Abstract
Purpose
The purpose of the study is to evaluate the effect of renal impairment (RI) and end-stage renal disease (ESRD) on the pharmacokinetics (PK) of isavuconazole and the inactive cleavage product, BAL8728.
Methods
A single intravenous dose of the prodrug isavuconazonium sulfate (372 mg, equivalent to 200 mg isavuconazole and 75 mg of BAL8728 cleavage product) was administered to healthy controls (parts 1 and 2) and participants with mild, moderate, or severe RI (part 2) or ESRD (part 1); ESRD participants received two doses of 200 mg isavuconazole, 1 h post-dialysis (day 1) and prior to dialysis (day 15). Plasma PK parameters for isavuconazole included maximum concentration (C max), area under the concentration–time curve (AUC) from time of dose to 72 h (AUC72), AUC extrapolated to infinity (AUC∞), AUC to last measurable concentration (AUClast), half-life (t ½ h), volume of distribution (V z), and total clearance (CL), for the healthy control group versus those with mild, moderate, or severe RI or ESRD.
Results
Isavuconazole C max values were 4% higher in mild RI and 7, 14, and 21% lower in participants with moderate RI, severe RI, or ESRD versus the healthy control group, respectively. When hemodialysis occurred post-dose (day 15), participants with ESRD had a 30% increase in AUC72 for isavuconazole in parallel with reduction of extracellular volume induced by dialysis. Exposure (AUC∞ and AUClast) was not significantly different for participants with mild, moderate, or severe RI versus healthy controls although there was considerable variability. The t1/2 (day 1) was 125.5 ± 63.6 h (healthy control group), 204.5 ± 82.6 h (ESRD group) in part 1, and 140.5 ± 77.7 h (healthy control group), 117.0 ± 66.2 h (mild RI), 158.5 ± 56.4 h (moderate RI), and 145.8 ± 65.8 L/h (severe RI) in part 2. CL was 2.4 ± 0.8 L/h (healthy control group) and 2.9 ± 1.3 L/h (ESRD group) in part 1 and 2.4 ± 1.2 L/h (healthy control group), 2.5 ± 1.0 L/h (mild RI), 2.2 ± 0.8 L/h (moderate RI), and 2.4 ± 0.8 L/h (severe RI) in part 2. The V z was 382.6 ± 150.6 L in the healthy control group and 735.6 ± 277.3 L in ESRD patients on day 1 in part 1 of the study. In part 2 of the study, V z was 410.8 ± 89.7 L in the healthy control group, 341.6 ± 72.3 L in mild RI, 509.1 ± 262.2 L in moderate RI, and 439.4 L in severe RI.
Conclusions
Based on the findings of this study, dose adjustments of isavuconazole are unlikely to be required in individuals with RI or in those with ESRD who receive hemodialysis.
Electronic supplementary material
The online version of this article (doi:10.1007/s00228-017-2213-7) contains supplementary material, which is available to authorized users.
Keywords: End-stage renal disease, Isavuconazole, Pharmacokinetics, Renal impairment
Introduction
Invasive fungal diseases (IFD), predominantly aspergillosis, are a prevalent cause of morbidity and mortality in immunocompromised patients, such as those with hematological malignancies or those undergoing transplantation [1–4]. Renal impairment (RI) is an independent risk factor for mortality in both hematopoietic stem cell transplant and solid organ transplant patients with invasive aspergillosis (IA) [5]. In intensive care units, 43% of patients with IA infections experience acute renal failure, which contributes to the mortality associated with IA [6]. The renal excretion of drugs and/or their metabolites may be hindered in patients with RI, and this could lead to an excessive accumulation of the drug in the body [7]. Conversely, hemodialysis may result in removal of some drugs, and thereby, additional doses may be required to prevent underdosing [8]. Triazole antifungal agents are pivotal in the treatment of IA [9]; however, their use may be restricted in patients with RI [10, 11]. Voriconazole and posaconazole may have restricted use in patients with moderate-to-severe RI due to the accumulation of the vehicle cyclodextrin used in their intravenous (IV) formulations [10–12]. Caution is also recommended for the use of itraconazole in patients with RI due to limited data on the use of this drug in this patient population [13]. Therefore, there is a requirement for potent antifungal agents that are efficacious and well tolerated to combat IFD in patients with RI.
Isavuconazonium sulfate is a water-soluble prodrug of the novel, broad-spectrum, triazole antifungal agent isavuconazole, which was developed to facilitate IV administration without the need for nephrotoxic excipients [14, 15]. Isavuconazonium sulfate is rapidly converted in plasma to the active triazole isavuconazole and the inactive cleavage product BAL8728. The per-oral (PO) capsules and cyclodextrin-free IV formulations of the prodrug are approved for the primary treatment of adults with IA and invasive mucormycosis by the US Food and Drug Administration (FDA) [16]. Isavuconazole is also approved by the European Medicines Agency (EMA) for the treatment of IA and treatment of invasive mucormycosis when amphotericin B is inappropriate [17].
A formal renal study using the final formulation, isavuconazonium sulfate, was conducted in accordance with the FDA and the EMA guidance on the evaluation of the pharmacokinetics (PK) of medicines in patients with impaired renal function [18, 19]. The objective of this study was to evaluate the effect of RI (mild, moderate, or severe) and end-stage renal disease (ESRD) on the PK of isavuconazole compared with the PK in healthy participants with normal renal function.
Methods
Study design
This was a phase I, open-label, single-dose parallel group study in male and female participants conducted in two parts (ClinicalTrials.gov NCT01555866 covering parts 1 and 2). Part 1 was a single-center study of isavuconazole administered to healthy participants with renal function in the normal range (referred to as the healthy control group) and those with ESRD requiring dialysis. Part 2 was a multi-center study of isavuconazole administered to a healthy control group and those with mild, moderate, or severe RI.
Both parts of the study were conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Guidelines for Good Clinical Practice. For all sites, approval of the protocol (9766-CL-0018) was obtained from the governmental authorities and Institutional Review Board(s).
Eligibility
Male and female participants aged 18–65 years, weighing ≥45 kg, and with a body mass index of 18–35 kg/m2 were enrolled. At screening, RI was based on the Cockcroft–Gault (CG) formula and adjusted for body surface area (BSA), then grouped as healthy control group (creatinine clearance (CLcr) >80 mL/min/1.73 m2), participants with ESRD and requiring hemodialysis (CLcr < 15 mL/min/1.73 m2), and participants with RI: mild (CLcr 50–80 mL/min/1.73 m2), moderate (CLcr 30–<50 mL/min/1.73 m2), and severe CLcr (<30 mL/min/1.73 m2). Participants were selected by age, sex, weight, and smoking status so that the ranges were similar between the healthy control group and each of the groups with RI.
Assessments
Each participant in part 1 and part 2 of the study received a single 1-h IV infusion of isavuconazonium sulfate 372 mg (equivalent to 200 mg isavuconazole) on day 1 (approximately 1 h after completion of their routine hemodialysis procedure in participants with ESRD). Participants with ESRD in part 1 of the study received an additional dose just prior to dialysis on day 15.
Blood samples for isavuconazole and BAL8728 plasma concentrations were collected pre-dose to 72 h post-dose on days 1 and 15 for ESRD participants and pre-dose to 72 h post-dose on day 1 for the healthy control group and RI participants. Single blood samples were taken from ESRD and RI participants on days 6, 8, 11, 13, and 15. During dialysis, samples were collected simultaneously at the inlet and outlet sides of the dialyzer as well as from the dialysate. For all participants, an additional blood sample was obtained at 4 h post-dose on day 1 for analysis of isavuconazole fraction unbound (fu).
In participants who produced urine, samples for the bioanalysis of isavuconazole and BAL8728 were collected up to 72 h post-dose on day 1. Renal function was assessed using the CG method adjusted for BSA using the following formula:
where Scr is serum creatinine.
Estimated glomerular filtration rate (eGFR) using the abbreviated Modification of Diet in Renal Disease (MDRD) formula was calculated using the following formula:
Pharmacokinetic assessments
Due to the extensive protein binding of isavuconazole to plasma proteins, the PK parameters reported in this study were based on total isavuconazole concentrations in plasma. Plasma PK sampling time points included pre-dose (prior to start of infusion), upon completion of infusion (obtained 1 min prior to end of infusion), 1.5, 2, 3, 4, 5, 6, 8, 12, 24 (day 2), 36 (day 2), 48 (day 3), 72 (day 4), 120 (day 6), 168 (day 8), 240 (day 11), 288 (day 13), 336 (day 15) h after the start of infusion. The primary plasma PK parameters for isavuconazole were area under the concentration–time curve (AUC) from time of dosing to 72 h (AUC72) and maximum concentration (C max) for the healthy control group compared with participants with ESRD, AUC from time of dosing extrapolated to infinity (AUC∞), AUC from time of dosing to last measurable plasma concentration (AUClast), and C max for the healthy control group compared with participants with mild, moderate, or severe RI. Additional PK parameters for isavuconazole included time to reach C max (t max), total clearance (CL), half-life (t ½), and volume of distribution (V z). PK parameters for BAL8728 included: healthy, ESRD, and RI participants (day 1): AUC∞, AUC72, AUClast, C max, t max, t 1/2, V z, and CL: ESRD participants (day 15): AUC72, C max, and t max.
Urine was collected for all able subjects over the following time intervals: day 1 pre-dose (−2 to 0) 0–6, 6–12, 12–24, 24–48, and 48–72 h after start of infusion. PK parameters included the amount and percentage of drug excreted unchanged in the urine (Aelast/Ae72, for the dialysis comparisons) for all participants and renal clearance (CLR calculated as Aelast/AUClast) at day 1 for the healthy control group and participants with mild, moderate, or severe RI; dialysis clearance (CLD) at day 15 was also assessed for participants with ESRD.
Plasma PK parameters were calculated using WinNonlin® version 5.2 or higher (Certara, Princeton, NJ, USA).
Safety assessments
Treatment-emergent adverse events (TEAEs; defined as adverse events that started any time after the first dose of study drug was administered through the follow-up visit) were assessed for all participants. The number and percentage of participants with TEAEs were summarized for each renal function group by system organ class.
Statistical analysis
A sample size of 16 participants (8 per group) in part 1 of the study and 32 participants (8 per group) in part 2 of the study was determined based on the precedent set by other PK studies similar in design. No formal sample size calculation was performed.
The PK analyses used two approaches: One approach compared PK between each renal impaired group (CG method CLcr), and a second approach compared the relationship between PK and eGFR (MDRD method). Descriptive statistics (number of participants, mean, and standard deviation, minimum, median, and maximum) were used to summarize continuous variables. Descriptive statistics used for categorical variables consisted of frequency and percentage of participants in each category. In addition, for PK parameters, geometric mean and coefficient of variation were also determined.
To assess the effect of RI on the PK of isavuconazole and BAL8728, an analysis of covariance (ANCOVA) was performed on natural log-transformed AUC∞, AUClast, and C max with renal function group (mild, moderate, or severe RI and healthy control group) as a fixed effect and age, sex, and current smoking status as covariates. The effect of time of dialysis relative to dosing on the PK of isavuconazole and BAL8728 was assessed using ANCOVA on the natural log-transformed AUC72 and C max between day 1 and day 15 (calculated using pre-dialysis access line concentrations), while subjects were on dialysis from the ESRD group with the visit as a fixed effect (day 1 and day 15), the subject as a random effect, and weight on day 1 and day 15 as a covariate. The 90% confidence intervals (CIs) around the geometric least square mean (LSM) ratios (day 15/day 1) of AUC72 and C max were constructed.
Covariates were assessed at the 0.1 significance level and removed from the model if insignificant. The 90% CIs around the geometric LSM ratios (severe/healthy control group, moderate/healthy control group, and mild/healthy control group) of AUC∞, AUClast, and C max were constructed. No effect of RI on PK was declared if the corresponding CIs for the ratio fell completely within the interval (70%, 143%) for all three parameters of isavuconazole.
Safety data were analyzed using descriptive statistics. All statistical analyses were performed using SAS® version 9.1 or higher (Statistical Analysis Software, Cary, NC, USA).
Results
Patient characteristics
A total of 20 participants were enrolled, and 19 completed part 1 of the study; 29 participants were enrolled and completed part 2 of the study (Table 1). Only five participants were enrolled in the severe RI group due to slow recruitment.
Table 1.
Demographics and characteristics of participants
| Study part 1 | Study part 2 | |||||
|---|---|---|---|---|---|---|
| Healthy control group (n = 9) | ESRD (n = 11) | Healthy control group (n = 8) | Mild RI (n = 8) | Moderate RI (n = 8) | Severe RI (n = 5) | |
| Age [years], median (range) | 48 (19–64) | 52 (20–64) | 51 (34–57) | 63 (51–65) | 56 (34–61) | 59 (50–62) |
| Males, n (%) | 5 (55.6) | 5 (45.5) | 5 (62.5) | 5 (62.5) | 3 (37.5) | 5 (100) |
| Race, n (%) | ||||||
| White | 8 (88.9) | 1 (9.1) | 6 (75.0) | 8 (100) | 4 (50.0) | 3 (60.0) |
| Black or African American | 0 | 10 (90.9) | 2 (25.0) | 0 | 3 (37.5) | 2 (40.0) |
| Other | 1 (11.1) | 0 | 0 | 0 | 1 (12.5) | 0 |
| Ethnicity, n (%) | ||||||
| Not Hispanic or Latino | 9 (100) | 11 (100) | 4 (50.0) | 3 (37.5) | 5 (62.5) | 3 (60.0) |
| eGFR-CG, mean ± SDa
eGFR-MDRD, mean ± SDb |
104.1 ± 18.6 90.7 ± 9.1 |
8.7 ± 2.3 6.6 ± 1.8 |
104.6 ± 17.9 94.7 ± 19.8 |
67.7 ± 7.7 64.1 ± 12.4 |
39.9 ± 5.7 32.1 ± 5.9 |
18.5 ± 5.4 14.8 ± 5.2 |
CG Cockcroft–Gault method, eGFR estimated glomerular filtration rate, ESRD end-stage renal disease, MDRD modification of diet in renal disease, RI renal impairment, SD standard deviation
aeGFR-CG (mL/min/1.73 m2) × Body surface area/1.73
beGFR-MDRD (mL/min/1.73 m2)
Dialysis and PK of isavuconazole and BAL8728
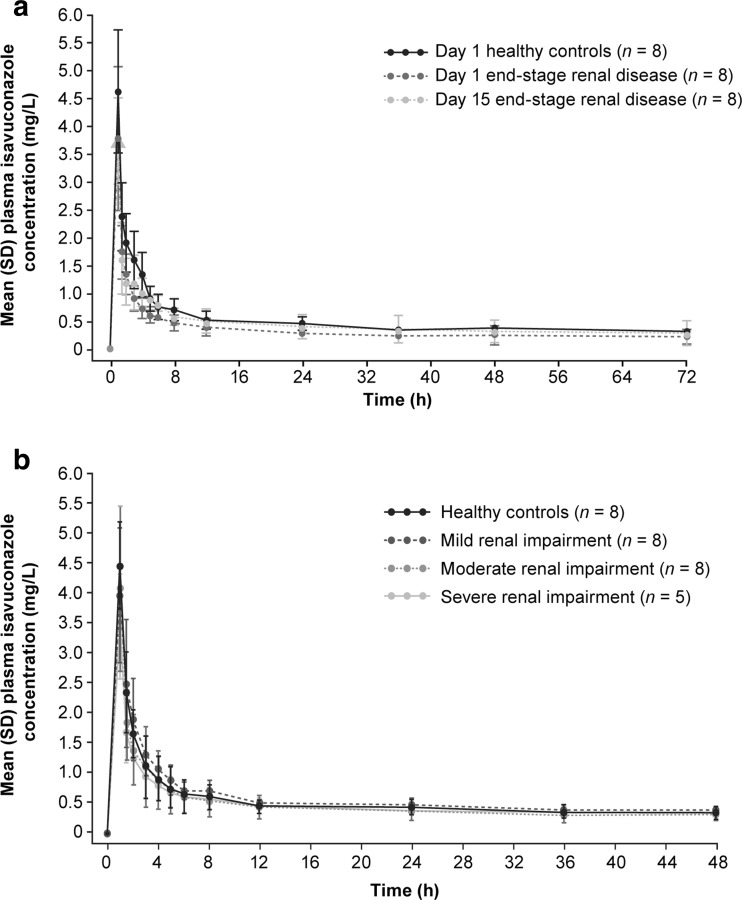
Mean plasma concentration–time profiles for isavuconazole in participants with ESRD compared with the healthy control group are shown in Fig. 1. More than 99.9% of isavuconazole was bound to protein in samples from all treatment groups. On day 1, when isavuconazonium sulfate was administered as a 1-h IV infusion post-hemodialysis in ESRD participants, there was a 34% decrease in AUC72 of isavuconazole and a 21% decrease in C max compared with the healthy control group dosed under similar conditions (Table 2). BAL8728 C max values were 2% lower in participants with ESRD, compared with the healthy control group. The AUC72 for isavuconazole increased by 30% and the AUC72 for BAL8728 decreased by 22% (Table 2) with dosing of isavuconazole prior to dialysis in participants with ESRD. The day 15 result was similar to the AUC72 results obtained for the healthy control group on day 1 (Table 3). The mean t 1/2 of total isavuconazole was approximately 1.6-fold longer in participants with ESRD versus the healthy control group (Table 3). Less than 1% of the administered isavuconazole was recovered in dialysate fluid, consistent with the low dialysis clearance (CLD) of 292 mL/h.
Fig. 1.
Mean (standard deviation [SD]) plasma concentration–time profiles for isavuconazole in a healthy control group (day 1) versus participants with end-stage renal disease on both day 1 and day 15 and b for participants with mild, moderate, and severe RI versus the healthy control group
Table 2.
Geometric LSM ratios for isavuconazole and BAL8728 in patients with ESRD versus healthy controls and for ESRD at day 15 versus day 1
| Parameter | Ratio study group/study group | Geometric LSM ratio % | 90% CI |
|---|---|---|---|
| Isavuconazole | |||
| AUC72
C max |
ESRD/healthy controls ESRD/healthy controls |
66.3 79.3 |
50.8–86.7 60.6–103.9 |
| AUC72 | ESRD day 15/ESRD day 1 | 130.5 | 122.8–138.6 |
| BAL8728 | |||
| AUC72
C max |
ESRD/healthy controls ESRD/healthy controls |
110.3 97.7 |
85.4–142.4 76.0–125.7 |
| AUC72 | ESRD day 15/ESRD day 1 | 78.2 | 68.6–89.0 |
AUC 72 area under the concentration curve at 72 h, CI confidence intervals, C max maximum plasma concentration, ESRD end-stage renal disease, LSM least square mean
Table 3.
Isavuconazole and BAL8728 pharmacokinetic parameters for participants with ESRD and healthy control group
| Parameter | Isavuconazole | BAL8728 | ||||
|---|---|---|---|---|---|---|
| Healthy control group (n = 8)a | ESRD day 1 (n = 8)b | ESRD day 15 (n = 8) | Healthy control group (n = 8)a | ESRD day 1 (n = 8)b | ESRD day 15 (n = 8) | |
| AUC72, mg*h/L | 36.9 ± 9.5 | 25.1 ± 10.0 | 32.3 ± 15.4 | 1.2 ± 0.4 | 1.3 ± 0.3 | 0.9 ± 0.2 |
| C max, mg/L | 4.6 ± 1.1 | 3.7 ± 1.3 | 3.7 ± 0.8 | 0.9 ± 0.2 | 0.9 ± 0.3 | 0.9 ± 0.2 |
| AUC∞, mg*h/L | 94.7 ± 32.3 | 95.7 ± 78.6 | – | 1.2 ± 0.4 | 1.3 ± 0.3 | – |
| AUClast, mg*h/L | 77.9 ± 22.1 | 62.0 ± 40.2 | – | 1.1 ± 0.3 | 1.2 ± 0.3 | – |
| t max, h | 1.0 (1.0–1.1) | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 1.0 (1.0–1.1) | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) |
| t ½, h | 125.5 ± 63.3 | 204.5 ± 82.6 | – | 1.3 ± 0.1 | 1.5 ± 0.3 | – |
| V z, L | 386.2 ± 150.5 | 735.6 ± 277.3 | – | 133.3 ± 35.2 | 144.3 ± 64.3 | – |
| CL, L/h | 2.4 ± 0.8 | 2.9 ± 1.3 | – | 70.5 ± 23.0 | 64.5 ± 23.9 | – |
| Aelast, % | 0.5 ± 0.2 | – | – | – | – | – |
| CLR, mL/h | 12.5 ± 5.5 | – | – | – | – | – |
| CLD, mL/h | – | 291.7 ± 87.4 | – | – | – | – |
All PK data expressed as mean ± standard deviation, except t max, which is expressed as median (range)
Ae last cumulative amount of unchanged isavuconazole excreted in the urine, AUC area under the concentration–time curve, AUC 72 AUC from time of dosing until 72 h, AUC ∞ AUC extrapolated to infinity, AUC last AUC to last measurable plasma concentration, C max maximum concentration of isavuconazole, CL total clearance of isavuconazole, CL D dialysis clearance of isavuconazole, CL R renal clearance of isavuconazole from plasma, ESRD end-stage renal disease, t max time to reach maximum concentration, t ½ half-life of isavuconazole
aOne participant discontinued on day 1
bPharmacokinetic results for three participants with ESRD were unavailable due to a handling error during sample collection resulting in the contamination of C max values
Isavuconazole and BAL8728 PK in renal impairment
There were no consistent changes in t 1/2 of isavuconazole or BAL8728 plasma concentrations observed in participants with mild-to-severe RI versus healthy control group (Table 4; Supplementary Table S1). Compared with the healthy control group, plasma BAL8728 AUC72 for subjects with ESRD was 10% higher, whereas the plasma AUC∞ in mild, moderate, or severe RI groups were 29, 4, and 24% higher, respectively. Ae % and CLR for both isavuconazole and BAL8728 decreased with increasing RI (mild to severe). BAL8728 C max values in participants with mild and severe RI were 16 and 11% higher, respectively, and 3% lower in participants with moderate RI.
Table 4.
Isavuconazole pharmacokinetic parameters for day 1 for healthy participants compared with individuals with renal impairment
| Parameter | Healthy control group (n = 8) | Mild RI (n = 8) | Moderate RI (n = 8) | Severe RI (n = 5) |
|---|---|---|---|---|
| AUC∞, mg*h/L | 98.8 ± 50.5 | 96.2 ± 46.9 | 97.2 ± 26.3 | 98.8 ± 53.9 |
| AUClast, mg*h/L | 75.8 ± 22.9 | 77.0 ± 22.8 | 74.0 ± 20.1 | 73.6 ± 19.9 |
| t max, h | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) |
| t ½, h | 140.5 ± 77.7 | 117.0 ± 66.2 | 158.5 ± 56.4 | 145.8 ± 65.8 |
| C max, mg/L | 4.4 ± 0.7 | 3.9 ± 1.1 | 4.1 ± 1.4 | 3.4 ± 0.9 |
| CL, L/h | 2.4 ± 1.2 | 2.5 ± 1.0 | 2.2 ± 0.8 | 2.4 ± 0.8 |
| Aelast, % | 0.4 ± 0.2 | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.03 |
| CLR, mL/h | 14.0 ± 13.3 | 6.8 ± 4.3 | 3.4 ± 2.7 | 2.0 ± 0.9 |
| V z, L | 410.8 ± 89.7 | 341.6 ± 72.3 | 509.1 ± 262.2 | 439.4 ± 65.4 |
All data are expressed as mean ± standard deviation, except t max which is expressed as median (range)
Ae last cumulative amount of unchanged isavuconazole excreted in the urine, AUC area under the concentration–time curve, AUC 72 AUC from time of dosing until 72 h, AUC ∞ AUC extrapolated to infinity, AUC last AUC to last measurable plasma concentration, CL total clearance of isavuconazole, CL R renal clearance of isavuconazole from plasma, RI renal impairment, t max time to reach maximum concentration, t ½ half-life of isavuconazole, V z volume of distribution
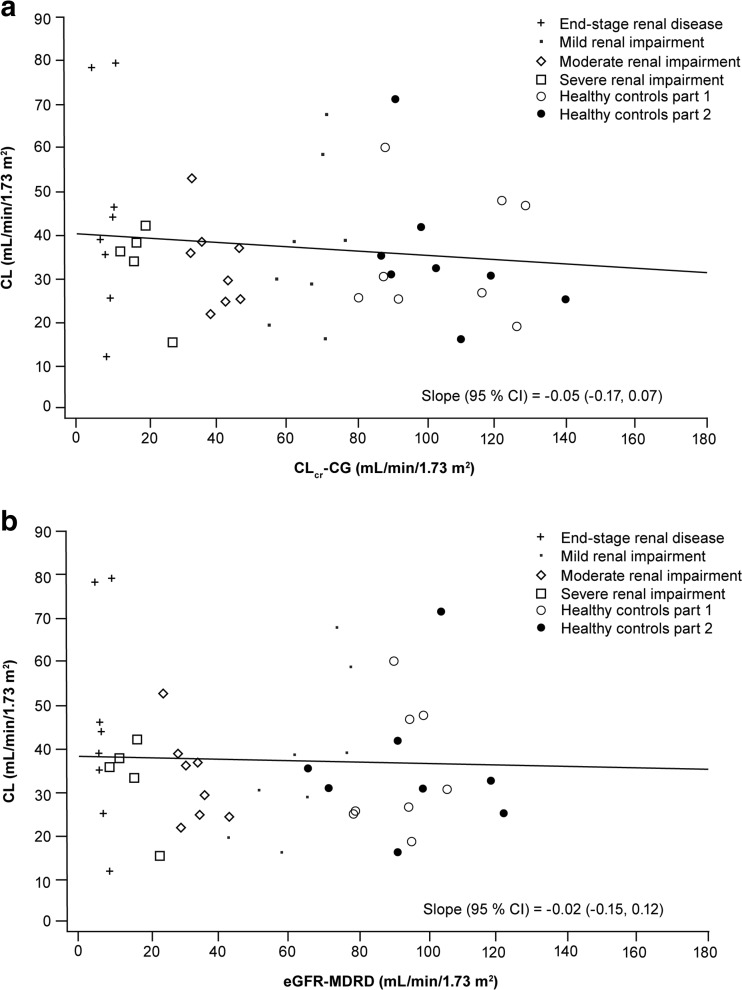
There was no significant relationship between total plasma isavuconazole PK parameters (C max and CL) with continuous markers of renal function CLcr and eGFR using either the CG or the abbreviated MDRD formula (Fig. 2, Supplementary Fig. S1). No correlation was identified between BAL8728 PK parameters and markers of renal function (data not shown).
Fig. 2.
The relationship between total clearance of isavuconazole (CL) and renal function in relation to creatinine clearance (CLcr) by Cockcroft–Gault (CG) method (a) and estimated glomerular filtration rate (eGFR) by the Modification of Diet in Renal Disease (MDRD) method (b). CI as confidence intervals
Urinary excretion
Isavuconazole urinary clearance decreased in parallel with a decrease in renal function (Supplementary Table S2). The amount of isavuconazole excreted unchanged in urine samples was 0.07% of the total dose in patients with severe RI compared with 0.44% in the healthy control group. The small volume of the dialysis clearance in participants with ESRD was consistent with the highly albumin-bound nature of isavuconazole (Supplementary Table S2). BAL8728 was not detected in dialysate samples.
Safety
Most TEAEs were considered mild. No participant experienced a TEAE that was considered severe, and no deaths were reported during the course of the study. However, one healthy participant in part 1 of the study experienced a TEAE (chest discomfort) during IV administration of isavuconazole that was considered related to the study drug and led to discontinuation from the study (Table 5).
Table 5.
Summary of treatment-emergent adverse eventsa
| Parameter n (%) | Study part 1 | Study part 2 | ||||
|---|---|---|---|---|---|---|
| Healthy control group (n = 9) | ESRD (n = 11) | Healthy control group (n = 8) | Mild RI (n = 8) | Moderate RI (n = 8) | Severe RI (n = 5) | |
| TEAEs | 7 (77.8) | 7 (63.6) | 4 (50.0) | 5 (62.5) | 5 (62.5) | 4 (80.0) |
| Drug-related TEAEs | 7 (77.8) | 7 (63.6) | 4 (50.0) | 4 (50.0) | 5 (62.5) | 3 (60.0) |
| TEAEs leading to study discontinuation | 1 (11.1)b | 0 | 0 | 0 | 0 | 0 |
| Most common TEAEsc | ||||||
| General disorders and administration site conditions | 6 (66.7) | 4 (36.4) | 2 (25.0) | 2 (25.0) | 3 (37.5) | 3 (60.0) |
| Nervous system disorders | 3 (33.3) | 4 (36.4) | 2 (25.0) | 2 (25.0) | 4 (50.0) | 1 (20.0) |
| Gastrointestinal disorders | 0 | 3 (27.3) | 1 (12.5) | 1 (12.5) | 2 (25.0) | 2 (40.0) |
| Infections and infestations | 0 | 2 (18.2) | 0 | 0 | 0 | 1 (20.0) |
| Skin and subcutaneous tissue | 0 | 2 (18.2) | 1 (12.5) | 0 | 0 | 0 |
ESRD end-stage renal disease, MedDRA medical dictionary for regulatory activities, RI renal impairment, TEAE treatment-emergent adverse event
aBy MedDRA version 12.1 system organ class
bTEAE was considered to be drug-related in this patient
cTEAEs occurring in ≥2 patients overall
Discussion
This study showed that there was no significant impact of renal function measured by either CLcr or eGFR on isavuconazole AUC and C max values. The AUC∞ and AUClast of plasma isavuconazole in participants with mild, moderate, or severe RI were not significantly different compared with healthy participants with normal renal function. The PK parameters of isavuconazole in plasma were similar between healthy participants with normal renal function and participants with mild, moderate, or severe RI.
Accurate assessment of kidney function is essential for determining appropriate drug dosing regimens [20]. Therefore, eGFR-MDRD equations have been developed to more accurately assess renal function and renal impairment and appropriate drug dosage adjustments [20]. Historically, the CG equation was the method most commonly used to assess drug dosage adjustments in renally impaired patients in clinical practice [20]. Currently, eGFR using the MDRD approach is an alternative approach to the CG method to determine drug dosages in patients with renal impairment [20–22]. However, previous studies have shown discordance rates of up to 40% and significant difference in drug dosing regimens between the MDRD and CG methods [21, 22]. We found no significant relationship between either total plasma isavuconazole C max or total body clearance from plasma and renal function assessed by CLcr (CG) or eGFR (MDRD). This is consistent with a population PK study which showed that eGFR used as a covariate did not have a significant effect on clearance of isavuconazole [23]. These findings add further support that dose adjustments of isavuconazole are unlikely to be required in individuals with RI or in those with ESRD who are receiving hemodialysis.
Two approaches were used in the analysis of data: The first approach examined renal function by categorically grouping the severity of impairment (mild, moderate, severe or ESRD as defined by CLcr by CG), and the second approach examined renal function as a continuous variable (eGFR or CLcr) related to PK parameters to the measure of renal function. Grouping by severity of renal impairment parallels the clinical approach found in national and international guidelines and is relevant to clinicians familiar with these guidelines [24–29]. The continuous variable approach was objective and independent of empirical classification. Consistency between the two approaches adds robustness to the findings of this study and provides support to its conclusions.
In participants with ESRD, the decrease in AUC and C max and wide variability for each when dialysis preceded drug dosing were influenced by intercompartmental fluid shifts intrinsic to hemodialysis and post-dialysis recovery. The clearance of drugs by conventional hemodialysis is predominantly a passive diffusional process driven by unbound concentration gradient between plasma water and dialysate [8, 30]. As the binding of drug to plasma proteins increases, removal of drug by dialysis will decrease [31]. Therefore, hemodialysis did not clear isavuconazole from the plasma of individuals with ESRD due to the high protein binding of isavuconazole (>99.9%) predominantly to albumin. However, the longer half-life and volume of distribution of isavuconazole in individuals with ESRD may also be due to decreased plasma binding by albumin due to uremia which may impact drug metabolism by the liver [32]. The increase in AUC in dialysis patients may be due to the displacement of isavuconazole from albumin by heparin while patients are on dialysis which has been reported for some other drugs [33]. In view of the low and intermittent dialytic clearance of isavuconazole from plasma, it can be concluded that clearance of isavuconazole by thrice weekly dialysis is unlikely to have any appreciable effects on the PK of isavuconazole in ESRD patients. Therefore, post-dialysis supplementation of isavuconazole is unlikely to be required. Conversely, if isavuconazole is inadvertently overdosed, the overdose cannot be effectively managed by hemodialysis.
Analysis of isavuconazole PK across renal function as a continuous variable showed no significant impact of renal function measured by eGFR on C max and AUC. Although differences in the renal excretion of isavuconazole were observed among groups with differing levels of renal impairment, the overall level of renal excretion was quite small and the observed differences would not be expected to impact on the PK of isavuconazole in any significant way.
In this study, a single IV infusion of isavuconazole was generally well tolerated by individuals with normal renal function; those with mild, moderate, or severe RI; and those with ESRD. The number and percentage of participants experiencing TEAEs were low and generally similar between groups, and most TEAEs were considered mild. However, more individuals in the ESRD and severe RI groups experienced gastrointestinal disorders compared with those with normal renal function. However, for those receiving hemodialysis, consideration should be given to administering isavuconazole pre-dialysis. Based on the findings of this study, dose adjustments of isavuconazole are unlikely to be required in individuals with RI or in those with ESRD who are receiving hemodialysis.
Electronic supplementary material
(DOC 33 kb)
(DOC 31 kb)
The relationship between maximum concentration (Cmax) of isavuconazole and creatinine clearance (CLcr) by the Cockcroft Gault (CG) method (a) and estimated Glomerular Filtration Rate (eGFR) by the Modification of Diet in Renal Disease (b) (GIF 51 kb)
Acknowledgements
Isavuconazonium sulfate was co-developed by Astellas Pharma Global Development, Inc., Northbrook, IL, USA, and Basilea Pharmaceutica International Ltd., Basel, Switzerland.
Compliance with ethical standards
Conflict of interest
R.W. Townsend, D.L. Kowalski, S. Mujais, and A.V. Desai are all employees of Astellas Pharma Global Development, Inc. S. Akhtar was an employee of Astellas Pharma Global Development, Inc. at the time of the study. H. Alcorn and J. Berg are employees of DaVita Clinical Research who were contracted by Astellas Pharma Global Development, Inc. to perform this trial. This study was funded by Astellas Pharma Global Development, Inc. Editorial support was provided by John Clarke, Envision Pharma Group, Horsham, UK, funded by Astellas Pharma Global Development, Inc.
References
- 1.Akan H, Antia VP, Kouba M, Sinko J, Tanase AD, Vrhovac R, et al. Preventing invasive fungal disease in patients with haematological malignancies and the recipients of haematopoietic stem cell transplantation: practical aspects. J Antimicrob Chemother. 2013;68(Suppl 3):iii5–ii16. doi: 10.1093/jac/dkt389. [DOI] [PubMed] [Google Scholar]
- 2.Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 2010;50(8):1091–1100. doi: 10.1086/651263. [DOI] [PubMed] [Google Scholar]
- 3.Marr KA, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34(7):909–917. doi: 10.1086/339202. [DOI] [PubMed] [Google Scholar]
- 4.Pagano L, Caira M, Candoni A, Offidani M, Fianchi L, Martino B, et al. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica. 2006;91(8):1068–1075. [PubMed] [Google Scholar]
- 5.Baddley JW, Andes DR, Marr KA, Kontoyiannis DP, Alexander BD, Kauffman CA, et al. Factors associated with mortality in transplant patients with invasive aspergillosis. Clin Infect Dis. 2010;50(12):1559–1567. doi: 10.1086/652768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vandewoude KH, Blot SI, Benoit D, Colardyn F, Vogelaers D. Invasive aspergillosis in critically ill patients: attributable mortality and excesses in length of ICU stay and ventilator dependence. J Hosp Infect. 2004;56(4):269–276. doi: 10.1016/j.jhin.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Verbeeck RK, Musuamba FT. Pharmacokinetics and dosage adjustment in patients with renal dysfunction. Eur J Clin Pharmacol. 2009;65(8):757–773. doi: 10.1007/s00228-009-0678-8. [DOI] [PubMed] [Google Scholar]
- 8.Bohler J, Donauer J, Keller F. Pharmacokinetic principles during continuous renal replacement therapy: drugs and dosage. Kidney Int Suppl. 1999;72:S24–S28. doi: 10.1046/j.1523-1755.56.s.72.2.x. [DOI] [PubMed] [Google Scholar]
- 9.Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46(3):327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 10.Ader F, Bienvenu AL, Rammaert B, Nseir S. Management of invasive aspergillosis in patients with COPD: rational use of voriconazole. Int J Chron Obstruct Pulmon Dis. 2009;4:279–287. doi: 10.2147/COPD.S4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girmenia C. New generation azole antifungals in clinical investigation. Expert Opin Investig Drugs. 2009;18(9):1279–1295. doi: 10.1517/13543780903176407. [DOI] [PubMed] [Google Scholar]
- 12.von Mach MA, Burhenne J, Weilemann LS. Accumulation of the solvent vehicle sulphobutylether beta cyclodextrin sodium in critically ill patients treated with intravenous voriconazole under renal replacement therapy. BMC Clin Pharmacol. 2006;6:6. doi: 10.1186/1472-6904-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janssen Pharmaceutica N.V (2001) Sporanox® (itraconazole) prescribing information. Available at: http://www.janssen.com/us/sites/www_janssen_com_usa/files/products-documents/pi-sporanoxcapsules.pdf; visited 16 Feb 2017
- 14.Miceli MH, Kauffman CA. Isavuconazole: a new broad-spectrum triazole antifungal agent. Clin Infect Dis. 2015;61(10):1558–1565. doi: 10.1093/cid/civ571. [DOI] [PubMed] [Google Scholar]
- 15.Seyedmousavi S, Verweij PE, Mouton JW. Isavuconazole, a broad-spectrum triazole for the treatment of systemic fungal diseases. Expert Rev Anti-Infect Ther. 2015;13(1):9–27. doi: 10.1586/14787210.2015.990382. [DOI] [PubMed] [Google Scholar]
- 16.Astellas Pharma US I (2015) CRESEMBA® (isavuconazonium sulfate) prescribing information. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207500Orig1s000lbl.pdf; visited 16 Feb 2017
- 17.European Medicines Agency (2015) Cresemba Isavuconazole. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002734/human_med_001907.jsp&mid=WC0b01ac058001d124; visited 16 Feb 2017
- 18.US Food and Drug Administration (2010) Guidance for industry: pharmacokinetics in patients with impaired renal function—study design, data analysis, and impact on dosing and labeling. Draft guidance [revision 1]. Available at: http://www.fda.gov/downloads/Drugs/.../Guidances/UCM204959.pdf; visited 16 Feb 2017
- 19.European Medicines Agency Committee for Medicinal Products for Human Use (CHMP) (2004) Note for guidance on the evaluation of the pharmacokinetics of medicinal products in patients with impaired renal function. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003123.pdf; visited 9 Dec 2015
- 20.Nyman HA, Dowling TC, Hudson JQ, Peter WL, Joy MS, Nolin TD (2011) Comparative evaluation of the Cockcroft-Gault equation and the Modification of Diet in Renal Disease (MDRD) study equation for drug dosing: an opinion of the Nephrology Practice and Research Network of the American College of Clinical Pharmacy. Pharmacotherapy 31(11):1130–1144. doi:10.1592/phco.31.11.1130 [DOI] [PubMed]
- 21.Golik MV, Lawrence KR (2008) Comparison of dosing recommendations for antimicrobial drugs based on two methods for assessing kidney function: Cockcroft-Gault and Modification of Diet in Renal Disease. Pharmacotherapy 28(9):1125–1132. doi:10.1592/phco.28.9.1125 [DOI] [PubMed]
- 22.Hermsen ED, Maiefski M, Florescu MC, Qiu F, Rupp ME (2009) Comparison of the Modification of Diet in Renal Disease and Cockcroft-Gault equations for dosing antimicrobials. Pharmacotherapy 29(6):649–655. doi:10.1592/phco.29.6.649 [DOI] [PubMed]
- 23.Kovanda LL, Desai AV, Lu Q, Townsend RW, Akhtar S, Bonate P, et al. Isavuconazole population pharmacokinetic analysis using nonparametric estimation in patients with invasive fungal disease (results from the VITAL study) Antimicrob Agents Chemother. 2016;60(8):4568–4576. doi: 10.1128/AAC.00514-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chertow GM, Lee J, Kuperman GJ, Burdick E, Horsky J, Seger DL, et al. Guided medication dosing for inpatients with renal insufficiency. JAMA. 2001;286(22):2839–2844. doi: 10.1001/jama.286.22.2839. [DOI] [PubMed] [Google Scholar]
- 25.Drenth-van Maanen AC, van Marum RJ, Jansen PA, Zwart JE, van Solinge WW, Egberts TC. Adherence with dosing guideline in patients with impaired renal function at hospital discharge. PLoS One. 2015;10(6):e0128237. doi: 10.1371/journal.pone.0128237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munar MY, Singh H. Drug dosing adjustments in patients with chronic kidney disease. Am Fam Physician. 2007;75(10):1487–1496. [PubMed] [Google Scholar]
- 27.Nolin TD, Aronoff GR, Fissell WH, Jain L, Madabushi R, Reynolds K, et al. Pharmacokinetic assessment in patients receiving continuous RRT: perspectives from the Kidney Health Initiative. Clin J Am Soc Nephrol. 2015;10(1):159–164. doi: 10.2215/CJN.05630614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swan SK, Bennett WM. Drug dosing guidelines in patients with renal failure. West J Med. 1992;156(6):633–638. [PMC free article] [PubMed] [Google Scholar]
- 29.van Dijk EA, Drabbe NR, Kruijtbosch M, De Smet PA. Drug dosage adjustments according to renal function at hospital discharge. Ann Pharmacother. 2006;40(7–8):1254–1260. doi: 10.1345/aph.1G742. [DOI] [PubMed] [Google Scholar]
- 30.Pea F, Viale P, Pavan F, Furlanut M. Pharmacokinetic considerations for antimicrobial therapy in patients receiving renal replacement therapy. Clin Pharmacokinet. 2007;46(12):997–1038. doi: 10.2165/00003088-200746120-00003. [DOI] [PubMed] [Google Scholar]
- 31.Trotman RL, Williamson JC, Shoemaker DM, Salzer WL. Antibiotic dosing in critically ill adult patients receiving continuous renal replacement therapy. Clin Infect Dis. 2005;41(8):1159–1166. doi: 10.1086/444500. [DOI] [PubMed] [Google Scholar]
- 32.Nolin TD. Altered nonrenal drug clearance in ESRD. Curr Opin Nephrol Hypertens. 2008;17(6):555–559. doi: 10.1097/MNH.0b013e3283136732. [DOI] [PubMed] [Google Scholar]
- 33.Desmond PV, Roberts RK, Wood AJ, Dunn GD, Wilkinson GR, Schenker S. Effect of heparin administration on plasma binding of benzodiazepines. Br J Clin Pharmacol. 1980;9(2):171–175. doi: 10.1111/j.1365-2125.1980.tb05829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 33 kb)
(DOC 31 kb)
The relationship between maximum concentration (Cmax) of isavuconazole and creatinine clearance (CLcr) by the Cockcroft Gault (CG) method (a) and estimated Glomerular Filtration Rate (eGFR) by the Modification of Diet in Renal Disease (b) (GIF 51 kb)