impairment resolution), and surgery to RTS clearance (α=0.05). We analyzed joint angles and moments, muscle forces, and medial tibiofemoral contact forces using 2×2 ANOVAs (α=0.05) with limb (involved=limb of primary ACLR) and group (ACLx1 vs. ACLx2) as within and between group factors.
Results
Demographics & Timing
Athletes who sustained a second ACL injury had higher body mass index and received surgery, had impairment resolution, and met criterion-based return-to-sport clearance earlier after injury than athletes who returned to sport without second injury (p<0.05; Table 1). All athletes (N=7) within the ACLx2 group had hamstring autografts while there was a mixture of bone patellar-tendon bone (N=3) and hamstring autografts (N=4) within the ACLx1 group. (ACLx2 athletes who sustained graft ruptures were matched by autograft type [i.e., hamstring].)
Gait Kinematics & Kinetics
There was no significant difference in gait velocity, although a trend (p=0.063) was noted toward faster velocity in the ACLx2 group (ACLx1: 1.47 ± .16 m/s vs. ACLx2: 1.58 ± .13 m/s) with a moderate effect size43 (Cohen's d=0.77). There was also a trend (p=0.089; Table 2) toward group differences in peak knee flexion angle with the ACLx2 athletes walking with larger pKFA as compared to ACLx1 in both limbs (Cohen's d=0.69). The involved limb difference of 5.9° exceeded the minimal detectable change (MDC) of 2.9°42 while the uninvolved limb difference (2.7°) did not meet the MDC.42 ACLx2 subjects demonstrated inter-limb symmetry in pKFA (ACLx2 involved – uninvolved = 0.2°) while ACLx1 subjects walked with meaningful42 inter-limb differences in pKFA (ACLx1 involved – uninvolved = −3.0°). There were no differences in pKAA, pKEM, or pKAM (Table 2).
Table 2.
Biomechanical variables of interest between group and limb
| Variable | ACLx1 | ACLx2 | P-values | |||||
|---|---|---|---|---|---|---|---|---|
| INV | UN | INV | UN | Model | Interaction | Group | Limb | |
| pKFA (°) | −17.4 ± 8.0 | −20.5 ± 5.1 | −23.3 ± 5.1 | −23.1 ± 6.9 | .294 | .513 | .089^ | .564 |
| pKAA (°) | −0.8 ± 2.3 | 0.0 ± 1.3 | 0.6 ± 3.4 | 1.0 ± 3.4 | .647 | .830 | .269 | .559 |
| pKEM (Nm/Kg*m) | 0.35 ± 0.21 | 0.42 ± 0.16 | 0.43 ± 0.10 | 0.54 ± 0.18 | .235 | .769 | .113 | .197 |
| pKAM (Nm/Kg*m) | −0.23 ± 0.07 | −0.28 ± 0.08 | −0.29 ± 0.08 | −0.27 ± 0.10 | .604 | .268 | .502 | .720 |
| pEXT (BW) | 2.1 ± 1.0 | 2.4 ± 0.7 | 2.5 ± 0.5 | 3.0 ± 0.8 | .210 | .771 | .126 | .145 |
| FLEX @ pKEM (BW) | 1.1 ± 0.6 | 1.3 ± 0.4 | 1.0 ± 0.4 | 1.0 ± 0.2 | .452 | .694 | .142 | .612 |
| pFLEX (BW) | 1.8 ± 0.6 | 2.0 ± 0.7 | 1.1 ± 1.4 | 1.7 ± 0.5 | .036* | .445 | .021* | .080^ |
| pMCCF (BW) | 2.9 ± 0.7 | 3.1 ± 0.6 | 3.0 ± 0.5 | 2.9 ± 0.6 | .977 | .737 | .872 | .813 |
Abbreviations: INV=involved limb; UN=uninvolved limb; pKFA=peak knee flexion angle (negative value indicates flexion); pKAA=peak knee adduction angle (positive value indicates adduction); pKEM=peak internal knee extension moment; pKAM=peak internal knee adduction moment; pEXT=peak knee extensor muscle forces; FLEX @ pKEM=knee flexor muscle forces at pKEM; pFLEX=peak knee flexor muscle forces; pMCCF=peak medial compartment tibiofemoral contact force; BW=body weight (units)
p<0.05
p<0.10.
Muscle & Joint Contact Forces
During the loading phase (i.e., first half) of stance, moderate effect sizes43 were present for both peak knee extensor muscle forces (p=0.126, Cohen's d = 0.60) and flexor muscle forces at pKEM (p=0.145, Cohen's d=0.65), with larger knee extensor and smaller knee flexor muscles forces in ACLx2 compared to ACLx1 subjects. There was no difference between groups for the occurrence of pEXT (average: 21 ± 2% of stance, full model p=0.819) or peak medial tibiofemoral joint contact force (Table 2).
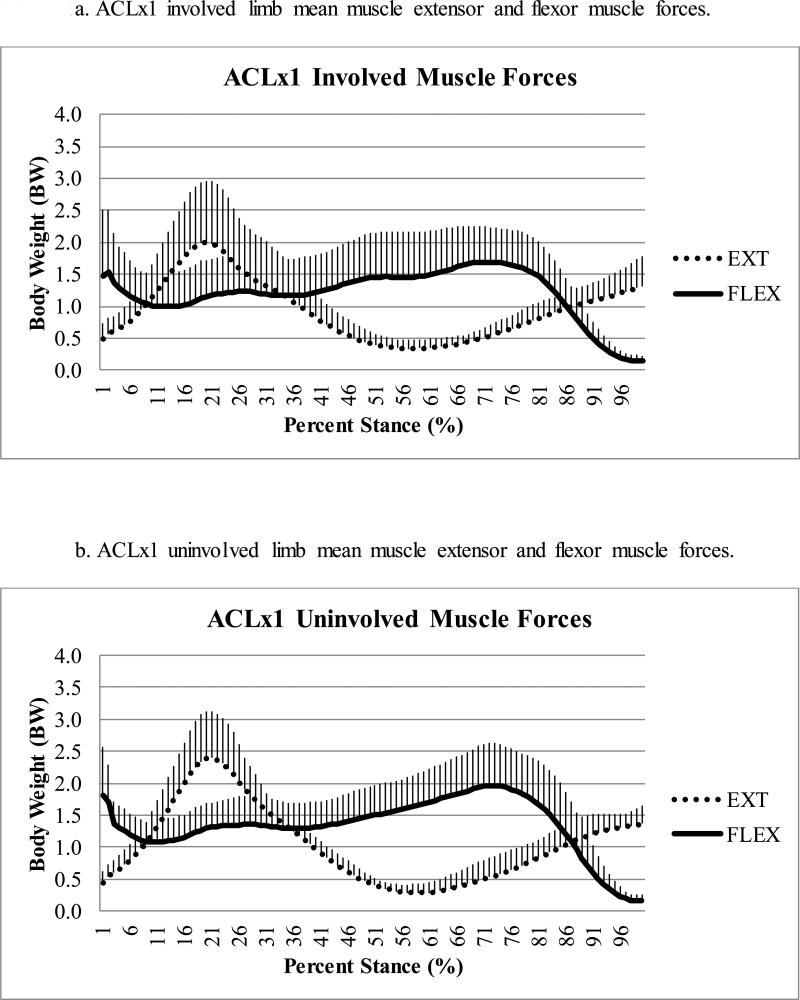
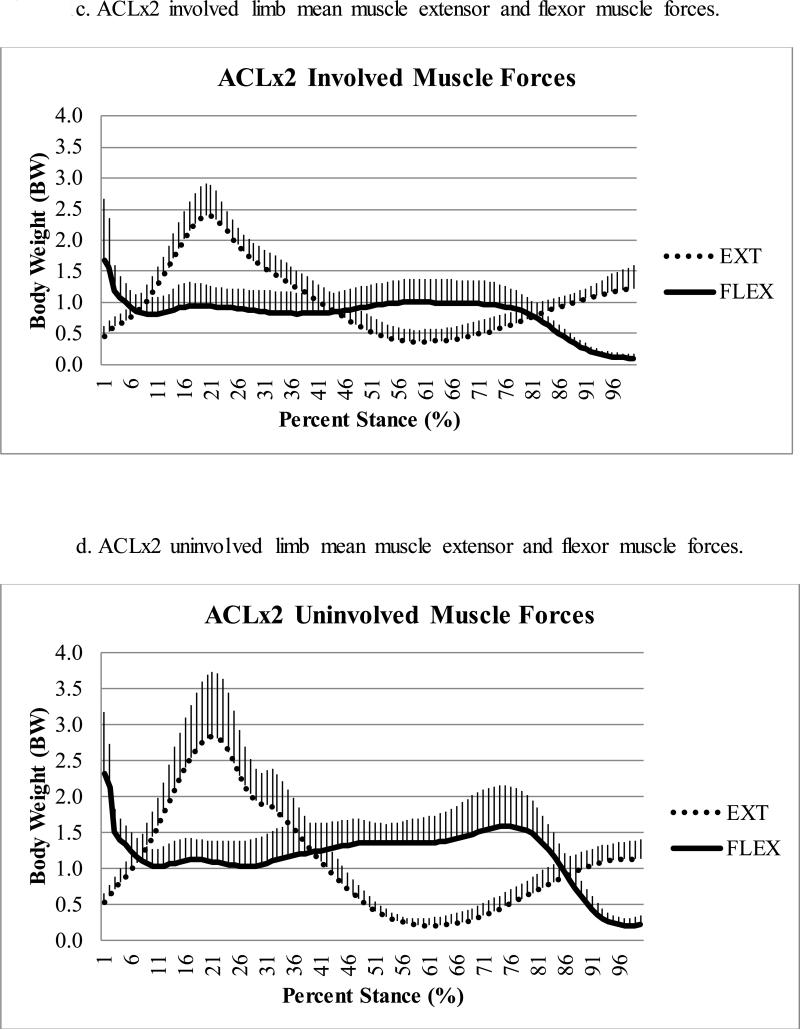
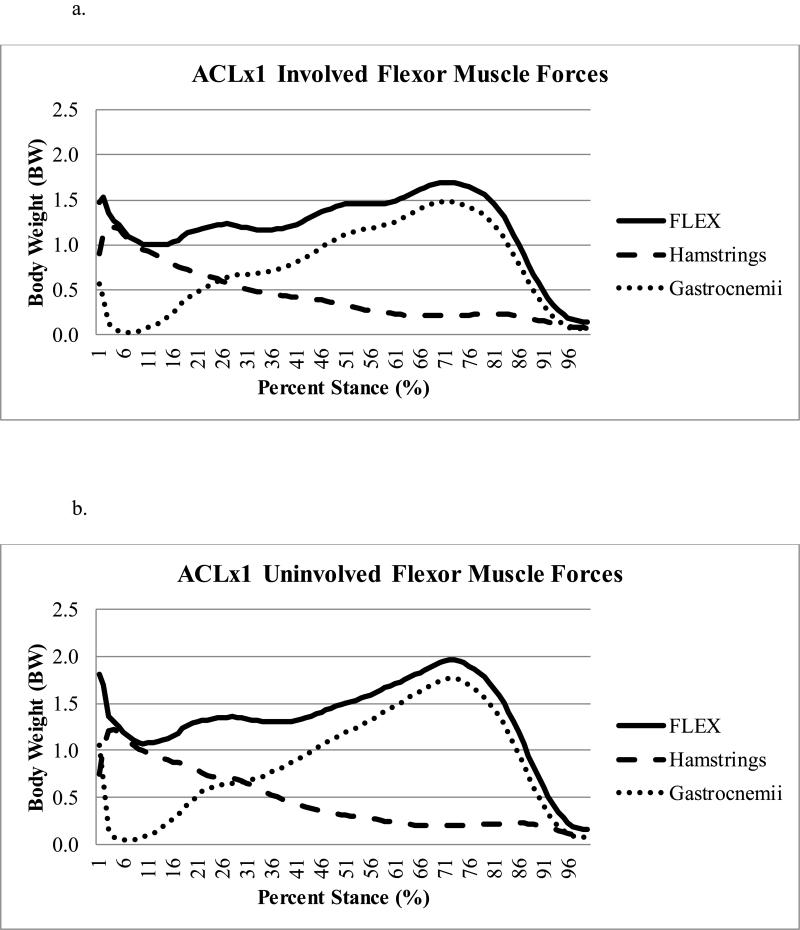
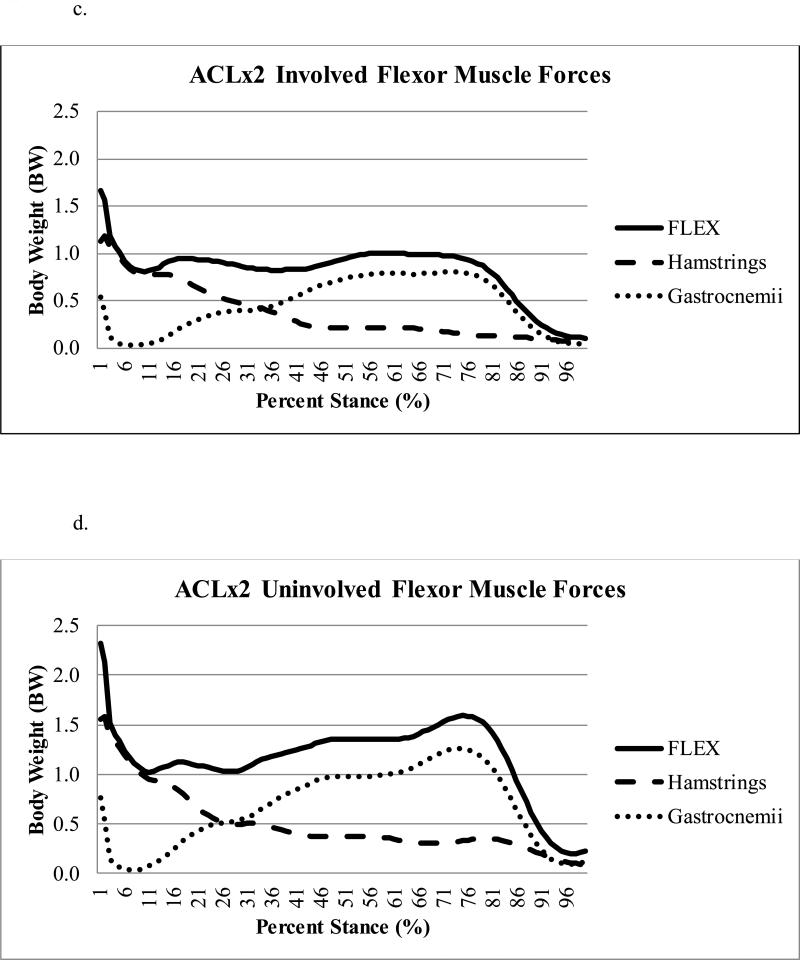
There was a statistically significant difference (p=0.021) in peak knee flexor muscle forces during the second half of stance regardless of limb, with ACLx2 subjects demonstrating lesser flexor forces compared to ACLx1 subjects (Table 2); this effect size (Cohen's d=0.90) was large.43 There was a trend (p=0.080) toward lesser peak knee flexor muscle forces in the involved as compared to uninvolved limb (Figure 1a-d), with a moderate43 effect size (Cohen's d=0.64). There was no difference in pFLEX occurrence (average: 69 ± 9% of stance, full model p=0.253), which was driven primarily by the gastrocnemii (Figure 2a-d).
Figure 1a-d.
Comparison of mean extensor (EXT) and flexor (FLEX) muscle forces during stance for ACLx1 (1a-b) and ACLx2 (1c-d) subjects (whiskers are standard deviations).
Figure 2a-d.
Contribution of the gastrocnemii versus hamstrings muscle forces to the total knee flexor (FLEX) muscle forces during stance for the ACLx1 involved (Figure 2a) and uninvolved (Figure 2b) and ACLx2 involved (Figure 2c) and uninvolved (Figure 2d) limbs.
Discussion
The purpose of this study was to investigate gait biomechanics, including modeling the muscle and joint contact forces, in a matched cohort of young female athletes after primary ACLR who either returned to sport without re-injury (ACLx1) or sustained a second ACL rupture within two years of surgery (ACLx2). We found that ACLx2 athletes received surgery more quickly after injury and met objective criteria for enrollment and RTS clearance more quickly than ACLx1 subjects. Yet despite being tested sooner after primary ACLR than ACLx1 subjects, ACLx2 subjects demonstrated a more normal gait pattern. Our findings suggest that even in the absence of clinical or gait impairments, returning to sports early after primary ACLR may place young female athletes at greater risk for second ACL injury.
The ACLx2 group demonstrated more normal gait biomechanics and symmetry than ACLx1 subjects: larger knee flexion angles, more symmetrical knee flexion angles, and less co-contraction. The inter-limb symmetry and magnitude of peak knee flexion angle of the ACLx2 athletes resembled the gait mechanics of healthy controls,44–46 stronger subjects after ACLR,46 and ACL-deficient subjects who have returned to pre-injury activity levels without reconstruction.44,45 ACLx2 subjects tended to walk with larger knee extensor muscle forces and smaller knee flexor muscle forces during the loading phase of gait regardless of limb; this muscle strategy is characteristic of a cyclical pattern of muscle recruitment associated with healthy control subjects and ACL-injured subjects who return to high level activities with minimal impairments.45 In contrast, during the loading phase of gait, ACLx1 subjects walked with smaller knee extensor and larger knee flexor muscle forces, which is indicative of co-contraction. ACLx1 subjects’ smaller pKFA, inter-limb pKFA asymmetry, and muscle strategies resemble subjects following ACL injury who have chronic knee instability, poorer function, and muscle co-contraction during gait.45
Gait kinematics and joint contact forces may also have implications for the development of post-traumatic osteoarthritis.24,25 Ironically, the magnitudes of pKFA of the ACLx2 subjects are more consistent with subjects who do not develop radiographic osteoarthritis five years after ACLR, while the pKFA of the ACLx1 subjects is similar to those who do develop radiographic osteoarthritis.25 However, both groups walked with peak medial compartment contact forces similar in magnitude to subjects six months after ACLR who do not develop radiographic osteoarthritis by five years after ACLR.24 Neither ACLx1 nor ACLx2 subjects exhibited the medial compartment joint unloading characteristic of (a separate cohort of) subjects who develop medial compartment osteoarthritis by five years after ACLR.24 More research is needed to understand better which individuals are most at risk for the development of post-traumatic osteoarthritis and/or second ACL injury.
In light of the above, our findings suggest that earlier return-to-sport time frames—even in athletes with more normal gait mechanics—are associated with second ACL injuries. One possible explanation is that earlier resolution of impairments is linked to both better gait patterns and earlier passing of objective RTS criteria, thus enabling the “best” subjects to resume sports more quickly after ACLR, placing them at greater risk for second ACL injury. In a recently published study, Grindem and colleagues found that returning to level I27 sport prior to nine months post-ACLR and asymmetrical quadriceps strength were independent predictors of knee re-injury.15 The present study corroborates the temporal findings in a separate cohort: ACLx2 athletes returned to sport nearly three months earlier than ACLx1 athletes (6.8 vs. 9.5 months). However, all of our athletes (i.e., ACLx1 and ACLx2 subjects) returned to high level sports only after meeting stringent RTS criteria including at least 90% quadriceps limb symmetry index. Our study adds in-depth biomechanical analysis with no observed gait impairments within the ACLx2 group. Biological healing time frames may be an important consideration above and beyond both functional criteria15,47 and gait mechanics when determining RTS clearance. Even in the absence of functional or gait impairments, our data corroborate recent evidence that suggests delaying return-to-sport until at least nine months or more postoperatively may decrease second ACL injury risk.15,47
Athletes who sustained a second ACL injury also had higher body mass index (BMI) than ACLx1 subjects. Interestingly, in a large cohort study investigating risk factors for revision ACLR, a BMI of less than 30 kg/m2 was associated with increased risk of revision surgery.37 All but one (ACLx2 subject: BMI of 30.7 kg/m2) of our athletes had a BMI of less than 30 kg/m2 – classifying 13 of 14 subjects in this higher risk cohort. Among athletes within this high-risk cohort (i.e., BMI < 30 kg/m2) who are returning to high level sports, our data suggest that higher, rather than lower, BMI may be associated with second injury, although more research with larger sample sizes is needed.
One factor which merits further discussion is graft type. All subjects in both groups received autografts for their primary ACLR. While all ACLx2 subjects underwent primary ACLR using a hamstring autograft, three of the ACLx1 subjects who were matched to ACLx2 subjects with contralateral second injuries had BPTB autografts. Although it may seem that these differences in graft type could influence the results48, we expect this discrepancy to have a minimal influence on our interpretation, for the following primary reason: the statistically significant differences in peak knee flexor muscle forces (pFLEX) occurred in the second half of stance, during which the gastrocnemii, and not the hamstrings, are contributing primarily to the total knee flexor muscle forces. For the ACLx2 group, the contribution of gastrocnemii to total knee flexor muscle forces was about 79% (involved=79%, uninvolved=79%). Similarly, for the ACLx1 group, the contribution of gastrocnemii to total knee flexor muscle forces was about 89% (involved=88%, uninvolved=90%). Also, during the first half of stance, similarities across limbs were noted: FLEX @ pKEM were similar across limbs (p=0.612) and did not interact between group and limb (p=0.694). Moreover, for ACLx2 subjects, FLEX @ pKEM were similar in both limbs (involved=0.97 body weight, uninvolved=0.99 body weight). The relative contribution of the hamstrings to the total FLEX @ pKEM was likewise similar across groups (ACLx2 involved=65%, ACLx2 uninvolved=57%; ACLx1 involved=59%, ACLx1 uninvolved=58%). Finally, all subjects in the present study were well-rehabilitated prior to study enrollment and motion analysis testing, which occurred at a time when functional regeneration of hamstrings harvested as grafts has been shown to occur.49 These findings suggest that the hamstring muscles were functioning similarly across limbs in both ACLx2 and ACLx1 subjects at this time within our cohort. Therefore, while graft type is a limitation of this study, the data suggest it had minimal influence on the results.
There are several other limitations to our study. First, the sample size is relatively small; however, we have a well-matched cohort of cases and controls in terms of age, sex, and sport. Due to all our athletes being young women, caution must be taken in generalizing the findings to men or women older than age 20. Second, our follow-up period was limited to two years; however, re-injury risk is most common in the first seven months after ACLR 12 and increases only marginally from one to two years after RTS.5,7 Third, athletic exposures were not accounted for, thus we are unable to conclude whether or not exposure moderated second injury risk. All ACLx1 athletes did, however, return to their pre-injury competition level of sport by one year after surgery. Fourth, we analyzed subjects during gait, thus it is unclear how the results could differ if athletes were tested on more demanding sport maneuvers. Finally, muscle and joint contact forces were estimated by the musculoskeletal model, not directly measured. Direct in vivo measurement is, however, not feasible; the musculoskeletal model employed in this study is patient-specific and previously validated;40,41 and, it is the first study to investigate muscle forces and joint loading and second ACL injury risk.
The present study is also unable to answer directly why these second injuries occurred. The authors suspect that early return to high level sport is the primary modifiable reason for second injury in these athletes (as all participants met rigorous functional and performance criteria prior to RTS). This assertion is supported by recent findings by Grindem et al. in a separate cohort of subjects, indicating that early RTS greatly increases re-injury risk after ACLR.15 We suspect insufficient graft healing as the most likely explanation for ipsilateral (i.e., graft) ruptures. For both contralateral and ipsilateral injuries, biology is a plausible explanation. All subjects sustained ACL injuries once—with the majority occurring through a noncontact mechanism—suggesting they have some predispositions.11,50 They are at high risk for contralateral ruptures,7,11 and this predisposition was not altered by their rehabilitation for the first injury. Further investigation is warranted.
In conclusion, athletes who sustained a second ACL injury received primary ACLR earlier, met enrollment and RTS criteria more quickly, had higher BMI, and walked with lower knee flexor muscle forces during the second half of stance than ACLx1 athletes. Additionally, ACLx2 subjects walked with a more normal gait strategy, including larger and symmetrical peak knee flexion angles and a more refined strategy of cyclical muscle contraction patterns. Our findings are in concurrence with and add an in-depth biomechanical analysis to recent literature suggesting delaying RTS until at least nine months.15 Although there are important limitations (i.e., graft type differences, testing time-point, small sample size, case-control series design) to consider when interpreting these findings, our data provide more evidence to delay RTS clearance in young female athletes. Delaying RTS clearance after ACLR even in the absence of clinical or biomechanical gait impairments may mitigate second ACL injury risk in young female athletes.
Statement of Clinical Significance.
Delayed return-to-sport clearance even in the absence of gait or clinical impairments following primary ACL reconstruction may be necessary to mitigate second ACL injury risk in young women.
Acknowledgments
Thank you to Martha Callahan and the Delaware Rehabilitation Institute Research Core; the University of Delaware Physical Therapy Clinic; and the National Institutes of Health (NIH). This project was funded by the following NIH grants: R01-AR048212, P30-GM103333, U54-GM104941, T32-HD00749, and R01-HD087459.
Footnotes
Publisher's Disclaimer: This is the accepted version of the following article: Capin, J. J., Khandha, A., Zarzycki, R., Manal, K., Buchanan, T. S. and Snyder-Mackler, L. (2016), Gait mechanics and second ACL rupture: Implications for delaying return-to-sport. J. Orthop. Res.. doi:10.1002/jor.23476, which has been published in final form at http://onlinelibrary.wiley.com/doi/10.1002/jor.23476/full.
Author Contributions: JJC contributed to research design, data acquisition, analysis, and interpretation, drafting the manuscript, and incorporating revisions. AK contributed to data acquisition, analysis, interpretation, and critical review. RZ contributed to data interpretation and critical review. KM contributed to data analysis, interpretation, and critical review. TSB and LSM contributed to research design, data interpretation, and critical review. All authors read and approved the final version prior to submission.
Disclosure Statement: No competing financial interests exist for any of the authors.
References
- 1.Griffin LY, Albohm MJ, Arendt E a, et al. Understanding and preventing noncontact anterior cruciate ligament injuries: a review of the Hunt Valley II meeting, January 2005. Am. J. Sports Med. 2006;34(9):1512–1532. doi: 10.1177/0363546506286866. [DOI] [PubMed] [Google Scholar]
- 2.Mall NA, Chalmers PN, Moric M, et al. Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am. J. Sports Med. 2014;42(10):2363–2370. doi: 10.1177/0363546514542796. [DOI] [PubMed] [Google Scholar]
- 3.Kim S, Bosque J, Meehan JP, et al. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J. Bone Jt. Surg. 2011;93(11):994–1000. doi: 10.2106/JBJS.I.01618. [DOI] [PubMed] [Google Scholar]
- 4.Ardern CL, Taylor NF, Feller JA, Webster KE. Fifty-five per cent return to competitive sport following anterior cruciate ligament reconstruction surgery: an updated systematic review and meta-analysis including aspects of physical functioning and contextual factors. Br. J. Sports Med. 2014;48(21):1543–52. doi: 10.1136/bjsports-2013-093398. [DOI] [PubMed] [Google Scholar]
- 5.Paterno MV, Rauh MJ, Schmitt LC, et al. Incidence of contralateral and ipsilateral anterior cruciate ligament (ACL) injury after primary ACL reconstruction and return to sport. Clin. J. Sport. Med. 2012;22(2):116–21. doi: 10.1097/JSM.0b013e318246ef9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shelbourne KD, Gray T, Haro M. Incidence of subsequent injury to either knee within 5 years after anterior cruciate ligament reconstruction with patellar tendon autograft. Am. J. Sports Med. 2009;37(2):246–251. doi: 10.1177/0363546508325665. [DOI] [PubMed] [Google Scholar]
- 7.Paterno MV, Rauh MJ, Schmitt LC, et al. Incidence of second ACL injuries 2 years after primary ACL reconstruction and return to sport. Am. J. Sports Med. 2014;42(7):1567–1573. doi: 10.1177/0363546514530088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Webster KE, Feller JA, Leigh WB, Richmond AK. Younger patients are at increased risk for graft rupture and contralateral injury after anterior cruciate ligament reconstruction. Am. J. Sports Med. 2014;42(3):641–647. doi: 10.1177/0363546513517540. [DOI] [PubMed] [Google Scholar]
- 9.Grassi A, Ardern CL, Muccioli G, et al. Does revision ACL reconstruction measure up to primary surgery? A meta-analysis comparing patient-reported and clinician-reported outcomes, and radiographic results. Br. J. Sports Med. 2016 doi: 10.1136/bjsports-2015-094948. Epub ahead. [DOI] [PubMed] [Google Scholar]
- 10.Wiggins AJ, Grandhi RK, Schneider DK, et al. Risk of Secondary Injury in Younger Athletes After Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-analysis. Am. J. Sports Med. 2016;44(7):1861–76. doi: 10.1177/0363546515621554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaeding CC, Pedroza a. D, Reinke EK, et al. Risk factors and predictors of subsequent ACL injury in either knee after ACL reconstruction: Prospective analysis of 2488 primary ACL reconstructions from the MOON cohort. Am. J. Sports Med. 2015;43(7):1583–1590. doi: 10.1177/0363546515578836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laboute E, Savalli L, Puig P, et al. Analysis of return to competition and repeat rupture for 298 anterior cruciate ligament reconstructions with patellar or hamstring tendon autograft in sportspeople. Ann. Phys. Rehabil. Med. 2010;53(10):598–614. doi: 10.1016/j.rehab.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Paterno MV, Schmitt LC, Ford KR, et al. Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. Am. J. Sports Med. 2010;38(10):1968–1978. doi: 10.1177/0363546510376053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paterno MV, Kiefer AW, Bonnette S, et al. Prospectively identified deficits in sagittal plane hip–ankle coordination in female athletes who sustain a second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. Clin. Biomech. 2015;30(10):1094–1101. doi: 10.1016/j.clinbiomech.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grindem H, Snyder-Mackler L, Moksnes H, et al. Simple decision rules can reduce reinjury risk by 84% after ACL reconstruction: the Delaware-Oslo ACL cohort study. Br. J. Sports Med. 2016;50(13):804–808. doi: 10.1136/bjsports-2016-096031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hewett TE, Stasi SL, Di, Myer GD. Current concepts for injury prevention in athletes after anterior cruciate ligament reconstruction. Am. J. Sports Med. 2013;41(1):216–224. doi: 10.1177/0363546512459638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Stasi SL, Logerstedt D, Gardinier ES, Snyder-Mackler L. Gait patterns differ between ACL-reconstructed athletes who pass return-to-sport criteria and those who fail. Am. J. Sports Med. 2013;41:1310–1318. doi: 10.1177/0363546513482718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardinier ES, Di Stasi S, Manal K, et al. Knee contact force asymmetries in patients who failed return-to-sport readiness criteria 6 months after anterior cruciate ligament reconstruction. Am J Sport. Med. 2014;42(12):2917–2925. doi: 10.1177/0363546514552184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lloyd DG, Besier TF. An EMG-driven musculoskeletal model to estimate muscle forces and knee joint moments in vivo. J. Biomech. 2003;36(6):765–776. doi: 10.1016/s0021-9290(03)00010-1. [DOI] [PubMed] [Google Scholar]
- 20.Winby CR, Lloyd DG, Besier TF, Kirk TB. Muscle and external load contribution to knee joint contact loads during normal gait. J. Biomech. 2009;42(14):2294–2300. doi: 10.1016/j.jbiomech.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 21.Kumar D, Rudolph KS, Manal KT. EMG-driven modeling approach to muscle force and joint load estimations: Case study in knee osteoarthritis. J. Orthop. Res. 2012;30(3):377–383. doi: 10.1002/jor.21544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shao Q, Bassett DN, Manal K, Buchanan TS. An EMG-driven model to estimate muscle forces and joint moments in stroke patients. Comput Biol Med. 2009;39(12):1083–1088. doi: 10.1016/j.compbiomed.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gardinier E, Manal K, Thomas B, Snyder-Mackler L. Gait and neuomuscular asymmetries after acute ACL rupture.Med Sci Sport. Exerc. 2012;44(8):1490–1496. doi: 10.1249/MSS.0b013e31824d2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wellsandt E, Gardinier ES, Manal K, et al. Decreased knee joint loading associated with early knee osteoarthritis after anterior cruciate ligament injury. Am J Sport. Med. 2016;44(1):143–151. doi: 10.1177/0363546515608475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khandha A, Manal K, Wellsandt E, et al. A comparison of knee gait mechanics in subjects with and without medial compartment knee osteoarthritis five years after anterior cruciate ligament reconstruction. J. Orthop. Res. 2016 Apr;:1–29. doi: 10.1002/jor.23261. Epub ahead. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saxby DJ, Bryant AL, Modenese L, et al. Tibiofemoral Contact Forces in the Anterior Cruciate Ligament-Reconstructed Knee. Med. Sci. Sports Exerc. 2016 doi: 10.1249/MSS.0000000000001021. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Daniel DM, Lou Stone M, Dobson BE, et al. Fate of the ACL-injured patient. A prospective outcome study. Am. J. Sports Med. 1994;22:632–644. doi: 10.1177/036354659402200511. [DOI] [PubMed] [Google Scholar]
- 28.Hefti F, Muller W, Jakob RP, Staubli HU. Evaluation of knee ligament injuries with the IKDC form. Knee Surgery, Sport. Traumatol. Arthrosc. 1993;1:226–234. doi: 10.1007/BF01560215. [DOI] [PubMed] [Google Scholar]
- 29.Sturgill LP, Snyder-Mackler L, Manal TJ, Axe MJ. Interrater reliability of a clinical scale to assess knee joint effusion. J Orthop Sport. Phys Ther. 2009;39(12):845–849. doi: 10.2519/jospt.2009.3143. [DOI] [PubMed] [Google Scholar]
- 30.White K, Di Stasi SL, Smith AH, Snyder-Mackler L. Anterior cruciate ligament-specialized post-operative return-to-sports (ACL-SPORTS) training: a randomized control trial. BMC Musculoskelet. Disord. 2013;14(108) doi: 10.1186/1471-2474-14-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adams D, Logerstedt D, Hunter-Giordano A, et al. Current concepts for anterior cruciate ligament reconstruction: a criterion-based rehabilitation progression. J. Orthop. Sports Phys. Ther. 2012;42(7):601–614. doi: 10.2519/jospt.2012.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noyes F, Barber S, Mangine R. Abnormal lower limb symmetry determined by function hop tests after anterior cruciate ligament rupture. Am. J. Sports Med. 1991;19(5):513–518. doi: 10.1177/036354659101900518. [DOI] [PubMed] [Google Scholar]
- 33.Reid A, Birmingham T, Stratford PW, et al. Hop testing provides a reliable valid outcome measure during rehab after ACLR. Phys. Ther. 2007;87(3):337–349. doi: 10.2522/ptj.20060143. [DOI] [PubMed] [Google Scholar]
- 34.Irrgang JJ, Snyder-Mackler L, Wainner RS, et al. Development of a patient-reported measure of function of the knee. J Bone Jt. Surg Am. 1998;80(8):1132–45. doi: 10.2106/00004623-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Nelson IR, Chen J, Love R, et al. A comparison of revision and rerupture rates of ACL reconstruction between autografts and allografts in the skeletally immature. Knee Surgery, Sport. Traumatol. Arthrosc. 2016;24(3):773–779. doi: 10.1007/s00167-016-4020-6. [DOI] [PubMed] [Google Scholar]
- 36.Mohtadi N, Chan D, Barber R, Paolucci EO. Reruptures, reinjuries, and revisions at a minimum 2-year follow-up: A randomized clinical trial comparing 3 graft types for ACL reconstruction. Clin. J. Sport Med. 2016;26(2):96–107. doi: 10.1097/JSM.0000000000000209. [DOI] [PubMed] [Google Scholar]
- 37.Maletis GB, Chen J, Inacio MC, Funahashi TT. Age-Related Risk Factors for Revision Anterior Cruciate Ligament Reconstruction: A Cohort Study of 21,304 Patients From the Kaiser Permanente Anterior Cruciate Ligament Registry. Am. J. Sports Med. 2016;44(2):331–336. doi: 10.1177/0363546515614813. [DOI] [PubMed] [Google Scholar]
- 38.Sanders TL, Pareek A, Hewett TE, et al. Long-term rate of graft failure after ACL reconstruction: a geographic population cohort analysis. Knee Surgery, Sport. Traumatol. Arthrosc. 2016 doi: 10.1007/s00167-016-4275-y. Epub ahead. [DOI] [PubMed] [Google Scholar]
- 39.Moisio KC, Sumner DR, Shott S, Hurwitz DE. Normalization of joint moments during gait: A comparison of two techniques. J. Biomech. 2003;36(4):599–603. doi: 10.1016/s0021-9290(02)00433-5. [DOI] [PubMed] [Google Scholar]
- 40.Manal K, Buchanan TS. An electromyogram-driven musculoskeletal model of the knee to predict in vivo joint contact forces during normal and novel gait patterns. J. Biomed. Eng. 2013;135(2):021014. doi: 10.1115/1.4023457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buchanan TS, Lloyd DG, Manal K, Besier TF. Neuromusculoskeletal modeling: estimation of muscle forces and joint moments and movements from measurements of neural command. J Appl Biomech. 2004;20(4):367–395. doi: 10.1123/jab.20.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gardinier ES, Manal K, Buchanan TS, Snyder-Mackler L. Minimum detectable change for knee joint contact force estimates using an EMG-driven model. Gait Posture. 2013;38(4):1051–1053. doi: 10.1016/j.gaitpost.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd Edition Lawrence Erlbaum Associates; Hillsdale, New Jersey: 1988. p. 567. [Google Scholar]
- 44.Rudolph KS, Eastlack ME, Axe MJ, Snyder-Mackler L. 1998 Basmajian Student Award Paper Movement patterns after anterior cruciate ligament injury: A comparison of patients who compensate well for the injury and those who require operative stabilization. J. Electromyogr. Kinesiol. 1998;8(6):349–362. doi: 10.1016/s1050-6411(97)00042-4. [DOI] [PubMed] [Google Scholar]
- 45.Rudolph KS, Axe MJ, Buchanan TS, et al. Dynamic stability in the anterior cruciate ligament deficient knee.Knee Surgery, Sport. Traumatol. Arthrosc. 2001;9(2):62–71. doi: 10.1007/s001670000166. [DOI] [PubMed] [Google Scholar]
- 46.Lewek M, Rudolph K, Axe M, Snyder-Mackler L. The effect of insufficient quadriceps strength on gait after anterior cruciate ligament reconstruction. Clin. Biomech. 2002;17(1):56–63. doi: 10.1016/s0268-0033(01)00097-3. [DOI] [PubMed] [Google Scholar]
- 47.Joreitz R, Lynch A, Rabuck S, et al. Patient-specific and surgery-specific factors that affect return to sport after ACL reconstruction. Int. J. Sports Phys. Ther. 2016;11(2):264–78. [PMC free article] [PubMed] [Google Scholar]
- 48.Konrath JM, Vertullo CJ, Kennedy BA, et al. Morphologic characteristics and strength of the hamstring muscles remain altered at 2 years after use of a hamstring tendon graft in anterior cruciate ligament reconstruction. Am. J. Sports Med. 2016 doi: 10.1177/0363546516651441. Epub ahead. [DOI] [PubMed] [Google Scholar]
- 49.Williams GN, Snyder-Mackler L, Barrance PJ, et al. Muscle and tendon morphology after reconstruction of the anterior cruciate ligament with autologous semitendinosus-gracilis graft. J. Bone Jt. Surg. Am. 2004;86-A(9):1936–46. doi: 10.2106/00004623-200409000-00012. [DOI] [PubMed] [Google Scholar]
- 50.Brophy RH, Silvers HJ, Mandelbaum BR. Anterior cruciate ligament injuries: etiology and prevention. Sports Med. Arthrosc. 2010;18(1):2–11. doi: 10.1097/JSA.0b013e3181cdd195. [DOI] [PubMed] [Google Scholar]