Abstract
Death receptor-mediated apoptosis is a key mechanism for the control of immune responses and dysregulation of this pathway may lead to autoimmunity. Cellular FLICE-inhibitory proteins (c-FLIPs) are known as inhibitors of death receptor-mediated apoptosis. The only short murine c-FLIP splice variant is c-FLIPRaji (c-FLIPR). To investigate the functional role of c-FLIPR in the immune system, we used the vavFLIPR mouse model constitutively expressing murine c-FLIPR in all hematopoietic compartments. Lymphocytes from these mice are protected against CD95-mediated apoptosis and activation-induced cell death. Young vavFLIPR mice display normal lymphocyte compartments, but the lymphocyte populations alter with age. We identified reduced levels of T cells and slightly higher levels of B cells in 1-year-old vavFLIPR mice compared with wild-type (WT) littermates. Moreover, both B and T cells from aged vavFLIPR animals show activated phenotypes. Sera from 1-year-old WT and transgenic animals were analysed for anti-nuclear antibodies. Notably, elevated titres of these autoantibodies were detected in vavFLIPR sera. Furthermore, tissue damage in kidneys and lungs from aged vavFLIPR animals was observed, indicating that vavFLIPR mice develop a systemic lupus erythematosus-like phenotype with age. Taken together, these data suggest that c-FLIPR is an important modulator of apoptosis and enforced expression leads to autoimmunity.
Keywords: c-FLIP, apoptosis, CD95, autoimmunity
It is crucial that excessive lymphocytes are deleted by apoptosis after efficient antigen clearance to maintain cell homeostasis.1 Moreover, insufficient apoptosis of autoreactive immune cells may result in autoimmune diseases.1 The extrinsic apoptosis pathway is triggered by ligand binding of death receptors, such as CD95 (Fas/APO-1), and regulates the removal of unwanted lymphocytes.2 Elimination of T cells is achieved by activation-induced cell death (AICD), which is triggered upon restimulation of T cells without co-stimulation.2 Notably, the CD95 death receptor pathway was shown to be important for AICD.3, 4, 5, 6 Furthermore, dysregulation of the CD95 signalling pathway was reported to result in severely altered lymphoproliferation (Lpr) and autoimmunity in both lpr and generalised lymphoproliferative disorder (gld) mice, which have mutated CD95 receptor or ligand, respectively, as well as in patients suffering from the homologous human disease autoimmune lymphoproliferative syndrome.7, 8 CD95 is the best-characterised death receptor and it is activated by binding of its cognate ligand CD95L.9 Ligand-binding results in receptor oligomerisation and formation of the death-inducing signalling complex (DISC) through recruitment of the proteins Fas-associated death domain-containing protein (FADD), procaspase-8, -10 and cellular FLICE-inhibitory protein (c-FLIP).10 Autoproteolytic processing of procaspase-8 at the DISC generates active caspase-8,11, 12 which cleaves and activates caspase-3 and -7. The effector caspases process further substrates, eventually leading to apoptosis.13, 14 The c-FLIPs inhibit apoptosis by competing with procaspase-8 for binding sites at the DISC and additionally interfere with caspase-8 processing.15, 16, 17 So far, three c-FLIP isoforms expressed on the protein level have been reported: c-FLIPLong (c-FLIPL), c-FLIPShort (c-FLIPS) and c-FLIPRaji (c-FLIPR).15, 17, 18 The long isoform resembles caspase-8, but lacks catalytic function because of substitution of amino-acid residues critical for enzymatic activity.18, 19 The two short isoforms are distinguished by a functional nucleotide polymorphism and mainly consist of the death effector domains with unique C-terminal tails.20 The structure of the murine Cflar (caspase-8 and FADD-like apoptosis regulator) gene differs from the human gene locus, in that only c-FLIPL and c-FLIPR can be expressed.21 Although the short c-FLIP isoforms have been described as anti-apoptotic, c-FLIPL was reported to act both pro- and anti-apoptotic depending on expression level and strength of receptor stimulation.22, 23, 24 Transgenic overexpression of c-FLIPL in mice resulted in autoimmunity, although only in the Balb/c background.25 In contrast, mice overexpressing human c-FLIPS did not develop autoimmune disease.26, 27 Not much is known about the physiological function of c-FLIPR. To investigate the functional role of c-FLIPR in the immune system, we used a mouse model, called vavFLIPR, with constitutive expression of murine c-FLIPR in all hematopoietic compartments. We previously reported that these transgenic mice have a better clearance of the Gram-positive bacteria Listeria monocytogenes compared with infected wild-type (WT) mice.28 Here we show that aged mice constitutively expressing murine c-FLIPR have altered lymphocyte populations with higher levels of activated B and T cells compared with WT littermates. Moreover, vavFLIPR animals spontaneously develop autoimmunity with age, showing features of systemic lupus erythematosus (SLE).
Results
vavFLIPR mice do not recapitulate the lpr/gld phenotype
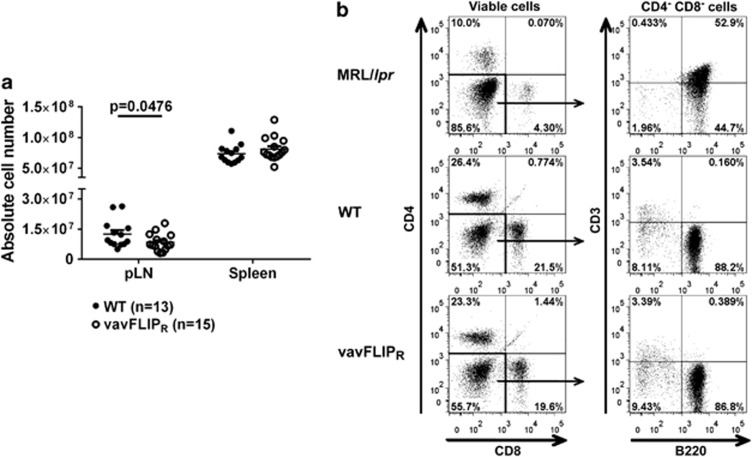
Insufficient cell death can lead to autoimmunity as a result of altered immune cell populations. Lpr and gld mice, with mutations in the CD95 receptor and ligand, respectively, develop Lpr and autoimmunity.7, 29 Moreover, these mice accumulate unusual CD4– CD8– double negative (DN) B220+ αβ T cells.7, 30 c-FLIPR inhibits apoptosis by competing with caspase-8 for binding sites at the DISC.17, 21 The mouse model vavFLIPR, in which murine c-FLIPR is under control of the vav-promoter to ensure expression in all hematopoietic compartments,28 was used to study the functional role of murine c-FLIPR in the immune system. As thymocytes as well as B and T cells from vavFLIPR mice were protected against CD95-induced apoptosis,28 we investigated if the impaired lymphocyte apoptosis in vavFLIPR mice would lead to elevated numbers of lymphocytes and altered lymphocyte populations with age. No Lpr was identified in 12–14 months old vavFLIPR animals. In fact, the number of cells in the peripheral lymph nodes (pLNs) from vavFLIPR mice was reduced compared with WT littermates (Figure 1a). Furthermore, pLN cells of WT and vavFLIPR mice were analysed for DN B220+ T cells compared with 3 months old MRL/lpr mice as controls. The characteristic DN B220+ population was identified in MRL/lpr mice, but was not observed in either WT or vavFLIPR animals (Figure 1b). The lack of DN B220+ T cells in vavFLIPR mice is similar to the phenotype of c-FLIPL transgenic mice31 and mice with transgenic expression of human c-FLIPS.27 Thus, vavFLIPR mice do not develop an lpr/gld-like phenotype.
Figure 1.
One-year-old vavFLIPR mice display normal cellularity in lymphoid organs and do not accumulate DN B220+ cells. (a) Absolute cell numbers in pLNs and spleens of WT (n=13) and vavFLIPR (n=15) mice at 12–14 months of age. Symbols represent individual mice, horizontal lines display the mean±S.E.M. pooled from five independent experiments. Statistical analysis was performed with two-tailed nonparametric Mann–Whitney U-test. (b) Analysis of DN B220+ cells in pLNs from 1-year-old WT and vavFLIPR animals. Representative dot plots are shown for MRL/lpr (n=2), WT (n=6) and vavFLIPR (n=7) mice. Three months old MRL/lpr mice develop the characteristic DN B220+ cells (upper panel). This subset could not be identified in either WT (mid panel) or vavFLIPR mice (lower panel)
Altered lymphocyte populations in aged vavFLIPR mice
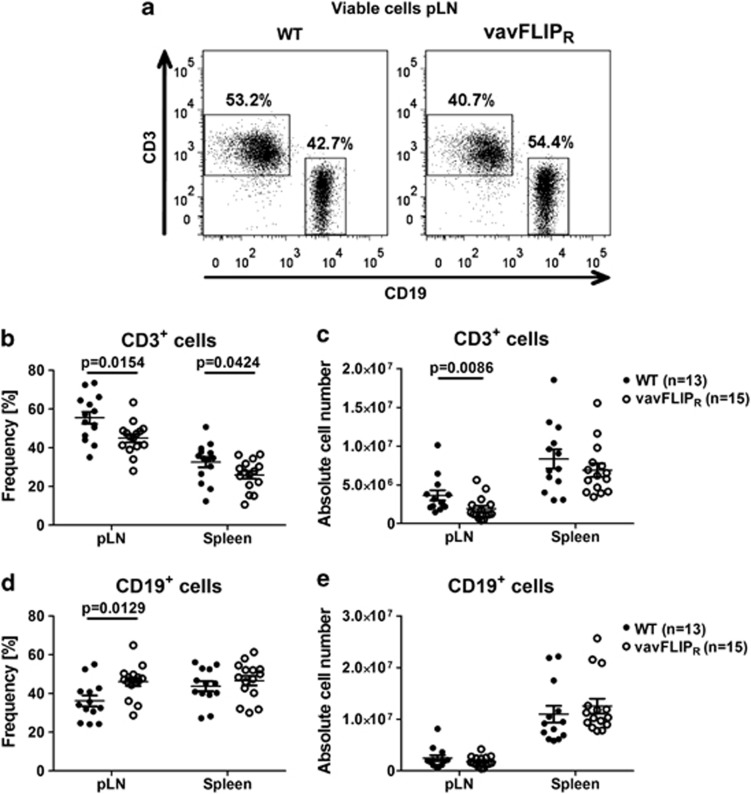
Young vavFLIPR mice displayed normal lymphocyte populations.28 We analysed aged WT and vavFLIPR mice to investigate if the constitutive expression of c-FLIPR alters lymphocyte populations over time. Dendritic cells (DCs), macrophages and granulocytes have been reported to be regulated through death receptors and c-FLIP proteins.32, 33 We therefore analysed the frequencies of CD11c+ DCs, CD49b+ natural killer (NK) cells, F4/80+ macrophages and Gr1+ granulocytes in 1-year-old mice. The populations of CD11c+, CD49b+, F4/80+ and Gr1+ cells from WT and vavFLIPR were comparable (Supplementary Table 1). Flow cytometry analyses of lymphocyte sub-populations in spleen and pLNs from 12 to 14 months old mice revealed reduced frequencies of vavFLIPR CD3+ T cells compared with WT CD3+ T cells (Figures 2a and b), consistent with reduced absolute CD3+ T-cell number in pLNs from vavFLIPR animals (Figure 2c). Furthermore, an increased percentage of vavFLIPR CD19+ B cells in pLNs compared with WT was observed (Figures 2a and d). Frequencies and absolute numbers of CD19+ B cells were not significantly altered in the spleen (Figure 2e). Neither did we detect alterations in germinal centre formation in aged transgenic mice (data not shown). The CD4+/CD8+ cell profiles within the CD3+ T-cell compartment were comparable between WT and vavFLIPR mice (Supplementary Figure 1). Nevertheless, lower frequencies of splenic CD8+ T cells were observed as well as reduced absolute cell numbers of CD8+ cells in pLNs (Supplementary Figure 1b). As we previously reported that thymocyte development in vavFLIPR mice is normal,28 we suggest that c-FLIPR expression regulates homeostasis of peripheral CD8+ T cells. Taken together, the B- and T-cell populations are altered in aged vavFLIPR mice, whereas other haematopoietic cells are unaffected.
Figure 2.
Altered lymphocyte populations in 12–14 months old vavFLIPR mice. (a) Representative dot plots of CD3- and CD19-stained WT and vavFLIPR pLN cells. Frequency and absolute cell number of CD3+ cells (b and c) and CD19+ cells (d and e) in freshly isolated pLNs and spleens from WT (n=13) and vavFLIPR (n=15) littermates. Individual mice are represented as separate symbols. Horizontal lines show the mean pooled from five independent experiments; error bars display the S.E.M. Statistical analyses were performed with two-tailed nonparametric Mann–Whitney U-tests
Activated phenotypes of T and B cells from vavFLIPR mice
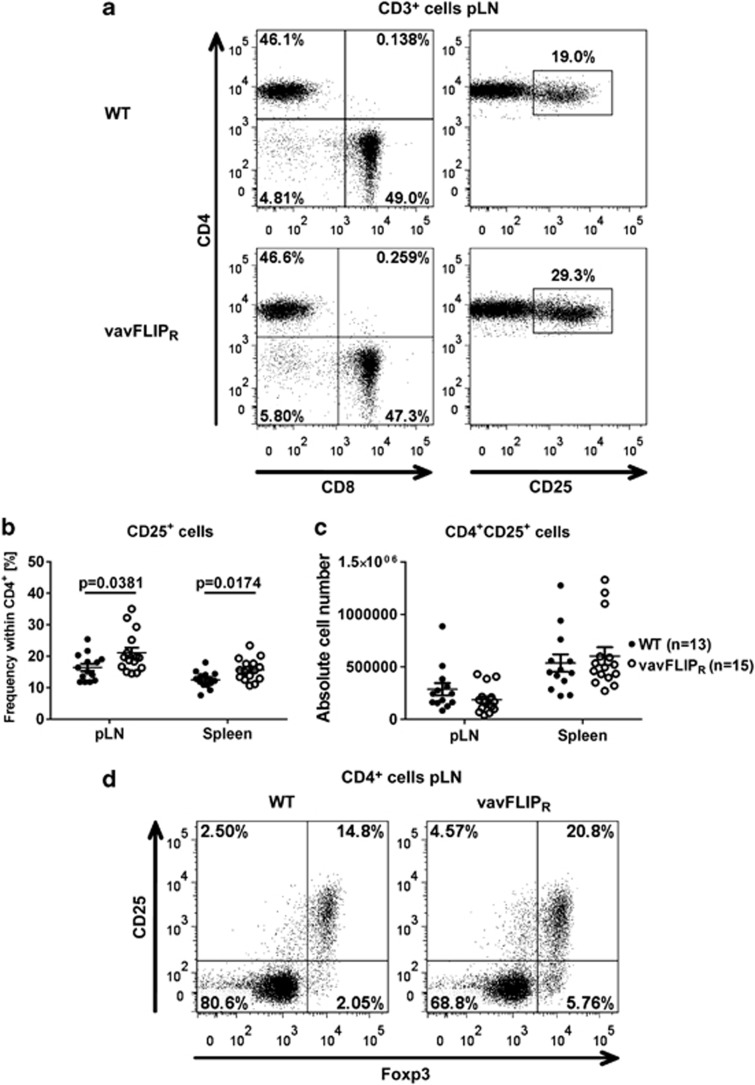
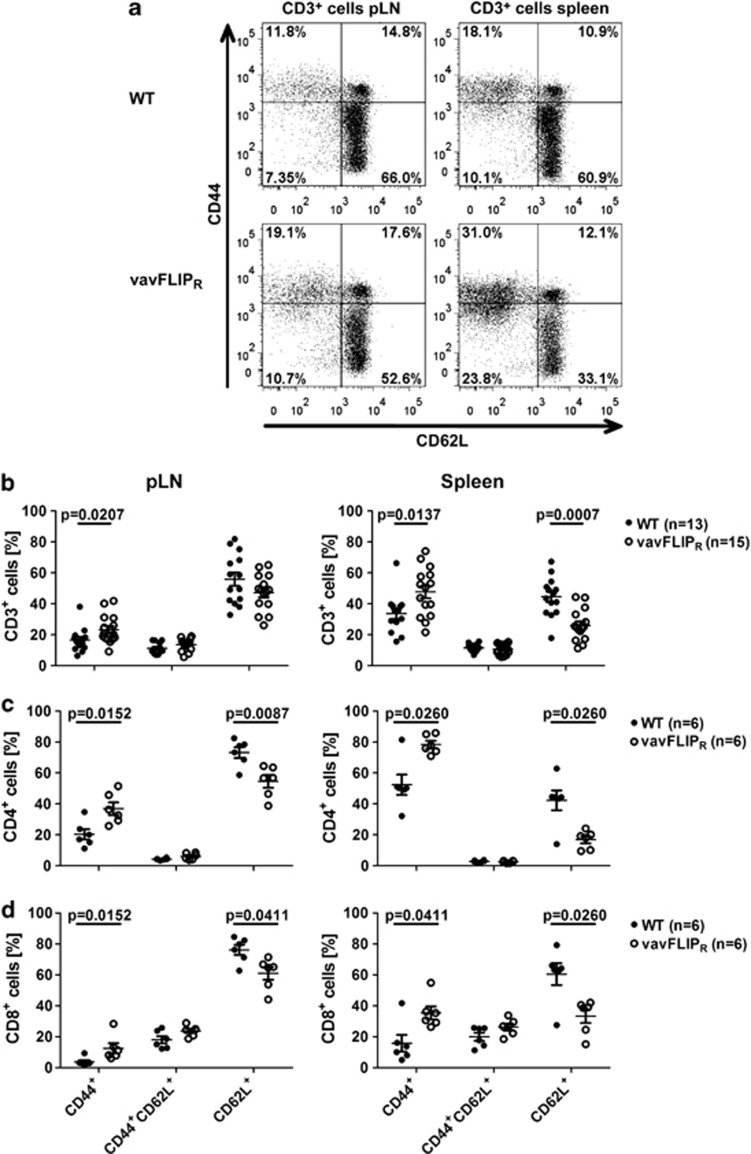
The activation status of T cells in young vavFLIPR mice was comparable to WT littermates.28 As AICD is impaired in T cells from vavFLIPR mice,28 we investigated if the activation status changed with age. We observed higher percentages of vavFLIPR CD4+ cells expressing the activation marker CD25 in comparison with WT CD4+ cells (Figures 3a and b), with absolute cell numbers being similar between 12 and 14 months old WT and vavFLIPR mice (Figure 3c). As CD25 is not only a marker for activated T cells, but is also highly expressed on regulatory T (Treg) cells,34 we analysed the expression of the master transcription factor of Treg cells, Foxp3.35 Indeed, most of the CD25+ CD4+ T cells co-expressed Foxp3 indicating that 1-year-old vavFLIPR mice have higher levels of Treg cells in both pLNs (Figure 3d) and spleen (data not shown) compared with WT littermates. Interestingly, we also observed an increase in CD25+ T cells that were negative for Foxp3 suggesting that conventional T cells may indeed be more activated (Figure 3d). Therefore, the memory/naive T-cell profile was analysed in 12–14 months old WT and vavFLIPR animals. Strikingly, elevated frequencies of CD44+ antigen-experienced cells within the CD3+ compartment were identified in both pLNs and spleen from vavFLIPR mice (Figures 4a and b). We also observed reduced numbers of CD3+CD62L+ naive T cells in vavFLIPR animals (Supplementary Figure 2a). Notably, both within the CD4+ helper T-cell population and the CD8+ cytotoxic T-cell population from vavFLIPR pLNs and spleens, higher levels of antigen-experienced (CD44+) cells and lower levels of naive (CD62L+) cells were identified compared with WT (Figures 4c and d). The absolute cell numbers of CD62L+ cells were reduced for both CD4+ and CD8+ T-cell populations (Supplementary Figures 2b and c). These data indicate that T cells from vavFLIPR mice are highly activated in comparison with WT T cells.
Figure 3.
Increased levels of CD25+ T cells and Treg cells in 1-year-old vavFLIPR animals. (a) Representative dot plots of WT (upper panel) and vavFLIPR (lower panel) CD3+ cells in pLNs. Frequency (b) and absolute cell number (c) of CD4+CD25+ cells in WT (n=13) and vavFLIPR (n=15) pLN and spleens. Symbols represent individual mice. Horizontal lines display the mean±S.E.M. Statistical analyses were performed with two-tailed nonparametric Mann–Whitney U-tests. (d) Dot plots showing CD25+ and Foxp3+ T cells within the CD4+ compartment representative for three WT (left) and four vavFLIPR (right) mice
Figure 4.
T cells from vavFLIPR mice at 12–14 months of age are highly activated. (a) Representative dot plots of pLN cells (left panel) and splenocytes (right panel) from WT (upper panel) and vavFLIPR animals (lower panel). Frequencies of CD44+, CD44+CD62L+ and CD62L+ cells within CD3+ cells (b), CD4+ cells (c) and CD8+ cells (d) from pLN and spleen. (b-d) Symbols represent individual WT (b: n=13, c and d: n=6) and vavFLIPR (b: n=15, c and d: n=6) mice. Horizontal lines represent (b) the mean pooled from five independent experiments or (c and d) the mean of one experiment representative for two independent experiments; error bars display S.E.M. Statistical analyses were performed with two-tailed nonparametric Mann–Whitney U-tests
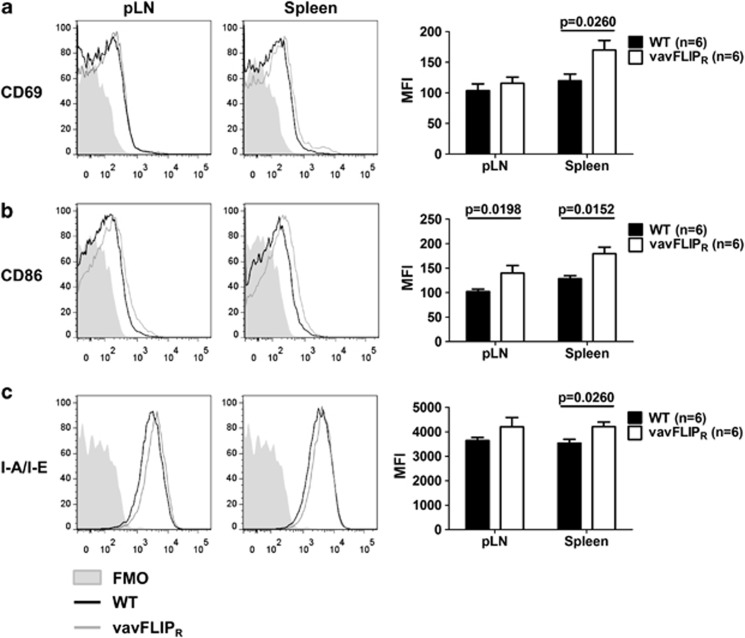
Similar to T cells, B cells from vavFLIPR mice are protected against apoptosis.28 The activation status of B cells was examined by flow cytometry. CD40, CD80 and CD95 had similar expression levels in vavFLIPR and WT animals (data not shown), whereas analysis of the activation markers CD69, CD86 and major histocompatibility complex class II molecule I-A/I-E revealed an increased expression of these markers in vavFLIPR B cells compared with WT B cells (Figure 5). This indicates that B cells from 12 to 14 months old vavFLIPR mice are more activated in comparison with B cells from WT animals at 1 year of age. The activated phenotypes of B and T cells identified in 1-year-old vavFLIPR mice are consistent with a previous study, which reported that B and T cells from c-FLIPL transgenic mice on a Balb/c background were highly activated.25
Figure 5.
vavFLIPR B cells from 12 to 14 months old mice show an activated phenotype. (a–c) pLN cells and splenocytes from WT and vavFLIPR mice (n=6) were isolated and analysed by flow cytometry. The activation status of CD19+ B cells was assayed by the markers CD69 (a), CD86 (b) and I-A/I-E (c). Representative histograms with fluorescent minus one (FMO) as control (left panel) and diagrams displaying the mean fluorescent intensity (MFI; right panel)±S.E.M. are shown. Statistical analyses were performed with two-tailed nonparametric Mann–Whitney U-tests
Disease progression in MOG-EAE is comparable between WT and vavFLIPR mice
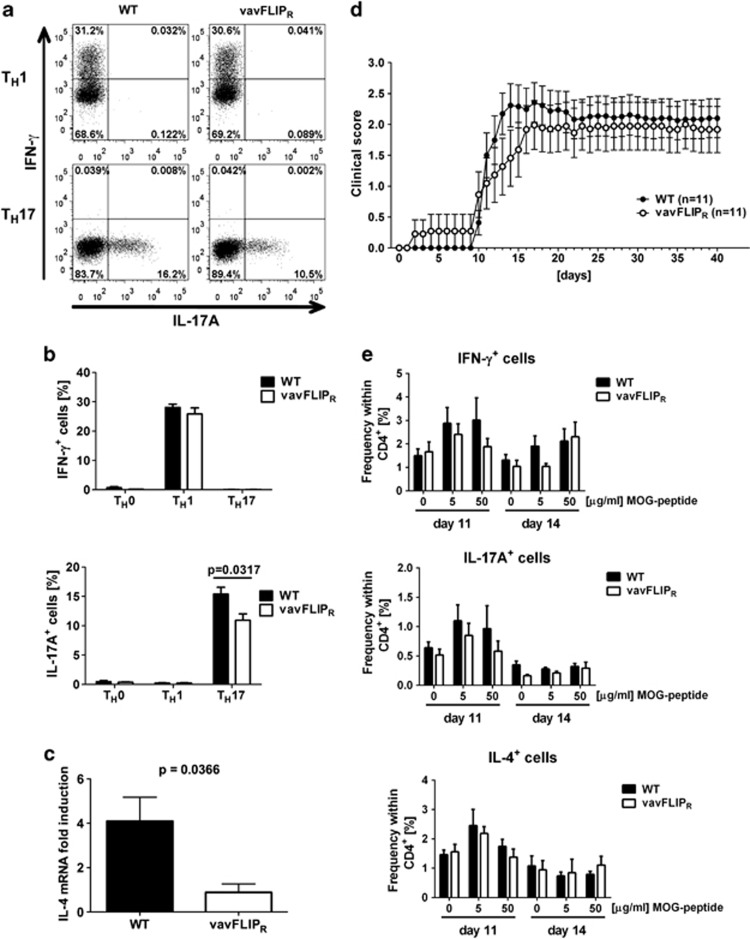
As constitutive expression of c-FLIPR alters lymphocyte populations and activation status with age, we investigated if this has functional relevance in T-cell-mediated autoimmune disease. Experimental autoimmune encephalomyelitis (EAE) is a widely accepted animal model for studying the human demyelinating inflammatory disorder multiple sclerosis (MS).36 TH1 and TH17 effector T cells have been shown to contribute to the pathology in EAE37 and c-FLIPL transgenic mice were reported to be resistant to myelin oligodendrocyte glycoprotein (MOG)-EAE because of enhanced TH2, and thus diminished TH1, effector responses.38 To assess if c-FLIPR influences the differentiation of TH0, TH1, TH2 or TH17 cells, we cultured naive WT and vavFLIPR T cells under TH1, TH2 or TH17 polarising conditions in vitro. The frequencies of interferon-γ (IFN-γ)-producing TH1 cells were comparable between WT and vavFLIPR differentiated T cells (Figures 6a and b). Interestingly, frequencies in IL-17A-producing TH17 cells and IL-4 mRNA induction in TH2 cells were lower in vavFLIPR T cells cultured under the respective polarising conditions (Figures 6a, b and c).
Figure 6.
Comparable disease progression in MOG35-55-peptide-induced EAE in WT and vavFLIPR mice. (a–c) Naive T cells from WT and vavFLIPR mice were isolated and cultured under TH0, TH1, TH2 and TH17 polarising conditions, followed by flow cytometry analysis of IFN-γ and IL-17A. (a) Representative dot plots of TH1 and TH17 differentiated cells are shown. (b) Data are represented as the mean±S.E.M. from two independent experiments (n=5 for WT and vavFLIPR cells). Statistical analyses were performed with two-tailed nonparametric Mann–Whitney U-tests. (c) Quantitative real-time PCR analysis of IL-4 mRNA expression in TH2 differentiated WT and vavFLIPR T cells. Data are represented as the mean±S.E.M. from two independent experiments (n=4 for WT and n=5 for vavFLIPR). (d) EAE was induced by injecting WT and vavFLIPR mice (n=11) with the MOG35-55-peptide and two further injections of pertussis toxin. Subsequently, the clinical score was monitored for 40 days. Data are pooled from two independent experiments and represented as the mean±S.E.M. (e) WT and vavFLIPR animals were injected with the MOG35-55-peptide in complete Freund's adjuvant. pLN cells isolated 11 (WT n=4, vavFLIPR n=4) and 14 (WT n=5, vavFLIPR n=3) days after injection were restimulated with the indicated concentrations of MOG peptide for 24 h, followed by flow cytometry analysis of IFN-γ, IL-17A and IL-4-producing T cells
WT and vavFLIPR mice were immunised with the MOG35-55 peptide in complete Freund's adjuvant in conjunction with pertussis toxin to investigate if constitutive murine c-FLIPR expression in T cells, macrophages and DCs influences EAE disease. However, both WT and vavFLIPR animals developed disease with neurological defects resulting in paralysis of the tail and muscle weakness with comparable severity of disease. Only a slightly slower disease progression was observed in vavFLIPR mice (Figure 6d). To investigate whether T-cell effector responses were affected by the transgenic expression of c-FLIPR in vivo, we analysed cytokine production in MOG-peptide restimulated T cells from MOG-immunised mice. We observed slightly reduced frequencies of IFN-γ+ and IL-17A+ cells from vavFLIPR pLNs in comparison with WT 11 days after immunisation (Figure 6e). This effect was levelled out at higher MOG peptide concentrations on day 14 (Figure 6e). The frequencies of IL-4-producing T cells in pLNs from vavFLIPR and WT mice were comparable (Figure 6e). Similar results were obtained when T cells were restimulated in a polyclonal manner, that is, with anti-CD3 and anti-CD28 (Supplementary Figure 3). In summary, our data indicate a minor defect in vavFLIPR effector T-cell responses that results in a slight delay in EAE disease progression in vavFLIPR mice.
Elevated ANA-titres and tissue damage of kidneys and lungs in aged vavFLIPR mice
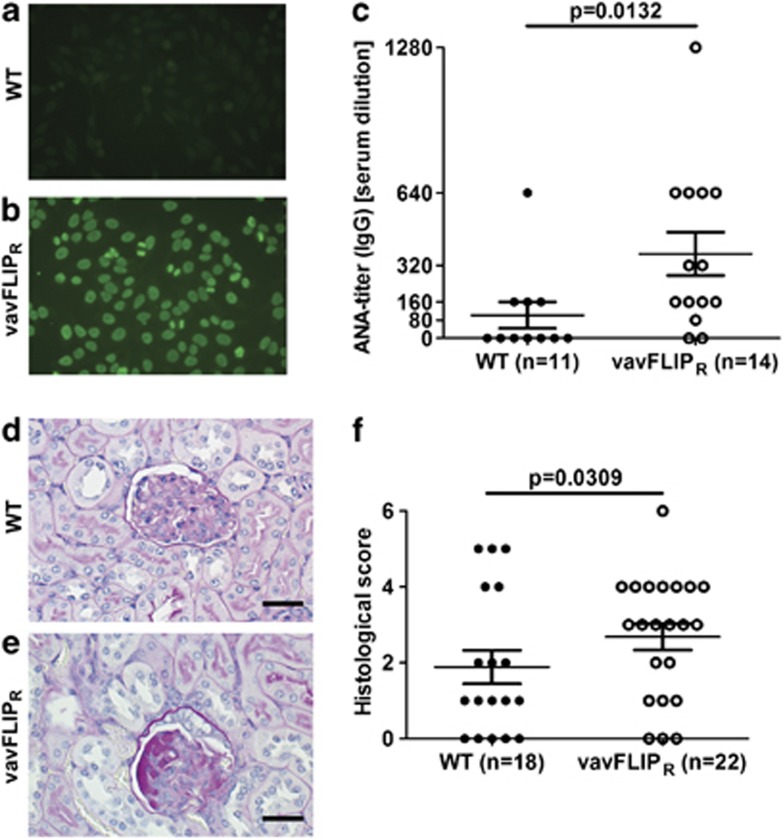
B-cell-mediated autoimmunity is characterised by autoantibodies targeting the body's own tissues. As vavFLIPR mice had slightly higher B-cell levels with activated phenotype, we analysed sera from 1-year-old WT and vavFLIPR mice for anti-nuclear antibodies (ANAs) using an indirect immunofluorescence assay with HEp-2 cells as source of nuclear antigens. Negative fluorescent pattern was most often observed when sera from WT mice were incubated on HEp-2 cells (Figure 7a), whereas homogenous fluorescent pattern was detected for the majority of sera from vavFLIPR mice even at high serum dilution (Figure 7b). Hence, significantly more autoantibodies were identified in sera from vavFLIPR mice compared with WT mice (Figure 7c).
Figure 7.
Autoantibody production and nephropathy in aged vavFLIPR mice. (a–c) Quantification of ANAs in sera from 1-year-old WT (n=11) and vavFLIPR (n=14) littermates. Representative examples of (a) negative fluorescent pattern (WT) and (b) homogenous ANA pattern (vavFLIPR). The ANA-IgG titre is shown in (c). Symbols represent individual mice. Horizontal lines represent the mean; error bars display S.E.M. Statistical analysis was performed with two-tailed nonparametric Mann–Whitney U-test. (d–f) Kidneys from 1-year-old WT (n=18) and vavFLIPR (n=22) animals were analysed by histology. (d and e) Representative histological sections of paraffin-embedded kidneys stained with PAS are shown for (d) WT glomerulus with mild thickening of the Bowman's capsule and (e) vavFLIPR glomerulus with moderate thickening of the Bowman's capsule and moderate glomerular protein deposition. Scale bar represents 25 μm. (f) Histological score of PAS-stained kidneys. Individual mice are displayed as separate symbols. Horizontal lines represent the mean; error bars show S.E.M. Animals were divided up into groups of normal alterations (histological score <3) and moderate-to-severe alterations (histological score 3 or higher), followed by statistical analysis with Fisher's exact test
Multiple organs can be affected in systemic autoimmune diseases. We therefore examined several organs from 1-year-old WT and vavFLIPR animals by histology. No differences between WT and vavFLIPR mice could be observed in brains, spleens and pancreas stained with haematoxylin and eosin (H&E) and in spleens stained for B cells and macrophages (data not shown). Kidneys from aged mice were stained with periodic acid–schiff (PAS) followed by histological scoring. The majority of glomeruli in kidneys from WT mice were normal (Figure 7d). In contrast, thickening of the Bowman's capsule and protein deposition in glomeruli were observed in kidneys from vavFLIPR mice, indicating nephropathy (Figure 7e). Glomerular deposition of PAS-positive material is common in aging mice.39 However, significantly more tissue damage was detected in vavFLIPR kidneys with 63.6% of the kidney sections displaying a histological score of three or higher, implying mild-to-moderate alterations (Figure 7f). In comparison, only 27.8% of kidneys from WT mice manifested similar damage (Figure 7f). Thus, vavFLIPR mice have a greater degree of nephropathy compared with WT animals at 12–14 months of age.
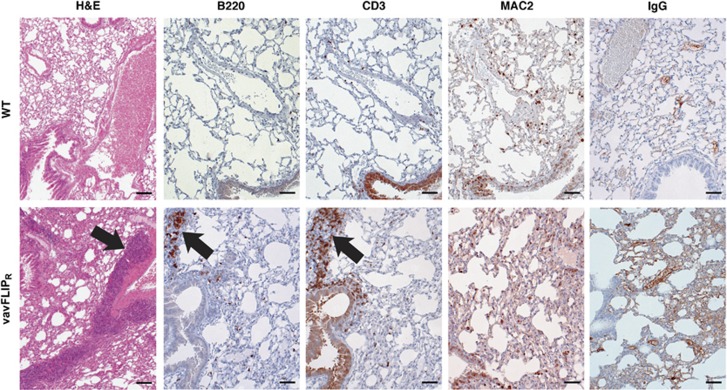
The histological analyses of lungs from aged mice revealed that three of six vavFLIPR lungs stained with H&E displayed marked focal interstitial pneumonia compared with one of five WT lungs. Increase in bronchial-associated lymphoid tissue (BALT) has been described in aging mice.40 Nevertheless, the amount of BALT was massively increased in four of six vavFLIPR animals (67%) in comparison with two of five WT mice (40%). Although the composition of BALT was not obviously altered, we detected more IgG-containing immune complexes (Figure 8). Infiltrating macrophages were observed in regions of interstitial pneumonia by immunohistochemical staining for B cells (B220), T cells (CD3) and macrophages (MAC2) in vavFLIPR lungs (Figure 8). Of note, interstitial pneumonia has occasionally been described for SLE.41
Figure 8.
Histological alterations in lungs of aged vavFLIPR mice. Lungs were prepared from 1-year-old WT (n=5) and vavFLIPR (n=6) mice and paraffin embedded. Sections were stained with H&E, the B-cell marker B220, the T-cell marker CD3 and the macrophage marker MAC2, respectively. Deposition of IgG-containing immune complexes was detected with goat-anti-mouse antibodies. Representative tissue sections are shown. Arrows indicate increased BALT and interstitial pneumonia, respectively. Scale bars represents 200 μm for H&E section and 50 μm for all other sections
Taken together, the elevated levels of ANAs and tissue damage in kidneys and lungs are symptoms similar to human SLE.41, 42 Hence, the kidney damage may be related to the ANAs and represent a mild form of SLE nephropathy.
Discussion
CD95-mediated apoptosis has a crucial role in controlling immune responses and preventing autoimmune diseases.1 c-FLIP proteins protect cells against CD95-induced apoptosis at the level of the DISC.2 Although the isoforms c-FLIPL and c-FLIPS have been thoroughly characterised, not much is known about the functional role of c-FLIPR in the immune system. In this study, we report that constitutive expression of murine c-FLIPR results in altered lymphocyte populations and lupus-like symptoms with age.
Lpr and gld mice with mutations in the CD95 receptor and ligand, respectively, develop lymphoproliferative disease and autoimmunity with accumulation of unusual DN B220+ T cells.7, 29, 30, 43 c-FLIP proteins inhibit the same apoptosis pathway by preventing caspase-8 cleavage at the DISC.15, 16 Indeed, T-cell-specific c-FLIPL transgenic mice were reported to develop autoimmunity when bred on Balb/c, but not C57BL/6, background.25 However, neither altered Lpr nor accumulation of DN B220+ T cells was identified in up to 1-year-old vavFLIPR mice, consistent with transgenic mice with T-cell-specific expression of murine c-FLIPL or human c-FLIPS in the C57BL/6 background.26, 27, 31 Hence, neither constitutive expression nor overexpression of c-FLIP proteins, at least in the C57BL/6 background, is sufficient for recapitulation of the lpr/gld phenotype.
The autoimmune inflammation in EAE is mainly caused by autoreactive TH1 and TH17 effector cells.37 Lpr mice showed a prolonged and enhanced inflammation in the central nervous system (CNS) upon EAE induction.44 Hence, CD95/CD95L interactions are of importance to delete disease-initiating, autoreactive T cells in the CNS in the course of EAE. Both long and short c-FLIP isoforms have been reported to be upregulated in activated T cells from the cerebral spinal fluid in patients with active MS.45, 46 Interestingly, transgenic mice overexpressing human c-FLIPL were protected against EAE because of enhanced TH2 effector responses and consequently reduced TH1 effector responses.38 Transgenic expression of murine c-FLIPR did not alter the cytokine production of T cells in a similar way, which may be one reason why the enforced expression of c-FLIPR only had a minor effect on the progression of EAE. Yu et al.47 reported high expression levels of c-FLIPL in TH17 cells, which protected these cells against AICD. This was proposed to be a mechanism for the high pathogenicity of TH17 cells in autoimmune diseases. Expression levels of c-FLIPR in TH17 cells was not investigated in this study, nevertheless, the increased expression of c-FLIPL in this cell population may override the effect of the constitutive c-FLIPR expression in vavFLIPR mice in the course of EAE. However, the impaired ability of naive vavFLIPR T cells to differentiate into the TH17 subset in vitro and the minor reduction of effector T-cell responses observed in MOG-immunised vavFLIPR mice could possibly explain the slightly slower disease progression in vavFLIPR mice compared with WT littermates.
B- and T-cell numbers and distribution were normal in young vavFLIPR mice28 and no differences in DCs, macrophages, granulocytes or NK cells could be identified even in mice at 1 year of age. Interestingly, aged vavFLIPR mice had altered lymphocyte populations with lower levels of T cells and higher frequencies of B cells compared with WT littermates. Moreover, reduced levels of CD8+ cells were observed, which is consistent with a report of Wu et al.48 where reduced peripheral CD8+ cell numbers were described in mice with transgenic expression of the viral FLIP MC159 under control of the CD2 enhancer cassette. Furthermore, alterations in the ratio of antigen experienced to naive T cells were identified, most probably because of defective AICD. Human c-FLIPS is upregulated in the activation and expansion phase of an immune response, but is thereafter downregulated to enable AICD and elimination of effector T cells.49 We previously described a similar kinetic for murine c-FLIPR, which explains the defect in AICD upon constitutive c-FLIPR expression.28 In this study, increased frequencies of activated T helper cells and antigen-experienced T cells as well as lower frequencies and numbers of naive T cells in vavFLIPR mice compared with WT mice were observed. This is consistent with a previous report where an increased memory T-cell pool was identified in c-FLIPS transgenic mice compared with WT animals after immunisation with the superantigen staphylococcal enterotoxin B.27 Similarly to the activated T-cell phenotype, vavFLIPR B cells expressed activation markers to a higher extent than WT B cells. Strikingly, histological analyses of kidneys and lungs from 12 to 14 months old mice revealed a higher degree of nephropathy in vavFLIPR mice and a larger proportion of vavFLIPR animals displayed focal interstitial pneumonia and an increased amount of BALT compared with WT littermates. Moreover, elevated levels of anti-nuclear autoantibodies were identified in vavFLIPR sera, indicating that vavFLIPR mice develop a lupus-like phenotype with age. Hence, our findings imply that the altered lymphocyte populations with activated phenotypes in aged vavFLIPR mice are of functional relevance. Most probably the autoimmune disease observed in vavFLIPR animals is caused by a combination of autoreactive B and T cells, with persisting CD4+ helper T cells, which may prime B cells to produce autoantibodies, and increased levels of B cells. In support of this notion, lupus-like disease was described in c-FLIPL transgenic mice on the Balb/c background and the development of autoimmunity in this study was shown to require CD4+ T cells, which were proposed to result from impaired thymic selection.25 Notably, elevated gene expression of CFLAR in CD33+ myeloid cells, CD4+ T cells and CD19+ B cells was reported in SLE patients.50 Furthermore, Xu et al.51 observed that human lupus T cells did not downregulate c-FLIPS after initial activation, which suggests that c-FLIP proteins are of importance in the development of SLE disease. The higher level of Treg cells in aged vavFLIPR mice is presumably a compensatory mechanism because of the observed autoimmunity. Imbalance of Treg cells has been described in various autoimmune diseases.52 Reduced levels or conflicting data have been reported in patients with, for example, juvenile idiopathic arthritis, autoimmune liver disease and myasthenia gravis.52 Consistent with our findings, increased levels of Treg cells were identified in patients with primary Sjögren's syndrome53 and in experimental arthritis in mice.54 The expanded Treg cell population observed in 1-year-old vavFLIPR animals most probably dampens the severity of the SLE-like phenotype.
We previously reported that vavFLIPR mice are better protected against Listeria monocytogenes infection compared with WT littermates.28 Hence, c-FLIPR expression is beneficial in an acute infection, whereas we in this study demonstrate that chronic expression results in autoimmunity. To conclude, aged vavFLIPR mice have altered lymphocyte populations with increased levels of antigen-experienced T cells and higher activation status of both B and T cells. Moreover, these animals develop lupus-like symptoms with age. Our results show that c-FLIPR has an important role in the control of autoimmunity.
Materials and Methods
Mice
vavFLIPR mice were previously described.28 WT littermates were used as control animals. All animals were kept in a specific pathogen-free environment in the animal facility of Helmholtz Centre for Infection Research, Braunschweig.55 C57BL/6 mice were purchased from Harlan Laboratories (Indianapolis, IN, USA) and Charles River (Wilmington, MA, USA). MRL/lpr mice were a kind gift from Dr Detlef Neumann, Hannover Medical School, Hannover, Germany. All breeding and experiments were performed in accordance with the guidelines of national and local authorities.
Cell preparation
Lymphoid organs (pLNs, mesenteric lymph nodes, spleen and thymus) were isolated from mice killed via CO2. Organs were homogenised through a 70 μm nylon mesh and washed with PBS. Erythrocytes were removed by 2-min incubation in ACK lysis buffer (0.15 M NH4Cl, 1 mM KHCO3, 0.1 mM EDTA, pH adjusted to 7.3 with NaOH) at room temperature followed by a further washing step. Primary murine cells were cultured in RPMI 1640 (Gibco – Life technologies, Grand Island, NY, USA) supplemented with 10% FCS (PAA, Pasching, Austria), 50 μg/ml of penicillin and streptomycin (Gibco – Life technologies), 1% non-essential amino acids (Gibco – Life technologies), 2 mM L-glutamine (Gibco – Life technologies) and 1 mM sodium pyruvate (Gibco – Life technologies).
In vitro generation of T helper cell subsets
For differentiation of T helper subsets, CD4+CD62LhighCD25− naive T cells were sorted by using a FACS Aria II (BD Biosciences, Heidelberg, Germany) or MoFlo (Beckman and Coulter, Indianapolis, IN, USA). Cells were seeded directly after sorting with 2 × 105 cells per well in 96-well plates and activated with plate-bound anti-CD3 and anti-CD28 in the presence of priming cytokines and inhibitory antibodies according to the respective T helper subset: TH0: 2 μg/ml anti-CD3 (145-2C11, BioLegend, San Diego, CA, USA), 2 μg/ml anti-CD28 (37.51, BioLegend), 10 μg/ml anti-IL-4 (11B11, self-purified) and 10 μg/ml anti-IFN-γ (XMG1.2, self-purified); TH1: 2 μg/ml anti-CD3 (145-2C11, BioLegend), 2 μg/ml anti-CD28 (37.51, BioLegend), 10 μg/ml anti-IL-4 (11B11, self-purified) and 10 ng/ml IL-12 (R&D Systems, Minneapolis, MN, USA); TH17: 3 μg/ml anti-CD3 (145-2C11, BioLegend), 5 μg/ml anti-CD28 (37.51, BioLegend), 10 μg/ml anti-IL-2 (JES6-1A12, BioLegend), 10 μg/ml anti-IFN-γ (XMG1.2, self-purified), 2 ng/ml pTGF-β (R&D Systems), 30 ng/ml IL-6 (R&D Systems), 10 ng/ml IL-1-β (R&D Systems) and 20 ng/ml TNF-α (Preprotech, Rocky Hill, NJ, USA). TH2 cells were differentiated as follows: CD4+CD62LhighCD25− naive T cells were sorted as above. In all, 2 × 106 cells were seeded after sorting in 24-well plates and activated with plate-bound 5 μg/ml anti-CD3 (145-2C11, BioLegend) and 2 μg/ml anti-CD28 (37.51, BioLegend). After 3 days, 2 × 105 cells were transferred to 96-well plate in the presence of the following priming cytokines and inhibitory antibodies and cultured for additional 3 days: 10 ng/ml murine IL-2 (402-ML, R&D Systems), 10 ng/ml IL-4 (11B11, self-purified), 5 μg/ml anti-IFN-γ (XMG1.2, self-purified) and 5 μg/ml anti-IL12p40 (C17.8, R&D Systems). Subsequently, the differentiated cells were stained for CD4 and either IFN-γ, IL-4 or IL-17A (see staining procedure in the next paragraph) followed by flow cytometry analysis after 4 days of differentiation.
Flow cytometry
Cells were stained with Live/Dead near IR or blue fluorescent reactive dyes (Life Technologies) by incubation for 30 min in PBS at 4 °C. Thereafter, cells were washed and Fcγ III/II receptors were blocked by incubation with anti-CD16/CD32 (2.4G2, BD Biosciences). Subsequently, cells were washed and stained with antibodies in PBS containing 2% BSA for 20 min at 4 °C. The following antibodies were used: CD3-HorizonV450 (17A2), CD3-FITC (145-2C11), CD4-HorizonV500 (RM4-5), CD8-FITC (53-6.7), CD19-FITC (1D3), CD25-PE Cy7 (PC61.5) (all from BD Biosciences); CD4-Pacific Blue (RM4-5), CD8-APC (53-6.7), CD86-APC (GL-1), I-A/I-E-Alexa Fluor 700 (M5/114.15.2) (all from BioLegend); CD11c-APC eFluor780 (N418), CD19-PerCP Cy5.5 (1D3), CD44-PE (IM7), CD45R (B220)-APC (RA3-6B2), CD49b-APC (Dx5), CD62L-PerCP Cy5.5 (MEL-14), CD69-PE Cy7 (H1.2F3), F4/80-PE (BM8) and Gr1-Pacific Blue (RB6-8C5) (all from eBiosciences, San Diego, CA, USA). Samples were analysed by LSRII or LSRFortessa flow cytometers (BD Biosciences). The FlowJo software (TreeStar, Ashland, OR, USA) was used to analyse the data.
Intracellular proteins were stained after surface marker staining. For staining of Foxp3-Alexa Fluor 488, cells were fixed and permeabilised for intracellular cytokine staining by using the Foxp3 Staining Buffer Set (Miltenyi Biotec, Auburn, CA, USA) according to the manufacturer's protocol. For intracellular cytokine staining of IFN-γ-FITC (XMG1.2, BioLegend), IL-4-PE (11B11, eBioscience) and IL-17A-APC (eBio17B7, eBioscience), cells were stimulated for 4 h with PMA and ionomycin (Sigma Aldrich, Munich, Germany), with addition of Brefeldin A (Sigma Aldrich) for the last 2 h, before the staining was performed as for Foxp3.
Real-time detection PCR
cDNA was used as a template for real-time PCR using SYBR Green (Roche, Mannheim, Germany). Ubiquitin C (UBC) was used as reference gene for normalisation. Measurements were performed in duplicates in the LightCycler 96 system (Roche) using the following primers: UBC fwd 5′-AAGAGAATCCACAAGGAATTGAATG-3′ UBC rev 5′-CAACAGGACCTGCTGAACACTG-3′ IL4 fwd 5′-CATCGGCATTTTGAACGAG-3′ IL4 rev 5′-CGAGCTCACTCTCTGTGGTG-3′.
Experimental autoimmune encephalomyelitis
EAE was induced in 10–12 weeks old WT and vavFLIPR mice by injecting 200 μg MOG35-55-peptide (MEVGWYRSPFSRVVHLYRNGK) together with 4 mg/ml mycobacteria in complete Freund's adjuvant subcutaneously at four sites. In all, 200 ng pertussis toxin was injected intraperitoneally to open the blood–brain-barrier. The pertussis toxin injection was repeated 2 days later. Mice were monitored daily for clinical signs of EAE from days 3 to 40 with 0 – no symptoms, 0.5 – partial limp tail, 1 – limp tail, 2 – delayed rotation from dorsal position, 2.5 – hindleg weakness, 3 – complete hindleg paralysis, 3.5 – starting foreleg weakness, 4 – paralysis of one foreleg, 5 – moribund, death. Mice with a score higher than 3 were killed.
For analysis of cytokine-producing T cells, mice were immunised with MOG35-55-peptide as above, but without pertussis toxin to prevent the migration of primed T cells into the brain. pLNs were isolated from mice killed 11 and 14 days after injection. In all, 5 × 105 cells were seeded per well in 96-well plates and either left untreated or restimulated with plate-bound anti-CD3 (2 μg/ml; 145-2C11, BioLegend) and anti-CD28 (2 μg/ml; 37.51, BioLegend) or up to 50 μg/ml MOG35-55-peptide for 24 h with addition of 10 μg/ml Brefeldin A (Sigma Aldrich) for the last 2 h. IFN-γ-, IL-4- and IL-17A-producing T cells were analysed by flow cytometry.
Analysis of ANAs
ANAs in sera from 1-year-old WT and vavFLIPR mice were analysed semiquantitatively by incubating HEp-2 cells seeded on microscope slides (Generic Assays, Dahlewitz, Germany) with sera diluted 1 : 80–1 : 1280 for 30 min at room temperature. Slides were washed two times 5 min in PBS followed by incubation with FITC-conjugated donkey anti-mouse IgG (Dianova, Hamburg, Germany) for 30 min in the dark. Thereafter, slides were washed two times 5 min in PBS, transferred to cover slips and sealed. Slides were analysed by fluorescence microscopy where a homogenous pattern was considered as positive for ANAs.
Histology
Kidneys, lungs, livers, spleens, pancreas and brains were isolated from 1-year-old WT and vavFLIPR mice. Organs were fixed in 4% neutrally buffered formaldehyde and embedded in paraffin for histological analysis. In all, 3 μm sections from organs were stained with H&E. The degree of focal interstitial pneumonia and BALT was determined in H&E-stained lung sections. Lungs were immunohistochemically stained with B220 (RA3-6B2; BD Biosciences), CD3 (SP7; Neo Markers, Fremont, CA, USA) and MAC2 (M3/38; CEDARLANE, Burlington, ON, Canada) to determine lymphocyte and macrophage infiltration. IgG complexes in lung sections were stained with goat-anti-mouse (TM-060-BN, Thermo Fisher Scientific, Waltham, MA, USA). Kidney sections were stained with PAS staining and the sections were analysed for thickening of the Bowman's capsule and protein casts in glomeruli. Scoring was performed in a blinded manner for each criterion as follows: 0 – no alteration, 1 – low grade (slightly visible), 2 – moderate (clearly visible), 3 – severe (dominant finding). Scores were added to a total score for each organ.
Statistical analyses
Statistical analyses were performed by nonparametric Mann–Whitney U-test or Fisher's exact test using GraphPad Prism software (Graph Pad Software, La Jolla, CA, USA). Data are presented as the mean with S.E.M. and S.D. as error bars.
Acknowledgments
We are grateful to Sabrina Schumann, Dominique Gollasch and Lena Seela for expert technical assistance, Anne-Marie Matthies for help with Ig ELISAs and Dr Marc Schuster for critically reading the manuscript. We thank Dr Detlef Neumann for providing us with MRL/lpr mice and Dr Jochen Huehn for various reagents. Moreover, we appreciate the excellent support from the FACS facility, especially Dr Lothar Groebe, and the animal facility, in particular David Dettbarn, at the Helmholtz Centre for Infection Research. We also thank Dr Frank Klawonn for assistance with statistical analyses. FE, MA and CP-S were supported by the President's Initiative and Networking Fund of the Helmholtz Association of German Research Centers (HGF) under contract number VH-GS-202. This project was funded by the DFG (SCHM1586/2-1 and SCHM1586/2-2).
Glossary
- AICD
activation-induced cell death
- ANA
anti-nuclear antibody
- BALT
bronchial-associated lymphoid tissue
- Cflar
caspase-8 and FADD-like apoptosis regulator
- c-FLIPL
cellular FLICE-inhibitory protein, long isoform
- c-FLIPS
cellular FLICE-inhibitory protein, short isoform
- c-FLIPR
cellular FLICE-inhibitory protein, Raji isoform
- CNS
central nervous system
- DC
dendritic cell
- DISC
death-inducing signalling complex
- DN
double negative
- EAE
experimental autoimmune encephalomyelitis
- FADD
Fas-associated death domain-containing protein
- Gld
generalised lymphoproliferative disorder
- IFN-γ
interferon-γ
- Lpr
lymphoproliferation
- MOG
myelin oligodendrocyte glycoprotein
- MS
multiple sclerosis
- SLE
systemic lupus erythematosus
- WT
wild type
Footnotes
Supplementary Information accompanies this paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by M Leverkus
The authors declare no conflict of interest.
Supplementary Material
References
- Bouillet P, O'Reilly LA. CD95, BIM and T cell homeostasis. Nat Rev Immunol 2009; 9: 514–519. [DOI] [PubMed] [Google Scholar]
- Krammer PH, Arnold R, Lavrik IN. Life and death in peripheral T cells. Nat Rev Immunol 2007; 7: 532–542. [DOI] [PubMed] [Google Scholar]
- Alderson MR, Tough TW, Davis-Smith T, Braddy S, Falk B, Schooley KA et al. Fas ligand mediates activation-induced cell death in human T lymphocytes. J Exp Med 1995; 181: 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhein J, Walczak H, Baumler C, Debatin KM, Krammer PH. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95). Nature 1995; 373: 438–441. [DOI] [PubMed] [Google Scholar]
- Brunner T, Mogil RJ, LaFace D, Yoo NJ, Mahboubi A, Echeverri F et al. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature 1995; 373: 441–444. [DOI] [PubMed] [Google Scholar]
- Ju ST, Panka DJ, Cui H, Ettinger R, el-Khatib M, Sherr DH et al. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature 1995; 373: 444–448. [DOI] [PubMed] [Google Scholar]
- Cohen PL, Eisenberg RA. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol 1991; 9: 243–269. [DOI] [PubMed] [Google Scholar]
- Sneller MC, Straus SE, Jaffe ES, Jaffe JS, Fleisher TA, Stetler-Stevenson M et al. A novel lymphoproliferative/autoimmune syndrome resembling murine lpr/gld disease. J Clin Invest 1992; 90: 334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter ME, Krammer PH. The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ 2003; 10: 26–35. [DOI] [PubMed] [Google Scholar]
- Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH et al. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J 1995; 14: 5579–5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boatright KM, Renatus M, Scott FL, Sperandio S, Shin H, Pedersen IM et al. A unified model for apical caspase activation. Mol Cell 2003; 11: 529–541. [DOI] [PubMed] [Google Scholar]
- Donepudi M, Mac Sweeney A, Briand C, Grutter MG. Insights into the regulatory mechanism for caspase-8 activation. Mol Cell 2003; 11: 543–549. [DOI] [PubMed] [Google Scholar]
- Li J, Yuan J. Caspases in apoptosis and beyond. Oncogene 2008; 27: 6194–6206. [DOI] [PubMed] [Google Scholar]
- Fischer U, Janicke RU, Schulze-Osthoff K. Many cuts to ruin: a comprehensive update of caspase substrates. Cell Death Differ 2003; 10: 76–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi C, Schmitz I, Krammer PH, Peter ME. The role of c-FLIP in modulation of CD95-induced apoptosis. J Biol Chem 1999; 274: 1541–1548. [DOI] [PubMed] [Google Scholar]
- Krueger A, Schmitz I, Baumann S, Krammer PH, Kirchhoff S. Cellular FLICE-inhibitory protein splice variants inhibit different steps of caspase-8 activation at the CD95 death-inducing signaling complex. J Biol Chem 2001; 276: 20633–20640. [DOI] [PubMed] [Google Scholar]
- Golks A, Brenner D, Fritsch C, Krammer PH, c-FLIPR Lavrik IN. a new regulator of death receptor-induced apoptosis. J Biol Chem 2005; 280: 14507–14513. [DOI] [PubMed] [Google Scholar]
- Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V et al. Inhibition of death receptor signals by cellular FLIP. Nature 1997; 388: 190–195. [DOI] [PubMed] [Google Scholar]
- Fuentes-Prior P, Salvesen GS. The protein structures that shape caspase activity, specificity, activation and inhibition. Biochem J 2004; 384(Pt 2): 201–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueffing N, Singh KK, Christians A, Thorns C, Feller AC, Nagl F et al. A single nucleotide polymorphism determines protein isoform production of the human c-FLIP protein. Blood 2009; 114: 572–579. [DOI] [PubMed] [Google Scholar]
- Ueffing N, Keil E, Freund C, Kuhne R, Schulze-Osthoff K, Schmitz I. Mutational analyses of c-FLIPR, the only murine short FLIP isoform, reveal requirements for DISC recruitment. Cell Death Differ 2008; 15: 773–782. [DOI] [PubMed] [Google Scholar]
- Fricker N, Beaudouin J, Richter P, Eils R, Krammer PH, Lavrik IN. Model-based dissection of CD95 signaling dynamics reveals both a pro- and antiapoptotic role of c-FLIPL. J Cell Biol 2010; 190: 377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheau O, Thome M, Schneider P, Holler N, Tschopp J, Nicholson DW et al. The long form of FLIP is an activator of caspase-8 at the Fas death-inducing signaling complex. J Biol Chem 2002; 277: 45162–45171. [DOI] [PubMed] [Google Scholar]
- Chang DW, Xing Z, Pan Y, Algeciras-Schimnich A, Barnhart BC, Yaish-Ohad S et al. c-FLIP(L) is a dual function regulator for caspase-8 activation and CD95-mediated apoptosis. EMBO J 2002; 21: 3704–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao G, Li Z, Minto AW, Shia J, Yang L, Bao L et al. Altered thymic selection by overexpressing cellular FLICE inhibitory protein in T cells causes lupus-like syndrome in a BALB/c but not C57BL/6 strain. Cell Death Differ 2010; 17: 522–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw-Makepeace J, Huston G, Fortner KA, Russell JQ, Holoch D, Swain S et al. c-FLIP(S) reduces activation of caspase and NF-kappaB pathways and decreases T cell survival. Eur J Immunol 2008; 38: 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehme I, Neumann F, Bosser S, Zornig M. Transgenic overexpression of the Caspase-8 inhibitor FLIP(short) leads to impaired T cell proliferation and an increased memory T cell pool after staphylococcal enterotoxin B injection. Eur J Immunol 2005; 35: 1240–1249. [DOI] [PubMed] [Google Scholar]
- Telieps T, Ewald F, Gereke M, Annemann M, Rauter Y, Schuster M et al. Cellular-FLIP, Raji isoform (c-FLIPR) modulates cell death induction upon T-cell activation and infection. Eur J Immunol 2013; 43: 1499–1510. [DOI] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature 1992; 356: 314–317. [DOI] [PubMed] [Google Scholar]
- Yasutomo K, Maeda K, Nagata S, Nagasawa H, Okada K, Good RA et al. Defective T cells from gld mice play a pivotal role in development of Thy-1.2+B220+ cells and autoimmunity. J Immunol 1994; 153: 5855–5864. [PubMed] [Google Scholar]
- Lens SM, Kataoka T, Fortner KA, Tinel A, Ferrero I, MacDonald RH et al. The caspase 8 inhibitor c-FLIP(L) modulates T-cell receptor-induced proliferation but not activation-induced cell death of lymphocytes. Mol Cell Biol 2002; 22: 5419–5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverkus M, Walczak H, McLellan A, Fries HW, Terbeck G, Brocker EB et al. Maturation of dendritic cells leads to up-regulation of cellular FLICE-inhibitory protein and concomitant down-regulation of death ligand-mediated apoptosis. Blood 2000; 96: 2628–2631. [PubMed] [Google Scholar]
- Huang QQ, Perlman H, Huang Z, Birkett R, Kan L, Agrawal H et al. FLIP: a novel regulator of macrophage differentiation and granulocyte homeostasis. Blood 2010; 116: 4968–4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell 2000; 101: 455–458. [DOI] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003; 299: 1057–1061. [DOI] [PubMed] [Google Scholar]
- Iglesias A, Bauer J, Litzenburger T, Schubart A, Linington C. T- and B-cell responses to myelin oligodendrocyte glycoprotein in experimental autoimmune encephalomyelitis and multiple sclerosis. Glia 2001; 36: 220–234. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Lalor SJ, Sweeney CM, Tubridy N, Mills KH. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Exp Immunol 2010; 162: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseveleki V, Bauer J, Taoufik E, Ruan C, Leondiadis L, Haralambous S et al. Cellular FLIP (long isoform) overexpression in T cells drives Th2 effector responses and promotes immunoregulation in experimental autoimmune encephalomyelitis. J Immunol 2004; 173: 6619–6626. [DOI] [PubMed] [Google Scholar]
- Maronpot RR BG, Gaul BW. Pathology of the Mouse. Cache River Press: St Louis, MO, USA, 1999. p 699. [Google Scholar]
- Pettan-Brewer C, Treuting PM. Practical pathology of aging mice. Pathobiol Aging Age Related Dis 2011; 1 doi:10.3402/pba.v1i0.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheema GS, Quismorio FP Jr. Interstitial lung disease in systemic lupus erythematosus. Curr Opin Pulm Med 2000; 6: 424–429. [DOI] [PubMed] [Google Scholar]
- Munoz LE, Gaipl US, Franz S, Sheriff A, Voll RE, Kalden JR et al. SLE—a disease of clearance deficiency? Rheumatology (Oxford) 2005; 44: 1101–1107. [DOI] [PubMed] [Google Scholar]
- Budd RC, Van Houten N, Clements J, Mixter PF. Parallels in T lymphocyte development between lpr and normal mice. Semin Immunol 1994; 6: 43–48. [DOI] [PubMed] [Google Scholar]
- Suvannavejh GC, Dal Canto MC, Matis LA, Miller SD. Fas-mediated apoptosis in clinical remissions of relapsing experimental autoimmune encephalomyelitis. J Clin Invest 2000; 105: 223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharief MK. Increased cellular expression of the caspase inhibitor FLIP in intrathecal lymphocytes from patients with multiple sclerosis. J Neuroimmunol 2000; 111: 203–209. [DOI] [PubMed] [Google Scholar]
- Semra YK, Seidi OA, Sharief MK. Overexpression of the apoptosis inhibitor FLIP in T cells correlates with disease activity in multiple sclerosis. J Neuroimmunol 2001; 113: 268–274. [DOI] [PubMed] [Google Scholar]
- Yu Y, Iclozan C, Yamazaki T, Yang X, Anasetti C, Dong C et al. Abundant c-Fas-associated death domain-like interleukin-1-converting enzyme inhibitory protein expression determines resistance of T helper 17 cells to activation-induced cell death. Blood 2009; 114: 1026–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Roberts M, Porter M, Walker F, Wherry EJ, Kelly J et al. Viral FLIP impairs survival of activated T cells and generation of CD8+ T cell memory. J Immunol 2004; 172: 6313–6323. [DOI] [PubMed] [Google Scholar]
- Kirchhoff S, Muller WW, Li-Weber M, Krammer PH. Up-regulation of c-FLIPshort and reduction of activation-induced cell death in CD28-costimulated human T cells. Eur J Immunol 2000; 30: 2765–2774. [DOI] [PubMed] [Google Scholar]
- Hutcheson J, Scatizzi JC, Siddiqui AM, Haines GK 3rd, Wu T, Li QZ et al. Combined deficiency of proapoptotic regulators Bim and Fas results in the early onset of systemic autoimmunity. Immunity 2008; 28: 206–217. [DOI] [PubMed] [Google Scholar]
- Xu L, Zhang L, Yi Y, Kang HK, Datta SK. Human lupus T cells resist inactivation and escape death by upregulating COX-2. Nat Med 2004; 10: 411–415. [DOI] [PubMed] [Google Scholar]
- Dejaco C, Duftner C, Grubeck-Loebenstein B, Schirmer M. Imbalance of regulatory T cells in human autoimmune diseases. Immunology 2006; 117: 289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottenberg JE, Lavie F, Abbed K, Gasnault J, Le Nevot E, Delfraissy JF et al. CD4 CD25high regulatory T cells are not impaired in patients with primary Sjogren's syndrome. J Autoimmun 2005; 24: 235–242. [DOI] [PubMed] [Google Scholar]
- Monte K, Wilson C, Shih FF. Increased number and function of FoxP3 regulatory T cells during experimental arthritis. Arthritis Rheum 2008; 58: 3730–3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehr M, Greweling MC, Tischer S, Singh M, Blocker H, Monner DA et al. Charles River altered Schaedler flora (CRASF) remained stable for four years in a mouse colony housed in individually ventilated cages. Lab Anim 2009; 43: 362–370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.