ABSTRACT
Trypanosoma cruzi is the agent of Chagas disease, and the finding that this parasite possesses a mitochondrial calcium uniporter (TcMCU) with characteristics similar to that of mammalian mitochondria was fundamental for the discovery of the molecular nature of MCU in eukaryotes. We report here that ablation of TcMCU, or its paralog TcMCUb, by clustered regularly interspaced short palindromic repeat (CRISPR)/Cas9 led to a marked decrease in mitochondrial Ca2+ uptake without affecting the membrane potential of these cells, whereas overexpression of each gene caused a significant increase in the ability of mitochondria to accumulate Ca2+. While TcMCU-knockout (KO) epimastigotes were viable and able to differentiate into trypomastigotes, infect host cells, and replicate normally, ablation of TcMCUb resulted in epimastigotes having an important growth defect, lower rates of respiration and metacyclogenesis, more pronounced autophagy changes under starvation, and significantly reduced infectivity. Overexpression of TcMCUb, in contrast to what was proposed for its mammalian ortholog, did not result in a dominant negative effect on TcMCU.
KEYWORDS: calcium signaling, mitochondria, Trypanosoma, uniporter
IMPORTANCE
The finding of a mitochondrial calcium uniporter (MCU) in Trypanosoma cruzi was essential for the discovery of the molecular nature of this transporter in mammals. In this work, we used the CRISPR/Cas9 technique that we recently developed for T. cruzi to knock out two components of the uniporter: MCU, the pore subunit, and MCUb, which was proposed as a negative regulator of MCU in human cells. In contrast to what occurs in human cells, MCU is not essential, while MCUb is essential for growth, differentiation, and infectivity; has a bioenergetic role; and does not act as a dominant negative subunit of MCU.
INTRODUCTION
Trypanosoma cruzi is the etiologic agent of Chagas disease, an enormous burden on human health in the American continent, which has four major developmental stages that alternate between an insect vector and a mammalian host. Two are replicative forms, the epimastigote found in the insect vector intestine and the intracellular mammalian form or amastigote, and two are nonreplicative, the metacyclic trypomastigote found in the rectum and urine of the vector and the bloodstream trypomastigote found in the mammalian host. All these forms appear to have functional mitochondria with an active oxidative metabolism (1, 2).
The finding that mitochondrial Ca2+ transport in T. cruzi is electrogenic, has low affinity and high capacity, and is inhibited by ruthenium red, as occurs with vertebrate mitochondria, identified the presence of a mitochondrial calcium uniporter (MCU) in trypanosomatids (3, 4). The presence of MCU in trypanosomes together with its absence in yeast (5) led to the identification, first, of the gene encoding an MCU modulator (mitochondrial calcium uptake 1 [MICU1]) (6) and then of the gene encoding the MCU of mammalian cells (7–9). In recent work, we demonstrated that the MCU of Trypanosoma brucei, which belongs to the group of parasites that produce African trypanosomiasis or sleeping sickness, is essential for the regulation of the bioenergetics of the parasite and its growth and infectivity (10).
After the discovery of the molecular identity of MCU, other components of the MCU complex were described (11, 12). One of them, MCUb, was shown to exert a dominant negative effect in HeLa cell mitochondria, reducing the mitochondrial Ca2+ increase evoked by agonist stimulation (13).
In this work, we report that knockout of the T. cruzi MCU (TcMCU) gene by clustered regularly interspaced short palindromic repeat (CRISPR)/Cas9 in T. cruzi epimastigotes (14) abolishes mitochondrial calcium uptake without affecting their mitochondrial membrane potential (ΔΨm) and reduces growth in low-glucose medium. However, epimastigotes conserve their ability to differentiate into metacyclic trypomastigotes and infect mammalian cells. In contrast to a previous report on HeLa cells (13), overexpression of TcMCUb does not have a dominant negative effect but increases mitochondrial Ca2+ uptake without affecting the ΔΨm. Knockout of TcMCUb by CRISPR/Cas9 abolishes mitochondrial Ca2+ transport, reduces respiration, has a significant effect on epimastigote growth, and increases autophagy. These cells have a reduced ability to differentiate into metacyclic trypomastigotes and are unable to efficiently infect cells, underscoring the relevance of TcMCUb for the parasite life cycle.
RESULTS
Ca2+ uptake by TcMCU and TcMCUb knockouts.
After the recent characterization of MCU as the channel-forming subunit of the mitochondrial calcium uniporter complex (15), several pore regulators were reported, among them mitochondrial calcium uptake 1 (MICU1), MICU2, MCUb, essential MCU regulator (EMRE), and MCU regulator 1 (MCUR1) (11). It has been suggested that MCUb is a dominant negative regulator of the uniporter complex (13). However, evidence of its influence on MCU regulation is lacking. MCUb has been identified in the T. cruzi genome, and similarly to its paralog MCU, it has two predicted transmembrane domains (16). Therefore, we aimed at investigating the effect of downregulation and overexpression of TcMCUb on the physiological role of the MCU complex in T. cruzi.
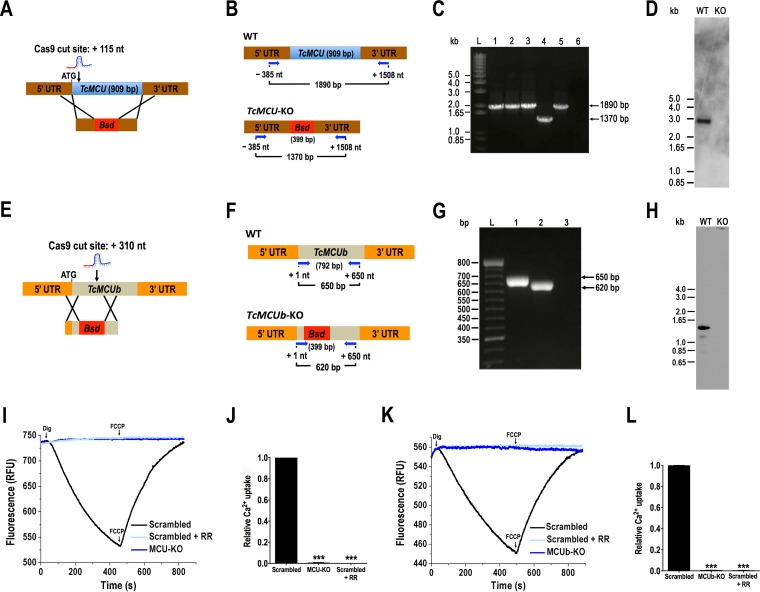
We used the recently developed CRISPR/Cas9 system with a DNA donor cassette for DNA repair (14) to knock out TcMCU (Fig. 1A and B) and TbMCUb (Fig. 1E and F) in epimastigotes. After 5 to 6 weeks of selection under G418 and blasticidin, we obtained resistant populations of epimastigotes transfected with specific single guide RNAs (sgRNAs) and blasticidin cassettes. These were constructed with long (~500-bp) flanking untranslated regions (UTRs) cloned in pGEM-T Easy vector to obtain TcMCU-KO cells or using long oligonucleotides (ultramers) with shorter homology regions (100 bp) to obtain the TcMCUb-KO cell line. Primers (see Table S1 in the supplemental material) were used in PCR experiments to check TcMCU and TcMCUb disruption in G418/blasticidin-resistant cells. As shown in Fig. 1C, TcMCU was ablated and replaced by the blasticidin resistance gene in TcMCU-KO cells. Disruption of the TcMCUb gene was also demonstrated using specific primers (Fig. 1G). In addition, Southern blot analyses demonstrated that TcMCU (Fig. 1D) and TcMCUb (Fig. 1H) were absent in genomic DNA of the KO cell lines.
FIG 1 .
Ca2+ uptake by TcMCU and TcMCUb knockouts. (A) Schematic representation of the strategy used to generate a TcMCU-KO mutant by CRISPR/Cas9-induced homologous recombination. A double-stranded gDNA break was produced by Cas9 at nt +115 of the TcMCU ORF. DNA was repaired with a blasticidin S-deaminase (Bsd) cassette containing ~500-bp homologous regions from the TcMCU locus. (B) Primers (arrows) that were used to verify gene disruption by PCR. The intact locus generates a PCR product of 1,890 bp, while the replaced locus generates a fragment of 1,370 bp. (C) TcMCU was ablated at its genomic locus and replaced in genomic DNA of the KO cell line. Lanes: L, 1-kb plus ladder; 1, wild type; 2, pTREX-n control cells; 3, Cas9/pTREX-n control cells; 4, TcMCU-KO mutant cell line; 5, scrambled sgRNA/Cas9/pTREX-n control cells; 6, PCR negative control. (D) Southern blot analysis of wild-type (WT) and TcMCU-KO (KO) epimastigotes. The blot was hybridized with a 100-bp radiolabeled region of TcMCU. (E) Schematic representation of the strategy used to generate a TcMCUb-KO mutant by CRISPR/Cas9-induced homologous recombination. A double-stranded gDNA break was produced by Cas9 at nt +310 of the TcMCUb ORF (792 bp). DNA was repaired with a blasticidin S-deaminase (Bsd) cassette containing 100-bp homologous regions spanning from nt −20 to +80 and from nt +510 to +610 of the TcMCUb locus. (F) Primers (arrows) used to verify gene disruption by PCR. The intact locus generates a PCR product of 650 bp, while the disrupted locus generates a fragment of 620 bp. (G) TcMCUb was disrupted at its genomic locus where the Bsd gene replaced a 430-bp fragment in the KO cell line. Lanes: L, 1-kb plus ladder; 1, wild type; 2, TcMCUb-KO mutant cell line; 3, PCR negative control. (H) Southern blot analysis of wild-type (WT) and TcMCUb-KO (KO) epimastigotes. The blot was hybridized with a biotin-labeled probe corresponding to 430 bp of TcMCUb (nt +80 to +510). (I) Ca2+ uptake by digitonin-permeabilized epimastigotes in relative fluorescence units (RFU). MCU-KO, TcMCU-KO cells; Scrambled, scrambled control cells in absence or presence (+ RR) of 5 µM ruthenium red. The reaction was started after adding 50 µM digitonin in the presence of 20 µM CaCl2. Where indicated, 4 µM FCCP was added. (J) Quantification of data in panel I. Relative Ca2+ uptake at 400 s compared with epimastigotes transfected with scrambled control. (K) Ca2+ uptake by digitonin-permeabilized epimastigotes in relative fluorescence units (RFU). MCUb-KO, TcMCU-KO cells; Scrambled, scrambled control cells in absence or presence (+ RR) of 5 µM ruthenium red. The reaction was started after adding 50 µM digitonin in the presence of 20 µM CaCl2. Where indicated, 4 µM FCCP was added. (L) Quantification of data in panel K. Relative Ca2+ uptake at 400 s compared with epimastigotes transfected with scrambled control. Values in panels J and L are means ± SD (n = 3). ***, P < 0.001.
Oligonucleotides used in this work. Download TABLE S1, DOCX file, 0.1 MB (110.9KB, docx) .
Copyright © 2017 Chiurillo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
To determine the capacity of TcMCU-KO and TcMCUb-KO cell lines to take up Ca2+, we monitored Ca2+ uptake with calcium green-5N probe in digitonin-permeabilized epimastigotes. A decrease in fluorescence indicates decreasing medium Ca2+ or increasing vesicular Ca2+. Figures 1I and K show that addition of 50 µM digitonin in the presence of 5 mM succinate and 20 µM Ca2+ produced a fast decrease in Ca2+ concentration starting after a period of about 60 s, which continued until addition of the uncoupler p-trifluoromethoxyphenylhydrazone (FCCP), which released the mitochondrial Ca2+ taken up. This Ca2+-transporting activity was fully eliminated by the addition of 5 µM ruthenium red, indicating that it is due to the uniporter. Knockout of TcMCU (Fig. 1I and J) or TcMCUb (Fig. 1K and L) abolished the T. cruzi mitochondrial ability to take up Ca2+.
Coimmunoprecipitation of TcMCU and TcMCUb and Ca2+ uptake by TcMCU- and TcMCUb-overexpressing cells.
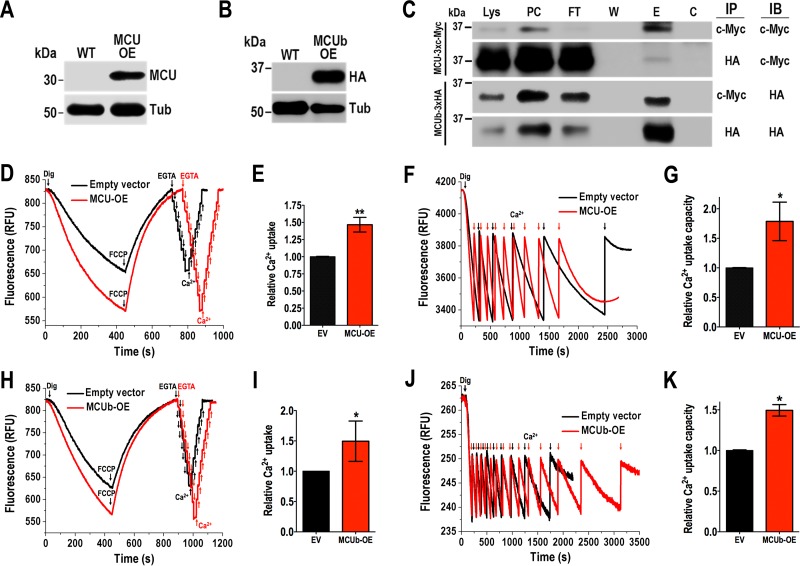
We also generated cell lines overexpressing TcMCU (TcMCU-OE) or TcMCUb (TcMCUb-OE) cloned in pTREX-n vector, as described in Materials and Methods. We recently demonstrated that endogenously tagged TcMCU localizes to the mitochondria of T. cruzi (17). TcMCU-OE protein also localized to the mitochondria, as demonstrated by immunofluorescence microscopy (see Fig. S1A). Antibody to T. brucei MCU (10) colocalized with antibody against the voltage-dependent anion channel (TbVDAC), which is a major protein of the outer mitochondrial membrane of eukaryotes. Western blot analysis detected a band of the expected size (32 kDa) in the overexpressing cell line (Fig. 2A). The band was not visible in wild-type (WT) cells, probably as a consequence of its low expression levels. TcMCU-OE and TcMCUb-OE (Fig. S1B) cells exhibited the same growth rate as control cells transfected with the pTREX-n empty vector. Western blot analysis of TcMCUb-OE cells using antibodies against its hemagglutinin (HA) tag confirmed its expression (Fig. 2B), and immunofluorescence analysis demonstrated its mitochondrial localization (Fig. S1C).
FIG 2 .
Coimmunoprecipitation of TcMCU and TcMCUb and Ca2+ uptake by TcMCU- and TcMCUb-overexpressing cells. (A) Western blot analysis of TcMCU in total protein extracts of wild-type (WT) and TcMCU-OE (MCU-OE) epimastigotes, using anti-TbMCU antibodies. Anti-α-tubulin antibodies were used as a loading control. (B) Western blot analysis of total protein extracts of wild-type (WT) and TcMCUb-3×HA-OE (MCUb OE) epimastigotes using anti-HA antibodies. Anti-α-tubulin antibodies were used as a loading control. (C) Anti-c-Myc and anti-HA immunoprecipitations (IP) using lysates from epimastigotes overexpressing TcMCUb-3×HA and TcMCU-3×c-Myc. Immunoblot assays (IB) were done with anti-c-Myc and anti-HA antibodies. Analyzed fractions were as follows: Lys, total lysate; PC, precleared lysate; FT, flowthrough; W, last wash; E, eluate; C, control agarose. (D) Ca2+ uptake by digitonin-permeabilized empty vector (pTREX-n) and TcMCU-OE (MCU-OE) cells. Other additions were as in Fig. 1I. EGTA (4 µM) and Ca2+ (2 µM pulses) were added where indicated. (E) Quantification of data in panel D. Relative Ca2+ uptake at 400 s compared with empty vector (EV). (F) Similar conditions as in panel D, except that reaction buffer contained 0.2% bovine serum albumin and further CaCl2 pulses (6 µM each time) were added to empty vector and MCU-OE cells to show the high mitochondrial capacity to take up Ca2+. (G) Quantification of data in panel F. Relative Ca2+ uptake capacity (number of Ca2+ pulses) compared with that of empty vector cells. (H) Ca2+ uptake by digitonin-permeabilized empty vector (pTREX-n) and MCUb-OE cells. Other additions were as in Fig. 1K. EGTA (4 µM) and Ca2+ (2 µM pulses) were added where indicated. (I) Quantification of data in panel H. Relative Ca2+ uptake at 400 s compared with empty vector. (J) Similar conditions as in panel H except that reaction buffer contained 0.2% bovine serum albumin and further CaCl2 additions (6 µM each time) were added to empty vector and MCUb-OE cells to show the high mitochondrial capacity to take up Ca2+. (K) Quantification of data in panel J. Relative Ca2+ uptake capacity (number of Ca2+ pulses) compared with that of empty vector cells. Values in panels E, G, I, and K are means ± SD (n = 3). *, P < 0.05; **, P < 0.01.
Localization and growth of TcMCU-OE and TcMCUb-OE epimastigotes. (A) Fluorescence microscopy of TcMCU-OE epimastigotes (MCU) using anti-TbMCU antibodies (green) and anti-TbVDAC (VDAC) antibodies as a mitochondrial marker (red). Nuclei and kinetoplast were labeled with DAPI (blue). Colocalization of TcMCU-OE and TcVDAC is shown in yellow (merge). A differential interference contrast (DIC) image is shown in the left panel. (B) Growth curve of TcMCU-OE (MCU-OE) epimastigotes, TcMCUb-OE (MCUb-OE) epimastigotes, and epimastigotes transfected with pTREX-n empty vector. (C) Colocalization of TcMCUb-3×HA-OE using anti-HA antibodies (HA) with MitoTracker (MT). (D) Western blot analysis of WT, TcMCU-3×c-Myc, and TcMCU-3×c-Myc/pTREX-n-TcMCUb-3×HA cell lines. Anti-c-Myc and anti-HA antibodies detect TcMCU-3×c-Myc (predicted size, 35 kDa) and TcMCUb-3×HA (predicted size, 31 kDa), respectively, whereas both bands are absent in WT parasites. Anti-α-tubulin antibody was used as a loading control. The TcMCU-3×c-Myc cell line was obtained by CRISPR/Cas9-mediated endogenous C-terminal tagging as described previously (15). Antibodies are indicated on the right side of the blots, and molecular weights are on the left side. There was no significant difference in intensity of bands corresponding to TcMCU-3×c-Myc detected with anti-c-Myc antibodies between TcMCU-3×c-Myc and TcMCU-3×c-Myc/pTREX-n-TcMCUb-3×HA cell lines. Bars in panels A and C, 10 µm. Values in panel B are means ± SD (n = 3; no significant difference). Download FIG S1, TIF file, 1.4 MB (1.5MB, tif) .
Copyright © 2017 Chiurillo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
To investigate whether TcMCU and TcMCUb could form oligomers as previously described with their orthologs in HeLa cells (13), we used a T. cruzi cell line that coexpresses the c-Myc-tagged TcMCU (TcMCU-3×c-Myc) and the HA-tagged TcMCUb (TcMCUb-3×HA), as described in Materials and Methods. Cells were lysed, and immunoprecipitation was done with either anti-c-Myc or anti-HA antibody. Western blot analyses showed that the anti-HA antibody immunoprecipitated TcMCU-3×c-Myc and the anti-c-Myc antibody immunoprecipitated TcMCUb-3×HA, revealing the MCU-MCUb interaction in situ (Fig. 2C).
TcMCU-OE (Fig. 2D and E) and TcMCUb-OE (Fig. 2H and I) epimastigotes showed increased Ca2+ uptake. The mitochondria of permeabilized control epimastigotes were capable of buffering multiple pulses of exogenously added Ca2+, and overexpression of TcMCU (Fig. 2F and G) or TcMCUb (Fig. 2J and K) increased significantly the ability of their mitochondria to accumulate Ca2+ in response to Ca2+ pulses. Overexpression of TcMCUb in epimastigotes possessing endogenously tagged TcMCU did not induce its overexpression (Fig. S1D).
Analysis of the mitochondrial membrane potential (ΔΨm) of mutant cell lines.
Safranin O was used to measure ΔΨm in digitonin-permeabilized epimastigotes in the presence of succinate as the mitochondrial substrate. To be certain that the ΔΨm was not affected in the mutants, we calibrated the ΔΨm using valinomycin and potassium as we described before (18) and expressed the changes in millivolts instead of fluorescence arbitrary units. The magnitude of this membrane potential of epimastigote mitochondria respiring on succinate was 177 ± 2.0, 179.6 ± 2.8, 177.6 ± 2.3, 177.7 ± 1.6, and 177.9 ± 1.7 mV for wild-type, TcMCU-KO, TcMCU-OE, TcMCUb-KO, and TcMCUb-OE cells, respectively (n = 3). When using safranin O, an increase in fluorescence after addition of digitonin indicated stacking of the dye to the energized inner mitochondrial membrane (Fig. S2A to C). Addition of ADP produced the expected small decrease in membrane potential, indicating ADP phosphorylation. ΔΨm returned to its initial level after addition of the adenine nucleotide translocator inhibitor carboxyatractyloside (CAT). Addition of FCCP collapsed the membrane potential. Neither knockout nor overexpression of either TcMCU (Fig. S2A to C) or TcMCUb (Fig. S2D to F) affected the ΔΨm at the steady state or ADP phosphorylation.
Mitochondrial membrane potential in mutant epimastigotes (A and B). Changes in mitochondrial membrane potential (ΔΨm) of digitonin-permeabilized epimastigotes as detected by changes in safranin O fluorescence in epimastigotes transfected with scrambled sgRNA/Cas9/pTREX-n (Scrambled) or TcMCU-KO (MCU-KO) (A) and empty vector or TcMCU-OE (MCU-OE) (B). Cells (5 × 107) were added to the reaction buffer (2 ml) containing 0.2% bovine serum albumin, 2 mM succinate, and 5 µM safranin O. The reaction was started with 50 µM digitonin, and 250 µM ADP, 20 µM carboxyatractyloside (CAT), and 4 µM carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP) were added where indicated. (C) Quantification of changes in ΔΨm in panels A and B. (D and E) Changes in ΔΨm in digitonin-permeabilized epimastigotes as detected by changes in safranin O fluorescence in epimastigotes transfected with scrambled sgRNA/Cas9/pTREX-n (Scrambled) or TcMCUb-KO (MCUb-KO) (D) and empty vector or TcMCUb-OE (MCUb-OE) (E). Other conditions are as in panels A and B. (F) Quantification of changes in ΔΨm in panels D and E. Values in panels C and F are means ± SD (n = 3; no significant differences, Student’s t test). Download FIG S2, TIF file, 1.4 MB (1.5MB, tif) .
Copyright © 2017 Chiurillo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Growth of TcMCU and TcMCUb mutants.
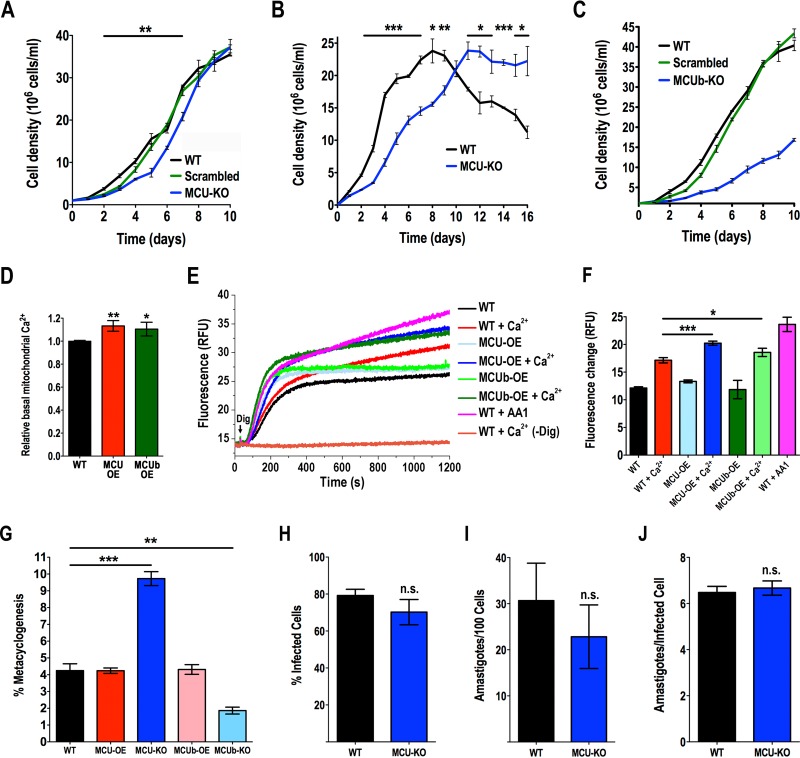
We initially evaluated the growth rate of epimastigotes in liver infusion tryptose (LIT) medium. TcMCU-KO cells grew at a slightly lower initial rate than wild-type cells or cells transfected with a scrambled sgRNA (Fig. 3A). We then tested their growth in low-glucose LIT medium. In this case, the initial growth rate of TcMCU-KO cells was significantly lower. Wild-type epimastigotes reached the stationary phase earlier (day 8) and then started dying, while TcMCU-KO cells reached the stationary phase later (day 11) and continued at steady state for a longer period (Fig. 3B). The longer survival of TcMCU-KO cells in low-glucose medium suggested that these cells could have access to an energy reserve. Amino acids or fatty acids derived from triglycerides have been proposed as a possible source of energy of epimastigotes under low-glucose conditions (1). We therefore investigated the presence of lipid droplets in TcMCU-KO cells under these conditions. Lipid droplets were more abundant in TcMCU-KO cells grown in LIT medium or in low-glucose LIT medium than in wild-type cells at the beginning of the stationary phase, suggesting triglycerides as a potential energy reserve of these cells (Fig. S3A and B).
FIG 3 .
Phenotypic changes in TcMCU and TcMCUb mutant epimastigotes. (A) Growth of wild-type (WT) and TcMCU-KO epimastigotes (MCU-KO) and epimastigotes transfected with scrambled sgRNA (Scrambled) in LIT medium. (B) Growth of WT and MCU-KO epimastigotes in low-glucose LIT medium. (C) Growth curve of wild-type (WT) and TcMCUb-KO (MCUb-KO) epimastigotes and epimastigotes transfected with a scrambled sgRNA grown in LIT medium (n = 3). (D) Relative Rhod-2 fluorescence in control cells (WT) compared with MCU-OE or MCUb-OE cells. (E) Mitochondrial oxidative stress as measured with MitoSOX Red in control (WT), TcMCU-OE (MCU-OE), and TcMCUb-OE (MCUb-OE) epimastigotes permeabilized with 50 μM digitonin in the presence or absence of 0.5 mM Ca2+. Controls were treated with 2 mM antimycin A1 (WT + AA1) or were epimastigotes exposed to 0.5 mM Ca2+ in the absence of digitonin [WT + Ca2+ (-Dig)]. (F) Quantification of changes in panel E at 1,200 s. (G) Percentage of metacyclic trypomastigotes in epimastigote cultures after incubation in TAU 3AAG medium. Differentiation of epimastigotes to metacyclic trypomastigotes was quantified by staining with DAPI to distinguish the position of the kinetoplast by fluorescence microscopy. (H and I) Effect of TcMCU knockout on trypomastigote infection of Vero cells. There were no significant differences in percentages of infected Vero cells (H) or in the number of intracellular parasites per 100 host cells (I). (J) Effect of TcMCU knockout in amastigote replication after 48 h. Differences were not significant. Values in panels A to D and F to J are means ± SD (n = 3). *, P < 0.05; **, P < 0.01; ***, P < 0.001; n.s., not significant.
Lipid droplets in epimastigotes and PCR analysis of TcMCU-KO trypomastigotes. (A) Staining with Nile red (red) of lipid droplets in wild-type (WT) and TcMCU-KO epimastigotes cultured in LIT or low-glucose LIT medium. T. cruzi epimastigotes were incubated in PBS containing 1.5 μg of Nile red (Sigma)/ml for 30 min at 28°C. Fluorescence optical images were captured with excitation at 510 nm and emission at 580 nm. Nuclei and kinetoplasts were labeled with DAPI (blue). Bars, 10 µm. (B) Number of lipid droplets detected per cell in LIT or low-glucose medium. At least 200 cells from three experiments with 20 random fields/experiment were analyzed (means ± SD; n = 3; ***, P < 0.001, Student’s t test). (C) PCR used to confirm TcMCU-KO genotype in trypomastigotes isolated after host cell infection, as described in Materials and Methods. The intact locus generates a PCR product of 909 bp in wild-type cells that is absent in TcMCU-KO trypomastigotes. Download FIG S3, TIF file, 2.2 MB (2.2MB, tif) .
Copyright © 2017 Chiurillo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
In contrast to TcMCU-KO cells, TcMCUb-KO cells grew in LIT medium at a significantly lower rate than wild-type cells or cells transfected with a scrambled sgRNA (Fig. 3C).
Oxidative stress in TcMCU-OE and TcMCUb-OE cells.
Overexpression of TcMCU or TcMCUb resulted in higher basal mitochondrial Ca2+ concentration, as measured by Rhod-2 fluorescence (Fig. 3D). Mitochondrial Ca2+ overload is known to generate oxidative stress. Accordingly, reactive oxygen species (ROS) generation was increased in digitonin-permeabilized TcMCU-OE or TcMCUb-OE epimastigotes compared with control cells (wild type [WT]) when in the presence of 0.5 mM Ca2+ (Fig. 3E and F). They reached levels comparable to those produced by addition of antimycin A1, a known complex III inhibitor of the electron transport chain (Fig. 3E and F).
Differentiation and infectivity of mutant cells.
To induce the differentiation of mutant epimastigotes into infective metacyclic trypomastigotes (metacyclogenesis), we incubated these cells in triatome artificial urine (TAU) medium as described in Materials and Methods. TcMCU-KO cells were able to differentiate to metacyclic trypomastigotes in a higher proportion than wild-type or TcMCU-OE cells (Fig. 3G). The TcMCU-KO metacyclic trypomastigotes were able to infect host cells. After several cycles of infection to obtain a sufficient amount of culture-derived trypomastigotes, we conducted in vitro infection assays to determine the percentage of infected cells after incubation with wild-type or TcMCU-KO trypomastigotes. Figures 3H and I show that there was no significant difference between infections with wild-type and TcMCU-KO trypomastigotes, and Fig. 3J shows that replication of amastigotes was not significantly affected by TcMCU knockout, demonstrating that TcMCU is not essential for differentiation, infectivity, or intracellular replication. PCR analysis of the TcMCU-KO trypomastigotes obtained from these tissue cultures confirmed the absence of TcMCU in these cells (Fig. S3C). In contrast, induction of metacyclogenesis in TcMCUb-OE cells was similar to that of wild-type cells but greatly diminished in TcMCUb-KO cells (Fig. 3G). Several attempts (n = 4) to infect tissue culture cells with TcMCUb-KO differentiated cells failed to recover significant amounts of trypomastigotes, suggesting that TcMCUb is important for infectivity.
Complementation of the KO mutants.
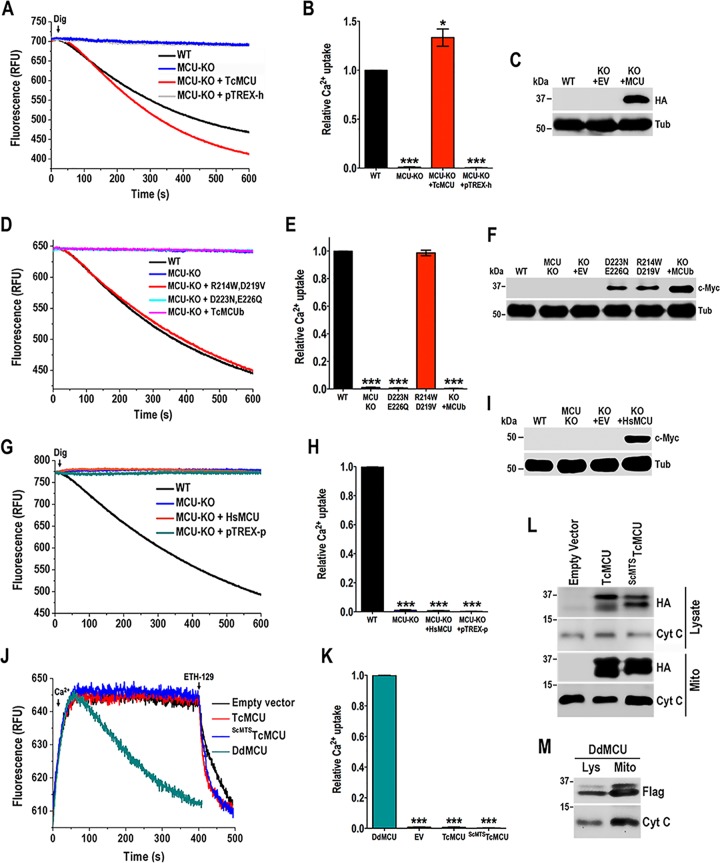
To exclude the possibility that the TcMCU silencing phenotype is due to off-target effects, we investigated whether an exogenous TcMCU gene could complement the ablation of endogenous TcMCU. First, we demonstrated that the exogenous TcMCU (with changes in the PAM sequence to prevent disruption by CRISPR/Cas9), bearing a hemagglutinin (HA) epitope tag (TcMCU-PAM-HA), was targeted to the epimastigote mitochondrion. Immunofluorescence assays in Fig. S4A show that exogenous TcMCU-HA colocalizes with MitoTracker in the mitochondria. Then, we observed that mitochondrial Ca2+ transport in permeabilized TcMCU-KO epimastigotes expressing exogenous TcMCU-HA was significantly higher than that of wild-type cells (Fig. 4A and B). The expression of TcMCU-HA was confirmed by Western blot analysis (Fig. 4C). Then, we introduced mutations in highly conserved residues to evaluate their ability to restore Ca2+ transport in TcMCU-KO cells (Fig. S4B). Complementation with a TcMCUR214W,D219V mutant, but not with the TcMCUD223N,E226Q mutant of the DIME region of TcMCU, was able to restore mitochondrial Ca2+ uptake (Fig. 4D and E), indicating the importance of Asp223 and Glu226 of the DIME domain for Ca2+ transport. TcMCU-KO epimastigotes overexpressing TcMCUb failed to rescue Ca2+ uptake, suggesting that TcMCUb cannot replace TcMCU. Western blot analysis confirmed the expression of these proteins (Fig. 4F). We also performed the complementation of TcMCU-KO cells with human MCU (Homo sapiens MCU [HsMCU]), and we found that it was not effective in restoring mitochondrial Ca2+ transport (Fig. 4G and H), although the protein colocalized with MitoTracker in the mitochondria of epimastigotes (Fig. S4C) and Western blot analysis confirmed its expression (Fig. 4I).
FIG 4 .
Complementation of the endogenous TcMCU and reconstitution of Ca2+ transport in yeast. (A) Ca2+ uptake reconstitution in digitonin-permeabilized TcMCU-KO epimastigotes transfected with pTREX-h and pTREX-h-TcMCU-PAM-HA (TcMCU). Experimental conditions were as in Fig. 1I. (B) Quantification of data in panel A. Relative Ca2+ uptake at 600 s compared with WT. (C) Western blot analysis of total protein extracts of wild-type (WT), TcMCU-KO plus pTREX-h empty vector (KO + EV), and TcMCU-KO plus TcMCU-PAM-HA (KO + MCU) epimastigotes, using anti-HA antibodies. Anti-α-tubulin antibodies were used as a loading control. (D) Ca2+ uptake reconstitution in digitonin-permeabilized TcMCU-KO epimastigotes transfected with TcMCUR214W,D219V (MCU-KO + R214W,D219V), TcMCUD223N,E226Q (MCU-KO + D223N,E226Q), or TcMCUb (MCU-KO + TcMCUb). Experimental conditions were as in Fig. 1I. (E) Quantification of data in panel D. Relative Ca2+ uptake at 600 s compared with WT (TcMCUR214W,D219V [shown as R214W,D219V] and TcMCUD223N,E226Q [shown as D223N,E226Q]). (F) Western blot analysis of total protein extracts of wild-type (WT), TcMCU-KO (MCU-KO), TcMCU-KO plus pTREX-h empty vector (KO + EV), TcMCUD223N,E226Q (D223N,E226Q), TcMCUR214W,D219V (R214W,D219V), and TcMCUb (KO + MCUb) epimastigotes, using anti-c-Myc antibodies. Anti-α-tubulin antibodies were used as a loading control. (G) Ca2+ uptake reconstitution in digitonin-permeabilized TcMCU-KO epimastigotes transfected with HsMCU or pTREX-p. Experimental conditions were as in Fig. 1I. (H) Quantification of data in panel G. Relative Ca2+ uptake at 600 s compared with WT. (I) Western blot analysis of total protein extracts of WT, TcMCU-KO (MCU-KO), TcMCU-KO plus pTREX-p empty vector (KO + EV), and TcMCU-KO plus HsMCU (KO + HsMCU) epimastigotes, using anti-c-Myc antibodies. Anti-α-tubulin antibodies were used as a loading control. (J) Ca2+ uptake by spheroplasts from yeast transformed with pACT2 empty vector, TcMCU, ScMTSTcMCU, or DdMCU. Experimental conditions were as described in Materials and Methods. (K) Quantification of data in panel J. Relative Ca2+ uptake at 350 s compared with DdMCU. (L) Western blot analysis of lysates and mitochondrial fractions of yeast complemented with empty vector, TcMCU-HA (TcMCU), or ScMTSTcMCU-HA (ScMTSTcMCU) using anti-HA antibodies. Anti-cytochrome c antibodies were used as a loading control. (M) Western blot analysis of lysates and mitochondrial fractions of yeast complemented with DdMCU-Flag (DdMCU) using anti-Flag antibodies. Anti-cytochrome c antibodies were used as a loading control. Values in panels B, E, H, and K are means ± SD (n = 3). *, P < 0.05; ***, P < 0.001.
Mitochondrial localization of TcMCU-HA and HsMCU-3×c-Myc in epimastigotes. (A) Fluorescence microscopy of exogenous TcMCU-HA expressed in TcMCU-KO epimastigotes using antibodies against HA (green) and MitoTracker as a mitochondrial marker (red). Nuclei and kinetoplast were labeled with DAPI (blue). Colocalization is shown in yellow (merge). (B) Schematic representation of MCU subunit domains. Sequence alignment of the DIME motif and flanking residues from MCU and MCUb. Numbers of critical conserved and amino acid substitutions are shown. TM, transmembrane domain; DIME, functional DIME motif. (C) Colocalization of HsMCU-3×c-Myc with MitoTracker using antibodies to c-Myc. Bars in panels A and C, 10 µm. Download FIG S4, TIF file, 1.6 MB (1.7MB, tif) .
Copyright © 2017 Chiurillo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Reconstitution of the mitochondrial calcium uniporter in Saccharomyces cerevisiae.
Saccharomyces cerevisiae does not possess an MCU, which makes it an ideal in vivo reconstitution system for studying the uniporter in a physiologically relevant organellar membrane (5, 19). Because the minimal components sufficient for in vivo MCU activity in trypanosomatids are unknown, we transformed S. cerevisiae with TcMCU to assess whether this protein is able to reconstitute yeast mitochondrial calcium uniporter activity on its own. We transformed yeast with MCU from Dictyostelium discoideum (DdMCU) to use as a positive control, as this protein was reported to be sufficient alone to reconstitute mitochondrial calcium uniporter activity in yeast (19). To ensure expression of TcMCU in yeast mitochondria, we created a chimeric gene, consisting of the mitochondrial targeting sequence (MTS) of yeast cytochrome oxidase 4 (ScCox4p) fused to TcMCU lacking its predicted MTS (ScMTSTcMCU) to obtain the expression of TcMCU in the yeast mitochondrial inner membrane. Extramitochondrial calcium levels were monitored with calcium green-5N, and we observed that mitochondria of yeast spheroplasts were able to take up Ca2+ in the presence of mitochondrial substrates (5 mM succinate, 5 mM malate, 5 mM pyruvate, 5 mM α-ketoglutarate, and 1 mM glutamate) only when complemented with DdMCU (Fig. 4J and K). However, neither TcMCU- nor ScMTSTcMCU-transformed cells were able to take up Ca2+ under the experimental conditions tested, although the cells were able to take up Ca2+ after addition of the membrane-potential-dependent calcium ionophore ETH-129 (19) (Fig. 4J). The enrichment of these proteins in the mitochondrial fraction was confirmed by Western blot analysis (Fig. 4L and M). Our results suggest that TcMCU alone is unable to reconstitute mitochondrial Ca2+ uptake in yeast.
Respiration of TcMCU-KO and TcMCUb-KO mutants.
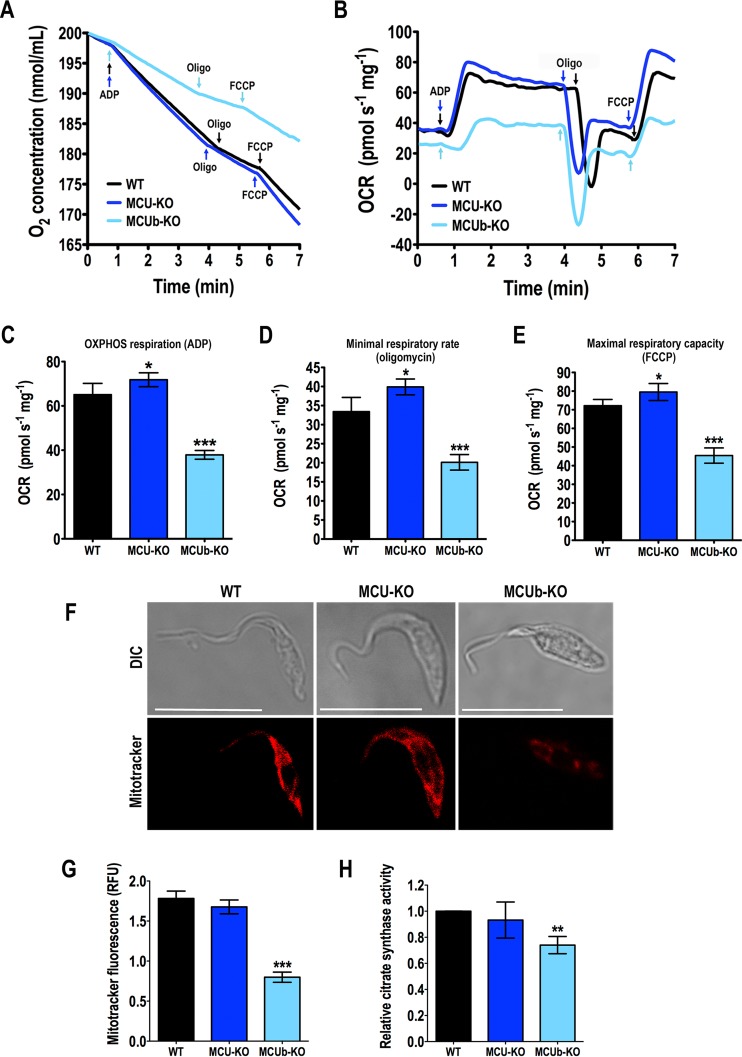
We measured oxygen uptake (Fig. 5A) and oxygen consumption rate (OCR) (Fig. 5B) under basal (state 2), ADP-stimulated (state 3), oligomycin-inhibited (state 4), and FCCP-stimulated (state 3u) conditions in wild-type and mutant digitonin-permeabilized cells in the presence of succinate. Wild-type and mutant mitochondria showed well-coupled respiration, although OCRs in the presence of ADP, oligomycin, and FCCP were significantly higher in TcMCU-KO and significantly lower in TcMCUb-KO mitochondria (Fig. 5C to E). Respiratory control rates (state 3/state 4) were 1.93 ± 0.05, 1.79 ± 0.09, and 1.92 ± 0.07 for WT, TcMCU-KO, and TcMCUb-KO cells, respectively (n = 4). The lower OCR of TcMCUb-KO mitochondria correlated with lower staining of the mitochondria with MitoTracker Deep Red FM, a dye currently used to estimate mitochondrial mass (20) (Fig. 5F and G), as well as with lower activity of citrate synthase, another mitochondrial mass indicator (21) (Fig. 5H), suggesting a lower mitochondrial mass in these cells.
FIG 5 .
Mitochondrial changes in mutant epimastigotes. (A and B) Representative traces of oxygen uptake (A) and oxygen consumption rate (OCR) (B) by digitonin-permeabilized wild-type (WT), TcMCU-KO (MCU-KO), and TcMCUb-KO (MCUb-KO) epimastigotes. Additions were 100 μM ADP, 1 µg/ml oligomycin (Oligo), and 1.0 µM FCCP. (C to E) OCR after addition of ADP (respiration stimulated by oxidative phosphorylation [OXPHOS]), oligomycin (minimal respiratory rate), and FCCP (maximal respiratory capacity), respectively (n = 4), using the test system as in panels A and B. (F) Fluorescence microscopy of representative wild-type (WT), TcMCU-KO (MCU-KO), and TcMCUb-KO (MCUb-KO) epimastigotes labeled with MitoTracker Deep Red FM for estimation of mitochondrial mass. (G) Quantification of data in panel F. Relative fluorescence of MitoTracker Deep Red FM-labeled cells. Over 200 cells from three experiments with 20 random fields/experiment were analyzed (n = 3). (H) Relative citrate synthase activity of wild-type and mutant parasites, measured for enzymatic estimation of mitochondrial mass, considering Vmax values of WT to be 1.0 (n = 5). Values in panels C to E, G, and H are means ± SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Autophagy in TcMCUb-KO cells.
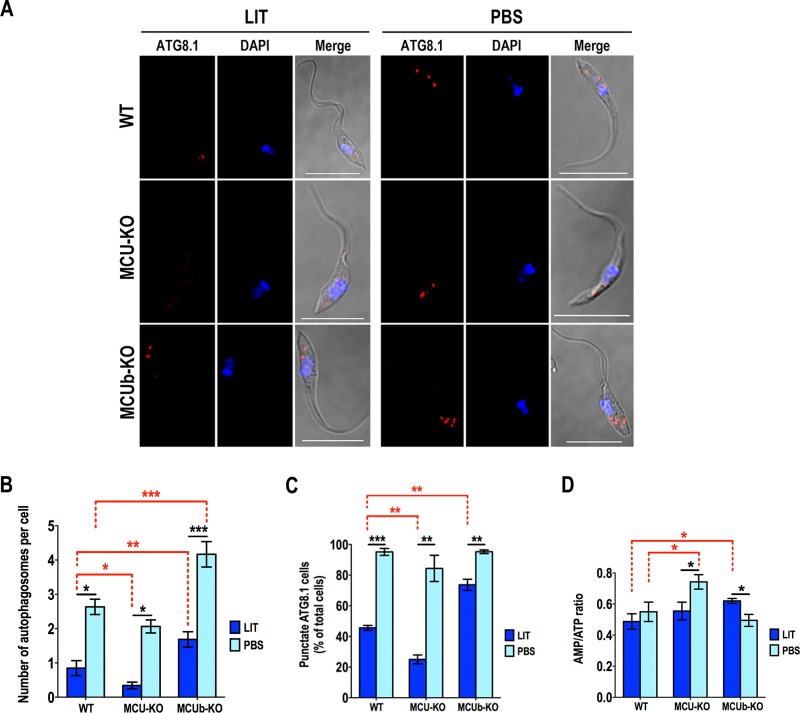
Autophagy has been reported as a starvation response in different trypanosomatids (22, 23). In addition, block of mitochondrial Ca2+ uptake was shown to increase autophagy as a survival mechanism (24, 25). Huang et al. (10) recently showed that knockdown of MCU in T. brucei procyclic forms increased the expression of autophagy markers under starvation condition. We evaluated autophagy in these cells by immunofluorescence microscopy (Fig. 6A) using antibodies against the autophagy marker Atg8.1, which is the ortholog of LC3-II in mammalian cells (26). Then, we quantified the number of autophagosomes per cell (Fig. 6B) and the number of cells containing autophagosomes (Fig. 6C). TcMCUb-KO cells had significantly increased numbers of autophagosomes per cell when incubated under starvation conditions, while lower levels were found in TcMCU-KO cells than in wild-type cells (Fig. 6A to C). Moreover, TcMCUb-KO cells showed an increased number of autophagosomes/cell and a higher percentage of cells with autophagosomes even when cultured in rich medium. It has been proposed that in vertebrate cells a lower mitochondrial Ca2+ transport capacity leads to an increase of the AMP/ATP ratio and stimulation of the AMP-dependent protein kinase (AMPK) signaling axis that stimulates autophagy (24). However, knockout of both TcMCU and TcMCUb abolished mitochondrial Ca2+ transport while only TcMCUb-KO cells showed increased autophagy. In agreement with these results, there was no correlation between the AMP/ATP ratio of these cells (Fig. 6D) and autophagy (Fig. 6A to C), ruling out this potential mechanism of autophagy stimulation in T. cruzi.
FIG 6 .
Autophagy changes in mutant epimastigotes. (A) Representative fluorescence microscopy images of wild-type (WT), TcMCU-KO (MCU-KO), and TcMCUb-KO (MCUb-KO) epimastigotes labeled with anti-TcATG8.1 antibody (red) after incubation in LIT medium or PBS for 16 h. DAPI staining (blue) and merged images on a differential interference contrast (DIC) background (Merge) are also shown. Bars, 10 µm. (B) Number of autophagosomes per cell under different conditions shown in panel A. (C) Percentage of cells with autophagosomes under conditions shown in panel A. (D) Comparison of AMP/ATP ratios between WT, MCU-KO, and MCUb-KO epimastigotes incubated in LIT medium or PBS for 16 h. For panels B to D, over 200 cells from wild-type (WT), TcMCU-KO (MCU-KO), and TcMCUb-KO (MCUb-KO) epimastigotes from three experiments with 20 random fields/experiment were analyzed. Values in panels B to D are means ± SD (n = 3). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
DISCUSSION
Our studies have shown that the mitochondrial calcium uniporter (TcMCU) is not essential for T. cruzi epimastigote growth in rich medium, for differentiation (metacyclogenesis) under well-established starvation conditions, and for trypomastigote host cell infection and intracellular replication, although it is needed for optimal exponential growth of epimastigotes. No mitochondrial Ca2+ transport was detected in TcMCU-KO epimastigotes, while the mitochondrial membrane potential (ΔΨm) was unaffected in these cells. In contrast to these results, TcMCUb was required for optimal growth in rich medium, for metacyclogenesis under starvation conditions, and for trypomastigote host cell infection. However, as occurs with TcMCU-KO cells, mitochondria of TcMCUb-KO epimastigotes were unable to transport Ca2+ while their ΔΨm was not altered. Additional differences between these KO cells were also found. TcMCUb-KO cells had lower respiratory activity, a higher AMP/ATP ratio in rich medium, reduced mitochondrial mass, and increased autophagy, suggesting that MCUb has additional bioenergetic roles independent of Ca2+ transport. Overexpression of TcMCUb or TcMCU resulted in higher rates of Ca2+ transport, no effects on ΔΨm, and no dominant negative effect of TcMCUb on mitochondrial Ca2+ transport, in contrast to previous results described for HeLa cells (13). The two proteins interact, as shown by their coimmunoprecipitation.
MICU1 has been shown to have a gatekeeping effect on MCU (27, 28), and the increased matrix Ca2+ observed after overexpression of TcMCU could be the result of a change in the stoichiometry of MCU/MICU1. A similar situation could be occurring in TcMCUb-overexpressing cells.
The lack of essentiality of MCU in epimastigotes grown in rich medium and their increased survival in stationary phase under low-glucose conditions is interesting. It has been previously reported that epimastigotes use glucose from the medium during their exponential phase of growth and that once glucose is depleted and cells enter the stationary phase, they use tricarboxylic acid (TCA) cycle intermediates (29). All the main stages of T. cruzi have a high endogenous rate of respiration, suggesting that the organisms contain an energy reserve. Early reports ruled out the presence of a carbohydrate polymer (1, 30). Oliveira et al. (31) first suggested the possibility that triglycerides could be the energy reserve, which would be similar to the situation in another stercorarian trypanosome, Trypanosoma lewisi, which also has a high endogenous rate of respiration and high triglyceride content (32). However, we found that lipid droplets increase, not decrease, in TcMCU-KO cells. As the presence of lipid droplets was evaluated at the beginning of the stationary phase, one possibility is that they could allow TcMCU-KO cells to survive longer because they were not consumed yet at that point. On the other hand, it is known that epimastigotes have a large pool of free amino acids that they use to maintain their osmolarity (33) and that protein degradation occurs under starvation conditions (1). Increased amino acid oxidation through the activity of the TCA cycle would bypass the need for the Ca2+-regulated formation of acetyl coenzyme A (CoA) from pyruvate (10). The higher ATP production via this pathway would explain the better survival of the cells when grown in low-glucose medium. The intracellular amastigotes appear to use fatty acids as an energy source, taking into account the increased levels of fatty acid oxidation enzymes in these stages (34). Amastigotes could then also bypass the need for the Ca2+-regulated pyruvate dehydrogenase to obtain energy through the TCA cycle. The predominance of fatty acid oxidation over glycolysis in the intracellular stages would explain the nonessential role of TcMCU for infection and replication.
Metacyclogenesis is usually stimulated under glucose-depleted conditions, and we detected a higher rate in TcMCU-KO cells. This higher rate of differentiation could be related to the lower ability of these cells to use endogenous substrates in low-glucose medium in contrast to TcMCU-OE cells. The increased metacyclogenesis observed in TcMCU-KO cells could also explain its longer survival during the stationary phase in low-glucose medium. On the other hand, the reduced metacyclogenesis of TcMCUb-KO cells correlated well with their inefficient ability to invade host cells.
To our knowledge, we report for the first time the complementation of a gene knockout (TcMCU-KO) obtained by CRISPR/Cas9 in T. cruzi. Complementation of TcMCU-KO cells with an exogenous copy of TcMCU but not with the gene mutated in the nucleotides encoding amino acids Asp223 and Glu226 of the DIME domain was able to restore mitochondrial Ca2+ transport, confirming that as demonstrated in mammals, these two conserved acidic residues of the DIME motif are also key for TcMCU-mediated Ca2+ uptake (8, 13). However, a mutant including a substitution at the acidic residue Asp219 (TcMCUR214W/D219V; Glu257 in HsMCU), which is highly conserved within MCU orthologs and has been proposed as a critical residue for Ca2+ transport (8, 13), rescued Ca2+ transport in TcMCU-KO epimastigotes. Similarly, a recent report showed that the HsMCUE257A mutant was able to rescue Ca2+ transport in HEK-293T MCU-KO cells, which is consistent with the predicted nuclear magnetic resonance (NMR)/electron microscopy (EM) structure of the MCU that exposes this residue outside the channel entrance (35).
Moreover, human MCU was unable to restore mitochondrial Ca2+ transport in TcMCU-KO cells. TcMCU alone, in contrast to DdMCU (19), could not reconstitute mitochondrial Ca2+ transport in yeast mitochondria. As T. cruzi lacks orthologs of EMRE (36) or MCUR1 (37, 38), this result suggests that, as in the case of human MCU (19), other, still-unknown subunits of the complex might be necessary for full reconstitution of mitochondrial Ca2+ transport in yeast by TcMCU.
An increase in autophagy was observed in TcMCUb-KO but not in TcMCU-KO cells when incubated under either rich or starvation conditions. In vertebrate cells, the lack of MCU-mediated Ca2+ uptake promotes autophagy (37, 38). An increase in the AMP/ATP ratio produced by downregulation of the TCA cycle by inhibition of the MCU could stimulate the phosphorylation of the AMP-dependent kinase (AMPK) and promote autophagy (24). However, we did not find such a correlation in T. cruzi, as TcMCU-KO cells have higher AMP/ATP ratios under starvation conditions and no difference in autophagy while TcMCUb-KO cells do not have differences in AMP/ATP ratios but exhibit higher autophagy than wild-type cells. These results suggest that this pathway might not be operative in T. cruzi and are in agreement with a reported AMPK-independent autophagy pathway reported recently in T. brucei (39).
It is possible that phenotypic changes in the TcMCUb-KO mutants may have been caused indirectly by adaptation to the loss of the gene that were not seen in the TcMCU-KO mutants because of the occurrence of more beneficial compensatory mutations in these cells. However, our attempts to complement TcMCUb-KO mutants with an exogenous TcMCUb gene devoid of the PAM motif did not result in selected parasites, probably due to the fragility of these cells and their inability to resist the electroporation stress (data not shown).
The mitochondria of TbMCU-OE epimastigotes have an increased ability for Ca2+ uptake, which results in Ca2+ overload and oxidative stress, and similar results were observed in TcMCUb-OE cells. These results agree with those obtained in HeLa cells (7) and T. brucei (10). Mitochondrial Ca2+ overload has also been proposed as the link between complement deposition and triggering of cell death in T. cruzi epimastigotes (40). Accumulation of Ca2+ in the mitochondrion leads to a decrease in cell respiration, dissipation of the inner membrane potential, and increased reactive oxygen species production (40).
In contrast with the results obtained in HeLa cells (13), where overexpression of MCUb has a dominant negative effect on mitochondrial Ca2+ transport, overexpression of TcMCUb led to increased Ca2+ uptake without affecting the mitochondrial membrane potential. Knockout of TcMCUb abolished mitochondrial Ca2+ transport, and the cells had a lower growth rate than control cells. Moreover, as mentioned above, we introduced in TcMCU amino acid substitutions (R214W/D219V) that are conserved in its paralog TcMCUb and considered critical to determine the dominant negative effect of the mammalian protein (13). However, we found that TcMCUR214W/D219V, but not TcMCUb, was able to restore mitochondrial Ca2+ transport in TcMCU-KO cells, suggesting that these substitutions are not enough to abolish Ca2+ transport.
In summary, our results indicate that TcMCU is not essential for growth, differentiation, infectivity, and intracellular replication of T. cruzi, although it could be important under stress situations, such as for growth in low-glucose medium. This could be relevant for epimastigotes grown in the insect’s gut, in which glucose is supposed to be scarce (41). In contrast to what has been described in some mammalian cells, where MCUb acts as a dominant negative subunit of the MCU complex (13), mitochondrial Ca2+ transport is enhanced in TcMCUb-OE epimastigotes and abolished in TcMCUb-KO cells. TcMCUb-KO cells have additional phenotypic alterations that suggest other bioenergetic roles besides mitochondrial Ca2+ transport.
MATERIALS AND METHODS
Culture methods.
T. cruzi epimastigotes (Y strain) were grown at 28°C in liver infusion tryptose (LIT) medium (42) (5.4 mM KCl, 150 mM NaCl, 24 mM glucose, 5% [vol/vol] liver extract, 0.02% [wt/vol] hemin, 2% [wt/vol] yeast extract, 1.5% [wt/vol] tryptose), supplemented with 10% heat-inactivated fetal bovine serum (FBS). To obtain a low-glucose LIT medium, glucose was not added. We determined the growth rate of T. cruzi epimastigotes by counting cells in a Neubauer chamber with a starting culture of 1 × 106 epimastigotes. Tissue culture cell-derived trypomastigotes were obtained from Vero cells infected with metacyclic trypomastigotes obtained as described below. T. cruzi trypomastigote forms were collected from the culture medium of infected host cells, using a modification of the method of Schmatz and Murray (43) as described previously (44). Vero cells were grown in RPMI supplemented with 10% fetal bovine serum and maintained at 37°C with 5% CO2.
Metacyclogenesis.
We followed the protocol described by Bourguignon et al. (45) with minor modifications. Epimastigotes were obtained after 4 days in LIT medium and submitted to a stress (incubation for 2 h in a medium containing 190 mM NaCl, 17 mM KCl, 2 mM MgCl2, 2 mM CaCl2, 0.035% sodium bicarbonate, 8 mM phosphate, pH 6.9, at room temperature; triatome artificial urine [TAU] medium). After this stress, parasites were incubated for 96 h in TAU 3AAG medium (which consists of the above-described TAU medium supplemented with 10 mM l-proline, 50 mM sodium l-glutamate, 2 mM sodium l-aspartate, and 10 mM glucose). To increase the number of metacyclic forms to infect Vero cells, the contents of the flask were collected and resuspended in medium containing fresh fetal bovine serum and incubated at 37°C for 20 h. The complement in the FBS kills epimastigotes while metacyclic trypomastigotes survive. Samples were harvested from the TAU 3AAG plus FBS-containing medium at days 5 and 10 of cultivation.
In vitro infection assay.
Gamma-irradiated (2,000-rad) Vero cells (4.5 × 105 cells) were plated onto sterile coverslips in a 12-well plate and incubated overnight at 35°C, 7% CO2, in RPMI medium plus 10% fresh fetal bovine serum. Tissue culture-derived trypomastigote collections were incubated at 4°C overnight to allow amastigotes to settle from swimming trypomastigotes. Trypomastigotes from the supernatants of these collections were counted and used to infect the coverslips at a ratio of 50 parasites to 1 host cell. At 4 h postinfection, coverslips were washed extensively with Dulbecco’s Hanks’ solution, followed by phosphate-buffered saline (PBS), pH 7.4, to remove any extracellular parasites. Coverslips were fixed immediately in 4% paraformaldehyde in PBS, pH 7.4, at 4°C for 30 min. Coverslips were washed once with PBS and mounted onto glass slides in Fluoromount G containing 15 µg/ml of 2-(4-aminophenyl)-1H-indole-6-carboxamidine (DAPI), which stains host and parasite DNA. Coverslips were viewed on an Olympus BX60 microscope to quantify the number of host cells that contained intracellular parasites and the number of intracellular parasites per cell in randomly selected fields. Three hundred host cells were counted per sample in three independent experiments. To quantify amastigote replication, the following modifications were used: host cells were infected at a ratio of 10 parasites to 1 host cell, and coverslips were allowed to incubate for 48 h postinfection at 35°C, 7% CO2, prior to fixation and DAPI staining.
TcMCU-KO.
Chimera single guide RNA (sgRNA) sequences to target the TcMCU gene (TryTripDB identifier [ID] TcCLB.503893.120) were PCR amplified from plasmid pUC_sgRNA, containing the sgRNA backbone sequence (82 bp) (46). One specific protospacer was included in the forward primer (primer 1 [see Table S1 in the supplemental material]) while using a common reverse primer (primer 2 [Table S1]) for sgRNA amplification. These primers also contained a BamHI restriction site for cloning into Cas9/pTREX-n (14) upstream of the HX1 transsplicing signal (47) to generate the TcMCU-sgRNA/Cas9/pTREX-n construct. The sgRNA orientation was verified by PCR using the specific TcMCU-sgRNA forward primer and the HX1 reverse primer (14), which annealed on the HX1 region of the vector (primer 3 [Table S1]). Positive clones that generate a 190-bp PCR fragment were also sequenced. A scrambled sgRNA (Sc-sgRNA/Cas9/pTREX-n), obtained with primers 2 and 4 (Table S1), was used as a control. The Cas9/pTREX-n vector contains the Streptococcus pyogenes Cas9 sequence with a twice-repeated simian virus 40 (SV40) nuclear localization signal (2×NLS) and green fluorescent protein (GFP) (14), thus generating mutant cell lines with green fluorescent nuclei after transfection. The molecular construct (DNA donor) to promote homologous directed repair and ablation of the target gene after the double-strand break (DSB) induced by Cas9 was obtained using a recombinant PCR strategy. Briefly, the 5′ and 3′ UTRs of the TcMCU gene (490 and 529 bp, respectively) were amplified by PCR using T. cruzi genomic DNA as the template (primers 7 to 10 [Table S1]). The open reading frame (ORF) of the blasticidin S-deaminase gene (Bsd) was also amplified by PCR from pTREX-b vector as the template using primers containing 20 nucleotides (nt) overlapping with the 5′ and 3′ flanking sequences of the TcMCU gene (primers 5 and 6 [Table S1]). The three PCR fragments (5′-TcMCU flanking sequence, blasticidin-resistant gene, and 3′-TcMCU flanking sequence) were linked together by sequential PCR using primers 7 and 10 (Table S1). The final PCR product was cloned into pGEM-T Easy vector (Promega). After sequencing verification of positive clones, circular constructs TcMCU-sgRNA/Cas9/pTREX-n and the Bsd cassette in pGEM-T Easy were used to cotransfect T. cruzi epimastigotes. After 5 weeks of selection with 250 µg/ml G418 and 10 µg/ml blasticidin, TcMCU gene ablation was verified by PCR using primers 11 and 12 (Table S1). Alternatively, to confirm TcMCU gene KO in trypomastigotes obtained from infected cells, we amplified the entire TcMCU ORF (909 bp) by PCR (primers 15 and 19 [Table S1]).
TcMCUb-KO.
An sgRNA to target the sequence coding for the hypothetical TcMCUb protein (TryTripDB ID TcCLB.416883.9) was amplified by PCR (primers 31 and 2 [Table S1]). Following the same strategy mentioned above for TcMCU, we obtained the TcMCUb-sgRNA/Cas9/pTREX-n construct. The DNA donor cassette designed to promote homologous directed repair and disruption of TcMCUb was obtained by PCR using a set of long primers (ultramers) containing 120 nucleotides, from which 100 nucleotides correspond to the region from nt −20 to +80 (forward ultramer) and nt +510 to +610 (reverse ultramer) of the TcMCUb ORF. Each one of the ultramers had 20 nt annealing on the Bsd gene (primers 32 and 33 [Table S1]). Gene disruption of TcMCUb was verified by PCR using primers 34 and 35 (Table S1).
Generation of TcMCU/TcMCUb-overexpressing cell lines in T. cruzi.
For gain-of-function analysis of TcMCU/TcMCUb, T. cruzi epimastigotes were transfected with the trypanosome expression vector pTREX-n (47, 48) containing the corresponding genes. The molecular construct for TcMCU overexpression was made as follows: the full sequence of TcMCU was PCR amplified using primers 15 and 16 (Table S1) and T. cruzi gDNA as the template. The purified fragment of 909 bp was digested with EcoRI and HindIII restriction enzymes and cloned into the pTREX-n vector linearized with the same enzymes. The molecular construct for TcMCUb overexpression was made as follows: the full sequence of TcMCUb was PCR amplified using primers 38 and 39 (Table S1) and T. cruzi gDNA as the template. For this purpose, we used the genomic sequence (KB205522 from nucleotide 139,788 to 140,579, TryTripDB) from T. cruzi strain Esmeraldo to design the reverse primer, because the MCUb gene 3′ end and 3′ UTRs are missing in the TcCLB.416883.9 sequence. The purified fragment of 792 bp was digested with XbaI and SalI restriction enzymes to be cloned into a version of the pTREX-n vector that includes a 3×HA epitope tag coding sequence. The pTREX-n vector was linearized with XbaI and XhoI enzymes, and the TcMCUb gene was cloned in frame with the 3×HA sequence for C-terminal tagging, allowing the detection of the overexpressed protein with anti-HA antibody. For this purpose, we previously cloned the 3×HA tag sequence, excised from pMOTag-4H vector (49) with XhoI and SalI enzymes, into pTREX-n by using the XhoI restriction site.
Molecular constructs to complement TcMCU-KO mutants.
To revert the phenotype exhibited by TcMCU-knockout epimastigotes, we obtained the TcMCU-PAM-HA construct to reconstitute the ablated TcMCU gene. Following a two-step-PCR strategy, we eliminated the PAM sequence (CGG to CCT) specific for the TcMCU-sgRNA used to obtain the TcMCU-KO cells, therefore avoiding constitutively expressed Cas9 to target the inserted sequence (Table S1, primers 15 and 17 to 19). The fragment obtained by overlap extension PCR was digested with EcoRI and HindIII and then cloned into pTREX-h (which confers hygromycin resistance) digested with the same enzymes. We also included a C-terminal HA tag in order to detect the overexpressed protein using anti-HA antibody.
Furthermore, we attempted to reconstitute the ablated TcMCU gene in TcMCU-KO mutants with the following. For TcMCUb, primers 38 and 39 (see Table S1 in the supplemental material) were used to amplify from T. cruzi gDNA the TcMCUb gene that was digested with XbaI and SalI enzymes and cloned in frame with the 3×c-Myc tag sequence into pTREX-h digested with XbaI and XhoI. For TcMCUD223N,E226Q and TcMCUR14W,D219N, the generation of pTREX-h-TcMCUD223N,E226Q-3×c-Myc was done by overlap extension PCR using the TcMCU-PAM-HA plasmid as a template and primers 20 to 23 (Table S1). Following a similar strategy, the pTREX-h-TcMCUR14W,D219N-3×c-Myc was obtained using primers 20, 21, 24, and 25 (Table S1). For the human MCU gene, we purchased from GenScript (USA) a cDNA clone corresponding to the Homo sapiens MCU variant 1 (GenBank accession number NM_138357) of 1,100 bp. The HsMCU fragment amplified with primers 26 and 27 (Table S1) was digested and cloned into pTREX-p (which confers puromycin resistance) by EcoRI and XhoI restriction sites in frame with the 3×c-Myc tag sequence.
For this purpose, we first generated pTREX-h and pTREX-p vectors that were not available (Table S1, primers 40 to 48). As templates to amplify GAPDH UTRs and hygromycin and puromycin genes, we used pTREX-n, pMOTag-4H (49), and pMOTag-23M (49) plasmids, respectively. The 3×c-Myc tag was excised from the pMOTag-23M vector (49) with XhoI and SalI enzymes and subsequently cloned into pTREX-h and pTREX-p by the XhoI restriction site.
Statistical analysis.
Values indicated in the figure legends are means ± standard deviations (SD) of n biological experiments (n is indicated in the figure legends), and the significance of their differences was evaluated by the Student t test.
Supplemental methods. Download TEXT S1, DOCX file, 0.1 MB (100.9KB, docx) .
Copyright © 2017 Chiurillo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Full blots shown in the article. Download FIG S5, TIF file, 2.6 MB (2.6MB, tif) .
Copyright © 2017 Chiurillo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
ACKNOWLEDGMENTS
We declare that we have no competing financial interests.
The funding agencies had no role in the study design, data collection and interpretation, or the decision to submit the work for publication. Opinions contained in this publication do not reflect the opinions of the funding agencies.
We thank Vanina Alvarez, and we thank Minu Chauduri for antibodies, Vamsi Mootha for DdMCU and the yeast strain, and the staff of the Life Sciences Core Facility (LaCTAD) at the State University of Campinas for the assistance in the acquisition of the confocal microscopy images. We also thank Guozhong Huang for technical advice with yeast manipulation and Roger Castilho and Tiago Figueira for the advice on experiments with membrane potential and respiration.
Funding for this work was provided by the São Paulo Research Foundation (FAPESP), Brazil (2013/50624-0), and the U.S. National Institutes of Health (grant AI107663). N.L. and M.A.C. are postdoctoral fellows of FAPESP (grants 2014/08995-4 and 2014/13148-9, respectively). M.S.B. is a Master’s fellow of FAPESP (grant 2015/25709-8).
Footnotes
Citation Chiurillo MA, Lander N, Bertolini MS, Storey M, Vercesi AE, Docampo R. 2017. Different roles of mitochondrial calcium uniporter complex subunits in growth and infectivity of Trypanosoma cruzi. mBio 8:e00574-17. https://doi.org/10.1128/mBio.00574-17.
REFERENCES
- 1.Rogerson GW, Gutteridge WE. 1979. Oxidative metabolism in mammalian and culture forms of Trypanosoma cruzi. Int J Biochem 10:1019–1023. doi: 10.1016/0020-711X(79)90083-1. [DOI] [PubMed] [Google Scholar]
- 2.Rogerson GW, Gutteridge WE. 1980. Catabolic metabolism in Trypanosoma cruzi. Int J Parasitol 10:131–135. doi: 10.1016/0020-7519(80)90024-7. [DOI] [PubMed] [Google Scholar]
- 3.Docampo R, Vercesi AE. 1989. Characteristics of Ca2+ transport by Trypanosoma cruzi mitochondria in situ. Arch Biochem Biophys 272:122–129. doi: 10.1016/0003-9861(89)90202-6. [DOI] [PubMed] [Google Scholar]
- 4.Docampo R, Vercesi AE. 1989. Ca2+ transport by coupled Trypanosoma cruzi mitochondria in situ. J Biol Chem 264:108–111. [PubMed] [Google Scholar]
- 5.Carafoli E, Balcavage WX, Lehninger AL, Mattoon JR. 1970. Ca2+ metabolism in yeast cells and mitochondria. Biochim Biophys Acta 205:18–26. doi: 10.1016/0005-2728(70)90057-5. [DOI] [PubMed] [Google Scholar]
- 6.Perocchi F, Gohil VM, Girgis HS, Bao XR, McCombs JE, Palmer AE, Mootha VK. 2010. MICU1 encodes a mitochondrial EF hand protein required for Ca2+ uptake. Nature 467:291–296. doi: 10.1038/nature09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Stefani D, Raffaello A, Teardo E, Szabò I, Rizzuto R. 2011. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, Koteliansky V, Mootha VK. 2011. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Docampo R, Lukeš J. 2012. Trypanosomes and the solution to a 50-year mitochondrial calcium mystery. Trends Parasitol 28:31–37. doi: 10.1016/j.pt.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang G, Vercesi AE, Docampo R. 2013. Essential regulation of cell bioenergetics in Trypanosoma brucei by the mitochondrial calcium uniporter. Nat Commun 4:2865. doi: 10.1038/ncomms3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamer KJ, Mootha VK. 2015. The molecular era of the mitochondrial calcium uniporter. Nat Rev Mol Cell Biol 16:545–553. doi: 10.1038/nrm4039. [DOI] [PubMed] [Google Scholar]
- 12.De Stefani D, Rizzuto R, Pozzan T. 2016. Enjoy the trip: calcium in mitochondria back and forth. Annu Rev Biochem 85:161–192. doi: 10.1146/annurev-biochem-060614-034216. [DOI] [PubMed] [Google Scholar]
- 13.Raffaello A, De Stefani D, Sabbadin D, Teardo E, Merli G, Picard A, Checchetto V, Moro S, Szabò I, Rizzuto R. 2013. The mitochondrial calcium uniporter is a multimer that can include a dominant-negative pore-forming subunit. EMBO J 32:2362–2376. doi: 10.1038/emboj.2013.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lander N, Li ZH, Niyogi S, Docampo R. 2015. CRISPR/Cas9-induced disruption of paraflagellar rod protein 1 and 2 genes in Trypanosoma cruzi reveals their role in flagellar attachment. mBio 6:e01012. doi: 10.1128/mBio.01012-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaudhuri D, Sancak Y, Mootha VK, Clapham DE. 2013. MCU encodes the pore conducting mitochondrial calcium currents. Elife 2:e00704. doi: 10.7554/eLife.00704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Docampo R, Vercesi AE, Huang G. 2014. Mitochondrial calcium transport in trypanosomes. Mol Biochem Parasitol 196:108–116. doi: 10.1016/j.molbiopara.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lander N, Chiurillo MA, Storey M, Vercesi AE, Docampo R. 2016. CRISPR/Cas9-mediated endogenous C-terminal tagging of Trypanosoma cruzi genes reveals the acidocalcisome localization of the inositol 1,4,5-trisphosphate receptor. J Biol Chem 291:25505–25515. doi: 10.1074/jbc.M116.749655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vercesi AE, Bernardes CF, Hoffmann ME, Gadelha FR, Docampo R. 1991. Digitonin permeabilization does not affect mitochondrial function and allows the determination of the mitochondrial membrane potential of Trypanosoma cruzi in situ. J Biol Chem 266:14431–14434. [PubMed] [Google Scholar]
- 19.Kovács-Bogdán E, Sancak Y, Kamer KJ, Plovanich M, Jambhekar A, Huber RJ, Myre MA, Blower MD, Mootha VK. 2014. Reconstitution of the mitochondrial calcium uniporter in yeast. Proc Natl Acad Sci U S A 111:8985–8990. doi: 10.1073/pnas.1400514111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cottet-Rousselle C, Ronot X, Leverve X, Mayol JF. 2011. Cytometric assessment of mitochondria using fluorescent probes. Cytometry 79:405–425. doi: 10.1002/cyto.a.21061. [DOI] [PubMed] [Google Scholar]
- 21.Wredenberg A, Wibom R, Wilhelmsson H, Graff C, Wiener HH, Burden SJ, Oldfors A, Westerblad H, Larsson NG. 2002. Increased mitochondrial mass in mitochondrial myopathy mice. Proc Natl Acad Sci U S A 99:15066–15071. doi: 10.1073/pnas.232591499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alvarez VE, Kosec G, Sant’Anna C, Turk V, Cazzulo JJ, Turk B. 2008. Autophagy is involved in nutritional stress response and differentiation in Trypanosoma cruzi. J Biol Chem 283:3454–3464. doi: 10.1074/jbc.M708474200. [DOI] [PubMed] [Google Scholar]
- 23.Brennand A, Rico E, Michels PA. 2012. Autophagy in trypanosomatids. Cells 1:346–371. doi: 10.3390/cells1030346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cárdenas C, Miller RA, Smith I, Bui T, Molgó J, Müller M, Vais H, Cheung KH, Yang J, Parker I, Thompson CB, Birnbaum MJ, Hallows KR, Foskett JK. 2010. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell 142:270–283. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smaili SS, Pereira GJ, Costa MM, Rocha KK, Rodrigues L, do Carmo LG, Hirata H, Hsu YT. 2013. The role of calcium stores in apoptosis and autophagy. Curr Mol Med 13:252–265. doi: 10.2174/1566524011313020003. [DOI] [PubMed] [Google Scholar]
- 26.Li FJ, Shen Q, Wang C, Sun Y, Yuan AY, He CY. 2012. A role of autophagy in Trypanosoma brucei cell death. Cell Microbiol 14:1242–1256. doi: 10.1111/j.1462-5822.2012.01795.x. [DOI] [PubMed] [Google Scholar]
- 27.Mallilankaraman K, Doonan P, Cárdenas C, Chandramoorthy HC, Müller M, Miller R, Hoffman NE, Gandhirajan RK, Molgó J, Birnbaum MJ, Rothberg BS, Mak DO, Foskett JK, Madesh M. 2012. MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca2+ uptake that regulates cell survival. Cell 151:630–644. doi: 10.1016/j.cell.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Csordás G, Golenár T, Seifert EL, Kamer KJ, Sancak Y, Perocchi F, Moffat C, Weaver D, de la Fuente Perez S, Bogorad R, Koteliansky V, Adijanto J, Mootha VK, Hajnóczky G. 2013. MICU1 controls both the threshold and cooperative activation of the mitochondrial Ca2+ uniporter. Cell Metab 17:976–987. doi: 10.1016/j.cmet.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Docampo R, de Boiso JF, Stoppani AO. 1978. Tricarboxylic acid cycle operation at the kinetoplast-mitochondrion complex of Trypanosoma cruzi. Biochim Biophys Acta 502:466–476. doi: 10.1016/0005-2728(78)90079-8. [DOI] [PubMed] [Google Scholar]
- 30.Ryley JF. 1956. Studies on the metabolism of the Protozoa. 7. Comparative carbohydrate metabolism of eleven species of trypanosome. Biochem J 62:215–222. doi: 10.1042/bj0620215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oliveira MM, Timm SL, Costa SC. 1977. Lipid composition of Trypanosoma cruzi. Comp Biochem Physiol B 58:195–199. doi: 10.1016/0305-0491(77)90109-2. [DOI] [PubMed] [Google Scholar]
- 32.Dixon H, Williamson J. 1970. The lipid composition of blood and culture forms of Trypanosoma lewisi and Trypanosoma rhodesiense compared with that of their environment. Comp Biochem Physiol 33:111–128. doi: 10.1016/0010-406X(70)90487-1. [DOI] [PubMed] [Google Scholar]
- 33.Rohloff P, Rodrigues CO, Docampo R. 2003. Regulatory volume decrease in Trypanosoma cruzi involves amino acid efflux and changes in intracellular calcium. Mol Biochem Parasitol 126:219–230. doi: 10.1016/S0166-6851(02)00277-3. [DOI] [PubMed] [Google Scholar]
- 34.Atwood JA III, Weatherly DB, Minning TA, Bundy B, Cavola C, Opperdoes FR, Orlando R, Tarleton RL. 2005. The Trypanosoma cruzi proteome. Science 309:473–476. doi: 10.1126/science.1110289. [DOI] [PubMed] [Google Scholar]
- 35.Oxenoid K, Dong Y, Cao C, Cui T, Sancak Y, Markhard AL, Grabarek Z, Kong L, Liu Z, Ouyang B, Cong Y, Mootha VK, Chou JJ. 2016. Architecture of the mitochondrial calcium uniporter. Nature 533:269–273. doi: 10.1038/nature17656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sancak Y, Markhard AL, Kitami T, Kovács-Bogdán E, Kamer KJ, Udeshi ND, Carr SA, Chaudhuri D, Clapham DE, Li AA, Calvo SE, Goldberger O, Mootha VK. 2013. EMRE is an essential component of the mitochondrial calcium uniporter complex. Science 342:1379–1382. doi: 10.1126/science.1242993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mallilankaraman K, Cárdenas C, Doonan PJ, Chandramoorthy HC, Irrinki KM, Golenár T, Csordás G, Madireddi P, Yang J, Müller M, Miller R, Kolesar JE, Molgó J, Kaufman B, Hajnóczky G, Foskett JK, Madesh M. 2012. MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism. Nat Cell Biol 14:1336–1343. doi: 10.1038/ncb2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomar D, Dong Z, Shanmughapriya S, Koch DA, Thomas T, Hoffman NE, Timbalia SA, Goldman SJ, Breves SL, Corbally DP, Nemani N, Fairweather JP, Cutri AR, Zhang X, Song J, Jaña F, Huang J, Barrero C, Rabinowitz JE, Luongo TS, Schumacher SM, Rockman ME, Dietrich A, Merali S, Caplan J, Stathopulos P, Ahima RS, Cheung JY, Houser SR, Koch WJ, Patel V, Gohil VM, Elrod JW, Rajan S, Madesh M. 2016. MCUR1 is a scaffold factor for the MCU complex function and promotes mitochondrial bioenergetics. Cell Rep 15:1673–1685. doi: 10.1016/j.celrep.2016.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li FJ, Xu ZS, Soo AD, Lun ZR, He CY. 2017. ATP-driven and AMPK-independent autophagy in an early-branching eukaryotic parasite. Autophagy 13:715–729. doi: 10.1080/15548627.2017.1280218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Irigoín F, Inada NM, Fernandes MP, Piacenza L, Gadelha FR, Vercesi AE, Radi R. 2009. Mitochondrial calcium overload triggers complement-dependent superoxide-mediated programmed cell death in Trypanosoma cruzi. Biochem J 418:595–604. doi: 10.1042/BJ20081981. [DOI] [PubMed] [Google Scholar]
- 41.Maugeri DA, Cannata JJ, Cazzulo JJ. 2011. Glucose metabolism in Trypanosoma cruzi. Essays Biochem 51:15–30. doi: 10.1042/bse0510015. [DOI] [PubMed] [Google Scholar]
- 42.Bone GJ, Steinert M. 1956. Isotopes incorporated in the nucleic acids of Trypanosoma mega. Nature 178:308–309. doi: 10.1038/178308a0. [DOI] [PubMed] [Google Scholar]
- 43.Schmatz DM, Murray PK. 1982. Cultivation of Trypanosoma cruzi in irradiated muscle cells: improved synchronization and enhanced trypomastigote production. Parasitology 85:115–125. doi: 10.1017/S0031182000054202. [DOI] [PubMed] [Google Scholar]
- 44.Moreno SN, Silva J, Vercesi AE, Docampo R. 1994. Cytosolic-free calcium elevation in Trypanosoma cruzi is required for cell invasion. J Exp Med 180:1535–1540. doi: 10.1084/jem.180.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bourguignon SC, de Souza W, Souto-Padrón T. 1998. Localization of lectin-binding sites on the surface of Trypanosoma cruzi grown in chemically defined conditions. Histochem Cell Biol 110:527–534. doi: 10.1007/s004180050314. [DOI] [PubMed] [Google Scholar]
- 46.Larson MH, Gilbert LA, Wang X, Lim WA, Weissman JS, Qi LS. 2013. CRISPR interference (CRISPRi) for sequence-specific control of gene expression. Nat Protoc 8:2180–2196. doi: 10.1038/nprot.2013.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vazquez MP, Levin MJ. 1999. Functional analysis of the intergenic regions of TcP2beta gene loci allowed the construction of an improved Trypanosoma cruzi expression vector. Gene 239:217–225. doi: 10.1016/S0378-1119(99)00386-8. [DOI] [PubMed] [Google Scholar]
- 48.Lorenzi HA, Vazquez MP, Levin MJ. 2003. Integration of expression vectors into the ribosomal locus of Trypanosoma cruzi. Gene 310:91–99. doi: 10.1016/S0378-1119(03)00502-X. [DOI] [PubMed] [Google Scholar]
- 49.Oberholzer M, Morand S, Kunz S, Seebeck T. 2006. A vector series for rapid PCR-mediated C-terminal in situ tagging of Trypanosoma brucei genes. Mol Biochem Parasitol 145:117–120. doi: 10.1016/j.molbiopara.2005.09.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Oligonucleotides used in this work. Download TABLE S1, DOCX file, 0.1 MB (110.9KB, docx) .
Copyright © 2017 Chiurillo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Localization and growth of TcMCU-OE and TcMCUb-OE epimastigotes. (A) Fluorescence microscopy of TcMCU-OE epimastigotes (MCU) using anti-TbMCU antibodies (green) and anti-TbVDAC (VDAC) antibodies as a mitochondrial marker (red). Nuclei and kinetoplast were labeled with DAPI (blue). Colocalization of TcMCU-OE and TcVDAC is shown in yellow (merge). A differential interference contrast (DIC) image is shown in the left panel. (B) Growth curve of TcMCU-OE (MCU-OE) epimastigotes, TcMCUb-OE (MCUb-OE) epimastigotes, and epimastigotes transfected with pTREX-n empty vector. (C) Colocalization of TcMCUb-3×HA-OE using anti-HA antibodies (HA) with MitoTracker (MT). (D) Western blot analysis of WT, TcMCU-3×c-Myc, and TcMCU-3×c-Myc/pTREX-n-TcMCUb-3×HA cell lines. Anti-c-Myc and anti-HA antibodies detect TcMCU-3×c-Myc (predicted size, 35 kDa) and TcMCUb-3×HA (predicted size, 31 kDa), respectively, whereas both bands are absent in WT parasites. Anti-α-tubulin antibody was used as a loading control. The TcMCU-3×c-Myc cell line was obtained by CRISPR/Cas9-mediated endogenous C-terminal tagging as described previously (15). Antibodies are indicated on the right side of the blots, and molecular weights are on the left side. There was no significant difference in intensity of bands corresponding to TcMCU-3×c-Myc detected with anti-c-Myc antibodies between TcMCU-3×c-Myc and TcMCU-3×c-Myc/pTREX-n-TcMCUb-3×HA cell lines. Bars in panels A and C, 10 µm. Values in panel B are means ± SD (n = 3; no significant difference). Download FIG S1, TIF file, 1.4 MB (1.5MB, tif) .
Copyright © 2017 Chiurillo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Mitochondrial membrane potential in mutant epimastigotes (A and B). Changes in mitochondrial membrane potential (ΔΨm) of digitonin-permeabilized epimastigotes as detected by changes in safranin O fluorescence in epimastigotes transfected with scrambled sgRNA/Cas9/pTREX-n (Scrambled) or TcMCU-KO (MCU-KO) (A) and empty vector or TcMCU-OE (MCU-OE) (B). Cells (5 × 107) were added to the reaction buffer (2 ml) containing 0.2% bovine serum albumin, 2 mM succinate, and 5 µM safranin O. The reaction was started with 50 µM digitonin, and 250 µM ADP, 20 µM carboxyatractyloside (CAT), and 4 µM carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP) were added where indicated. (C) Quantification of changes in ΔΨm in panels A and B. (D and E) Changes in ΔΨm in digitonin-permeabilized epimastigotes as detected by changes in safranin O fluorescence in epimastigotes transfected with scrambled sgRNA/Cas9/pTREX-n (Scrambled) or TcMCUb-KO (MCUb-KO) (D) and empty vector or TcMCUb-OE (MCUb-OE) (E). Other conditions are as in panels A and B. (F) Quantification of changes in ΔΨm in panels D and E. Values in panels C and F are means ± SD (n = 3; no significant differences, Student’s t test). Download FIG S2, TIF file, 1.4 MB (1.5MB, tif) .
Copyright © 2017 Chiurillo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Lipid droplets in epimastigotes and PCR analysis of TcMCU-KO trypomastigotes. (A) Staining with Nile red (red) of lipid droplets in wild-type (WT) and TcMCU-KO epimastigotes cultured in LIT or low-glucose LIT medium. T. cruzi epimastigotes were incubated in PBS containing 1.5 μg of Nile red (Sigma)/ml for 30 min at 28°C. Fluorescence optical images were captured with excitation at 510 nm and emission at 580 nm. Nuclei and kinetoplasts were labeled with DAPI (blue). Bars, 10 µm. (B) Number of lipid droplets detected per cell in LIT or low-glucose medium. At least 200 cells from three experiments with 20 random fields/experiment were analyzed (means ± SD; n = 3; ***, P < 0.001, Student’s t test). (C) PCR used to confirm TcMCU-KO genotype in trypomastigotes isolated after host cell infection, as described in Materials and Methods. The intact locus generates a PCR product of 909 bp in wild-type cells that is absent in TcMCU-KO trypomastigotes. Download FIG S3, TIF file, 2.2 MB (2.2MB, tif) .
Copyright © 2017 Chiurillo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Mitochondrial localization of TcMCU-HA and HsMCU-3×c-Myc in epimastigotes. (A) Fluorescence microscopy of exogenous TcMCU-HA expressed in TcMCU-KO epimastigotes using antibodies against HA (green) and MitoTracker as a mitochondrial marker (red). Nuclei and kinetoplast were labeled with DAPI (blue). Colocalization is shown in yellow (merge). (B) Schematic representation of MCU subunit domains. Sequence alignment of the DIME motif and flanking residues from MCU and MCUb. Numbers of critical conserved and amino acid substitutions are shown. TM, transmembrane domain; DIME, functional DIME motif. (C) Colocalization of HsMCU-3×c-Myc with MitoTracker using antibodies to c-Myc. Bars in panels A and C, 10 µm. Download FIG S4, TIF file, 1.6 MB (1.7MB, tif) .
Copyright © 2017 Chiurillo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Supplemental methods. Download TEXT S1, DOCX file, 0.1 MB (100.9KB, docx) .
Copyright © 2017 Chiurillo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Full blots shown in the article. Download FIG S5, TIF file, 2.6 MB (2.6MB, tif) .
Copyright © 2017 Chiurillo et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.