Abstract
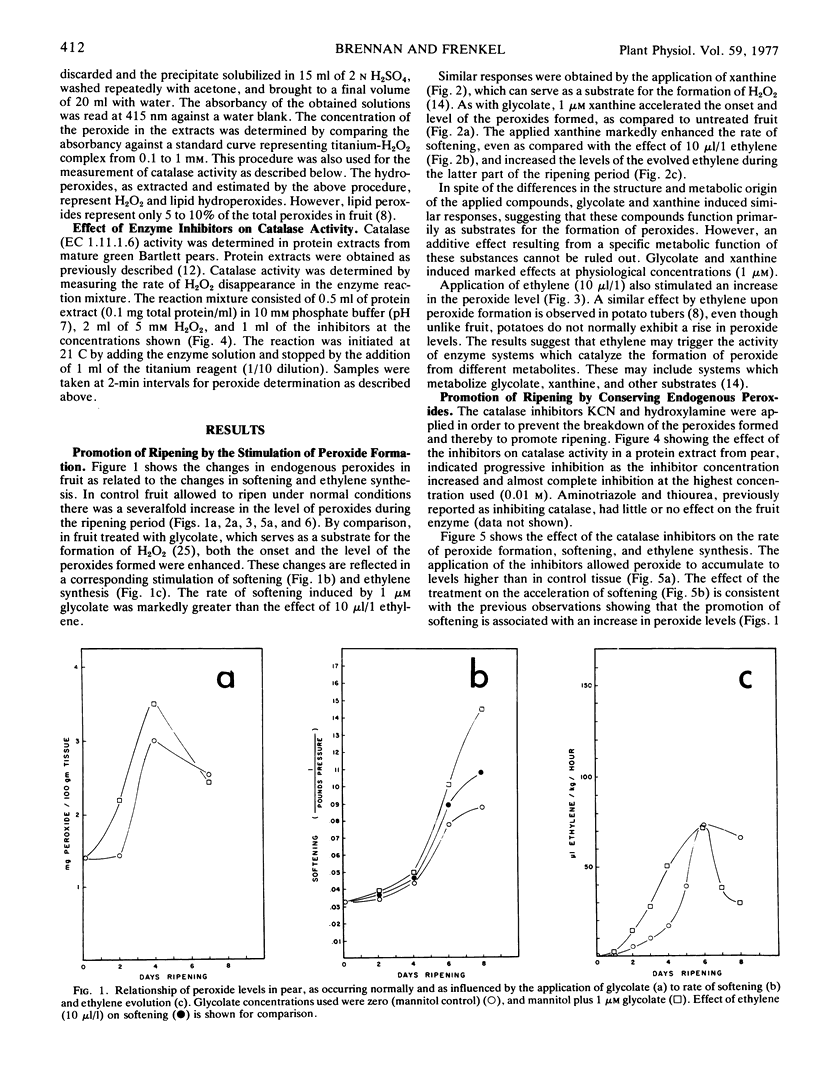
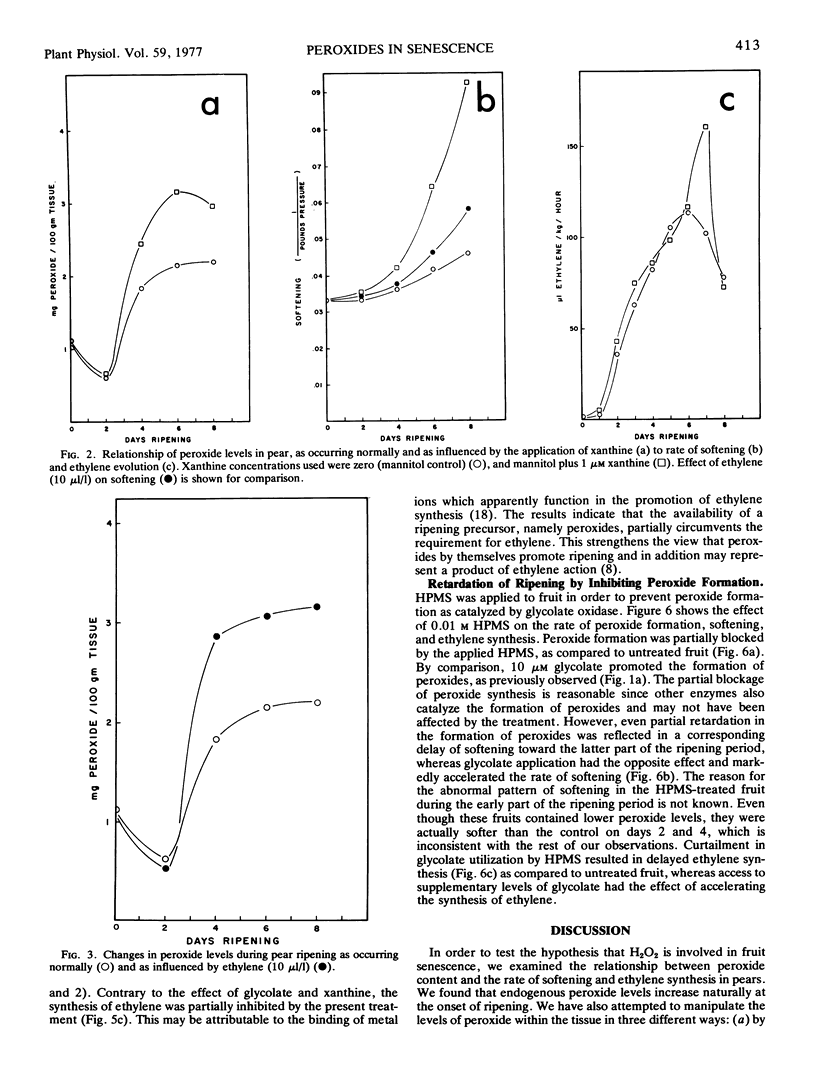
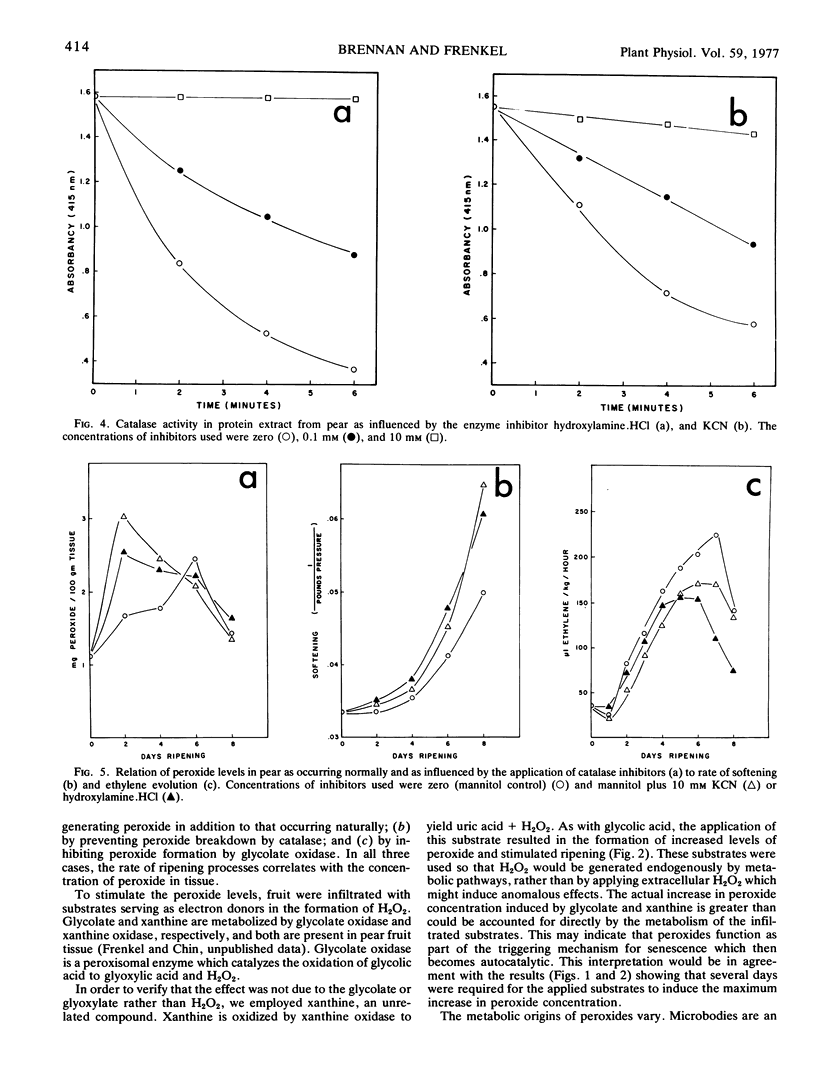
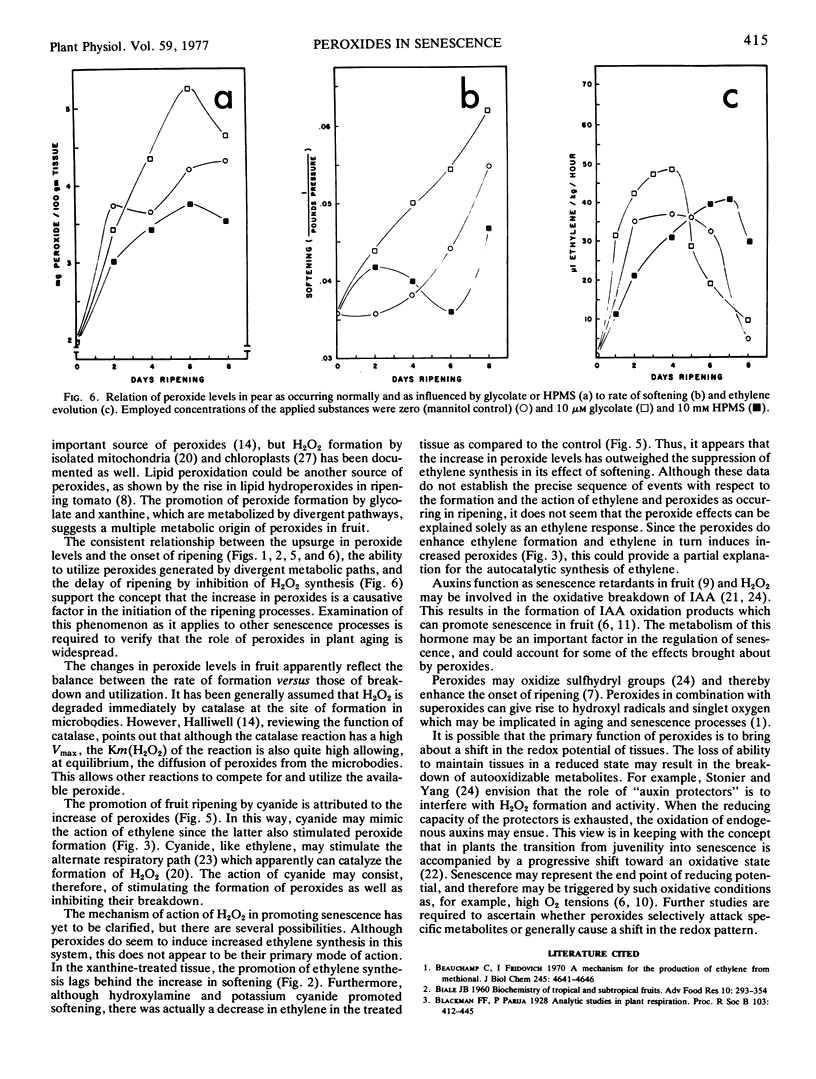
Endogenous peroxide levels in pear fruit (Pyrus communis) were measured using a titanium assay method, and were found to increase during senescence in both Bartlett and Bosc varieties. Application of glycolic acid or xanthine, serving as substrates for the formation of H2O2, increased the peroxide content of the tissue and accelerated the onset of ripening, as measured by increased softening and ethylene evolution. Application of ethylene also induced increased peroxide levels. Ripening processes were similarly promoted when peroxides were conserved by inhibiting the activity of catalase with hydroxylamine or potassium cyanide. By comparison, the inhibition of glycolate oxidase with alphahydroxy-2-pyridinemethanesulfonic acid decreased the peroxide content of the tissue and delayed the onset of ripening. These results indicate that the onset of ripening correlates with the peroxide content of fruit tissues as occurring under normal conditions or as influenced by the treatments. Hydrogen peroxide may be involved in oxidative processes required in the initiation and the promotion of ripening.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beauchamp C., Fridovich I. A mechanism for the production of ethylene from methional. The generation of the hydroxyl radical by xanthine oxidase. J Biol Chem. 1970 Sep 25;245(18):4641–4646. [PubMed] [Google Scholar]
- Frenkel C. Involvement of Peroxidase and Indole-3-acetic Acid Oxidase Isozymes from Pear, Tomato, and Blueberry Fruit in Ripening. Plant Physiol. 1972 May;49(5):757–763. doi: 10.1104/pp.49.5.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel C., Klein I., Dilley D. R. Protein synthesis in relation to ripening of pome fruits. Plant Physiol. 1968 Jul;43(7):1146–1153. doi: 10.1104/pp.43.7.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel C. Oxidative turnover of auxins in relation to the onset of ripening in bartlett pear. Plant Physiol. 1975 Mar;55(3):480–484. doi: 10.1104/pp.55.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel C. Promotion of softening and ethylene synthesis in bartlett pears by 3-methylene oxindole. Plant Physiol. 1975 Nov;56(5):647–649. doi: 10.1104/pp.56.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARMAN D. Role of free radicals in mutation, cancer, aging, and the maintenance of life. Radiat Res. 1962 May;16:753–763. [PubMed] [Google Scholar]
- Jackson A. O., Larkins B. A. Influence of Ionic Strength, pH, and Chelation of Divalent Metals on Isolation of Polyribosomes from Tobacco Leaves. Plant Physiol. 1976 Jan;57(1):5–10. doi: 10.1104/pp.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar M., Mishra D. Catalase, Peroxidase, and Polyphenoloxidase Activities during Rice Leaf Senescence. Plant Physiol. 1976 Feb;57(2):315–319. doi: 10.1104/pp.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M., Kunishi A. T. Ethylene production from methionine. Biochem J. 1965 Nov;97(2):449–459. doi: 10.1042/bj0970449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich P. R., Boveris A., Bonner W. D., Jr, Moore A. L. Hydrogen peroxide generation by the alternate oxidase of higher plants. Biochem Biophys Res Commun. 1976 Aug 9;71(3):695–703. doi: 10.1016/0006-291x(76)90887-1. [DOI] [PubMed] [Google Scholar]
- SIEGEL S. M., GALSTON A. W. Peroxide genesis in plant tissues and its relation to indoleacetic acid destruction. Arch Biochem Biophys. 1955 Jan;54(1):102–113. doi: 10.1016/0003-9861(55)90012-6. [DOI] [PubMed] [Google Scholar]
- Solomos T., Laties G. G. Effects of Cyanide and Ethylene on the Respiration of Cyanide-sensitive and Cyanide-resistant Plant Tissues. Plant Physiol. 1976 Jul;58(1):47–50. doi: 10.1104/pp.58.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stonier T., Yang H. M. Studies on Auxin Protectors: XI. Inhibition of Peroxidase-Catalyzed Oxidation of Glutathione by Auxin Protectors and o-Dihydroxyphenols. Plant Physiol. 1973 Feb;51(2):391–395. doi: 10.1104/pp.51.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelitch I. Comparison of the effectiveness of glycolic Acid and glycine as substrates for photorespiration. Plant Physiol. 1972 Jul;50(1):109–113. doi: 10.1104/pp.50.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]


