Abstract
The Research Domain Criteria (RDoC) initiative provides a large-scale, dimensional framework for the integration of research findings across traditional diagnoses, with the long-term aim of improving existing psychiatric treatments. A neurodevelopmental perspective is essential to this endeavor. However, few papers synthesizing research findings across childhood and adolescent disorders exist. Here, we discuss how the RDoC framework may be applied to the study of childhood and adolescent impulsive and addictive disorders in order to improve neurodevelopmental understanding and to enhance treatment development. Given the large scope of RDoC, we focus on a single construct highly relevant to addictive and impulsive disorders – initial responsiveness to reward attainment. Findings from genetic, molecular, neuroimaging and other translational research methodologies are highlighted.
Keywords: dopamine, development, substance use, impulsivity, fMRI, reward sensitivity
Introduction
The National Institute of Mental Health (NIMH) Research Domain Criteria (RDoC) project represents the first large-scale attempt to create a classification system based on measurable and dimensional biobehavioral characteristics for psychiatric research. Based on the essential observation that multiple different systems (e.g., reward responses, inhibitory control) are often implicated in a single disorder - and that a single system is often implicated across different disorders (e.g., substance addiction, attention deficit hyperactivity disorder (ADHD), bipolar disorder) - RDoC provides a systematic, transdiagnostic framework for psychiatry research aimed at integrating research findings across multiple domains (e.g., molecular, genetic, behavioral) and disorders with the long-term aim of improving treatments. While RDoC aims to cut across diagnoses, it also recognizes that existing diagnostic categories may be helpful starting points from which to synthesize current findings (Insel, et al., 2010; Insel, 2014). Here, we provide an overview of the RDoC framework as it relates to childhood and adolescent impulsive and addictive disorders and discuss how different aspects of this framework may be used to improve understanding of the development of these disorders and to improve treatments.
The first guiding principle of the RDoC project is that it is: ‘conceived as a dimensional system ... spanning the range from normal to abnormal’ (NIMH, 2011, p. 2). Although not explicitly stated, this dimensional approach may be considered fundamentally developmental in nature, as it encompasses the full range - or progression - of a given system from normal to abnormal. Thus, the RDoC approach is fully complementary to ongoing research efforts aimed at identifying the etiology of psychiatric disorders within a neurodevelopmental framework - and RDoC itself can be strengthened by the successful integration of neurodevelopmental research to the overall framework (Casey, Oliveri, & Insel, 2014). In addition, application of the RDoC approach to childhood and adolescent research may provide insights that cut across traditional diagnoses, offering novel opportunities for successful therapeutic intervention and ultimately improving existing treatments.
As shown in Figure 1, the RDoC framework is provided by a matrix composed of ‘rows’ that are hierarchically organized into domains, constructs and subconstructs (Insel, et al., 2010). Importantly, in order for a construct to be included in the RDoC matrix it must be empirically linked to ‘a specific biological system, such as a brain circuit’ (Cuthbert & Insel, 2013). Constructs and subconstructs may be studied under the ‘columns’ that are organized into seven units of analysis (genes, molecules, cells, circuits, physiology, behavior, self-reports) and one additional column for paradigms (e.g., monetary incentive delay tasks to assess reward responses). The organization of RDoC therefore provides an incredibly rich framework for the incorporation of research findings across different levels of analysis.
Figure 1. Schematic diagram depicting organization of Initial Responsiveness to Reward Attainment within the RDoC matrix.
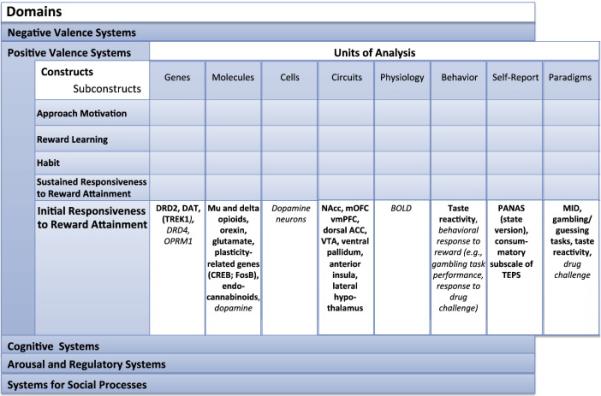
Matrix overview: The RDoC matrix is composed of five domains organized into rows. Each domain (e.g., Positive Valence Systems) is hierarchically organized into constructs and subconstructs (due to space limitations, subconstructs are not shown above) also conceptualized as rows. Constructs and subconstructs may be studied across different units of analysis, conceptualized as columns.
Initial Responsiveness to Reward Attainment: Within the matrix for initial responsiveness to reward attainment (a construct within Positive Valence Systems), items shown in bold font correspond to items currently included in the online RDoC matrix. Items shown in italics correspond to items proposed by the authors which are not currently included under this construct in the online matrix (http://www.nimh.nih.gov/research-priorities/rdoc/rdoc-constructs.shtml#initial_responsiveness; November 2015).
NAcc = nucleus accumbens, mOFC=medial orbitofrontal cortex, vmPFC=ventromedial prefrontal cortex, ACC=anterior cingulate cortex, VTA=ventral tegmental area, BOLD=blood oxygenation level dependent, PANAS=Positive and Negative Affect Scale, TEPS=Temporal Experience of Pleasure Scale, MID=Monetary Incentive Delay task
The items included within different elements of the RDoC matrix were generated during workshop proceedings conducted between 2010 and 2012 and should not be considered exhaustive; further details on individual workshop proceedings are found on the NIMH website (NIMHa). The general inclusionary criteria for inclusion in the RDoC matrix given to the workshops were: “(1) evidence for a functional behavioral or psychological construct, (2) evidence for a neural system or circuit that plays a major role in implementing the function, and (3) a putative relationship to some clinically significant problem or symptom” (NIMHb).
As the current matrix includes five domains, each comprised of a number of constructs and subconstructs and each warranting its own review, we will here focus on data relating to the single construct of ‘initial responsiveness to reward attainment’ (a construct within the domain of Positive Valence Systems, highlighted in Figure 1), as it relates to human studies of childhood and adolescent addictive and/or impulsive disorders. In theory, a similar approach may be applied to other RDoC constructs, and the current focus on ‘initial responsiveness to reward attainment’ was selected because of its importance to adolescent addictive and/or impulsive disorders. However, other constructs both within Positive Valence Systems (e.g., approach motivation, sustained responsiveness to reward attainment, reward learning) as well as within other domains (i.e., Negative Valence Systems, Cognitive Systems, Systems for Social Process, Arousal and Regulatory Systems) are also relevant to the development of adolescent addictive and impulsive disorders. Thus, further literature reviews aimed at ‘filling-in’ other elements of the RDoC matrix in relation to these constructs are also needed.
Initial responsiveness to reward attainment
The NIMH workshop on Positive Valence Systems (June 2011) defined ‘initial responsiveness to reward attainment’ as the ‘mechanisms/processes associated with hedonic responses—as reflected in subjective experiences, behavioral responses, and/or engagement of the neural systems to a positive reinforcer—and culmination of reward seeking’ (NIMH, 2011, p.4). Importantly, this definition emphasizes not only immediate hedonic responses, but also the ‘culmination of reward seeking’. As such, we will here discuss aspects of reward responses related to both initial responses to reward as well as those more generally related to approach behaviors involved in reward seeking (e.g., incentive salience; development of prefrontal cortical (PFC) inhibitory control mechanisms), although we appreciate that some of these latter constructs may map more directly onto other constructs within the RDoC matrix (e.g., approach motivation, cognitive control). We will also discuss aspects of normative brain development (e.g., maturation of white- and gray-matter structures) as these are essential starting points for understanding RDoC constructs – including initial responsiveness to reward attainment - from a developmental perspective. We have adopted this relatively inclusive approach in order to allow for synthesis of existing data as they relate to the development of circuit-related factors influencing reward responses during childhood and adolescence with the long-term goal of synthesizing findings to improve existing treatments. Finally, given the biological emphasis of the RDoC framework, we will primarily focus on data relating to Genes, Molecules and Circuits.
The complete list of the systems proposed in the RDoC matrix on initial responsiveness to reward attainment is shown in Figure 1. The items included in this matrix were generated by the Reward Seeking and Consummatory Behavior Group, a subgroup of the Positive Valence Systems workshop, which also generated the constructs and associated matrix elements for approach motivation and sustained/longer-term responsiveness to reward attainment (NIMH, 2011). As the proceedings from this group specifically notes that some potential matrix elements may have been omitted due to time constraints (NIMH, 2011), Figure 1 also lists some provisional items (shown in italics) that are not included in the current matrix but which are proposed as relevant to this construct. For further details on workshop proceedings relevant to this and other constructs within Positive Valence Systems, see (NIMH, 2011).
Circuits and Physiology1
The current RDoC matrix on initial responsiveness to reward lists a number of brain regions under the heading of ‘Circuits‘: medial orbitofrontal cortex (mOFC), ventromedial prefrontal cortex (vmPFC), anterior insula, dorsal anterior cingulate, nucleus accumbens (NAcc), ventral tegmental area (VTA), ventral pallidum and lateral hypothalamus. While no specific ‘circuit’ is currently listed, many of the brain regions listed may be rather broadly classified as part of the mesocorticolimbic system. The processes associated with these regions as they relate to childhood and adolescent addictive and impulsive disorders are presented in Table 1 along with selected neurodevelopmental evidence from structural and functional MRI studies.
Table 1.
Selected evidence in support of brain regions proposed in the RDoC matrix on initial responsiveness to reward attainment
| Development | Processes | |
|---|---|---|
| ‘Top-down’ cortical regions | ||
| Anterior insula | Structural MRI: linear maturation ongoing during adolescence (Shaw, et al., 2008); Functional MRI: Decreased BOLD in children and adolescents in comparison to adults during risky decision-making (Paulsen, Carter, Platt, Huettel, & Brannon, 2011) | Emotion processing, interoceptive awareness (Paulus & Stein, 2006); goal-directed behavior (Naqvi, Gaznick, Tranel, & Bechara, 2014), reward and aversion-based learning (Lasiter, Deems, Oetting, & Garcia, 1985; Lawrence, et al., 2014) |
| Dorsal anterior cingulate | Structural MRI: inverted ‘U-shaped’ maturation of anterior cingulate with development ongoing into adulthood (Shaw, et al., 2008); Functional MRI: Decreased BOLD in children and adolescents in comparison to adults during error processing (Velanova, Wheeler, & Luna, 2008) | Inhibitory control, behavioral monitoring (Goldstein & Volkow, 2011) |
| Medial OFC and vmPFC | Structural MRI: GM development ongoing during adolescence into adulthood (Giedd, 2004; Gogtay, et al., 2004); Functional MRI: OFC development protracted in comparison to subcortical (NAcc) development (Galvan et al., 2006; Casey et al., 2008) | Incentive salience, value encoding, decision-making, emotion regulation (Goldstein & Volkow, 2011) |
| ‘Bottom-up’ subcortical regions | ||
| Striatum (Nacc and VTA) | Structural MRI: typically thought to stabilize by middle to late adolescence; this view is challenged by recent evidence of protracted development during adolescence (Dennison, et al., 2013; Raznahan, et al., 2014). Functional MRI: Earlier maturation in comparison to OFC (Galvan et al., 2006; Casey et al., 2008) |
Reward ‘hotspot’ (Berridge, et al., 2009); reward learning (Creed, Ntamati, & Tan, 2014); encoding of reward and aversion (Volman, et al., 2013) |
| Ventral pallidum | Structural MRI: Protracted development during adolescence (Dennison, et al., 2013; Raznahan, et al., 2014). | Encoding of hedonic reward value (‘liking’) (Berridge, et al., 2009) |
| Lateral hypothalamus | Structural MRI: Linear decrease in thalamus volume during childhood and adolescence (Sowell, Trauner, Gamst, & Jernigan, 2002) | Reward-seeking (Harris, Wimmer, & Aston-Jones, 2005), motivational encoding and goal-directed behaviors (Boutrel, Cannella, & de Lecea, 2010) |
NAcc = nucleus accumbens, mOFC=medial orbitofrontal cortex, vmPFC=ventromedial prefrontal cortex, VTA= ventral tegmental area
Collectively, these regions (and the circuits they comprise) are sometimes described as pertaining to ‘top-down’ and ‘bottom-up’ processes. Within this framework, the term ‘bottom-up’ generally refers to the encoding of reward and incentive salience by subcortical limbic and related neurocircuitry (e.g., NAcc, VTA, ventral pallidum) with reciprocal projections to the PFC (D'Ardenne, McClure, Nystrom, & Cohen, 2008; Everitt & Robbins, 2005; Matzeu, Zamora-Martinez, & Martin-Fardon, 2014; Schultz, 1997, 2000). Conversely, the term ‘top-down’ is used to refer to the control of subcortical limbic regions by primarily prefrontal cortical (PFC) brain regions involved in cognitive control and executive functioning processes (Goldstein & Volkow, 2011; Kober, et al., 2010; Taber, 1993; Volkow, et al., 2010). Such inhibitory processes are covered elsewhere in the matrix (e.g., ‘cognitive control’ is a construct within the domain of ‘Cognitive Systems’). However, in addition to inhibitory functions, ‘top-down’ regions such as the mOFC and vmPFC are also themselves implicated in the encoding of reward and salience (Goldstein & Volkow, 2011). These systems interact in a dynamic manner to influence a range of complex cognitive processes relevant to reward seeking and impulse control (e.g., encoding of incentive salience; cognitive control mechanisms) (Koob & Volkow, 2010; Volman, et al., 2013), and develop at different rates during childhood, adolescence and adulthood. Thus, one of the challenges for developmental research under RDoC will be to further quantify interactions between different domains over time.
Neurodevelopmental findings from longitudinal and large-scale cross-sectional imaging-based studies of healthy children and adults provide an essential starting point from which to interpret findings from studies conducted in psychiatric and at-risk populations. In the following sections we will therefore first review what is known about the developmental trajectories of white- and gray-matter structures. We will also discuss findings from functional magnetic resonance imaging (fMRI) studies that may be used to draw inferences about the neural functional development of top-down and bottom-up neurocircuitry. Finally, we will highlight recent findings from the field of connectomics and discuss how these factors may be studied within the context of adolescent impulsive and addictive disorders.
White matter
Generally speaking, most major white-matter tracts are characterized by increases in fractional anisotropy (FA) and decreases in mean diffusivity (MD) from childhood into early adulthood. Once thought to be linear, evidence from diffusion-weighted MRI studies suggests relatively nonlinear developmental trajectories for most major white-matter tracts (Lebel & Beaulieu, 2011; Lebel, et al., 2012; Lebel, Walker, Leemans, Phillips, & Beaulieu, 2008), as well as different developmental trajectories across different tracts; e.g. (Barnea-Goraly, et al., 2005; Hasan, et al., 2009; Lebel, et al., 2008). Within a developmental context, increases in axon density, myelination and straightening of fiber tracts likely contribute to increases in FA and decreases in MD during typical development (Paus, T., 2010), although it is important to note that other factors (e.g., increases in intracellular vs. extracellular fluid) may influence these measures. As such, further preclinical studies combining ex vivo and in vivo methods are essential to better characterize cellular factors influencing diffusion-based measures within a developmental context (Paus, T, 2010; Paus, Keshavan, & Giedd, 2008).
FA values within callosal regions such as the genu and splenium appear to stabilize by early adolescence, whereas FA values continue to increase within fronto-temporal-limbic tracts such as the cingulum, uncinate fasciculus and superior longitudinal fasciculus during late adolescence into early adulthood (Lebel & Beaulieu, 2011; Lebel, et al., 2012; Lebel, et al., 2008; Peters, et al., 2012). Age-related reductions in MD values also occur earlier in callosal versus fronto-temporal-limbic pathways, although generally these values stabilize slightly later than do FA values; e.g., (Lebel, et al., 2012; Lebel, et al., 2008). Together, these data demonstrate that maturation of pathways connecting prefrontal cortical and limbic structures – key pathways for the regulation and control of motivated behaviors - is ongoing throughout adolescence into adulthood.
Significant individual variation in the development of white-matter tracts exists. For example, whereas most individuals continue to exhibit increases in FA within fronto-temporal-limbic tracts during early adulthood, a subset of individuals exhibit decreases during this time (Lebel & Beaulieu, 2011). Given that alterations in FA have been reported among adolescents and young adults with bipolar disorder and ADHD and other individuals at-risk for addictions, e.g., (Adler, et al., 2006; Elofson, Gongvatana, & Carey, 2013; Hamilton, et al., 2008; Yip, Chandler, Rogers, Mackay, & Goodwin, 2013), it is likely that such individual difference factors relate to the development of impulsive and addictive disorders. Within the context of RDoC, longitudinal studies focused on identifying common and distinct aspects of white matter neurodevelopmental trajectories across disorders are needed.
Gray matter: evidence from structural and functional MRI
Generally speaking, gray-matter cortical and subcortical development is ongoing throughout childhood and adolescence into early adulthood, with tissue volumes increasing before puberty and decreasing after puberty to form an inverted-U-shaped trajectory (Giedd, 1999; Giedd, 2004; Gogtay, et al., 2004; Gogtay & Thompson, 2010; Sowell, et al., 2003; Sowell, Thompson, Tessner, & Toga, 2001). These volumetric reductions are thought to result from dendritic pruning and reorganization of neuronal fibers (as opposed to neuronal loss), possibly related to increases in intra-cortical mylenation (Giedd, 2004; Gogtay & Thompson, 2010; Paus, 2005; Sowell, et al., 2001).
Within the cortex, gray-matter maturation occurs hierarchically, with maturation of lower-order sensorimotor regions preceding maturation of higher-order association areas (Giedd, 2004; Gogtay, et al., 2004). Regions of the PFC involved in ‘top-down’ processes (e.g., executive function, response inhibition) develop throughout adolescence and do not fully reach adult levels of maturity until the mid 20s (Galvan, et al., 2006; Giedd, 2004). While most studies tend to focus on cortical rather than subcortical maturational changes, ‘bottom-up’ subcortical structures have typically been thought to reach adult maturational levels earlier (Casey, Jones, & Hare, 2008). However, it is important to note that gray-matter development within regions including the pallidum and striatum continues into adulthood (Giedd, 2004; Langen, et al., 2009; Raznahan, et al., 2014; Sowell, Thompson, Holmes, Jernigan, & Toga, 1999).
Findings of protracted development of subcortical limbic structures arguably challenge developmental models emphasizing an imbalance between the development of cortical and subcortical structures (Dennison, et al., 2013; Raznahan, et al., 2014). Notably, a recent study reporting an imbalance between subcortical and cortical development in adolescence did not find an association between this imbalance and self-reported risk-taking (Mills, Goddings, Clasen, Giedd, & Blakemore, 2014). Thus, an important future research goal of the RDoC initiative should involve determining the functional significance of cortical versus subcortical developmental differences during adolescence.
Findings from fMRI studies can also inform understanding of neurodevelopment, as these data can provide insight into the functional development of neural circuits; for reviews see (Casey, 2014; Galvan, 2010). Cross-sectional studies of reward-responses (e.g., as assessed using monetary incentive delay (MID) tasks) comparing neural responses between adolescents and adults have yielded equivocal findings, with both increased, e.g., (Galvan, et al., 2006; Van Leijenhorst, et al., 2010), and decreased, e.g., (Bjork, Smith, Chen, & Hommer, 2010), striatal responses reported among adolescents; for a review, see (Richards, Plate, & Ernst, 2012). A recent longitudinal fMRI study found relatively increased dorsal striatal activity during reward anticipation during late versus middle adolescence (Lamm, et al., 2014), consistent with the general hypothesis of increases in subcortical reward responses during normative development. However, further task-based functional MRI research incorporating multiple time points and larger sample sizes - as has been done in structural and resting state MRI studies, e.g., (Di Martino, A., et al., 2014; Raznahan, et al., 2014) - is needed to clarify the natural trajectory of reward-system activity across development.
For a number of years, findings from fMRI studies have been interpreted as supporting neurodevelopmental models emphasizing relative maturity of subcortical (e.g., ventral striatal) versus cortical (e.g., orbitofrontal cortex; OFC) regions; e.g., (Bunge & Wright, 2007; Galvan, et al., 2006). This imbalance has been invoked to explain heightened reward-seeking behaviors observed in adolescents (Casey, 2014; Crone & Dahl, 2012; Galvan, 2010). However, it is important to note that conflicting data also exist (Crone & Dahl, 2012; Galvan, 2010). For example, several studies indicate that, despite age-related differences in the type of conditions under which maximal PFC engagement is exhibited, magnitudes of maximal engagement may not differ between adolescents and adults; reviewed in (Crone & Dahl, 2012). Findings such as these suggest that maturation of cognitive-control processes during adolescence may involve complex functional reorganization of networks, rather than simple linear maturation, consistent with recent findings from resting-state studies employing graph-theory analytical methods (Crone & Dahl, 2012; Di Martino, A., et al., 2014).
A key area for further research will be to determine when and how different contextual factors (e.g., task-related factors, social and emotional factors) influence engagement of different neural networks across development (Braams, Peters, Peper, Guroglu, & Crone, 2014; Casey, 2014; Crone & Dahl, 2012). A complementary area requiring additional study and well-suited to an RDoC approach is the functional significance of increased reward responses during adolescence (Crone & Dahl, 2012). For example, findings from recent studies suggest that increased reward responsiveness (at least as defined by heightened striatal activity) may be an adaptive feature of adolescent development; e.g., (Barkley-Levenson & Galvan, 2014; Hauser, Iannaccone, Walitza, Brandeis, & Brem, 2015).
Systems-level changes: Insights from connectomics
In recent years, a rapidly growing body of research, generally referred to as connectomics, has focused on understanding the development of the human brain from the perspective of large-scale system dynamics via analysis of resting state fMRI and diffusion-weighted imaging data; e.g., (Behrens & Sporns, 2012; Collin & van den Heuvel, 2013; Dennis, et al., 2013; Di Martino, Adriana, et al., 2014; Dosenbach, et al., 2010; Grayson, et al., 2014; Ingalhalikar, et al., 2014; van den Heuvel & Sporns, 2011; Yang, et al., 2014). This line of inquiry has demonstrated that, while much of the macroscopic organization of the connectome is complete by age two (reviewed in (Collin & van den Heuvel, 2013; Di Martino, A., et al., 2014)), childhood and adolescence are characterized by a number of complex systems-level organizational changes (Di Martino, Adriana, et al., 2014). These include a shift from predominantly local connectivity during infancy (characterized by primarily short-range connections) to increasingly distributed connectivity (characterized by long-range connections) during adolescence into adulthood (although the most dramatic changes occur during infancy and very early childhood) (Di Martino, Adriana, et al., 2014; Dosenbach, et al., 2010; Fair, et al., 2009; Satterthwaite, et al., 2013). Other significant neurodevelopmental factors include changes in interhemispheric connectivity (Di Martino, Adriana, et al., 2014; Ingalhalikar, et al., 2014) and in the organization of connections (e.g., the development of ‘rich clubs’ or groups of highly connected nodes that are themselves interconnected) (Bullmore & Sporns, 2012; Di Martino, Adriana, et al., 2014; Grayson, et al., 2014). The overall picture is one of increasing efficiency of connectivity via a range of complex higher order systems-level refinements (e.g., increases in rich-clubness); for reviews see (Collin & van den Heuvel, 2013; Di Martino, A., et al., 2014).
The topological changes outlined above are thought to underlie the development of increasingly sophisticated cognitive processes (Grayson, et al., 2014). This line of research therefore has the potential to significantly enhance understanding of the pathophysiology of psychiatric disorders via similar assessment of atypical neural development (Bassett & Bullmore, 2009; Collin & van den Heuvel, 2013; Di Martino, A., et al., 2014; Grayson, et al., 2014). While specific topological properties (e.g., rich-clubness) are not explicitly listed in the current matrix, connectome-based approaches are consistent with the dimensional focus of the RDoC framework (Castellanos, Di Martino, Craddock, Mehta, & Milham, 2013). Further work is nonetheless required to understand interactions between specific topological properties and other units of analysis (e.g., genes, behavior). Publicly available large-scale neuroimaging datasets represent a powerful resource for future studies in this area; e.g., the Philadelphia Neurodevelopmental Cohort PDN (Satterthwaite, et al., 2015) and Pediatric Imaging, Neurocognition, and Genetics (PING) (Jernigan, et al., 2015) databases.
Individual variation in circuit development – relationship to reward responses under RDoC
As outlined above, neuroimaging data suggest significant inter-individual variation in the developmental trajectories of gray- and white-matter structures, and this may contribute to individual differences in reward processing and/or to vulnerabilities for addictive and impulsive disorders; e.g., (Dennison, et al., 2013; Rahman, Xu, & Potenza, 2014; Samanez-Larkin, Levens, Perry, Dougherty, & Knutson, 2012; Shaw, et al., 2011; Xu, et al., 2012). However, the precise relationship between individual variation in white-matter changes during typical development and factors such as impulsivity during adolescence remains unclear. For example, increased FA values have been associated with both decreased delay-discounting behaviors (Olson, et al., 2009) and increased risk-taking behaviors during adolescence (Berns, Moore, & Capra, 2009). These seemingly conflicting findings highlight the need for dimensional research studies assessing the relationship between factors such as FA and different measures related to reward responses. Within the specific framework of initial responsiveness to reward attainment, such research might, for example, assess the relationship between longitudinal changes in FA values within corticolimbic tracts and changes in: (i) taste reactivity (behavioral unit of analysis); (ii) scores on the consummatory subscale of the Temporal Experience of Pleasure Scale (TEPS) (Gard, Gard, Kring, & John, 2006) (self-report unit of analysis); and, (iii) neural responses during reward receipt (circuit-related unit of analysis).
Findings of alterations in white- and gray-matter structures among adolescents and young adults with disorders including ADHD, bipolar disorder and alcohol misuse, e.g., (Adleman, et al., 2012; Adler, et al., 2006; Elofson, et al., 2013; Hamilton, et al., 2008; Shaw, et al., 2007; Yip, et al., 2013), support the hypothesis of pathophysiological involvement of circuit development in conferring vulnerability to a range of impulsive and addictive disorders. Similarly, data from functional MRI studies have demonstrated blunted reward-related ventral-striatal responses among adolescents and young adults with substance-use disorders, bipolar disorder and ADHD; e.g., (Nymberg, et al., 2013; Peters, et al., 2011; Yip, Worhunsky, Rogers, & Goodwin, 2015). In contrast, data from two recent prospective studies comparing individuals early-on in substance-use experimentation (i.e., prior to dependence) suggest that increased reward-responses may be predictive of future substance-use problems (Dager, et al., 2014; Heitzeg, et al., 2014); for a review of findings from recent prospective studies, see (Heitzeg, Cope, Martz, & Hardee, 2015).
Further research is therefore needed to determine the relationship between neural reward responses across different stages of development and in relation to substance-use initiation and other risk factors. The importance of assessing interactions between different risk factors is illustrated by recent data indicating differential neural substrates of illicit substance-use initiation between adolescents with and without prenatal cocaine exposure (Yip, Lacadie, Sinha, Mayes, & Potenza, 2015). Similarly, given the presence of similar findings across a range of disorders, studies directly comparing neural structure and functional responses across different diagnostic categories are needed to examine the disease-specificity of existing data (Insel, 2014). Within a developmental framework, similar research could assess the utility of, for example, blunted reward responses during early childhood in predicting the development of different addictive and impulsive disorders by late adolescence, with the long-term goal of identifying clinically meaningful biomarkers.
Although not extensively reviewed here, it is important to note that current data suggest significant sex differences in the longitudinal development of both white- and gray-matter structures; e.g., (Dennison, et al., 2013; Ingalhalikar, et al., 2014; Perrin, et al., 2009; Raznahan, et al., 2014; Satterthwaite, et al., 2014). While further research is needed to understand the biological basis of sex differences in neural tissue development, existing data suggest that aspects of gray- (e.g., cortical thickness, tissue density) and white-matter development (e.g., axon diameter growth, mylenation) are influenced by the expression of sex hormones including estrogen and testosterone (Arevalo, Santos-Galindo, Bellini, Azcoitia, & Garcia-Segura, 2010; Bramen, et al., 2011; Brouwer, et al., 2015; Paus, 2013; Pesaresi, et al., 2015). Thus, a further area for research under RDoC will be the interaction between sex-specific neurodevelopmental trajectories and dimensional changes in responsiveness to reward attainment as measured across different units of analysis (including neural functional activity).
Circuits and treatment
Collectively, the above data indicate that similar alterations in neural development may contribute to vulnerability for multiple different but related childhood and adolescent disorders. These data also highlight the complex, dynamic trajectories of human neurodevelopment throughout childhood and adolescence and suggest multiple implications for the treatment and prevention of adolescent impulsive and addictive disorders. For example, given the ongoing development of prefrontal cortical regions involved in ‘top-down’ inhibitory processes during adolescence – in conjunction with evidence from adult studies suggesting involvement of these regions in treatment responses to behavioral therapies; e.g., (Brewer, Worhunsky, Carroll, Rounsaville, & Potenza, 2008; DeVito, et al., 2012) – future research could explore the efficacy of combining existing treatments with specific interventions aimed at strengthening inhibitory mechanisms or in reducing approach tendencies (e.g., working-memory training, cognitive-bias modification) (Eberl, et al., 2013; Wiers, Eberl, Rinck, Becker, & Lindenmeyer, 2011).
Recent data suggest that cognitive-bias modification may be effective in reducing subcortical neural responses to alcohol-cues associated with craving among abstinent adults (Wiers, et al., 2015). Thus, it is possible that cognitive-bias modification might be effective in reducing ‘bottom-up’ reward responses among adolescents at-risk for disorders such as addictions (Houben, Havermans, Nederkoorn, & Jansen, 2012), particularly when combined with existing treatments (Eberl, et al., 2013). A separate but complimentary line of research involves the indirect targeting of reward-related decision-making (e.g., as assessed using delay-discounting tasks) via cognitive training. For example, working-memory training has been found to reduce delay-discounting rates among adults with stimulant dependence (Bickel, Yi, Landes, Hill, & Baxter, 2011); for reviews, see (McClure & Bickel, 2014; Wesley & Bickel, 2014). Further research is needed to determine whether specific interventions aimed at altering approach tendencies (as in cognitive-bias modification) or at increasing ‘top-down’ executive functioning (as in working-memory training) (Eberl, et al., 2013; McClure & Bickel, 2014; Wesley & Bickel, 2014; Wiers, et al., 2011) might be efficacious in altering aberrant reward responses among adolescents. Within this context, fMRI might aid in the a priori identification of individuals with altered subcortical versus prefrontal cortical neurodevelopment who might benefit from different forms of cognitive training across a range of disorders. As discussed above, such research should aim to incorporate multiple measures of circuit-level functioning (e.g., task-based fMRI, resting-state connectivity, structural imaging).
Accumulating evidence from primarily adult studies suggests that individual differences in pretreatment neural structure and function are related to individual differences in treatment responses to behavioral therapies across a range of addictions, e.g., (Brewer, et al., 2008; Feldstein Ewing, Filbey, Sabbineni, Chandler, & Hutchison, 2011; Kober, Devito, Deleone, Carroll, & Potenza, 2014; Yip, et al., 2014); for reviews, see (Feldstein Ewing & Chung, 2013; Yip, Carroll, & Potenza, 2015). Further, findings from adult studies directly comparing pre- versus post-treatment fMRI data suggest that the efficacy of treatments such as cognitive behavioral therapy relate to its neuromodulatory effects on regions involved in cognitive control processes, such as the dorsolateral PFC (DeVito, et al., 2012; Goldapple, 2004).
Several recent studies conducted in adolescent populations suggest somewhat similar relationships between pretreatment cortical neural function and behavioral treatment responses; e.g., (Chung, Paulsen, Geier, Luna, & Clark, 2015; Feldstein Ewing, et al., 2013; Feldstein Ewing, et al., 2012; Krishnan-Sarin, et al., 2013). For example, increased pretreatment activations within regions including the inferior frontal gyrus and ventrolateral PFC are associated with better substance-use outcomes following treatments for cannabis and tobacco use in adolescents (Chung, et al., 2015; Feldstein Ewing, et al., 2013; Krishnan-Sarin, et al., 2013). However, data suggest that similar therapies may engage different brain regions in adults versus adolescents. For example, exposure to change-talk – a key component of motivational interviewing - is associated with activation of the striatum and PFC among adults (Feldstein Ewing, et al., 2011) and with activation of the inferior frontal gyrus and insula among adolescents (Feldstein Ewing, et al., 2013). Taken together, these data suggest possible mechanistic differences in the efficacy of behavioral therapies between adolescents and adults, highlighting the need for further research focusing on circuit-level factors and treatment responses specifically in adolescent populations.
In adults, associations between treatment outcomes and neural function during reward-task performance within subcortical regions have also been reported; e.g., (Jia, et al., 2011; Yip, et al., 2014). For example, a negative association between pretreatment ventral striatal activity during reward receipt and abstinence during treatment has been reported among adults with cocaine dependence (Jia, et al., 2011), and increased pretreatment caudate activity (during loss processing) has been reported among cannabis-dependent young adults who did not subsequently achieve a period of sustained abstinence during treatment (Yip, et al., 2014). Notably, there has been one report of a negative association between subcortical (amygdala, putamen and NAcc) activation early in treatment and symptom severity following treatment in an adolescent population (Chung, et al., 2015), suggesting that individual differences within subcortical regions may also contribute to variations in treatment responses among adolescents. Giving the growing body of evidence indicating that development of subcortical structures continues into adulthood, e.g., (Giedd, 2004; Langen, et al., 2009; Raznahan, et al., 2014; Sowell, et al., 1999), further research assessing age-related differences in subcortical structure and function in relation to treatment responses is needed.
Several lines of evidence suggest that pharmacological treatment interventions may impact on neurodevelopmental processes related to reward processing. Meta-analytic data from studies of ADHD suggest normalizing effects of dopaminergic medications on neural activations and gray-matter structural volumes (Hart, Radua, Nakao, Mataix-Cols, & Rubia, 2013; Nakao, Radua, Rubia, & Mataix-Cols, 2011). Pre-clinical data and data from human adult studies further suggest normalizing and/or ameliorative effects of mood stabilizers on white-matter structures (Benedetti, et al., 2011; Macritchie, et al., 2010; Makoukji, et al., 2012). These findings, in conjunction with data suggesting associations between treatment outcomes and white-matter microstructural characteristics (Xu, et al., 2010; Zhou, et al., 2011), suggest directions for further research, although the benefits of pharmacological treatment for childhood and adolescent disorders should be weighed against any associated risks.
To our knowledge, no studies have assessed the longitudinal effects of behavioral treatments for substance-use (via comparison of pre- versus post-treatment fMRI data) in an adolescent population. Such research will inform the current understanding of the neurobiological mechanisms underlying effective treatments and aid in further treatment development. Large-scale neuroimaging studies assessing the neural correlates of successful behavioral change following treatments (e.g., abstinence) across different developmental epochs (e.g., pre- versus post-puberty) are warranted. Such data could aid in the identification of critical periods for adolescent interventions wherein treatments might be most likely to exert sustained positive change (Casey, BJ, et al., 2014), as it is likely that the effectiveness of different treatments may change as a functioning of ongoing neurodevelopmental processes (e.g., dendritic pruning, myelination).
Summary of circuits
Unlike ligand-based molecular imaging modalities (reviewed below, see Molecules and Cells), functional, structural and diffusion-weighted MRI do not require the use of radiotracers and therefore provide a powerful, non-invasive means of assessing the developing human brain in vivo. In general, existing evidence suggests that the adolescent brain may be characterized by increased functional activation of ‘bottom-up' limbic reward regions (e.g., ventral striatum) relative to that of ‘top-down’ cortical inhibitory regions (e.g., OFC, dorsolateral PFC). While it has long been acknowledged that cortical development is ongoing into early adulthood, emerging data suggest that development of subcortical regions previously thought to stabilize prior to adulthood is also ongoing during this time. Further, significant individual differences in gray- and white-matter development exist, and these may relate to vulnerabilities for a range of disorders. Thus, further study using imaging-based methodologies is warranted to characterize the full range of ‘typical to atypical’ neurodevelopment (Casey, BJ, et al., 2014). In particular, studies conducted among children, adolescents and young adults either very early on in the disease course (e.g., prior to significant clinical intervention such as long-term pharmacotherapy) or even prior to disease onset (e.g., prodromal studies of individuals at increased risk for disorders) are needed to further identify possible biomarkers in order to aid treatment interventions. Ideally, these studies should incorporate different neuroimaging modalities (i.e., multi-modal imaging) to clarify brain structure-function relationships.
Genes
The current RDoC matrix highlights DRD2 and DAT12 as genes relevant to the construct of initial responsiveness to reward attainment. However, it is important to note that other genes including DRD4, OPRM1 and COMT are implicated in this construct; e.g., (Camara, et al., 2010; Dreher, Kohn, Kolachana, Weinberger, & Berman, 2009; Foti & Hajcak, 2012; Hendershot, Claus, & Ramchandani, 2014; Perez-Edgar, et al., 2014; Peters, et al., 2011; Ray & Hutchison, 2007; Ray, et al., 2014; Schacht, et al., 2013; Silveira, et al., 2014; Smith & Boettiger, 2012; Stice, Yokum, Bohon, Marti, & Smolen, 2010). Given the breadth of the existing literature in this area, the following section will focus on the relevance of those genes already listed in the RDoC matrix on initial responsiveness to reward attainment – namely, DRD2 and DAT1 - to childhood and adolescent addictive and impulsive disorders.
Initial responsiveness to reward attainment – DRD2 and DAT1
Family, twin and adoption studies have demonstrated significant heritability rates for addictive disorders, with estimates ranging from 48-66% for alcohol dependence and 23-54% for opioid dependence; for a review, see (Agrawal, et al., 2012). In addition, genetic association studies have identified multiple allelic variants putatively associated with addictive and impulsive disorders; e.g., (Demers, Bogdan, & Agrawal, 2014; Sklar, et al., 2002; Sun, Yuan, Shen, Xiong, & Wu, 2014). However, findings have often been inconsistent. For example, studies focusing on DRD2 and DAT1 – genes listed in the current RDoC matrix on initial responsiveness to reward attainment – have yielded equivocal results within the context of specific disorders including substance and behavioral addictions (Blomqvist, Gelernter, & Kranzler, 2000; Comings, Muhleman, Ahn, Gysin, & Flanagan, 1994; Comings, et al., 1996; Lohoff, et al., 2010; Moyer, et al., 2011; Sweitzer, Donny, & Hariri, 2012; Ueno, et al., 1999; van der Zwaluw, et al., 2009), bipolar disorder (Gomez-Casero, Perez de Castro, Saiz-Ruiz, Llinares, & Fernandez-Piqueras, 1996; Greenwood, et al., 2013; Greenwood, Schork, Eskin, & Kelsoe, 2006; Keikhaee, et al., 2005; Manki, et al., 1996; Massat, et al., 2002; Pinsonneault, et al., 2011; Wang, et al., 2014) and ADHD (Barr, et al., 2001; Chen, et al., 2003; Greenwood, et al., 2013; Kustanovich, et al., 2004; Lasky-Su, et al., 2008; Nyman, et al., 2007).
Given the somewhat conflicting findings from disorder-specific studies, an alternative research approach has been to identify genetic variants associated with specific aspects of reward processing – e.g., responses to reward attainment. Such research has demonstrated that, in healthy adults, the DRD2 and DAT1 genes may mediate individual neural reward responses. For example, increased ventral striatal activations in response to rewarding stimuli have been reported among adult carriers of allelic variants associated with increased synaptic dopamine (DA); e.g., DRD2 141C Del, DAT1 9-repeat (Dreher, et al., 2009; Forbes, et al., 2009).
Data also suggest that variation in DAT1 may contribute to individual differences in neural reward responses among adolescents (Paloyelis, Mehta, Faraone, Asherson, & Kuntsi, 2012). However, replication using larger sample sizes and longitudinal designs is needed, as the relative contribution of genetic factors may not be constant over the course of development. For example, differential contributions of genes including DAT1 and DRD2 to alcohol use behaviors have been reported across different stages of development; e.g., (Guo, Wilhelmsen, & Hamilton, 2007; Hopfer, et al., 2005). Thus, an important next step will be to characterize the natural developmental trajectory of reward responses across childhood and adolescence among individuals with and without different genetic variants found to impact reward responses. Such research may allow for the identification of critical periods wherein treatment interventions might be most effective for individuals at increased genetic risk for a range of disorders (Casey, BJ, et al., 2014). Additionally, as DRD2 has been found to be in linkage disequilibrium with ANKK1 and certain addictive behaviors may link more closely to ANKK1 than to DRD2 (Dick, et al., 2007), additional research should investigate the extent to which ANKK1 variation may relate to adolescent reward processing and addiction vulnerability.
A complementary line of research involves the study of gene-by-environment interactions (GxE), which may be efficacious in understanding how functional genetic polymorphisms interact with other factors to confer vulnerability for a variety of complex behavioral phenotypes, such as addictive and impulsive disorders. Examples of this line of inquiry include data demonstrating increased ADHD-related symptoms among adolescent boys homozygous for the DAT1 10-repeat allele with high levels of psychosocial adversity, but not among those with different genotypes or who have experienced lower levels of adversity (Laucht, Skowronek, Becker, & et al., 2007). GxE data also suggest interactions between the 10-repeat allele, timing of substance use behaviors during early adolescence, and levels of cigarette and alcohol consumption during late adolescence (Schmid, et al., 2009). In addition, two recent studies suggest significant interaction effects between parenting behaviors and DRD2 genotype on adolescent drinking behaviors (Pieters, et al., 2012; van der Zwaluw, et al., 2010). Studies using this type of GxE approach may be helpful in identifying critical periods for intervention and in reconciling seemingly conflicting findings of associations between specific gene variants in some but not all studies of a given disorder, as they suggest that genetics alone may be insufficient to explain complex behavioral phenotypes (e.g., substance use), which likely relate to multiple different systems (units of analysis).
Despite the potential of GxE research it should nonetheless be noted that findings from studies of locus-specific GxE (such as those highlighted above) are somewhat controversial, e.g., (Duncan & Keller, 2011; Munafo, Zammit, & Flint, 2014a, 2014b; Rutter, 2014), as findings have proved difficult to replicate and are not always borne out in genome-wide-association studies (GWAS) (Duncan & Keller, 2011; Munafo, Durrant, Lewis, & Flint, 2009; Munafo & Flint, 2009).
Relationship to treatment
A growing body of research has explored the relationship between genetic factors and treatment responses for addictive and impulsive disorders among young adults. Examples of this work include studies on the relationship between responses to behavioral treatments for cannabis and alcohol use and genes encoding for aspects of both serotonergic (5HT2A) and dopaminergic (DRD4) functioning; e.g., (Feldstein Ewing, LaChance, Bryan, & Hutchison, 2009; Feldstein Ewing, et al., 2012). However, relatively few studies have assessed the influence of DRD2 and DAT1 genotypes on treatment outcomes for childhood and adolescent addictive and impulsive disorders. One notable exception is the relationship between DAT1 genotype and responses to methylphenidate among children and adolescents with ADHD. However, findings have not been consistent across studies, with some data suggesting that homozygosity for the DAT1 10-repeat allele is associated with a reduced response to methylphenidate treatment, e.g., (Cheon, Ryu, Kim, & Cho, 2005; Roman, 2002; Winsberg & Comings, 1999) and other data suggesting that homozygosity for the 9-repeat allele is associated with a poorer response, e.g. (Joober, et al., 2007; Stein, 2005). Thus, a recent meta-analysis concluded that DAT1 genotype alone was insufficient to predict methylphenidate responses (Kambeitz, Romanos, & Ettinger, 2014).
To our knowledge, there are no studies that have examined the relationship between DRD2 or DAT1 genotype and treatment responses for addictions in a child or adolescent population. However, recent data suggest an interaction between DRD2 genotype and participation in an alcohol prevention program on subsequent drinking outcomes in youth: In a study of over 900 adolescents, individuals with a DRD2 risk variant who were not assigned to a drinking prevention program had increased alcohol-use at 2-year follow-up in comparison to individuals with the same risk variant who received a preventative intervention and in comparison to youth without the risk variant (Brody, Chen, & Beach, 2013). Consistent with this, findings from some adult studies further suggest possible involvement of DRD2 in treatment outcomes. For example, increased relapse rates following 12-Step treatment for alcohol dependence have been reported among individuals with the DRD2 Taq1A allele (Dahlgren, et al., 2011) and increased Taq1A allelic frequency has been associated with poorer treatment responses among individuals with opioid dependence (Doehring, et al., 2009; Lawford, et al., 2000), although conflicting reports for the latter finding also exist (Barratt, Coller, & Somogyi, 2006; Crettol, et al., 2008). As in the case of disorder-specific genetic association studies, seemingly contrary research findings may relate to small single gene effects or insufficient characterization of ultimately highly heterogeneous behavioral phenotypes (in this case, phenotypes such as ‘responders’ vs. ‘nonresponders’) (Falcone, et al., 2013; Hutchison, 2010).
Multimodal studies combining genotyping with other translational research methods such as fMRI suggest that DRD2 and DAT1 allelic variation may relate to individual differences in treatment responses via neural responses during abstinence and cue-induced craving. For example, using perfusion-based MRI, Wang and colleagues (2008) demonstrated greater abstinence-induced changes in regional cerebral blood flow (rCBF) among adult smokers with DRD2 141C Del allele within regions associated with subjective experiences of craving; e.g., ventral striatum, dorsolateral PFC (Wang, et al., 2008). FMRI data further suggest a positive association between the DAT1 9-repeat allele and increased ventral striatal and dorsolateral PFC responses to smoking-related cues among adult smokers (Franklin, et al., 2009; Franklin, et al., 2011). In a separate study, Moeller and colleagues reported a significant interaction effect between recency of cocaine use, DAT1 genotype and neural activations, such that individuals with the 9-repeat allele and recent (≤72 hours) cocaine use had increased responses to drug cues in comparison to individuals with a different genotype or without recent cocaine use (Moeller, et al., 2013). Together, these data suggest that genes encoding for DA-related moieties may influence treatment responses via neural reward responses and demonstrate the importance of assessing the multiple systems in relation to a single construct or disorder.
Molecules and Cells3
Relevance of dopamine and dopamine neurons
Despite the inclusion of genes encoding for DA-related moieties in the current RDoC matrix on initial responsiveness to reward attainment, DA itself is not included under ‘Molecules’. One possible reason for this omission is that research has suggested that the DA system does not contribute importantly to the encoding of hedonic responses to food; e.g., as assessed in preclinical studies using taste reactivity paradigms (see ‘Behavior’ for more on taste reactivity). This and other observations have led to a distinction between the encoding of ‘liking’ versus ‘wanting’, with preclinical research indicating that hedonic food ‘liking’ is primarily encoded by opioids, endocannabinoids, orexins and glutamate; for reviews, see (Berridge, Robinson, & Aldridge, 2009; Pecina, Smith, & Berridge, 2006; Wilmouth & Spear, 2009). Notably, relatively little is known about the postnatal development of these systems, including how aspects of these systems may differ during adolescence versus adulthood (Wilmouth & Spear, 2009).
Despite the preclinical distinction described above, data indicate that DA is involved in multiple aspects of reward processing, including performance of tasks currently listed under ‘Paradigms’ in the initial responsiveness to reward attainment matrix (e.g., monetary incentive delay and gambling tasks), and in hedonic responses to drugs of abuse ( e.g., (Berridge, 1998; Koob & Volkow, 2010; Schott, et al., 2008; Schultz, 1992, 1997)), including ‘drug-liking’ in humans (Volkow, et al., 1999; Volkow, et al., 2002).
The mesocorticolimbic DA system is further involved in processes including the encoding of reward value, incentive salience and motivational drives (Volman, et al., 2013; Wahlstrom, White, & Luciana, 2010b) and has been highlighted in neurodevelopmental models of addictive and impulsive disorders; e.g., (Chambers, Taylor, & Potenza, 2003; Phillips, Ladouceur, & Drevets, 2008; Potenza, 2013; Sonuga-Barke, 2005). In addition, development of the human DA system is ongoing throughout childhood and adolescence, with aspects of this system not reaching mature levels until adulthood; Figure 1; reviewed in (Wahlstrom, Collins, White, & Luciana, 2010a). As such, we will here focus on what is known about the development of the DA system in relation to reward responses and childhood and adolescent addictive and impulsive disorders. Although we will focus primarily on findings from human studies, preclinical literature related to environmental influences on DA development will also be briefly discussed. For reviews on other molecules listed in Figure 1, see (Aston-Jones, et al., 2010; Baimel, et al., 2014; Berridge, et al., 2009; Pecina, et al., 2006; Wilmouth & Spear, 2009).
Developmental trajectory of the human DA system
Molecular research methods including ligand-based imaging - positron emission tomography (PET); single-photon emission computed tomography (SPECT) - are able to provide information about the endogenous functioning of neurochemical systems at high spatial resolution and can therefore be used for the in vivo quantification of specific neurochemical factors including receptor density and neurotransmitter release. However, given the reliance of these methodologies on radiotracers, very few ligand-based studies of healthy human development exist. Nonetheless, existing PET data (Jucaite, Forssberg, Karlsson, Halldin, & Farde, 2010) – in conjunction with evidence from human post-mortem and preclinical studies - suggest that the development of the DA system is ongoing throughout childhood and adolescence (Figure 2); for reviews, see (Wahlstrom, et al., 2010a; Wahlstrom, et al., 2010b).
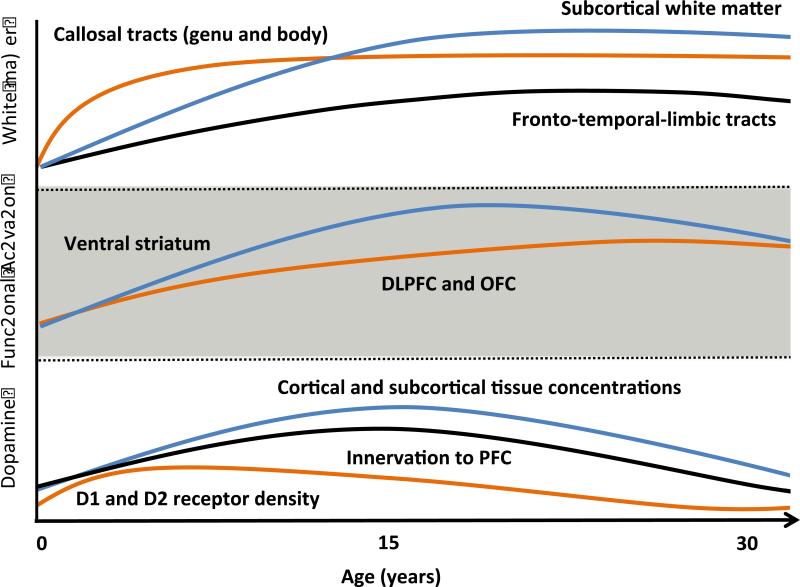
Figure 2. Theorized postnatal development of selected aspects of the human reward system warranting further study.
Figure 2 is a schematic diagram depicting the theorized longitudinal developmental trajectories for selected white-matter tissue tracts, functional responses and aspects of dopaminergic development hypothesized to relate to initial responsiveness to reward attainment (RDoC) and to the development of childhood and adolescent addictive and impulsive disorders. Trajectories are based on data reported in (Casey, 2014; Galvan, et al., 2006; Giedd, 2004; Gogtay, et al., 2004; Lebel & Beaulieu, 2011; Lebel, et al., 2008; Raznahan, et al., 2014; Wahlstrom, et al., 2010b).
DLPFC = dorsolateral prefrontal cortex; OFC = orbitofrontal cortex; PFC = prefrontal cortex
Tissue concentrations of both cortical and subcortical DA are elevated during adolescence in comparison to childhood and adulthood (Goldman-Rakic & Brown, 1982; Haycock, et al., 2003). By contrast, cortical and subcortical densities of both D1-like and D2-like receptors4 peak during childhood; however, they remain elevated during adolescence with respect to adulthood (Lidow, Goldman-Rakic, & Rakic, 1991; Montague, Lawler, Mailman, & Gilmore, 1999; Seeman, et al., 1987; Wahlstrom, et al., 2010b). Dopaminergic innervation to regions of the PFC also increases during adolescence, with peak axonal lengths of DA neurons found during this time (Lambe, Krimer, & Goldman-Rakic, 2000; Wahlstrom, et al., 2010b). Collectively, these data suggest heightened dopaminergic activity during adolescence, which, taken together with ongoing changes in white- and gray-matter tissues (discussed above; see Circuits and Physiology), likely contribute to the increased rates of impulsive behaviors observed during adolescence (Chambers, et al., 2003; Potenza, 2013; Wahlstrom, et al., 2010a; Wahlstrom, et al., 2010b). Similarly, it is likely that the alterations in dopaminergic activity associated with many adult psychiatric disorders may first become manifest during – or may be traced back to - this time of dynamic neurochemical development.
Evidence of dopamine involvement in initial responsiveness to reward attainment and in addictive and impulsive disorders
Data from adult ligand-based studies suggests that individual differences in subjective responses to drugs of abuse (e.g. ‘drug liking’) are associated with DA-related factors including the availability of DA D2-like receptors (Volkow, et al., 1999; Volkow, et al., 2002). Together with findings from pharmaceutical challenge studies indicating that individual differences in subjective reward responses relate to differences in addiction vulnerability (Davidson, Finch, & Schenk, 1993; Schuckit, 1994; Schuckit & Smith, 1996; Yip, et al., 2012), these data suggest that alterations in reward responses relating to aspects of DA functioning may confer increased vulnerability for addictive disorders. This interpretation is further supported by PET studies demonstrating reduced availability of DA D2-like receptors and reduced striatal DA release among adults with cocaine and other stimulant abuse or dependence; e.g., (Lee, et al., 2009; Martinez, et al., 2004; Volkow, et al., 2001; Volkow, et al., 1993). However, prospective assessment (prior to initiation of substance-use) would be required to determine whether alterations in aspects of DA functioning are a precursor to, or a consequence of, substance-use.
Reductions in D2-like receptor availability have also been reported among non-substance-addicted adults with high levels of impulsivity, including individuals with ADHD, as well as those with morbid obesity (Volkow, et al., 2009; Volkow, et al., 2007; Volkow, et al., 2008), suggesting that reduced D2-like receptor availability and blunted DA release may be a more general marker of increased impulsivity conferring vulnerability for addictions and other impulse-control-related disorders (Trifilieff & Martinez, 2014). Partially consistent with this hypothesis, Casey and colleagues recently reported blunted striatal DA release to acute amphetamine – but no alterations in receptor availability - among young adults at very high risk for addiction (based on both a positive family-history and current personal use) in comparison to individuals matched for current use without a positive family history and in comparison to drug-naïve controls (Casey, KF, et al., 2014), suggesting that decreased DA release may be a vulnerability factor for – as opposed to a consequence of – addictions.
Treatment implications and future directions
While further research is needed in adolescent populations, findings from adult studies suggest that individual differences in DA functioning may be important mediators of treatment responses. For example, both decreased DA D2/3 receptor availability and blunted methylphenidate-induced striatal DA release are associated with poorer treatment responses following contingency management (CM) among adults with cocaine dependence (Martinez, et al., 2011). Thus, given the dynamic development of different aspects of the DA system throughout childhood and adolescence, it is likely that individuals at different developmental stages may differ in treatment responses to reward-based (and possibly other) therapies. It will therefore be important for future research to further characterize the human DA system across different ages in relation to different disorders in order to guide effective treatment interventions.
However, as mentioned previously, the widespread assessment of in vivo DA (and other neurotransmitter) functioning among children and adolescents is limited by the human subjects concerns surrounding current radio-ligand-dependent methodologies. Thus, a remaining challenge is how best to study and understand typical versus atypical DA development in humans. Pre-clinical research can provide a wealth of complex data; however, given evidence of species-specific trajectories for aspects of DA functioning (Wahlstrom, et al., 2010b), the ability of pre-clinical data to directly inform understanding of the human DA system may be somewhat limited. Thus, further human studies using methods such as pharmaceutical challenge (Wahlstrom, et al., 2010b) and post-mortem tissue analysis (e.g., via the NIH NeuroBioBank; https://neurobiobank.nih.gov/) are needed to better characterize DA development.
Early environment and development of the DA system: Insights from preclinical research
Preclinical research indicates effects of the early environment on the development of DA (and other neurotransmitter) systems; reviewed in (Strathearn, 2011; Suri, Teixeira, Cagliostro, Mahadevia, & Ansorge, 2015). For example, rats reared in isolation exhibit increased levels of extracellular DA (Hall, et al., 1998; Jones, Hernandez, Kendall, Marsden, & Robbins, 1992) and decreases in DA transporter availability (Meaney, Brake, & Gratton, 2002). Isolation-rearing and maternal separation are further associated with increased stress responses, sensitization to cocaine (Li, Robinson, & Bhatnagar, 2003; Meaney, et al., 2002) and hyperactivity (Einon & Morgan, 1978; Hall, et al., 1998; Meaney, et al., 2002; Wilkinson, et al., 1994). Thus, maternal deprivation has been proposed as one environmental regulator of DA development that may predispose to drug abuse (Meaney, et al., 2002). As reviewed above, collective data suggest species-specific trajectories for aspects of DA functioning, reviewed in (Wahlstrom, et al., 2010b); thus a further important direction for future research will be the incorporation of measures of maternal attachment and caregiving within the context of reward system development in humans. Given recent data suggesting methylation-dependent regulation of drug-seeking behaviors related to DA functioning in mice (Wright, et al., 2015), further research into epigenetic effects on DA development as related to substance-use initiation is also needed.
Behavior, Paradigms and Self-Reports
One of the fundamental tenets of RDoC is that it is based on the relationship between biology (e.g., molecules, genes, neural circuits) and behavior (Casey, BJ, et al., 2014). As shown in Figure 1, taste reactivity is proposed as a behavioral measure relevant to the construct of initial responsiveness to reward attainment. First used to study the reaction of human infants to sweet and bitter tastes, taste-reactivity paradigms have since been widely used in studies of non-human animals (for a review, see (Berridge, 2000)) and generally indicate increased hedonic responses to positive tastes – interpreted as increased reward responsiveness - during adolescence in comparison to adulthood (Wilmouth & Spear, 2009). Although no direct analogue to taste-reactivity tasks exist for the study of humans, possible complementary data may be obtained using cue-reactivity or pharmaceutical challenge-type paradigms (e.g., subjective responses to alcohol or drugs of abuse in adults or to highly palatable foods in adolescents) either alone or in combination with fMRI scanning; e.g., (Brumback, et al., 2015; Filbey, et al., 2008; Gilman, Ramchandani, Crouss, & Hommer, 2012; Schuckit, Smith, & Kalmijn, 2014; Small, 2001; Tapert, et al., 2003; Yip, et al., 2012).
Although not currently included in the RDoC matrix (and thus not extensively reviewed here) on initial responsiveness to reward attainment, other behavioral measures of relevance to this construct include performance on gambling and decision-making tasks (such as those listed under ‘Paradigms’ in the current matrix; Figure 1); see (Casey, 2014; Hamilton, et al., 2015; Steinberg, 2008). However, as psychological research suggests differing developmental trajectories for related but distinct constructs such as sensation-seeking and impulsivity (Steinberg, 2008), further research into how development of these constructs may confer vulnerability to the development of addictive and adolescent disorders within the context of RDoC is needed. Such research could, for example, explore the interaction between performance on behavioral measures of reward responsiveness and self-report measures (discussed below) and initiation of illicit substance use during adolescence, in order to identify critical periods for intervention (Casey, BJ, et al., 2014).
Self-report measurements are relatively low-cost and thus may be more widely and frequently administered than molecular, genetic and neuroimaging measures. They therefore represent a powerful tool for large-scale, longitudinal assessments of reward-related constructs. However, to our knowledge no studies have yet utilized either of the self-report measures shown related to initial responsiveness to reward attainment – the consummatory subscale of the Temporal Experience of Pleasure Scale (TEPS) (Gard, et al., 2006) and the state version of the Positive and Negative Affect Scale (PANAS) (Watson, Clark, & Tellegen, 1988) - to study reward responses within a longitudinal developmental framework. Such data would provide an important starting point from which to interpret disorder- or symptom-specific findings.
Summary, future directions and challenges
RDoC provides a unique transdiagnostic framework from which to approach developmental neuropsychiatric research. Recently proposed complementary translational research efforts, such as the Alcohol Addiction RDoC (AARDoC) (Litten, et al., 2015), may also be helpful in integrating developmental findings. Significant challenges nonetheless remain. The first challenge involves the synthesis of existing data in relation to different constructs and units of analysis. Thus, further narrative and systematic review is warranted to ‘fill-in’ other matrix elements.
A second challenge will be the acquisition of multi-system data in an integrative manner. In particular, further work is needed to understand the interactions between – and even within - different systems (Casey, BJ, et al., 2014). To take the example of circuits, neuroimaging studies should aim to collect multiple types of data from each subject so that interactions between functional and structural neural features may be explored. It will also be important to apply advanced neuroimaging analysis methods (e.g., independent component analysis (Calhoun, 2001a, 2001b); intrinsic connectivity distribution (Scheinost, et al., 2012)) to examine interactions between different functional networks in relation to different constructs. Similarly, further genetic research is needed to understand how different genetic polymorphisms interact with environmental factors to influence neural responses. Additionally, genes relating to neurotransmitter systems beyond dopamine should be examined in relationship to reward and development, and additional approaches (e.g., utilizing genetic risk scores generated from large-scale studies involving assessments of multiple allelic variants) (Belsky, et al., 2013; Dudbridge, 2013) should be used to understand developmental aspects of reward as related to addiction and impulse control. Large-scale initiatives utilizing longitudinal designs such as the Adolescent Brain Cognitive Development (ABCD) (http://addictionresearch.nih.gov/adolescent-brain-cognitive-development-study) will be essential to this endeavor.
A neurodevelopmental approach will be essential to such future research, as characterization of the specific trajectories of different systems along a developmental continuum is needed to truly elucidate interactions between systems, as well as to identify possible ‘critical periods’ for effective interventions (Casey, BJ, et al., 2014). Correspondingly, application of an RDoC framework to existing neurodevelopmental research efforts will inform the understanding of the shared and unique biological factors underlying traditional psychiatric diagnoses, and this may be used to ultimately improve existing treatments.
Highlights.
Adolescence represents a neurodevelopmental period of vulnerability for impulse-control and addictive behaviors.
Reward sensitivity represents an important construct relating to addictive behaviors and impulse control.
Considering reward sensitivity within a Research-Domain-Criteria framework has important implications for adolescent health.
Acknowledgments
The authors would like to thank Drs. Patrick Worhunsky and Iris Balodis for consultations regarding figures. Dr. Marci Mitchell for helpful conversations regarding preclinical literature.
Funding: Dr. Yip's involvement was supported by the National Institute On Drug Abuse (NIDA) grant (DA007238-23) and CASAColumbia. Dr. Potenza's involvement was supported by the National Institute On Drug Abuse (NIDA) grants P50 DA09241, P20 DA027844 and R01 DA035058, CASAColumbia and a Center of Excellence grant from the National Center for Responsible Gaming. The contents of the manuscript were generated independently from individuals within the funding agencies and may not represent the views of the funding agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Although no systems are currently identified under ‘Physiology’ in the RDoC matrix on initial responsiveness to reward attainment, findings from fMRI studies may also be considered under this unit of analysis as blood-oxygenation-level-dependent (BOLD) signals are listed under ‘Physiology’ in other rows of the matrix (e.g., ‘Attention’).
TREK1 is also included in parentheses in the online matrix; see Figure 1.
No systems are currently identified under ‘Cells’ in the RDoC matrix on initial responsiveness to reward attainment. We propose that dopamine neurons may be a possible candidate cell related to this construct.
Based on structural and functional similarities across the two different receptor types; D1-like refers to D1 and D5 receptors; D2-like refers to D2, D3 and D4 receptors
Conflict of Interest and Disclosures: The authors report no conflicts of interest with respect to the content of this manuscript. Dr. Potenza has received financial support or compensation for the following: Dr. Potenza has consulted for and advised Somaxon, Boehringer Ingelheim, Lundbeck, Ironwood, Shire, INSYS and RiverMend Mental Health; has received research support from the National Institutes of Health, Veteran`s Administration, Mohegan Sun Casino, the National Center for Responsible Gaming, and Forest Laboratories, Ortho-McNeil, Oy-Control/Biotie, Glaxo-SmithKline, and Psyadon pharmaceuticals; has participated in surveys, mailings or telephone consultations related to drug addiction, impulse control disorders or other health topics; has consulted for legal and gambling entities on issues related to impulse control disorders; provides clinical care in the Connecticut Department of Mental Health and Addiction Services Problem Gambling Services Program; has performed grant reviews for the National Institutes of Health and other agencies; has edited journals or journal sections; has given academic lectures in grand rounds, CME events and other clinical or scientific venues; and has generated books or book chapters for publishers of mental health texts.
References
- Adleman NE, Fromm SJ, Razdan V, Kayser R, Dickstein DP, Brotman MA, Pine DS, Leibenluft E. Cross-sectional and longitudinal abnormalities in brain structure in children with severe mood dysregulation or bipolar disorder. J Child Psychol Psychiatry. 2012;53:1149–56. doi: 10.1111/j.1469-7610.2012.02568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler CM, Adams J, DelBello MP, Holland SK, Schmithorst V, Levine A, Jarvis K, Strakowski SM. Evidence of White Matter Pathology in Bipolar Disorder Adolescents Experiencing Their First Episode of Mania: A Diffusion Tensor Imaging Study. American Journal of Psychiatry. 2006;163:322–24. doi: 10.1176/appi.ajp.163.2.322. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Verweij KJ, Gillespie NA, Heath AC, Lessov-Schlaggar CN, Martin NG, Nelson EC, Slutske WS, Whitfield JB, Lynskey MT. The genetics of addiction-a translational perspective. Transl Psychiatry. 2012;2:e140. doi: 10.1038/tp.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arevalo MA, Santos-Galindo M, Bellini MJ, Azcoitia I, Garcia-Segura LM. Actions of estrogens on glial cells: Implications for neuroprotection. Biochim Biophys Acta. 2010;1800:1106–12. doi: 10.1016/j.bbagen.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, Richardson KA. Lateral hypothalamic orexin/hypocretin neurons: A role in reward-seeking and addiction. Brain Res. 2010;1314:74–90. doi: 10.1016/j.brainres.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baimel C, Bartlett SE, Chiou LC, Lawrence AJ, Muschamp JW, Patkar O, Tung LW, Borgland SL. Orexin/hypocretin role in reward: implications for opioid and other addictions. British Journal of Pharmacology. 2014 doi: 10.1111/bph.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley-Levenson E, Galvan A. Neural representation of expected value in the adolescent brain. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:1646–51. doi: 10.1073/pnas.1319762111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, Dant CC, Reiss AL. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex. 2005;15:1848–54. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Barr CL, Xu C, Kroft J, Feng Y, Wigg K, Zai G, Tannock R, Schachar R, Malone M, Roberts W, Nothen MM, Grunhage F, Vandenbergh DJ, Uhl G, Sunohara G, King N, Kennedy JL. Haplotype study of three polymorphisms at the dopamine transporter locus confirm linkage to attention-deficit/hyperactivity disorder. Biol Psychiatry. 2001;49:333–9. doi: 10.1016/s0006-3223(00)01053-2. [DOI] [PubMed] [Google Scholar]
- Barratt DT, Coller JK, Somogyi AA. Association between the DRD2 A1 allele and response to methadone and buprenorphine maintenance treatments. Am J Med Genet B Neuropsychiatr Genet. 2006;141b:323–31. doi: 10.1002/ajmg.b.30319. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Bullmore ET. Human brain networks in health and disease. Curr Opin Neurol. 2009;22:340–7. doi: 10.1097/WCO.0b013e32832d93dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Sporns O. Human connectomics. Curr Opin Neurobiol. 2012;22:144–53. doi: 10.1016/j.conb.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky DW, Moffitt TE, Sugden K, Williams B, Houts R, McCarthy J, Caspi A. Development and evaluation of a genetic risk score for obesity. Biodemography Soc Biol. 2013;59:85–100. doi: 10.1080/19485565.2013.774628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F, Absinta M, Rocca MA, Radaelli D, Poletti S, Bernasconi A, Dallaspezia S, Pagani E, Falini A, Copetti M, Colombo C, Comi G, Smeraldi E, Filippi M. Tract-specific white matter structural disruption in patients with bipolar disorder. Bipolar Disorders. 2011;13:414–24. doi: 10.1111/j.1399-5618.2011.00938.x. [DOI] [PubMed] [Google Scholar]
- Berns GS, Moore S, Capra CM. Adolescent engagement in dangerous behaviors is associated with increased white matter maturity of frontal cortex. PLoS ONE. 2009;4:e6773. doi: 10.1371/journal.pone.0006773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge K, Robinson TE. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Res Rev. 1998;28:309–69. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev. 2000;24:173–98. doi: 10.1016/s0149-7634(99)00072-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: 'liking', 'wanting', and learning. Curr Opin Pharmacol. 2009;9:65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Landes RD, Hill PF, Baxter C. Remember the future: working memory training decreases delay discounting among stimulant addicts. Biol Psychiatry. 2011;69:260–5. doi: 10.1016/j.biopsych.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Chen G, Hommer DW. Adolescents, Adults and Rewards: Comparing Motivational Neurocircuitry Recruitment Using fMRI. PLoS ONE. 2010;5:e11440. doi: 10.1371/journal.pone.0011440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomqvist O, Gelernter J, Kranzler HR. Family-based study of DRD2 alleles in alcohol and drug dependence. Am J Med Genet. 2000;96:659–64. doi: 10.1002/1096-8628(20001009)96:5<659::aid-ajmg12>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Boutrel B, Cannella N, de Lecea L. The role of hypocretin in driving arousal and goal-oriented behaviors. Brain Res. 2010;1314:103–11. doi: 10.1016/j.brainres.2009.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braams BR, Peters S, Peper JS, Guroglu B, Crone EA. Gambling for self, friends, and antagonists: differential contributions of affective and social brain regions on adolescent reward processing. NeuroImage. 2014;100:281–9. doi: 10.1016/j.neuroimage.2014.06.020. [DOI] [PubMed] [Google Scholar]
- Bramen JE, Hranilovich JA, Dahl RE, Forbes EE, Chen J, Toga AW, Dinov ID, Worthman CM, Sowell ER. Puberty influences medial temporal lobe and cortical gray matter maturation differently in boys than girls matched for sexual maturity. Cereb Cortex. 2011;21:636–46. doi: 10.1093/cercor/bhq137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. Pretreatment Brain Activation During Stroop Task Is Associated with Outcomes in Cocaine-Dependent Patients. Biol Psychiatry. 2008;64:998–1004. doi: 10.1016/j.biopsych.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Chen YF, Beach SR. Differential susceptibility to prevention: GABAergic, dopaminergic, and multilocus effects. J Child Psychol Psychiatry. 2013;54:863–71. doi: 10.1111/jcpp.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer RM, Koenis MM, Schnack HG, van Baal GC, van Soelen IL, Boomsma DI, Hulshoff Pol HE. Longitudinal development of hormone levels and grey matter density in 9 and 12-year-old twins. Behav Genet. 2015;45:313–23. doi: 10.1007/s10519-015-9708-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumback T, Squeglia LM, Jacobus J, Pulido C, Tapert SF, Brown SA. Adolescent heavy drinkers' amplified brain responses to alcohol cues decrease over one month of abstinence. Addict Behav. 2015;46:45–52. doi: 10.1016/j.addbeh.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. The economy of brain network organization. Nat Rev Neurosci. 2012;13:336–49. doi: 10.1038/nrn3214. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Wright SB. Neurodevelopmental changes in working memory and cognitive control. Curr Opin Neurobiol. 2007;17:243–50. doi: 10.1016/j.conb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Calhoun V, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Human Brain Mapping. 2001a;14:140–51. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun V, Adali T, Pearlson GD, Pekar JJ. Spatial and temporal independent component analysis of functional MRI data containing a pair of task-related waveforms. Human Brain Mapping. 2001b;13:43–53. doi: 10.1002/hbm.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camara E, Kramer UM, Cunillera T, Marco-Pallares J, Cucurell D, Nager W, Mestres-Misse A, Bauer P, Schule R, Schols L, Tempelmann C, Rodriguez-Fornells A, Munte TF. The effects of COMT (Val108/158Met) and DRD4 (SNP -521) dopamine genotypes on brain activations related to valence and magnitude of rewards. Cereb Cortex. 2010;20:1985–96. doi: 10.1093/cercor/bhp263. [DOI] [PubMed] [Google Scholar]
- Casey BJ. Beyond Simple Models of Self-Control to Circuit-Based Accounts of Adolescent Behavior. Annu Rev Psychol. 2014;66:295–315. doi: 10.1146/annurev-psych-010814-015156. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann N Y Acad Sci. 2008;1124:111–26. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Oliveri ME, Insel T. A Neurodevelopmental Perspective on The Research Domain Criteria (RDoC) Framework. Biol Psychiatry. 2014;76:350–53. doi: 10.1016/j.biopsych.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Casey KF, Benkelfat C, Cherkasova MV, Baker GB, Dagher A, Leyton M. Reduced dopamine response to amphetamine in subjects at ultra-high risk for addiction. Biol Psychiatry. 2014;76:23–30. doi: 10.1016/j.biopsych.2013.08.033. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Di Martino A, Craddock RC, Mehta AD, Milham MP. Clinical applications of the functional connectome. NeuroImage. 2013;80:527–40. doi: 10.1016/j.neuroimage.2013.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Taylor J, Potenza M. Developmental Neurocircuitry of Motivation in Adolescence: A Critical Period of Addiction Vulnerability. American Journal of Psychiatry. 2003;160:1041–52. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CK, Chen SL, Mill J, Huang YS, Lin SK, Curran S, Purcell S, Sham P, Asherson P. The dopamine transporter gene is associated with attention deficit hyperactivity disorder in a Taiwanese sample. Mol Psychiatry. 2003;8:393–6. doi: 10.1038/sj.mp.4001238. [DOI] [PubMed] [Google Scholar]
- Cheon KA, Ryu YH, Kim JW, Cho DY. The homozygosity for 10-repeat allele at dopamine transporter gene and dopamine transporter density in Korean children with attention deficit hyperactivity disorder: relating to treatment response to methylphenidate. Eur Neuropsychopharmacol. 2005;15:95–101. doi: 10.1016/j.euroneuro.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Chung T, Paulsen DJ, Geier CF, Luna B, Clark DB. Regional brain activation supporting cognitive control in the context of reward is associated with treated adolescents' marijuana problem severity at follow-up: A preliminary study. Dev Cogn Neurosci. 2015 doi: 10.1016/j.dcn.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin G, van den Heuvel MP. The ontogeny of the human connectome: development and dynamic changes of brain connectivity across the life span. Neuroscientist. 2013;19:616–28. doi: 10.1177/1073858413503712. [DOI] [PubMed] [Google Scholar]
- Comings DE, Muhleman D, Ahn C, Gysin R, Flanagan SD. The dopamine D2 receptor gene: a genetic risk factor in substance abuse. Drug and Alcohol Dependence. 1994;34:175–80. doi: 10.1016/0376-8716(94)90154-6. [DOI] [PubMed] [Google Scholar]
- Comings DE, Rosenthal RJ, Lesieur HR, Rugle LJ, Muhleman D, Chiu C, Dietz G, Gade R. A study of the dopamine D2 receptor gene in pathological gambling. Pharmacogenetics. 1996;6:223–34. doi: 10.1097/00008571-199606000-00004. [DOI] [PubMed] [Google Scholar]
- Creed MC, Ntamati NR, Tan KR. VTA GABA neurons modulate specific learning behaviors through the control of dopamine and cholinergic systems. Front Behav Neurosci. 2014;8:8. doi: 10.3389/fnbeh.2014.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crettol S, Besson J, Croquette-Krokar M, Hammig R, Gothuey I, Monnat M, Deglon JJ, Preisig M, Eap CB. Association of dopamine and opioid receptor genetic polymorphisms with response to methadone maintenance treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1722–7. doi: 10.1016/j.pnpbp.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat Rev Neurosci. 2012;13:636–50. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ardenne K, McClure SM, Nystrom LE, Cohen JD. BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science. 2008;319:1264–7. doi: 10.1126/science.1150605. [DOI] [PubMed] [Google Scholar]
- Dager AD, Anderson BM, Rosen R, Khadka S, Sawyer B, Jiantonio-Kelly RE, Austad CS, Raskin SA, Tennen H, Wood RM, Fallahi CR, Pearlson GD. Functional magnetic resonance imaging (fMRI) response to alcohol pictures predicts subsequent transition to heavy drinking in college students. Addiction. 2014;109:585–95. doi: 10.1111/add.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren A, Wargelius HL, Berglund KJ, Fahlke C, Blennow K, Zetterberg H, Oreland L, Berggren U, Balldin J. Do alcohol-dependent individuals with DRD2 A1 allele have an increased risk of relapse? A pilot study. Alcohol Alcohol. 2011;46:509–13. doi: 10.1093/alcalc/agr045. [DOI] [PubMed] [Google Scholar]
- Davidson ES, Finch JF, Schenk S. Variability in subjective responses to cocaine: initial experiences of college students. Addict Behav. 1993;18:445–53. doi: 10.1016/0306-4603(93)90062-e. [DOI] [PubMed] [Google Scholar]
- Demers CH, Bogdan R, Agrawal A. The Genetics, Neurogenetics and Pharmacogenetics of Addiction. Curr Behav Neurosci Rep. 2014;1:33–44. doi: 10.1007/s40473-013-0004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EL, Jahanshad N, McMahon KL, de Zubicaray GI, Martin NG, Hickie IB, Toga AW, Wright MJ, Thompson PM. Development of brain structural connectivity between ages 12 and 30: a 4-Tesla diffusion imaging study in 439 adolescents and adults. NeuroImage. 2013;64:671–84. doi: 10.1016/j.neuroimage.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison M, Whittle S, Yucel M, Vijayakumar N, Kline A, Simmons J, Allen NB. Mapping subcortical brain maturation during adolescence: evidence of hemisphere- and sex-specific longitudinal changes. Dev Sci. 2013;16:772–91. doi: 10.1111/desc.12057. [DOI] [PubMed] [Google Scholar]
- DeVito EE, Worhunsky PD, Carroll KM, Rounsaville BJ, Kober H, Potenza MN. A preliminary study of the neural effects of behavioral therapy for substance use disorders. Drug and Alcohol Dependence. 2012;122:228–35. doi: 10.1016/j.drugalcdep.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Fair Damien A, Kelly C, Satterthwaite Theodore D., Castellanos FX, Thomason Moriah E., Craddock RC, Luna B, Leventhal Bennett L., Zuo X-N, Milham Michael P. Unraveling the Miswired Connectome: A Developmental Perspective. Neuron. 2014;83:1335–53. doi: 10.1016/j.neuron.2014.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Fair DA, Kelly C, Satterthwaite TD, Castellanos FX, Thomason ME, Craddock RC, Luna B, Leventhal BL, Zuo XN, Milham MP. Unraveling the miswired connectome: a developmental perspective. Neuron. 2014;83:1335–53. doi: 10.1016/j.neuron.2014.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Wang JC, Plunkett J, Aliev F, Hinrichs A, Bertelsen S, Budde JP, Goldstein EL, Kaplan D, Edenberg HJ, Nurnberger J, Jr., Hesselbrock V, Schuckit M, Kuperman S, Tischfield J, Porjesz B, Begleiter H, Bierut LJ, Goate A. Family-based association analyses of alcohol dependence phenotypes across DRD2 and neighboring gene ANKK1. Alcohol Clin Exp Res. 2007;31:1645–53. doi: 10.1111/j.1530-0277.2007.00470.x. [DOI] [PubMed] [Google Scholar]
- Doehring A, Hentig N, Graff J, Salamat S, Schmidt M, Geisslinger G, Harder S, Lotsch J. Genetic variants altering dopamine D2 receptor expression or function modulate the risk of opiate addiction and the dosage requirements of methadone substitution. Pharmacogenet Genomics. 2009;19:407–14. doi: 10.1097/FPC.0b013e328320a3fd. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, Nelson SM, Wig GS, Vogel AC, Lessov-Schlaggar CN, Barnes KA, Dubis JW, Feczko E, Coalson RS, Pruett JR, Jr., Barch DM, Petersen SE, Schlaggar BL. Prediction of individual brain maturity using fMRI. Science. 2010;329:1358–61. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher JC, Kohn P, Kolachana Weinberger, D., Berman KF. Variation in dopamine genes influences responsivity of the human reward system. PNAS. 2009;106:617–22. doi: 10.1073/pnas.0805517106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudbridge F. Power and predictive accuracy of polygenic risk scores. PLoS Genet. 2013;9:e1003348. doi: 10.1371/journal.pgen.1003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. Am J Psychiatry. 2011;168:1041–9. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl C, Wiers RW, Pawelczack S, Rinck M, Becker ES, Lindenmeyer J. Approach bias modification in alcohol dependence: do clinical effects replicate and for whom does it work best? Dev Cogn Neurosci. 2013;4:38–51. doi: 10.1016/j.dcn.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einon DF, Morgan MJ. Early isolation produces enduring hyperactivity in the rat, but no effect upon spontaneous alternation. Q J Exp Psychol. 1978;30:151–6. doi: 10.1080/14640747808400663. [DOI] [PubMed] [Google Scholar]
- Elofson J, Gongvatana W, Carey KB. Alcohol use and cerebral white matter compromise in adolescence. Addict Behav. 2013;38:2295–305. doi: 10.1016/j.addbeh.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt B, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature neuroscience. 2005;8:1481–89. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, Schlaggar BL, Petersen SE. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone M, Smith RM, Chenoweth MJ, Kumar Bhattacharjee A, Kelsoe JR, Tyndale RF, Lerman C. Neuroimaging in psychiatric pharmacogenetics research: the promise and pitfalls. Neuropsychopharmacology. 2013;38:2327–37. doi: 10.1038/npp.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Chung T. Neuroimaging mechanisms of change in psychotherapy for addictive behaviors: emerging translational approaches that bridge biology and behavior. Psychol Addict Behav. 2013;27:329–35. doi: 10.1037/a0031491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Filbey FM, Sabbineni A, Chandler LD, Hutchison KE. How Psychosocial Alcohol Interventions Work: A Preliminary Look at What fMRI Can Tell Us. Alcoholism: Clinical and Experimental Research. 2011;35:643–51. doi: 10.1111/j.1530-0277.2010.01382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, LaChance HA, Bryan A, Hutchison KE. Do genetic and individual risk factors moderate the efficacy of motivational enhancement therapy? Drinking outcomes with an emerging adult sample. Addict Biol. 2009;14:356–65. doi: 10.1111/j.1369-1600.2009.00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, McEachern AD, Yezhuvath U, Bryan AD, Hutchison KE, Filbey FM. Integrating brain and behavior: evaluating adolescents' response to a cannabis intervention. Psychol Addict Behav. 2013;27:510–25. doi: 10.1037/a0029767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Mead HK, Yezhuvath U, DeWitt S, Hutchison KE, Filbey FM. A preliminary examination of how serotonergic polymorphisms influence brain response following an adolescent cannabis intervention. Psychiatry Research: Neuroimaging. 2012;204:112–16. doi: 10.1016/j.pscychresns.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Claus E, Audette AR, Niculescu M, Banich MT, Tanabe J, Du YP, Hutchison KE. Exposure to the taste of alcohol elicits activation of the mesocorticolimbic neurocircuitry. Neuropsychopharmacology. 2008;33:1391–401. doi: 10.1038/sj.npp.1301513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Brown SM, Kimak M, Ferrell RE, Manuck SB, Hariri AR. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Mol Psychiatry. 2009;14:60–70. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Hajcak G. Genetic variation in dopamine moderates neural response during reward anticipation and delivery: evidence from event-related potentials. Psychophysiology. 2012;49:617–26. doi: 10.1111/j.1469-8986.2011.01343.x. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Lohoff FW, Wang Z, Sciortino N, Harper D, Li Y, Jens W, Cruz J, Kampman K, Ehrman R, Berrettini W, Detre JA, O'Brien CP, Childress AR. DAT genotype modulates brain and behavioral responses elicited by cigarette cues. Neuropsychopharmacology. 2009;34:717–28. doi: 10.1038/npp.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Li Y, Suh JJ, Goldman M, Lohoff FW, Cruz J, Hazan R, Jens W, Detre JA, Berrettini W, O'Brien CP, Childress AR. Dopamine transporter genotype modulation of neural responses to smoking cues: confirmation in a new cohort. Addict Biol. 2011;16:308–22. doi: 10.1111/j.1369-1600.2010.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A. Adolescent development of the reward system. Front Hum Neurosci. 2010;4:6. doi: 10.3389/neuro.09.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26:6885–92. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Gard MG, Kring AM, John OP. Anticipatory and consummatory components of the experience of pleasure: A scale development study. Journal of Research in Personality. 2006;40:1086–102. [Google Scholar]
- Giedd J, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neurosci. 1999;2:861–63. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural Magnetic Resonance Imaging of the Adolescent Brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Ramchandani VA, Crouss T, Hommer DW. Subjective and neural responses to intravenous alcohol in young adults with light and heavy drinking patterns. Neuropsychopharmacology. 2012;37:467–77. doi: 10.1038/npp.2011.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8174–79. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Thompson PM. Mapping gray matter development: Implications for typical development and vulnerability to psychopathology. Brain Cogn. 2010;72:6–15. doi: 10.1016/j.bandc.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldapple K, Segal Z, Garson C, Lau M, Bieling P, Kennedy S, Mayberg H. Modulation of cortical-limbic pathways in major depression: treatment specific effects of cognitive behavioral therapy compared to paroxetine. Archives of General Psychiatry. 2004;61:34–41. doi: 10.1001/archpsyc.61.1.34. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Brown RM. Postnatal development of monoamine content and synthesis in the cerebral cortex of rhesus monkeys. Brain Res. 1982;256:339–49. doi: 10.1016/0165-3806(82)90146-8. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–69. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Casero E, Perez de Castro I, Saiz-Ruiz J, Llinares C, Fernandez-Piqueras J. No association between particular DRD3 and DAT gene polymorphisms and manic-depressive illness in a Spanish sample. Psychiatr Genet. 1996;6:209–12. doi: 10.1097/00041444-199624000-00007. [DOI] [PubMed] [Google Scholar]
- Grayson DS, Ray S, Carpenter S, Iyer S, Dias TG, Stevens C, Nigg JT, Fair DA. Structural and functional rich club organization of the brain in children and adults. PLoS ONE. 2014;9:e88297. doi: 10.1371/journal.pone.0088297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood TA, Joo E-J, Shekhtman T, Sadovnick AD, Remick RA, Keck PE, McElroy SL, Kelsoe JR. Association of dopamine transporter gene variants with childhood ADHD features in bipolar disorder. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2013;162:137–45. doi: 10.1002/ajmg.b.32108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood TA, Schork NJ, Eskin E, Kelsoe JR. Identification of additional variants within the human dopamine transporter gene provides further evidence for an association with bipolar disorder in two independent samples. Mol Psychiatry. 2006;11:125–33. 15. doi: 10.1038/sj.mp.4001764. [DOI] [PubMed] [Google Scholar]
- Guo G, Wilhelmsen K, Hamilton N. Gene-lifecourse interaction for alcohol consumption in adolescence and young adulthood: five monoamine genes. Am J Med Genet B Neuropsychiatr Genet. 2007;144b:417–23. doi: 10.1002/ajmg.b.30340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FS, Wilkinson LS, Humby T, Inglis W, Kendall DA, Marsden CA, Robbins TW. Isolation rearing in rats: pre- and postsynaptic changes in striatal dopaminergic systems. Pharmacol Biochem Behav. 1998;59:859–72. doi: 10.1016/s0091-3057(97)00510-8. [DOI] [PubMed] [Google Scholar]
- Hamilton KR, Mitchell MR, Wing VC, Balodis IM, Bickel WK, Fillmore M, Lane SD, Lejuez CW, Littlefield AK, Luijten M, Mathias CW, Mitchell SH, Napier TC, Reynolds B, Schutz CG, Setlow B, Sher KJ, Swann AC, Tedford SE, White MJ, Winstanley CA, Yi R, Potenza MN, Moeller FG. Choice impulsivity: Definitions, measurement issues, and clinical implications. Personal Disord. 2015;6:182–98. doi: 10.1037/per0000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton LS, Levitt JG, O'Neill J, Alger JR, Luders E, Phillips OR, Caplan R, Toga AW, McCracken J, Narr KL. Reduced white matter integrity in attention-deficit hyperactivity disorder. Neuroreport. 2008;19:1705–8. doi: 10.1097/WNR.0b013e3283174415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris G, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437 doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70:185–98. doi: 10.1001/jamapsychiatry.2013.277. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Iftikhar A, Kamali A, Kramer LA, Ashtari M, Cirino PT, Papanicolaou AC, Fletcher JM, Ewing-Cobbs L. Development and aging of the healthy human brain uncinate fasciculus across the lifespan using diffusion tensor tractography. Brain Res. 2009;1276:67–76. doi: 10.1016/j.brainres.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser TU, Iannaccone R, Walitza S, Brandeis D, Brem S. Cognitive flexibility in adolescence: neural and behavioral mechanisms of reward prediction error processing in adaptive decision making during development. NeuroImage. 2015;104:347–54. doi: 10.1016/j.neuroimage.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycock JW, Becker L, Ang L, Furukawa Y, Hornykiewicz O, Kish SJ. Marked disparity between age-related changes in dopamine and other presynaptic dopaminergic markers in human striatum. J Neurochem. 2003;87:574–85. doi: 10.1046/j.1471-4159.2003.02017.x. [DOI] [PubMed] [Google Scholar]
- Heitzeg MM, Cope LM, Martz ME, Hardee JE. Neuroimaging Risk Markers for Substance Abuse: Recent Findings on Inhibitory Control and Reward System Functioning. Curr Addict Rep. 2015;2:91–103. doi: 10.1007/s40429-015-0048-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzeg MM, Villafuerte S, Weiland BJ, Enoch MA, Burmeister M, Zubieta JK, Zucker RA. Effect of GABRA2 genotype on development of incentive-motivation circuitry in a sample enriched for alcoholism risk. Neuropsychopharmacology. 2014;39:3077–86. doi: 10.1038/npp.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot CS, Claus ED, Ramchandani VA. Associations of OPRM1 A118G and alcohol sensitivity with intravenous alcohol self-administration in young adults. Addict Biol. 2014 doi: 10.1111/adb.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfer CJ, Timberlake D, Haberstick B, Lessem JM, Ehringer MA, Smolen A, Hewitt JK. Genetic influences on quantity of alcohol consumed by adolescents and young adults. Drug and Alcohol Dependence. 2005;78:187–93. doi: 10.1016/j.drugalcdep.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Houben K, Havermans RC, Nederkoorn C, Jansen A. Beer a no-go: learning to stop responding to alcohol cues reduces alcohol intake via reduced affective associations rather than increased response inhibition. Addiction. 2012;107:1280–7. doi: 10.1111/j.1360-0443.2012.03827.x. [DOI] [PubMed] [Google Scholar]
- Hutchison KE. Substance Use Disorders: Realizing the Promise of Pharmacogenomics and Personalized Medicine. Annual Review of Clinical Psychology. 2010;6:577–89. doi: 10.1146/annurev.clinpsy.121208.131441. [DOI] [PubMed] [Google Scholar]
- Ingalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott MA, Ruparel K, Hakonarson H, Gur RE, Gur RC, Verma R. Sex differences in the structural connectome of the human brain. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:823–8. doi: 10.1073/pnas.1316909110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–51. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Insel TR. The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. Am J Psychiatry. 2014;171:395–7. doi: 10.1176/appi.ajp.2014.14020138. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Brown TT, Hagler DJ, Jr., Akshoomoff N, Bartsch H, Newman E, Thompson WK, Bloss CS, Murray SS, Schork N, Kennedy DN, Kuperman JM, McCabe C, Chung Y, Libiger O, Maddox M, Casey BJ, Chang L, Ernst TM, Frazier JA, Gruen JR, Sowell ER, Kenet T, Kaufmann WE, Mostofsky S, Amaral DG, Dale AM. The Pediatric Imaging, Neurocognition, and Genetics (PING) Data Repository. NeuroImage. 2015 doi: 10.1016/j.neuroimage.2015.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z, Worhunsky PD, Carroll KM, Rounsaville BJ, Stevens MC, Pearlson GD, Potenza MN. An Initial Study of Neural Responses to Monetary Incentives as Related to Treatment Outcome in Cocaine Dependence. Biol Psychiatry. 2011;70:553–60. doi: 10.1016/j.biopsych.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GH, Hernandez TD, Kendall DA, Marsden CA, Robbins TW. Dopaminergic and serotonergic function following isolation rearing in rats: study of behavioural responses and postmortem and in vivo neurochemistry. Pharmacol Biochem Behav. 1992;43:17–35. doi: 10.1016/0091-3057(92)90635-s. [DOI] [PubMed] [Google Scholar]
- Joober R, Grizenko N, Sengupta S, Amor LB, Schmitz N, Schwartz G, Karama S, Lageix P, Fathalli F, Torkaman-Zehi A, Ter Stepanian M. Dopamine transporter 3′-UTR VNTR genotype and ADHD: a pharmaco-behavioural genetic study with methylphenidate. Neuropsychopharmacology. 2007;32:1370–6. doi: 10.1038/sj.npp.1301240. [DOI] [PubMed] [Google Scholar]
- Jucaite A, Forssberg H, Karlsson P, Halldin C, Farde L. Age-related reduction in dopamine D1 receptors in the human brain: from late childhood to adulthood, a positron emission tomography study. Neuroscience. 2010;167:104–10. doi: 10.1016/j.neuroscience.2010.01.034. [DOI] [PubMed] [Google Scholar]
- Kambeitz J, Romanos M, Ettinger U. Meta-analysis of the association between dopamine transporter genotype and response to methylphenidate treatment in ADHD. Pharmacogenomics J. 2014;14:77–84. doi: 10.1038/tpj.2013.9. [DOI] [PubMed] [Google Scholar]
- Keikhaee MR, Fadai F, Sargolzaee MR, Javanbakht A, Najmabadi H, Ohadi M. Association analysis of the dopamine transporter (DAT1)-67A/T polymorphism in bipolar disorder. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2005;135B:47–49. doi: 10.1002/ajmg.b.30174. [DOI] [PubMed] [Google Scholar]
- Kober H, Devito EE, Deleone CM, Carroll KM, Potenza MN. Cannabis Abstinence During Treatment and One-Year Follow-up: Relationship to Neural Activity in Men. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN. Prefrontal–striatal pathway underlies cognitive regulation of craving. Proceedings of the National Academy of Sciences. 2010;107:14811–16. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Balodis IM, Kober H, Worhunsky PD, Liss T, Xu J, Potenza MN. An exploratory pilot study of the relationship between neural correlates of cognitive control and reduction in cigarette use among treatment-seeking adolescent smokers. Psychol Addict Behav. 2013;27:526–32. doi: 10.1037/a0032479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustanovich V, Ishii J, Crawford L, Yang M, McGough JJ, McCracken JT, Smalley SL, Nelson SF. Transmission disequilibrium testing of dopamine-related candidate gene polymorphisms in ADHD: confirmation of association of ADHD with DRD4 and DRD5. Mol Psychiatry. 2004;9:711–7. doi: 10.1038/sj.mp.4001466. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Krimer LS, Goldman-Rakic PS. Differential postnatal development of catecholamine and serotonin inputs to identified neurons in prefrontal cortex of rhesus monkey. J Neurosci. 2000;20:8780–7. doi: 10.1523/JNEUROSCI.20-23-08780.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Benson BE, Guyer AE, Perez-Edgar K, Fox NA, Pine DS, Ernst M. Longitudinal study of striatal activation to reward and loss anticipation from mid-adolescence into late adolescence/early adulthood. Brain Cogn. 2014;89:51–60. doi: 10.1016/j.bandc.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langen M, Schnack HG, Nederveen H, Bos D, Lahuis BE, de Jonge MV, van Engeland H, Durston S. Changes in the developmental trajectories of striatum in autism. Biol Psychiatry. 2009;66:327–33. doi: 10.1016/j.biopsych.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Lasiter PS, Deems DA, Oetting RL, Garcia J. Taste discriminations in rats lacking anterior insular gustatory neocortex. Physiol Behav. 1985;35:277–85. doi: 10.1016/0031-9384(85)90350-6. [DOI] [PubMed] [Google Scholar]
- Lasky-Su J, Neale BM, Franke B, Anney RJL, Zhou K, Maller JB, Vasquez AA, Chen W, Asherson P, Buitelaar J, Banaschewski T, Ebstein R, Gill M, Miranda A, Mulas F, Oades RD, Roeyers H, Rothenberger A, Sergeant J, Sonuga-Barke E, Steinhausen HC, Taylor E, Daly M, Laird N, Lange C, Faraone SV. Genome-wide association scan of quantitative traits for attention deficit hyperactivity disorder identifies novel associations and confirms candidate gene associations. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2008;147B:1345–54. doi: 10.1002/ajmg.b.30867. [DOI] [PubMed] [Google Scholar]
- Laucht M, Skowronek MH, Becker K, et al. Interacting effects of the dopamine transporter gene and psychosocial adversity on attention-deficit/hyperactivity disorder symptoms among 15-year-olds from a high-risk community sample. Archives of General Psychiatry. 2007;64:585–90. doi: 10.1001/archpsyc.64.5.585. [DOI] [PubMed] [Google Scholar]
- Lawford BR, Young RM, Noble EP, Sargent J, Rowell J, Shadforth S, Zhang X, Ritchie T. The D(2) dopamine receptor A(1) allele and opioid dependence: association with heroin use and response to methadone treatment. Am J Med Genet. 2000;96:592–8. doi: 10.1002/1096-8628(20001009)96:5<592::aid-ajmg3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Lawrence EJ, Su L, Barker GJ, Medford N, Dalton J, Williams SCR, Birbaumer N, Veit R, Ranganatha S, Bodurka J, Brammer M, Giampietro V, David AS. Self-regulation of the anterior insula: Reinforcement learning using real-time fMRI neurofeedback. NeuroImage. 2014;88:113–24. doi: 10.1016/j.neuroimage.2013.10.069. [DOI] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. Longitudinal Development of Human Brain Wiring Continues from Childhood into Adulthood. The Journal of Neuroscience. 2011;31:10937–47. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage. 2012;60:340–52. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. NeuroImage. 2008;40:1044–55. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J, Bilder RM, Brody AL, Mandelkern MA. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci. 2009;29:14734–40. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Robinson TE, Bhatnagar S. Effects of maternal separation on behavioural sensitization produced by repeated cocaine administration in adulthood. Brain Res. 2003;960:42–7. doi: 10.1016/s0006-8993(02)03752-6. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Goldman-Rakic PS, Rakic P. Synchronized overproduction of neurotransmitter receptors in diverse regions of the primate cerebral cortex. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:10218–21. doi: 10.1073/pnas.88.22.10218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Ryan ML, Falk DE, Reilly M, Fertig JB, Koob GF. Heterogeneity of alcohol use disorder: understanding mechanisms to advance personalized treatment. Alcohol Clin Exp Res. 2015;39:579–84. doi: 10.1111/acer.12669. [DOI] [PubMed] [Google Scholar]
- Lohoff FW, Bloch PJ, Hodge R, Nall AH, Ferraro TN, Kampman KM, Dackis CA, O'Brien CP, Pettinati HM, Oslin DW. Association analysis between polymorphisms in the dopamine D2 receptor (DRD2) and dopamine transporter (DAT1) genes with cocaine dependence. Neurosci Lett. 2010;473:87–91. doi: 10.1016/j.neulet.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macritchie KAN, Lloyd AJ, Bastin ME, Vasudev K, Gallagher P, Eyre R, Marshall I, Wardlaw JM, Ferrier IN, Moore PB, Young AH. White matter microstructural abnormalities in euthymic bipolar disorder. The British Journal of Psychiatry. 2010;196:52–58. doi: 10.1192/bjp.bp.108.058586. [DOI] [PubMed] [Google Scholar]
- Makoukji J, Belle M, Meffre D, Stassart R, Grenier J, Shackleford GG, Fledrich R, Fonte C, Branchu J, Goulard M, de Waele C, Charbonnier F, Sereda MW, Baulieu E-E, Schumacher M, Bernard S, Massaad C. Lithium enhances remyelination of peripheral nerves. Proceedings of the National Academy of Sciences. 2012;109:3973–78. doi: 10.1073/pnas.1121367109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manki H, Kanba S, Muramatsu T, Higuchi S, Suzuki E, Matsushita S, Ono Y, Chiba H, Shintani F, Nakamura M, Yagi G, Asai M. Dopamine D2, D3 and D4 receptor and transporter gene polymorphisms and mood disorders. J Affect Disord. 1996;40:7–13. doi: 10.1016/0165-0327(96)00035-3. [DOI] [PubMed] [Google Scholar]
- Martinez D, Broft A, Foltin RW, Slifstein M, Hwang DR, Huang Y, Perez A, Frankle WG, Cooper T, Kleber HD, Fischman MW, Laruelle M. Cocaine dependence and d2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuropsychopharmacology. 2004;29:1190–202. doi: 10.1038/sj.npp.1300420. [DOI] [PubMed] [Google Scholar]
- Martinez D, Carpenter K, Liu F, Slifstein M, Broft A, Friedman A, Kumar D, Van Heertum R, Kleber H, Nunes E. Imaging dopamine transmission in cocaine dependence: link between neurochemistry and response to treatment. Am J Psychiatry. 2011;168:634–41. doi: 10.1176/appi.ajp.2010.10050748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massat I, Souery D, Del-Favero J, Van Gestel S, Serretti A, Macciardi F, Smeraldi E, Kaneva R, Adolfsson R, Nylander PO, Blackwood D, Muir W, Papadimitriou GN, Dikeos D, Oruc L, Segman RH, Ivezic S, Aschauer H, Ackenheil M, Fuchshuber S, Dam H, Jakovljevic M, Peltonen L, Hilger C, Hentges F, Staner L, Milanova V, Jazin E, Lerer B, Van Broeckhoven C, Mendlewicz J. Positive association of dopamine D2 receptor polymorphism with bipolar affective disorder in a European Multicenter Association Study of affective disorders. Am J Med Genet. 2002;114:177–85. [PubMed] [Google Scholar]
- Matzeu A, Zamora-Martinez ER, Martin-Fardon R. The paraventricular nucleus of the thalamus is recruited by both natural rewards and drugs of abuse: recent evidence of a pivotal role for orexin/hypocretin signaling in this thalamic nucleus in drug-seeking behavior. Front Behav Neurosci. 2014;8:117. doi: 10.3389/fnbeh.2014.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Bickel WK. A dual-systems perspective on addiction: contributions from neuroimaging and cognitive training. Ann N Y Acad Sci. 2014;1327:62–78. doi: 10.1111/nyas.12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Brake W, Gratton A. Environment regulation of the development of mesolimbic dopamine systems: A neurobiological mechanism for vulnerability to drug abuse? Psychoneuroendocrinology. 2002;27:127–38. doi: 10.1016/s0306-4530(01)00040-3. [DOI] [PubMed] [Google Scholar]
- Mills KL, Goddings AL, Clasen LS, Giedd JN, Blakemore SJ. The developmental mismatch in structural brain maturation during adolescence. Dev Neurosci. 2014;36:147–60. doi: 10.1159/000362328. [DOI] [PubMed] [Google Scholar]
- Moeller SJ, Parvaz MA, Shumay E, Beebe-Wang N, Konova AB, Alia-Klein N, Volkow ND, Goldstein RZ. Gene x abstinence effects on drug cue reactivity in addiction: multimodal evidence. J Neurosci. 2013;33:10027–36. doi: 10.1523/JNEUROSCI.0695-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague DM, Lawler CP, Mailman RB, Gilmore JH. Developmental regulation of the dopamine D1 receptor in human caudate and putamen. Neuropsychopharmacology. 1999;21:641–9. doi: 10.1016/S0893-133X(99)00062-7. [DOI] [PubMed] [Google Scholar]
- Moyer RA, Wang D, Papp AC, Smith RM, Duque L, Mash DC, Sadee W. Intronic Polymorphisms Affecting Alternative Splicing of Human Dopamine D2 Receptor Are Associated with Cocaine Abuse. Neuropsychopharmacology. 2011;36:753–62. doi: 10.1038/npp.2010.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo MR, Durrant C, Lewis G, Flint J. Gene X environment interactions at the serotonin transporter locus. Biol Psychiatry. 2009;65:211–9. doi: 10.1016/j.biopsych.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Flint J. Replication and heterogeneity in gene x environment interaction studies. Int J Neuropsychopharmacol. 2009;12:727–9. doi: 10.1017/S1461145709000479. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Zammit S, Flint J. Commentary: Response to commentary by Rutter on Munafo et al. (2014). J Child Psychol Psychiatry. 2014a;55:1105–6. doi: 10.1111/jcpp.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo MR, Zammit S, Flint J. Practitioner review: A critical perspective on gene-environment interaction models--what impact should they have on clinical perceptions and practice? J Child Psychol Psychiatry. 2014b;55:1092–101. doi: 10.1111/jcpp.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao T, Radua J, Rubia K, Mataix-Cols D. Gray matter volume abnormalities in ADHD: voxel-based meta-analysis exploring the effects of age and stimulant medication. Am J Psychiatry. 2011;168:1154–63. doi: 10.1176/appi.ajp.2011.11020281. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Gaznick N, Tranel D, Bechara A. The insula: a critical neural substrate for craving and drug seeking under conflict and risk. Ann N Y Acad Sci. 2014;1316:53–70. doi: 10.1111/nyas.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIMH. Positive Valence Systems: Workshop Proceedings. NIMH; 2011. [Google Scholar]
- NIMHa . Development of the RDoC Framework. NIMH; 2015. [Google Scholar]
- NIMHb . RDoC Frequently Asked Questions. NIMH; 2015. [Google Scholar]
- Nyman ES, Ogdie MN, Loukola A, Varilo T, Taanila A, Hurtig T, Moilanen IK, Loo SK, McGough JJ, Jarvelin MR, Smalley SL, Nelson SF, Peltonen L. ADHD candidate gene study in a population-based birth cohort: association with DBH and DRD2. J Am Acad Child Adolesc Psychiatry. 2007;46:1614–21. doi: 10.1097/chi.0b013e3181579682. [DOI] [PubMed] [Google Scholar]
- Nymberg C, Jia T, Lubbe S, Ruggeri B, Desrivieres S, Barker G, Buchel C, Fauth-Buehler M, Cattrell A, Conrod P, Flor H, Gallinat J, Garavan H, Heinz A, Ittermann B, Lawrence C, Mann K, Nees F, Salatino-Oliveira A, Paillere Martinot ML, Paus T, Rietschel M, Robbins T, Smolka M, Banaschewski T, Rubia K, Loth E, Schumann G. Neural mechanisms of attention-deficit/hyperactivity disorder symptoms are stratified by MAOA genotype. Biol Psychiatry. 2013;74:607–14. doi: 10.1016/j.biopsych.2013.03.027. [DOI] [PubMed] [Google Scholar]
- Olson EA, Collins PF, Hooper CJ, Muetzel R, Lim KO, Luciana M. White Matter Integrity Predicts Delay Discounting Behavior in 9-to 23-Year-Olds: A Diffusion Tensor Imaging Study. Journal of Cognitive Neuroscience. 2009;21:1406–21. doi: 10.1162/jocn.2009.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paloyelis Y, Mehta MA, Faraone SV, Asherson P, Kuntsi J. Striatal Sensitivity During Reward Processing in Attention-Deficit/Hyperactivity Disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2012;51:722–32. e9. doi: 10.1016/j.jaac.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen DJ, Carter RM, Platt ML, Huettel SA, Brannon EM. Neurocognitive development of risk aversion from early childhood to adulthood. Front Hum Neurosci. 2011;5:178. doi: 10.3389/fnhum.2011.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An Insular View of Anxiety. Biological Psychiatry. 2006;60:383–87. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9:60–8. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Paus T. Growth of white matter in the adolescent brain: Myelin or axon? Brain Cogn. 2010;72:26–35. doi: 10.1016/j.bandc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Paus T. Growth of white matter in the adolescent brain: myelin or axon? Brain Cogn. 2010;72:26–35. doi: 10.1016/j.bandc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Paus T. How environment and genes shape the adolescent brain. Horm Behav. 2013;64:195–202. doi: 10.1016/j.yhbeh.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–57. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina S, Smith KS, Berridge KC. Hedonic hot spots in the brain. Neuroscientist. 2006;12:500–11. doi: 10.1177/1073858406293154. [DOI] [PubMed] [Google Scholar]
- Perez-Edgar K, Hardee JE, Guyer AE, Benson BE, Nelson EE, Gorodetsky E, Goldman D, Fox NA, Pine DS, Ernst M. DRD4 and striatal modulation of the link between childhood behavioral inhibition and adolescent anxiety. Soc Cogn Affect Neurosci. 2014;9:445–53. doi: 10.1093/scan/nst001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin JS, Leonard G, Perron M, Pike GB, Pitiot A, Richer L, Veillette S, Pausova Z, Paus T. Sex differences in the growth of white matter during adolescence. NeuroImage. 2009;45:1055–66. doi: 10.1016/j.neuroimage.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Pesaresi M, Soon-Shiong R, French L, Kaplan DR, Miller FD, Paus T. Axon diameter and axonal transport: In vivo and in vitro effects of androgens. NeuroImage. 2015;115:191–201. doi: 10.1016/j.neuroimage.2015.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters BD, Szeszko PR, Radua J, Ikuta T, Gruner P, DeRosse P, Zhang JP, Giorgio A, Qiu D, Tapert SF, Brauer J, Asato MR, Khong PL, James AC, Gallego JA, Malhotra AK. White matter development in adolescence: diffusion tensor imaging and meta-analytic results. Schizophr Bull. 2012;38:1308–17. doi: 10.1093/schbul/sbs054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Bromberg U, Schneider S, Brassen S, Menz M, Banaschewski T, Conrod PJ, Flor H, Gallinat J, Garavan H, Heinz A, Itterman B, Lathrop M, Martinot J-L, Paus T, Poline J-B, Robbins TW, Rietschel M, Smolka M, Ströhle A, Struve M, Loth E, Schumann G, Büchel C. Lower Ventral Striatal Activation During Reward Anticipation in Adolescent Smokers. Am J Psychiatry. 2011;168:540–49. doi: 10.1176/appi.ajp.2010.10071024. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:833–57. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieters S, Van Der Zwaluw CS, Van Der Vorst H, Wiers RW, Smeets H, Lambrichs E, Burk WJ, Engels RC. The moderating effect of alcohol-specific parental rule-setting on the relation between the dopamine D2 receptor gene (DRD2), the mu-opioid receptor gene (OPRM1) and alcohol use in young adolescents. Alcohol Alcohol. 2012;47:663–70. doi: 10.1093/alcalc/ags075. [DOI] [PubMed] [Google Scholar]
- Pinsonneault JK, Han DD, Burdick KE, Kataki M, Bertolino A, Malhotra AK, Gu HH, Sadee W. Dopamine Transporter Gene Variant Affecting Expression in Human Brain is Associated with Bipolar Disorder. Neuropsychopharmacology. 2011;36:1644–55. doi: 10.1038/npp.2011.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN. Biological Contributions to Addictions in Adolescents and Adults: Prevention, Treatment, and Policy Implications. Journal of Adolescent Health. 2013;52:S22–S32. doi: 10.1016/j.jadohealth.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman AS, Xu J, Potenza MN. Hippocampal and amygdalar volumetric differences in pathological gambling: a preliminary study of the associations with the behavioral inhibition system. Neuropsychopharmacology. 2014;39:738–45. doi: 10.1038/npp.2013.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray L, Hutchison K. Effects of naltrexone on alcohol sensitivity and genetic moderators of medication response: A double-blind placebo-controlled study. Archives of General Psychiatry. 2007;64:1069–77. doi: 10.1001/archpsyc.64.9.1069. [DOI] [PubMed] [Google Scholar]
- Ray LA, Courtney KE, Hutchison KE, Mackillop J, Galvan A, Ghahremani DG. Initial evidence that OPRM1 genotype moderates ventral and dorsal striatum functional connectivity during alcohol cues. Alcohol Clin Exp Res. 2014;38:78–89. doi: 10.1111/acer.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Shaw PW, Lerch JP, Clasen LS, Greenstein D, Berman R, Pipitone J, Chakravarty MM, Giedd JN. Longitudinal four-dimensional mapping of subcortical anatomy in human development. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:1592–7. doi: 10.1073/pnas.1316911111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JM, Plate RC, Ernst M. Neural systems underlying motivated behavior in adolescence: implications for preventive medicine. Prev Med. 2012;55(Suppl):S7–s16. doi: 10.1016/j.ypmed.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman T, Szobot C, Martins S, Biederman J, Rohde LA, Hutz MH. Dopamine transporter gene and response to methylphenidate in attention-deficit/hyperactivity disorder. Pharmacogenetics. 2002;12:497–99. doi: 10.1097/00008571-200208000-00011. [DOI] [PubMed] [Google Scholar]
- Rutter M. Commentary: G x E in child psychiatry and psychology: a broadening of the scope of enquiry as prompted by Munafo et al. (2014). J Child Psychol Psychiatry. 2014;55:1102–4. doi: 10.1111/jcpp.12309. [DOI] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Levens SM, Perry LM, Dougherty RF, Knutson B. Frontostriatal White Matter Integrity Mediates Adult Age Differences in Probabilistic Reward Learning. The Journal of Neuroscience. 2012;32:5333–37. doi: 10.1523/JNEUROSCI.5756-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Connolly JJ, Ruparel K, Calkins ME, Jackson C, Elliott MA, Roalf DR, Ryan Hopsona KP, Behr M, Qiu H, Mentch FD, Chiavacci R, Sleiman PM, Gur RC, Hakonarson H, Gur RE. The Philadelphia Neurodevelopmental Cohort: A publicly available resource for the study of normal and abnormal brain development in youth. NeuroImage. 2015 doi: 10.1016/j.neuroimage.2015.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Vandekar S, Wolf DH, Ruparel K, Roalf DR, Jackson C, Elliott MA, Bilker WB, Calkins ME, Prabhakaran K, Davatzikos C, Hakonarson H, Gur RE, Gur RC. Sex differences in the effect of puberty on hippocampal morphology. J Am Acad Child Adolesc Psychiatry. 2014;53:341–50. doi: 10.1016/j.jaac.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Ruparel K, Erus G, Elliott MA, Eickhoff SB, Gennatas ED, Jackson C, Prabhakaran K, Smith A, Hakonarson H, Verma R, Davatzikos C, Gur RE, Gur RC. Heterogeneous impact of motion on fundamental patterns of developmental changes in functional connectivity during youth. NeuroImage. 2013;83:45–57. doi: 10.1016/j.neuroimage.2013.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Voronin KE, Randall PK, Li X, Henderson S, Myrick H. Interacting effects of naltrexone and OPRM1 and DAT1 variation on the neural response to alcohol cues. Neuropsychopharmacology. 2013;38:414–22. doi: 10.1038/npp.2012.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D, Benjamin J, Lacadie CM, Vohr B, Schneider KC, Ment LR, Papademetris X, Constable RT. The intrinsic connectivity distribution: a novel contrast measure reflecting voxel level functional connectivity. NeuroImage. 2012;62:1510–9. doi: 10.1016/j.neuroimage.2012.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid B, Blomeyer D, Becker K, Treutlein J, Zimmermann US, Buchmann AF, Schmidt MH, Esser G, Banaschewski T, Rietschel M, Laucht M. The interaction between the dopamine transporter gene and age at onset in relation to tobacco and alcohol use among 19-year-olds. Addict Biol. 2009;14:489–99. doi: 10.1111/j.1369-1600.2009.00171.x. [DOI] [PubMed] [Google Scholar]
- Schott BH, Minuzzi L, Krebs RM, Elmenhorst D, Lang M, Winz OH, Seidenbecher CI, Coenen HH, Heinze HJ, Zilles K, Duzel E, Bauer A. Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. J Neurosci. 2008;28:14311–9. doi: 10.1523/JNEUROSCI.2058-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry. 1994;151:184–89. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. An 8-year follow-up of 450 sons of alcoholic and control subjects. Archives of General Psychiatry. 1996;53:202–10. doi: 10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Kalmijn JA. The patterns of drug and alcohol use and associated problems over 30 years in 397 men. Alcohol Clin Exp Res. 2014;38:227–34. doi: 10.1111/acer.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Apicella P, Scarnati E, Ljungberg T. Neuronal activity in monkey ventral striatum related to the expectation of reward. J Neurosci. 1992;12:4595–610. doi: 10.1523/JNEUROSCI.12-12-04595.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague RR. A neural substrate of prediction and reward. Science. 1997;275:1593–99. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cerebral Cortex. 2000;10:272–84. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Seeman P, Bzowej NH, Guan HC, Bergeron C, Becker LE, Reynolds GP, Bird ED, Riederer P, Jellinger K, Watanabe S, et al. Human brain dopamine receptors in children and aging adults. Synapse. 1987;1:399–404. doi: 10.1002/syn.890010503. [DOI] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, Clasen L, Evans A, Giedd J, Rapoport JL. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19649–54. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Gilliam M, Liverpool M, Weddle C, Malek M, Sharp W, Greenstein D, Evans A, Rapoport J, Giedd J. Cortical development in typically developing children with symptoms of hyperactivity and impulsivity: support for a dimensional view of attention deficit hyperactivity disorder. Am J Psychiatry. 2011;168:143–51. doi: 10.1176/appi.ajp.2010.10030385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN, Wise SP. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–94. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira PP, Portella AK, Kennedy JL, Gaudreau H, Davis C, Steiner M, Soares CN, Matthews SG, Sokolowski MB, Dube L, Loucks EB, Hamilton J, Meaney MJ, Levitan RD. Association between the seven-repeat allele of the dopamine-4 receptor gene (DRD4) and spontaneous food intake in pre-school children. Appetite. 2014;73:15–22. doi: 10.1016/j.appet.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar P, Gabriel SB, McInnis MG, Bennett P, Lim Y, Tsan G, Schaffner S, Kirov G, Jones I, Owen M, Craddock N, DePaulo JR, Lander ES. Family-based association study of 76 candidate genes in bipolar disorder: BDNF is a potential risk locus. Brain-derived neutrophic factor. Mol Psychiatry. 2002;7:579–93. doi: 10.1038/sj.mp.4001058. [DOI] [PubMed] [Google Scholar]
- Small D, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 2001;124:1720–33. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- Smith CT, Boettiger CA. Age modulates the effect of COMT genotype on delay discounting behavior. Psychopharmacology (Berl) 2012;222:609–17. doi: 10.1007/s00213-012-2653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biol Psychiatry. 2005;57:1231–8. doi: 10.1016/j.biopsych.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga A. Mapping cortical change across the human life span. Nat Neurosci. 2003;3:309–15. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999;2:859–61. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. J Neurosci. 2001;21:8819–29. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev Med Child Neurol. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Stein M, Waldman ID, Sarampote CS, Seymour KE, Robb AS, Conlon C, Kim S-J, Cook EH. Dopamine transporter genotype and methylphenidate dose response in children with ADHD. Neuropsychophamacol. 2005;30:1374–82. doi: 10.1038/sj.npp.1300718. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A Social Neuroscience Perspective on Adolescent Risk-Taking. Dev Rev. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Bohon C, Marti N, Smolen A. Reward circuitry responsivity to food predicts future increases in body mass: moderating effects of DRD2 and DRD4. NeuroImage. 2010;50:1618–25. doi: 10.1016/j.neuroimage.2010.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L. Maternal neglect: oxytocin, dopamine and the neurobiology of attachment. J Neuroendocrinol. 2011;23:1054–65. doi: 10.1111/j.1365-2826.2011.02228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Yuan F, Shen X, Xiong G, Wu J. Role of COMT in ADHD: a systematic meta-analysis. Mol Neurobiol. 2014;49:251–61. doi: 10.1007/s12035-013-8516-5. [DOI] [PubMed] [Google Scholar]
- Suri D, Teixeira CM, Cagliostro MK, Mahadevia D, Ansorge MS. Monoamine-sensitive developmental periods impacting adult emotional and cognitive behaviors. Neuropsychopharmacology. 2015;40:88–112. doi: 10.1038/npp.2014.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer MM, Donny EC, Hariri AR. Imaging genetics and the neurobiological basis of individual differences in vulnerability to addiction. Drug and Alcohol Dependence. 2012;123(1):S59–71. doi: 10.1016/j.drugalcdep.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber M, Fibiger HC. Electrical stimulation of the medial prefrontal cortex increases dopamine release in the striatum. Neuropsychophamacol. 1993;9:271–75. doi: 10.1038/npp.1993.63. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Cheung EH, Brown GG, Frank LR, Paulus MP, Schweinsburg AD, Meloy MJ, Brown SA. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Archives of General Psychiatry. 2003;60:727–35. doi: 10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- Trifilieff P, Martinez D. Imaging addiction: D2 receptors and dopamine signaling in the striatum as biomarkers for impulsivity. Neuropharmacology. 2014;76(Pt B):498–509. doi: 10.1016/j.neuropharm.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S, Nakamura M, Mikami M, Kondoh K, Ishiguro H, Arinami T, Komiyama T, Mitsushio H, Sano A, Tanabe H. Identification of a novel polymorphism of the human dopamine transporter (DAT1) gene and the significant association with alcoholism. Mol Psychiatry. 1999;4:552–7. doi: 10.1038/sj.mp.4000562. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O. Rich-club organization of the human connectome. J Neurosci. 2011;31:15775–86. doi: 10.1523/JNEUROSCI.3539-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zwaluw CS, Engels RC, Buitelaar J, Verkes RJ, Franke B, Scholte RH. Polymorphisms in the dopamine transporter gene (SLC6A3/DAT1) and alcohol dependence in humans: a systematic review. Pharmacogenomics. 2009;10:853–66. doi: 10.2217/pgs.09.24. [DOI] [PubMed] [Google Scholar]
- van der Zwaluw CS, Engels RC, Vermulst AA, Franke B, Buitelaar J, Verkes RJ, Scholte RH. Interaction between dopamine D2 receptor genotype and parental rule-setting in adolescent alcohol use: evidence for a gene-parenting interaction. Mol Psychiatry. 2010;15:727–35. doi: 10.1038/mp.2009.4. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SA, Crone EA. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cereb Cortex. 2010;20:61–9. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, Luna B. Maturational Changes in Anterior Cingulate and Frontoparietal Recruitment Support the Development of Error Processing and Inhibitory Control. Cerebral Cortex. 2008;18:2505–22. doi: 10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001;158:377–82. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang G-J, Telang F, Logan J, Jayne M, Ma Y, Pradhan K, Wong C, Swanson JM. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. NeuroImage. 2010;49:2536–43. doi: 10.1016/j.neuroimage.2009.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DL, Dewey SL, Wolf AP. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–77. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Gifford A, Hitzemann R, Ding YS, Pappas N. Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. Am J Psychiatry. 1999;156:1440–3. doi: 10.1176/ajp.156.9.1440. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Thanos PP, Logan J, Gatley SJ, Gifford A, Ding YS, Wong C, Pappas N. Brain DA D2 receptors predict reinforcing effects of stimulants in humans: replication study. Synapse. 2002;46:79–82. doi: 10.1002/syn.10137. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Kollins SH, Wigal TL, Newcorn JH, Telang F, Fowler JS, Zhu W, Logan J, Ma Y, Pradhan K, Wong C, Swanson JM. Evaluating dopamine reward pathway in ADHD: clinical implications. JAMA. 2009;302:1084–91. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Newcorn J, Telang F, Solanto MV, Fowler JS, Logan J, Ma Y, Schulz K, Pradhan K, Wong C, Swanson JM. Depressed dopamine activity in caudate and preliminary evidence of limbic involvement in adults with attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 2007;64:932–40. doi: 10.1001/archpsyc.64.8.932. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Thanos PK, Logan J, Alexoff D, Ding YS, Wong C, Ma Y, Pradhan K. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. NeuroImage. 2008;42:1537–43. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volman SF, Lammel S, Margolis EB, Kim Y, Richard JM, Roitman MF, Lobo MK. New insights into the specificity and plasticity of reward and aversion encoding in the mesolimbic system. J Neurosci. 2013;33:17569–76. doi: 10.1523/JNEUROSCI.3250-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstrom D, Collins P, White T, Luciana M. Developmental changes in dopamine neurotransmission in adolescence: behavioral implications and issues in assessment. Brain Cogn. 2010a;72:146–59. doi: 10.1016/j.bandc.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstrom D, White T, Luciana M. Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neurosci Biobehav Rev. 2010b;34:631–48. doi: 10.1016/j.neubiorev.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TY, Lee SY, Chen SL, Chang YH, Chen SH, Huang SY, Tzeng NS, Wang CL, Yeh PH, Chen KC, Lee IH, Yeh TL, Yang YK, Lu RB. Gender-specific association of the SLC6A4 and DRD2 gene variants in bipolar disorder. Int J Neuropsychopharmacol. 2014;17:211–22. doi: 10.1017/S1461145713001296. [DOI] [PubMed] [Google Scholar]
- Wang Z, Ray R, Faith M, Tang K, Wileyto EP, Detre JA, Lerman C. Nicotine abstinence-induced cerebral blood flow changes by genotype. Neurosci Lett. 2008;438:275–80. doi: 10.1016/j.neulet.2008.04.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wesley MJ, Bickel WK. Remember the future II: meta-analyses and functional overlap of working memory and delay discounting. Biol Psychiatry. 2014;75:435–48. doi: 10.1016/j.biopsych.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiers CE, Stelzel C, Gladwin TE, Park SQ, Pawelczack S, Gawron CK, Stuke H, Heinz A, Wiers RW, Rinck M, Lindenmeyer J, Walter H, Bermpohl F. Effects of cognitive bias modification training on neural alcohol cue reactivity in alcohol dependence. Am J Psychiatry. 2015;172:335–43. doi: 10.1176/appi.ajp.2014.13111495. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Eberl C, Rinck M, Becker ES, Lindenmeyer J. Retraining automatic action tendencies changes alcoholic patients' approach bias for alcohol and improves treatment outcome. Psychol Sci. 2011;22:490–7. doi: 10.1177/0956797611400615. [DOI] [PubMed] [Google Scholar]
- Wilkinson LS, Killcross SS, Humby T, Hall FS, Geyer MA, Robbins TW. Social isolation in the rat produces developmentally specific deficits in prepulse inhibition of the acoustic startle response without disrupting latent inhibition. Neuropsychopharmacology. 1994;10:61–72. doi: 10.1038/npp.1994.8. [DOI] [PubMed] [Google Scholar]
- Wilmouth CE, Spear LP. Hedonic sensitivity in adolescent and adult rats: Taste reactivity and voluntary sucrose consumption. Pharmacology Biochemistry and Behavior. 2009;92:566–73. doi: 10.1016/j.pbb.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsberg BG, Comings DE. Association of the dopamine transporter gene (DAT1) with poor methylphenidate response. J Am Acad Child Adolesc Psychiatry. 1999;38:1474–7. doi: 10.1097/00004583-199912000-00006. [DOI] [PubMed] [Google Scholar]
- Wright KN, Hollis F, Duclot F, Dossat AM, Strong CE, Francis TC, Mercer R, Feng J, Dietz DM, Lobo MK, Nestler EJ, Kabbaj M. Methyl supplementation attenuates cocaine-seeking behaviors and cocaine-induced c-Fos activation in a DNA methylation-dependent manner. 2015;35:8948–58. doi: 10.1523/JNEUROSCI.5227-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, EE D, F, W. P., KM C, BJ R, Potenza MN. White matter integrity is associated with treatment outcome measures in cocaine dependence. Neuropsychopharmacology. 2010;35:1541–49. doi: 10.1038/npp.2010.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kober H, Carroll KM, Rounsaville BJ, Pearlson GD, Potenza MN. White matter integrity and behavioral activation in healthy subjects. Human Brain Mapping. 2012;33:994–1002. doi: 10.1002/hbm.21275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Chang C, Xu T, Jiang L, Handwerker DA, Castellanos FX, Milham MP, Bandettini PA, Zuo XN. Connectivity trajectory across lifespan differentiates the precuneus from the default network. NeuroImage. 2014;89:45–56. doi: 10.1016/j.neuroimage.2013.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip S, Carroll K, Potenza M. Feldstein Ewing S, Witkiewitz K, Filbey F, editors. An overview of translational approaches to the treatment of addictions. Neuroimaging and Psychosocial Addiction Treatment: An Integrative Guide for Researchers and Clinicians: Palgrave. 2015 [Google Scholar]
- Yip S, Chandler R, Rogers R, Mackay C, Goodwin G. White matter alterations in antipsychotic- and mood stabilizer-naive individuals with bipolar II/NOS disorder. Neuroimage Clinical. 2013;3:271–8. doi: 10.1016/j.nicl.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip S, Devito E, Kober H, Worhunsky P, Carroll K, Potenza M. Pretreatment measures of brain structure and reward-processing brain function in cannabis dependence: An exploratory study of relationships with abstinence during behavioral treatment. Drug and Alcohol Dependence. 2014;140:33–41. doi: 10.1016/j.drugalcdep.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip S, Doherty J, Wakeley J, Saunders K, Tzagarakis C, de Wit H, Goodwin GM, Rogers RD. Reduced Subjective Response to Acute Ethanol Administration Among Young Men with a Broad Bipolar Phenotype. Neuropsychopharmacology. 2012;37:1808–15. doi: 10.1038/npp.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip S, Lacadie CM, Sinha R, Mayes LC, Potenza MN. Prenatal cocaine exposure, illicit-substance use and stress and craving processes during adolescence. Drug & Alcohol Dependence. 2015 doi: 10.1016/j.drugalcdep.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip S, Worhunsky PD, Rogers RD, Goodwin GM. Hypoactivation of the Ventral and Dorsal Striatum During Reward and Loss Anticipation in Antipsychotic and Mood Stabilizer-Naive Bipolar Disorder. Neuropsychopharmacology. 2015;40:658–66. doi: 10.1038/npp.2014.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Qin L.-d., Chen J, Qian L.-j., Tao J, Fang Y.-r., Xu J.-r. Brain microstructural abnormalities revealed by diffusion tensor images in patients with treatment-resistant depression compared with major depressive disorder before treatment. European Journal of Radiology. 2011;80:450–54. doi: 10.1016/j.ejrad.2010.06.041. [DOI] [PubMed] [Google Scholar]