Abstract
Neem leaf extract (NLE) has medicinal properties, which have been attributed to its limonoid content. We identified the NLE tetranorterpenoid, nimbolide, as being the key limonoid responsible for the cytotoxicity of NLE in various preclinical models of human B-lymphocyte cancer. Of the models tested, Waldenströms macroglobulinemia (WM) cells were most sensitive to nimbolide, undergoing significant mitochondrial mediated apoptosis. Notably, nimbolide toxicity was also observed in drug-resistant (bortezomib or ibrutinib) WM cells. To identify putative targets of nimbolide, relevant in WM, we used chemoinformatics-based approaches comprised of virtual in silico screening, molecular modeling and target–ligand reverse docking. In silico analysis revealed the antiapoptotic protein BCL2 was the preferential binding partner of nimbolide. The significance of this finding was further tested in vitro in RS4;11 (BCL2-dependent) tumor cells, in which nimbolide induced significantly more apoptosis compared with BCL2 mutated (Jurkat BCL2Ser70-Ala) cells. Lastly, intraperitoneal administration of nimbolide in WM tumor xenografted mice, significantly reduced tumor growth and IgM secretion in vivo, while modulating the expression of several proteins as seen on immunohistochemistry. Overall, our data demonstrate that nimbolide is highly active in WM cells, as well as other B-cell cancers, and engages BCL2 to exert its cytotoxic activity.
Introduction
Waldenströms macroglobulinemia (WM) is an indolent Non-Hodgkin's lymphoma characterized by clonal proliferation of lymphoplasmacytoid B-cells in the bone marrow, spleen and the lymph nodes. Patients can present with signs or symptoms associated with cytopenias, lymphadenopathy, splenomegaly and/or hepatomegaly. The malignant WM tumor clone characteristically produces and secretes excessive amounts of immunoglobulin M (IgM), which can cause hyperviscosity syndrome.1
The pathological behavior of WM cells relies on aberrant B-cell receptor and Toll-like receptor signaling and is intricately supported by malfunctions in initiation of apoptosis.2, 3 In this dynamic, a complex shift between functionally active pro-apoptotic and anti-apoptotic proteins from the BCL2 family occurs and bestows the malignant cells with a significant survival advantage.3, 4, 5 This defective apoptotic signaling is associated with aggressive clinical behavior, chemoresistance and a poor prognosis in many B-cell cancers, including WM.3, 4, 5, 6, 7 Thus, B-cell receptor- or Toll-like receptor-induced proliferation and abnormal apoptotic signaling act in concert for the assurance of the malignant WM cells survival.
There are currently no Food and Drug Administration approved treatments for WM, and the disease remains incurable. Management of symptomatic malignancy is currently conducted using therapeutics commonly employed for other lymphoid cancers, such as alkylating agents (cyclophosphamide), purine analogs (fludarabine), monoclonal antibodies (CD20 targeting rituximab) and the use of the proteasome inhibitor, bortezomib. However none of these strategies is curative and the median survival of WM patients is ~5 years.8
Over the last three decades ~70% of the Food and Drug Administration approved drugs have been developed from natural sources.9 Naturally occurring compounds remain a critical reservoir for drug development. Several are currently under investigation for cancer therapy either as whole compounds or their components.10, 11 Neem is one such natural herb with demonstrable anti-cancer properties and is a source of several limonoids, which are a class of oxygenated triterpenes called tetranorterpenoids. These limonoids are responsible for the anti-tumor effects of neem leaf extract (NLE).12 Of the NLE tetranorterpenoids, it has been reported that nimbolide is the most cytotoxic; however, its mechanism of action remains to be conclusively established. Being a primary constituent of NLE, nimbolide, is anticipated to trigger multiple cell death pathways through activation of apoptosis machinery (through BCL2 family proteins),13 induction of heat shock proteins and the tumor suppressor TP53.13, 14, 15, 16
Unfortunately, because of their mechanistic ambiguity, many phytochemicals with observable anti-tumor efficacy are often precluded from being developed as viable therapeutic agents. The experimental deduction of a precise biological target for a particular ligand is a complicated and time-consuming process. Analogous to experimental target finding techniques (affinity chromatography, nuclear magnetic resonance), an emerging technology that capitalizes on computational approaches is increasingly being implemented to determine the biological target(s) for a given chemical compound of interest.17, 18, 19 This in silico approach relies on the mapping of the compounds biochemical structure into known structure–activity relationship space by querying large biologically annotated protein databases (virtual screening). Using this technique and large chemogenomic repositories that contain the three-dimensional crystal structures of numerous biological proteins, a molecular model can be designed where the bioactive compound is ‘reverse docked' to the protein to determine its binding site and preferred orientation.18 This in turn can be used to predict the binding affinity between the compound and the protein, which is quantitatively relayed by a scoring function.18 The utility of these rapid and cost-effective virtual methods has been applied to elucidate the ligand–target (including unperceived off-target) interactions for synthetic therapeutics such as PRIMA-1,20 4 H-tamoxifen,21 torcetrapib22 as well as organic compounds such as vitamin E,21 catechin (a polyphenol)23 and baicalein (a flavonoid).24
Through a systematic approach combining in silico computational techniques and experimental methods, we investigated nimbolide to ascertain its exact protein targets and decipher its mechanism of anti-tumor activity in B-cancer cells. As such, this is the first mechanistic analysis of nimbolide demonstrating 1) its activity in WM cancer cells in vitro, 2) the use of virtual screening methods to classify its molecular targets and 3) its ability to be safely administered and produce anti-tumor effects in an in vivo model of hematologic malignancy.
Materials and methods
Cell lines, cell culture and animal experiments
Heparinized peripheral blood was obtained from healthy human donors (n=3). Peripheral blood mononuclear cells from healthy human donors were isolated as previously described.13 WM (BCWM.1, MWCL-1 and RPCI-WM1) cell lines, multiple myeloma cell lines (U266, KMS11 and OPM2) and their unique corresponding bortezomib resistant (BR) or ibrutinib-resistant (IR) clones (BCWM.1/BR, BCWM.1/IR, MWCL-1/BR, U266/BR, OPM2/BR and KMS11/BR), which were developed in our laboratory, were used in experiments. In addition, BCL2-dependent leukemia cells (RS4;11), BCL2 mutated cells (Jurkat Bcl2Ser70-Ala) and Burkitts lymphoma cells (Raji) were also used in this study and purchased from ATCC (Manassas, VA, USA). All cell lines were cultured in RPMI-1640 containing 10% FBS and penicillin (100U ml−1) and streptomycin (100 ug ml−1). Culture medium was replaced every three days. Cell viability was maintained at>90% and was measured by trypan blue exclusion assay using ViCell-XR viability counter.
All animal experiments were performed with the approval of the Institutional Animal Care and Use Committee of Mayo Clinic. A xenograft model of WM was established as previously described.25, 26
Please see the Supplemental Materials & Methods for additional information.
Results
NLE induces cell death in preclinical models of B-cell cancers and its cytotoxic effects are primarily mediated through nimbolide in a tumor-specific manner
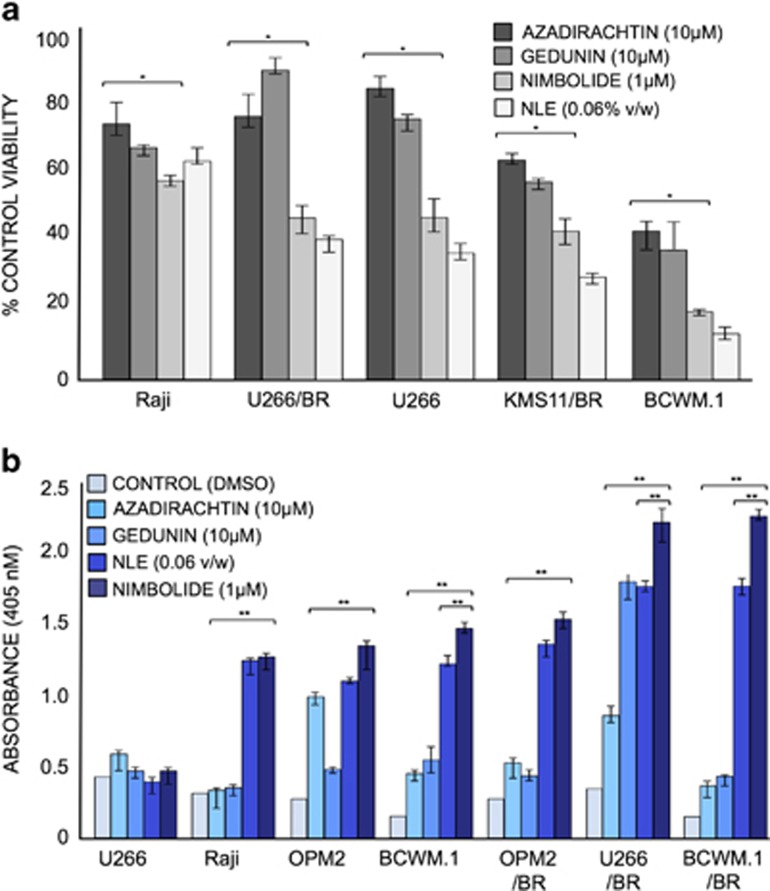
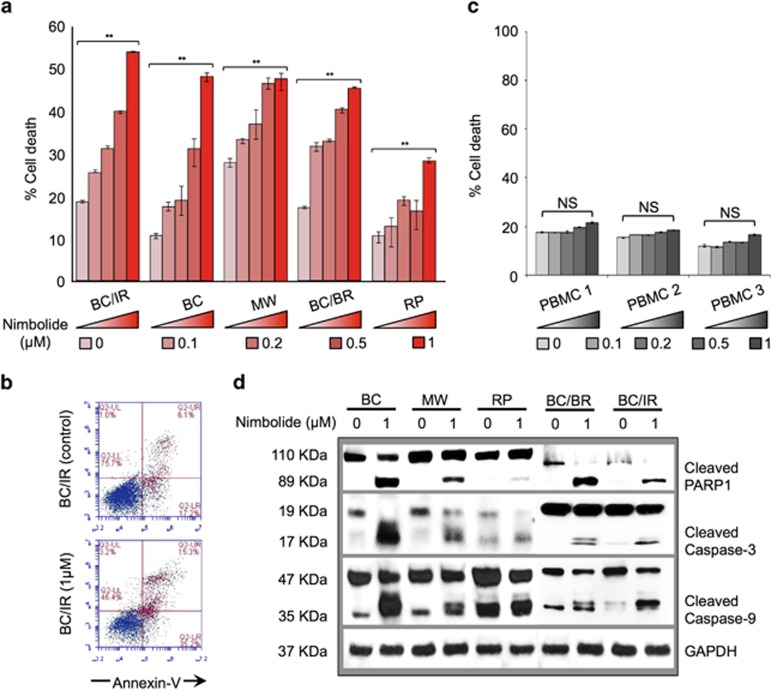
We recently reported the proapoptotic effects of NLE in malignant CLL patient cells.13 To investigate if this effect was also evident in other B-cell cancers, we evaluated the activity of NLE and its individual limonoids in cancer cell lines representing human MM, Non-Hodgkin's lymphoma and WM in vitro. Tumor cell viability was determined by trypan blue exclusion assay after treating the cancer cell lines with NLE and its individual limonoids, nimbolide, azadirachtin or gedunin. In comparing the antineoplastic effects of the three limonoids with NLE, we observed that cell proliferation was most significantly inhibited by NLE and that this effect was most comparably mimicked by nimbolide (Figure 1a). As compared with azadirachtin and gedunin whose IC50 values in BCWM.1 cells were ~13 and 3 μM, respectively, the IC50 of nimbolide was considerably lower (200 nM) (Supplementary Figure 1). Apoptosis ELISA confirmed that nimbolide was the most potent inducer of apoptotic cell death, and this was independent of the disease model studied (Figure 1b). This effect was evident even in the drug-resistant models; bortezomib sensitive cell lines as well as BR derivatives were equally susceptible to nimbolide. Although cell viability (Figure 1a) was most reduced in presence of NLE, programmed cell death (apoptosis ELISA using cell culture supernatants) was more evident in nimbolide treated cells, with WM models showing the greatest sensitivity. Observing that apoptosis was the primary means of nimbolide-induced WM cell death, we treated WM cells with different concentrations of nimbolide ranging from 0.1 to 1 μM for 6 h and conducted annexin-V and PI staining followed by flow cytometry to assess the degree of apoptosis across the different models. Nimbolide was able to produce apoptotic cell death in a dose-dependent manner (Figure 2a). Using our most drug-resistant model (RPCI-WM1 that was established from a therapy-resistant and terminal WM patient),26 we noted significant induction of apoptosis with nimbolide (26% apoptosis). Interestingly, the ibrutinib-resistant (IR) WM subclones were the most sensitive with ~53% of the cells undergoing apoptosis (Figure 2b). Under similar experimental conditions, nimbolide exerted minimal effect on the induction of apoptosis in peripheral blood mononuclear cells from healthy donors (Figure 2c). Lastly, tumor cell apoptosis was also confirmed on immunoblot analysis by detection of PARP-1, initiator caspase-9 and executioner caspase-3 cleavage, signifying involvement of the intrinsic (death receptor independent) apoptotic system (Figure 2d). All together, these experiments confirmed that nimbolide is the most potent component of NLE and it induces cytotoxicity through apoptotic mechanisms.
Figure 1.
Nimbolide significantly reduces cell viability and induces apoptotic cell death in B-cell cancer models. MM (U266, U266/BR, KMS, OPM2, OPM2/BR), WM (BCWM.1, BCWM.1/BR) and Non-Hodgkin's lymphoma (Raji) cell lines were treated with NLE or its limonoid components for 24 h. (a) Cell viability was determined by trypan blue exclusion assay. All cell lines showed significant sensitivity to nimbolide with maximum effect noted in the WM model. Cells from each representative model were treated with DMSO and used as a control. (b) MM, WM and Non-Hodgkin's lymphoma cells were treated with indicated concentrations of NLE or its limonoid components and apoptosis was measured by ELISA (Roche, Indianapolis, IN, USA). All plasma cell cancer models demonstrated significant apoptosis in presence of nimbolide. Notably the proapoptotic effect of nimbolide was most significant in BR models. *P<0.05; **P<0.01; BR; bortezomib-resistant model.
Figure 2.
Nimbolide induces dose-dependent apoptosis in WM cells. (a and b) WM cell lines were treated with nimbolide at different concentrations for 6 h. Cell death was analyzed by annexin V staining followed by flow cytometry. Nimbolide induced apoptosis was dose dependent with maximum cell death at 1 μM, most notably in ibrutinib-resistant (BC/IR) cells (51% cell death). (c) Peripheral blood mononuclear cells from healthy human donors (n=3) were treated with indicated concentrations of nimbolide and stained with annexin V to assess apoptosis. Toxicity from nimbolide was minimal ranging between 0.15 and 4.7% of % control cell death, with the latter only seen at the highest concentration tested (1 μM). (d) Western blot analysis of WM cells treated with nimbolide at 0 and 1 μM confirms apoptosis by cleavage of caspases 3, 9 and PARP-1. All experiments were performed in triplicate. NS, Not statistically significant; **P<0.01. WM tumor cell lines: BC, BCWM.1; BC/IR, BCWM.1/IR; BC/BR, BCWM.1/BR; MW, MWCL-1; RP, RPCI-WM1
The mitochondrial transmembrane potential of WM cells is altered in presence of nimbolide
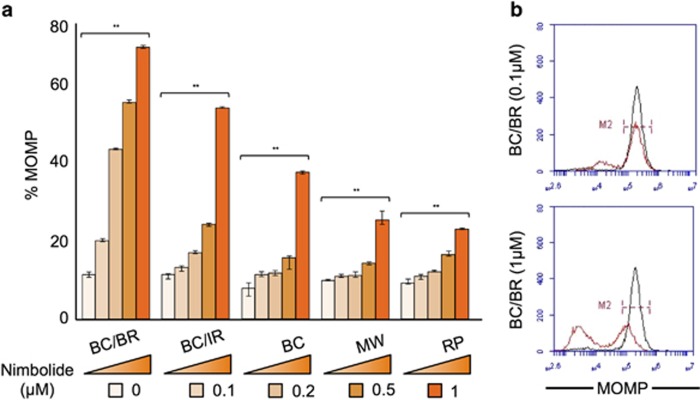
To further understand the mechanism of apoptosis induced by nimbolide in WM cell lines in vitro, we focused our study on the effect of nimbolide on mitochondrial integrity. Disturbance of the mitochondrial transmembrane potential (Δψm), through an increase in mitochondrial outer membrane permeability (MOMP) is a hallmark of apoptosis that occurs autonomous of death receptor activation.27 Thus we investigated whether Δψm was altered in the presence of nimbolide. Wild-type, BR and IR WM cell lines (total n=7) were treated with increasing concentrations of nimbolide for 6 h followed by incubation with tetramethylrhodamine methyl ester (TMRM) for 15 min. MOMP was measured by examining TMRM fluorescence in tumor cells versus control cells (% MOMP) using flow cytometry. Nimbolide treatment resulted in dose-dependent MOMP activation in all the WM cell lines tested (Figure 3a). Nimbolide-treated cells showed substantially less TMRM fluorescence, which specified disruption of the Δψm and suggested mitochondrial leakiness and caspase release from mitochondria into the cytoplasm. To further confirm the consequence of compromised mitochondrial integrity, we investigated the activation of caspases 9 and 3, which signify the complete activation of the intrinsic apoptotic pathway. Both initiator caspase-9 and executioner caspase-3 cleavage was significantly increased in cells treated with nimbolide as compared with control untreated cells (see Figure 2d).
Figure 3.
Nimbolide alters MOMP in WM cells. (a and b) WM cell lines were treated with nimbolide at different concentrations for 6 h and subsequently incubated with 20 nM TMRM for 15 min followed by flow cytometry for analysis of MOMP. MOMP was most activated at 1 μM, particularly bortezomib-resistant (BC/BR) WM cells (69% MOMP). All experiments were performed in triplicate. **P<0.01. WM tumor cell lines: BC, BCWM.1; BC/IR, BCWM.1/IR; BC/BR, BCWM.1/BR; MW, MWCL-1; RP, RPCI-WM1.
Nimbolide restores the cytotoxic activity of bortezomib or ibrutinib in drug-resistant WM models
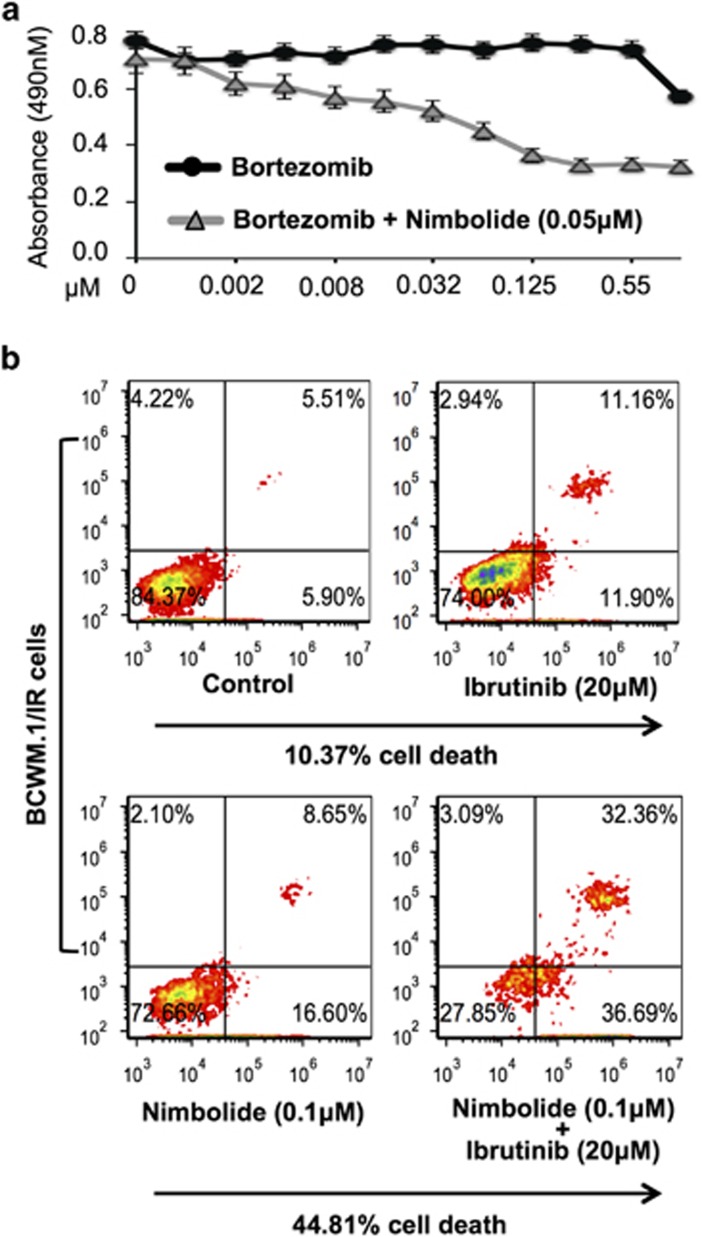
We previously demonstrated that the proapoptotic effects of nimbolide are maintained despite drug resistance. In our BR model system we investigated if nimbolide could restore bortezomib sensitivity. On a 48 h MTS assay, we noted that nimbolide significantly decreased the established EC50 of bortezomib (50-fold) in BCWM.1/BR cells (Figure 4a). Similar effects were noted in our IR model, wherein; we assessed the ability of nimbolide to restore the IR cells sensitivity toward ibrutinib. We examined cell death in BCWM.1/IR cells using escalating doses of ibrutinib (5 –20 μM). Even at the highest dose tested (20 μM), ibrutinib could only achieve 10.37% cell death. We observed that when a sub-lethal concentration of nimbolide (0.1 μM) was added to ibrutinib-treated BCWM.1/IR cells, the combination activity significantly increased BCWM.1/IR cell death (44.81%) compared with when either agent was used alone (Figure 4b). These observations demonstrate (a) the ability of nimbolide to reverse drug resistance and (b) its capacity to be successfully combined with either a proteasome inhibitor (bortezomib) or a BTK-inhibitor (ibrutinib) for enhanced anti-WM tumor kill.
Figure 4.
Acquired resistance to bortezomib or ibrutinib is reversed in presence of nimbolide. (a) Bortezomib-resistant (BCWM.1/BR) WM cells (IC50 >0.5 μM) were treated with indicated concentrations of nimbolide for 48 h with increasing concentrations of bortezomib and cell growth measured by MTS assay. Nimbolide significantly lowered the EC50 of bortezomib from 0.585 to 0.0010 μM. (b) Ibrutinib-resistant (BCWM.1/IR) (IC50 ~45 μM) cells were treated with DMSO or ibrutinib ranging from 10–50 μM (20 μM concentration shown); separately, BCWM.1/IR cells were treated with nimbolide at a suboptimal concentration (0.1 μM) alone or in combination with ibrutinib (10–50 μM) and stained with annexin-V for analysis of cell death. At a 20 μM concentration, ibrutinib treatment resulted in 10.37% cell death. However, when nimbolide was combined with ibrutinib (20 μM), significantly more apoptosis was observed (~44%) over ibrutinib (~10%) or nimbolide treatment alone (~12%).
Chemoinformatics and molecular modeling of nimbolide–target interactions identifies BCL2 as a primary binding partner of nimbolide
The rapid and potent cytotoxic effect of nimbolide on all the B-cell cancer cell lines tested was intriguing and indicated that the limonoid targets important survival pathways biologically relevant to lymphoid cells and independent of acquired resistance to bortezomib or ibrutinib. To further understand the intracellular targets of nimbolide, we performed a chemoinformatics analysis to determine its biochemical and molecular properties (Supplementary Table 1). Briefly, nimbolide is a 466.52 Dalton small molecule with an atomic formula of C27H30O7 (chemical structure shown in Supplementary Figure 2), which has the IUPAC nomenclature methyl-[(2 aR,5 aR,6 S,6 aR,8 R,9 aR,10 aS,10bR,10 cR)-8-(3-furyl)-2a,5a,6a,7-tetramethyl-2,5 dioxo-2a,5a,6,6a,8,9,9a,10a,10b,10c-decahydro-2 H,5 H cyclopenta[b]furo[2',3',4':4,5]naphtho[2,3-d]furan-6-yl]acetate. It has a polar surface area of 92.04 Å2, solvent accessible surface area of 651.41 Å2 and calculated geometric volume of 417.32 Å2. In addition, nimbolide has excellent characteristics exemplified by zero violations of either Lipinski Rule of 5 or Jorgensen Rule of 3.
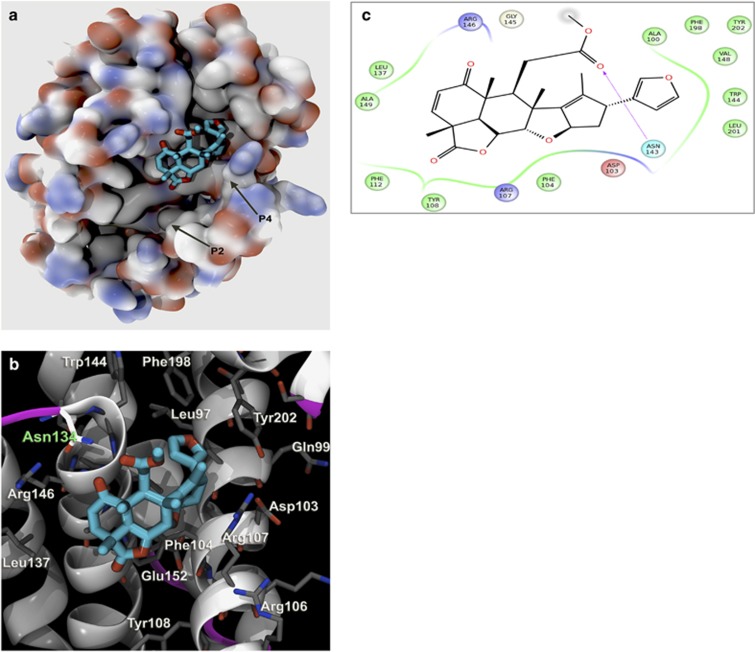
Next, we conducted virtual screening using in silico target identification coupled with detailed all-atom molecular docking experiments (reverse docking) to identify targets of nimbolide relevant in WM. Molecular optimization algorithms to determine relative ranking of protein affinity with nimbolide were utilized.28, 29, 30, 31, 32, 33, 34, 35, 36, 37 Using the pre-docking filter LASSO (Ligand Activity by Surface Similarity Order) as a broad screen, we first identified a pool of over 50 proteins with which nimbolide potentially interacted (top 3 proteins shown in Supplementary Table 2). Next we conducted a docking analysis on all proteins of interest and their different isoforms (total protein targets, n=86) from the pre-docking screen. Using comprehensive algorithms (see Supplementary Methods section) that ensured statistical mechanics output and thermodynamic validity, we identified the BCL2 protein proper as displaying the highest affinity for nimbolide with a binding energy of -84.86 (MM-GBSA score). Nimbolide interacts with highest affinity within the P4 pocket of BCL2 (Figure 5a) and uses optimal binding energy at particular residues (Figure 5b). These BCL2 residues react with both furan moieties and carbonyl oxygens of nimbolide (Figure 5c). There is a strong interaction with Asn143 and the methyl carboxylic acid at the carbonyl oxygen, which is at the closest opening of the cavity facing the BCL2 molecule. Overall, nimbolide–BCL2 binding is a dynamic process and the kinetics of the system are such that the optimal substrate will occupy the enzyme binding site most frequently, but it will still undergo dissociation and rebinding (see Supplementary Movie 1). The regions and thermodynamic interactions that are contributing the most energy for nimbolide–BCL2 binding are shown in Supplementary Figures 3A and B. Thus taken together, this unique approach of using in silico docking technologies for determining protein affinity validated prior reports on the role of these potential nimbolide targets and prompted further experimental investigation.
Figure 5.
Detailed interaction of nimbolide with BCL2 as identified by in silico docking analysis. (a) Nimbolide binds with BCL2 in reported P4-binding pocket. Hydrophobic binding pocket termed P4 is shown with nimbolide bound. The protein is shown with solvent-accessible surface threshold attenuated to reveal crevices and side chain details, and carbon atoms are colored gray, oxygen atoms red and nitrogen atoms blue. A box denoting the P4 pocket is shown at higher resolution in panel (b) where nimbolide is shown in detail with nearby residues, in particular Asn134 as part of a loop segment at the end of the helix. (c) Nimbolide 2D structure is shown with chirality. The residues Ala100, Asp103, Phe104, Arg107, Tyr108, Phe112, Leu137, Trp144, Gly145, Arg146, Val148, Ala149, Phe198, Leu201 and Tyr202 all form close proximity with nimbolide. The interactions are given as hydrophobic in green, charge in blue or blue and dashed arrow indicate H-bond
BCL2 protein levels are modulated by nimbolide in vitro as well as the protein profiles of additional nimbolide-related interactions
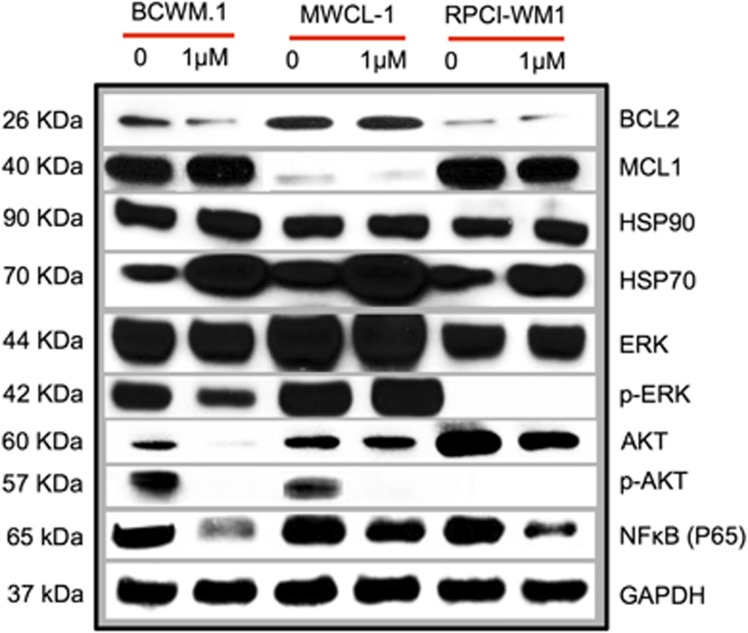
We were encouraged to find that our in silico analysis corroborated prior findings that BCL2 is an important and preferential target of nimbolide.13, 14, 15, 16 This finding was validated in WM cells, where BCL2 protein levels were assessed by western blot and appeared to decrease after cell exposure to the limonoid (Figure 6). Recognizing that nimbolide is a promiscuous molecule (as many phytochemicals tend to be), with additional targets,38 we sought to examine other potential binding partners identified in the virtual screening campaign. Whereas BCL2 ranked highest in terms of binding affinity, HSP90 and PI3K (p110γ) ranked second and third most likely by MM-GBSA calculations to interact with nimbolide, respectively (see Supplementary Table 2). In nimbolide treated WM cells, we did not observe a direct shift in HSP90; however, a marked induction of HSP70 and a decrease in MAPK signaling (p-ERK1/2) was observed, which was indicative of HSP90 inhibition.39, 40 Next we looked for evidence of PI3K (p110γ) modulation in nimbolide-treated cells and found its downstream targets, protein kinase B (AKT) and p-AKT, were notably decreased. In addition to its well-known effects on AKT, PI3K inhibition has been noted to mitigate NFκB signaling.41 We found a marked decrease in NFκB (p65) expression following nimbolide treatment of WM cells, which is consistent with reports by other investigators.42 Overall, these effects were evident in all WM cell lines (to varying extent) and show that BCL2 and other potential nimbolide targets are modulated in response to the limonoid.
Figure 6.
Nimbolide shifts expression of BCL2 and proteins associated with other potential targets, identified on in silico screen. WM cells (BCWM.1, MWCL-1 and RPCI-WM1) were treated with different concentrations of nimbolide for 6 h and western blot analysis was performed. Among the concentrations tested, nimbolide effects were most evident at 1 μM (nimbolide 0.1 and 0.5 μM data not shown).
BCL2-dependent cells are highly sensitive to the cytotoxic effects of nimbolide
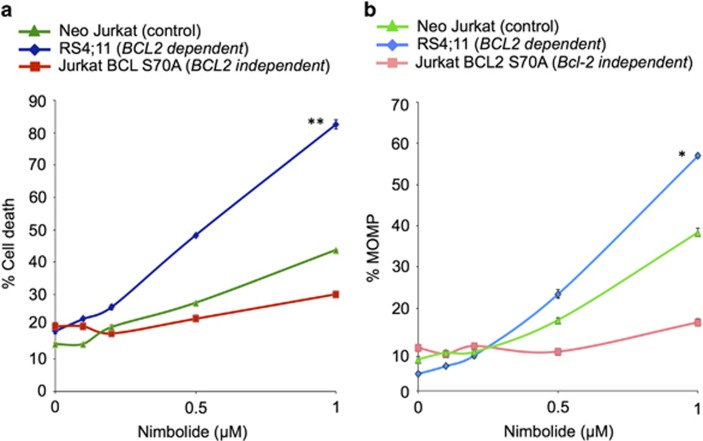
With virtual screening and immunoblot analysis pointing toward BCL2 as a primary target of nimbolide, we hypothesized that the effects of the limonoid would be catastrophic in tumor cells that are completely reliant on BCL2. Conversely, we postulated that activity of the limonoid would be diminished in BCL2-independent cells. To test this, we used RS4;11 tumor cells, which are BCL2-dependent43 and Jurkat BCL2Ser70-Ala cells, which served as a BCL2-independent model. Neo Jurkat cells were used as a neutral control. As anticipated, RS4;11 cells were sensitive to nimbolide, undergoing significant apoptosis (P<0.00008, 82% apoptosis at 1 μM) and activation of MOMP in a dose-dependent manner as compared with Jurkat BCL2Ser70-Ala cells (28% apoptosis at 1 μM) (Figures 7a and b). These corroborated our in silico observations that BCL2 is a central mediator of nimbolide cytotoxicity.
Figure 7.
BCL2-dependent tumor cells are highly sensitive to nimbolide whereas BCL2-independent tumor cells are not. (a) RS4;11, Neo Jurkat and Jurkat BCL2Ser70-Ala tumor cells were treated with different concentrations of nimbolide for 6 h and apoptosis was measured by annexin V staining and flow cytometry. BCL2-dependent cells (RS4;11) were significantly sensitive to 1 μM nimbolide (82% cell death) as compared with BCL2-independent Jurkat BCL2Ser70-Ala tumor cells (28% cell death, P<0.00008) or Neo Jurkat cells (40% cell death, P<0.0001). (b) MOMP activation was also more significant in RS4;11 cells as compared with Jurkat BCL2Ser70-Ala or Neo Jurkat cells. *P<0.0001, **P<0.00008.
Nimbolide significantly inhibits tumor growth in a xenograft model of WM
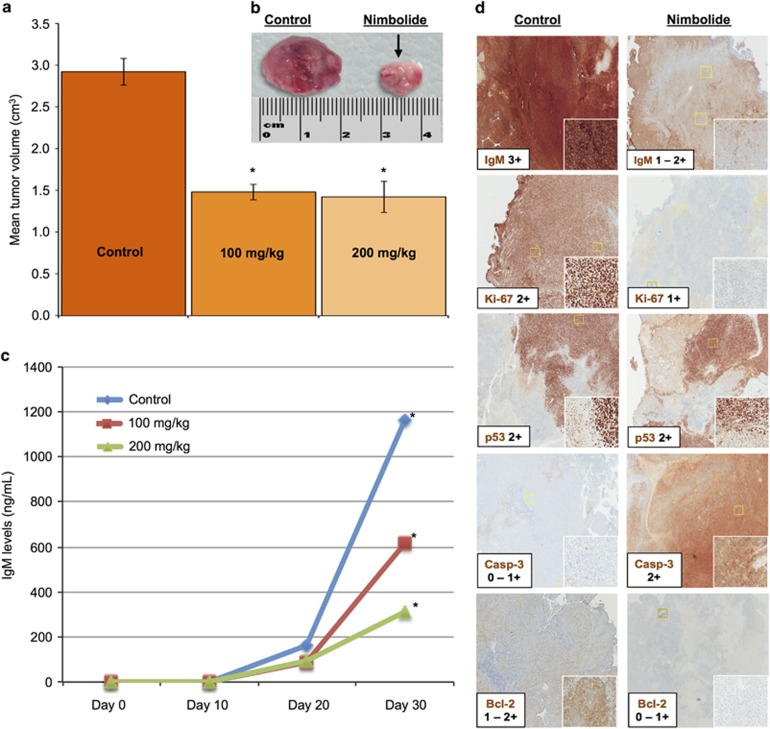
Although nimbolide has been examined in murine models of various cancers,14, 42, 44 its activity and effects on specific protein targets have not been assessed in in vivo models of WM (or any B-cell cancer). We hypothesized that systemically delivered nimbolide would engage its target(s) in vivo to induce WM cell death at pharmacologically achievable doses. Using our murine xenograft model of WM,26 we administered nimbolide via intraperitoneal injection in two separate cohorts of mice, testing two doses, 100 and 200 mg kg−1 (see Supplementary section). Compared with the control group (DMSO-treated mice), we observed a 48% reduction in tumor volume in the nimbolide treated mice (100 mg kg−1 cohort, P<0.05; Figures 8a and b and Supplementary Figure 4) and a corresponding decrease in human IgM secreted by the xenografted WM cells (P<0.05) (Figure 8c). A higher dose (200 mg kg−1) had a more pronounced impact on IgM reduction but not on tumor volume (vs that observed in the 100 mg kg−1 mice cohort). Immunohistochemistry (IHC) analysis was conducted on tumor tissues obtained from the animals treated with nimbolide (100 mg kg−1) or DMSO alone. In vivo activity was validated by a notable decrease of IgM and BCL2 in nimbolide treated mice vs control. Likewise, an increase in tumor cell apoptosis (cleaved caspase-3 stain) and decrease in cell proliferation (Ki-67 stain) was noted in these tissues (Figure 8d). These data provide sufficient evidence that nimbolide can be safely administered in WM xenografted mice, elicit a significant anti-tumor effect and modulate important WM cell death and apoptotic pathways.
Figure 8.
Nimbolide exerts anti-tumor activity in a human WM xenograft model. Female SCID mice (n=20) were subcutaneously implanted with 1 × 106 RPCI-WM1 cells (as described in the Supplementary methods). Progressive increase in IgM was measured in the serum and this corresponded with tumor growth in a time-dependent fashion. On average, WM tumor tissues grew to a median of 3 cm and IgM levels reached a median of 823 ng ml−1 by Day 30 post-implantation. (a) Average tumor reduction in the nimbolide 100 mg kg−1 cohort was 48% with no additional anti-WM benefit noted in the 200 mg kg−1 cohort. (b) Tumors from two representative mice (control and nimbolide 100 mg kg−1) show a significant reduction in nimbolide-treated mice (~50%). (c) Human IgM levels measured by ELISA were significantly lower in nimbolide treated mice vs control (DMSO). Data from 15 mice is shown as representatives of the 3 different treatment groups. *P<0.005. (d) IHC analysis of tumor samples taken from DMSO and nimbolide (100 mg kg−1) treated mice shows changes in IgM, Ki-67 and BCL2. The TP53 protein did not appear changed. Cleaved caspase-3 (Casp-3) was notably increased in nimbolide-treated mice.
Discussion
Nimbolide is a potent limonoid triterpene that is derived from the neem plant. It has been shown to produce anti-tumor responses in various preclinical solid tumor cancer models including melanoma,45 osteosarcoma,46 choriocarcinoma,47 cervical cancer,48 breast cancer,49 prostate adenocarcinoma,15, 50 colorectal carcinoma42, 51 and glioblastoma.16 However, the study of its activity in hematologic malignancies is limited; it has only been examined in MM, T-cell leukemia, myeloid leukemia and monocytic leukemia/lymphoma cell lines.45, 52 Our report provides critical insight into the mechanisms of nimbolide's cytotoxic potential in lymphoid cells and reveals that malignant WM (lymphoplasmacytic) cells are highly sensitive to the limonoid.
We have previously demonstrated that the anti-leukemic capabilities of NLE are mediated through caspase activation and engagement of the intrinsic apoptotic pathway.13 Being a powerful component of NLE, we anticipated that nimbolide would catalyze a similar cascade of death receptor-independent apoptotic signaling events in WM cells in vitro, as evidenced by cleavage of caspase-9. For activation of initiator caspase-9, destabilization of mitochondrial membrane permeability must occur.53 As such, we detected a dose-dependent increase in MOMP (indicating loss of Δψm) in nimbolide treated WM cells and this correlated with nimbolide-mediated cytotoxicity. Although we identified caspase-9 induction and cleavage in nimbolide treated cells, nimbolide has been shown to also engage the death receptor-dependent apoptotic pathway.14, 42, 49 These observations were made in preclinical models of breast, colon and buccal pouch carcinoma and attest to the unique properties of this limonoid in inducing (perhaps simultaneously) both intrinsic and extrinsic apoptotic signaling cascades. However, the degree to which nimbolide distinctly activates the respective apoptotic systems and their overall contribution toward imminent cell death maybe tumor-cell type dependent and remains to be investigated in cells of lymphocytic lineage.
Currently, management of WM entails the use of bortezomib-based regimens54 with reports of promising activity of the BTK inhibitor, ibrutinib, in relapsed/refractory WM patients.55 However, as in most B-cell cancers, drug resistance eventually develops and remains a daunting yet inevitable clinical challenge. As such, we were encouraged to find that nimbolide's cytotoxic effects were maintained in bortezomib or ibrutinib-resistant cell lines (BR and IR models, respectively) underscoring its potential role in these clinical settings. More intriguingly, BCWM.1/IR cells appeared most susceptible to nimbolide therapy exhibiting notably more apoptosis compared to ibrutinib sensitive (BCWM.1, MWCL-1) models. Using our IR model, we also observed that ibrutinib resistance could be ‘reversed' by treating BCWM.1/IR cells with ibrutinib combined with a suboptimal dose of nimbolide. Together, the two agents induced significantly more apoptosis (48% P<0.05), which indicates that nimbolide distinctively disrupts key oncogenic cellular networks engaged in ibrutinib resistance.
The capability of nimbolide to concurrently modulate several biological pathways has been previously described in solid tumor models where NFκB, TP53 and BCL2 associated proteins have been proposed to be the primary mediators of nimbolide's cytotoxicity.14, 42, 48, 56 It is likely that the anti-tumor effects of nimbolide are based on increased dependence of the tumor cell toward one (or more) of these pro-survival molecular pathways that are cell type-specific and are more vulnerable to modification by the limonoid. With compounds such as nimbolide, where the exact biological target is not known and the possibility of multiple oncotargets exists, the in silico technique of virtual screening is being increasingly utilized for its rapid and accurate prediction of compound–ligand interactions.18 Thus, to identify potential endogenous binding partners of nimbolide, we used a chemoinformatics-based approach to reverse dock all known human biological proteins that were similar in shape, structure and biochemical properties (descriptors) to nimbolide and with which it could potentially associate with. Others and we have previously reported that NLE modulates the expression of BCL2, an anti-apoptotic protein that plays a major role in preventing malignant B-cell (including WM) death.13, 14, 15, 16 We hypothesized that the anti-tumor activity of nimbolide might also be mediated through similar interactions. Our analysis suggests the BCL2–nimbolide interaction is critical for the antitumor activity of nimbolide and for the first time predicts the exact amino acid residues along with the thermodynamic and molecular mechanical exchanges that occur between these two molecules.
BCL2 functional biology is complex and tightly regulated by a number of post-translational modifications, which include phosphorylation, proteolytic cleavage, ubiquitination and proteasomal degradation.57 Phosphorylation of BCL2 in tumor cells has been shown to prolong the stability of BCL2, enhance its antiapoptotic activity and delay G1/S transition of the cell cycle.58, 59 Thus a post-translational modification, such as a phosphorylation-disrupting event, could destabilize BCL2 and render it vulnerable to proteolytic destruction, potentially explaining its partial downregulation in nimbolide treated cells. BCL2 phosphorylation can occur at multiple sites in the flexible loop domain, but it occurs notably at Ser70, Ser87, Thr56 and Thr74. However, its phosphorylation at Ser70 is widely accepted to support malignant cell survival and resistance to therapy. Because in silico screening identified BCL2 as the prime target we sought to validate the results in vitro by testing the cytotoxicity of nimbolide in a tumor cell model where the BCL2 protein is absolutely critical for cell survival, comparing against a model where the function of BCL2 has been attenuated by mutation and is not detrimental to tumor subsistence. We used RS4;11 tumor cells, which have been shown to express increased BCL2.43 Using a BH3 profiling technique, it was demonstrated that RS4;11 cells functionally rely on BCL2 (versus other BCL2 antiapoptotic members) for survival and were most sensitive to the anti-tumor effects of the BH3 mimetic ABT-737.43 For comparison, we also utilized Jurkat BCL2Ser70-Ala cells in which the Ser70 residue of the BCL2 flexible domain has been mutated to alanine and prevents full phosphorylation of BCL2, in effect reducing the antiapoptotic potential of the protein. Our finding that BCL2 mutated cells tolerated nimbolide treatment significantly (P<0.00008) more so than RS4;11 cells provides further support to the notion that nimbolide affects BCL2 and that the phosphorylational capacity of the protein indeed impacts the ability of the limonoid to produce an apoptotic reaction.
Lastly, we resolved to determine whether nimbolide could mitigate WM cell tumor expansion in vivo and how this effect was carried out. In vivo kinetics and toxicity profiling of nimbolide has been described previously, but no data exists in tumor models of lymphoid malignancy. The LD50 of nimbolide administered via intraperitoneal route in adult female mice has been reported as 280 mg kg−1 body weight.60 As such, for our initial study we opted to use two doses of nimbolide: a lower dose that was ~65% of the LD50 (100 mg kg−1) and a higher dose 200 mg kg−1, which was ~35% of the LD50 reported. Using these doses, we anticipated minimal toxicity with preliminary indication of anti-tumor efficacy, which was more prominent in the higher dose cohort. However, we did not observe any additional tumor volume reduction in the 200 mg kg−1 arm; rather this dose was less tolerated resulting in the death of two mice. In mice treated with the 100 mg kg−1 dose, we noted an approximate 50% decrease in tumor volume and IgM. This was an impressive finding and indicated that nimbolide had the potency to significantly (P<0.005) kill WM cells in vivo, particularly in a xenograft model that contains tumor cells developed from a terminally refractory WM patient. Additionally, IHC analysis provided valuable insight into the putative in vivo targets of nimbolide. A notable decrease in BCL2 was evident but we did not find any change in TP53 in nimbolide treated mice. This is largely consistent with our in vitro findings that indicated a negligible decrease in TP53 protein, which was observed on immunoblot (data not shown). The ability of nimbolide to act through TP53-independent and TP53-dependent mechanisms14, 48, 56 is not entirely surprising as it is a derivative of NLE. We previously demonstrated that NLE is equally active in TP53 intact and TP53–/– CD19+ CLL cells and TP53 protein expression is decreased by NLE treatment.13 With TP53 mutations and deletions being prevalent in the more aggressive stages of cancer, this finding warrants attention because an anti-cancer compound with activity in TP53 defective patients is highly desirable.
In summary, we have extensively examined the biochemical features of nimbolide in preclinical models of B-cell cancer. Our data unequivocally demonstrate that its anti-tumor effects, most notably in WM cancer cells in vitro and in vivo, are mediated through the intrinsic apoptotic pathway. Nimbolide directly targets BCL2 and this appears to be a central mechanism for its cytotoxic activity in lymphoid cancer cells. Other potential targets such as HSP90 and PI3K (p110γ), identified through in silico reverse docking, may also be affected as indicated by modulation of their associated proteins. Ongoing studies will examine their significance and interplay through a more in depth manner. Our investigations also show that nimbolide is capable of being combined with established anti-WM therapeutics and sensitizes drug resistant WM cells to the effects of bortezomib or ibrutinib. Collectively, the data provided in this report lay the foundational studies and provide a firm rationale for additional testing of nimbolide and its consideration for further development as an anti-WM therapeutic.
Acknowledgments
The experiments and analysis carried out in this study were supported by funding from the Leukemia and Lymphoma Society (AC-K is a Leukemia and Lymphoma Scholar in Clinical Research) and the Daniel Foundation of Alabama (AC-K). We thank Mrs Kelly Viola for her editorial assistance.
Footnotes
Supplementary Information accompanies this paper on Blood Cancer Journal website (http://www.nature.com/bcj)
The authors declare no conflict of interest.
Supplementary Material
References
- Owen RG, Treon SP, Al-Katib A, Fonseca R, Greipp PR, McMaster ML et al. Clinicopathological definition of Waldenstrom's macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom's Macroglobulinemia. Semin Oncol 2003; 30: 110–115. [DOI] [PubMed] [Google Scholar]
- Ansell SM, Hodge LS, Secreto FJ, Manske M, Braggio E, Price-Troska T et al. Activation of TAK1 by MYD88 L265P drives malignant B-cell Growth in Non-Hodgkin lymphoma. Blood Cancer J 2014; 4: e183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus A, Chitta K, Akhtar S, Personett D, Miller KC, Thompson KJ et al. AT-101 downregulates BCL2 and MCL1 and potentiates the cytotoxic effects of lenalidomide and dexamethasone in preclinical models of multiple myeloma and Waldenstrom macroglobulinaemia. Br J Haematol 2014; 164: 352–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JC. Bcl-2 family proteins: regulators of apoptosis and chemoresistance in hematologic malignancies. Semin Hematol 1997; 34: 9–19. [PubMed] [Google Scholar]
- Thomas A, El Rouby S, Reed JC, Krajewski S, Silber R, Potmesil M et al. Drug-induced apoptosis in B-cell chronic lymphocytic leukemia: relationship between p53 gene mutation and bcl-2/bax proteins in drug resistance. Oncogene 1996; 12: 1055–1062. [PubMed] [Google Scholar]
- Ailawadhi S, Miecznikowski J, Gaile DP, Wang D, Sher T, Mulligan G et al. Bortezomib mitigates adverse prognosis conferred by Bcl-2 overexpression in patients with relapsed/refractory multiple myeloma. Leuk Lymphoma 2012; 53: 1174–1182. [DOI] [PubMed] [Google Scholar]
- Iqbal J, Meyer PN, Smith LM, Johnson NA, Vose JM, Greiner TC et al. BCL2 predicts survival in germinal center B-cell-like diffuse large B-cell lymphoma treated with CHOP-like therapy and rituximab. Clin Cancer Res 2011; 17: 7785–7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansell SM, Kyle RA, Reeder CB, Fonseca R, Mikhael JR, Morice WG et al. Diagnosis and management of Waldenstrom macroglobulinemia: Mayo stratification of macroglobulinemia and risk-adapted therapy (mSMART) guidelines. Mayo Clin Proc 2010; 85: 824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod 2012; 75: 311–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan K, Wierda WG, Keating MJ, Gandhi V. Gossypol, a BH3 mimetic, induces apoptosis in chronic lymphocytic leukemia cells. Blood 2008; 112: 1971–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanafelt TD, Call TG, Zent CS, LaPlant B, Bowen DA, Roos M et al. Phase I trial of daily oral Polyphenon E in patients with asymptomatic Rai stage 0 to II chronic lymphocytic leukemia. J Clin Oncol 2009; 27: 3808–3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Prasad M, Sah NK. Anticancer biology of Azadirachta indica L (neem): a mini review. Cancer Biol Ther 2011; 12: 467–476. [DOI] [PubMed] [Google Scholar]
- Chitta KS, Khan AN, Ersing N, Swaika A, Masood A, Paulus A et al. Neem leaf extract induces cell death by apoptosis and autophagy in B-chronic lymphocytic leukemia cells. Leuk Lymphoma 2013; 55: 652–661. [DOI] [PubMed] [Google Scholar]
- Harish Kumar G, Vidya Priyadarsini R, Vinothini G, Vidjaya Letchoumy P, Nagini S. The neem limonoids azadirachtin and nimbolide inhibit cell proliferation and induce apoptosis in an animal model of oral oncogenesis. Invest New Drugs 2010; 28: 392–401. [DOI] [PubMed] [Google Scholar]
- Raja Singh P, Arunkumar R, Sivakamasundari V, Sharmila G, Elumalai P, Suganthapriya E et al. Anti-proliferative and apoptosis inducing effect of nimbolide by altering molecules involved in apoptosis and IGF signalling via PI3K/Akt in prostate cancer (PC-3) cell line. Cell Biochem Funct 2013; 32: 217–228. [DOI] [PubMed] [Google Scholar]
- Karkare S, Chhipa RR, Anderson J, Liu X, Henry H, Gasilina A et al. Direct inhibition of retinoblastoma phosphorylation by nimbolide causes cell-cycle arrest and suppresses glioblastoma growth. Clin Cancer Res 2014; 20: 199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CP. Finding the target after screening the phenotype. Drug Discov Today 2005; 10: 513–519. [DOI] [PubMed] [Google Scholar]
- Kar S, Roy K. How far can virtual screening take us in drug discovery? Expert Opin Drug Discov 2013; 8: 245–261. [DOI] [PubMed] [Google Scholar]
- Wang L, Ma C, Wipf P, Liu H, Su W, Xie XQ. TargetHunter: an in silico target identification tool for predicting therapeutic potential of small organic molecules based on chemogenomic database. AAPS J 2013; 15: 395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinter SZ, Liang Y, Huang SY, Hyder SM, Zou X. An inverse docking approach for identifying new potential anti-cancer targets. J Mol Graph Model 2011; 29: 795–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YZ, Zhi DG. Ligand-protein inverse docking and its potential use in the computer search of protein targets of a small molecule. Proteins 2001; 43: 217–226. [DOI] [PubMed] [Google Scholar]
- Fan S, Geng Q, Pan Z, Li X, Tie L, Pan Y et al. Clarifying off-target effects for torcetrapib using network pharmacology and reverse docking approach. BMC Syst Biol 2012; 6: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng R, Chen TS, Lu T. A comparative reverse docking strategy to identify potential antineoplastic targets of tea functional components and binding mode. Int J Mol Sci 2011; 12: 5200–5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Fang JS, Bai XY, Zhou D, Wang YT, Liu AL et al. In silico target fishing for the potential targets and molecular mechanisms of baicalein as an antiparkinsonian agent: discovery of the protective effects on NMDA receptor-mediated neurotoxicity. Chem Biol Drug Des 2013; 81: 675–687. [DOI] [PubMed] [Google Scholar]
- Paulus A, Akhtar S, Kuranz M, Ailawadhi S, Miller K, Rivera CE et al. Development of a human IgM secreting model of Rpci-WM1 through xenografting In SCID mice for in vivo drug evaluation. Blood 2013; 122: 3081. [Google Scholar]
- Chitta KS, Paulus A, Ailawadhi S, Foster BA, Moser MT, Starostik P et al. Development and characterization of a novel human Waldenstrom macroglobulinemia cell line: RPCI-WM1, Roswell Park Cancer Institute—Waldenstrom Macroglobulinemia 1. Leuk Lymphoma 2013; 54: 387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev 2007; 87: 99–163. [DOI] [PubMed] [Google Scholar]
- Zhang YJ, Caulfield T, Xu YF, Gendron TF, Hubbard J, Stetler C et al. The dual functions of the extreme N-terminus of TDP-43 in regulating its biological activity and inclusion formation. Hum Mol Genet 2013; 22: 3112–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivoli M, Caulfield TR, Martinez-Mayorga K, Johnson AT, Jiao GS, Lindberg I. Inhibition of prohormone convertases PC1/3 and PC2 by 2,5-dideoxystreptamine derivatives. Mol Pharmacol 2012; 81: 440–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Franco JL, Caulfield T. Advances in the computational development of DNA methyltransferase inhibitors. Drug Discov Today 2011; 16: 418–425. [DOI] [PubMed] [Google Scholar]
- Caulfield TR, Devkota B, Rollins GC. Examinations of tRNA Range of Motion Using Simulations of Cryo-EM Microscopy and X-Ray Data. J Biophys 2011; 2011: 219515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield TR. Inter-ring rotation of apolipoprotein A-I protein monomers for the double-belt model using biased molecular dynamics. J Mol Graph Model 2011; 29: 1006–1014 [DOI] [PubMed] [Google Scholar]
- Caulfield T, Medina-Franco JL. Molecular dynamics simulations of human DNA methyltransferase 3B with selective inhibitor nanaomycin A. J Struct Biol 2011; 176: 185–191. [DOI] [PubMed] [Google Scholar]
- Caulfield T, Devkota B. Motion of transfer RNA from the A/T state into the A-site using docking and simulations. Proteins 2012; 80: 2489–2500. [DOI] [PubMed] [Google Scholar]
- Ash PE, Bieniek KF, Gendron TF, Caulfield T, Lin WL, Dejesus-Hernandez M et al. Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron 2013; 77: 639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdul-Hay SO, Lane AL, Caulfield TR, Claussin C, Bertrand J, Masson A et al. Optimization of peptide hydroxamate inhibitors of insulin-degrading enzyme reveals marked substrate-selectivity. J Med Chem 2013; 56: 2246–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan AA, Berthoux P, El Deeb S, Bonnevial L, Cecillon S, Berthoux F. Impact of hepatitis C on renal transplantation: a long-term study. Saudi J Kidney Dis Transpl 1999; 10: 487–492. [PubMed] [Google Scholar]
- Bodduluru LN, Kasala ER, Thota N, Barua CC, Sistla R. Chemopreventive and therapeutic effects of nimbolide in cancer: The underlying mechanisms. Toxicol In Vitro 2014; 28: 1026–1035. [DOI] [PubMed] [Google Scholar]
- Nimmanapalli R, O'Bryan E, Kuhn D, Yamaguchi H, Wang HG, Bhalla KN. Regulation of 17-AAG-induced apoptosis: role of Bcl-2, Bcl-XL, and Bax downstream of 17-AAG-mediated down-regulation of Akt, Raf-1, and Src kinases. Blood 2003; 102: 269–275. [DOI] [PubMed] [Google Scholar]
- Banerjee Mustafi S, Chakraborty PK, Raha S. Modulation of Akt and ERK1/2 pathways by resveratrol in chronic myelogenous leukemia (CML) cells results in the downregulation of Hsp70. PLoS One 2010; 5: e8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloo B, Nagel D, Pfeifer M, Grau M, Duwel M, Vincendeau M et al. Critical role of PI3K signaling for NF-kappaB-dependent survival in a subset of activated B-cell-like diffuse large B-cell lymphoma cells. Proc Natl Acad Sci USA 2011; 108: 272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SC, Prasad S, Sethumadhavan DR, Nair MS, Mo YY, Aggarwal BB. Nimbolide, a limonoid triterpene, inhibits growth of human colorectal cancer xenografts by suppressing the proinflammatory microenvironment. Clin Cancer Res 2013; 19: 4465–4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Gaizo Moore V, Schlis KD, Sallan SE, Armstrong SA, Letai A. BCL-2 dependence and ABT-737 sensitivity in acute lymphoblastic leukemia. Blood 2008; 111: 2300–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priyadarsini RV, Manikandan P, Kumar GH, Nagini S. The neem limonoids azadirachtin and nimbolide inhibit hamster cheek pouch carcinogenesis by modulating xenobiotic-metabolizing enzymes, DNA damage, antioxidants, invasion and angiogenesis. Free Radic Res 2009; 43: 492–504. [DOI] [PubMed] [Google Scholar]
- Roy MK, Kobori M, Takenaka M, Nakahara K, Shinmoto H, Isobe S et al. Antiproliferative effect on human cancer cell lines after treatment with nimbolide extracted from an edible part of the neem tree (Azadirachta indica). Phytother Res 2007; 21: 245–250. [DOI] [PubMed] [Google Scholar]
- Cohen E, Quistad GB, Casida JE. Cytotoxicity of nimbolide, epoxyazadiradione and other limonoids from neem insecticide. Life Sci 1996; 58: 1075–1081. [DOI] [PubMed] [Google Scholar]
- Harish Kumar G, Chandra Mohan KV, Jagannadha Rao A, Nagini S. Nimbolide a limonoid from Azadirachta indica inhibits proliferation and induces apoptosis of human choriocarcinoma (BeWo) cells. Invest New Drugs 2009; 27: 246–252. [DOI] [PubMed] [Google Scholar]
- Priyadarsini RV, Murugan RS, Sripriya P, Karunagaran D, Nagini S. The neem limonoids azadirachtin and nimbolide induce cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells. Free Radic Res 2010; 44: 624–634. [DOI] [PubMed] [Google Scholar]
- Elumalai P, Gunadharini DN, Senthilkumar K, Banudevi S, Arunkumar R, Benson CS et al. Induction of apoptosis in human breast cancer cells by nimbolide through extrinsic and intrinsic pathway. Toxicol Lett 2012; 215: 131–142. [DOI] [PubMed] [Google Scholar]
- Wu Q, Kohli M, Bergen III HR, Cheville JC, Karnes RJ, Cao H et al. Preclinical evaluation of the supercritical extract of Azadirachta indica (neem) leaves in vitro and in vivo on inhibition of prostate cancer tumor growth. Mol Cancer Ther 2014; 13: 1067–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babykutty S, S PP, J NR, Kumar MA, Nair MS, Srinivas P et al. Nimbolide retards tumor cell migration, invasion, and angiogenesis by downregulating MMP-2/9 expression via inhibiting ERK1/2 and reducing DNA-binding activity of NF-kappaB in colon cancer cells. Mol Carcinog 2012; 51: 475–490. [DOI] [PubMed] [Google Scholar]
- Gupta SC, Prasad S, Reuter S, Kannappan R, Yadav VR, Ravindran J et al. Modification of cysteine 179 of IkappaBalpha kinase by nimbolide leads to down-regulation of NF-kappaB-regulated cell survival and proliferative proteins and sensitization of tumor cells to chemotherapeutic agents. J Biol Chem 2010; 285: 35406–35417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengartner MO. The biochemistry of apoptosis. Nature 2000; 407: 770–776. [DOI] [PubMed] [Google Scholar]
- Boswell EN, Chuma S, Banwait R, Hanlon C, Leblebjian H, Warren D et al. Phase I/II Trial Of Everolimus, Bortezomib and Rituximab in relapsed or relapsed/refractory Waldenstrom's macroglobulinemia. Blood 2013; 122: 4402. [Google Scholar]
- Tripsas CK, Yang G, Cao Y, Xu L, Hunter Z, Cropper SJ et al. A prospective multicenter study of the Bruton's Tyrosine Kinase inhibitor Ibrutinib in patients with relapsed or refractory Waldenstrom's macroglobulinemia. Blood 2013; 122: 251. [Google Scholar]
- Gupta SC, Reuter S, Phromnoi K, Park B, Hema PS, Nair M et al. Nimbolide sensitizes human colon cancer cells to TRAIL through reactive oxygen species- and ERK-dependent up-regulation of death receptors, p53, and Bax. J Biol Chem 2011; 286: 1134–1146. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell 2006; 10: 375–388. [DOI] [PubMed] [Google Scholar]
- Deng X, Gao F, Flagg T, May WS Jr. Mono- and multisite phosphorylation enhances Bcl2's antiapoptotic function and inhibition of cell cycle entry functions. Proc Natl Acad Sci USA 2004; 101: 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Galan P, Roue G, Lopez-Guerra M, Nguyen M, Villamor N, Montserrat E et al. BCL-2 phosphorylation modulates sensitivity to the BH3 mimetic GX15-070 (Obatoclax) and reduces its synergistic interaction with bortezomib in chronic lymphocytic leukemia cells. Leukemia 2008; 22: 1712–1720. [DOI] [PubMed] [Google Scholar]
- Glinsukon T, Somjaree R, Piyachaturawat P, Thebtaranonth Y. Acute toxicity of nimbolide and nimbic acid in mice, rats and hamsters. Toxicol Lett 1986; 30: 159–166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.