Abstract
Aims/hypothesis
Transcription factor 7-like 2 (TCF7L2) is a high mobility group (HMG) box-containing transcription factor and downstream effector of the Wnt signalling pathway. SNPs in the TCF7L2 gene have previously been associated with an increased risk of type 2 diabetes in genome-wide association studies. In animal studies, loss of Tcf7l2 function is associated with defective islet beta cell function and survival. Here, we explore the role of TCF7L2 in the control of the counter-regulatory response to hypoglycaemia by generating mice with selective deletion of the Tcf7l2 gene in pancreatic alpha cells.
Methods
Alpha cell-selective deletion of Tcf7l2 was achieved by crossing mice with floxed Tcf7l2 alleles to mice bearing a Cre recombinase transgene driven by the preproglucagon promoter (PPGCre), resulting in Tcf7l2AKO mice. Glucose homeostasis and hormone secretion in vivo and in vitro, and islet cell mass were measured using standard techniques.
Results
While glucose tolerance was unaffected in Tcf7l2AKO mice, glucose infusion rates were increased (AUC for glucose during the first 60 min period of hyperinsulinaemic–hypoglycaemic clamp test was increased by 1.98 ± 0.26-fold [p < 0.05; n = 6] in Tcf7l2AKO mice vs wild-type mice) and glucagon secretion tended to be lower (plasma glucagon: 0.40 ± 0.03-fold vs wild-type littermate controls [p < 0.01; n = 6]). Tcf7l2AKO mice displayed reduced fasted plasma glucose concentration. Glucagon release at low glucose was impaired in islets isolated from Tcf7l2AKO mice (0.37 ± 0.02-fold vs islets from wild-type littermate control mice [p < 0.01; n = 6). Alpha cell mass was also reduced (72.3 ± 20.3% [p < 0.05; n = 7) in Tcf7l2AKO mice compared with wild-type mice.
Conclusions/interpretation
The present findings demonstrate an alpha cell-autonomous role for Tcf7l2 in the control of pancreatic glucagon secretion and the maintenance of alpha cell mass and function.
Keywords: Alpha cell, Diabetes, Gene, Glucagon, GWAS, Islet
Introduction
Personalised treatments for type 2 diabetes are moving closer to reality with the information made available [1] through genome-wide association studies (GWAS) and the technical advances that are making genome sequencing much more affordable. One of the major challenges post-GWAS is in understanding precisely how, at the cellular level, the risk variants contribute to disease risk. Such information is likely to be critical for the rational design of therapies [2]. Frequently, the implicated SNPs occur in non-coding regions in the genome, making it difficult to assess how they lead to disease [2]. A prime example is the intronic SNP rs7903146 in the gene encoding transcription factor 7 like-2 (TCF7L2). Risk allele carriers have a ~1.5-fold higher risk for type 2 diabetes per allele [3–6] and for latent autoimmune diabetes in later life [7]. Individuals carrying the rs7903146 risk allele display defective beta cell function, with evidence of reduced beta cell mass and survival [3–14]. There is a growing body of literature on TCF7L2 in the context of glucose homeostasis and diabetes [1–35], with much effort dedicated to elucidating how the SNP alters TCF7L2 expression and how a change in TCF7L2 content in pancreatic islets affects beta cell function [8, 10–16, 19–21, 26–30, 32, 34, 35]. Existing evidence suggests that reduced levels of TCF7L2 in the beta cell [28] lead to impaired insulin secretion [2]. Thus, although TCF7L2 variants have been proposed in one study to act through the liver [10], both clinical data [4–6, 10, 14, 29] and our own [15, 16, 28] and others’ [11, 20, 21] findings using gene silencing in isolated islets, targeted recombination or expression of dominant-negative TCF7L2 in mice are more consistent with an action largely through the endocrine pancreas. Taken together, the available literature thus points to the SNP leading to a loss of TCF7L2 function in pancreatic islets. This may be due to either the increased expression of a tissue-specific, dominant-negative variant of TCF7L2 [26, 29–33], or a lowering in the expression of a more active isoform, perhaps generated by alternative splicing between exons 13 and 14 and expressed selectively in the beta (and alpha) cell [19, 34, 35].
While extensive efforts have been made by us [15, 16, 28] and others [8, 11, 12, 17–23, 25–27] to examine the role of TCF7L2 in pancreatic beta cell function, little is known about the role of this factor in the other pancreatic islet cell types. Here, we describe the consequences of alpha cell-specific deletion of Tcf7l2 in the mouse. We chose to use C57BL/6 mice for our study as this mouse strain has been extensively used for the study of glucose homeostasis in the context of the study of diabetes in humans. Our hypothesis is that Tcf7l2 function in the alpha cell is important for the control of glucagon release and the maintenance of glucose homeostasis.
Methods
Materials
Unless otherwise stated all materials were obtained from Sigma (Poole, UK).
Generation and maintenance of alpha cell-selective Tcf7l2-knockout mice
Mice carrying conditional null alleles of Tcf7l2 (Tcf7l2 fl/fl) were generated as described in [16] and bred into a C57BL/6 background. Tcf7l2 fl/fl mice were crossed with mice expressing Cre under the control of the a 0.6 kB fragment of the preproglucagon promoter (PPGCre mice [36]; provided by P. Herrera, University of Geneva, Switzerland), which had been crossed into a C57BL/6 background to generate PPGCre:Tcf7l2 fl/fl mice (herein referred to as Tcf7l2AKO mice), in which there is deletion of Tcf7l2 in pancreatic alpha cells and limited expression of Tcf7l2 in extrapancreatic tissue [36–39]. Tcf7l2AKO mice were born at the expected Mendelian ratios and male mice were phenotyped at 8–20 weeks of age. Genotyping was performed by PCR using DNA from ear biopsies. Wild-type littermate control (Tcf7l2 fl/fl) mice lacked the PPGCre allele. Possession of the latter allele exerted no effects on glucose tolerance or glucagon secretion compared with wild-type mice, as previously reported [38]. All mouse lines were maintained on a C57BL/6 background. Mice were housed in groups of two to five per individually ventilated cage in a pathogen-free facility with 12 h light–dark cycle and were fed ad libitum with a standard mouse chow diet. All in vivo procedures described were performed at the Imperial College Central Biomedical Service and approved by the local ethical committee and UK Home Office according to the Animals (Scientific Procedures) Act 1986 of the UK (PPL 70/7971).
In vivo physiology
IPGTT and insulin tolerance test
Mice fasted for 16 h (with free access to water) were injected intraperitoneally with 1 g glucose/kg, and glucose levels in tail-vein blood were measured with an automatic glucometer (Accuchek Compact Plus; Roche, Burgess Hill, UK) [28]. Insulin tolerance was assessed by i.p. injection of insulin (0.75 U/kg; ActRapid, NovoNordisk, London, UK), which was administered to mice that had been subjected to a 5 h fast. Plasma was collected and centrifuged (2000 g, 5 min) in heparin-coated tubes (Microvette; Sarstedt, Leicester, UK) and plasma glucagon and glucagon-like peptide-1 (GLP-1) were assessed by radioimmunoassay (Millipore/Linco, Watford, UK). Hyperinsulinaemic–hypoglycaemic clamp tests were performed by perfusion of insulin and glucose solutions through a jugular catheter, as described [39].
Islet isolation, in vitro glucose-stimulated glucagon secretion and real-time PCR analysis
After mice were euthanised by cervical dislocation, islets were purified on histopaque gradients and hand-picked as described [40]. Islets were cultured in RPMI medium (Gibco, Paisley, UK) supplemented with 2 mmol/l glutamine, 100 U/ml of penicillin, 100 U/ml of streptomycin and 10% (vol./vol.) heat-inactivated FBS for 24 h. Secretion from islets (10 per condition, size-matched) was measured in 0.5 ml KRB solution containing 3 or 10 mmol/l glucose as described [41, 42].
Total RNA was extracted in Trizol (Invitrogen, Paisley, UK) from 100 mouse islets and real-time PCR analysis of Gcg and Mafb was conducted as previously described [43].
Immunohistochemistry
Beta and alpha cell masses were assessed as previously described [43] in pancreases from 20-week-old mice. Briefly, isolated pancreases were fixed in 10% buffered formalin and embedded in paraffin wax within 24 h of removal. Head-to-tail sections (5 μm lengthwise) were cut and incubated overnight at 37°C on superfrost slides. Slides were submerged sequentially in Histochoice followed by decreasing concentrations of industrial methylated spirits for removal of paraffin wax. TCF7L2 protein content in pancreatic alpha cells was assessed by immunohistochemistry (anti-TCF7L2 antibody [SC-8631]; 1:50 dilution; Santa Cruz, Heidelberg, Germany), as per the manufacturer’s instructions. Images were captured on a Zeiss Axio Observer.Z1 Motorised Inverted Widefield Microscope (Zeiss, Cambridge, UK) fitted with a Hamamatsu Flash 4.0 Camera (Hamamatsu Photonics, Welwyn Garden City, UK) using Plan-Apochromat 20×/0.8 M27 air objective (Zeiss) with Colibri.2 LED illumination. Data acquisition was controlled by Zeiss Zen Blue 2012 software configured at a bit depth of 16-bit and binning mode 2 × 2 (Zeiss). Whole-tissue tiled preview scans were obtained using an EC Plan-Neofluar 10x/0.3 Ph1 air objective with phase contrast (Zeiss). Excitation intensities and exposure times were kept constant for all images. Image analysis was performed using Volocity (PerkinElmer, Beaconsfield, UK) and Fiji (https://fiji.sc/, accessed 25 June 2015) [43]. Experimenters were blinded to the group assignment for assessment of islet cell mass.
Laser capture microdissection and real-time PCR analysis
Laser capture microdissection was performed on pancreatic slices essentially as described [44]. Alpha and beta cells were identified by fluorescent staining as described in the methods for ‘immunohistochemistry’. Cells were extracted from ten pancreatic slices from three separate pancreases from Tcf7l2AKO or wild-type littermate control mice, and pooled for RNA extraction. Real-time quantitative PCR was conducted to analyseTcf7l2, Gcg, Ins2 and Mafb expression, as previously described [43].
Statistical analysis
Samples were not randomised. No data, samples or animals were excluded. Data are expressed as means ± SEM. Significance was tested by two sample unpaired or paired Student’s t test using Excel (Microsoft, Reading, UK). A value of p < 0.05 was considered significant.
Results
Generation of mice deleted for Tcf7l2 selectively in the pancreatic alpha cell
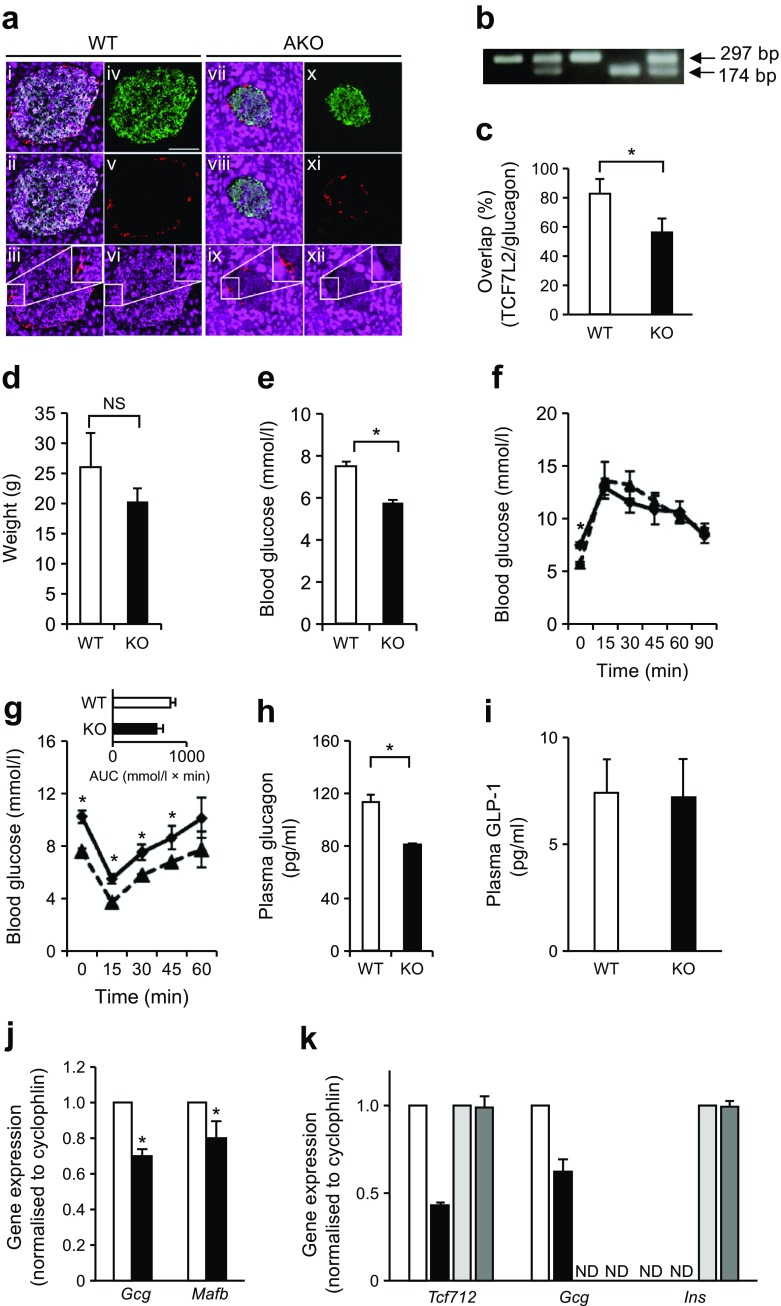
Cross-breeding of mice with floxed Tcf7l2 alleles with mice expressing Cre recombinase under the control of the preproglucagon (PPG) gene promoter [36] was predicted to lead to recombination selectively in pancreatic islet alpha cells (generating Tcf7l2AKO mice). We used immunohistochemistry to assess TCF7L2 protein content in alpha cells because of the low abundance of alpha cells in rodent islets (~20% of all cells) [45, 46], and expected 20–50% deletion with the PPGCre used here [46]. Correspondingly, immunohistochemical analysis revealed a 56.7 ± 9.5% overlap (vs 83.4 ± 10.6% in pancreases from wild-type littermate control mice) of signal from anti-TCF7L2 antibody with the signal from glucagon in Tcf7l2AKO islets (Fig. 1a, c).
Fig. 1.
Tcf7l2AKO mice display reduced blood glucose, insulin intolerance and plasma glucagon concentration. Tcf7l2 was knocked out using an alpha cell-selective Cre [36]. (a) Representative images of immunohistochemical analysis are shown to confirm knockout by labelling pancreases from wild-type (WT; panels i–vi) and Tcf7l2AKO (panels vii–xii) mice with anti-glucagon (red), anti-insulin (green) and anti-TCF7L2 (magenta) antibodies. Images i and vii show overlay of all three channels, ii and viii show overlay of insulin with TCF7L2, iii and ix show overlay of glucagon with TCF7L2, and iv–vi and x–xii show the individual channels. Inset panels show magnified images of the indicated areas. Scale bar, 50 μm and applies to all micrographs in part (a). (b) PCR genotyping gel to confirm the presence of the wild-type (WT; 174 bp) and conditional knockout (AKO; 297 bp) allele. (c) Graph showing quantification of the degree of overlap between glucagon-positive alpha cells and TCF7L2-positive cells in pancreases from WT and Tcf7l2AKO mice. (d) Tcf7l2AKO mice exhibit normal weight. (e–g) i.p. glucose and insulin tolerance tests were conducted on 8–9-week-old mice on a normal chow diet. (e) Fasting glucose but not (f) overall glucose tolerance was altered in Tcf7l2AKO mice compared with WT mice. (g) Insulin tolerance, (h) fasting (16 h) plasma glucagon and (i) plasma GLP-1 were also measured in Tcf7l2AKO mice. (j) Real-time PCR analysis of islets of Langerhans from 20-week-old Tcf7l2AKO mice and WT littermate control mice on normal chow diet. (k) Real-time quantitative PCR analysis of cells captured by laser microdissection for measurements of the indicated genes. In (a–j): white bars and solid lines, WT mice; black bars and dashed lines, Tcf7l2AKO mice. In (k): white bars, glucagon-positive cells from WT mice; black bars, glucagon-positive cells from Tcf7l2AKO mice; light grey bars, insulin-positive cells from WT mice; dark grey bars, insulin-positive cells from Tcf7l2AKO mice. For (a–i), n = 5; for (j) and (k), n = 3. ND, non-detectable (i.e. Gcg and Ins expression was undetectable in insulin- and glucagon-positive cells, respectively), NS, non-significant. *p < 0.05
Pancreatic alpha cell-selective deletion of Tcf7l2 leads to reduced fasting glucose, normal insulin tolerance but impaired counter-regulatory response
There were no significant differences in weight between Tcf7l2AKO mice and wild-type littermate control mice (Fig. 1d). Fasting plasma glucose (measured at 09:00 hours following a 16 h fast, Fig. 1e, f) was lower in Tcf7l2AKO mice compared with wild-type littermate control mice, while glucose tolerance (as assessed by IPGTT) was unaffected (Fig. 1f). Tcf7l2AKO mice exhibited normal tolerance to i.p insulin (Fig. 1g). While plasma glucose levels were significantly higher at all except one time point sampled during the period of the test, there was no significant difference between genotypes in the area under the curve (AUC) for the period of the test (Fig. 1g, inset). Tcf7l2AKO mice exhibited reduced fasting plasma glucagon levels (Fig. 1h), suggestive of defective counter-regulatory responses. Plasma GLP-1 levels were unchanged (Fig. 1i), while islet glucagon (Gcg) and Mafb gene expression were significantly reduced in islets from Tcf7l2AKO vs wild-type littermate control mice (Fig. 1j). Gene expression analysis of pooled glucagon-positive cells captured by laser capture microdissection demonstrated a 56.8 ± 5.45% and 37.2 ± 6.52% decrease in Tcf7l2 and Gcg gene expression, respectively, in cells from Tcf7l2AKO vs wild-type littermate control mice (Fig. 1k). In contrast, neither Tcf7l2 nor Ins gene expression were significantly affected in insulin-positive cells captured by laser capture microdissection (Fig. 1k).
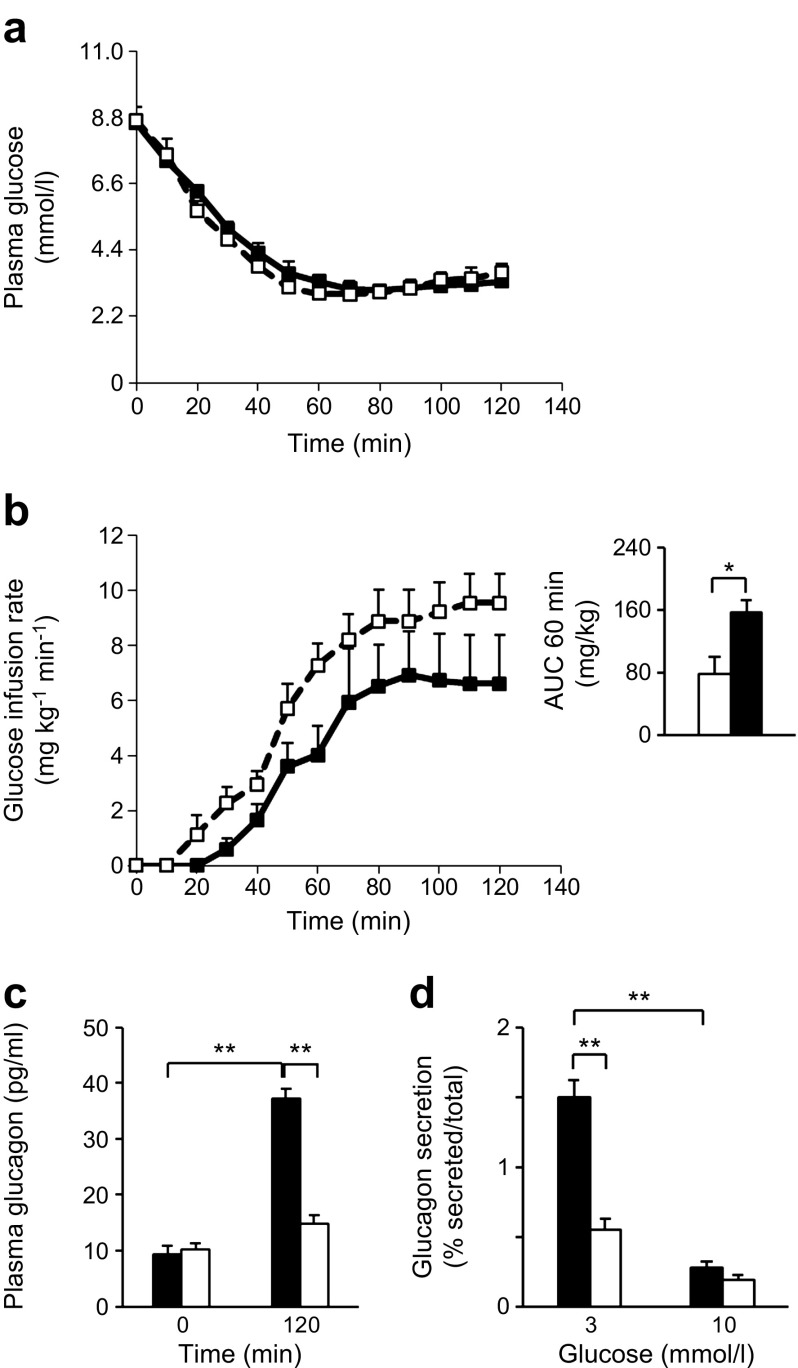
To determine whether the above changes result in impaired glucagon release in vivo we performed hyperinsulinaemic–hypoglycaemic clamp tests. During the time period 20–120 min after the start of insulin infusion, when blood glucose levels were similar in both groups (Fig. 2a), it was necessary to infuse glucose more rapidly into Tcf7l2AKO vs wild-type littermate control mice to maintain blood glucose levels (Fig. 2b). Thus, a significant increase (1.98 ± 0.26-fold; p < 0.05) in the AUC for glucose was observed during the first 60 min period (Fig. 2b [inset]), and plasma glucagon was reduced at 120 min (0.40 ± 0.03 fold; p < 0.01; Fig. 2c) vs wild-type littermate control mice following glucose infusion, confirming a defective counter-regulatory response in these mice. Likewise, glucagon secretion from isolated islets was significantly reduced in response to low glucose (3 mmol/l; Fig. 2d), while total islet glucagon content was not significantly different between Tcf7l2AKO vs islets from wild-type littermate control mice (12.1 ± 0.8 vs 13.6 ± 0.9 ng per ten islets, respectively).
Fig. 2.
Tcf7l2AKO mice display impaired counter-regulatory response to hypoglycaemia. (a) Plasma glucose concentration and (b) glucose infusion rates for hyperinsulinaemic–hypoglycaemic clamp tests in 20-week-old wild-type (solid line/black squares and black bars) and Tcf7l2AKO (dashed lines/white squares and white bars) mice. Insulin was infused at time 0 min; inset in (b) shows AUC for glucose infusion rates. (c) Plasma glucagon levels in mice undergoing hyperinsulinaemic–hypoglycaemic clamp tests at 0 and 120 min of the glucose infusion protocol. (d) Levels of glucagon secretion from isolated islets of Langerhans exposed to glucose at the indicated concentrations. Black bars, wild-type mice; white bars, Tcf7l2AKO mice. n = 6 mice for all;*p < 0.05 and **p < 0.01
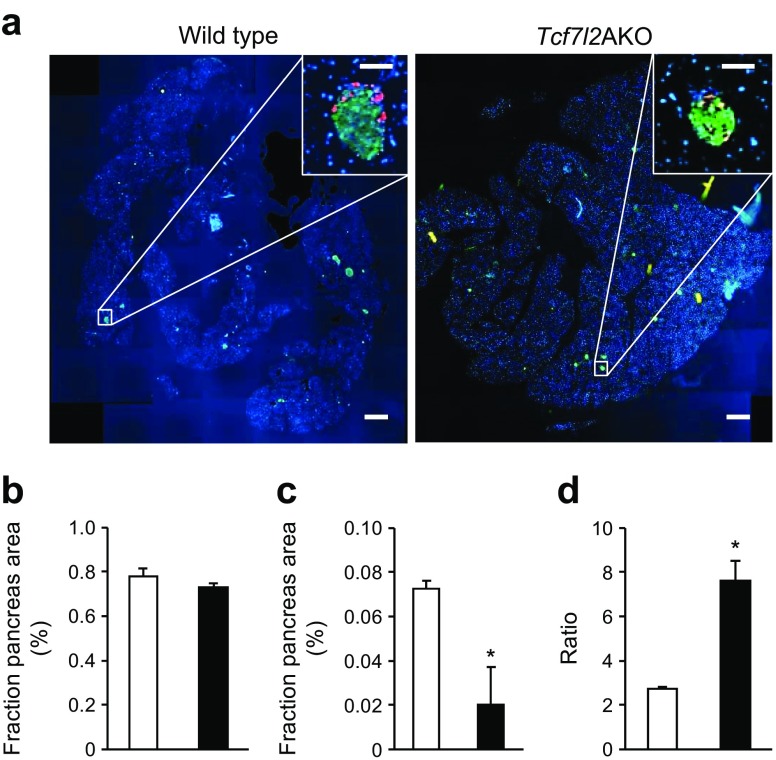
Pancreatic alpha cell mass is reduced in Tcf7l2AKO mice
Immunohistochemical analysis revealed no change in beta cell mass (Fig. 3b) but a 72.3 ± 20.3% (p < 0.05) decrease in alpha cell mass in Tcf7l2AKO vs wild-type littermate control mice (Fig. 3c), resulting in a 2.8 ± 0.09-fold increase in beta/alpha cell mass ratio (Fig. 3d).
Fig. 3.
Tcf7l2AKO mice display reduced alpha cell mass. (a) Representative images from pancreatic sections from wild-type and Tcf7l2AKO mice are shown. Scale bar in large micrographs, 500 μm; scale bar in insets, 100 μm. (b) Beta cell (defined as insulin-positive cells, in green) and (c) alpha cell (defined as glucagon-positive cells, in red) mass, and (d) beta/alpha cell ratio from 20-week-old mice were quantified [44]. White bars, wild-type mice; black bars, Tcf7l2AKO mice. n = 7 mice for all; *p < 0.05
Discussion
It was recently demonstrated that individuals without diabetes bearing rs7903146 risk variants display an increased pancreatic alpha/beta cell ratio [10]. We [15, 16, 28] and others [8, 11, 12, 17–23, 25–27], have previously demonstrated that loss of Tcf7l2 from pancreatic islets [16] or selectively from beta cells [28] leads to decreased beta cell mass and increased beta cell apoptosis [11–13, 16, 21, 28, 29, 32, 47]. The observation that alpha cell mass was not altered in mice with beta cell-specific deletions in Tcf7l2 and glucose intolerance [28] suggests that the increase in this variable in risk allele carriers may be due to a cell-autonomous role for TCF7L2 in the alpha cell.
As a means of testing this hypothesis directly, we provide here the first description of mice with pancreatic alpha cell-specific deletion of Tcf7l2. Tcf7l2AKO mice present with a robust reduction of TCF7L2 protein content (Fig. 1a, c) and Tcf7l2 expression (Fig. 1k) in alpha cells, reflecting the expected efficiency of the PPGCre strain (which recombines in 20–50% of alpha cells; see [39] and references therein). In addition, we demonstrated that the expression of two alpha cell-specific genes, Gcg and Mafb, was reduced in pancreatic islets (Fig. 1j), although interestingly these changes did not result in an apparent lowering of islet glucagon content (described further below). The reason(s) for this discordance between changes at the mRNA and protein level are unclear.
We note that although other Cre driver lines under the control of the glucagon promoter result in more complete recombination in alpha cells [48, 49]. However, these also recombine in the brain and in intestinal L cells, complicating the interpretation of results obtained through their use. This is particularly relevant as TCF7L2 has recently been shown to regulate gut and brain proglucagon gene expression and glucose homeostasis [22]. However, unlike the Gcg promoter used in the Shao study [22], the PPGCre strain we use in this study has previously been shown to exhibit no recombination in the brain and <5% recombination in the small intestine, with no effect on plasma GLP-1 content [37]. Here, we show that plasma GLP-1 levels were unchanged (Fig. 1i) in Tcf7l2AKO mice in comparison with wild-type littermate controls, indicating that deletion of Tcf7l2 in cells in which the PPG promoter is active did not significantly alter GLP-1 production or release.
One of our key findings was that 8–9-week-old Tcf7l2AKO mice have lower fasted (Fig. 1e, f) blood glucose concentrations. While glucose (Fig. 1f) and insulin tolerance (Fig. 1g) was unaffected in Tcf7l2AKO mice, Tcf7l2AKO mice displayed lower fasting plasma glucagon (Fig. 1h) compared with wild-type littermate controls. These data point to a defective counter-regulatory response (where, in health, an increase in glucagon release from pancreatic alpha cells leads to avoidance of hypoglycaemia) which we confirmed by performing hyperinsulinaemic–hypoglycaemic clamps (Fig. 2). Glucagon secretion from isolated islets in response to low glucose levels (3 mmol/l) was similarly impaired (Fig. 2d), while islet glucagon content was unaltered in Tcf7l2AKO vs islets from wild-type littermate control mice, and alpha cell mass was decreased (Fig. 3). Thus, the loss of counter-regulatory response in Tcf7l2AKO mice would appear to reflect, at least in large part, decreases in both alpha cell mass and function. The downstream targets of Tcf7l2 that may mediate the molecular mechanisms that lead to the loss of alpha cell mass and function remain to be determined.
The present findings thus extend our published data on Tcf7l2 function in pancreatic beta cells [28], indicating that TCF7L2 has a cell-autonomous function in pancreatic islet alpha cells through modulation of cell mass, function and the expression of Gcg and other genes in these cells. The molecular mechanisms that underlie these changes remain, however, to be characterised in detail, though actions on membrane potential or calcium dynamics, as reported in beta cells deficient in Tcf7l2 [15], are likely possibilities.
Intriguingly, the present and previous [14] data indicate that loss of TCF7L2 function, as anticipated in carriers of the risk allele rs7903146 as a result of increases in the expression of dominant-negative isoforms of the protein, leads to decreases in both alpha and beta [28] cell mass. The combined effect is an overall larger impact on islet function, potentially having an impact on pathways that are involved in islet cell regeneration in disease conditions. Importantly a decrease of similar magnitude in the functional mass of both cell types is expected to reduce antihyperglycaemic drives postprandially, when alpha cells are largely inactive in individuals without diabetes, thus increasing diabetes risk. Data are currently unavailable on the effect of risk alleles on the expression of Tcf7l2 mRNA levels and splicing in alpha cells. Of note, we have previously hypothesised that risk variants may not act on TCF7L2 activity in the liver if the affected splice variant containing exons 13, 13b and 14 is not present in this tissue [19, 28, 34, 35, 50]. Studies on purified human alpha cell populations will be required to address this question.
Interestingly, we have also recently shown [39] that loss of the secretory granule zinc transporter ZnT8, encoded by the type 2 diabetes GWAS gene SLC30A8 [5] from the murine alpha cell, leads to exaggerated glucagon release in response to low glucose levels, though alpha cell mass was not altered in the latter model. Conversely, overexpression of ZnT8 in alpha cells stimulates glucagon secretion [51]. The latter findings demonstrate that the increased abundance of type 2 diabetes in risk allele carriers might, in both cases, involve alterations in glucagon release, consistent with the ‘dual hormone’ model for this disease [52]. Whether personalised treatments of type 2 diabetes based on genotype at either locus may usefully target these changes in glucagon release may be worthy of exploration in the future. Likewise, variation in the association SNPs in both alleles might be useful in the context of type 1 diabetes as a predictor of counter-regulatory responses to hypoglycaemia.
Acknowledgements
We thank L. Lawrence (Histology Services) and S. Rothery (FILM) for help with immunohistochemistry. We also thank P. Kemp and R. Farre Garros (NHLI) for help with laser capture microdissection.
Abbreviations
- GLP-1
Glucagon-like peptide-1
- GWAS
Genome-wide association studies
- PPG
Preproglucagon
- TCF7L2
Transcription factor 7 like-2
- Tcf7l2fl/fl
Mice carrying conditional null alleles of Tcf7l2
- Tcf7l2AKO
Alpha cell-specific deletion of Tcf7l2 in the mouse
- PPGCre
Cre under the control of the a 0.6 kB fragment of the preproglucagon promoter
Data availability
Data are available on request from the authors.
Funding
GdSX thanks Diabetes UK (BDA 13/0004672), EFSD-MSD and Rosetrees Trust for Project grants. This work was funded by grants to GAR from Diabetes UK (Project BDA 11/0004210), the Wellcome Trust (Programme 081958/Z/07/Z; Senior Investigator Award WT098424AIA), and the MRC (UK; Project GO401641; Programme MR/J0003042/1). The work leading to this publication also received support from the Innovative Medicines Initiative Joint Undertaking under grant agreement no. 155005 (IMIDIA) (GAR, CM, PLH), resources of which are composed of a financial contribution from the European Union’s Seventh Framework Programme (FP7/2007–2013) and EFPIA companies’ in kind contribution.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
GdSX and GAR conceived and designed the studies and co-wrote the article. PLH provided PPGCre mice, advice and help in the generation of the Tcf7l2AKO mice and interpretation of the data. AM and GdSX designed, generated, genotyped and characterised the conditional knockout mice, and contributed to the interpretation of the data. AM, VM and GdSX performed immunohistochemical analysis and analysed the data. CM, JD and CC-G performed hypoglycaemic clamps and analysed and interpreted the data. All authors revised the article critically for important intellectual content and approved the final version to be published. GdSX is responsible for the integrity of the work as a whole.
References
- 1.Marullo L, El-Sayed Moustafa JS, Prokopenko I. Insights into the genetic susceptibility to type 2 diabetes from genome-wide association studies of glycaemic traits. Curr Diab Rep. 2014;14:551. doi: 10.1007/s11892-014-0551-8. [DOI] [PubMed] [Google Scholar]
- 2.Rutter GA. Dorothy Hodgkin lecture 2014. Understanding genes identified by genome-wide association studies for type 2 diabetes. Diabet Med. 2014;31:1480–1487. doi: 10.1111/dme.12579. [DOI] [PubMed] [Google Scholar]
- 3.Scott LJ, Mohlke KL, Bonnycastle LL, et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grant SF, Thorleifsson G, Reynisdottir I, et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006;38:320–323. doi: 10.1038/ng1732. [DOI] [PubMed] [Google Scholar]
- 5.Zeggini E, Weedon MN, Lindgren CM, et al. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316:1336–1341. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sladek R, Rocheleau G, Rung J, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 7.Andersen MK, Sterner M, Forsen T, et al. Type 2 diabetes susceptibility gene variants predispose to adult-onset autoimmune diabetes. Diabetologia. 2014;57:1859–1868. doi: 10.1007/s00125-014-3287-8. [DOI] [PubMed] [Google Scholar]
- 8.Villareal DT, Robertson H, Bell GI, et al. TCF7L2 variant rs7903146 affects the risk of type 2 diabetes by modulating incretin action. Diabetes. 2010;59:479–485. doi: 10.2337/db09-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pilgaard K, Jensen CB, Schou JH, et al. The T allele of rs7903146 TCF7L2 is associated with impaired insulinotropic action of incretin hormones, reduced 24 h profiles of plasma insulin and glucagon, and increased hepatic glucose production in young healthy men. Diabetologia. 2009;52:1298–1307. doi: 10.1007/s00125-009-1307-x. [DOI] [PubMed] [Google Scholar]
- 10.Le BO, Kerr-Conte J, Gargani S, et al. TCF7L2 rs7903146 impairs islet function and morphology in non-diabetic individuals. Diabetologia. 2012;55:2677–2681. doi: 10.1007/s00125-012-2660-8. [DOI] [PubMed] [Google Scholar]
- 11.Shu L, Sauter NS, Schulthess FT, Matveyenko AV, Oberholzer J, Maedler K. Transcription factor 7-like 2 regulates beta-cell survival and function in human pancreatic islets. Diabetes. 2008;57:645–653. doi: 10.2337/db07-0847. [DOI] [PubMed] [Google Scholar]
- 12.Shu L, Matveyenko AV, Kerr-Conte J, Cho JH, McIntosh CH, Maedler K. Decreased TCF7L2 protein levels in type 2 diabetes mellitus correlate with downregulation of GIP- and GLP-1 receptors and impaired beta-cell function. Hum Mol Genet. 2009;18:2388–2399. doi: 10.1093/hmg/ddp178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shu L, Zien K, Gutjahr G, et al. TCF7L2 promotes beta cell regeneration in human and mouse pancreas. Diabetologia. 2012;55:3296–3307. doi: 10.1007/s00125-012-2693-z. [DOI] [PubMed] [Google Scholar]
- 14.Lyssenko V, Lupi R, Marchetti P, et al. Mechanisms by which common variants in the TCF7L2 gene increase risk of type 2 diabetes. J Clin Invest. 2007;117:2155–2163. doi: 10.1172/JCI30706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.da Silva XG, Loder MK, McDonald A, et al. TCF7L2 regulates late events in insulin secretion from pancreatic islet beta-cells. Diabetes. 2009;58:894–905. doi: 10.2337/db08-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.da Silva XG, Mondragon A, Sun G, et al. Abnormal glucose tolerance and insulin secretion in pancreas-specific Tcf7l2-null mice. Diabetologia. 2012;55:2667–2676. doi: 10.1007/s00125-012-2600-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boj SF, van Es JH, Huch M, et al. Diabetes risk gene and Wnt effector Tcf7l2/TCF4 controls hepatic response to perinatal and adult metabolic demand. Cell. 2012;151:1595–1607. doi: 10.1016/j.cell.2012.10.053. [DOI] [PubMed] [Google Scholar]
- 18.Korinek V, Barker N, Moerer P, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 19.Prokunina-Olsson L, Welch C, Hansson O, et al. Tissue-specific alternative splicing of TCF7L2. Hum Mol Genet. 2009;18:3795–3804. doi: 10.1093/hmg/ddp321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Z, Habener JF. Glucagon-like peptide-1 activation of TCF7L2-dependent Wnt signaling enhances pancreatic beta cell proliferation. J Biol Chem. 2008;283:8723–8735. doi: 10.1074/jbc.M706105200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takamoto I, Kubota N, Nakaya K, et al. TCF7L2 in mouse pancreatic beta cells plays a crucial role in glucose homeostasis by regulating beta cell mass. Diabetologia. 2014;57:542–553. doi: 10.1007/s00125-013-3131-6. [DOI] [PubMed] [Google Scholar]
- 22.Shao W, Wang D, Chiang YT, et al. The Wnt signaling pathway effector TCF7L2 controls gut and brain proglucagon gene expression and glucose homeostasis. Diabetes. 2013;62:789–800. doi: 10.2337/db12-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norton L, Fourcaudot M, Abdul-Ghani MA, et al. Chromatin occupancy of transcription factor 7-like 2 (TCF7L2) and its role in hepatic glucose metabolism. Diabetologia. 2011;54:3132–3142. doi: 10.1007/s00125-011-2289-z. [DOI] [PubMed] [Google Scholar]
- 24.Savic D, Ye H, Aneas I, Park SY, Bell GI, Nobrega MA. Alterations in TCF7L2 expression define its role as a key regulator of glucose metabolism. Genome Res. 2011;21:1417–1425. doi: 10.1101/gr.123745.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaulton KJ, Nammo T, Pasquali L, et al. A map of open chromatin in human pancreatic islets. Nat Genet. 2010;42:255–259. doi: 10.1038/ng.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Y, Zhang E, Berggreen C, et al. Survival of pancreatic beta cells is partly controlled by a TCF7L2-p53-p53INP1-dependent pathway. Hum Mol Genet. 2012;21:196–207. doi: 10.1093/hmg/ddr454. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Y, Park SY, Su J, et al. TCF7L2 is a master regulator of insulin production and processing. Hum Mol Genet. 2014;23:6419–6431. doi: 10.1093/hmg/ddu359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchell RK, Mondragon A, Chen L, et al. Selective disruption of Tcf7l2 in the pancreatic beta cell impairs secretory function and lowers beta cell mass. Hum Mol Genet. 2015;24:1390–1399. doi: 10.1093/hmg/ddu553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Bacquer O, Shu L, Marchand M, et al. TCF7L2 splice variants have distinct effects on beta-cell turnover and function. Hum Mol Genet. 2011;20:1906–1915. doi: 10.1093/hmg/ddr072. [DOI] [PubMed] [Google Scholar]
- 30.Locke JM, da Silva XG, Rutter GA, Harries LW. An alternative polyadenylation signal in TCF7L2 generates isoforms that inhibit T cell factor/lymphoid-enhancer factor (TCF/LEF)-dependent target genes. Diabetologia. 2011;54:3078–3082. doi: 10.1007/s00125-011-2290-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duval A, Rolland S, Tubacher E, Bui H, Thomas G, Hamelin R. The human T cell transcription factor-4 gene: structure, extensive characterization of alternative splicings, and mutational analysis in colorectal cancer cell lines. Cancer Res. 2000;60:3872–3879. [PubMed] [Google Scholar]
- 32.Mondal AK, Das SK, Baldini G, et al. Genotype and tissue-specific effects on alternative splicing of the transcription factor 7-like 2 gene in humans. J Clin Endocrinol Metab. 2010;95:1450–1457. doi: 10.1210/jc.2009-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weise A, Bruser K, Elfert S, et al. Alternative splicing of Tcf7l2 transcripts generates protein variants with differential promoter-binding and transcriptional activation properties at Wnt/beta-catenin targets. Nucleic Acids Res. 2010;38:1964–1981. doi: 10.1093/nar/gkp1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hansson O, Zhou Y, Renstrom E, Osmark P. Molecular function of TCF7L2: consequences of TCF7L2 splicing for molecular function and risk for type 2 diabetes. Curr Diab Rep. 2010;10:444–451. doi: 10.1007/s11892-010-0149-8. [DOI] [PubMed] [Google Scholar]
- 35.Osmark P, Hansson O, Jonsson A, Ronn T, Groop L, Renstrom E. Unique splicing pattern of the TCF7L2 gene in human pancreatic islets. Diabetologia. 2009;52:850–854. doi: 10.1007/s00125-009-1293-z. [DOI] [PubMed] [Google Scholar]
- 36.Herrera PL. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127:2317–2322. doi: 10.1242/dev.127.11.2317. [DOI] [PubMed] [Google Scholar]
- 37.Kawamori D, Kurpad AJ, Hu J, et al. Insulin signaling in alpha cells modulates glucagon secretion in vivo. Cell Metab. 2009;9:350–361. doi: 10.1016/j.cmet.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quoix N, Cheng-Xue R, Guiot Y, Herrera PL, Henquin JC, Gilon P. The GluCre-ROSA26EYFP mouse: a new model for easy identification of living pancreatic alpha-cells. FEBS Lett. 2007;581:4235–4240. doi: 10.1016/j.febslet.2007.07.068. [DOI] [PubMed] [Google Scholar]
- 39.Solomou A, Meur G, Bellomo E, et al. The zinc transporter Slc30a8/ZnT8 is required in a subpopulation of pancreatic alpha-cells for hypoglycemia-induced glucagon secretion. J Biol Chem. 2015;290:21432–21442. doi: 10.1074/jbc.M115.645291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ravier MA, Rutter GA. Glucose or insulin, but not zinc ions, inhibit glucagon secretion from mouse pancreatic alpha-cells. Diabetes. 2005;54:1789–1797. doi: 10.2337/diabetes.54.6.1789. [DOI] [PubMed] [Google Scholar]
- 41.da Silva XG, Farhan H, Kim H, et al. Per-arnt-Sim (PAS) domain-containing protein kinase is downregulated in human islets in type 2 diabetes and regulates glucagon secretion. Diabetologia. 2011;54:819–827. doi: 10.1007/s00125-010-2010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Semplici F, Mondragon A, Macintyre B, et al. Cell type-specific deletion in mice reveals roles for PAS kinase in insulin and glucagon production. Diabetologia. 2016;59:1938–1947. doi: 10.1007/s00125-016-4025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mondragon A, Davidsson D, Kyriakidou S, et al. Divergent effects of liraglutide, exendin-4, and sitagliptin on beta-cell mass and indicators of pancreatitis in a mouse model of hyperglycaemia. PLoS One. 2014;9:e104873. doi: 10.1371/journal.pone.0104873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mohan D, Lewis A, Patel MS, et al. Using laser capture microdissection to study fiber specific signalling in locomotor muscle in COPD: a pilot study. Muscle Nerve. 2016 doi: 10.1002/mus.25423. [DOI] [PubMed] [Google Scholar]
- 45.Elayat AA, el-Naggar MM, Tahir M. An immunocytochemical and morphometric study of the rat pancreatic islets. J Anat. 1995;186(Pt 3):629–637. [PMC free article] [PubMed] [Google Scholar]
- 46.Sun G, da Silva XG, Gorman T, et al. LKB1 and AMPKalpha1 are required in pancreatic alpha cells for the normal regulation of glucagon secretion and responses to hypoglycemia. Mol Metab. 2015;4:277–286. doi: 10.1016/j.molmet.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mitchell KJ, Tsuboi T, Rutter GA. Role for plasma membrane-related Ca2+-ATPase-1 (ATP2C1) in pancreatic β-cell Ca2+ homeostasis revealed by RNA silencing. Diabetes. 2004;53:393–400. doi: 10.2337/diabetes.53.2.393. [DOI] [PubMed] [Google Scholar]
- 48.Parker HE, Adriaenssens A, Rogers G, et al. Predominant role of active versus facilitative glucose transport for glucagon-like peptide-1 secretion. Diabetologia. 2012;55:2445–2455. doi: 10.1007/s00125-012-2585-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soedling H, Hodson DJ, Adrianssens AE, et al. Limited impact on glucose homeostasis of leptin receptor deletion from insulin- or proglucagon-expressing cells. Mol Metab. 2015;4:619–630. doi: 10.1016/j.molmet.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rutter GA. Understanding GWAS genes for type 2 diabetes. Diabet Med. 2014;31:1480–1487. doi: 10.1111/dme.12579. [DOI] [PubMed] [Google Scholar]
- 51.Solomou A, Philippe E, Chabosseau P, et al. Over-expression of Slc30a8/ZnT8 selectively in the mouse α cell impairs glucagon release and responses to hypoglycemia. Nutr Metab. 2016;13:46. doi: 10.1186/s12986-016-0104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Unger RH, Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet. 1975;1:14–16. doi: 10.1016/S0140-6736(75)92375-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on request from the authors.