Abstract
Ongoing climate change is expected to shift tree species distribution and therefore affect forest biodiversity and ecosystem services. To assess and project tree distributional shifts, researchers may compare the distribution of juvenile and adult trees under the assumption that differences between tree life-stages reflect distributional shifts triggered by climate change. However, the distribution of tree life-stages could differ within the lifespan of trees, therefore we hypothesize that currently observed distributional differences could represent shifts over ontogeny as opposed to climatically driven changes. Here we test this hypothesis with data from 1435 plots resurveyed after more than three decades across the Western Carpathians. We compared seedling, sapling and adult distribution of 12 tree species along elevation, temperature and precipitation gradients. We analyzed i) temporal shifts between the surveys and ii) distributional differences between tree life-stages within both surveys. Despite climate warming, tree species distribution of any life-stage did not shift directionally upward along elevation between the surveys. Temporal elevational shifts were species-specific and an order of magnitude lower than differences among tree life-stages within the surveys. Our results show that the observed range shifts among tree life-stages are more consistent with ontogenetic differences in the species’ environmental requirements than with responses to recent climate change. The distribution of seedlings substantially differed from saplings and adults, while the distribution of saplings did not differ from adults, indicating a critical transition between seedling and sapling tree life-stages. Future research has to take ontogenetic differences among life-stages into account as we found that distributional differences recently observed worldwide may not reflect climate change but rather the different environmental requirements of tree life-stages.
Keywords: elevational range shift, tree ontogeny, realized niche, semi-permanent plots, temperate forests, tree life-stages, vegetation resurvey
Introduction
In response to climate change, tree species are expected to shift their distribution toward higher elevations and latitudes. However, ambiguous or even contradictory distributional shifts have been found. While some studies showed upslope tree migration (e.g. Kelly & Goulden, 2008; Lenoir et al., 2008), others found disparity in tree distribution shifts or even downslope migration (e.g. Crimmins et al., 2011; Rabasa et al., 2013). In addition, changes in tree species distributions across latitudes are not directional (e.g. Zhu et al., 2012, Woodall et al., 2013). These inconsistencies in species range shifts are usually attributed to differences in individual species biology (Chen et al., 2011; Lenoir & Svenning, 2015; Gibson-Reinemer & Rahel, 2015). In forests, the buffering effect of the tree canopy can contribute to the observed disparity in species distributional shifts (De Frenne et al., 2013; Dobrowski et al., 2015; Stevens et al., 2015). Other non-climatic factors, such as habitat modification, biotic interactions and species traits, can also be responsible for the species-specific distributional shifts (Lenoir et al., 2010; Bodin et al., 2012; Madrigal-González et al., 2014, Grytnes et al., 2014).
The migration of tree species in response to climate change depends on successful regeneration in new habitats (Davis & Shaw, 2001). Therefore researchers often compare the distribution of juvenile and adult trees under the assumption that global warming triggered tree recruitment at higher elevations and latitudes (Table 1). However, the results are rather idiosyncratic. While Lenoir et al., (2009) and Vitasse et al., (2012) found tree seedlings at higher elevations than adult trees, Rabasa et al., (2013) showed that the differences between juveniles and adults varied non-directionally among European tree species. Similarly, both northward and southward tree range shifts were found in North America (Zhu et al., 2012; Boisvert-Marsh et al., 2014).
Table 1.
Overview of studies comparing the distribution of tree life-stages along environmental gradients (elevation, latitude, climate) in the context of climate change. Most studies found differences between tree life-stages and interpreted them as an effect of climate change. However, most of them relied on snapshot, temporally not replicated data. When replicated data were used, they spanned relatively short intervals (given in parentheses).
| Study | Location | Difference between tree life-stages distribution |
Temporally replicated data | |||
|---|---|---|---|---|---|---|
| evident | gradient |
|||||
| elevation | latitude | climate | ||||
| Lenoir et al., 2009 | France | yes | ● | no | ||
| Ewald, 2012 | Germany | yes | ● | no | ||
| Vitasse et al., 2012 | Switzerland | yes | ● | no | ||
| Rabasa et al., 2013 | Europe | no | ● | no | ||
| Benavides et al., 2013 | Spain | yes | ● | ● | no | |
| Monleon & Lintz, 2015 | western USA | yes | ● | ● | ● | no |
| Woodall et al., 2009 | eastern USA | yes | ● | no | ||
| Zhu et al., 2012 | eastern USA | no | ● | no | ||
| Urbieta et al., 2011 | southern Spain | yes | ● | no | ||
| Bell et al., 2013 | western USA | yes | ● | no | ||
| Zhu et al., 2013 | eastern USA | yes | ● | no | ||
| Dobrowski et al., 2015 | western USA | yes | ● | no | ||
| Urli et al., 2014 | Spain | yes | ● | yes (10 yrs) | ||
| Woodall et al., 2013 | eastern USA | no | ● | yes (5 yrs) | ||
| Boisvert-Marsh et al., 2014 | Canada (Québec) | yes | ● | yes (ca. 24 yrs) | ||
However, climate is not the only driver of species distribution and the same species can have various environmental requirements within its distributional range (Diekmann & Lawesson, 1999, Coudun & Gégout, 2005). These distributional differences are mostly driven by interactions with other species and local adaptations leading to spatial and temporal heterogeneity in realized species niche (Silvertown, 2004; Pearman et al., 2008). Interestingly, it was shown that the realized species niche could differ also between juvenile and adult life-stages (Young et al., 2005; Miriti, 2006) and can change even through the ontogeny of trees (e.g. Stohlgren et al., 1998; Bertrand et al., 2011). Moreover, tree life-stages also differ in their physiology as seedlings are more sensitive to various environmental factors, such as frost, drought or shade than adult trees of the same species (e.g. Valladares & Niinemets, 2008; Lloret et al., 2009; Mérian & Lebourgeois, 2011; Vittase et al., 2014; Bennett et al., 2015).
It is therefore possible that the frequently observed differences between seedling and adult tree species distribution reflect these ontogenetic differences rather than temporal shifts triggered by climate change. However, this hypothesis has not yet been tested, particularly because it requires detailed temporally replicated data covering large spatial scales and long environmental gradients.
To test this hypothesis, we analyzed a large dataset of 1435 forest vegetation plots resurveyed after more than three decades of changing climate across the Western Carpathians. We asked the following questions: (1) Did tree species shift their elevational distribution during ongoing climate change? (2) Did the distribution along elevational and climatic gradients differ between tree life-stages and are these differences stable in time? and (3) How large are the ontogenetic differences between tree life-stages compared to temporal shifts potentially triggered by climate change?
Material and Methods
Study area
We explored tree distribution in Slovakia, East-Central Europe. The region is topographically diverse and consists of the Carpathian Mountains and surrounding lowlands. Elevation ranges from 94 to 2656 m. The climate is temperate with mean annual temperatures from 0 °C to 10 °C and annual precipitation sum from 500 to 2000 mm (Faško & Šťastný, 2002; Šťastný et al., 2002).
Forests cover approximately 41% of Slovakia. Broadleaved deciduous species dominate up to approximately 1300 m a.s.l., represented mostly by Quercus spp. and Carpinus betulus in lowlands and Fagus sylvatica in submontane regions. Fraxinus excelsior, Acer spp. and Tilia spp. are more abundant at sites with rocky soils, screes and ravines. Conifers, such as Abies alba, are admixed at higher elevations. Above 1300 m a.s.l., Picea abies forests prevail. The treeline is at ca. 1500 m a.s.l. Sites with extreme conditions due to drought, low nutrients or shallow soils are occupied mainly by Pinus sylvestris.
Tree species data
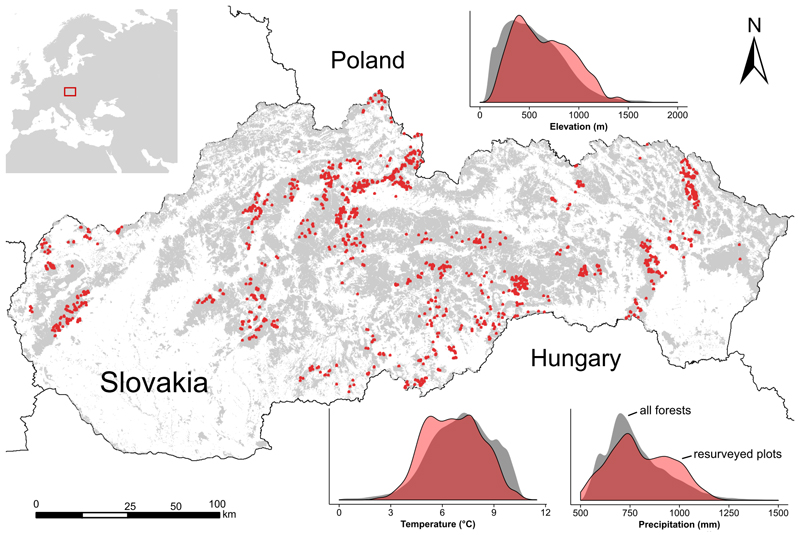
We used data from 1435 forest vegetation plots repeatedly sampled in Slovakia (Fig. 1). These plots were established between 1966 and 1979 and resampled using original methods in 2005–2007 (Vladovič et al., 2014). Each plot had an area of 500 m2. Field records contained a standardized description of local conditions and a complete list of vascular plant species. Trees were assigned to several vertically defined vegetation layers. All resampled plots were located in forest stands not affected by heavy-cutting or large disturbances between surveys (details in Appendix S1). This exceptional dataset covers the entire elevational gradient from the lowlands up to the treeline and sufficiently represents the climatic variability of Slovakian forests (Fig. 1).
Fig. 1.
Distribution of 1435 forest vegetation plots resurveyed after more than three decades (old survey 1966–1979, new survey 2005–2007) across Slovakia, East-Central Europe. The location of resurveyed plots (indicated as dots) are shown on the background of forest area. The inserted density plots show the relative frequency of elevation, annual mean temperature and annual precipitation of the 1435 resurveyed plots compared to the relative frequency of the same variables in all Slovakian forests (details in Appendix S2).
To compare the distribution of tree-life stages, we analyzed tree species presence within three vertical layers representing different tree life-stages. The seedling stage comprised all trees ≤1.3m height. The sapling stage represented young trees with a height between 1.3 m and the bottom of tree crowns creating the main stand canopy. This relative limit therefore depends on the height of the stand determined by the local conditions. Finally, the adult stage consisted of mature trees forming the stand canopy.
Climatic data
We calculated ecologically meaningful and commonly used climatic variables, mean annual temperature and annual precipitation sum (e.g. Benavides et al., 2013; Zhu et al., 2013; Monleon & Lintz, 2015). The calculation was done for each plot and each survey through interpolating of the daily data from 91 meteorological stations of the Slovak Hydrometeorological Institute. We used the interpolation that accounted for spatially and temporary variable relationships between climate and elevation. For each plot, daily mean temperature and precipitation sum was calculated from distance weighted measurements at nearby meteorological stations and subsequently adjusted with locally derived elevation lapse-rates (Štěpánek et al., 2011). To account for inter-annual climatic variability and lag in vegetation response to climate, we used climatic data from five years preceding vegetation sampling (Gottfried et al., 2012; De Frenne et al., 2013).
Data analysis
We analyzed the distribution of all tree species for which all three life-stages (seedling, sapling and adult) occurred on at least 20 plots within each survey, namely Abies alba Mill., Acer platanoides L., A. pseudoplatanus L., Carpinus betulus L., Fagus sylvatica L., Fraxinus excelsior L., Picea abies (L.) H. Karst., Pinus sylvestris L., Quercus cerris L., Q. petraea (refers to Q. petraea agg. which includes Q. dalechampii Ten., Q. petraea (Matt.) Liebl., Q. polycarpa Schur), Tilia cordata Mill. and Ulmus glabra Huds. For most species there were much more than 20 plots with the occurrence of a particular life-stage (Table S1). We used this threshold – in accordance with previous studies – to balance sample size and uncertainty (Rabasa et al., 2013; Dobrowski et al., 2015; Monleon & Lintz, 2015).
To test whether tree life-stages have different distributions along environmental gradients, we used a permutation test implemented with the oneway_test function from the coin R-package (Hothorn et al., 2008). To account for differences among species, we used 9999 permutations restricted to blocks defined by species. We used this test to explore 1) overall temporal range shifts of particular life-stages between sampling periods and 2) ontogenetic range differences between life-stages within old and new surveys separately.
To test species-specific distributional differences between life-stages or temporal shifts between surveys, we used a Kruskal-Wallis test comparing pairs of life-stages, i.e. seedlings vs. adults, seedlings vs. saplings and saplings vs. adults. For each species, we tested the difference between elevation, mean annual temperature and precipitation sum for plots where a particular life-stage was present and the values for plots where other life-stages were present. The same procedure was used to test the temporal shift of each life-stage between the surveys, for example elevation values of plots with seedling occurrence in the old survey vs. elevation values of plots with seedling occurrence in the new survey.
To characterize species distribution along environmental gradients (elevation, mean annual temperature and precipitation sum), we calculated the mean, the 10th percentile and the 90th percentile of the environmental values of the plots with the presence of focal species in each particular survey. We used the 10th percentile as the lower and the 90th percentile as the upper distributional margins to exclude extreme occurrences at both ends of the environmental gradient. The difference between the upper and lower distributional margins represents the species distributional range – i.e. environmental conditions considered as to be suitable for the long-term persistence of the given species persistence (e.g. Lenoir et al., 2009; Bertrand et al., 2011; Rabasa et al., 2013).
Results
Distributional range shifts between surveys
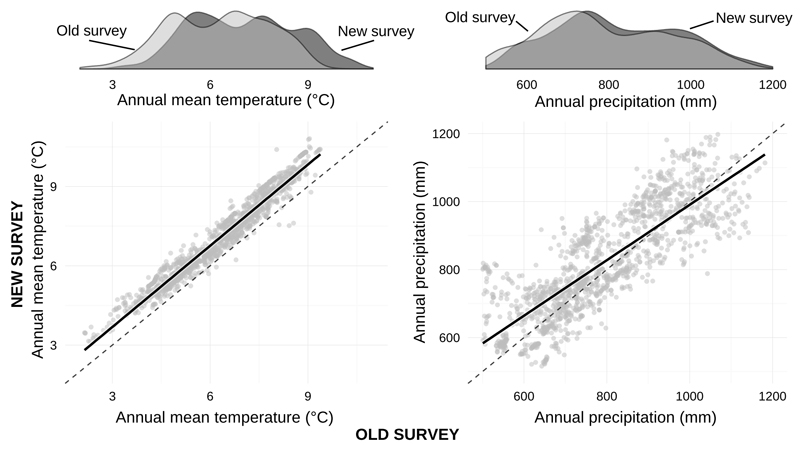
Between surveys, mean annual temperature increased along the whole elevational gradient – on average by 0.76 °C (Fig. 2). Annual precipitation sum also increased between surveys (+33.6 mm on average), but the changes were more variable – 62% plots, mostly at lower elevations, experienced higher precipitation (Fig. 2).
Fig. 2.
Climate change on resurveyed plots between the old and new surveys. Mean annual temperature substantially increased along entire temperature gradient. Annual precipitation sum also increased, particularly at lower elevations. The dashed line represents 1:1 reference, the solid line represents linear relationship fitted to the data. The density curves show the distribution of temperature and precipitation in the old and the new survey separately.
Despite this climate change, the pattern of tree species distribution along an elevational gradient was temporally stable (Fig. 3). Distribution along elevation was statistically not different between the old and new surveys for all life-stages, i.e. for adults (Z = 0.67, p-value = 0.5), saplings (Z = 0.31, p-value = 0.8) and seedlings (Z = 0.13, p-value = 0.9). At the individual species-level, we found no statistically significant temporal shifts for any adult or sapling life-stage (Table S1). Temporal shifts of seedling life-stage of four species were statistically significant (Table S1). However, these results should be interpreted with caution – we performed 72 individual tests (12 species × 3 life-stages × 3 environmental gradients) and this substantially increased the probability of a Type I error – i.e. to find statistically significant shifts even when in fact the distributional changes were random.
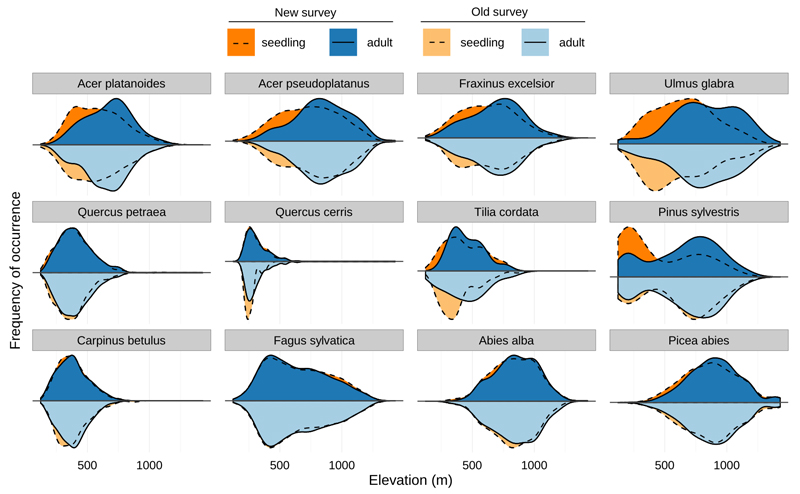
Fig. 3.
Distribution of seedling and adult trees along an elevational gradient in the old (1966–1979) and new (2005–2007) surveys. While the distribution of individual life-stages remained similar despite ongoing climate change, the seedlings of most species occurred at lower elevations than adult trees in both survey periods.
The distributional parameters of individual species did not shift directionally. The mean elevation of most species slightly moved upslope across life-stages. Adults of eight out of twelve species showed upslope shifts (Abies alba, Acer platanoides, Fraxinus excelsior, Quercus cerris, Q. petraea, Picea abies, Tilia cordata, Ulmus glabra), with species level shifts ranging from −13.0 (Pinus sylvestris) to +33.7 (Ulmus glabra) and an all-species average of 9.6 ± 15.8 m (± SD). Seedlings of nine out of twelve species also moved upslope (Abies alba, Acer platanoides, Carpinus betulus, Fagus sylvatica, Quercus cerris, Q. petraea, Picea abies, Tilia cordata, Ulmus glabra), with species level shifts ranging from −154.2 (Pinus sylvestris) to +58.2 (Tilia cordata). Due to the substantial downward shift of Pinus sylvestris the average shift of mean elevation of seedlings was −0.9 ± 54.6 m. The mean elevation of saplings of six species showed upslope shifts, while saplings of six other species moved downslope, with species level shifts ranging from −55.9 (Ulmus glabra) to +19.1 (Picea abies) and with an all-species average shift of −5.4 ± 21.2 m (for individual species see Table S1). The upper distributional margins were temporally more stable than the lower distributional margins, especially for adult trees (Table S1). Due to an upward shift in their lower distributional margin, some species (e.g. Ulmus glabra, Tilia cordata) experienced a substantial contraction of their distributional range.
Ontogenetic differences among life-stages
In both surveys, seedlings of almost all species occurred at lower elevations than adults (Fig. 4, Table S1). In the old survey, the average difference between seedling and adult elevational mean was 46.4 m and the distributional difference between the two life-stages was highly significant (Z = −4.14, p-value = 0.0001). In the new survey, the average difference between seedlings and adults was 56.8 m and the distributional difference was again highly significant (Z = −4.93, p-value = 0.0001).
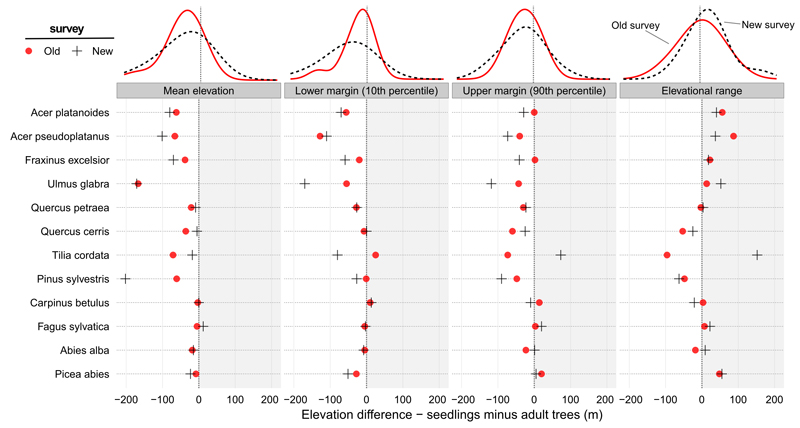
Fig. 4.
Differences between seedling and adult tree distribution along an elevational gradient. Despite ongoing climate change, seedlings of most species occurred at lower elevations than adult trees in both surveys. Each symbol represents the difference between a particular distributional parameter of each species in the old and the new surveys separately. The density curves above each panel show the distribution of individual species values in the old and the new survey separately.
The differences in distributional margins were smaller than differences in elevational means (Table S1). For the upper distributional margin, the difference between seedlings and adults was similar in both surveys (23.1 m old, 25.8 m new). For the lower margin, the difference in the old survey was similar to the upper margin difference (24.6 m), but in the new survey the difference was twice as high (49.2 m). The increasing difference was caused by a shift in the lower margin of adults into higher elevations between surveys (Table S1). Consequently, the distributional range of seedlings was also higher than that of adults in the new survey (23.4 m), while there was almost no difference between life-stage ranges in the old survey (1.5 m).
To find out more about distributional shifts over ontogeny, we also compared the sapling life-stage with seedlings and adults. While there was no difference in distribution between adults and saplings (old survey: Z = 0.90, p-value = 0.4; new survey Z = 0.44, p-value = 0.7), the distribution of saplings significantly differed from that of seedlings (old survey: Z = 4.88, p-value = 0.0001; new survey: Z = 4.57, p-value = 0.0001; individual species-levels in Table S2). This showed the consecutive trend of elevational shift over ontogeny in both surveys and suggested that the critical transition occurs between seedling and sapling stage.
Ontogenetic differences among tree life-stages were species-specific. While the elevational distribution considerably differed among life-stages of Acer platanoides, A. pseudoplatanus, Fraxinus excelsior or Ulmus glabra, there was a substantially smaller difference for Quercus petraea, Carpinus betulus, Fagus sylvatica or Abies alba (Fig. 3, 4; Table S2).
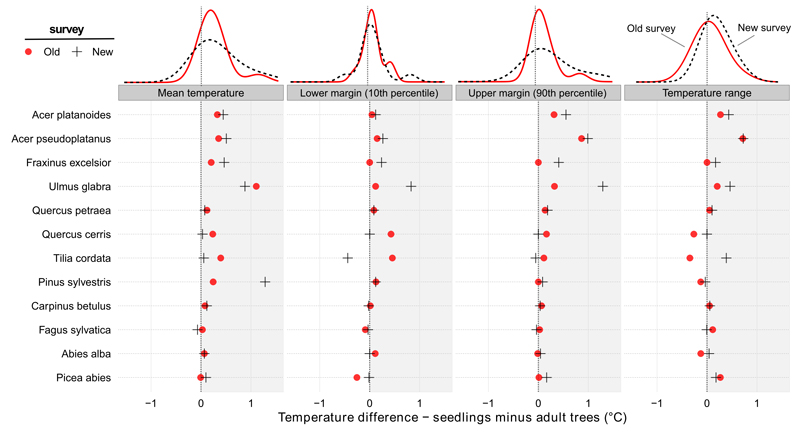
Regarding climatic conditions, we found that due to ontogenetic differences in life-stage distribution along an elevational gradient and a highly significant correlation between climate and elevation (Pearson correlation coefficient between altitude and mean annual temperature was −0.96 and −0.95 in the old and new surveys, respectively, and between altitude and annual precipitation sum 0.79 and 0.78), seedlings generally grew in warmer (Fig. 5) and drier conditions than adults (Fig. S1). The average of seedling temperature mean was 0.26 °C and 0.33 °C higher in the old and in the new survey, respectively, than the average of adult mean. The average of seedling precipitation mean was lower than that for adults by 25.1 mm in the old and 24.6 mm in the new survey.
Fig. 5.
Differences between seedling and adult tree distribution along a temperature gradient. Seedlings of most species occurred in warmer conditions than adult trees of the same species in both surveys. Each symbol represents the difference between a particular parameter of each species in the old and the new surveys separately. The density curves above each panel show the distribution of individual species values in the old and the new survey separately.
Discussion
Temporal shifts
Although climate in the study region substantially changed (Melo et al., 2013), tree species distribution did not directionally shift along an elevational gradient. Instead, we found considerable variability in the temporal shifts of individual species. This finding corresponds with other studies reporting species-specific reactions to climate change (e.g. Chen et al., 2011; Rabasa et al., 2013; Gibson-Reinemer & Rahel, 2015).
Species whose mean elevations shifted upwards typically showed shifts in their lower rather than upper margins (Table S1). This process has been considered as an early stage of species distributional shift in response to climate change (Lenoir & Svenning, 2015). Similarly to Kelly & Goulden, (2008) and Woodall et al., (2013) in North America, we found the most pronounced range contraction for adult trees, likely induced by adult tree mortality at lower elevations. This mortality is most probably related to factors directly affecting species physiology, such as drought or insect outbreaks, which are closely connected to temperature (Breshears et al., 2005; van Mantgem et al., 2009; Allen et al., 2010).
Upward shift of upper distributional margins in response to climate change is expected for tree seedlings (Davis & Shaw, 2001; Ibáñez et al., 2009). However, we found a weak upward distributional shift only for Quercus petraea, Q. cerris, Tilia cordata and Abies alba (Table S1). Except for Abies alba these species could be considered as thermophilous trees typical for lower elevations of study area. Successful colonization of new habitats is limited by many factors (Honnay et al., 2002; Ibáñez et al., 2009). These limiting factors are particularly important in mountain landscapes due to climatic unsuitability or the absence of mature trees facilitating regeneration above the treeline, for example by the seed accumulation in tree wells, the creation of favorable microclimate or by seedbeds on decaying deadwood (Körner, 2003; Bell et al., 2014; Wild et al., 2014). Interestingly, the distribution of seedlings was rather stable despite the fact that their frequency substantially increased between the surveys (Table S1). Other studies from central Europe also noted the increasing frequency of tree seedlings, which they attributed to changes in forest management (Hédl et al., 2010; Verheyen et al., 2012; Kopecký et al., 2013).
The observed distributional shifts could be induced by climate change. However, for several species, distributional shifts may have been the result of other drivers. For example, the upward shift of the lower margin of Ulmus glabra was most probably caused by Dutch elm disease (Gibs, 1978; Brasier, 1991), which resulted in a serious decline of Ulmus at lower elevations (Jamnický, 1976). Picea abies is another species that demonstrated an upward shift of its lower distributional margin. Picea abies has been widely planted out of its natural distributional range and during the past few decades it suffered from drought and bark-beetle outbreaks particularly at lower elevations (Akselsson et al., 2004; Hlásny & Turčáni, 2013). Among all studied species, Picea abies is the only one which could shift upwards without our ability to detect it, because our plots were situated below the treeline. However, the upward shift of Picea abies in the Alps was attributed to land-abandonment rather than to climate change (Gehrig-Fasel et al., 2007). Similarly to the Alps, natural treeline can be rarely observed in Slovakia due to the long-term and intensive pasturing of alpine meadows and forests. The rapid decline of this traditional land-use during the past few decades – and not climate change – may have potentially induced the upward shift in the upper distributional margin of Picea abies. Another species whose upward distributional shift could be confounded by non-climatic factors is Abies alba. It was heavily affected by air-born pollution in the 1970s and 1980s, but since 1990s it has shown substantial growth recovery across Europe, probably due to the sharp decline of SO2-immissions and to climate warming (Bošeľa et al., 2014; Büntgen et al., 2014).
On the other hand, broadleaved tree species typical for lower elevations, namely Quercus cerris, Q. petraea and Tilia cordata, moved slightly upslope and climate change is probable the driver behind these distributional shifts. The upward shift of the lower margin of these species in the adult life-stage could be related to summer drought, which is the main limiting factor of their southern distribution (Brewer et al., 2002; Radoglou et al., 2008). Indeed, summer droughts became more frequent in the lowlands of the study region (Faško et al., 2008). Furthermore, the upward shift of upper margins in seedling life-stage might be the result of warming, because the production of fertile seeds is controlled by temperature (Pigott & Huntley, 1981). Similar temporal change in distribution was observed by Gray & Grist, (2000) for Tilia spp. and by Urli et al., (2014) for Quercus petraea.
Previous studies which compared the distribution of seedlings and adult trees (Table 1), including those based on temporally replicated data (Woodall et al., 2013, Boisvert-Marsh et al., 2014; Urli et al., 2014), found species-specific responses to climate change. This suggests that range shifts depend on species biology and more knowledge at the individual species level is needed to reasonably predict future distributional changes (Clark et al., 2011; Chen et al., 2011; Ehrlén & Morris, 2015).
Ontogenetic differences
Several earlier studies focused on the differences between tree life-stages distribution along environmental gradients in the context of climate change (Table 1). These studies usually found significant differences between life-stages and directly attributed these differences to climate change. However, our results showed that mismatches among life-stages are not explicable in terms of recent climate change. Rather, apparent range shifts are more consistent with studies showing that different life-stages of tree and shrub species have different realized niches (Bertrand et al., 2011; Eriksson, 2002; Miriti, 2006; Stohlgren et al., 1998). This suggests that distributional differences among tree life-stages are ontogenetically fixed and cannot be used as evidence of climate change effects.
Variability observed in ontogenetically fixed differences among tree species is probably related to species-specific traits affecting reproduction and competitive ability (Nakashizuka, 2001).We found the largest ontogenetically fixed differences for early successional species (e.g. Acer spp., Fraxinus excelsior) that need more light – and therefore more severe disturbances – for successful regeneration (Petritan et al., 2007; Yamamoto, 2000). At the same time, highly competitive shade-tolerant species (e.g. Fagus sylvatica, Abies alba) have smaller distributional differences among life-stages. This species-specific variability of ontogenetically fixed differences among life-stages is likely related also to the dispersal of seeds and interactions between juveniles and neighboring adult trees (Young et al., 2005; Miriti, 2006). We found that seedlings of tree species adapted to seed dispersal by wind generally grow at lower elevations than adults, while the difference was substantially smaller for large-seeded, shade-tolerant trees. Early-successional species have effective long-distance seed dispersal, so that they are able to reach a greater variety of site conditions and colonize appropriate locations. However, if shade-tolerant species established at a site, they usually outcompete early-successional species because they have higher growth-rate and survivorship in low light conditions (Niinemets, 2006). This can explain why we found large differences between the distributions of life-stages for early-successional species with better seed dispersal, but we found only small differences for shade-tolerant, large-seeded tree species.
We found that the distribution of seedlings substantially differed from saplings and adults, while the distribution of saplings did not differ from adults. This indicates that a critical transition occurs between the seedling and sapling life-stages. By contrast, Bertrand et al., (2011) found more important differences between saplings and adults. Unfortunately these results are not directly comparable because different definitions of tree life-stages were used in these studies. Clearly, there is a need for more studies exploring ontogenetically fixed differences among tree life-stages and possible mechanisms behind this striking pattern. According to our results, special regard should be given to early-successional species, which have larger distributional differences among life-stages. As climate change has already affected tree distribution in many places, the historical data about tree species composition capturing tree species distribution before recent climate change are especially valuable. To better understand mechanisms behind ontogenetically fixed differences, we highly recommend further analyses of these historical and long-term datasets.
Ontogenetic differences and climate change effects
For the first time, we simultaneously explored distributional differences between tree life-stages and temporal range shifts potentially driven by climate change. We found that the distribution of tree life-stages systematically differs in both surveys despite ongoing climate change. Comparing these ontogenetic shifts with temporal ones, we conclude that previous studies may have misattributed distributional differences among life-stages to climate change. Future research has to take these ontogenetic differences into account as we found that recently observed distributional differences did not reflect climate change but rather the different environmental requirements of tree life-stages.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Acknowledgements
We thank all researchers who spent countless hours in the field sampling vegetation plots that allowed us to perform this study. We also thank Carl Salk and three anonymous reviewers for very useful comments and Péter Szabó for language revision. This work was supported by the agency APVV under projects SK-CZ-0123-11, APVV-14-0086, APVV-0593-12 and by projects VEGA 1/0362/13, MŠMT CZ.1.07/2.3.00/20.0267 and RVO 67985939. The research leading to these results also received funding from the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007-2013)/ERC Grant agreement no 278065.
References
- Akselsson C, Ardö J, Sverdrup H. Critical loads of acidity for forest soils and relationship to forest decline in the northern Czech Republic. Environmental Monitoring and Assessment. 2004;98:363–79. doi: 10.1023/b:emas.0000038196.53204.ab. [DOI] [PubMed] [Google Scholar]
- Allen CD, Macalady AK, Chenchouni H, et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecology and Management. 2010;259:660–684. [Google Scholar]
- Bell DM, Bradford JB, Lauenroth WK. Mountain landscapes offer few opportunities for high elevation tree species migration. Global Change Biology. 2014;20:1441–1451. doi: 10.1111/gcb.12504. [DOI] [PubMed] [Google Scholar]
- Bell DM, Bradford JB, Lauenroth WK. Early indicators of change: divergent climate envelopes between tree life stages imply range shifts in the western United States. Global Ecology and Biogeography. 2013;23:168–180. [Google Scholar]
- Benavides R, Rabasa SG, Granda E, et al. Direct and indirect effects of climate on demography and early growth of Pinus sylvestris at the rear edge: changing roles of biotic and abiotic factors. PloS ONE. 2013;8:e59824. doi: 10.1371/journal.pone.0059824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett AC, McDowell NG, Allen CD, Anderson-Teixeira KJ. Larger trees suffer most during drought in forests worldwide. Nature Plants. 2015;1:15139. doi: 10.1038/nplants.2015.139. [DOI] [PubMed] [Google Scholar]
- Bertrand R, GJ, Gégout JC, Bontemps J. Niches of temperate tree species converge towards nutrient-richer conditions over ontogeny. Oikos. 2011;120:1479–1488. [Google Scholar]
- Bodin J, Badeau V, Bruno E, Cluzeau C, Moisselin J-M, Walther G-R, Dupouey J-L. Shifts of forest species along an elevational gradient in Southeast France: climate change or stand maturation? Journal of Vegetation Science. 2012;24:269–283. [Google Scholar]
- Boisvert-Marsh L, Périé C, de Blois S. Shifting with climate? Evidence for recent changes in tree species distribution at high latitudes. Ecosphere. 2014;5:1–33. [Google Scholar]
- Bošeľa M, Petráš R, Sitková Z, et al. Possible causes of the recent rapid increase in the radial increment of silver fir in the Western Carpathians. Environmental Pollution. 2014;184:211–221. doi: 10.1016/j.envpol.2013.08.036. [DOI] [PubMed] [Google Scholar]
- Brasier CM. Ophiostoma novo-ulmi sp. nov., causative agent of current Dutch elm disease pandemics. Mycopathologia. 1991;115:151–161. [Google Scholar]
- Breshears DD, Cobb NS, Rich PM, et al. Regional vegetation die-off in response to global-change-type drought. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:15144–15148. doi: 10.1073/pnas.0505734102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer S, Cheddadi R, de Beaulieu JL, et al. The spread of deciduous Quercus throughout Europe since the last glacial period. Forest Ecology and Management. 2002;156:27–48. [Google Scholar]
- Büntgen U, Tegel W, Kaplan JO, et al. Placing unprecedented recent fir growth in a European-wide and Holocene-long context. Frontiers in Ecology and the Environment. 2014;12:100–106. [Google Scholar]
- Chen I, Hill JK, Ohlemüller R, Roy DB, Thomas CD. Rapid range shifts of species of climate warming. Science. 2011;333:1024–1026. doi: 10.1126/science.1206432. [DOI] [PubMed] [Google Scholar]
- Clark JS, Bell DM, Hersh MH, et al. Individual-scale variation, species-scale differences: inference needed to understand diversity. Ecology Letters. 2011;14:1273–1287. doi: 10.1111/j.1461-0248.2011.01685.x. [DOI] [PubMed] [Google Scholar]
- Coudun C, Gégout JC. Ecological behaviour of herbaceous forest species along a pH gradient: a comparison between oceanic and semicontinental regions in northern France. Global Ecology and Biogeography. 2005;14:263–270. [Google Scholar]
- Crimmins S, Dobrowski S, Greenberg J, Abatzoglou J, Mynsberge AR. Changes in climatic water balance drive downhill shifts in plant species’ optimum elevations. Science. 2011;331:324–327. doi: 10.1126/science.1199040. [DOI] [PubMed] [Google Scholar]
- Davis MB, Shaw RG. Range shifts and adaptive responses to Quaternary climate change. Science. 2001;292:673–679. doi: 10.1126/science.292.5517.673. [DOI] [PubMed] [Google Scholar]
- De Frenne P, Rodríguez-Sánchez F, Coomes DA, et al. Microclimate moderates plant responses to macroclimate warming. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:18561–18565. doi: 10.1073/pnas.1311190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekmann M, Lawesson JE. Shifts in ecological behaviour of herbaceous forest species along a transect from northern central to north Europe. Folia Geobotanica. 1999;34:127–141. [Google Scholar]
- Dobrowski SZ, Swanson AK, Abatzoglou JT, Holden ZA, Safford HD, Schwartz MK, Gavin DG. Forest structure and species traits mediate projected recruitment declines in western US tree species. Global Ecology and Biogeography. 2015 doi: 10.1111/geb.12302. [DOI] [Google Scholar]
- Ehrlén J, Morris WF. Predicting changes in the distribution and abundance of species under environmental change. Ecology Letters. 2015;18:303–314. doi: 10.1111/ele.12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson O. Ontogenetic niche shifts and their implications for recruitment in three clonal Vaccinium shrubs: Vaccinium myrtillus, Vaccinium vitis-idaea, and Vaccinium oxycoccos. Canadian Jounal of Botany. 2002;80:635–641. [Google Scholar]
- Ewald J. Vegetation databases provide a close-up on altitudinal tree species distribution in the Bavarian Alps. In: Dengler J, Oldeland J, Jansen F, Chytrý M, Ewald J, Finckh M, Glöckler F, Lopez-Gonzalez G, Peet RK, Schaminée JHJ, editors. Vegetation databases for the 21st century, Biodiversity & Ecology. Vol. 4. 2012. pp. 41–48. [Google Scholar]
- Faško P, Lapin M, Pecho J. 20-year extraordinary climatic period in Slovakia. Meteorologický časopis – Meterological journal. 2008;11:99–105. [Google Scholar]
- Faško P, Štastný P. Landscape Atlas of the Slovak Republic. Ministry of Environment of the Slovak Republic, Bratislava, Slovak Environmental Agency; Banská Bystrica: 2002. Mean annual precipitation totals; p. 99. [Google Scholar]
- Gehrig-Fasel J, Guisan A, Zimmermann NE. Tree line shifts in the Swiss Alps: Climate change or land abandonment? Journal of Vegetation Science. 2007;18:571–582. [Google Scholar]
- Gibson-Reinemer DK, Rahel FJ. Inconsistent range shifts within species highlight idiosyncratic responses to climate warming. PLoS ONE. 2015;10:e0132103. doi: 10.1371/journal.pone.0132103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried M, Pauli H, Futschik A, et al. Continent-wide response of mountain vegetation to climate change. Nature Climate Change. 2012;2:111–115. [Google Scholar]
- Gray RKS, Grist NR. Natural regeneration of limes (Tilia spp.) in Scotland: Locally widespread and more numerous in 1999. Glasgow Naturalist. 2000;23:13–16. [Google Scholar]
- Grytnes JA, Kapfer J, Jurasinski G, et al. Identifying the driving factors behind observed elevational range shifts on European mountains. Global Ecology and Biogeography. 2014;23:876–884. [Google Scholar]
- Hédl R, Kopecký M, Komárek J. Half a century of succession in a temperate oakwood: from species-rich community to mesic forest. Diversity and Distributions. 2010;16:267–276. [Google Scholar]
- Hlásny T, Turčáni M. Persisting bark beetle outbreak indicates the unsustainability of secondary Norway spruce forests: Case study from Central Europe. Annals of Forest Science. 2013;70:481–491. [Google Scholar]
- Honnay O, Verheyen K, Butaye J, Jacquemyn H, Bossuyt B, Hermy M. Possible effects of habitat fragmentation and climate change on the range of forest plant species. Ecology Letters. 2002;5:525–530. [Google Scholar]
- Hothorn T, Hornik K, van de Wiel M, Zeileis A. Implementing a class of permutation tests: The coin package. Journal of Statistical Software. 2008;28:1–23. [Google Scholar]
- Ibáñez I, Clark JS, Dietze MC. Estimating colonization potential of migrant tree species. Global Change Biology. 2009;15:1173–1188. [Google Scholar]
- Jamnický J. Vplyv grafiózy na zastúpenie brestov v oblasti slovenských nížin a pahorkatín. Lesnícky časopis. 1976;22:399–406. [Google Scholar]
- Kelly AE, Goulden ML. Rapid shifts in plant distribution with recent climate change. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:11823–11826. doi: 10.1073/pnas.0802891105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecký M, Hédl R, Szabó P. Non-random extinctions dominate plant community changes in abandoned coppices. Journal of Applied Ecology. 2013;50:79–87. doi: 10.1111/1365-2664.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körner C. Alpine plant life: functional plant ecology of high mountain ecosystems. Springer Science & Business Media; Heidelberg: 2003. [Google Scholar]
- Lenoir J, Gégout J-C, Guisan A, et al. Going against the flow: potential mechanisms for unexpected downslope range shifts in a warming climate. Ecography. 2010;33:295–303. [Google Scholar]
- Lenoir J, Gégout JC, Marquet PA, de Ruffray P, Brisse H. A significant upward shift in plant species optimum elevation during the 20th century. Science. 2008;320:1768–1771. doi: 10.1126/science.1156831. [DOI] [PubMed] [Google Scholar]
- Lenoir J, Gégout J-C, Pierrat J-C, Bontemps J-D, Dhôte J-F. Differences between tree species seedling and adult altitudinal distribution in mountain forests during the recent warm period (1986–2006) Ecography. 2009;32:765–777. [Google Scholar]
- Lenoir J, Svenning J-C. Climate-related range shifts - a global multidimensional synthesis and new research directions. Ecography. 2015;38:15–38. [Google Scholar]
- Lloret F, Peñuelas J, Prieto P, Llorens L, Estiarte M. Plant community changes induced by experimental climate change: Seedling and adult species composition. Perspectives in Plant Ecology, Evolution and Systematics. 2009;11:53–63. [Google Scholar]
- Van Mantgem PJ, Stephenson NL, Byrne JC, et al. Widespread increase of tree mortality rates in the western United States. Science. 2009;323:521–524. doi: 10.1126/science.1165000. [DOI] [PubMed] [Google Scholar]
- Madrigal-González J, García-Rodríguez JA, Zavala MA. Shrub encroachment shifts the bioclimatic limit between marcescent and sclerophyllous oaks along an elevation gradient in west-central Spain. Journal of Vegetation Science. 2014;25:514–524. [Google Scholar]
- Melo M, Lapin M, Kapolková H, Pecho J. Climate Trends in the Slovak Part of the Carpathians. In: Kozak J, Ostapowicz K, Bytnerowicz A, Wyżga B, editors. The Carpathians: Integrating Nature and Society Towards Sustainability. Springer-Verlag; Berlin Heidelberg: 2013. pp. 131–150. [Google Scholar]
- Mérian P, Lebourgeois F. Size-mediated climate–growth relationships in temperate forests: a multi-species analysis. Forest Ecology and Management. 2011;261:1382–1391. [Google Scholar]
- Miriti MN. Ontogenetic shift from facilitation to competition in a desert shrub. Journal of Ecology. 2006;94:973–979. [Google Scholar]
- Monleon VJ, Lintz HE. Evidence of tree species’ range shifts in a complex landscape. PLoS ONE. 2015;10:e0118069. doi: 10.1371/journal.pone.0118069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashizuka T. Species coexistence in temperate, mixed, deciduous forests. Trends in Ecology and Evolution. 2001;16:205–210. doi: 10.1016/s0169-5347(01)02117-6. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü. The controversy over traits conferring shade-tolerance in trees: ontogenetic changes revisited. Journal of Ecology. 2006;94:464–470. [Google Scholar]
- Pearman PB, Guisan A, Broennimann O, Randin CF. Niche dynamics in space and time. Trends in Ecology and Evolution. 2008;23:149–158. doi: 10.1016/j.tree.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Petritan MA, Von Lupke B, Petritan IC. Effects of shade on growth and mortality of maple (Acer pseudoplatanus), ash (Fraxinus excelsior) and beech (Fagus sylvatica) saplings. Forestry. 2007;80:397–412. [Google Scholar]
- Pigott CD, Huntley JP. Factors controlling the distribution of Tilia cordata at the northern limits of its geographical range. III. Nature and causes of seed sterility. New Phytologist. 1981;87:817–839. [Google Scholar]
- Rabasa SG, Granda E, Benavides R, et al. Disparity in elevational shifts of European trees in response to recent climate warming. Global Change Biology. 2013;19:2490–2499. doi: 10.1111/gcb.12220. [DOI] [PubMed] [Google Scholar]
- Radoglou K, Dobrowolska D, Spyroglou G, Nicolecu VN. A review on the ecology and silviculture of limes. (Tilia cordata Mill., Tilia platyphyllos Scop. and Tilia tomentosa Moench.) in Europe. Die Bodenkultur. 2008;60:1–29. [Google Scholar]
- Silvertown J. Plant coexistence and the niche. Trends in Ecology & Evolution. 2004;19:605–611. [Google Scholar]
- Šťastný P, Nieplová E, Melo M. Landscape Atlas of the Slovak Republic. Vol. 98. Ministry of Environment of the Slovak Republic, Bratislava, Slovak Environmental Agency; Banská Bystrica: 2002. Mean annual air temperature. [Google Scholar]
- Štěpánek P, Zahradníček P, Huth R. Interpolation techniques used for data quality control and calculation of technical series: an example of Central European daily time series. Idöjárás. 2011;115:87–98. [Google Scholar]
- Stevens JT, Stafford HD, Harrison S, Latimer AM. Forest disturbance accelerates thermophilization of understorey plant communities. Journal of Ecology. 2015;103:1253–1263. [Google Scholar]
- Stohlgren TJ, Bachand RR, Onami Y, Binkley D. Species-environment relationships and vegetation patterns: effects of spatial scale and tree life-stage. Plant Ecology. 1998;135:215–228. [Google Scholar]
- Urbieta IR, García LV, Zavala MA, Marañón T. Mediterranean pine and oak distribution in southern Spain: Is there a mismatch between regeneration and adult distribution? Journal of Vegetation Science. 2011;22:18–31. [Google Scholar]
- Urli M, Delzon S, Eyermann A, Couallier V, García-Valdés R, Zavala MA, Porté AJ. Inferring shifts in tree species distribution using asymmetric distribution curves: a case study in the Iberian mountains. Journal of Vegetation Science. 2014;25:147–159. [Google Scholar]
- Valladares F, Niinemets Ü. Shade tolerance, a key plant feature of complex nature and consequences. Annual Review of Ecology, Evolution, and Systematics. 2008;39:237–257. [Google Scholar]
- Verheyen K, Baeten L, De Frenne P, et al. Driving factors behind the eutrophication signal in understorey plant communities of deciduous temperate forests. Journal of Ecology. 2012;100:352–365. [Google Scholar]
- Vitasse Y, Hoch G, Randin CF, Lenz A, Kollas C, Körner C. Tree recruitment of European tree species at their current upper elevational limits in the Swiss Alps. Journal of Biogeography. 2012;39:1439–1449. [Google Scholar]
- Vitasse Y, Lenz A, Hoch G, Körner C. Earlier leaf-out rather than difference in freezing resistance puts juvenile trees at greater risk of damage than adult trees. Journal of Ecology. 2014;102:981–988. [Google Scholar]
- Vladovič J, Merganič J, Máliš F, et al. The response of forest vegetation diversity to changes in edaphic-climatic conditions in Slovakia. Technical University in Zvolen; 2014. [Google Scholar]
- Wiens JJ, Graham CH. Niche conservatism: integrating evolution, ecology, and conservation biology. Annual Review of Ecology, Evolution, and Systematics. 2005;36:519–539. [Google Scholar]
- Wild J, Kopecký M, Svoboda M, Zenáhlíková J, Edwards-Jonášová M, Herben T. Spatial patterns with memory: tree regeneration after stand-replacing disturbance in Picea abies mountain forests. Journal of Vegetation Science. 2014;25:1327–1340. [Google Scholar]
- Woodall CW, Oswalt CM, Westfall JA, Perry CH, Nelson MD, Finley AO. An indicator of tree migration in forests of the eastern United States. Forest Ecology and Management. 2009;257:1434–1444. [Google Scholar]
- Woodall CW, Zhu K, Westfall JA, Oswalt CM, D’Amato WA, Walters BF, Lintz HE. Assessing the stability of tree ranges and influence of disturbance in eastern US forests. Forest Ecology and Management. 2013;291:172–180. [Google Scholar]
- Yamamoto SI. Forest gap dynamics and tree regeneration. Journal of Forest Research. 2000;5:223–229. [Google Scholar]
- Young TP, Petersen DA, Clary JJ. The ecology of restoration: historical links, emerging issues and unexplored realms. Ecology Letters. 2005;8:662–673. [Google Scholar]
- Zhu K, Woodall CW, Clark JS. Failure to migrate: lack of tree range expansion in response to climate change. Global Change Biology. 2012;18:1042–1052. [Google Scholar]
- Zhu K, Woodall CW, Ghosh S, Gelfand AE, Clark JS. Dual impacts of climate change: forest migration and turnover through life history. Global Change Biology. 2013;20:251–264. doi: 10.1111/gcb.12382. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.