Abstract
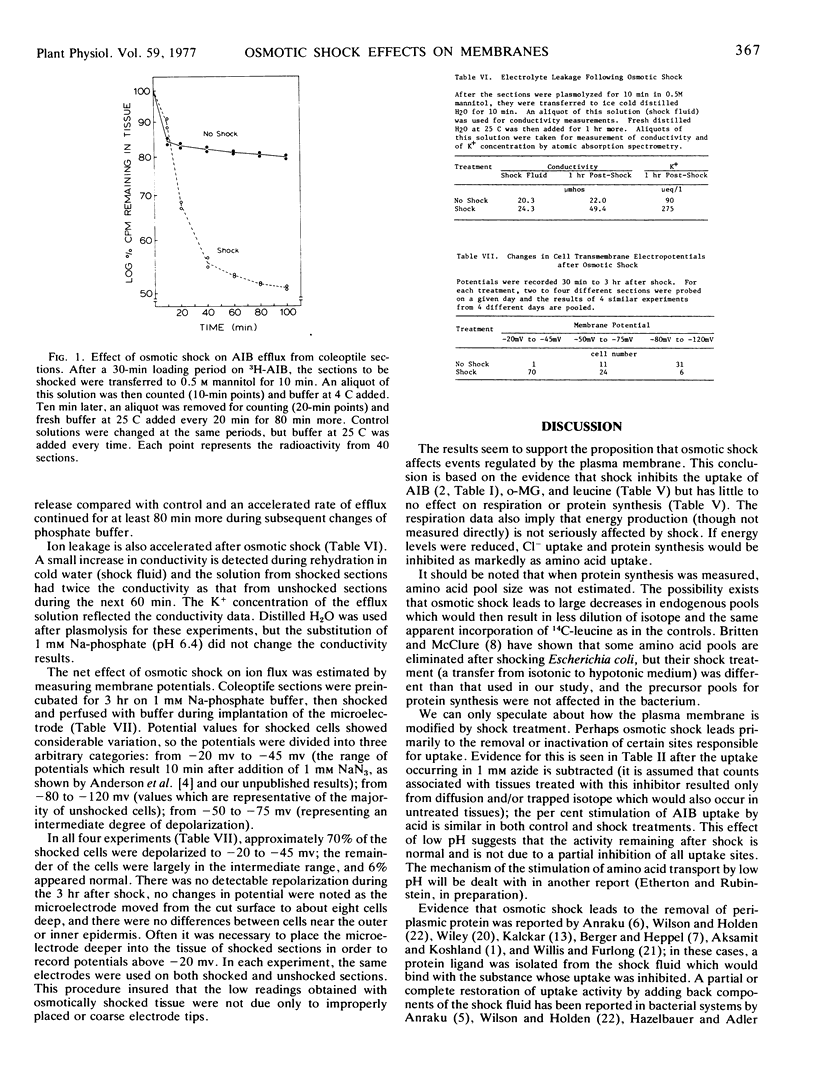
Oat coleoptile sections (Avena sativa L. cv. “Garry”) were osmotically shocked with 0.5 m mannitol followed by 1 mm Na-phosphate (pH 6.4) at 4 C. This treatment reduced uptake of α-aminoisobutyric acid, 3-o-methyl glucose, and leucine by 75 to 90% but inhibited 36Cl− uptake only 30%. Some recovery was observed 1 to 3 hours later. Respiration rates were unaffected by osmotic shock and protein synthesis was reduced 11%.
Osmotic shock also stimulated efflux of α-aminoisobutyric acid and K+ and led to an increase in conductivity of the solution bathing shocked sections. The transmembrane electropotential of 75% of the shocked cells fell to −20 mv to −45 mv compared with the majority of unshocked cells at −80 mv to −120 mv.
We concluded that osmotic shock selectively modifies the plasma membrane. The inhibitions of uptake could be due to removal of specific components of the plasma membrane and/or to the lowered electropotential.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aksamit R., Koshland D. E., Jr A ribose binding protein of Salmonella typhimurium. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1348–1353. doi: 10.1016/0006-291x(72)90860-1. [DOI] [PubMed] [Google Scholar]
- Amar L., Reinhold L. Loss of membrane transport ability in leaf cells and release of protein as a result of osmotic shock. Plant Physiol. 1973 Apr;51(4):620–625. doi: 10.1104/pp.51.4.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson W. P., Hendrix D. L., Higinbotham N. The effect of cyanide and carbon monoxide on the electrical potential and resistance of cell membranes. Plant Physiol. 1974 Nov;54(5):712–716. doi: 10.1104/pp.54.5.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anraku Y. The reduction and restoration of galactose transport in osmotically shocked cells of Escherichia coli. J Biol Chem. 1967 Mar 10;242(5):793–800. [PubMed] [Google Scholar]
- Anraku Y. Transport of sugars and amino acids in bacteria. I. Purification and specificity of the galactose- and leucine-binding proteins. J Biol Chem. 1968 Jun 10;243(11):3116–3122. [PubMed] [Google Scholar]
- BRITTEN R. J., McCLURE F. T. The amino acid pool in Escherichia coli. Bacteriol Rev. 1962 Sep;26:292–335. doi: 10.1128/br.26.3.292-335.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. A., Heppel L. A. A binding protein involved in the transport of cystine and diaminopimelic acid in Escherichia coli. J Biol Chem. 1972 Dec 10;247(23):7684–7694. [PubMed] [Google Scholar]
- Etherton B. Relationship of Cell Transmembrane Electropotential to Potassium and Sodium Accumulation Ratios in Oat and Pea Seedlings. Plant Physiol. 1963 Sep;38(5):581–585. doi: 10.1104/pp.38.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelbauer G. L., Adler J. Role of the galactose binding protein in chemotaxis of Escherichia coli toward galactose. Nat New Biol. 1971 Mar 24;230(12):101–104. doi: 10.1038/newbio230101a0. [DOI] [PubMed] [Google Scholar]
- Heppel L. A. Selective release of enzymes from bacteria. Science. 1967 Jun 16;156(3781):1451–1455. doi: 10.1126/science.156.3781.1451. [DOI] [PubMed] [Google Scholar]
- Kalckar H. M. The periplasmic galactose binding protein of Escherichia coli. Science. 1971 Nov 5;174(4009):557–565. doi: 10.1126/science.174.4009.557. [DOI] [PubMed] [Google Scholar]
- Leonard R. T., Hanson J. B. Induction and development of increased ion absorption in corn root tissue. Plant Physiol. 1972 Mar;49(3):430–435. doi: 10.1104/pp.49.3.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macklon A. E., Higinbotham N. Electropotential in excised pea epicotyls. Plant Physiol. 1968 Jun;43(6):888–892. doi: 10.1104/pp.43.6.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Oxender D. L. Membrane transport. Annu Rev Biochem. 1972;41(10):777–814. doi: 10.1146/annurev.bi.41.070172.004021. [DOI] [PubMed] [Google Scholar]
- Pitman M. G., Mertz S. M., Graves J. S., Pierce W. S., Higinbotham N. Electrical potential differences in cells of barley roots and their relation to ion uptake. Plant Physiol. 1971 Jan;47(1):76–80. doi: 10.1104/pp.47.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley W. R. Tryptophan transport in Neurospora crassa: a tryptophan-binding protein released by cold osmotic shock. J Bacteriol. 1970 Sep;103(3):656–662. doi: 10.1128/jb.103.3.656-662.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis R. C., Furlong C. E. Purification and properties of a ribose-binding protein from Escherichia coli. J Biol Chem. 1974 Nov 10;249(21):6926–6929. [PubMed] [Google Scholar]
- Wilson O. H., Holden J. T. Arginine transport and metabolism in osmotically shocked and unshocked cells of Escherichia coli W. J Biol Chem. 1969 May 25;244(10):2737–2742. [PubMed] [Google Scholar]


