ABSTRACT
Analysis of monoclonal antibodies (MAbs) derived from single B cell cloning has been highly beneficial for antimicrobial immunotherapy, vaccine design, and advancing our understanding of pathogen-triggered effects on the human immunoglobulin repertoire. Sequencing of variable domains of single B cells, and characterization of binding and functional activities of MAbs derived from those sequences, provides in-depth insight not only into sites of susceptibility for antibody-mediated neutralization or opsonization of the pathogen but also into the dynamics of protective antibody evolution during infection. This information can be utilized to rapidly develop novel immunotherapies of completely human origin and provides a roadmap for structure-based vaccine design that aims to elicit similar protective antibody responses. Here, we summarize recent aspects of the single B cell cloning approach.
KEYWORDS: antibody discovery, immune responses, immunotherapy, single B cell sorting
INTRODUCTION
Immunity from infection, natural or acquired via vaccination, requires protective memory immune responses, in many cases mediated by antibodies (Abs). The majority of microbial antigens trigger protective polyclonal antibody responses by stimulating B cells to produce protective amounts of pathogen-specific antibodies. The host's ability to combat a huge variety of microorganisms is based on the immense combinatorial repertoire of circulating B cells in the periphery and organs of an individual. The calculated number of 1018 possible B cell receptor (BCR) combinations, with diversified heavy- and light-chain pairs, greatly exceeds the total number of B cells in an average adult (1, 2). Nonetheless, the process of affinity maturation and clonal selection results in evolution of antigen-specific antibodies within weeks or months of infection or immunization (3). Although responses to pathogens in humans and animals are polyclonal and thus heterogeneous in nature, modern methodologies have allowed researchers to characterize the composite monoclonal antibodies (MAbs) by cloning and expressing variable domain regions from single B cells. The high-throughput nature of this approach, which often yields 50 to over 300 pathogen-specific MAbs, not only allows analysis of granular aspects of individual MAb-pathogen interactions but also provides a global view of human immune responses and how they may vary depending upon the invading pathogen. The antimicrobial human antibody repertoire can be studied by single B cell isolation and in-depth repertoire sequencing (Fig. 1); both aspects have proven to be powerful techniques for the characterization of immune responses to pathogen-specific antigens (4, 5). Following the isolation of peripheral blood mononuclear cells (PBMCs) from affected individuals, antigen-specific plasmablasts or memory B cells can be sorted by fluorescence-activated cell sorting (FACS) by labeling the cells with a fluorescently tagged antigen or by sorting of naive plasmablasts in acutely infected individuals (Fig. 1). Positive hits are distributed in microtiter plates at a density of a single cell per well. The variable heavy chains (VH) and variable light chains (VL) are recovered by nested reverse transcription-PCR (RT-PCR). The recovery rate of the VH and VL is mainly based on the primer set used and the quality/quantity of the intracellular mRNA/cDNA. After isotype-specific amplification using appropriate antisense primers for the CH1 region of, e.g., IgG, IgM, or IgA, VH and VL regions can be grafted onto the respective constant domains. Subsequently, antibodies can be recombinantly expressed and processed for further characterization (4, 6, 7). Thus, recombinant MAbs can be immediately obtained without the need for B cell immortalization or fusion with myeloma partners. In general, recombinant MAbs provide an advantage in terms of reliability and reproducibility relative to polyclonal antibodies or even MAbs isolated from hybridoma cell lines. The characterization of single MAbs in complex with the respective antigen provides insights into the identification of novel mechanisms and epitopes required for successful protection against the pathogen. Those MAbs with potent protective ability against a broad range of related pathogens or strains (broadly neutralizing antibodies [bNAbs]) are of great interest for antibody discovery and are promising candidates for immunotherapeutic development (4).
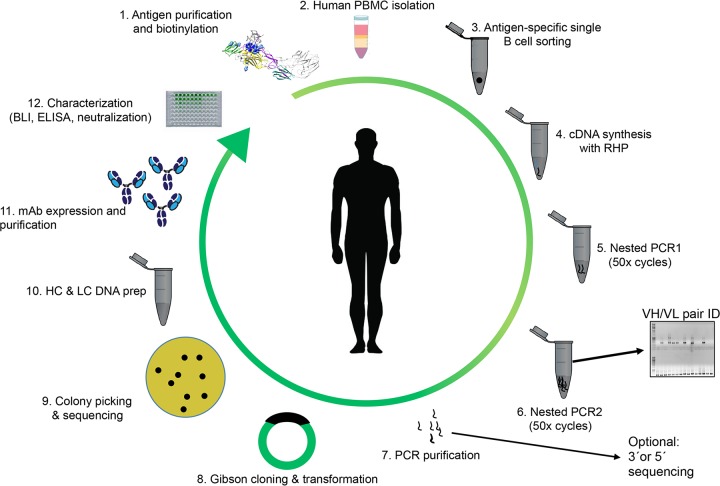
FIG 1.
Workflow for the isolation of monoclonal antibodies by antigen-dependent single B lymphocyte sorting (abbreviations: PBMC, peripheral blood mononuclear cells; RHP, random hexamer primers; VH, variable domain of the heavy chain; VL, variable domain of the light chain; HC, heavy chain; LC, light chain; MAb, monoclonal antibody; BLI, biolayer interferometry; ELISA, enzyme-linked immunosorbent assay).
Recently, several previously underappreciated or insufficiently characterized pathogens triggered severe global epidemics. Such events have the capacity to result in a large number of fatal cases or chronically ill patients or to cause local public health emergencies with global implications (8). While the spread of some pathogens, e.g., Mycobacterium tuberculosis and dengue virus (DENV), is ongoing, the epidemic spread of viruses such as LaCrosse virus, chikungunya virus, or Zika virus was difficult to predict despite previous experiences (9). Antibody-mediated immunotherapy, consisting of single MAbs or cocktails of multiple MAbs, provides an attractive platform for initial emergency responses. Further, such epidemics have raised scientific questions about the antigen-directed evolution of the human antibody response during microbial exposure (10, 11). Patients who had previously been exposed to viruses or bacteria usually show tremendous levels of highly affinity matured immunoglobulins against one or more antigens of the same pathogen. Studies on single B cells suggest that pathogen-induced affinity maturation can be site specific and that somatic hypermutation (SHM) is connected to the evolution of bNAbs (6, 12, 13). In this short article, we focus on recent discoveries in the field of antimicrobial antibody responses that were achieved by single B cell analysis.
Insights into B cell expansion and seroconversion from single B cell sorting.
Pathogen-mediated B cell receptor cross-linking triggers the activation of antigen-specific B cells (ASBCs) that subsequently undergo massive cell proliferation. During the course of an infection, the overall number of ASBCs in the periphery mainly depends on the elapsed time since exposure (6, 14, 15). Several studies recorded incremental changes in plasmablast counts to identify the ASBC-specific subpopulation by a time course-dependent flow cytometric analysis (14, 15). After acute DENV infection, the number of virus-specific plasmablasts peaks within the first week after antigen exposure (15, 16). For an acute DENV infection, the proportions of DENV-specific CD19+ CD3− CD20−/low CD38hi CD27hi plasmablasts rise on average from 0% to 47% (15). In total numbers, DENV infections boost the average basal level of plasmablasts from 3.7 × 105 to more than 1 × 106 per milliliter of blood (15). This result affirms the high immunogenicity of DENV in comparison to (for example) yellow fever virus, another flavivirus, which shows the highest peak at day 7 and day 11 with an overall level of <7% of that seen with reactive plasmablasts (15). These temporarily high numbers of B cells might induce high rates of acute and covalescent seroconversion in individuals over a long duration (6). For instance, a small population of patients that were vaccinated with the M. tuberculosis vaccine BCG displayed unusually high serum levels against the M. tuberculosis-derived antigen lipoarabinomannan (AM) even >10 years after vaccination (12). Flow cytometry analysis of AM-reactive B cells reveals a very low number of antigen-specific B cells that might be responsible for the production of significant amounts of monoclonal antibodies that are efficient enough to provide protection.
For a large number of approved vaccines (e.g., measles, mumps, rubella vaccine; yellow fever vaccine), antibody titers stay consistently protective in a large number of patients over the course of several years, while other vaccines require booster immunization (e.g., tetanus vaccine) (17–19). Antigen-mediated boosting triggers the differentiation of previously primed B cells and the generation of antigen-specific plasmablasts (20). A fraction of these plasmablasts then migrate into specialized bone marrow niches and contribute as long-lived plasma cells to the maintenance of the serum immunoglobulin levels (20, 21). Technological advances in mass spectrometry and single B cell cloning have facilitated a deeper understanding of the serological memory of vaccinations. To correlate levels of circulating or resting long-lived plasma cells with their actual activity with respect to immunoglobulin expression levels in the serum, next-generation sequencing (NGS) of the V gene repertoire can be paired with high-resolution liquid chromatography-tandem MS (MS/MS) (19, 22). To elicit a correlation between levels of existing plasmablasts and actual immunoglobulin expression, the peripheral repertoire of anti-tetanus antibodies was investigated before and after tetanus (TT) booster vaccination (19). Deep sequencing of the TT-positive (TT+) serum IgG repertoire of two donors recorded between 80 and 100 different TT+-specific clonotypes after the booster vaccination. There, three clonotypes represented >40% of the overall TT+-specific immunoglobulin levels in the serum. The remaining clonotypes were present at frequencies of less than 0.5% each. Comparison of the clonotypic repertoire to the expressed serum immunoglobulin repertoire revealed that only a small fraction of plasmablast clonotypes or memory B cells encode antibodies that contribute to the Ig serum 9 months after booster vaccination. This suggests that a large population of booster-responsive TT+ plasmablasts were not part of the long-lived serological memory (19). The high quality of selection for specific B cells is an important process that mainly facilitates an efficient immune response.
Affinity maturation during antigen exposure.
The goal of many vaccinations is to mimic protective aspects of pathogen-induced B cell responses. Over the time course of multiple vaccinations, antibodies are selected and affinity matured to improve specificity. However, hypervariable viruses such as influenza virus or HIV-1 that mutate at a high frequency have been particularly recalcitrant to vaccine development. Efficient protection against HIV-1 and influenza virus likely requires the induction of bNAbs which, even in actively viremic individuals, may be exceedingly rare or not present (23). Isolation of bNAbs by single sorting has revealed that a large portion of broadly neutralizing influenza virus, HIV-1, or hepatitis C virus (HCV) antibodies is derived from the VH1-69 germ line segment (23). The unexpectedly high representation of bNAbs from the VH1-69 germ line segment implies that viral infections can alter adaptive immune responses and bias or deregulate typical antibody germ line usage. Interestingly, VH1-69-derived antibodies show a high percentage of somatic hypermutations based on a critical polymorphism (23). It can be presumed that those specific B cells derive from germinal centers with high mutagenic activity (24). The reconstruction of genealogical trees of multimember clones from influenza virus-exposed and vaccinated individuals revealed mutation sites within complementarity-determining regions (CDRs) of isolated anti-hemagglutinin bNAbs that seem to be specifically favored during the process of bNAb evolution and are important sites for affinity maturation (23). Pappas et al. discovered five antibodies that were clonally related, based on the conserved heavy-chain CDR (HCDR) residues R30, A52, and Y98. Back-mutation of these residues to the germ line sequence did not abolish binding to the virus. However, it was shown that the presence of the germ line sequence of the antibody led to the reduction of neutralization of other H1 strains. This implies that site-specific evolution of certain residues during antibody development is essential for the development of broadly neutralizing activity. Site-specific affinity maturation was also shown to be important in broadening the specificity for other influenza virus antibodies (13). The 3I14 antibody derived from IGHV3-30 is able to bind the H3 and H1 subtypes but not H5 in its germ line configuration (D94). Interestingly, germ line reversion of the D94N somatic hypermutation significantly restores the binding to H5, underlining that certain residues are important for broad antiviral protection (13).
Understanding the site-specific SHMs that give rise to bNAbs in HIV-1 and influenza virus infections might therefore provide an opportunity for immunogen engineering as a way to properly guide affinity maturation toward protective bNAb responses by vaccination. For HIV-1 bNAb PGT121 and related members, it was previously shown that the maturation of these narrowly related bNAbs may be mainly based on specific interactions or avoidance of steric clashes with a high-mannose glycan on the HIV-1 trimer (25). It was demonstrated that SHMs on some of the PGT121-like MAbs promote CDR configurations to avoid nonproductive interactions with glycosylated residue N137, while in other cases, SHMs themselves contribute to direct interactions with the same glycan. Removal of glycosylated N137 from gp120 increased the binding affinity of all PGT121-related MAbs by significant amounts, suggesting that the presence of the glycan essentially shapes the maturation process of the antibody (25). In general, highly mutated HIV-1 antibodies have a significant higher potential to protect due to reoccurring exposure of the molecules to the antigen over time. It was shown that not only the virus but also the bNAbs evolve over time in long-term HIV-1 infection (26). Time-dependent sequencing of antibodies from the CH103 lineage paired with neutralization and binding studies demonstrated that unmutated antibodies can evolve to broadly neutralizing molecules within 34 months (26). Due to chronic exposure of the virus, SHM rates incrementally change from 0% to 17%, and study results show that the most extensively evolved antibody, CH103, neutralizes more than 50% of the tested HIV-1 pseudoviruses in comparison to clonally related antibodies with a lower rate of mutation (26). Interestingly, the maturation process focused on sites that were only minimally affected by viral escape mutations. This result implies a continual “host-virus” battle that favors antibodies that suppress a broad array of viruses.
For the elicitation of bNAbs by immunization, it is likely important that the vaccine is able to prime germ line B cell receptors, later inducing a mature bNAb lineage. This was achieved by Jardine et al., who remodeled the CD4 binding site on gp120 to target VRC01-class bNAb germ line precursors and then evaluated this immunogen as a multivalent immunonanoparticle-based eOD-GT6 vaccine (27). After cross-linking of a specific class of BCRs by the resurfaced CD4 binding site was performed, antigen-specific germ line B cells proliferated and matured to become broadly neutralizing VRC01-class B cells. VRC01-class bNAbs neutralized HIV-1, as they mimic CD4 by binding to gp120 (27). To identify the precursor VRC01-B cells, the affinity of the immunonanoparticles for VRC01-class-derived antibodies was optimized by yeast display panning against germ line-derived and mature VRC01 antibodies (28). The evolved eOD-GT8 immunogen was then used as a probe for epitope-specific B cell sorting. Highly stringent B cell sorting of samples from 15 donors revealed that only 1 in 2.4 million naive B cells were derived from the VRC01 lineage (28). This provides an explanation about the rarity of these antibodies but also demonstrates the precision of single B cell sorting performed with an optimized antigen.
Taking the data together, smart vaccine design can mainly improve the induction of broadly specific antibodies. However, sophisticated analysis of the evolution of single B cell populations and a detailed structural analysis have to be performed to design a rational concept for broadly protective vaccines (29).
Isolation of antigen-specific molecules for immunotherapy from single B cells.
In some cases, the application of vaccines is inappropriate or not manageable due to circumstances such as severe acute infections or cross-reactivity with other pathogens. In these cases, immunotherapies consisting of one or more MAbs provide an attractive therapeutic alternative. Furthermore, even for pathogens for which an effective vaccine exists, it may be desirable to have accompanying immunotherapies for use in cases where the vaccine is inaccessible or not adopted. Rapid development of immunotherapies for newly emerging pathogens, or analysis of antibody repertoires, depends on highly sophisticated technology platforms such as single B cell sorting or hybridoma technology. In acute cases, it is important to utilize the most rapid direct method for the isolation of potent human antibodies to generate therapeutic molecules for clinical applications—even within a few weeks (4). In comparison to hybridoma technology or immunization of transgenic animals (e.g., with humanized Ig loci), there are significant advantages of B cell sorting. Hybridoma technology and murine immunization require long-term screening and animal handling. Furthermore, it is important to consider that murine antibody responses might differ from human patterns. Murine antibodies were also shown to trigger immunogenic responses, provide less stability, and hamper downstream Fc effector functions. On the basis of all of these pitfalls, our laboratory and those of others use antigen-dependent B cell sorting for the isolation of a variety of different pathogen-specific molecules for a straightforward MAb isolation within a few weeks (4, 30). For the detection of antigen-specific B cell populations, the antigen must be labeled either by fluorescently labeled streptavidin or by another fluorescent tag. In our work, we observed that the selection and careful design of the antigen are critical to the quality of the isolated antibodies. Random biotinylation methods can either decrease the number of specific molecules or block important functional binding sites that lead to the exclusion of potential top hits. The configuration of the sorting antigen (e.g., trimeric, monomeric) is equally important. A large number of potent molecules for, e.g., dengue or Zika virus rely on quaternary epitopes that can be fished out only by mimicking the natural architecture of the viral surface (31, 32).
A successful isolation of a large number of human antibodies was performed for an Ebola virus (EBOV) patient with elevated glycoprotein (GPEBOV) serum responses. It was shown that 1% to 3% of memory B cells were GPEBOV reactive 3 months after infection (4). By using high-throughput single B cell sorting, Bornholdt et al. isolated 349 antibodies against different neutralization-relevant epitopes of GPEBOV from the survivor of the 2014 Ebola outbreak (4). Thereby, analysis of GPEBOV-reactive VH and VL revealed a widely distributed usage of clonal lineages, meaning that the isolated antibodies massively differ in V/J combinations, CDR3 loop lengths, and CDR3 sequence homologies (4). This result contrasts with studies isolating influenza virus- or HIV-1-specific antibodies that seem to favor particular germ line lineages depending on the epitope used (14, 33). Methods of downstream characterization of antigen-specific antibodies such as epitope mapping and binding affinity studies unravel novel epitopes and provide information about the immunogenicity or mechanisms of action of the antigen binding sites. Most of the human antigen responses to GPEBOV are directed against well-characterized GP epitopes such as the glycan cap or the GP1/GP2 interface (4). However, one of most potent GPEBOV antibodies from that study, ADI-15758, binds proximally to the viral membrane—a so far less extensively characterized epitope that was presumably hardly accessible—and demonstrated efficient protection against lethal doses of EBOV in mice. Based on the visualization of three ADI-15758 Fab molecules complexed with three GPEBOV, it was assumed that the HR2 region of the pre-GP might already exist as a three-helix bundle. This shows that information from single B cell isolation and epitope mapping provides valuable predictions of the structural function of the viral glycoprotein.
Similar studies were also performed for the isolation of broadly neutralizing dengue virus antibodies. Dengue virus infection, with approximately 400 million human infections per year, is the leading arthropod-transmitted viral disease in the world (34). The mature, prefusion glycoprotein E exists as a head-to-tail dimer organized into rafts with icosahedral geometry on the viral particle. Each E subunit contains three domains, DI, DII, and DIII. The postfusion E structure is a trimer with DIII, and the stem region is significantly relocated relative to DI and DII, so as to bring the host and viral membranes into proximity to facilitate viral membrane fusion. The members of one promising class of protective molecules are described as antibodies that bind the E dimer epitope (EDE) on the E protein, efficiently neutralizing multiple DENV serotypes (34). Dejnirattisai et al. sorted plasmablasts from seven acutely infected patients and isolated 145 antibodies by single B cell cloning (34). Antibodies to EDE were shown to be highly cross-reactive to multiple DENV strains (34). Epitope mapping by alanine scanning revealed that EDE either is an epitope that is contained within an E dimer or is shared by adjacent E dimers on the viral surface (34). For isolated EDE2 antibody 747(4)B7, it was shown that EDE-dependent neutralization does not necessarily depend on the cross-linking of two adjacent dimers. Cryo-electron microscopy of Fab 747(4)B7 showed that EDE was bound only at an intradimer epitope and not across dimers. However, other EDE antibodies such as EDE1 C8 use the binding across dimers to efficiently cross-neutralize dengue and Zika viruses in a highly efficient way (35).
Despite the very efficient generation of antibodies, there are many challenges for single B cell sorting. Because of the unbiased nature of this procedure, not all isolated antibodies express very well due to a certain V gene usage. Another pitfall is linked to the appropriate isolation of the correctly assembled constant region of the antibodies. Some applications require the right combination between the isotype and the variable region to trigger the appropriate downstream effector functions. So far, recent polymerase technologies have limited the isolation of antibodies to only the variable regions based on the limited half-life of the polymerases under conditions of excessive PCR cycle numbers. However, since protein engineering is rapidly evolving, this problem might be solved in the near future. A final limitation is that the B cell sorting method does not correlate with secreted MAbs and thus that the individual composite MAbs may not reflect the serum response. Nonetheless, all of the studies mentioned showed that high-throughput screening of PBMCs can lead to efficient generation of new molecules for immunotherapy and to a better understanding of the pathogen-triggered immune response. The isolation of monoclonal human antibodies is a powerful tool to map out important binding epitopes and unravel neutralization mechanisms for a large number of different pathogens in a short time.
ACKNOWLEDGMENTS
We thank Jose Quiroz (Einstein) and Laura Walker (Adimab) for helpful discussions.
J.R.L. gratefully acknowledges funding from the NIH (U19AI109762 and R41AI122403) and from the Irma T. Hirschl Charitable Trust.
REFERENCES
- 1.Hoehn KB, Fowler A, Lunter G, Pybus OG. 2016. The diversity and molecular evolution of B-cell receptors during infection. Mol Biol Evol 33:1147–1157. doi: 10.1093/molbev/msw015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeKosky BJ, Kojima T, Rodin A, Charab W, Ippolito GC, Ellington AD, Georgiou G. 2015. In-depth determination and analysis of the human paired heavy- and light-chain antibody repertoire. Nat Med 21:86–91. doi: 10.1038/nm.3743. [DOI] [PubMed] [Google Scholar]
- 3.French DL, Laskov R, Scharff MD. 1989. The role of somatic hypermutation in the generation of antibody diversity. Science 244:1152–1157. doi: 10.1126/science.2658060. [DOI] [PubMed] [Google Scholar]
- 4.Bornholdt ZA, Turner HL, Murin CD, Li W, Sok D, Souders CA, Piper AE, Goff A, Shamblin JD, Wollen SE, Sprague TR, Fusco ML, Pommert KB, Cavacini LA, Smith HL, Klempner M, Reimann KA, Krauland E, Gerngross TU, Wittrup KD, Saphire EO, Burton DR, Glass PJ, Ward AB, Walker LM. 2016. Isolation of potent neutralizing antibodies from a survivor of the 2014 Ebola virus outbreak. Science 351:1078–1083. doi: 10.1126/science.aad5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeKosky BJ, Lungu OI, Park D, Johnson EL, Charab W, Chrysostomou C, Kuroda D, Ellington AD, Ippolito GC, Gray JJ, Georgiou G. 2016. Large-scale sequence and structural comparisons of human naive and antigen-experienced antibody repertoires. Proc Natl Acad Sci U S A 113:E2636–E2645. doi: 10.1073/pnas.1525510113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Priyamvada L, Quicke KM, Hudson WH, Onlamoon N, Sewatanon J, Edupuganti S, Pattanapanyasat K, Chokephaibulkit K, Mulligan MJ, Wilson PC, Ahmed R, Suthar MS, Wrammert J. 2016. Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proc Natl Acad Sci U S A 113:7852–7857. doi: 10.1073/pnas.1607931113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murugan R, Imkeller K, Busse CE, Wardemann H. 2015. Direct high-throughput amplification and sequencing of immunoglobulin genes from single human B cells. Eur J Immunol 45:2698–2700. doi: 10.1002/eji.201545526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zumla A, George A, Sharma V, Herbert RH; Baroness Masham of Ilton, Oxley A, Oliver M. 2015. The WHO 2014 global tuberculosis report–further to go. Lancet Glob Health 3:e10–. doi: 10.1016/S2214-109X(14)70361-4. [DOI] [PubMed] [Google Scholar]
- 9.Foy BD, Kobylinski KC, Chilson Foy JL, Blitvich BJ, Travassos da Rosa A, Haddow AD, Lanciotti RS, Tesh RB. 2011. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis 17:880–882. doi: 10.3201/eid1705.101939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiu X, Audet J, Lv M, He S, Wong G, Wei H, Luo L, Fernando L, Kroeker A, Fausther Bovendo H, Bello A, Li F, Ye P, Jacobs M, Ippolito G, Saphire EO, Bi S, Shen B, Gao GF, Zeitlin L, Feng J, Zhang B, Kobinger GP. 2016. Two-mAb cocktail protects macaques against the Makona variant of Ebola virus. Sci Transl Med 8:329ra33. doi: 10.1126/scitranslmed.aad9875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith SA, Silva LA, Fox JM, Flyak AI, Kose N, Sapparapu G, Khomandiak S, Ashbrook AW, Kahle KM, Fong RH, Swayne S, Doranz BJ, McGee CE, Heise MT, Pal P, Brien JD, Austin SK, Diamond MS, Dermody TS, Crowe JE Jr. 2015. Isolation and characterization of broad and ultrapotent human monoclonal antibodies with therapeutic activity against chikungunya virus. Cell Host Microbe 18:86–95. doi: 10.1016/j.chom.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen T, Blanc C, Eder AZ, Prados-Rosales R, Souza AC, Kim RS, Glatman-Freedman A, Joe M, Bai Y, Lowary TL, Tanner R, Brennan MJ, Fletcher HA, McShane H, Casadevall A, Achkar JM. 2016. Association of human antibodies to arabinomannan with enhanced mycobacterial opsonophagocytosis and intracellular growth reduction. J Infect Dis 214:300–310. doi: 10.1093/infdis/jiw141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu Y, Zhang Z, Sheehan J, Avnir Y, Ridenour C, Sachnik T, Sun J, Hossain MJ, Chen LM, Zhu Q, Donis RO, Marasco WA. 2016. A broadly neutralizing anti-influenza antibody reveals ongoing capacity of haemagglutinin-specific memory B cells to evolve. Nat Commun 7:12780. doi: 10.1038/ncomms12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng NY, Mays I, Garman L, Helms C, James J, Air GM, Capra JD, Ahmed R, Wilson PC. 2008. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 453:667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wrammert J, Onlamoon N, Akondy RS, Perng GC, Polsrila K, Chandele A, Kwissa M, Pulendran B, Wilson PC, Wittawatmongkol O, Yoksan S, Angkasekwinai N, Pattanapanyasat K, Chokephaibulkit K, Ahmed R. 2012. Rapid and massive virus-specific plasmablast responses during acute dengue virus infection in humans. J Virol 86:2911–2918. doi: 10.1128/JVI.06075-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Priyamvada L, Cho A, Onlamoon N, Zheng NY, Huang M, Kovalenkov Y, Chokephaibulkit K, Angkasekwinai N, Pattanapanyasat K, Ahmed R, Wilson PC, Wrammert J. 2016. B cell responses during secondary dengue virus infection are dominated by highly cross-reactive, memory-derived plasmablasts. J Virol 90:5574–5585. doi: 10.1128/JVI.03203-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Markowitz LE, Preblud SR, Fine PE, Orenstein WA. 1990. Duration of live measles vaccine-induced immunity. Pediatr Infect Dis J 9:101–110. doi: 10.1097/00006454-199002000-00008. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. 2013. Vaccines and vaccination against yellow fever: WHO position paper—June 2013. Wkly Epidemiol Rec 88:269–284. [PubMed] [Google Scholar]
- 19.Lavinder JJ, Wine Y, Giesecke C, Ippolito GC, Horton AP, Lungu OI, Hoi KH, DeKosky BJ, Murrin EM, Wirth MM, Ellington AD, Dorner T, Marcotte EM, Boutz DR, Georgiou G. 2014. Identification and characterization of the constituent human serum antibodies elicited by vaccination. Proc Natl Acad Sci U S A 111:2259–2264. doi: 10.1073/pnas.1317793111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nutt SL, Hodgkin PD, Tarlinton DM, Corcoran LM. 2015. The generation of antibody-secreting plasma cells. Nat Rev Immunol 15:160–171. doi: 10.1038/nri3795. [DOI] [PubMed] [Google Scholar]
- 21.Ellebedy AH, Jackson KJ, Kissick HT, Nakaya HI, Davis CW, Roskin KM, McElroy AK, Oshansky CM, Elbein R, Thomas S, Lyon GM, Spiropoulou CF, Mehta AK, Thomas PG, Boyd SD, Ahmed R. 2016. Defining antigen-specific plasmablast and memory B cell subsets in human blood after viral infection or vaccination. Nat Immunol 17:1226–1234. doi: 10.1038/ni.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J, Boutz DR, Chromikova V, Joyce MG, Vollmers C, Leung K, Horton AP, DeKosky BJ, Lee CH, Lavinder JJ, Murrin EM, Chrysostomou C, Hoi KH, Tsybovsky Y, Thomas PV, Druz A, Zhang B, Zhang Y, Wang L, Kong WP, Park D, Popova LI, Dekker CL, Davis MM, Carter CE, Ross TM, Ellington AD, Wilson PC, Marcotte EM, Mascola JR, Ippolito GC, Krammer F, Quake SR, Kwong PD, Georgiou G. 2016. Molecular-level analysis of the serum antibody repertoire in young adults before and after seasonal influenza vaccination. Nat Med 22:1456–1464. doi: 10.1038/nm.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pappas L, Foglierini M, Piccoli L, Kallewaard NL, Turrini F, Silacci C, Fernandez-Rodriguez B, Agatic G, Giacchetto-Sasselli I, Pellicciotta G, Sallusto F, Zhu Q, Vicenzi E, Corti D, Lanzavecchia A. 2014. Rapid development of broadly influenza neutralizing antibodies through redundant mutations. Nature 516:418–422. doi: 10.1038/nature13764. [DOI] [PubMed] [Google Scholar]
- 24.De Silva NS, Klein U. 2015. Dynamics of B cells in germinal centres. Nat Rev Immunol 15:137–148. doi: 10.1038/nri3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garces F, Lee JH, de Val N, de la Pena AT, Kong L, Puchades C, Hua Y, Stanfield RL, Burton DR, Moore JP, Sanders RW, Ward AB, Wilson IA. 2015. Affinity maturation of a potent family of HIV antibodies is primarily focused on accommodating or avoiding glycans. Immunity 43:1053–1063. doi: 10.1016/j.immuni.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liao HX, Lynch R, Zhou T, Gao F, Alam SM, Boyd SD, Fire AZ, Roskin KM, Schramm CA, Zhang Z, Zhu J, Shapiro L; NISC Comparative Sequencing Program, Mullikin JC, Gnanakaran S, Hraber P, Wiehe K, Kelsoe G, Yang G, Xia SM, Montefiori DC, Parks R, Lloyd KE, Scearce RM, Soderberg KA, Cohen M, Kamanga G, Louder MK, Tran LM, Chen Y, Cai F, Chen S, Moquin S, Du X, Joyce MG, Srivatsan S, Zhang B, Zheng A, Shaw GM, Hahn BH, Kepler TB, Korber BT, Kwong PD, Mascola JR, Haynes BF. 2013. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature 496:469–476. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jardine J, Julien JP, Menis S, Ota T, Kalyuzhniy O, McGuire A, Sok D, Huang PS, MacPherson S, Jones M, Nieusma T, Mathison J, Baker D, Ward AB, Burton DR, Stamatatos L, Nemazee D, Wilson IA, Schief WR. 2013. Rational HIV immunogen design to target specific germline B cell receptors. Science 340:711–716. doi: 10.1126/science.1234150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jardine JG, Kulp DW, Havenar-Daughton C, Sarkar A, Briney B, Sok D, Sesterhenn F, Ereño-Orbea J, Kalyuzhniy O, Deresa I. 2016. HIV-1 broadly neutralizing antibody precursor B cells revealed by germline-targeting immunogen. Science 351:1458–1463. doi: 10.1126/science.aad9195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Correia BE, Bates JT, Loomis RJ, Baneyx G, Carrico C, Jardine JG, Rupert P, Correnti C, Kalyuzhniy O, Vittal V, Connell MJ, Stevens E, Schroeter A, Chen M, Macpherson S, Serra AM, Adachi Y, Holmes MA, Li Y, Klevit RE, Graham BS, Wyatt RT, Baker D, Strong RK, Crowe JE Jr, Johnson PR, Schief WR. 2014. Proof of principle for epitope-focused vaccine design. Nature 507:201–206. doi: 10.1038/nature12966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.von Boehmer L, Liu C, Ackerman S, Gitlin AD, Wang Q, Gazumyan A, Nussenzweig MC. 2016. Sequencing and cloning of antigen-specific antibodies from mouse memory B cells. Nat Protoc 11:1908–1923. doi: 10.1038/nprot.2016.102. [DOI] [PubMed] [Google Scholar]
- 31.Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, Bianchi S, Vanzetta F, Minola A, Jaconi S, Mele F, Foglierini M, Pedotti M, Simonelli L, Dowall S, Atkinson B, Percivalle E, Simmons CP, Varani L, Blum J, Baldanti F, Cameroni E, Hewson R, Harris E, Lanzavecchia A, Sallusto F, Corti D. 2016. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science 353:823–826. doi: 10.1126/science.aaf8505. [DOI] [PubMed] [Google Scholar]
- 32.Gallichotte EN, Widman DG, Yount BL, Wahala WM, Durbin A, Whitehead S, Sariol CA, Crowe JE Jr, de Silva AM, Baric RS. 2015. A new quaternary structure epitope on dengue virus serotype 2 is the target of durable type-specific neutralizing antibodies. mBio 6:e01461–. doi: 10.1128/mBio.01461-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheid JF, Mouquet H, Feldhahn N, Seaman MS, Velinzon K, Pietzsch J, Ott RG, Anthony RM, Zebroski H, Hurley A, Phogat A, Chakrabarti B, Li Y, Connors M, Pereyra F, Walker BD, Wardemann H, Ho D, Wyatt RT, Mascola JR, Ravetch JV, Nussenzweig MC. 2009. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature 458:636–640. doi: 10.1038/nature07930. [DOI] [PubMed] [Google Scholar]
- 34.Dejnirattisai W, Wongwiwat W, Supasa S, Zhang X, Dai X, Rouvinski A, Jumnainsong A, Edwards C, Quyen NT, Duangchinda T, Grimes JM, Tsai WY, Lai CY, Wang WK, Malasit P, Farrar J, Simmons CP, Zhou ZH, Rey FA, Mongkolsapaya J, Screaton GR. 2015. A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus. Nat Immunol 16:170–177. doi: 10.1038/ni.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barba-Spaeth G, Dejnirattisai W, Rouvinski A, Vaney MC, Medits I, Sharma A, Simon-Loriere E, Sakuntabhai A, Cao-Lormeau VM, Haouz A, England P, Stiasny K, Mongkolsapaya J, Heinz FX, Screaton GR, Rey FA. 2016. Structural basis of potent Zika-dengue virus antibody cross-neutralization. Nature 536:48–53. doi: 10.1038/nature18938. [DOI] [PubMed] [Google Scholar]