Abstract
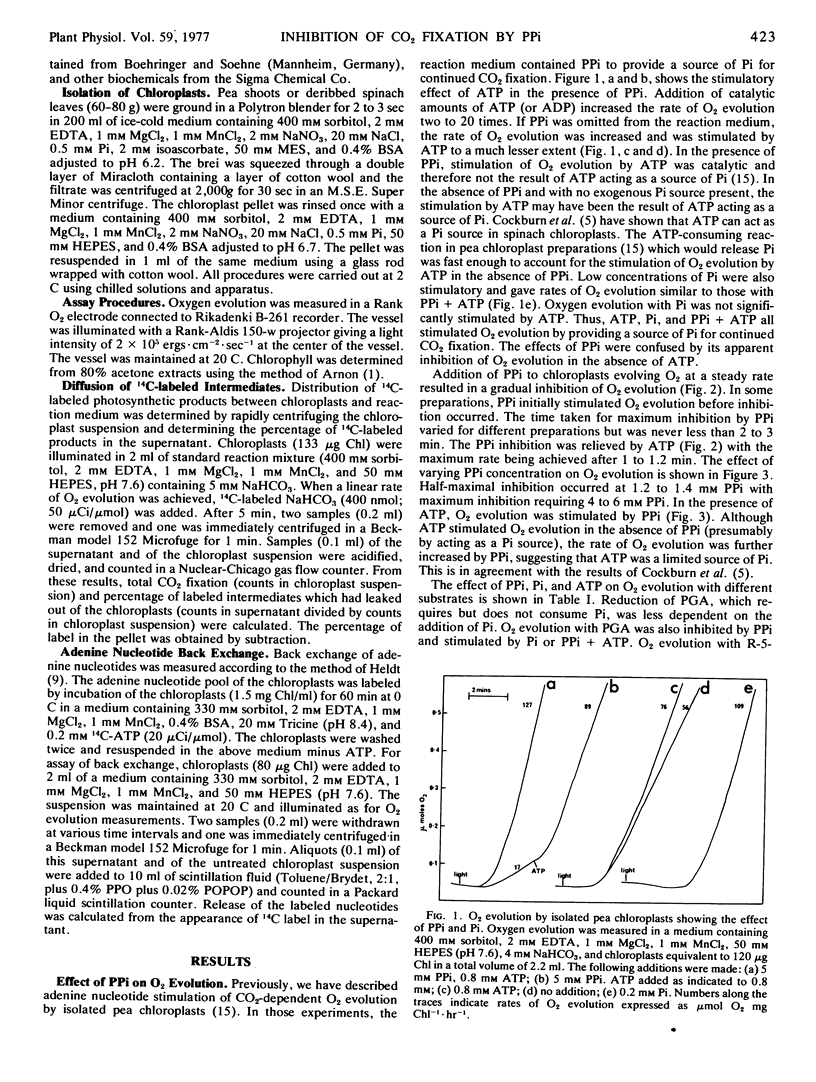
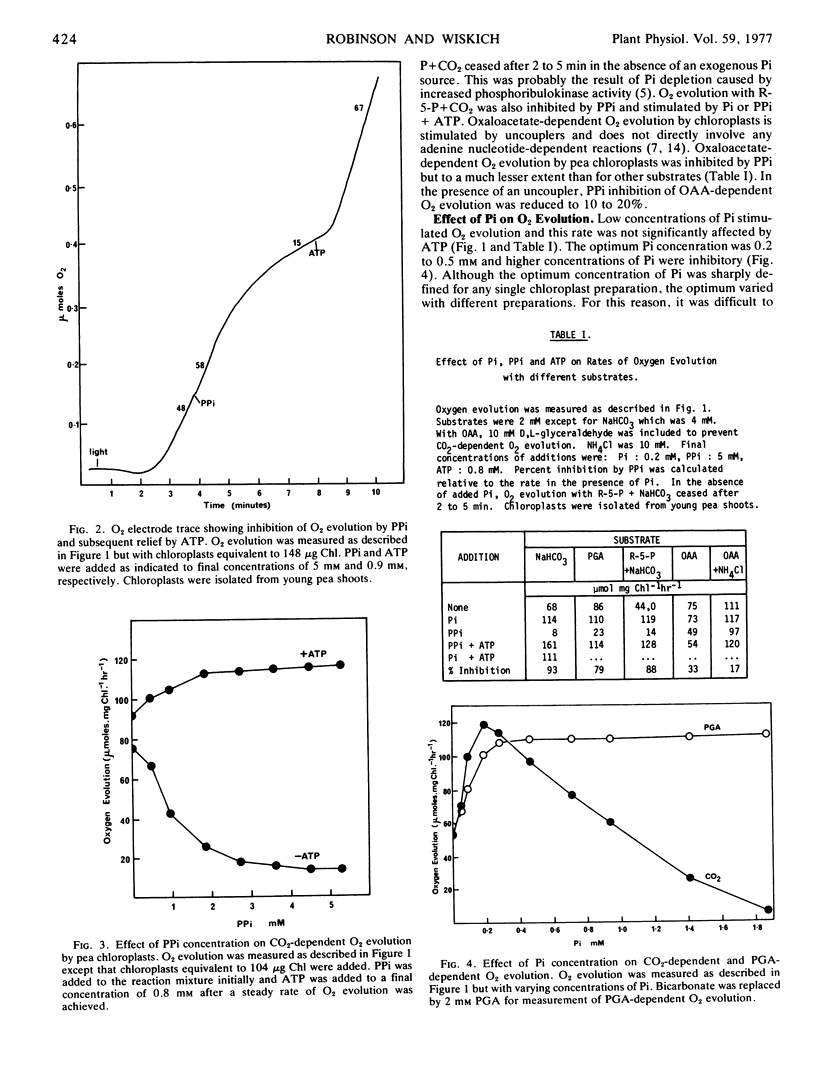
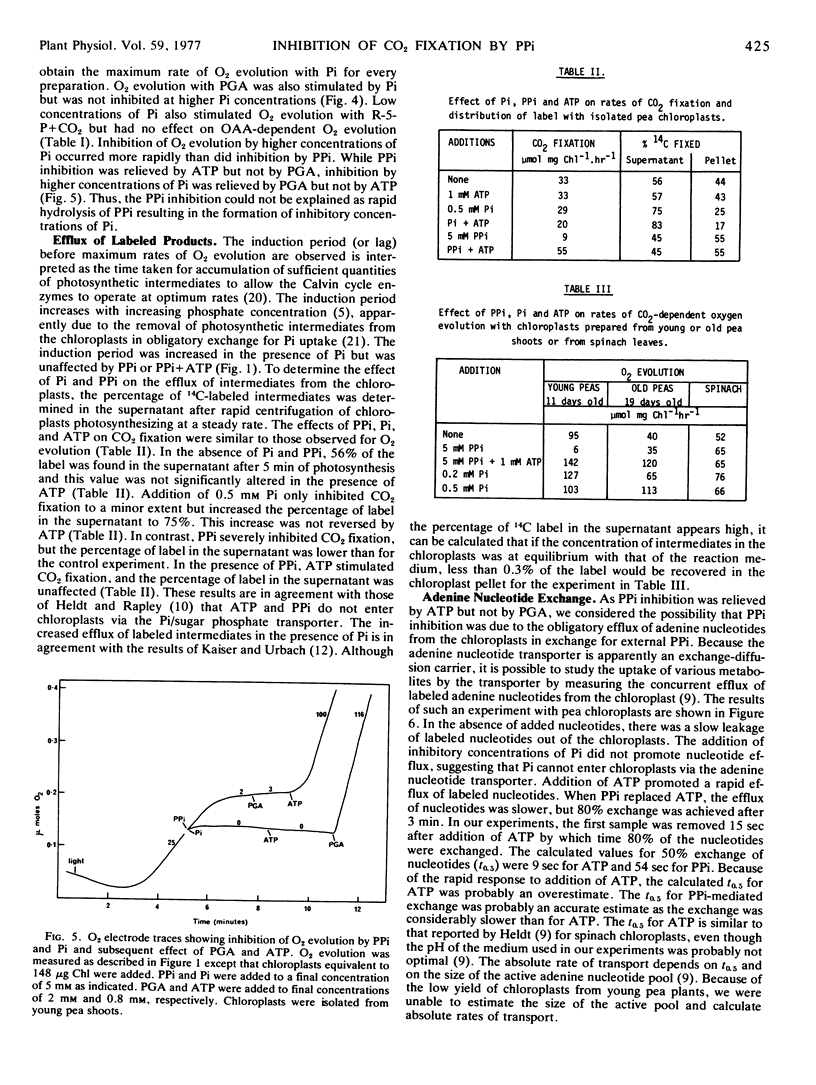
Carbon dioxide-dependent O2 evolution by isolated pea (Pisum sativum) chloroplasts was inhibited by inorganic pyrophosphate (PPi). Oxygen evolution was also inhibited by high concentrations of orthophosphate (Pi) and the inhibition was relieved by 3-phosphoglycerate. In contrast, the inhibition by PPi was not relieved by 3-phosphoglycerate, indicating that hydrolysis of PPi and accumulation of inhibitory concentrations of Pi were not occurring. In agreement with this suggestion, the percentage of 14C-labeled products diffusing out of the chloroplasts was increased by Pi but not by PPi. The inhibition of O2 evolution by PPi was reversed by ATP. The concentration of PPi required for 50% inhibition was 1.2 to 1.4 mm and the subsequent stimulation by ATP was half-maximal at 16 to 25 μm. Carbon dioxide-dependent O2 evolution by spinach chloroplasts, or chloroplasts isolated from older pea plants, was not significantly inhibited by PPi.
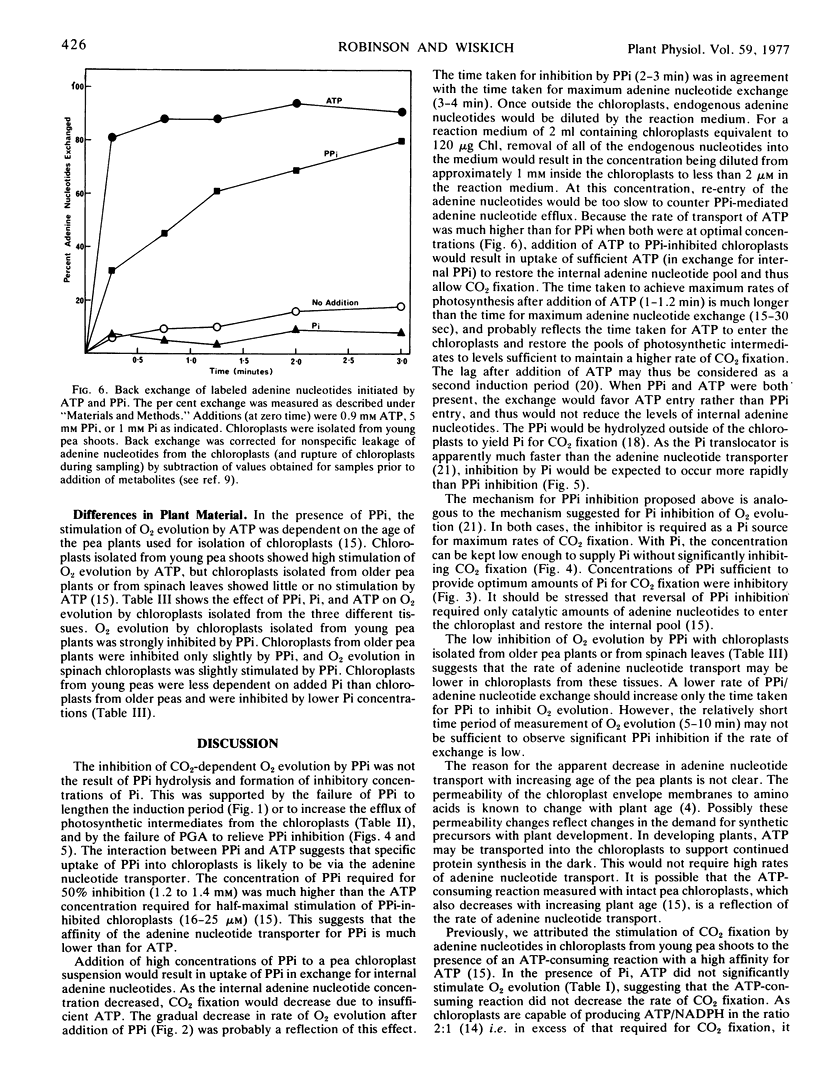
Chloroplasts were preloaded with 14C-ATP and release of the labeled nucleotides was measured to assess the activity of adenine nucleotide transport across the inner chloroplast envelope membrane. A rapid exchange was promoted by the addition of exogenous ATP. Addition of PPi also resulted in a release of endogenous nucleotides. We suggest that PPi inhibits CO2 fixation by entering the chloroplast in exchange for endogenous adenine nucleotides via the transporter on the inner envelope membrane. The subsequent depletion of the internal adenine nucleotide pool would result in decreased CO2 fixation due to insufficient ATP. Addition of ATP to PPi-inhibited chloroplasts apparently results in uptake of catalytic amounts of ATP and restoration of the internal adenine nucleotide pool thus relieving the inhibition of CO2 fixation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberger E. S., Ehrlich B. A., Gibbs M. The glyceraldehyde 3-phosphate and glycerate 3-phosphate shuttle and carbon dioxide assimilation in intact spinach chloroplasts. Plant Physiol. 1975 Jun;55(6):1023–1030. doi: 10.1104/pp.55.6.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassham J. A., Kirk M., Jensen R. G. Photosynthesis by isolated chloroplasts. I. Diffusion of labeled photosynthetic intermediates between isolated chloroplasts and suspending medium. Biochim Biophys Acta. 1968 Jan 15;153(1):211–218. doi: 10.1016/0005-2728(68)90162-x. [DOI] [PubMed] [Google Scholar]
- Cockburn W., Baldry C. W., Walker D. A. Some effects of inorganic phosphate on O2 evolution by isolated chloroplasts. Biochim Biophys Acta. 1967;143(3):614–624. doi: 10.1016/0005-2728(67)90067-9. [DOI] [PubMed] [Google Scholar]
- Heber U., Kirk M. R. Flexibility of coupling and stoichiometry of ATP formation in intact chloroplasts. Biochim Biophys Acta. 1975 Jan 31;376(1):136–150. doi: 10.1016/0005-2728(75)90212-1. [DOI] [PubMed] [Google Scholar]
- Heber U., Santarius K. A. Direct and indirect transfer of ATP and ADP across the chloroplast envelope. Z Naturforsch B. 1970 Jul;25(7):718–728. doi: 10.1515/znb-1970-0714. [DOI] [PubMed] [Google Scholar]
- Heldt H. W. Adenine nucleotide translocation in spinach chloroplasts. FEBS Lett. 1969 Sep;5(1):11–14. doi: 10.1016/0014-5793(69)80280-2. [DOI] [PubMed] [Google Scholar]
- Jensen R. G., Bassham J. A. Photosynthesis by isolated chloroplasts. 3. Light activation of the carboxylation reaction. Biochim Biophys Acta. 1968 Jan 15;153(1):227–234. doi: 10.1016/0005-2728(68)90164-3. [DOI] [PubMed] [Google Scholar]
- Kaiser W., Urbach W. Rates and properties of endogenous cyclic photophosphorylation of isolated intact chloroplasts measured by CO2 fixation in the presence of dihydroxyacetone phosphate. Biochim Biophys Acta. 1976 Jan 15;423(1):91–102. doi: 10.1016/0005-2728(76)90103-1. [DOI] [PubMed] [Google Scholar]
- Lilley R. M., Schwenn J. D., Walker D. A. Inorganic pyrophosphatase and photosynthesis by isolated chloroplasts. II. The controlling influence of orthophosphate. Biochim Biophys Acta. 1973 Dec 14;325(3):596–604. doi: 10.1016/0005-2728(73)90219-3. [DOI] [PubMed] [Google Scholar]
- Robinson S. P., Wiskich J. T. Factors affecting the ADP/O ratio in isolated chloroplasts. Biochim Biophys Acta. 1976 Jul 9;440(1):131–146. doi: 10.1016/0005-2728(76)90119-5. [DOI] [PubMed] [Google Scholar]
- Robinson S. P., Wiskich J. T. Stimulation of carbon dioxide fixation in isolated pea chloroplasts by catalytic amounts of adenine nucleotides. Plant Physiol. 1976 Aug;58(2):156–162. doi: 10.1104/pp.58.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarius K. A., Heber U. Changes in the intracellular levels of ATP, ADP, AMP and P1 and regulatory function of the adenylate system in leaf cells during photosynthesis. Biochim Biophys Acta. 1965 May 25;102(1):39–54. doi: 10.1016/0926-6585(65)90201-3. [DOI] [PubMed] [Google Scholar]
- Schwenn J. D., Lilley R. M., Walker D. A. Inorganic pyrophospatase and photosynthesis by isolated chloroplasts. I. Characterisation of chloroplast pyrophosphatase and its relation to the response to exogenous pyrophosphate. Biochim Biophys Acta. 1973 Dec 14;325(3):586–595. doi: 10.1016/0005-2728(73)90218-1. [DOI] [PubMed] [Google Scholar]
- Schürmann P., Buchanan B. B., Arnon D. I. Role of cyclic photophosphorylation in photosynthetic carbon dioxide assimilation by isolated chloroplasts. Biochim Biophys Acta. 1972 Apr 20;267(1):111–124. doi: 10.1016/0005-2728(72)90143-0. [DOI] [PubMed] [Google Scholar]


