Abstract
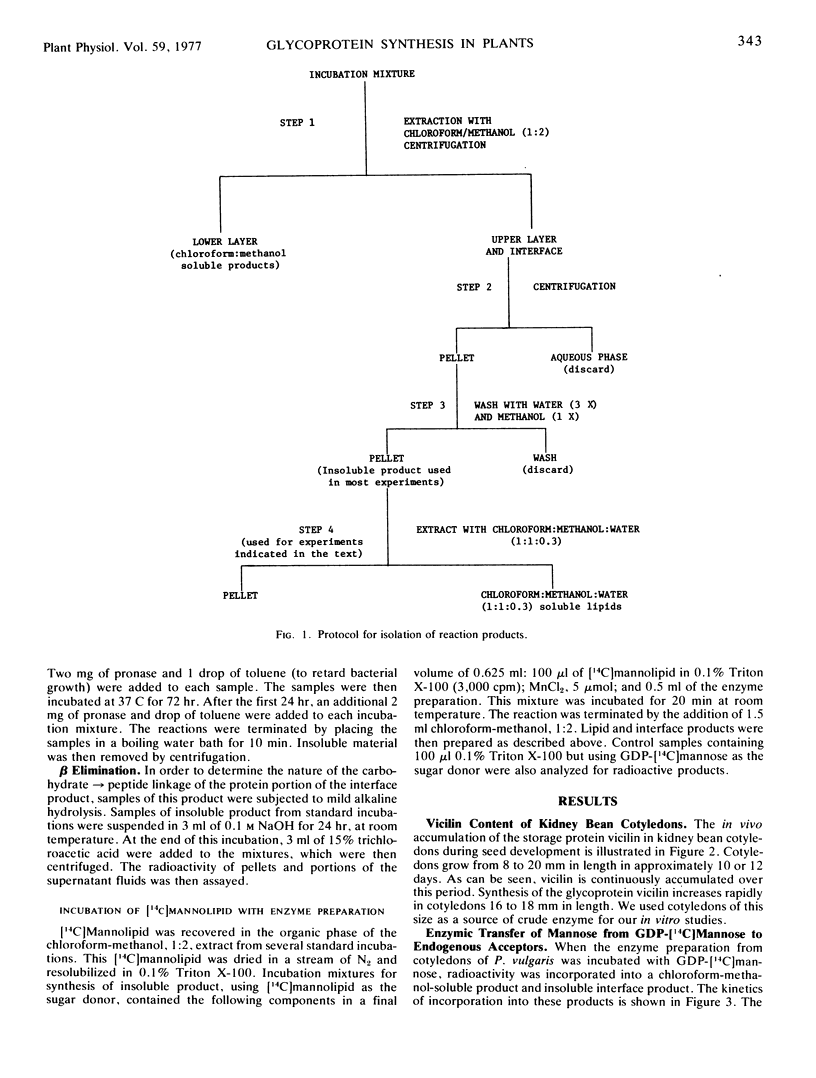
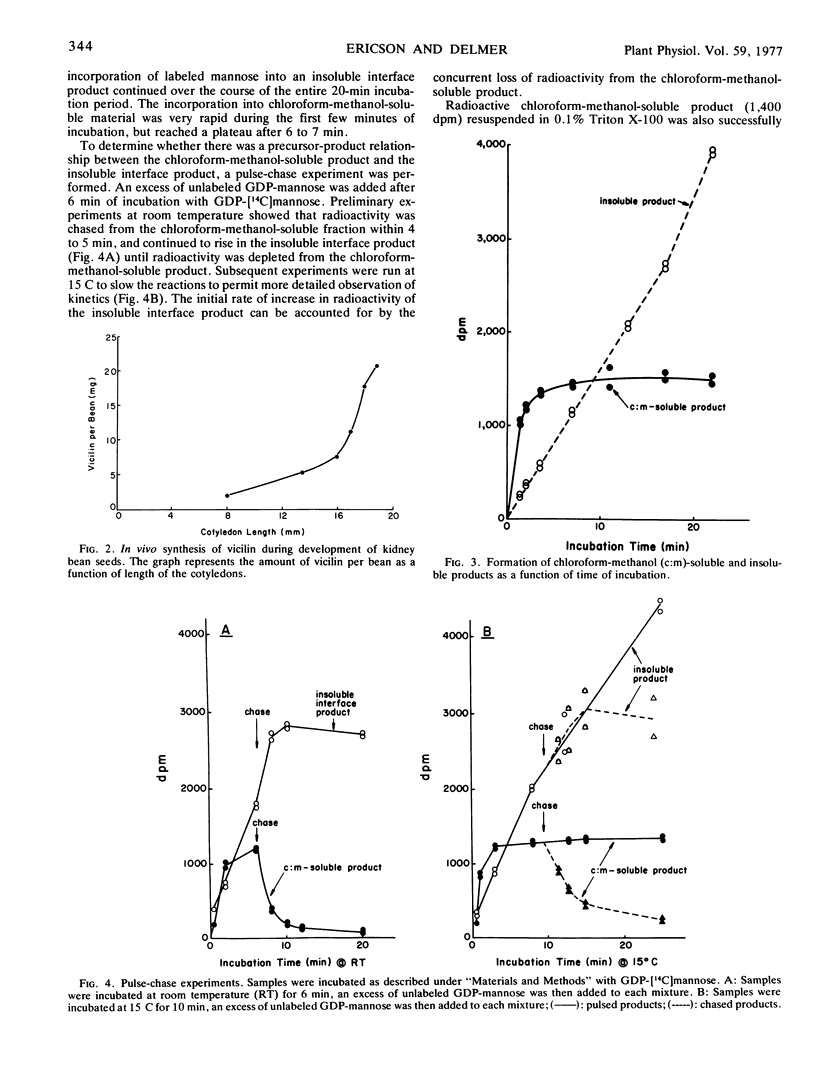
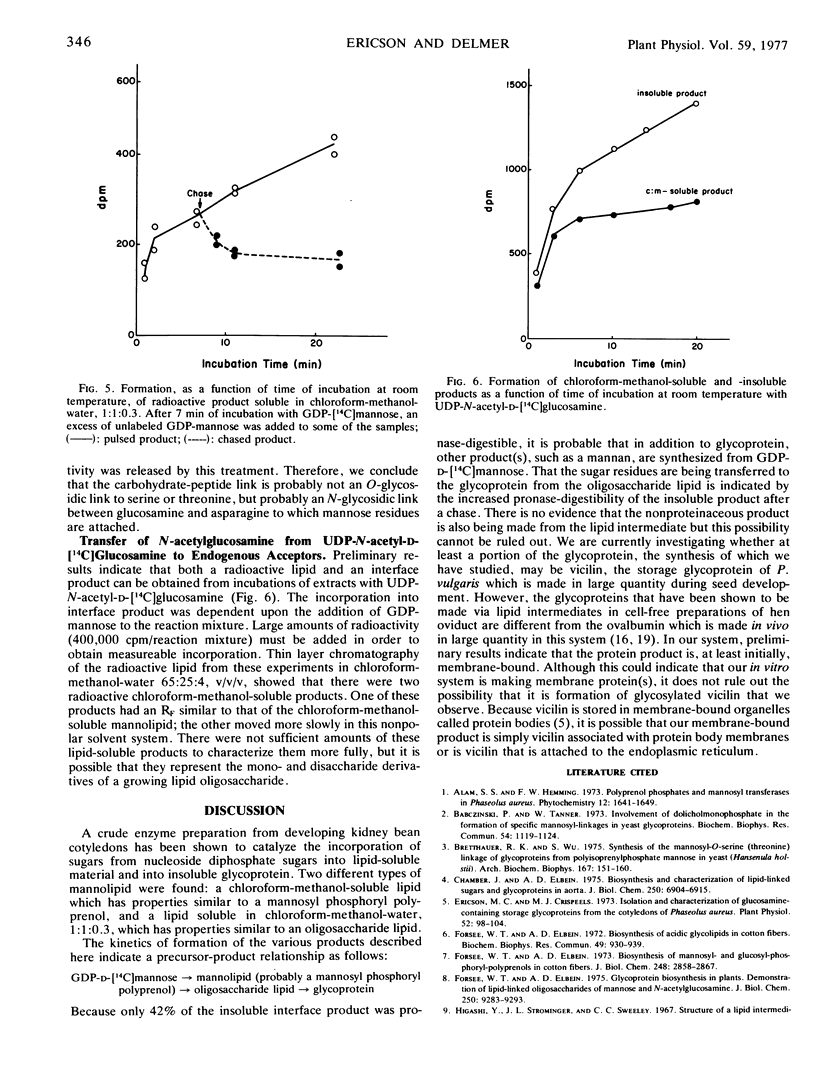
The enzymic processes involved in glycoprotein synthesis have been studied using crude extracts obtained from developing cotyledons of Phaseolus vulgaris harvested at the time of active deposition of vicilin. Radioactivity from GDP-[14C]mannose can be incorporated by crude extracts into a single chloroform-methanol-soluble product as well as into insoluble product(s). Mannose is the sole 14C-labeled constituent of the lipid. The kinetics of incorporation of 14C, as determined by pulse and pulse-chase experiments using GDP-[14C]mannose, as well as direct incorporation from added [14C]mannolipid, shows that the mannolipid is an intermediate in the synthesis of the insoluble product(s). The characteristics of the mannolipid are consistent with it being a mannosyl phosphoryl polyprenol. The mannose is apparently attached to the lipid via a monophosphate linkage. Of the radioactivity in the insoluble product(s), about 20% is pronase-digestible during a “pulse experiment.” After a chase with unlabeled GDP-mannose, about 40% is pronase-digestible; the other 60% is as yet uncharacterized. A radioactive product soluble in a mixture of chloroform-methanol-H2O can be extracted from the insoluble residue obtained during a pulse, but is no longer present after a chase. This product may be a lipid oligosaccharide, the final intermediate in glycoprotein synthesis. Data are presented on incorporation from UDP-N-[14C]acetylglucosamine into both chloroform-methanol-soluble and -insoluble product(s). The results are consistent with an involvement of lipid intermediates in the glycosylation of protein in this system, and support the concept that the mechanisms of glycoprotein synthesis in higher plants are similar to those which have been reported for mammalian systems.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babczinski P., Tanner W. Involvement of dolicholmonophosphate in the formation of specific mannosyl-linkages in yeast glycoproteins. Biochem Biophys Res Commun. 1973 Oct 1;54(3):1119–1124. doi: 10.1016/0006-291x(73)90808-5. [DOI] [PubMed] [Google Scholar]
- Bretthauer R. K., Wu S. Synthesis of the mannosyl-O-serine (threonine) linkage of glycoproteins from polyisoprenylphosphate mannose in yeast (Hansenula holstii). Arch Biochem Biophys. 1975 Mar;167(1):151–160. doi: 10.1016/0003-9861(75)90451-8. [DOI] [PubMed] [Google Scholar]
- Chambers J., Elbein A. D. Biosynthesis and characterization of lipid-linked sugars and glycoproteins in aorta. J Biol Chem. 1975 Sep 10;250(17):6904–6915. [PubMed] [Google Scholar]
- Ericson M. C., Chrispeels M. J. Isolation and Characterization of Glucosamine-containing Storage Glycoproteins from the Cotyledons of Phaseolus aureus. Plant Physiol. 1973 Aug;52(2):98–104. doi: 10.1104/pp.52.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsee W. T., Elbein A. D. Biosynthesis of acidic glycolipids in cotton fibers. Possible intermediates in cell wall synthesis. Biochem Biophys Res Commun. 1972 Nov 15;49(4):930–939. doi: 10.1016/0006-291x(72)90301-4. [DOI] [PubMed] [Google Scholar]
- Forsee W. T., Elbein A. D. Biosynthesis of mannosyl- and glucosyl-phosphoryl-polyprenols in cotton fibers. J Biol Chem. 1973 Apr 25;248(8):2858–2867. [PubMed] [Google Scholar]
- Forsee W. T., Elbein A. D. Glycoprotein biosynthesis in plants. Demonstration of lipid-linked oligosaccharides of mannose and N-acetylglucosamine. J Biol Chem. 1975 Dec 25;250(24):9283–9293. [PubMed] [Google Scholar]
- Jung P., Tanner W. Identification of the lipid intermediate in yeast mannan biosynthesis. Eur J Biochem. 1973 Aug 1;37(1):1–6. doi: 10.1111/j.1432-1033.1973.tb02949.x. [DOI] [PubMed] [Google Scholar]
- Lennarz W. J. Lipid linked sugars in glycoprotein synthesis. Science. 1975 Jun 6;188(4192):986–991. doi: 10.1126/science.167438. [DOI] [PubMed] [Google Scholar]
- Lennarz W. J., Talamo B. The chemical characterization and enzymatic synthesis of mannolipids in Micrococcus lysodeikticus. J Biol Chem. 1966 Jun 10;241(11):2707–2719. [PubMed] [Google Scholar]
- Lezica R. P., Brett C. T., Martinez P. R., Dankert M. A. A glucose acceptor in plants with the properties of an alpha-saturated polyprenyl-monophosphate. Biochem Biophys Res Commun. 1975 Oct 6;66(3):980–987. doi: 10.1016/0006-291x(75)90736-6. [DOI] [PubMed] [Google Scholar]
- Lucas J. J., Waechter J., Lennarz W. J. The participation of lipid-linked oligosaccharide in synthesis of membrane glycoproteins. J Biol Chem. 1975 Mar 25;250(6):1992–2002. [PubMed] [Google Scholar]
- Parodi A. J., Behrens N. H., Leloir L. F., Dankert M. Glucose transfer from dolichol monophosphat glucose. The lipid moiety of the endogenous microsomal acceptor. Biochim Biophys Acta. 1972 Aug 11;270(4):529–536. doi: 10.1016/0005-2760(72)90118-x. [DOI] [PubMed] [Google Scholar]
- Pless D. D., Lennarz W. J. A lipid-linked oligosaccharide intermediate in glycoprotein synthesis. Characterization of [Man-14C]glycoproteins labeled from [Man-14C]oligosaccharide-lipid and GDP-[14C]Man. J Biol Chem. 1975 Sep 10;250(17):7014–7019. [PubMed] [Google Scholar]
- Richards J. B., Hemming F. W. The transfer of mannose from guanosine diphosphate mannose to dolichol phosphate and protein by pig liver endoplasmic reticulum. Biochem J. 1972 Nov;130(1):77–93. doi: 10.1042/bj1300077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. M., Pollard W. E. The Incorporation of d-Glucosamine into Glycolipids and Glycoproteins of Membrane Preparations from Phaseolus aureus Hypocotyls. Plant Physiol. 1975 Mar;55(3):431–436. doi: 10.1104/pp.55.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J. C., Takayama K. The role of mannosylphosphorylpolyisoprenol in glycoprotein biosynthesis in Mycobacterium smegmatis. Biochim Biophys Acta. 1975 Jan 13;381(1):175–184. doi: 10.1016/0304-4165(75)90199-3. [DOI] [PubMed] [Google Scholar]


