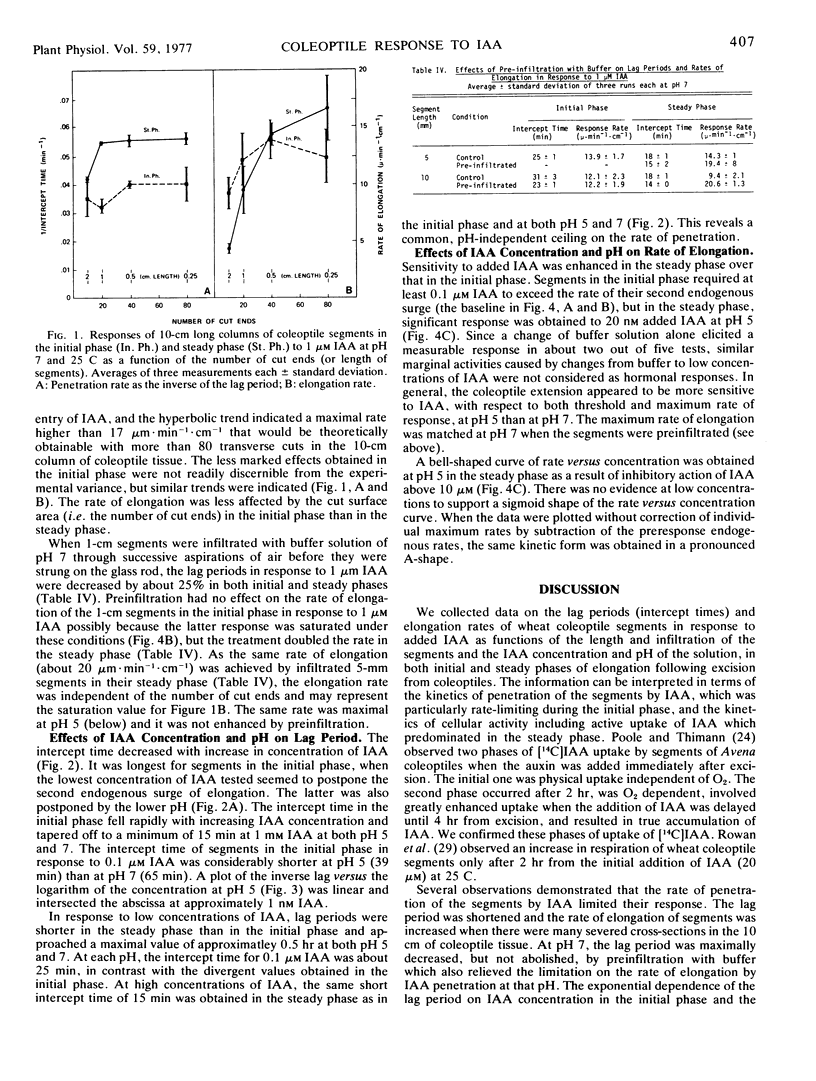
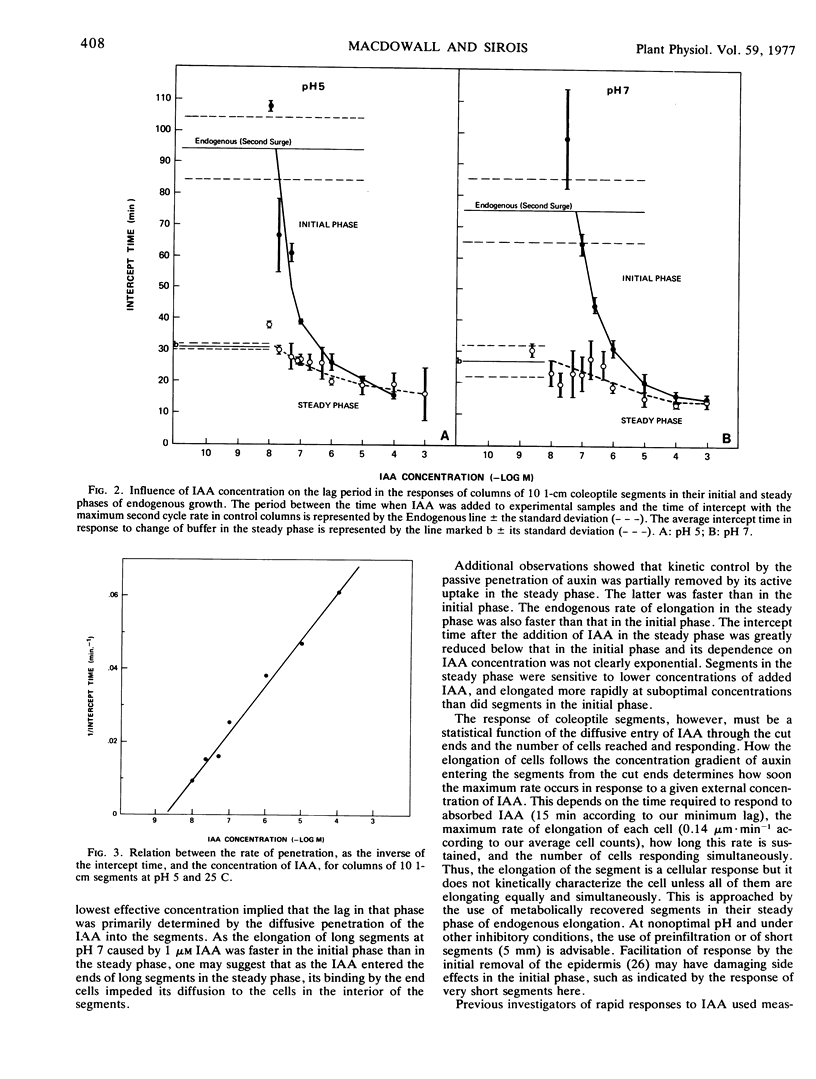
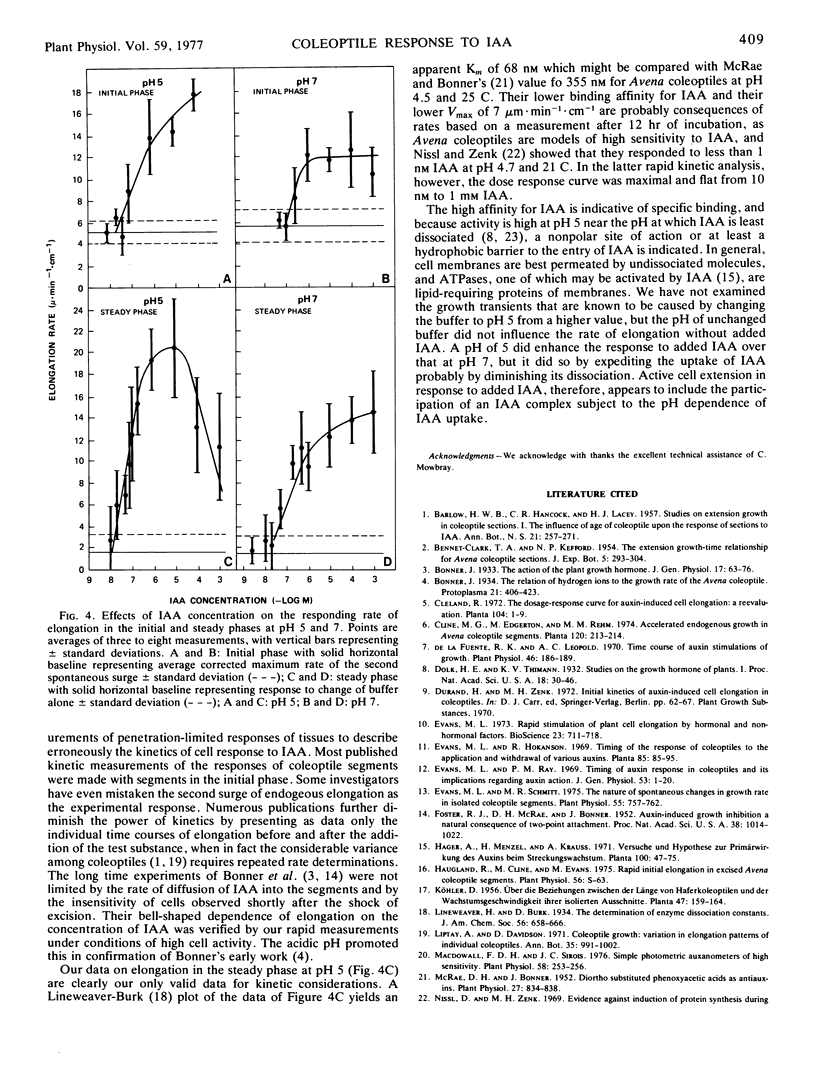
Abstract
Segments of coleoptiles of 3-day-old wheat (Triticum × Aestivum L. cv. Kharkov M.C. 22) grown at 24 C were strung on a glass rod and the kinetics of their elongation in 0.01 m K-phosphate buffer was examined photometrically. Measured rates of elongation in response to treatments were corrected by subtraction of endogenous rates. The customary practice of testing the effects of growth regulators added between the two endogenous surges of growth, that is, up to 3 hours after segments were excised from coleoptiles, gave erroneous kinetic data. Rates of response were then limited by the passive penetration of added auxin and the second endogenous surge interfered with late responses. It was necessary to wait for a phase of more rapid but more steady elongation after the second endogenous surge was over, about 4 hours for wheat at 25 C, to attain the active uptake required for nearly synchronous response through the segment. The more active uptake in this steady phase was confirmed with β-[2-14C]indoleacetic acid and it was greater at pH 5 than at pH 7. The degree of dissociation of indoleacetic acid added at pH 7 was an impediment to penetration that could be compensated for by removal of intercellular air. The pH did not influence the endogenous rate of elongation. The dependence of the rate of elongation on the concentration of indoleacetic acid added at pH 5 was bell-shaped with maximum rate at 10 μm indoleacetic acid, in confirmation of previous measurements made over long intervals of time. The relation between the response and suboptimal concentrations was not sigmoid but was indicative of greater binding affinity than previously reported.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dela Fuente R. K., Leopold A. C. Time course of auxin stimulations of growth. Plant Physiol. 1970 Aug;46(2):186–189. doi: 10.1104/pp.46.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolk H. E., Thimann K. V. Studies on the Growth Hormone of Plants: I. Proc Natl Acad Sci U S A. 1932 Jan;18(1):30–46. doi: 10.1073/pnas.18.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. L., Ray P. M. Timing of the auxin response in coleoptiles and its implications regarding auxin action. J Gen Physiol. 1969 Jan;53(1):1–20. doi: 10.1085/jgp.53.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. L., Schmitt M. R. The nature of spontaneous changes in growth rate in isolated coleoptile segments. Plant Physiol. 1975 Apr;55(4):757–762. doi: 10.1104/pp.55.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster R. J., McRae D. H., Bonner J. Auxin-Induced Growth Inhibition a Natural Consequence of Two-Point Attachment. Proc Natl Acad Sci U S A. 1952 Dec;38(12):1014–1022. doi: 10.1073/pnas.38.12.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdowall F. D., Sirois J. C. Simple photometric auxanometers of high sensitivity. Plant Physiol. 1976 Sep;58(3):253–256. doi: 10.1104/pp.58.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae D. H., Bonner J. DIORTHO SUBSTITUTED PHENOXYACETIC ACIDS AS ANTIAUXINS. Plant Physiol. 1952 Oct;27(4):834–838. doi: 10.1104/pp.27.4.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayle D. L., Evans M. L., Hertel R. Action of auxin on cell elongation. Proc Natl Acad Sci U S A. 1970 Jan;65(1):184–191. doi: 10.1073/pnas.65.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]


