Abstract
The gastrointestinal mucosa is an important site of HIV acquisition, viral replication and pathogenesis. Immune cells in mucosal tissues frequently differ in phenotype and function from their non-mucosal counterparts. Although perforin-mediated cytotoxicity as measured in blood is a recognized correlate of HIV immune control, its role in gastrointestinal tissues is unknown. We sought to elucidate the cytotoxic features of rectal mucosal CD8+ T-cells in HIV infected and uninfected subjects. Perforin expression and lytic capacity were significantly reduced in rectal CD8+ T-cells compared to their blood counterparts, regardless of HIV clinical status; granzyme B (GrzB) was reduced to a lesser extent. Mucosal perforin and GrzB expression were higher in participants not on antiretroviral therapy compared to those on therapy and controls. Reduction in perforin and GrzB was not explained by differences in memory/effector subsets. Expression of T-bet and Eomesodermin was significantly lower in gut CD8+ T-cells compared to blood, and in vitro neutralization of TGF-β partially restored perforin expression in gut CD8+ T-cells. These findings suggest that rectal CD8+ T-cells are primarily non-cytotoxic, and phenotypically shaped by the tissue microenvironment. Further elucidation of rectal immune responses to HIV will inform the development of vaccines and immunotherapies targeted to mucosal tissues.
INTRODUCTION
The healthy gastrointestinal (GI) tract maintains an immunosuppressive environment to limit inappropriate immune responses to food antigens and the gut microbiome. Thus, immune cells housed at mucosal sites often differ in phenotype and function from their counterparts in non-mucosal tissues1. For example, human intestinal macrophages display “inflammatory anergy” and tissue-resident T-cells display unique phenotypes driven in part by a local microenvironment rich in TGF-β2, 3, 4, 5, 6, 7. Because immune responses in tissues can differ from those in blood, and because the GI tract is an important site of HIV infection, understanding HIV-specific immune responses in the gut may be critical to the development of immune-based therapies and prophylactics8, 9.
Cytotoxic T-cells mainly use granule-mediated mechanisms to eliminate intracellular pathogens. Although several models exist, the pore-forming protein perforin is thought to disrupt plasma membranes and endosomal membranes, facilitating entry of granzymes into the cytosol and ultimately leading to target cell apoptosis10. Accordingly, perforin activity is thought to be essential for CD8+ T-cell mediated cytotoxicity. Perforin-mediated cytotoxicity, as measured in blood, is a consistent correlate of HIV immune control11, 12, 13, 14, 15, 16, 17. However, gastrointestinal CD8+ T-cells display low perforin expression, a phenomenon likely related to tissue localization, as similar observations have been made in lymphoid tissues18, 19 and for intestinal natural killer cells20. Attenuating cytotoxicity may be a protective measure to limit tissue damage; for example, cytotoxic CD8+ T-cells are implicated in development of relapsing colitis in normal mice21, and an influx of perforin+ CD8+ T-cells in duodenal mucosa during acute HIV infection correlates with epithelial apoptosis22. In contrast, gastrointestinal CD8+ T-cells exhibit strong cytokine and β-chemokine production, mechanisms that have also been implicated in HIV immune control23, 24, 41.
Whether low perforin expression in gastrointestinal CD8+ T-cells negatively impacts the host’s ability to eradicate HIV infection remains unclear. In this study, we set out to elucidate the cytotoxic capacity of intestinal CD8+ T-cells, understand the mechanistic basis for the difference in perforin expression between CD8+ T-cells in blood and gut, and clarify the role of gut CD8+ T-cells in host defense against chronic HIV infection.
RESULTS
Ex vivo perforin and granzyme B expression in resting CD8+ T-cells is reduced in rectal mucosa compared to blood, regardless of HIV status
We previously reported reduced frequencies of perforin and granzyme B (GrzB)-expressing CD8+ T-cells in rectal mucosa compared to peripheral blood in both chronically HIV-infected and seronegative participants19, 24. This was apparent in flow cytometry staining of isolated rectal CD8+ T-cells as well as immunohistochemistry and fluorescence microscopy of rectal tissue sections, and was not a consequence of mucosal cell purification protocols19. From these earlier studies, it was clear that the relatively low perforin expression detected in rectal mucosa was not limited to HIV-infected individuals. However, whether perforin and GrzB expression in rectal CD8+ T-cells varies by disease status or is affected by antiretroviral therapy, was unknown12, 13. To address these questions, we utilized flow cytometry to assess intracellular perforin and GrzB protein expression, and qPCR to examine mRNA levels. Unstimulated CD8+ T-cells from blood and rectal mucosa were evaluated in the following participant groups: HIV controllers (C); HIVpositive, viremic individuals not on antiretroviral therapy (V); HIV-positive individuals on antiretroviral therapy (Tx); early infection, HIV-positive individuals within the first year postdiagnosis (E); and seronegative controls (SN) (Table 1).
Table 1.
Participant Characteristics
| Participant Category |
Gender | Race | Plasma viral load, RNA copies/mL* [Median] |
CD4 count, Cells/mm3 [Median] |
Time Post HIV+ Diagnosis (Years) [Median] |
|---|---|---|---|---|---|
|
HIV Controllers (n = 11) |
M, 6 F, 3 T, 2 |
AA, 7 C, 3 Mx, 1 |
<40 | 746 | 20.2 |
|
Viremic Untreated (n = 16) |
M, 15 | AA, 7 C, 6 L, 2 |
20,544 | 421 | 10.8 |
|
Early Infection (n = 6) |
M, 6 | AA, 2 C, 4 |
97,107 | 460 | 0.21 |
|
ART Treated (n = 23) |
M, 20 F, 1 T, 2 |
AA, 5 C, 13 L, 3 Mx, 2 |
<40 | 598 | 16.5 |
|
HIV Seronegatives **(n = 19) |
M, 16 F, 1 T, 1 |
AA, 5 C, 10 L, 1 Mx, 1 NAm, 1 |
NA | 975 | NA |
M, Male; F, Female; T, Transgender.
AA, African American; C, Caucasian; L, Latino; Mx, Mixed; NAm, Native American. NA, Not applicable.
Median calculated using only detectable viral load data points.
One Seronegative participant’s demographic information was unavailable.
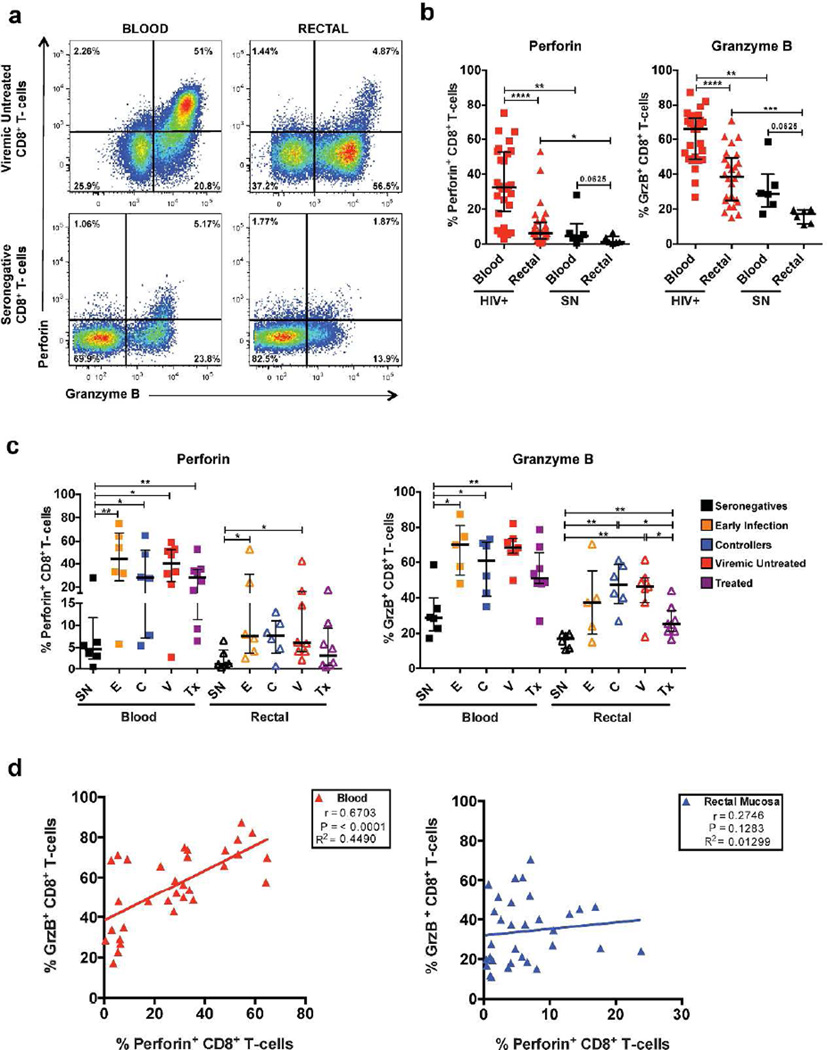
We observed three major trends: first, lower transcript levels and proportions of perforin+ and GrzB+ CD8+ T-cells in rectal mucosa compared to blood in all participant groups regardless of HIV disease status (Figure 1 a–c, Supplementary Figure S1); second, a higher proportion of perforin+ and GrzB+ CD8+ T-cells in all HIV+ groups compared to seronegatives (Figure 1b); and third, a discordance between perforin and GrzB expression in rectal mucosa (Figure 1d). In rectal mucosa, the frequencies of perforin+ and GrzB+ CD8+ T-cells were elevated in each HIV+ group compared to seronegatives, reaching statistical significance for viremic untreated and early infection groups as compared to seronegatives for perforin; and in controllers, viremic, and treated groups compared to seronegatives for GrzB. A similar trend was observed in blood (Figure 1c). The highest proportions of perforin+ and GrzB+ CD8+ T-cells in both tissues were detected in participants sampled during early infection. However, when comparing HIV+ subgroups to one another, significant differences were only noted in GrzB expression in rectal mucosa, with elevated frequencies of GrzB+ CD8+ T-cells in controllers and viremic untreated compared to treated participant groups (Figure 1c). In blood but not rectal mucosa, the proportions of perforin+ and GrzB+ CD8+ T-cells were positively correlated (Figure 1d). Thus, despite a universal reduction in perforin and GrzB in rectal mucosa compared to blood, expression of these cytotoxic effectors appeared discordant and was elevated in rectal mucosa during untreated HIV-infection.
Figure 1.
Varying perforin and GrzB expression between blood and rectal CD8+ T-cells and across HIV-infection. (a) Representative flow cytometry plot of intracellular perforin and GrzB expression in unstimulated ex vivo blood and rectal mucosal CD8+ T-cells from representative participants: viremic untreated (top) and seronegative (bottom). (b) Difference in intracellular perforin and GrzB expression between blood and rectal CD8+ T-cells in a consolidated group of all HIV+ participants compared to seronegative (SN) participants. (c) Difference in intracellular perforin and GrzB expression in rectal and blood CD8+ T-cells across HIV-disease status. (d) Spearman correlation analysis relating the frequency of GrzB+ CD8+ T-cells with the frequency of perforin+ CD8+ T-cells in rectal mucosa and blood. Wide horizontal bars represent medians; narrow whiskers indicate interquartile ranges; asterisks show level of significance as follows: * P <0.05, ** P <0.01, *** P <0.001, **** P <0.0001.
Perforin expression in response to TCR stimulation is reduced in rectal mucosa compared to blood
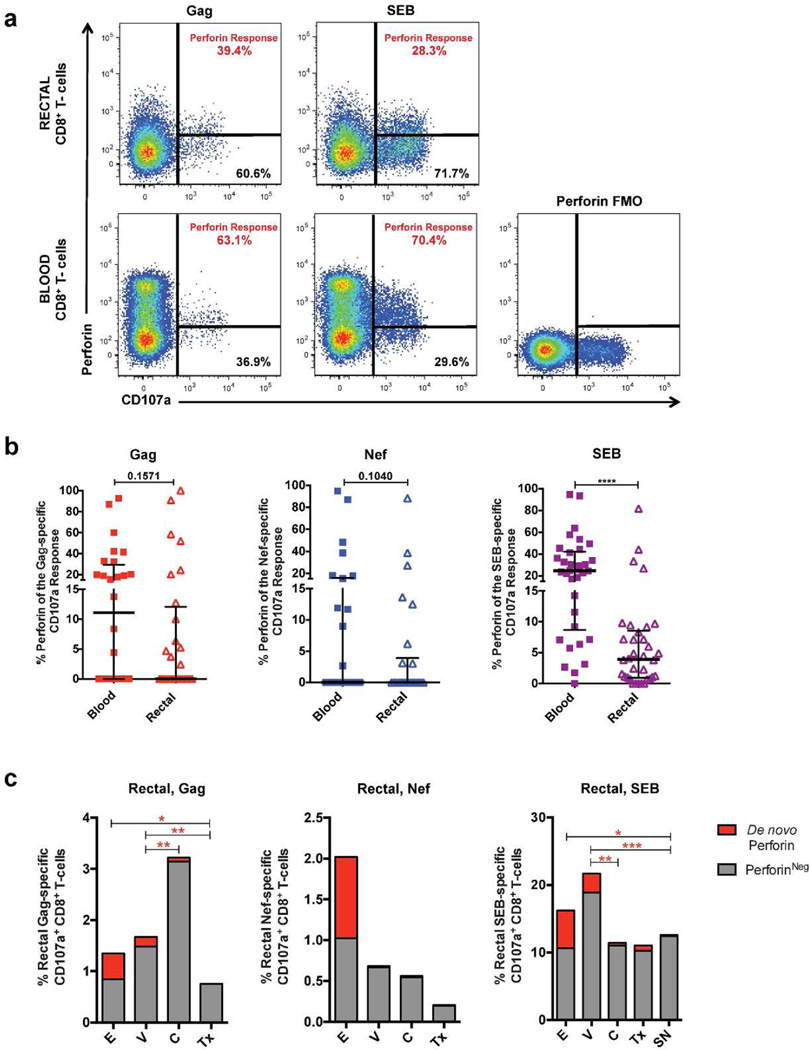
Because previous studies revealed that CD8+ T-cells must proliferate in order to reconstitute cellular perforin stores and because the rectal mucosa is a site of high antigen exposure, the apparent low perforin expression in rectal CD8+ T-cells might be due to frequent release of cytotoxic granules. It has been reported that CD8+ T-cells rapidly up-regulate de novo perforin production upon antigenic stimulation; this de novo perforin protein, not yet associated with cytotoxic granules, can be detected using a recently described monoclonal antibody25. We hypothesized that while rectal CD8+ T-cells maintain low levels of granule-associated perforin, they may rapidly express non-granule-associated perforin upon TCR stimulation. To test this hypothesis, we stimulated lymphocytes from blood and rectal mucosa with HIV peptide pools or Staphylococcal enterotoxin B (SEB) in a 5.5 hour ex vivo stimulation assay25. The de novo perforin response was calculated as the percentage of degranulating CD8+ T-cells (i.e., CD107a+) positive for perforin (Figure 2a).
Figure 2.
Perforin up-regulation in blood and rectal mucosal CD8+ T-cells following TCR stimulation. (a) Representative flow cytometry plot of the de novo perforin response in degranulating (CD107a+) CD8+ T-cells from blood and rectal mucosa. (b) Difference in perforin response to Gag, Nef, and SEB between blood and rectal degranulating (CD107a+) CD8+ T-cells in HIV+ participants (Gag, Nef), and in HIV+ and seronegative participants combined (SEB). (c) Perforin response to Gag, Nef, and SEB in degranulating (CD107a+) rectal CD8+ T-cells across HIV-disease status. Bar heights indicate mean values for each participant group. Wide horizontal bars represent medians; narrow whiskers indicate interquartile ranges; asterisks indicate level of significance as follows: * P <0.05, ** P <0.01, *** P <0.001, **** P <0.0001.
Regardless of antigenic stimulation, the de novo perforin response was generally reduced in rectal CD8+ T-cells compared to blood, reaching statistical significance only in response to SEB stimulation (Figure 2b). When analyzed by HIV disease status, it was apparent that viremic and early infection participant groups had unusually strong Gag-specific perforin responses (Figure 2c). Notably, although controllers displayed strong degranulation in response to Gag, they did not have stronger mucosal perforin responses than other subject groups. Instead, viremic participants had significantly stronger mucosal Gag-specific perforin responses than controllers and treated subjects, and participants in early infection had stronger perforin responses than treated subjects. A similar trend was observed for SEB stimulation (Figure 2c). Taken together, the results presented in Figures 1 and 2 demonstrate that despite strong degranulation, perforin expression is significantly reduced in rectal mucosal CD8+ T-cells relative to blood, both in resting cells and following TCR stimulation, but is elevated in the mucosa of participants with significant viremia.
CD8+ T-cell memory/effector subsets display reduced perforin and GrzB expression relative to blood
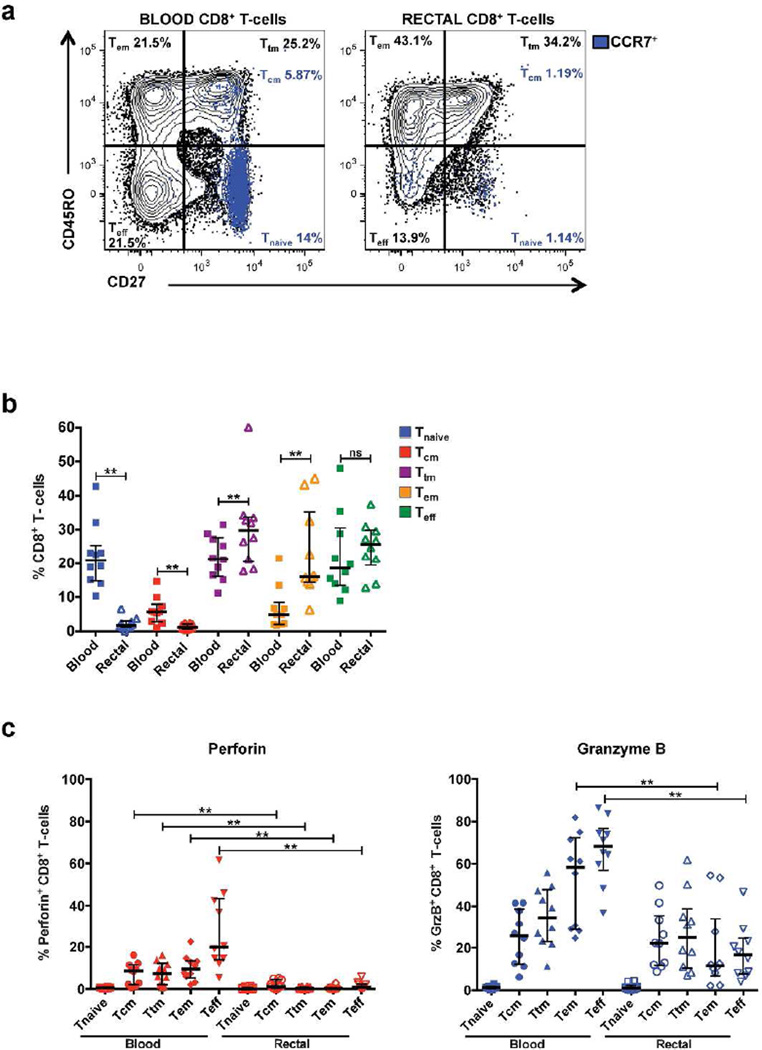
Based upon findings in peripheral blood, it is thought that expression of cytotoxic effector molecules increases with T-cell maturation status, with the highest levels observed in highly differentiated effector T-cells26, 27, 28. Therefore, the differences in perforin and GrzB expression described above might be attributable to the relative abundance of effector CD8+ T-cell memory subsets in rectal mucosa compared to blood. To address this question, we used differentiation markers CD45RO, CCR7, and CD27 to characterize memory subsets in unstimulated blood and rectal CD8+ T-cells as follows: naive (CD45RO− CD27+ CCR7+), central memory (CD45RO+ CD27+ CCR7+), transitional memory (CD45RO+ CD27+ CCR7−), effector memory (CD45RO+ CD27− CCR7−), and effector (CD45RO− CD27− CCR7−)29, 30. We then assessed expression of perforin and GrzB in each of these subsets, without prior stimulation.
No significant differences were observed in the frequencies of memory subsets between HIV-infected and seronegative participants in either compartment; accordingly, all participants were grouped together for further analysis (Supplementary Figure S2). As expected, rectal mucosa contained significantly lower proportions of Tnaive and Tcm and higher proportions of Ttm and Tem CD8+ T-cells compared to blood (Figure 3a–b). Although rectal mucosa displayed increased proportions of Teff, Tem, and Ttm subsets relative to blood, expression of perforin and GrzB within these subsets was consistently reduced compared to blood (Figure 3c). Thus, irrespective of differentiation status, CD8+ T-cells in rectal mucosa were uniformly low in perforin and GrzB compared to their blood counterparts.
Figure 3.
Reduced expression of perforin and GrzB in rectal mucosal CD8+ T-cell memory/effector populations compared to blood. (a) Representative flow cytometry plot displaying surface staining for the memory differentiation markers CD45RO, CD27, and CCR7 in blood and rectal CD8+ T-cells. (b) Frequency of memory/effector subsets within blood and rectal CD8+ T-cell populations in a consolidated group of HIV+ and seronegative participants. (c) Distribution of perforin and GrzB expression in blood and rectal CD8+ T-cell memory/effector subsets. Wide horizontal bars represent medians; narrow whiskers indicate interquartile ranges; asterisks indicate level of significance as follows: * P <0.05, ** P <0.01, *** P <0.001, **** P <0.0001.
CD8+ T-cell cytotoxic capacity is reduced in rectal mucosa compared to blood, irrespective of HIV disease status
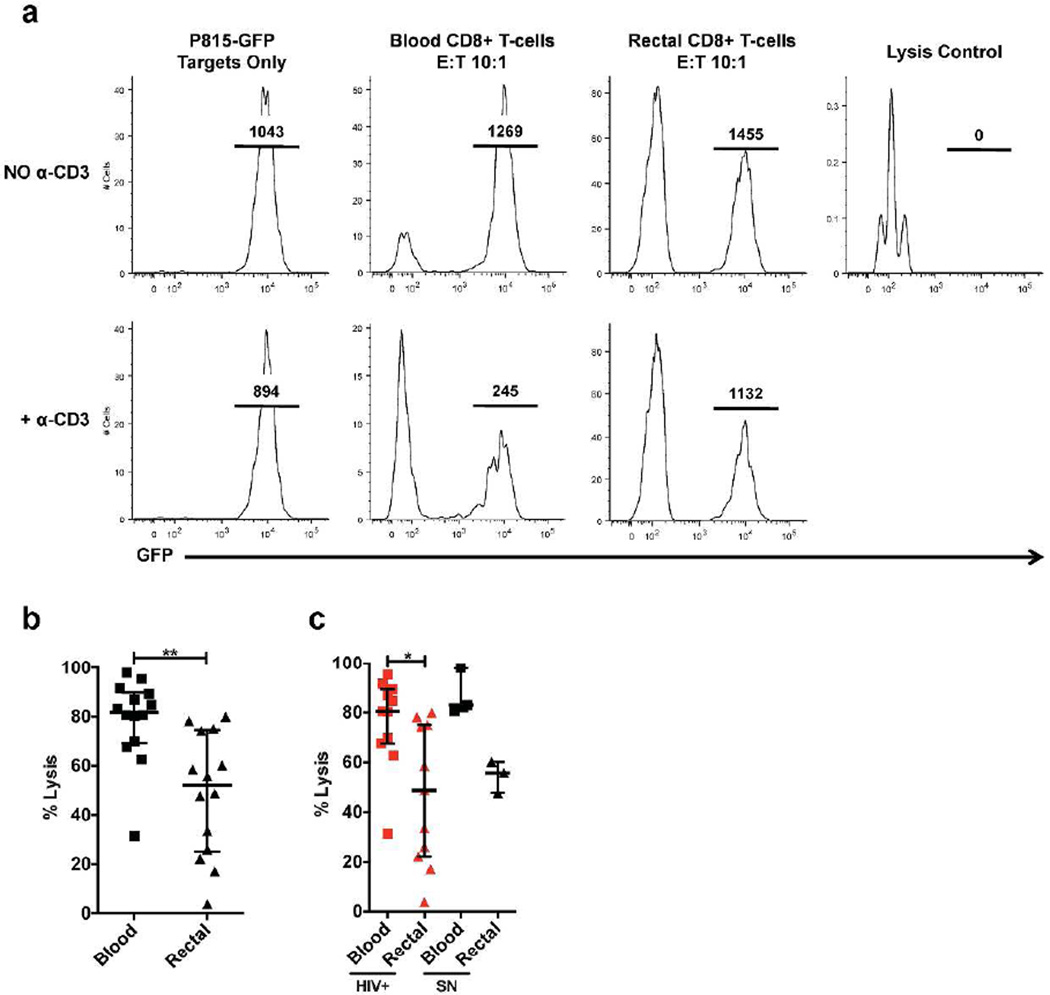
Low expression of perforin and GrzB in rectal CD8+ T-cells led us to hypothesize that rectal CD8+ T-cells might have reduced cytotoxic capacity relative to blood CD8+ T-cells. However, directly addressing this question with traditional cytotoxicity assays was problematic due to cell number: yields of viable CD8+ T-cells recovered from 20 rectal biopsies are in the range of 0.46–1.89 × 106 CD8+ T-cells (median 0.88 × 106; these authors, unpublished data). Therefore, to investigate CD8+ T-cell killing ability we utilized a flow cytometry-based redirected lysis assay31.
Both blood and rectal CD8+ T-cells exhibited redirected lysis of GFP-expressing target cells; however, target cell lysis was significantly reduced when rectal CD8+ T-cells were used as effector cells compared to blood CD8+ T-cells (Figure 4a–b). This trend was conserved across HIV disease status; no significant differences were observed between participant groups (data not shown), or between HIV+ and seronegative participants (Figure 4c). To control for any potential impact of the enzymatic digestion used to isolate mucosal CD8+ T-cells, we also performed these experiments using blood CD8+ T-cells that had been pre-treated with Liberase and did not detect a significant impact of this treatment on cytotoxicity (data not shown).
Figure 4.
Cytotoxicity was reduced in CD8+ T-cells from rectal mucosa compared to blood in a redirected lysis assay. (a) Representative flow cytometry plot of rectal and blood CD8+ T-cell-mediated lysis of GFP+ target cells at an E:T ratio of 10:1 in the presence of anti-CD3. Lysis is indicated by a reduction in P815 GFP+ target cells with the addition of anti-CD3. Histogram plots display the frequency of live cells expressing GFP. Numbers over bars indicate the count of P815 GFP+ target cells (b) Rectal CD8+ T-cells displayed a significant (P = 0.0052) reduction in killing ability compared to blood CD8+ T-cells. (c) Findings summarized by HIV status: HIV+ versus seronegative (SN). No significant differences were detected in killing capacity between HIV disease progression groups (viremics, controllers, ART-treated, seronegatives); however, this issue could not be fully explored due to subject recruitment limitations. Wide horizontal bars represent medians; narrow whiskers indicate interquartile ranges; asterisks indicate level of significance as follows: * P <0.05, ** P <0.01, *** P <0.001, **** P <0.0001.
Protein and mRNA levels of T-bet and Eomesodermin are reduced in rectal mucosa compared to blood, irrespective of disease status
To elucidate mechanisms contributing to reduced perforin and GrzB expression in rectal CD8+ T-cells, we investigated expression of T-bet and Eomesodermin (Eomes), two key transcription factors regulating expression of perforin and GrzB in cytotoxic CD8+ T-cells28, 32, 33. A previous study reported TGF-β-dependent reduction of T-bet expression in human gut CD4+ T-cells34; accordingly, we predicted that expression of T-bet and Eomes would likely be reduced in rectal CD8+ T-cells compared to blood.
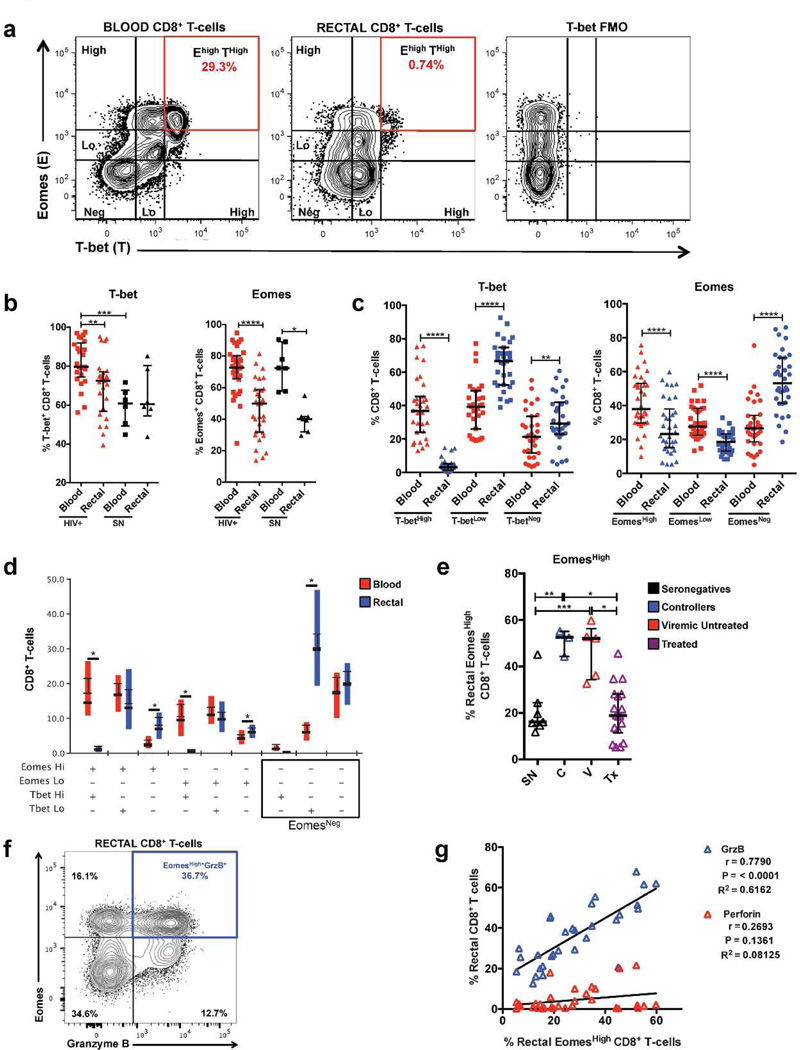
In agreement with earlier findings in blood35, flow cytometric analysis revealed CD8+ T-cell populations expressing high and low levels of T-bet; a similar trend was observed for Eomes (Figure 5a). In HIV+ participants, frequencies of T-bet+ and Eomes+ CD8+ T-cells were significantly reduced in rectal mucosa compared to blood. In seronegative participants, this trend was only observed for Eomes (Figure 5b). T-bet and Eomes transcript levels were also reduced in rectal CD8+ T-cells compared to blood (Supplementary Figure S1a–b). We next compared the frequencies of CD8+ T-cells expressing high or low levels of these transcription factors in blood and mucosa, consolidating all participant groups. In this analysis, the proportions of CD8+ T-cells expressing high levels of Eomes or T-bet were significantly reduced in rectal mucosa compared to blood; the difference in T-betHigh expression was particularly striking. The proportion of T-betLow rectal CD8+ T-cells was significantly greater than in blood. In contrast, the proportion of EomesLow CD8+ T-cells in rectal mucosa was reduced compared to blood (Figure 5c). Analysis of T-bet and Eomes co-expression patterns confirmed that, in contrast to blood, a large proportion of rectal CD8+ T-cells were T-betLowEomesNeg (Figure 5d). Thus, irrespective of HIV-disease status, rectal CD8+ T-cells displayed significantly reduced levels of T-bet and Eomes expression compared to blood.
Figure 5.
T-bet and Eomesodermin are differentially expressed in blood and rectal CD8+ T-cells. (a) Representative flow cytometry plot of data from a seronegative participant showing high and low fluorescence intensities of T-bet and Eomesodermin (Eomes) in blood and rectal CD8+ T-cells, highlighting the reduction in frequency of T-betHighEomesHigh CD8+ T-cells in rectal mucosa compared to blood. (b) Differences in the frequencies of total T-bet+ and Eomes+ CD8+ T-cells between blood and rectal mucosa and between HIV+ and seronegative participants. (c) Differences in the frequencies of CD8+ T-cells with T-bet and Eomes high and low expression intensities in blood and rectal mucosa. (d) Difference in T-bet and Eomes co-expression patterns in blood and rectal CD8+ T-cells. (e) Frequency of EomesHigh CD8+ T-cells in rectal mucosa across HIV disease status. Similar results were observed in blood (data not shown). (f) Representative flow cytometry plot showing co-expression of EomesHigh and GrzB in rectal mucosal CD8+ T-cells. (g) Spearman correlation analysis relating the frequency of EomesHigh CD8+ T-cells with the frequency of CD8+ T-cells expressing perforin or GrzB in rectal mucosa. Wide horizontal bars represent medians; narrow whiskers indicate interquartile ranges; asterisks indicate level of significance as follows: * P <0.05, ** P <0.01, *** P <0.001, **** P <0.0001.
A previous study observed an association between T-bet expression in blood CD8+ T-cells and HIV control35; accordingly, we looked for similar correlates in the rectal mucosa. We did not detect significant differences in T-bet expression in rectal CD8+ T-cells among HIV+ participant groups (data not shown). In contrast, the frequency of EomesHigh CD8+ T-cells in rectal mucosa was significantly greater in HIV controllers and viremic participants compared to treated and seronegative participants (Figure 5e).
We identified a strong positive correlation between the frequency of GrzB+ CD8+ T-cells and EomesHigh CD8+ T-cells in rectal mucosa; co-expression analysis confirmed that rectal GrzB+ CD8+ T-cells were mainly EomesHighT-betLowPerforinNeg, particularly in HIV+ participants (Figure 5f–g, Supplementary Figure S3. In contrast, there was no correlation between perforin and EomesHigh CD8+ T-cells in either blood or rectal mucosa (Figure 5f–g, Supplementary Figure S4). Blood perforin+ CD8+ T-cells were mainly GrzB+T-betHigh. Rectal perforin+ CD8+ T-cells were often GrzB+ but lacked a single definitive T-bet/Eomes co-expression pattern, likely due to ‘noise’ generated by the low frequencies of perforin+ CD8+ T-cells (Figure 5f–g, Supplementary Figure S3). Thus, although Eomes expression was reduced in rectal mucosa relative to blood, its expression was elevated in rectal mucosa of HIV-infected participants not on therapy and strongly correlated with expression of GrzB, but not perforin, in this tissue.
In vitro TGF-β neutralization increases perforin expression in rectal CD8+ T-cells
TGF-β is an immunomodulatory cytokine whose expression in the gastrointestinal mucosa is involved in establishing peripheral tolerance4, 5, 34, 36 and development of tissue-resident T-cells2, 37, 38. In conjunction with other cytokines, TGF-β expression induces the down-regulation of transcription factors T-bet and Eomes and up-regulation of the mucosal retention integrin αEβ72, 7, 37. In vitro data also suggest a role for TGF-β in reducing cytotoxicity of CD8+ T-cells39. Therefore, we hypothesized that reduction of T-bet, Eomes, Perforin, and GrzB in rectal CD8+ T-cells might be due in part to TGF-β. We tested whether in vitro neutralization of TGF-β in short-term cultures of rectal leukocytes would restore expression of cytotoxic effectors and transcription factors. Freshly isolated rectal mononuclear cells were incubated for 24h with PBS, IgG isotype control, or a ‘pan-neutralizing’ antibody directed towards TGF-β as previously described34. Following incubation, cells were stained intracellularly for effector molecules and analyzed via flow cytometry. Treatment with anti-TGF-β significantly increased both the frequency and fluorescence intensity of perforin in rectal CD8+ T-cells compared to PBS and the isotype-matched control antibody (Supplementary Figure S5). Interestingly, the increase in perforin protein was not accompanied by a detectable increase in expression of T-bet, Eomes, or GrzB (data not shown).
DISCUSSION
Numerous studies have suggested a relationship between CD8+ T-cell function and immune control of HIV infection9, 33. The vast majority of these studies have focused on blood T-cells; however, tissues including lymph nodes and the gastrointestinal mucosa are major sites of HIV replication, and T-cells in these tissues differ in phenotype and function from their blood counterparts18, 40. The GI tract is important in HIV pathogenesis for several reasons: first, it serves as a major portal of entry for HIV; second, perturbation of mucosal barrier integrity in acute HIV-infection leads to translocation of microbial products that increase immune activation; third, HIV persists in the GI mucosa throughout chronic infection8. Because perforin-mediated cytotoxicity is thought to be the major means by which CD8+ T-cells eradicate virally infected cells, characterizing this ability in rectal T-cells was the primary aim of this study.
In all participant categories studied, the proportion of CD8+ T-cells expressing perforin was reduced in rectal mucosa compared to blood. However, as a group, HIV+ individuals had higher proportions of perforin+ mucosal CD8+ T-cells than seronegative participants. In chronic HIV infection, Gag-specific mucosal perforin production was significantly greater among viremic individuals not on antiretroviral therapy (ART) compared to HIV controllers and participants on ART. This differs from published findings in which elite controllers displayed stronger HIV-specific perforin responses in blood compared to viremic controllers and chronic progressors12. Taken together, our data suggest several possible interpretations: first, during chronic HIV infection, perforin-mediated cytotoxicity may not be the major CD8+ T-cell defense mechanism within the gastrointestinal mucosa; second, and more broadly, antigen-specific CD8+ T-cells in the GI tract might rely on perforin-independent mechanisms, such as engagement of death receptors, to kill target cells; third, antigen-specific CD8+ T-cells in the GI tract might be intrinsically biased towards cytokine/chemokine release rather than robust cytotoxicity8, 9.
In support of the interpretation that mucosal CD8+ T-cells are skewed towards cytokine production rather than a cytotoxic response, previous studies detected robust polyfunctional HIV Gag-specific responses, including degranulation and production of cytokines (IL-2, IFNγ, TNFα) and chemokines (MIP-1β), by mucosal CD8+ T-cells; these responses were strongest in HIV controllers compared to viremic untreated participants and subjects on ART24, 41.
Individuals with early HIV infection had the highest median percentages of mucosal CD8+ T-cells expressing perforin in response to Gag stimulation. This observation parallels results from a SIV-infection model, in which perforin protein and mRNA peaked in the colon during early infection, returning to low levels during chronic infection40. Similar observations have been made in human duodenal mucosa during acute HIV infection22. The contrast in cytotoxic phenotype between HIV/SIV-specific mucosal cells during acute/early versus chronic infection suggests a model in which antigen-specific cells with a more cytotoxic phenotype migrate rapidly to the gut from the periphery during acute/early infection. After clearance of acute phase viremia, some of these cells may remain in the GI tract, where they are exposed to cytokines that induce a tissue resident memory phenotype. It should also be noted that our findings are limited by sample size, and larger enrollment might reveal additional differences between groups. Further studies of acute/early infection will be required to address these questions in greater detail.
Extending previous studies, we also observed a reduction in the proportion of rectal CD8+ T-cells expressing GrzB compared to blood24, but an increase in GrzB expression in the mucosa of HIV+ compared to seronegative participants. Site-specific contrasts in cytotoxic protein expression levels could not be explained by differences in the relative frequencies of effector T-cells (Teff and Tem) between the two compartments. In fact, although rectal mucosa had higher proportions of effector T-cells compared to blood, expression of perforin and GrzB within each memory subset was reduced in mucosa compared to blood. Taken together, these datasets suggest a model in which mucosal T-cells limit production of perforin, perhaps to avoid ‘collateral damage’ to sensitive mucosal tissues. Nevertheless, perforin expression in the mucosa can be increased in response to infection, as observed in HIV/SIV.
Interestingly, although expression of both perforin and GrzB in rectal CD8+ T-cells was low compared to blood, a higher proportion of rectal CD8+ T-cells expressed GrzB compared to perforin; a similar trend has been observed for granzyme A19. CD8+ T-cells expressing granzymes in the absence of perforin have been identified in blood and displayed impaired cytotoxicity compared to perforin+ cells27. As perforin is considered a limiting factor for cytotoxicity and GrzB does not induce apoptosis in the absence of perforin10, these observations highlight critical questions regarding the contents of cytotoxic granules and the role of the robust degranulation observed in rectal mucosal CD8+ T-cells. Although perforin and GrzB are important for classical, MHC class I-restricted cytotoxicity, alternative functions for granzymes have been postulated including cleavage of extracellular matrix proteins, facilitating cellular migration and promoting local inflammation42, 43, 44, 45. Cytotoxic granules also contain non-cytotoxic, anti-HIV molecules such as β-chemokines MIP-1α and MIP-1β33. The hypothesis that cytotoxic granule constituents function in host defense either independently of perforin or in the presence of sublytic levels of perforin in the rectal mucosa, is intriguing and warrants further study, particularly as the gastrointestinal mucosa is a tissue with high exposure to microbes and potential for perforin-mediated ‘collateral’ damage.
Rectal CD8+ T-cells displayed reduced killing capacity in a redirected lysis assay compared to blood, further supporting the interpretation that mucosal CD8+ T-cells are not ‘programmed’ primarily for cytotoxicity. However, rectal CD8+ T-cells did demonstrate some killing ability. Whether the observed lysis was mediated by serial killing by a few perforin+ effectors, levels of perforin below our limit of detection, or driven by other mechanisms involving engagement of death receptors, will require further study. It was not possible to directly test the ability of HIV-specific rectal CD8+ T-cells to kill target cells expressing HIV antigens, due to low numbers of cells obtained from rectal biopsies of human subjects.
Consistent with the interpretation that perforin-mediated cytotoxicity is suppressed in rectal mucosa, expression of two transcription factors critical for perforin expression, T-bet and Eomes, were also reduced in rectal CD8+ T-cells compared to blood. Notably, T-betHigh CD8+ T-cells were nearly absent from rectal mucosa, and the elevated perforin expression observed in early HIV infection and chronic viremia was not accompanied by an increase in T-bet expression. A large proportion of rectal CD8+ T-cells displayed a T-betLowEomesNeg phenotype, a profile characteristic of tissue-resident T-cells promoted in part by exposure to TGF-β37. Unlike T-bet expression, which did not vary with HIV-disease status, we observed an increase in the frequency of EomesHigh CD8+ T-cells in rectal mucosa of controllers and viremic untreated participants compared to subjects on ART and seronegatives. High Eomes expression in conjunction with PD-1 expression phenotypically represents a subset of terminally ‘exhausted’ T-cells46; accordingly, our observation may represent an increased proportion of exhausted CD8+ T-cells in rectal mucosa of HIV+ subjects not on ART and is the subject of ongoing research. High Eomes expression in untreated HIV+ subjects may explain greater GrzB expression in these individuals, as Eomes and GrzB were co-expressed and positively correlated. In contrast, no correlation was found between Eomes and perforin, supporting the notion that perforin and GrzB are regulated differently in the rectal mucosa.
Blocking TGF-β in culture increased perforin expression in rectal CD8+ T-cells with no observable difference in GrzB, T-bet, or Eomes expression, suggesting that although TGF-β is likely involved in perforin suppression, other cytokines, transcription factors, and/or post-transcriptional mechanisms are likely also involved in suppression of the cytotoxic phenotype in the rectal mucosa28, 47.
Taken together, this work suggests the majority of human rectal CD8+ T-cells may be ‘programmed’ primarily as non-cytotoxic effectors rather than as serial killers of infected host cells. The abundance of rectal mucosal CD8+ T-cells with a T-betLowEomesNeg phenotype suggests a large population of tissue resident cells in the rectal mucosa and may explain the phenotypic and functional differences between blood and rectal CD8+ T-cells. The work described in this paper has important implications for our understanding of adaptive immunity in the gastrointestinal tract, as well as for the design of vaccines targeted to mucosal tissues. It will be important to determine how to induce and modulate the responsiveness of these mucosal effector populations in order to design improved therapies and vaccines against mucosal pathogens.
METHODS
Participants and Sample Collection
HIV-1 positive and seronegative participants were enrolled through the SCOPE and Options studies based at San Francisco General Hospital. Participants were characterized as one of four groups defined by plasma viral load (VL) and antiretroviral therapy (ART) as follows: Controllers (C) maintained VL consistently < 2,000 copies/mL without ART; Viremic (V) subjects maintained VL ≥ 2,000 copies/mL without ART; ART treated (Tx) participants had VL < 40 copies/mL; and HIV-1 seronegative (N). Participants within the first year of HIV infection were characterized as Early Infection (E) regardless of viral load. Written informed consent for phlebotomy and rectal biopsy was obtained through protocols approved by the Committee on Human Subjects Research, School of Medicine, University of California, San Francisco [Protocols #10-01218, 10-00263 and 10-01330]. Twenty to 40 ml of blood was collected by sterile venipuncture. Twenty-four to 30 rectal biopsies were obtained by sigmoidoscopy approximately 10–30cm from the anal verge9. Biopsies were placed in 50mL conical tubes with 15mL of R-15 [RPMI-1640 containing fetal calf serum (15%), penicillin (100U/mL), streptomycin (100 mg/mL), and glutamine (2 mM)]. Blood and biopsy samples were transported at room temperature to the laboratory for immediate processing.
Blood and Tissue Processing
Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll-Paque ™ (Pfizer-Pharmacia, New York, NY) and rested overnight in R-15 at 37°C, 5% CO2. Rectal mucosal mononuclear cells (RMMCs) were isolated from biopsies using enzymatic and mechanical disruption as previously described48. Briefly, biopsies were subjected to shaking incubation at 37°C for 30 minutes in 25mg/mL Liberase DL (Roche, Indianapolis, IN) followed by passage through a 16-gauge blunt end needle to disrupt tissue. Following disruption, free cells were collected through a sterile 70µm cell strainer. The disruption process was repeated until all biopsy tissue was digested. Free RMMCs were washed three times in 20mL of R-15 and rested overnight at 37°C, 5% CO2 in R-15 supplemented with 200× Zozyn (Pfizer-Pharmacia).
CD8+ T-cell Isolation
Live CD8+ T-cells were isolated from fresh RMMCs and PBMCs using commercial on-column kits in accordance to manufacturers’ protocols. Briefly, live cells were isolated using the Dead Cell Removal Kit (Miltenyi Biotec, Auburn, CA). CD8+ T-cells were then negatively isolated from live RMMC and PBMCs using the CD8+ T-cell Isolation Kit, Human (Miltenyi).
P815 and P815-GFP Culture
P815 and GFP-labeled P815 were a gift from Drs. Norbert Kienzle (University of Queensland, Brisbane, Australia) and Dorothy Hudig (University of Nevada, Reno NV). Puromycin resistant GFP-labeled P815 mouse mastocytoma cells (P815-GFP) were maintained in DMEM [DMEM/F-12 (1:1) (Fischer, Waltham, MA), fetal calf serum (10%), penicillin (100U/ml), streptomycin (100 mg/ml), and glutamine (2 mM)] supplemented with 2ug/mL puromycin (Sigma Aldrich, St. Louis, MO). Unlabeled P815 cells were maintained in DMEM in the absence of puromycin. Thawed P815-GFP cells were grown in media alone for a two-day lag period before addition of puromycin to allow for re-expression of the puromycin resistance gene.31
Antibodies and Peptide Pools
The following fluorochrome-labeled monoclonal antibodies were used in flow cytometry: CD107a (H4A3: PE-Cy5, PE-Cy7), CD8 (SK1: APC-H7, FITC), CCR7 (3D12: PE-Cy7), Granzyme B (BG11: V450) from BD Biosciences (San Jose, CA); CD4 (T4D11: ECD) from Beckman Coulter (Brea, CA); CD4 (RPA-TA: BV785), CD45RO (UCHL1: BV785), CD27 (O323: BV650), Granzyme A (CB9: Ax647), CD3 (UCHT-1: Ax700), and T-bet (4B10: BV711) from Biolegend (San Diego, CA). CD3 (S4.1: Qdot655) was purchased from Invitrogen (Carlsbad, CA). T-bet (4B10: Ax647); unlabeled CD28 (L293), and CD49d (L25) were from BD Pharmingen (San Diego, CA). Eomesodermin (WD1928: PE-eFluor610) and unlabeled functional grade CD3 (OKT3) were from eBiosciences (San Diego, CA). Perforin (B-D48: PE) was from Cell Sciences (Canton, MA). Unlabeled pan anti-TGF-β (Cat No. T9429) and rabbit IgG (Cat No. I5006) were from Sigma Aldrich. The HIV Gag peptide pool (p55, HXB2 sequence) consisted of 15-mers with an 11 amino acid overlap (BD Biosciences). HIV Nef peptide pool (PepMix™ HIV NEF Ultra, JPT Peptide Technologies, Berlin, Germany) consisted of 150 15-mers with an 11 amino acid overlap. Staphylococcal enterotoxin B (SEB) was from Sigma Aldrich.
Antigen Stimulation and Intracellular Cytokine Staining (ICS)
Staining was performed as previously described with slight modifications24 on freshly isolated blood and rectal mononuclear cells or isolated CD8+ T-cells rested overnight at 37°C, 5% CO2. For stimulation assays, cells were incubated at a concentration of 2×10^6 cells per 200µL R-15 for 5.5 hours in the presence of CD28 (1µg/mL) and CD49d (1µg/mL) co-stimulation, anti-CD107a, 1µM GolgiStop™ (BD Biosciences), brefeldin A (5µg/mL, Sigma Aldrich) and the appropriate antigenic stimulation: Gag-peptide pool (3.5µg/mL), Nef-peptide pool (3.5µg/mL), Staphylococcal enterotoxin B (5µg/mL), or DMSO (peptide solvent) as the negative control. Following incubation, cells were stained for surface markers and viability (Aqua Dead cell stain kit, Invitrogen) for 20 minutes at room temperature. Cells were then fixed in 4% paraformaldehyde and permeabilized using FACS Perm 2 (BD Biosciences) prior to intracellular staining for CD3, perforin and GrzB. When staining for transcription factors T-bet and Eomesodermin, the Transcription Factor Buffer Set (BD Biosciences) was used as follows: cells were fixed with Transcription Factor Fix/Perm Buffer for 40 minutes at 4°C, washed twice in Transcription Factor Perm/Wash Buffer, and stained with antibodies for 40 minutes at 4°C in Transcription Factor Perm/Wash. Regardless of fixation and permeabilization method, cells were re-suspended in 1% paraformaldehyde and stored at 4°C in the dark until analysis.
TGF-β Neutralization
TGF-β neutralization was done as previously described, with slight modifications34. Five ×10^5 freshly isolated RMMCs were incubated for 24 hours at 37°C, 5% CO2 in 200µL R-15 in the presence of: 20µg/mL pan anti-TGF-β, 20µg/mL rabbit IgG isotype control, or 1× PBS (antibody diluent). Following incubation, cells were washed in cold 1× PBS and stained for surface and intracellular markers as described above, then stored in 1% paraformaldehyde until analysis.
Redirected Lysis Assay
Assays were performed on cells rested overnight at 37°C, 5% CO2 as previously described, with modifications31. Briefly, P815-GFP cells were incubated for 30 minutes at 4°C, protected from light, in the presence or absence of 5µg/mL functional grade anti-CD3. Anti-CD3-coated and uncoated P815-GFP target cells were co-incubated with CD8+ T-cell Effectors from blood or mucosal tissue at an E:T ratio of 10:1 in 500uL of R-15, for 5 hours at 37°C, 5% CO2. Targets incubated in the absence of Effectors or in the presence of 0.05% Tween-20 served as controls. Following incubation, the cells were washed in 1× PBS and stained for viability for 20 minutes at room temperature, then washed in FACS buffer [1× PBS, fetal calf serum (2%)], and stored in 1% paraformaldehyde at 4°C until analysis. The percent lysis was calculated as 100×[(number of live GFP+ cellsE:T −number of live GFP+ cellsE:T +αCD3) ÷ number of live GFP+ cellsE:T].
qRT-PCR
Negatively selected blood and rectal mucosa CD8+ T-cells underwent a 2.5-hour stimulation assay as described above. Following incubation, cells were washed once in cold 1× PBS and lysed per Qiagen RNA Mini kit protocol (Qiagen, Hilden, Germany). Cell lysates were submitted to the UC Davis Real-Time PCR Research and Diagnostics Core Facility for RNA extraction and real-time qPCR using TaqMan gene expression assays (Applied Biosystems, Foster City, CA) for perforin (Hs00169473_m1), Granzyme B (Hs01554355_m1), T-bet (Hs00203436_m1), Eomesodermin (Hs00172872_m1), and 18S rRNA (Hs99999901_s1). Transcript levels were determined using the delta Ct method with 18S rRNA as the reference gene49.
Data Acquisition and Analysis
Flow cytometry data were acquired using an LSR II running FACSDiva™ software (Becton Dickinson Immuno-cytometry Systems) within 24h of staining completion. Flow cytometric data analysis was performed using Flowjo software (Flowjo LLC, Ashland, Or). An in-house statistical algorithm was used to determine whether antigen-specific responses differed significantly from the negative control24. For responses deemed statistically significant, net responses were then calculated by subtracting the negative control values from antigen-specific responses. SPICE was used to visualize multifunctional T-cell populations50. Statistical analyses were performed with GraphPad Prism V.6 (Graphpad Software, San Diego, CA) or SPICE. Non-parametric statistical tests included Wilcoxon matched-pairs signed rank test for paired samples, Mann-Whitney test for unpaired samples, Spearman correlation and linear regression analysis.
Supplementary Material
Acknowledgments
The authors thank Rebecca Hoh, Montha Pao, Monika Deswal and the clinical staff at San Francisco General Hospital for their assistance with participant recruitment and sample collection. We thank the participants for their willingness to contribute to this study. We thank Drs. Norbert Kienzle, (University of Queensland, Brisbane Australia) and Dorothy Hudig (University of Nevada, Reno NV) for their gift of P815-GFP cells. This work was supported primarily by NIH/NIAID R01-AI057020 to BLS. The UC Davis Flow Cytometry Shared Resource Laboratory (FCSR) was supported by grants from the National Institutes of Health: NCI P30 CA0933730, and NIH NCRR C06-RR12088, S10-RR12964, S10-RR026825 and S10-OD018223-01A1; and from the James B. Pendleton Charitable Trust. We thank the FCSR staff members Ms. Bridget McLaughlin and Mr. Jonathan Van Dyke, for assistance with flow cytometry.
Footnotes
DISCLOSURE
The authors report no conflicts of interest that could be perceived to bias this work.
AUTHOR CONTRIBUTIONS
Project concept and direction: BLS with consultation from PWH and SGD. Characterization of participant cohort and procurement of clinical samples: MS, PWH, FH, SGD. Laboratory assays: BEK with assistance from AG and JWC. Data analysis: BEK, BLS. Manuscript writing and editing: BEK, BLS. All authors read and approved the manuscript.
Literature Cited
- 1.Thome JJ, et al. Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell. 2014;159:814–828. doi: 10.1016/j.cell.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casey KA, et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. Journal of immunology. 2012;188:4866–4875. doi: 10.4049/jimmunol.1200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masopust D, Vezys V, Wherry EJ, Barber DL, Ahmed R. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. Journal of immunology. 2006;176:2079–2083. doi: 10.4049/jimmunol.176.4.2079. [DOI] [PubMed] [Google Scholar]
- 4.Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nature reviews. Immunology. 2003;3:331–341. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- 5.Smythies LE, et al. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005;115:66–75. doi: 10.1172/JCI19229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huff KR, et al. Extracellular matrix-associated cytokines regulate CD4+ effector T-cell responses in the human intestinal mucosa. Mucosal Immunol. 2011;4:420–427. doi: 10.1038/mi.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schenkel JM, Masopust D. Tissue-resident memory T cells. Immunity. 2014;41:886–897. doi: 10.1016/j.immuni.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shacklett BL, Anton PA. HIV Infection and Gut Mucosal Immune Function: Updates on Pathogenesis with Implications for Management and Intervention. Curr Infect Dis Rep. 2010;12:19–27. doi: 10.1007/s11908-009-0072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shacklett BL, Ferre AL. Mucosal immunity in HIV controllers: the right place at the right time. Curr Opin HIV AIDS. 2011;6:202–207. doi: 10.1097/COH.0b013e3283453e2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thiery J, Lieberman J. Perforin: a key pore-forming protein for immune control of viruses and cancer. Subcell Biochem. 2014;80:197–220. doi: 10.1007/978-94-017-8881-6_10. [DOI] [PubMed] [Google Scholar]
- 11.Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. Journal of virology. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hersperger AR, et al. Perforin expression directly ex vivo by HIV-specific CD8 T-cells is a correlate of HIV elite control. PLoS pathogens. 2010;6:e1000917. doi: 10.1371/journal.ppat.1000917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Migueles SA, et al. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nature immunology. 2002;3:1061–1068. doi: 10.1038/ni845. [DOI] [PubMed] [Google Scholar]
- 14.Migueles SA, et al. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity. 2008;29:1009–1021. doi: 10.1016/j.immuni.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogg GS, et al. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 16.Saez-Cirion A, et al. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc Natl Acad Sci U S A. 2007;104:6776–6781. doi: 10.1073/pnas.0611244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shankar P, Xu Z, Lieberman J. Viral-specific cytotoxic T lymphocytes lyse human immunodeficiency virus-infected primary T lymphocytes by the granule exocytosis pathway. Blood. 1999;94:3084–3093. [PubMed] [Google Scholar]
- 18.Andersson J, et al. Perforin is not co-expressed with granzyme A within cytotoxic granules in CD8 T lymphocytes present in lymphoid tissue during chronic HIV infection. Aids. 1999;13:1295–1303. doi: 10.1097/00002030-199907300-00005. [DOI] [PubMed] [Google Scholar]
- 19.Shacklett BL, et al. Abundant expression of granzyme A, but not perforin, in granules of CD8+ T cells in GALT: implications for immune control of HIV-1 infection. Journal of immunology. 2004;173:641–648. doi: 10.4049/jimmunol.173.1.641. [DOI] [PubMed] [Google Scholar]
- 20.Reeves RK, et al. CD16- natural killer cells: enrichment in mucosal and secondary lymphoid tissues and altered function during chronic SIV infection. Blood. 2010;115:4439–4446. doi: 10.1182/blood-2010-01-265595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nancey S, et al. CD8+ cytotoxic T cells induce relapsing colitis in normal mice. Gastroenterology. 2006;131:485–496. doi: 10.1053/j.gastro.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 22.Epple HJ, et al. Acute HIV infection induces mucosal infiltration with CD4+ and CD8+ T cells, epithelial apoptosis, and a mucosal barrier defect. Gastroenterology. 2010;139:1289–1300. doi: 10.1053/j.gastro.2010.06.065. [DOI] [PubMed] [Google Scholar]
- 23.Ferre AL, et al. Immunodominant HIV-specific CD8+ T-cell responses are common to blood and gastrointestinal mucosa, and Gag-specific responses dominate in rectal mucosa of HIV controllers. Journal of virology. 2010;84:10354–10365. doi: 10.1128/JVI.00803-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Critchfield JW, et al. Magnitude and complexity of rectal mucosa HIV-1-specific CD8+ T-cell responses during chronic infection reflect clinical status. PloS one. 2008;3:e3577. doi: 10.1371/journal.pone.0003577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hersperger AR, Makedonas G, Betts MR. Flow cytometric detection of perforin upregulation in human CD8 T cells. Cytometry. Part A : the journal of the International Society for Analytical Cytology. 2008;73:1050–1057. doi: 10.1002/cyto.a.20596. [DOI] [PubMed] [Google Scholar]
- 26.Chattopadhyay PK, et al. The cytolytic enzymes granyzme A, granzyme B, perforin: expression patterns, cell distribution, and their relationship to cell maturity and bright CD57 expression. Journal of leukocyte biology. 2009;85:88–97. doi: 10.1189/jlb.0208107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harari A, Bellutti Enders F, Cellerai C, Bart PA, Pantaleo G. Distinct profiles of cytotoxic granules in memory CD8 T cells correlate with function, differentiation stage, and antigen exposure. Journal of virology. 2009;83:2862–2871. doi: 10.1128/JVI.02528-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pipkin ME, Rao A, Lichtenheld MG. The transcriptional control of the perforin locus. Immunological reviews. 2010;235:55–72. doi: 10.1111/j.0105-2896.2010.00905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamann D, et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186:1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mackay CR. Dual personality of memory T cells. Nature. 1999;401:659–660. doi: 10.1038/44309. [DOI] [PubMed] [Google Scholar]
- 31.Kienzle N, Olver S, Buttigieg K, Kelso A. The fluorolysis assay, a highly sensitive method for measuring the cytolytic activity of T cells at very low numbers. Journal of immunological methods. 2002;267:99–108. doi: 10.1016/s0022-1759(02)00150-3. [DOI] [PubMed] [Google Scholar]
- 32.Glimcher LH, Townsend MJ, Sullivan BM, Lord GM. Recent developments in the transcriptional regulation of cytolytic effector cells. Nature reviews. Immunology. 2004;4:900–911. doi: 10.1038/nri1490. [DOI] [PubMed] [Google Scholar]
- 33.Demers KR, Reuter MA, Betts MR. CD8(+) T-cell effector function and transcriptional regulation during HIV pathogenesis. Immunological reviews. 2013;254:190–206. doi: 10.1111/imr.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Sabatino A, et al. Blockade of transforming growth factor beta upregulates T-box transcription factor T-bet, and increases T helper cell type 1 cytokine and matrix metalloproteinase-3 production in the human gut mucosa. Gut. 2008;57:605–612. doi: 10.1136/gut.2007.130922. [DOI] [PubMed] [Google Scholar]
- 35.Hersperger AR, et al. Increased HIV-specific CD8+ T-cell cytotoxic potential in HIV elite controllers is associated with T-bet expression. Blood. 2011;117:3799–3808. doi: 10.1182/blood-2010-12-322727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oh SA, Li MO. TGF-beta: guardian of T cell function. Journal of immunology. 2013;191:3973–3979. doi: 10.4049/jimmunol.1301843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mackay LK, et al. T-box Transcription Factors Combine with the Cytokines TGF-beta and IL-15 to Control Tissue-Resident Memory T Cell Fate. Immunity. 2015;43:1101–1111. doi: 10.1016/j.immuni.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 38.Skon CN, et al. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol. 2013;14:1285–1293. doi: 10.1038/ni.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rich S, Seelig M, Lee HM, Lin J. Transforming growth factor beta 1 costimulated growth and regulatory function of staphylococcal enterotoxin B-responsive CD8+ T cells. Journal of immunology. 1995;155:609–618. [PubMed] [Google Scholar]
- 40.Quigley MF, et al. Perforin expression in the gastrointestinal mucosa is limited to acute simian immunodeficiency virus infection. Journal of virology. 2006;80:3083–3087. doi: 10.1128/JVI.80.6.3083-3087.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferre AL, et al. Mucosal immune responses to HIV-1 in elite controllers: a potential correlate of immune control. Blood. 2009;113:3978–3989. doi: 10.1182/blood-2008-10-182709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andrade F. Non-cytotoxic antiviral activities of granzymes in the context of the immune antiviral state. Immunological reviews. 2010;235:128–146. doi: 10.1111/j.0105-2896.2010.00909.x. [DOI] [PubMed] [Google Scholar]
- 43.Anthony DA, Andrews DM, Watt SV, Trapani JA, Smyth MJ. Functional dissection of the granzyme family: cell death and inflammation. Immunological reviews. 2010;235:73–92. doi: 10.1111/j.0105-2896.2010.00907.x. [DOI] [PubMed] [Google Scholar]
- 44.Sower LE, Klimpel GR, Hanna W, Froelich CJ. Extracellular Activities of Human Granzymes. Cell Immunol. 1996;171:159–163. [PubMed] [Google Scholar]
- 45.Wensink AC, Hack CE, Bovenschen N. Granzymes regulate proinflammatory cytokine responses. Journal of immunology. 2015;194:491–497. doi: 10.4049/jimmunol.1401214. [DOI] [PubMed] [Google Scholar]
- 46.Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36:265–276. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim N, et al. MicroRNA-150 regulates the cytotoxicity of natural killers by targeting perforin-1. J Allergy Clin Immunol. 2014;134:195–203. doi: 10.1016/j.jaci.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shacklett BL, Critchfield JW, Lemongello D. Isolating mucosal lymphocytes from biopsy tissue for cellular immunology assays. Methods Mol Biol. 2009;485:347–356. doi: 10.1007/978-1-59745-170-3_23. [DOI] [PubMed] [Google Scholar]
- 49.Bas A, Forsberg G, Hammarstrom S, Hammarstrom ML. Utility of the housekeeping genes 18S rRNA, beta-actin and glyceraldehyde-3-phosphate-dehydrogenase for normalization in real-time quantitative reverse transcriptase-polymerase chain reaction analysis of gene expression in human T lymphocytes. Scand J Immunol. 2004;59:566–573. doi: 10.1111/j.0300-9475.2004.01440.x. [DOI] [PubMed] [Google Scholar]
- 50.Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry. Part A : the journal of the International Society for Analytical Cytology. 2011;79:167–174. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.