Abstract
Interfering with memory reconsolidation has valuable potential to be used as a treatment for maladaptive memories and psychiatric disorders. Numerous studies suggest that reconsolidation-based therapies may benefit psychiatric populations, but much remains unanswered. After reviewing the literature in clinical and healthy human populations, we discuss some of the major limitations to reconsolidation studies and clinical application. Finally, we provide recommendations for developing improved reconsolidation-based treatments, namely exploiting known boundary conditions and focusing on a novel unconditioned stimulus-retrieval paradigm.
Keywords: reconsolidation, addiction, psychopathology, PTSD, schizophrenia, garcinol
1. Introduction1
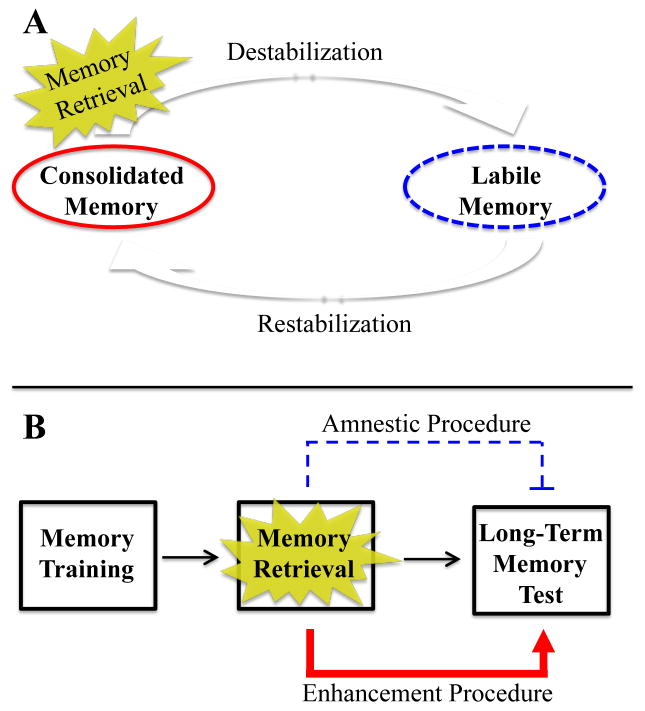
The traditional theory of memory consolidation holds that memories undergo one single consolidation process, a process that converts a short-term memory into a long-term memory (McGaugh 1966). Once this conversion occurs, the memories are thought to be permanent and resistant to change. However, the conventional theory has recently been displaced by a new theory, one that hypothesizes that whenever memories are reactivated they enter a period of lability, during which time the memories can be altered or updated (Fig. 1A). Notably, certain aspects of the memory (e.g., declarative or emotional components) may have the potential to be independently altered or updated. Following memory destabilization, memories must undergo another round of a consolidation-like process, which has been termed reconsolidation. If the reconsolidation process is interfered with, the memory may be permanently weakened or disrupted, a state that is also known as “amnesic deconsolidation” (Hong et al. 2011).
Figure 1. Memory Reconsolidation Process.
A, A schematic representation of memory reconsolidation. Upon memory retrieval, a consolidated memory destabilizes into a labile memory. The labile memory then restabilizes back into a consolidated memory. B, A schematic representation of paradigms used to study memory reconsolidation. Following memory training, participants are undergo an amnestic or enhancement procedure (e.g., behavioral procedure or administration of a pharmacological compound) before or after a memory retrieval session. Amnestic and enhancement procedures should decrease or increase memory retention, respectively, on a long-term memory test due to modulation of reconsolidation.
Memory reconsolidation was first explored in the 1960s, examining the effects of post-retrieval electroconvulsive shock on subsequent amnesia for a fear conditioning memory (Misanin et al. 1968; Schneider and Sherman 1968). Such findings called the consolidation theory into question (Lewis 1969), but interest in the so-called electroconvulsive shock-induced retrograde amnesia phenomenon soon died out. However, interest in reconsolidation was rekindled around the turn of the millennium (Nader et al. 2000; Przybyslawski et al. 1999; Przybyslawski and Sara 1997). In the past decade and a half, a plethora of research has since been carried out, and reconsolidation has become an intriguing topic with many proposed therapeutic effects for patients with psychiatric disorders, including post-traumatic stress disorder (PTSD) and addiction (see for recent review, Taylor and Torregrossa 2015).
In this review article, the clinical treatment potential of reconsolidation-based manipulations will be examined. First, behavioral procedures employed in human studies of reconsolidation will be introduced. Then, the literature on reconsolidation manipulations in clinical populations and in healthy individuals will be discussed. Next, some of the major limitations to reconsolidation studies and clinical application will be overviewed. Finally, we will provide recommendations for developing improved reconsolidation-based treatments, namely exploiting known boundary conditions and focusing on a novel unconditioned stimulus (US)-retrieval paradigm.
2. Reconsolidation Paradigms
Most paradigms used for experimentally studying reconsolidation follow the same basic structure (Fig. 1B). First, participants undergo a training session, during which a new memory is learned. After the memory is consolidated, participants undergo a memory reactivation session in which the memory is briefly reactivated through presentation of either a memory-related cue, in order to induce memory destabilization and lability. Before or after memory reactivation, a pharmacological agent (e.g., propranolol), behavioral procedure (e.g., stress), or non-invasive technique (e.g., transcranial magnetic stimulation; TMS) is administered to enhance or to interfere with reconsolidation. Finally, after the reconsolidation process has ended, a long-term memory test is performed, during which time memory retention is assessed.
Important controls should be included to verify that any effects of the manipulation on long-term memory are due to modulation of reconsolidation. No-reactivation control groups should receive the experimental and control manipulations in the absence of memory reactivation. Memory reconsolidation depends upon post-reactivation memory labilization, and, therefore, the experimental manipulation in the absence of memory retrieval should have no effect on long-term memory. Another control option is a delayed-treatment group, in which memory reactivation takes place but pharmacological or other treatment is administered after the memory has putatively restabilized and is no longer labile. Administration of the experimental manipulation outside of the post-reactivation window of lability should have no effect on long-term memory. In addition to the no-reactivation or delayed-treatment control groups, a short-term memory test can also be administered soon after memory reactivation, before the reconsolidation process has completed. Memory retention should be present in the short-term memory test but not the long-term memory test if the manipulation is interfering with the reconsolidation process. In addition, the inhibition of the memory should be long-lasting and not subject to renewal or spontaneous recovery if it has been altered by a reconsolidation-based mechanism.
In some studies, behavioral extinction sessions are used as a post-reactivation manipulation (Monfils et al. 2009; Xue et al. 2012; Schiller et al. 2010). In the retrieval-extinction paradigm, extinction sessions are administered at a time point at which the memory is thought to already be destabilized but not yet restabilized (Fig. 1A). These studies were originally hypothesized to interfere with reconsolidation, as extinction administration during the post-retrieval period of memory lability could putatively enable the extinction learning to be incorporated into the original memory trace upon restabilization. Recent reports call into question whether retrieval-extinction is, in fact, working through a reconsolidation-based mechanism (Hutton-Bedbrook and McNally 2013). Reconsolidation theory would posit that retrieval must come before extinction for the procedure to impair reinstatement. However, retrieval following extinction has also been shown to impair fear reinstatement (Baker et al. 2013), and in some instances extinction following retrieval paradoxically enhances fear behavior (Chan et al. 2010; Stafford et al. 2013). It also appears that multiple retrieval-extinction sessions may be required to achieve the desired mnemonic consequences in certain paradigms, though this has yet to be studied systematically and this will be discussed in detail later.
In experiments using the retrieval-extinction paradigm, an additional control must be included in which subjects receive extinction first followed by retrieval afterwards in order to shed more light on the mechanism of the retrieval-extinction effect (Torregrossa and Taylor 2012). Though the debate has yet to be resolved, we will include discussion of the retrieval-extinction literature in humans in the following sections.
3. Reconsolidation in Clinical Populations
3.1 Addiction
Examination of the treatment efficacy of reconsolidation manipulations in clinical populations of addiction has just recently begun. For example, in hazardous alcohol drinkers, post-retrieval ethanol cue reappraisal or counterconditioning blocked reconsolidation in a retrieval-dependent manner (Das et al. 2015b; Hon et al. 2015). Furthermore, in these studies, no behavioral effect was elicited when the design did not explicitly elicit a prediction error, which is thought to enhance memory lability memory and initiate reconsolidation (Exton-McGuinness et al. 2015; Torregrossa and Taylor 2016; Corlett and Taylor 2013). In another study, a retrieval-extinction paradigm administered to heroin addicts significantly reduced subsequent cue-induced craving and blood pressure changes, as tested at 1, 30, or 180 days following treatment (Xue et al. 2012). However, these results have yet to be replicated, and no control group was included that reversed the order of extinction and reactivation to verify that the mechanism of memory inhibition is based on reconsolidation mechanisms (Millan et al. 2013).
Also in abstinent heroin addicts, social stress or propranolol administered after recall of a learned word list impaired memory of the word list the next day (Zhao et al. 2011, 2009). Interestingly, subjects displayed a deficit in remembering words related to heroin, while their ability to recall neutral words was not affected, indicating that neutral and emotional memories may be differentially modulated by reconsolidation manipulation. Post-retrieval propranolol has also been reported to block cocaine-cue reactivity and to attenuate cue-elicited craving in non-treatment-seeking cocaine-dependent individuals (Saladin et al. 2013), and pre-reactivation propranolol can reduce tonic craving in treatment-seeking individuals with substance use disorders (Lonergan et al. 2016). However, neither of these two more recent studies included a no-reactivation or delayed-treatment control group, leaving open the possibility that propranolol may be acting through a mechanism unrelated to reconsolidation.
Two studies investigating the effect of reconsolidation manipulations on memories related to cigarette smoking failed to find an effect of either propranolol or the N-methyl-d-aspartate (NMDA) receptor antagonist memantine on such memories (Das et al. 2015a; Pachas et al. 2015). Each cigarette puff represents a single administration of nicotine, and multiple puffs per cigarette are compounded over multiple cigarettes per day; thus, there are many more drug administrations per day in most smokers than in heroin or cocaine addicts. The increased number of administrations could strengthen the cue-associated memory, making the smoking memory more resistant to reconsolidation disruption than cocaine or heroin memories.
One contrasting study in opioid-dependent patients on methadone maintenance reported that pre-reactivation propranolol unexpectedly increased cocaine-cue reactivity (Jobes et al. 2015). The reason for propranolol strengthening the memory as opposed to weakening it are unclear and are in contrast with all previous reports of propranolol and reconsolidation, which have found that propranolol either weakens a memory or has no effect. Despite this aberrant report, overall results are promising that interfering with the reconsolidation of drug-related memories in patients with substance use disorders may prove to be a viable treatment option (Milton 2012).
Overall, results are promising that reconsolidation-based manipulations may be a useful treatment for addiction (Torregrossa et al. 2011). With an optimized protocol, it is possible that retrieval of a drug-associated memory may labilize to such an extent that relapse will be reduced. Additional research is needed to examine the conditions under which reconsolidation-based treatments may be most beneficial and to rule out alternative explanations by including additional control groups.
3.2 PTSD
The first study of memory reconsolidation in patients with PTSD demonstrated that propranolol may effectively reduce the reconsolidation of certain aspects of traumatic memories (Brunet et al. 2008). Specifically, propranolol administered after reactivation of the traumatic memory through the reading of a previously prepared personalized script caused a decrease in physiological responding to the personalized script one week later. Another study found that the mammalian target of rapamycin (mTOR) inhibitor rapamycin administered prior to personalized script-driven traumatic memory reactivation decreased symptoms of PTSD in combat veterans (Surís et al. 2013). These effects were present at one but not three months following propranolol treatment and were found for veterans with recent (post-Vietnam era; mean illness duration of 7 years) but not old (Vietnam-era; mean illness duration of 41 years) trauma, indicating that PTSD may be more easily treated through reconsolidation manipulation in its early stages rather than its late stages. Neither of these studies included no-reactivation or delayed-treatment control groups. Thus, additional studies examining the effect of propranolol in the absence of reactivation are warranted to verify that the effects of propranolol are due to reconsolidation blockade as opposed to its non-specific anxiolytic effects.
An additional case report demonstrated a possible effect of post-reactivation electroconvulsive therapy (ECT) on reducing memories related to the reactivated trauma in a patient with PTSD (Gahr et al. 2014), circling back to early examinations of electroconvulsive shock-induced retrograde amnesia (Misanin et al. 1968; Rubin 1976; Schneider and Sherman 1968). Critically, memories related to a trauma that was not reactivated were spared, indicating that ECT was not inducing global memory impairments. Randomized-controlled trials investigating the effect of ECT on PTSD reconsolidation should be carried out to verify this reported effect.
The need for including appropriate control groups in human studies of reconsolidation is highlighted by one published study of reconsolidation in PTSD patient s that found no effect of pre-reactivation propranolol or mifepristone (a glucocorticoid antagonist) on physiological responding to traumatic imagery or on PTSD symptoms (Wood et al. 2015). The reason for the differing results as compared to the aforementioned study by Brunet and colleagues (2008) may be due to a difference in control groups. Whereas the earlier study compared participants who received pre-reactivation propranolol to participants who received pre-reactivation placebo, the study by Wood and colleagues compared participants who received pre-reactivation propranolol or mifepristone to participants who received propranolol or mifepristone two days prior to traumatic memory reactivation, essentially a delayed-treatment control group. Thus, all participants in the second study received propranolol or mifepristone at some point, and it is therefore possible that propranolol administration has non-specific effects on PTSD symptoms and physiological responding that are independent of memory recall or reconsolidation. Future work is needed to parse out these possibilities. Lastly, while reconsolidation-based manipulations may not induce complete amnesia of the traumatic event (and it may be argued that such manipulations should not induce complete amnesia of life events, as discussed below), interfering with the emotionality aspect of the memory may provide substantial therapeutic benefit.
3.3 Phobias
Emerging evidence implicates reconsolidation manipulation as a potential treatment for phobias. Specifically, in patients with spider phobia, cortisol administered before four reactivation sessions reduced participants’ self-reported fear towards a spider stimulus when assessed two days after the reactivation sessions concluded (Soravia et al. 2006). In this study, the phobia memory was reactivated through the brief presentation of a photograph of a spider. No control group was included that received cortisol in the absence of memory reactivation. A recent study, however, has demonstrated that propranolol administered following reactivation blocks fearful responding towards spiders in individuals with spider phobia and that propranolol given in the absence of reactivation has no effect on fear (Soeter and Kindt 2015). This study reactivated the memory through presentation of an actual tarantula and demonstrated that the effect of propranolol at reducing physiological responding and self-declared fear towards spider-related stimuli persists for at least one year. The long-lasting effects of propranolol treatment are extremely promising for clinical application. In addition to spider phobias, a report of a randomized controlled trial of the reconsolidation-modulating effects of propranolol on dental extraction phobia is also underway, though results are still forthcoming (Steenen et al. 2015).
3.4 Schizophrenia
In contrast to the previous disorders in which reconsolidation has been studied mainly as a treatment, aberrant reconsolidation has been implicated in the actual formation and persistence of delusions and psychosis (Corlett et al. 2009). Briefly, it has been hypothesized that patients with schizophrenia or psychotic symptoms may experience aberrant dopaminergic prediction error signaling, which inadvertently attributes salience to stimuli that would normally be ignored and which induces memory destabilization when destabilization would not normally occur (Corlett et al. 2010, 2009). Due to enhanced destabilization, a memory can then be inappropriately updated and reconsolidated. This inappropriate reconsolidation can lead to delusional beliefs, which persist because they are continuously reconsolidated and strengthened upon recall.
Consistent with this hypothesis, in a preclinical model of psychosis through acute ketamine administration, rats that received pre-reactivation ketamine demonstrated enhanced reconsolidation in a manner suggestive of increased destabilization (Honsberger et al. 2015). Importantly, ketamine had no effect in the absence of reactivation. In addition, in a human model of psychosis in which healthy participants received acute injections of ketamine, reconsolidation was also enhanced in pre-reactivation ketamine-treated individuals and positively correlated with prediction-error signaling in the brain (Corlett et al. 2013).
Due to the findings that reconsolidation manipulations can enhance fear in humans, studies attempting to block reconsolidation and decrease aberrant memories in humans (e.g., for addiction, PTSD, or phobias) should take extreme care to utilize methodology that will not unexpectedly enhance reconsolidation. Additionally, future work should examine reconsolidation in patients with schizophrenia and investigate whether treatments that impair reconsolidation may effectively decrease the strength of delusions.
4. Reconsolidation in Healthy Participants
Studies examining reconsolidation in healthy subjects can help shed light on optimal reconsolidation parameters and better inform potential reconsolidation-based psychiatric treatment. In healthy subjects, reconsolidation of many types of neutral memories can be impaired, including motor skill learning (de Beukelaar et al. 2014; Walker et al. 2003), declarative memory (Forcato et al. 2007, 2009), and episodic memory (Chan et al. 2009; Hupbach et al. 2007). The following subsections detail the most-investigated means of interfering with reconsolidation in healthy participants.
4.1 Effect of Propranolol on Reconsolidation in Healthy Participants
The role of β-adrenergic receptors in human memory reconsolidation has been extensively examined, and β-adrenergic blockade with propranolol frequently impairs the reconsolidation of negative or aversive memories. In healthy subjects, propranolol has been reported to interfere with the reconsolidation of negative memories, while having no effect on the reconsolidation of neutral memories (Schwabe et al. 2012a, 2012b). Propranolol has likewise been demonstrated to impair fear memory reconsolidation in healthy participants (Kindt et al. 2009; Sevenster et al. 2014; Soeter and Kindt 2011), even when the fear memory is strengthened by acquisition manipulations (Soeter and Kindt 2012). Propranolol disruption of reconsolidation can also impair the generalization of fear, which is critical for the translation to anxiety disorder treatment (Soeter and Kindt 2011). The effect of propranolol on fear memory reconsolidation was shown to have no effect on the declarative memory for the stimulus-fear association, though the emotional response to the stimulus was greatly reduced (Soeter and Kindt 2012).
These affirmative effects of propranolol on negative memories are not universal, however. One group found that propranolol administered before retrieval affected neither emotional nor neutral memories (Tollenaar et al. 2009a, 2009b), and two other groups recently reported no effect of propranolol on fear memory reconsolidation (Bos et al. 2014a; Spring et al. 2015). It is unclear why these findings were inconsistent with previous results, especially given that procedures were nearly identical to earlier studies (Kindt et al. 2009), but it suggests that even subtle methodological differences can have a profound impact on results. Further research is needed to determine optimal conditions for blocking reconsolidation with propranolol.
Despite these few disparate studies, a recent meta-analysis has found that propranolol significantly reduces the reconsolidation of emotional memories in humans across studies (Lonergan et al. 2012), indicating that propranolol should continue to be investigated as an effective tool for manipulating reconsolidation. The idea that propranolol more strongly inhibits memory reconsolidation of negative or fear-related memories as opposed to neutral memories has valuable implications for the clinical use of propranolol in the treatment of psychiatric disorders. Specifically, propranolol may be more effective at inhibiting aberrant aversive memories in disorders such as PTSD as opposed to aberrant reward-related memories in disorders such as substance use. This idea is supported by our preclinical findings that systemically administered propranolol does not impair the reconsolidation of cocaine-cue memories in a model of reinstatement (i.e., relapse) to cocaine-seeking behavior in rodents trained to self-administer cocaine (Dunbar and Taylor 2016).
4.2 Effect of Stress on Reconsolidation in Healthy Participants
Administration of a stressor following memory recall can have bidirectional effects on memory reconsolidation processes, which holds strong clinical implications for stress-related psychiatric disorders such as anxiety and PTSD. In some cases, administration of a stressor around the time of recall can inhibit memory reconsolidation (Marin et al. 2011; Strange et al. 2010; Tollenaar et al. 2008, 2009a; Zhao et al. 2009). For example, episodic memory reconsolidation is impaired when an emotionally aversive stimulus or a glucocorticoid antagonist is administered after memory retrieval (Marin et al. 2011; Strange et al. 2010). Additionally, administration of a stressor or of cortisol, which may pharmacologically mimic some components of stress, prior to retrieval of an emotional or neutral task impaired reconsolidation of both tasks in healthy subjects (Tollenaar et al. 2008, 2009a). One group found that a stressor given after memory reactivation impaired reconsolidation of neutral episodic memories but had no effect on emotional episodic memories (Schwabe and Wolf 2009, 2010). In support of this finding, administration of cortisol following reactivation also had no effect on emotional memory reconsolidation in healthy subjects (Tollenaar et al. 2009b).
Conversely, post-reactivation stressors can also serve to enhance memory reconsolidation in some paradigms. Mild stressors or reminders of negative emotional events just following memory recall improve reconsolidation of emotional or neutral declarative learning tasks (Bos et al. 2014b; Coccoz et al. 2011; Finn and Roediger 2011). Additionally, post-reactivation, but not pre-reactivation psychosocial stress can enhance episodic memory (Dongaonkar et al. 2013).
Whether stress affects reconsolidation in a positive or negative manner likely depends upon the severity of the stressor as well as the emotional characteristics of the memory that is reactivated. Some type of stress dose-response curve may exist for predicting whether stress will disrupt or enhance reconsolidation, which will be an intriguing area for future work to examine. Additional work should also address the effect of post-retrieval stress on reconsolidation in populations with stress-related psychiatric disorders.
4.3 Effect of Retrieval-Extinction on Reconsolidation in Healthy Participants
Inspired by a promising preclinical study (Monfils et al. 2009), a non-pharmacological retrieval-extinction paradigm for reducing fear memory reconsolidation was tested in humans (Schiller et al. 2010), which consisted of sessions of reactivating a fear memory immediately prior to multiple extinction trials in order to decrease reconsolidation. While this effect has been replicated in human fear conditioning experiments (Johnson and Casey 2015; Oyarzún et al. 2012) and extended to studies of drug-related memories in humans (Xue et al. 2012), other replications of this behavioral procedure have produced mixed results (Golkar et al. 2012; Kindt and Soeter 2011; Meir Drexler et al. 2014; Soeter and Kindt 2011). Additionally, the critical control group of extinction prior to retrieval is typically absent in human studies. Thus, it will be necessary to further investigate the retrieval-extinction paradigm in the context of psychiatric treatment and to ensure that future studies include proper control groups to confirm that this method depends on reconsolidation processes.
4.4 Other Modulators of Reconsolidation in Healthy Participants
Other studies using non-pharmacological reconsolidation manipulations found that TMS in the primary motor cortex blocks motor memory reconsolidation (Censor et al. 2014, 2010). Conversely, repetitive TMS or transcranial direct current stimulation of the prefrontal cortex enhances reconsolidation of an episodic or declarative memory, respectively (Sandrini et al. 2013; Javadi and Cheng 2012), indicating a neuroanatomical divergence in substrates involved in the strengthening or weakening of reconsolidation, as compared to the motor cortex. In addition, ECT administered following memory reactivation of an emotional episodic memory has been demonstrated to block reconsolidation (Kroes et al. 2014).
Behavioral manipulations also effectively interfere with memory reconsolidation. For example, initiation of new learning immediately following reactivation interferes with reconsolidation of motor, episodic, and declarative memories (Chan and LaPaglia 2013; de Beukelaar et al. 2014; Wichert et al. 2012). In contrast, engagement in a divided attention task during memory retrieval enhances memory reconsolidation as compared to engaging in full attention during memory retrieval (Kessler et al. 2014). Similar studies using non-invasive behavioral techniques should be carried out in the context of reconsolidation in psychiatric disorders, in order to determine whether such paradigms may be useful treatment options.
5. Limitations of the Clinical Application of Reconsolidation
It is necessary to note that due to the complicated ethical and methodological nature of manipulating memory reconsolidation in an experimental or clinical setting in humans, there are a number of factors that preclude a clear interpretation of the nature of reconsolidation in humans (Schiller and Phelps 2011). For example, some experimental designs may not control for experimental manipulation effects on retrieval versus reconsolidation (Kindt et al. 2009; Soeter and Kindt 2010) or for the effects of the experimental manipulation in the absence of memory reactivation. Additionally, the behavioral paradigms used in different studies are inconsistent and cannot be directly compared.
5.1 Pharmacological Experiments
In most experiments examining the effect of pharmacological treatment on reconsolidation, the control group receives a placebo treatment that does not induce any physiological effects. Notably, a placebo control may not be appropriate for compounds that induce anxiolytic interoceptive properties (e.g., propranolol and mifepristone). In such cases, the participant may be able to accurately guess whether they received pharmacological treatment or placebo, which would bias the study results. Such studies should include verification that participants are sufficiently blinded to their treatment. In cases where blinding is compromised, an appropriate pharmacological control that does not block reconsolidation but that does produce interoceptive effects should be utilized to increase the efficacy of participant blinding.
5.2 State-Dependency of Reconsolidated Memories
Recent reports have argued that paradigms thought to block memory reconsolidation may actually be inducing the integration of state-dependency into the previously consolidated memory (Flint et al. 2013; Gisquet-Verrier et al. 2015; Sierra et al. 2013). State-dependency is a long-studied phenomenon in which a memory learned in one state (e.g., under the influence of a drug) can only be recalled while in the same state but not in a different state (Girden and Culler 1937). With regards to reconsolidation, administration of a supposed amnestic agent at reactivation may induce an interoceptive state characteristic of that pharmacological agent. Because memory reactivation induces memory lability, the interoceptive state is then incorporated into the previously consolidated memory. Thus, the now state-dependent memory will only be recalled while in the same state as reactivation (i.e., when under the influence of the same pharmacological agent as at reactivation).
This hypothesis posits that the decreased memory present at the long-term memory test in most reconsolidation paradigms is not indicative of amnesia but is instead indicative only that the participant is not in the same reactivation state. Accordingly, if the subject is administered a long-term memory test in conjunction with the pharmacological agent from reactivation, the subject should demonstrate memory retention. Indeed, this hypothesis has been upheld in preclinical studies that demonstrated a reversal of the supposed amnestic effect of common pharmacological agents used to block reconsolidation, including the NMDA receptor antagonist MK-801 and the protein synthesis inhibitor cycloheximide, when the agents were administered both at reactivation and at the long-term memory test (Flint et al. 2013; Gisquet-Verrier et al. 2015).
Future studies of reconsolidation may consider using a control group that receives the supposed amnestic agent prior to the long-term memory test. Importantly, this could be accomplished through a within-subjects design and may not require additional subjects. The clinical relevance of the implications of state-dependency integration may, however, be minimal; as long as the individual avoids entering the state induced by the treatment agent, the aberrant memory should still be held at abeyance.
In addition, it is unlikely that all studies of reconsolidation blockade can be explained through the state-dependency integration hypothesis. Critically, this hypothesis cannot explain how certain compounds administered in conjunction with reactivation can strengthen a memory, even when the long-term memory test is administered in the absence of any pharmacology. Under the state-dependency integration hypothesis, even memory enhancement should only be present while in the same state as at reactivation. Thus, future work needs to be conducted to determine under what conditions a reconsolidated memory incorporates state-dependent information.
5.3 Ethics
Concerns have been raised as to whether it is ethical to alter the substance of a memory, as it may disrupt a sense of self (President’s Council on Bioethics 2003). However, others argue that, in individuals who may benefit from reconsolidation treatment, perhaps the sense of self is already disrupted due to the disabling psychological disabilities that interfere with daily life (Donovan 2010). Evidence shows that disruption of reconsolidation in humans impairs emotional response to memory while sparing declarative memory (Soeter and Kindt 2012), which suggests that an individual’s memory would not be entirely “erased” in the manner of a dystopic science-fiction plot, as might be feared. Although it is prudent to be cautious about the use of memory reconsolidation manipulations in human, the research suggests that the potential benefits may far outweigh the risks, and research should continue to be done in this area.
5.4 Boundary Conditions
Memories do not appear to be universally reconsolidated upon recall, which suggests the existence of certain boundary conditions that define whether or not a memory will undergo reconsolidation (Nader and Einarsson 2010; Reichelt and Lee 2013; Tronson and Taylor 2007). These boundary conditions that preclude reconsolidation are likely mirrored neurobiologically by downregulation of mechanisms that are critical for lability (Wang et al. 2009). Boundary conditions are not yet clearly defined, as for nearly every proposed boundary condition there exists a contradictory finding in the literature that calls the proposed boundary condition definition into question.
One of the most discussed boundary conditions is memory strength, which correlates positively with the strength of training and negatively with the age of the memory. Strong training has been shown to inhibit destabilization and preclude efficacy of reconsolidation manipulations (Liddie and Itzhak 2014; Robinson and Franklin 2010; Winters et al. 2009). Additionally, older memories are increasingly susceptible to reconsolidation as compared to newer memories (Robinson and Franklin 2010; Winters et al. 2009). In certain cases, however, robust memories showed no difference in comparison to weak memories in their ability to undergo reconsolidation (Debiec and LeDoux 2004; Lee et al. 2006).
The duration of reactivation session has also been proposed as a boundary condition. Short reactivation sessions appear to promote reconsolidation, whereas long reactivation sessions promote extinction (de Beukelaar et al. 2014; Pedreira and Maldonado 2003). As suggested by the trace dominance theory (Dudai 2004; Nader 2003), it has been proposed that conditions promoting extinction before memory lability occurs and before reconsolidation is initiated preclude reconsolidation mechanisms from engaging (Bustos et al. 2009; Eisenberg et al. 2003; Suzuki et al. 2004). In contrast, it has also been proposed that it is not actually the occurrence of extinction processes per se that imposes a boundary condition on reconsolidation, but, rather, it is the engagement of shared neurocircuitry in some reconsolidation and extinction paradigms that precludes reconsolidation (Duvarci et al. 2006).
Another boundary condition that has been proposed includes the experience of a prediction error, or a mismatch in what is expected and what is experienced, during reactivation session (Pedreira et al. 2004; Reichelt and Lee 2012; Sevenster et al. 2012). This can be accomplished by adding unexpected information or removing expected information during the reactivation session. Prediction-error signaling is thought to initiate reconsolidation in order to integrate the new information from the reactivation session into the existing memory (Exton-McGuinness et al. 2015; Torregrossa and Taylor 2016; Corlett and Taylor 2013). Evidence supporting the necessity of prediction-error signaling in memory destabilization is recent yet robust (Das et al. 2015a; Exton-McGuinness and Lee 2015; Exton-McGuinness et al. 2014; Hon et al. 2015; Sevenster et al. 2013, 2014).
5.5 Specificity of Reconsolidation Manipulations
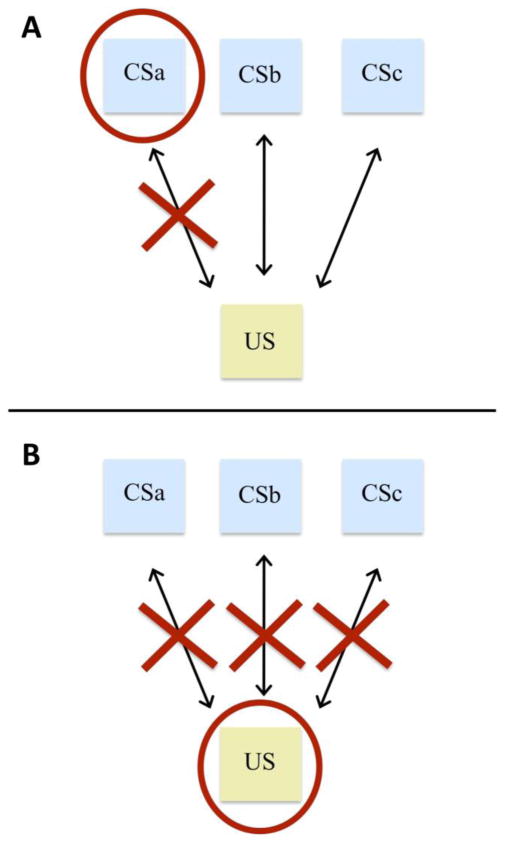
Conventional reconsolidation paradigms in which a manipulation is performed before or after conditioned stimulus (CS) reactivation specifically target the reactivated CS memory while sparing other memoires (Doyère et al. 2007). In a preclinical fear conditioning paradigm, for example, if two separate CSs are both paired with one aversive US, reactivation of one CS followed by intra-amygdalar NMDA-receptor antagonism weakens the association between the reactivated stimulus and fear, while the other non-reactivated CS continues to elicit a fear response (Doyère et al. 2007). While on one hand it is beneficial that reconsolidation manipulations do not indiscriminately impair unrelated memories, on the other hand, weakening the association between only one CS and the US may not have high clinical potential for disorders such as addiction, in which one US (i.e., the feeling of using the drug) is associated with numerous CSs (e.g., paraphernalia, internal states, daily activities, etc.).
Theoretically, a preferable treatment for such disorders would be one that disrupts the association between the US and all associated CSs, while sparing any memories that are unrelated to the US. Research using fear-conditioning paradigms has begun to show that this may, in fact, be feasible. First, LeDoux and colleagues demonstrated that a simple change in paradigm—reactivating the auditory fear memory through presentation of the US rather than the CS prior to pharmacological treatment—impairs the association of multiple CSs paired with the reactivated US after a single US retrieval session (Debiec et al. 2010; Díaz-Mataix et al. 2011). Importantly, the disruption of reconsolidation was specific to the CSs paired only with the reactivated US, as the association between a distinct CS and distinct non-reactivated US remained intact. Another group found that a paradigm consisting of US retrieval followed by extinction induced a long-lasting decrease in fear responding to multiple CSs in both rats and humans (Liu et al. 2014). Again, the manipulations were specific to the CSs paired with the reactivated US and does not globally disrupt the fear response, but no control group received extinction prior to reactivation to confirm that the effect resulted from a disruption of reconsolidation.
Evidence also suggests that US-evoked recall may also be effective in initiating a window of lability for drug-related memories (i.e., destabilizing the memories) during which time reconsolidation can be manipulated. For example, a single US-reactivation session followed by pharmacological treatment in a rodent model of cocaine self-administration blocks cue-induced reinstatement to multiple CSs paired with the drug (Dunbar and Taylor Under Review). The pharmacological agent used was garcinol, a naturally-occurring histone acetyltransferase inhibitor that can safely be given to humans and also blocks reconsolidation after CS reactivation (Monsey et al. 2016; Maddox et al. 2013). Importantly, US-reactivation destabilized the memory whether or not it was administered through the same route as during self-administration training, showing high translational potential for the development of clinical reconsolidation-based treatments for drug addiction, which is associated with several routes of drug administration. Whereas the conventional CS-reactivation procedure may recruit the neural correlates of only a single CS-US memory (Fig. 2A), US reactivation may recruit neural correlates of all CSs associated with the reactivated US, thus allowing for destabilization and, thus, disruption of all CS-US associations for the reactivated US (Fig. 2B).
Figure 2. Enhanced efficacy of the US-reactivation paradigm over the traditional CS-reactivation paradigm.
A, CS Reactivation: reactivation of CSa, followed by a reconsolidation manipulation, impairs the CSa-US association while sparing the associations between other CSs and the US. B, US Reactivation: reactivation of the US impairs the associations between the US and all CSs.
In addition, a longer protocol consisting of 12 days of US memory retrieval followed by 3-hour extinction sessions during the window of lability decreases reinstatement to the multiple CSs paired with a single cocaine US (Luo et al. 2015). Notably, the effect does not occur when extinction occurs prior to US retrieval, indicating that the mechanism is based on reconsolidation mechanisms. The US reactivation followed by pharmacological treatment may have higher translational potential in the clinical for treating addiction, as it requires only a single session lasting less than 10 minutes as opposed to the retrieval-extinction procedure that requires 12 sessions lasting longer than 3 hours each.
6. Recommendations
6.1 Exploiting Boundary Conditions
In designing studies to test reconsolidation manipulations in psychiatric populations, paradigms should be constructed to exploit the previously identified boundary conditions. For example, reconsolidation-based treatments will likely be more effective if administered early in the course of the disorder when the aberrant memory is weaker as compared to later in the disorder time course (Surís et al. 2013). Additionally, since many individuals do not seek treatment until later in the course of the disorder, behavioral (e.g., enhancement of prediction error) or pharmacological (e.g., d-cycloserine administration) adjuncts should be evaluated that may more effectively destabilize a stronger memory and make that memory susceptible to reconsolidation blockade. Enhancement of prediction error may be accomplished by altering the magnitude of the CS and/or US or adjusting the CS-US temporal contingency during memory reactivation (Díaz-Mataix et al. 2013; Exton-McGuinness et al. 2014).
6.2 US-Reactivation Paradigms
While only a handful of studies have specifically examined US reactivation procedures to block reconsolidation (Dunbar and Taylor Under Review; Debiec et al. 2010; Díaz-Mataix et al. 2011; Liu et al. 2014; Luo et al. 2015), these studies have demonstrated remarkable insight into a manner in which aberrant memories associated with multiple CSs may be diminished without globally disrupting unrelated memory processes. US reactivation confers an undeniable advantage over traditional CS reactivation procedures in that it a single reactivation session may activate the neural circuits associated with multiple CSs paired with a single CS, allowing for more efficient blockade of multiple aberrant cue memories. However, much remains to be elucidated about the US-reactivation procedure. In order to answer these questions and in light of the procedural advantage, we recommend that researchers interested in reconsolidation increase their focus on US-reactivation paradigms.
Most notably, more US-reactivation reconsolidation experiments should be conducted with humans, both in healthy and clinical populations. Only one study published thus far has examined a US-retrieval-extinction paradigm (Liu et al. 2014) in healthy subjects who were mildly fear conditioned. While this study found promising results despite not including an important extinction-retrieval control, no studies have examined a US-reactivation procedure in the context of drug use, in clinical populations, or using a post-US-reactivation pharmacological treatment. Such experiments are crucial for shedding light on the potential of this paradigm to be used as a potential treatment for psychopathology.
In addition, it will be important to directly examine whether the extinction or pharmacological treatment following US reactivation is more effective at blocking aberrant memories. Based on preclinical studies of drug memories, the pharmacological approach may be more advantageous as compared to retrieval-extinction, as it requires only one short treatment session (Dunbar and Taylor Under Review; Luo et al. 2015). Developing an effective clinical treatment that requires fewer sessions and less time will increase patient retention, decrease costs, and improve treatment outcomes. An exciting candidate to examine for the pharmacological approach is garcinol, which can be given safely to humans and which blocks reconsolidation in rodents (Dunbar and Taylor Under Review; Maddox et al. 2013; Monsey et al. 2016).
Another interesting empirical question is whether US reactivation generates a larger prediction error than CS reactivation. It is possible that since the US is associated with multiple CSs, a very large negative prediction error may occur when the US is reactivated in the absence of any CS. In contrast, a smaller negative prediction error may occur if the CS is reactivated in the absence of the US, since the CS is typically associated with just one US. If US reactivation does indeed promote an enhanced prediction error signal, the US reactivation procedure might block memories that may be more resistant to reconsolidation due to boundary conditions, such as older or stronger memories, and would increase the potential of this paradigm to treat strongly engrained psychopathologies.
7. Conclusion
Researchers have had success in translating the work investigated in animal models into human studies in both clinical and healthy populations, indicating promising therapeutic potential for reconsolidation therapies in the treatment of psychiatric disorders such as addiction and PTSD. Further work must be done to exploit the boundary conditions that apply to reconsolidation in a clinical setting and to apply the knowledge gained from studies conducted in healthy participants to clinical populations. In addition, clinical studies investigating the potential of the US-reactivation paradigm as a treatment for psychiatric disorders should also be prioritized. A better understanding of reconsolidation-manipulation paradigms that may effectively combat multiple aberrant learning associations is critical for future clinical application of reconsolidation-based treatment of psychopathology.
Highlights.
The reconsolidation literature in humans is reviewed.
Major limitations to the clinical application are discussed
Recommendations for improved reconsolidation-based treatments are offered.
Acknowledgments
This work was supported by DA015222 (JRT), the Charles B.G. Murphy Fund, the Connecticut Department of Mental Health and Addiction Services, and the NSF Graduate Research Fellowship Program.
Footnotes
Abbreviations: CS; conditioned stimulus; ECT: electroconvulsive therapy; mTOR: mammalian target of rapamycin; NMDA: N-methyl-d-aspartate; PTSD: post-traumatic stress disorder; TMS: transcranial magnetic stimulation; US: unconditioned stimulus
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker KD, McNally GP, Richardson R. Memory retrieval before or after extinction reduces recovery of fear in adolescent rats. Learn Mem Cold Spring Harb N. 2013;20:467–473. doi: 10.1101/lm.031989.113. [DOI] [PubMed] [Google Scholar]
- Bos MG, Beckers T, Kindt M. Noradrenergic blockade of memory reconsolidation: a failure to reduce conditioned fear responding. Front Behav Neurosci. 2014a;8:412. doi: 10.3389/fnbeh.2014.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos MG, Schuijer J, Lodestijn F, Beckers T, Kindt M. Stress enhances reconsolidation of declarative memory. Psychoneuroendocrinology. 2014b;46:102–113. doi: 10.1016/j.psyneuen.2014.04.011. [DOI] [PubMed] [Google Scholar]
- Brunet A, Orr SP, Tremblay J, Robertson K, Nader K, Pitman RK. Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. J Psychiatr Res. 2008;42:503–506. doi: 10.1016/j.jpsychires.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Bustos SG, Maldonado H, Molina VA. Disruptive effect of midazolam on fear memory reconsolidation: decisive influence of reactivation time span and memory age. Neuropsychopharmacology. 2009;34:446–457. doi: 10.1038/npp.2008.75. [DOI] [PubMed] [Google Scholar]
- Censor N, Dayan E, Cohen LG. Cortico-subcortical neuronal circuitry associated with reconsolidation of human procedural memories. Cortex. 2014;58:281–288. doi: 10.1016/j.cortex.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Censor N, Dimyan MA, Cohen LG. Modification of existing human motor memories is enabled by primary cortical processing during memory reactivation. Curr Biol. 2010;20:1545–1549. doi: 10.1016/j.cub.2010.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JC, LaPaglia JA. Impairing existing declarative memory in humans by disrupting reconsolidation. Proc Natl Acad Sci U S A. 2013;110:9309–9313. doi: 10.1073/pnas.1218472110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JC, Thomas AK, Bulevich JB. Recalling a witnessed event increases eyewitness suggestibility: the reversed testing effect. Psychol Sci. 2009;20:66–73. doi: 10.1111/j.1467-9280.2008.02245.x. [DOI] [PubMed] [Google Scholar]
- Chan WYM, Leung HT, Westbrook RF, McNally GP. Effects of recent exposure to a conditioned stimulus on extinction of Pavlovian fear conditioning. Learn Mem Cold Spring Harb N. 2010;17:512–521. doi: 10.1101/lm.1912510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccoz V, Maldonado H, Delorenzi A. The enhancement of reconsolidation with a naturalistic mild stressor improves the expression of a declarative memory in humans. Neuroscience. 2011;185:61–72. doi: 10.1016/j.neuroscience.2011.04.023. [DOI] [PubMed] [Google Scholar]
- Corlett PR, Cambridge V, Gardner JM, Piggot JS, Turner DC, Everitt JC, Arana FS, Morgan HL, Milton AL, Lee JL, et al. Ketamine effects on memory reconsolidation favor a learning model of delusions. PloS One. 2013;8:e65088. doi: 10.1371/journal.pone.0065088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlett PR, Krystal JH, Taylor JR, Fletcher PC. Why do delusions persist? Front Hum Neurosci. 2009;3:12. doi: 10.3389/neuro.09.012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlett PR, Taylor JR. Memory Reconsolidation. Academic Press; San Diego: 2013. [Accessed June 6, 2016]. Chapter 13 - The Translational Potential of Memory Reconsolidation A2 - Alberini, Cristina M. ppabcxyzpp273–292. http://www.sciencedirect.com/science/article/pii/B9780123868923000135. [Google Scholar]
- Corlett PR, Taylor JR, Wang X-J, Fletcher PC, Krystal JH. Toward a neurobiology of delusions. Prog Neurobiol. 2010;92:345–369. doi: 10.1016/j.pneurobio.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das RK, Hindocha C, Freeman TP, Lazzarino AI, Curran HV, Kamboj SK. Assessing the translational feasibility of pharmacological drug memory reconsolidation blockade with memantine in quitting smokers. Psychopharmacology (Berl) 2015a;232:3363–3374. doi: 10.1007/s00213-015-3990-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das RK, Lawn W, Kamboj SK. Rewriting the valuation and salience of alcohol-related stimuli via memory reconsolidation. Transl Psychiatry. 2015b;5:e645. doi: 10.1038/tp.2015.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beukelaar TT, Woolley DG, Wenderoth N. Gone for 60 seconds: reactivation length determines motor memory degradation during reconsolidation. Cortex. 2014;59:138–145. doi: 10.1016/j.cortex.2014.07.008. [DOI] [PubMed] [Google Scholar]
- Debiec J, Díaz-Mataix L, Bush DEA, Doyère V, LeDoux JE. The amygdala encodes specific sensory features of an aversive reinforcer. Nat Neurosci. 2010;13:536–537. doi: 10.1038/nn.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129:267–272. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Díaz-Mataix L, Debiec J, LeDoux JE, Doyère V. Sensory-specific associations stored in the lateral amygdala allow for selective alteration of fear memories. J Neurosci. 2011;31:9538–9543. doi: 10.1523/JNEUROSCI.5808-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Mataix L, Ruiz Martinez RC, Schafe GE, LeDoux JE, Doyère V. Detection of a temporal error triggers reconsolidation of amygdala-dependent memories. Curr Biol. 2013;23:467–472. doi: 10.1016/j.cub.2013.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dongaonkar B, Hupbach A, Gomez R, Nadel L. Effects of psychosocial stress on episodic memory updating. Psychopharmacology (Berl) 2013;226:769–779. doi: 10.1007/s00213-013-2998-8. [DOI] [PubMed] [Google Scholar]
- Donovan E. Propranolol use in the prevention and treatment of posttraumatic stress disorder in military veterans: forgetting therapy revisited. Perspect Biol Med. 2010;53:61–74. doi: 10.1353/pbm.0.0140. [DOI] [PubMed] [Google Scholar]
- Doyère V, Debiec J, Monfils M-H, Schafe GE, LeDoux JE. Synapse-specific reconsolidation of distinct fear memories in the lateral amygdala. Nat Neurosci. 2007;10:414–416. doi: 10.1038/nn1871. [DOI] [PubMed] [Google Scholar]
- Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- Dunbar AB, Taylor JR. Garcinol Blocks the Reconsolidation of Multiple Cocaine-Paired Cues After a Single Cocaine-Reactivation Session. Biol Psychiatry. doi: 10.1038/npp.2017.27. Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar AB, Taylor JR. Inhibition of Protein Synthesis but not β-Adrenergic Receptors Blocks Reconsolidation of a Cocaine-Associated Cue Memory. Learn Mem. 2016;23:391–308. doi: 10.1101/lm.042838.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S, Mamou CB, Nader K. Extinction is not a sufficient condition to prevent fear memories from undergoing reconsolidation in the basolateral amygdala. Eur J Neurosci. 2006;24:249–260. doi: 10.1111/j.1460-9568.2006.04907.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg M, Kobilo T, Berman DE, Dudai Y. Stability of retrieved memory: inverse correlation with trace dominance. Science. 2003;301:1102–1104. doi: 10.1126/science.1086881. [DOI] [PubMed] [Google Scholar]
- Exton-McGuinness MT, Lee JL. Reduction in Responding for Sucrose and Cocaine Reinforcement by Disruption of Memory Reconsolidation. eNeuro. 2015:2. doi: 10.1523/ENEURO.0009-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton-McGuinness MT, Lee JL, Reichelt AC. Updating memories--the role of prediction errors in memory reconsolidation. Behav Brain Res. 2015;278:375–384. doi: 10.1016/j.bbr.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Exton-McGuinness MT, Patton RC, Sacco LB, Lee JL. Reconsolidation of a well-learned instrumental memory. Learn Mem. 2014;21:468–477. doi: 10.1101/lm.035543.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn B, Roediger HL., 3rd Enhancing retention through reconsolidation: negative emotional arousal following retrieval enhances later recall. Psychol Sci. 2011;22:781–786. doi: 10.1177/0956797611407932. [DOI] [PubMed] [Google Scholar]
- Flint RW, Jr, Noble LJ, Ulmen AR. NMDA receptor antagonism with MK-801 impairs consolidation and reconsolidation of passive avoidance conditioning in adolescent rats: evidence for a state dependent reconsolidation effect. Neurobiol Learn Mem. 2013;101:114–119. doi: 10.1016/j.nlm.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Forcato C, Argibay PF, Pedreira ME, Maldonado H. Human reconsolidation does not always occur when a memory is retrieved: the relevance of the reminder structure. Neurobiol Learn Mem. 2009;91:50–57. doi: 10.1016/j.nlm.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Forcato C, Burgos VL, Argibay PF, Molina VA, Pedreira ME, Maldonado H. Reconsolidation of declarative memory in humans. Learn Mem. 2007;14:295–303. doi: 10.1101/lm.486107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahr M, Schönfeldt-Lecuona C, Spitzer M, Graf H. Electroconvulsive therapy and posttraumatic stress disorder: First experience with conversation-based reactivation of traumatic memory contents and subsequent ECT-mediated impairment of reconsolidation. J Neuropsychiatry Clin Neurosci. 2014;26:E38–E39. doi: 10.1176/appi.neuropsych.13070159. [DOI] [PubMed] [Google Scholar]
- Girden E, Culler E. Conditioned responses in curarized striate muscle in dogs. J Comp Psychol. 1937;23:261–274. [Google Scholar]
- Gisquet-Verrier P, Lynch JF, Cutolo P, Toledano D, Ulmen A, Jasnow AM, Riccio DC. Integration of new information with active memory accounts for retrograde amnesia: A challenge to the consolidation/reconsolidation hypothesis? J Neurosci. 2015;35:11623–11633. doi: 10.1523/JNEUROSCI.1386-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golkar A, Bellander M, Olsson A, Ohman A. Are fear memories erasable?-reconsolidation of learned fear with fear-relevant and fear-irrelevant stimuli. Front Behav Neurosci. 2012;6:80. doi: 10.3389/fnbeh.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hon T, Das RK, Kamboj SK. The effects of cognitive reappraisal following retrieval-procedures designed to destabilize alcohol memories in high-risk drinkers. Psychopharmacology (Berl) 2015 doi: 10.1007/s00213-015-4164-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong I, Kim J, Song B, Park S, Lee J, Kim J, An B, Lee S, Choi S. Modulation of fear memory by retrieval and extinction: a clue for memory deconsolidation. Rev Neurosci. 2011;22:205–229. doi: 10.1515/RNS.2011.023. [DOI] [PubMed] [Google Scholar]
- Honsberger MJ, Taylor JR, Corlett PR. Memories reactivated under ketamine are subsequently stronger: A potential pre-clinical behavioral model of psychosis. Schizophr Res. 2015;164:227–233. doi: 10.1016/j.schres.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupbach A, Gomez R, Hardt O, Nadel L. Reconsolidation of episodic memories: a subtle reminder triggers integration of new information. Learn Mem. 2007;14:47–53. doi: 10.1101/lm.365707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton-Bedbrook K, McNally GP. The promises and pitfalls of retrieval-extinction procedures in preventing relapse to drug seeking. Front Psychiatry. 2013;4:14. doi: 10.3389/fpsyt.2013.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javadi AH, Cheng P. Transcranial direct current stimulation (tDCS) enhances reconsolidation of long-term memory. Brain Stimulat. 2012 doi: 10.1016/j.brs.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Jobes ML, Aharonovich E, Epstein DH, Phillips KA, Reamer D, Anderson M, Preston KL. Effects of prereactivation propranolol on cocaine craving elicited by imagery script/cue sets in opioid-dependent polydrug users: A randomized study. J Addict Med. 2015;9:491–498. doi: 10.1097/ADM.0000000000000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DC, Casey BJ. Extinction during memory reconsolidation blocks recovery of fear in adolescents. Sci Rep. 2015;5:8863. doi: 10.1038/srep08863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler Y, Vandermorris S, Gopie N, Daros A, Winocur G, Moscovitch M. Divided attention improves delayed, but not immediate retrieval of a consolidated memory. PloS One. 2014;9:e91309. doi: 10.1371/journal.pone.0091309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt M, Soeter M. Reconsolidation in a human fear conditioning study: A test of extinction as updating mechanism. Biol Psychol. 2011 doi: 10.1016/j.biopsycho.2011.09.016. [DOI] [PubMed] [Google Scholar]
- Kindt M, Soeter M, Vervliet B. Beyond extinction: erasing human fear responses and preventing the return of fear. Nat Neurosci. 2009;12:256–258. doi: 10.1038/nn.2271. [DOI] [PubMed] [Google Scholar]
- Kroes MC, Tendolkar I, van Wingen GA, van Waarde JA, Strange BA, Fernández G. An electroconvulsive therapy procedure impairs reconsolidation of episodic memories in humans. Nat Neurosci. 2014;17:204–206. doi: 10.1038/nn.3609. [DOI] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci. 2006;26:5881–5887. doi: 10.1523/JNEUROSCI.0323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DJ. Sources of experimental amnesia. Psychol Rev. 1969;76:461–472. doi: 10.1037/h0028177. [DOI] [PubMed] [Google Scholar]
- Liddie S, Itzhak Y. Variations in the stimulus salience of cocaine reward influences drug-associated contextual memory. Addict Biol. 2014 doi: 10.1111/adb.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhao L, Xue Y, Shi J, Suo L, Luo Y, Chai B, Yang C, Fang Q, Zhang Y, et al. An unconditioned stimulus retrieval extinction procedure to prevent the return of fear memory. Biol Psychiatry. 2014;76:895–901. doi: 10.1016/j.biopsych.2014.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonergan MH, Olivera-Figueroa LA, Pitman RK, Brunet A. Propranolol’s effects on the consolidation and reconsolidation of long-term emotional memory in healthy participants: A meta-analysis. J Psychiatry Neurosci. 2012;37:120111. doi: 10.1503/jpn.120111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonergan MH, Saumier D, Tremblay J, Kieffer B, Brown TG, Brunet A. Reactivating addiction-related memories under propranolol to reduce craving: A pilot randomized controlled trial. J Behav Ther Exp Psychiatry. 2016;50:245–249. doi: 10.1016/j.jbtep.2015.09.012. [DOI] [PubMed] [Google Scholar]
- Luo Y, Xue Y, Liu J, Shi H, Jian M, Han Y, Zhu W, Bao Y, Wu P, Ding Z, et al. A novel UCS memory retrieval-extinction procedure to inhibit relapse to drug seeking. Nat Commun. 2015;6:7675. doi: 10.1038/ncomms8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox SA, Watts CS, Doyère V, Schafe GE. A naturally-occurring histone acetyltransferase inhibitor derived from Garcinia indica impairs newly acquired and reactivated fear memories. PloS One. 2013;8:e54463. doi: 10.1371/journal.pone.0054463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin MF, Hupbach A, Maheu FS, Nader K, Lupien SJ. Metyrapone administration reduces the strength of an emotional memory trace in a long-lasting manner. J Clin Endocrinol Metab. 2011;96:E1221–1227. doi: 10.1210/jc.2011-0226. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Time-dependent processes in memory storage. Science. 1966;153:1351–1358. doi: 10.1126/science.153.3742.1351. [DOI] [PubMed] [Google Scholar]
- Meir Drexler S, Merz CJ, Hamacher-Dang TC, Marquardt V, Fritsch N, Otto T, Wolf OT. Effects of postretrieval-extinction learning on return of contextually controlled cued fear. Behav Neurosci. 2014;128:474–481. doi: 10.1037/a0036688. [DOI] [PubMed] [Google Scholar]
- Millan EZ, Milligan-Saville J, McNally GP. Memory retrieval, extinction, and reinstatement of alcohol seeking. Neurobiol Learn Mem. 2013;101:26–32. doi: 10.1016/j.nlm.2012.12.010. [DOI] [PubMed] [Google Scholar]
- Milton AL. Drink, drugs and disruption: memory manipulation for the treatment of addiction. Curr Opin Neurobiol. 2012 doi: 10.1016/j.conb.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Misanin JR, Miller RR, Lewis DJ. Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science. 1968;160:554–555. doi: 10.1126/science.160.3827.554. [DOI] [PubMed] [Google Scholar]
- Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009;324:951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsey MS, Sanchez H, Taylor JR. The naturally-occurring compound Garcinia indica selectively impairs the reconsolidation of a cocaine-associated memory. Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K. Memory traces unbound. Trends Neurosci. 2003;26:65–72. doi: 10.1016/S0166-2236(02)00042-5. [DOI] [PubMed] [Google Scholar]
- Nader K, Einarsson EO. Memory reconsolidation: An update. Ann N Y Acad Sci. 2010;1191:27–41. doi: 10.1111/j.1749-6632.2010.05443.x. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Oyarzún JP, Lopez-Barroso D, Fuentemilla L, Cucurell D, Pedraza C, Rodriguez-Fornells A, de Diego-Balaguer R. Updating fearful memories with extinction training during reconsolidation: a human study using auditory aversive stimuli. PloS One. 2012;7:e38849. doi: 10.1371/journal.pone.0038849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachas GN, Gilman J, Orr SP, Hoeppner B, Carlini SV, Grasser EB, Loebl T, Nino J, Pitman RK, Evins AE. Single dose propranolol does not affect physiologic or emotional reactivity to smoking cues. Psychopharmacology (Berl) 2015;232:1619–1628. doi: 10.1007/s00213-014-3797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedreira ME, Maldonado H. Protein synthesis subserves reconsolidation or extinction depending on reminder duration. Neuron. 2003;38:863–869. doi: 10.1016/s0896-6273(03)00352-0. [DOI] [PubMed] [Google Scholar]
- Pedreira ME, Pérez-Cuesta LM, Maldonado H. Mismatch between what is expected and what actually occurs triggers memory reconsolidation or extinction. Learn Mem. 2004;11:579–585. doi: 10.1101/lm.76904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- President’s Council on Bioethics. Beyond therapy: Biotechnology and the pursuit of happiness. Dana Press; Washington, DC: 2003. [Google Scholar]
- Przybyslawski J, Roullet P, Sara SJ. Attenuation of emotional and nonemotional memories after their reactivation: role of beta adrenergic receptors. J Neurosci. 1999;19:6623–6628. doi: 10.1523/JNEUROSCI.19-15-06623.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybyslawski J, Sara SJ. Reconsolidation of memory after its reactivation. Behav Brain Res. 1997;84:241–246. doi: 10.1016/s0166-4328(96)00153-2. [DOI] [PubMed] [Google Scholar]
- Reichelt AC, Lee JL. Memory reconsolidation in aversive and appetitive settings. Front Behav Neurosci. 2013;7:118. doi: 10.3389/fnbeh.2013.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichelt AC, Lee JL. Over-expectation generated in a complex appetitive goal-tracking task is capable of inducing memory reconsolidation. Psychopharmacology (Berl) 2012 doi: 10.1007/s00213-012-2934-3. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Franklin KB. Reconsolidation of a morphine place preference: impact of the strength and age of memory on disruption by propranolol and midazolam. Behav Brain Res. 2010;213:201–207. doi: 10.1016/j.bbr.2010.04.056. [DOI] [PubMed] [Google Scholar]
- Rubin RD. Clinical use of retrograde amnesia produced by electroconvulsive shock. A conditioning hypothesis. Can Psychiatr Assoc J. 1976;21:87–90. doi: 10.1177/070674377602100205. [DOI] [PubMed] [Google Scholar]
- Saladin ME, Gray KM, McRae-Clark AL, Larowe SD, Yeatts SD, Baker NL, Hartwell KJ, Brady KT. A double blind, placebo-controlled study of the effects of post-retrieval propranolol on reconsolidation of memory for craving and cue reactivity in cocaine dependent humans. Psychopharmacology (Berl) 2013;226:721–737. doi: 10.1007/s00213-013-3039-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrini M, Censor N, Mishoe J, Cohen LG. Causal role of prefrontal cortex in strengthening of episodic memories through reconsolidation. Curr Biol. 2013;23:2181–2184. doi: 10.1016/j.cub.2013.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Monfils MH, Raio CM, Johnson DC, LeDoux JE, Phelps EA. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463:49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Phelps EA. Does reconsolidation occur in humans? Front Behav Neurosci. 2011;5:24. doi: 10.3389/fnbeh.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider AM, Sherman W. Amnesia: a function of the temporal relation of footshock to electroconvulsive shock. Science. 1968;159:219–221. doi: 10.1126/science.159.3811.219. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Nader K, Pruessner JC. β-Adrenergic blockade during reactivation reduces the subjective feeling of remembering associated with emotional episodic memories. Biol Psychol. 2012a:227–232. doi: 10.1016/j.biopsycho.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Nader K, Wolf OT, Beaudry T, Pruessner JC. Neural signature of reconsolidation impairments by propranolol in humans. Biol Psychiatry. 2012b;71:380–386. doi: 10.1016/j.biopsych.2011.10.028. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT. New episodic learning interferes with the reconsolidation of autobiographical memories. PloS One. 2009;4:e7519. doi: 10.1371/journal.pone.0007519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT. Stress impairs the reconsolidation of autobiographical memories. Neurobiol Learn Mem. 2010;94:153–157. doi: 10.1016/j.nlm.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Sevenster D, Beckers T, Kindt M. Prediction error demarcates the transition from retrieval, to reconsolidation, to new learning. Learn Mem. 2014;21:580–584. doi: 10.1101/lm.035493.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevenster D, Beckers T, Kindt M. Prediction error governs pharmacologically induced amnesia for learned fear. Science. 2013;339:830–833. doi: 10.1126/science.1231357. [DOI] [PubMed] [Google Scholar]
- Sevenster D, Beckers T, Kindt M. Retrieval per se is not sufficient to trigger reconsolidation of human fear memory. Neurobiol Learn Mem. 2012;97:338–345. doi: 10.1016/j.nlm.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Sierra RO, Cassini LF, Santana F, Crestani AP, Duran JM, Haubrich J, de Oliveira Alvares L, Quillfeldt JA. Reconsolidation may incorporate state-dependency into previously consolidated memories. Learn Mem Cold Spring Harb N. 2013;20:379–387. doi: 10.1101/lm.030023.112. [DOI] [PubMed] [Google Scholar]
- Soeter M, Kindt M. An abrupt transformation of phobic behavior after a post-retrieval amnesic agent. Biol Psychiatry. 2015;78:880–886. doi: 10.1016/j.biopsych.2015.04.006. [DOI] [PubMed] [Google Scholar]
- Soeter M, Kindt M. Disrupting reconsolidation: pharmacological and behavioral manipulations. Learn Mem. 2011;18:357–366. doi: 10.1101/lm.2148511. [DOI] [PubMed] [Google Scholar]
- Soeter M, Kindt M. Dissociating response systems: erasing fear from memory. Neurobiol Learn Mem. 2010;94:30–41. doi: 10.1016/j.nlm.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Soeter M, Kindt M. Erasing fear for an imagined threat event. Psychoneuroendocrinology. 2012;37:1769–1779. doi: 10.1016/j.psyneuen.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Soravia LM, Heinrichs M, Aerni A, Maroni C, Schelling G, Ehlert U, Roozendaal B, de Quervain DJ. Glucocorticoids reduce phobic fear in humans. Proc Natl Acad Sci U S A. 2006;103:5585–5590. doi: 10.1073/pnas.0509184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring JD, Wood NE, Mueller-Pfeiffer C, Milad MR, Pitman RK, Orr SP. Prereactivation propranolol fails to reduce skin conductance reactivity to prepared fear-conditioned stimuli. Psychophysiology. 2015;52:407–415. doi: 10.1111/psyp.12326. [DOI] [PubMed] [Google Scholar]
- Stafford JM, Maughan DK, Ilioi EC, Lattal KM. Exposure to a fearful context during periods of memory plasticity impairs extinction via hyperactivation of frontal-amygdalar circuits. Learn Mem Cold Spring Harb N. 2013;20:156–163. doi: 10.1101/lm.029801.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenen SA, van Wijk AJ, van Westrhenen R, de Lange J, de Jongh A. Effects of propranolol on fear of dental extraction: study protocol for a randomized controlled trial. Trials. 2015;16:536. doi: 10.1186/s13063-015-1065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Kroes MCW, Fan JE, Dolan RJ. Emotion causes targeted forgetting of established memories. Front Behav Neurosci. 2010;4:175. doi: 10.3389/fnbeh.2010.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surís A, Smith J, Powell C, North CS. Interfering with the reconsolidation of traumatic memory: sirolimus as a novel agent for treating veterans with posttraumatic stress disorder. Ann Clin Psychiatry. 2013;25:33–40. [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JR, Torregrossa MM. Pharmacological disruption of maladaptive memory. Handb Exp Pharmacol. 2015;228:381–415. doi: 10.1007/978-3-319-16522-6_13. [DOI] [PubMed] [Google Scholar]
- Tollenaar MS, Elzinga BM, Spinhoven P, Everaerd W. Immediate and prolonged effects of cortisol, but not propranolol, on memory retrieval in healthy young men. Neurobiol Learn Mem. 2009a;91:23–31. doi: 10.1016/j.nlm.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Tollenaar MS, Elzinga BM, Spinhoven P, Everaerd W. Long-term outcomes of memory retrieval under stress. Behav Neurosci. 2008;122:697–703. doi: 10.1037/0735-7044.122.3.697. [DOI] [PubMed] [Google Scholar]
- Tollenaar MS, Elzinga BM, Spinhoven P, Everaerd W. Psychophysiological responding to emotional memories in healthy young men after cortisol and propranolol administration. Psychopharmacology (Berl) 2009b;203:793–803. doi: 10.1007/s00213-008-1427-x. [DOI] [PubMed] [Google Scholar]
- Torregrossa MM, Corlett PR, Taylor JR. Aberrant learning and memory in addiction. Neurobiol Learn Mem. 2011;96:609–623. doi: 10.1016/j.nlm.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrossa MM, Taylor JR. Learning to forget: manipulating extinction and reconsolidation processes to treat addiction. Psychopharmacology (Berl) 2012 doi: 10.1007/s00213-012-2750-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrossa MM, Taylor JR. Neuroscience of learning and memory for addiction medicine: from habit formation to memory reconsolidation. Prog Brain Res. 2016;223:91–113. doi: 10.1016/bs.pbr.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci. 2007;8:262–275. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Hobson JA, Stickgold R. Dissociable stages of human memory consolidation and reconsolidation. Nature. 2003;425:616–620. doi: 10.1038/nature01930. [DOI] [PubMed] [Google Scholar]
- Wang SH, de Oliveira Alvares L, Nader K. Cellular and systems mechanisms of memory strength as a constraint on auditory fear reconsolidation. Nat Neurosci. 2009;12:905–912. doi: 10.1038/nn.2350. [DOI] [PubMed] [Google Scholar]
- Wichert S, Wolf OT, Schwabe L. Changing memories after reactivation: A one-time opportunity? Neurobiol Learn Mem. 2012 doi: 10.1016/j.nlm.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Winters BD, Tucci MC, DaCosta-Furtado M. Older and stronger object memories are selectively destabilized by reactivation in the presence of new information. Learn Mem. 2009;16:545–553. doi: 10.1101/lm.1509909. [DOI] [PubMed] [Google Scholar]
- Wood NE, Rosasco ML, Suris AM, Spring JD, Marin MF, Lasko NB, Goetz JM, Fischer AM, Orr SP, Pitman RK. Pharmacological blockade of memory reconsolidation in posttraumatic stress disorder: three negative psychophysiological studies. Psychiatry Res. 2015;225:31–39. doi: 10.1016/j.psychres.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Xue YX, Luo YX, Wu P, Shi HS, Xue LF, Chen C, Zhu WL, Ding Zb, Bao YP, Shi J, et al. A memory retrieval-extinction procedure to prevent drug craving and relapse. Science. 2012;336:241–245. doi: 10.1126/science.1215070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao LY, Sun LL, Shi J, Li P, Zhang Y, Lu L. Effects of β-adrenergic receptor blockade on drug-related memory reconsolidation in abstinent heroin addicts. Drug Alcohol Depend. 2011;118:224–229. doi: 10.1016/j.drugalcdep.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Zhao LY, Zhang XL, Shi J, Epstein DH, Lu L. Psychosocial stress after reactivation of drug-related memory impairs later recall in abstinent heroin addicts. Psychopharmacology (Berl) 2009;203:599–608. doi: 10.1007/s00213-008-1406-2. [DOI] [PMC free article] [PubMed] [Google Scholar]