ABSTRACT
Mycobacterium bovis BCG vaccination sensitizes cattle to bovine tuberculin, which compromises the use of the current bovine tuberculosis (TB) surveillance tests. Although the performance of a blood test (that utilizes antigens expressed by Mycobacterium bovis but not by BCG) capable of discriminating infected from vaccinated animals (DIVA interferon gamma test [DIT]) has been evaluated in naturally infected TB field reactors, there is a need to perform similar analysis in a BCG-vaccinated M. bovis-infected population. Furthermore, we explored different scenarios under which a DIT may be implemented alongside BCG vaccination: (i) serial testing to resolve potential false-positive skin test results or (ii) a standalone test to replace the single intradermal comparative cervical tuberculin (SICCT) skin test. Our results demonstrated significantly better relative test sensitivity when the DIT was evaluated in a serial test scenario. Direct comparison of pre- and post-skin test blood samples revealed that the SICCT test induced significant boosting of the gamma interferon response in M. bovis-infected animals to both the ESAT-6–CFP-10 and Rv3615c peptide cocktails that comprise the DIT, which persisted for the ESAT-6–CFP-10 reagent for at least 14 days. Importantly, no similar boosting effects were observed in noninfected BCG vaccinates, suggesting that DIVA blood testing after a recent skin test would have minimal impact on test specificity.
KEYWORDS: blood test, bovine tuberculosis, DIVA, gamma interferon, Mycobacterium bovis, tuberculin skin test
INTRODUCTION
Bovine tuberculosis (TB) in cattle, caused predominantly by infection with Mycobacterium bovis, poses a significant economic animal health problem, where it has been estimated that globally the disease costs $3 billion U.S. annually (1). In Great Britain, bovine TB control measures are based on a test and cull program using the single intradermal comparative cervical tuberculin (SICCT) skin test as a primary diagnostic test, but also under certain circumstances the whole-blood interferon gamma (IFN-γ) release assay as an ancillary test to maximize detection of M. bovis-infected cattle. Despite this, the annual number of herd breakdowns remains high in the West of England and Wales, and thus new control strategies, including cattle vaccination, are under consideration. Studies with cattle have shown that M. bovis BCG vaccination significantly protected cattle following experimental infection with M. bovis, with some animals displaying no evidence of disease pathology in either the lungs or associated lymph nodes (2–4). However, these studies also highlight that BCG is not fully protective in all vaccinated animals, and as such, should BCG vaccination be implemented as part of a control program, surveillance tests would still be required to identify BCG-vaccinated animals that develop bovine TB following exposure to M. bovis. BCG vaccination sensitizes a proportion of cattle to bovine tuberculin, which compromises the use of the current surveillance tests (SICCT and IFN-γ release assay) that use this test reagent. Thus, a complementary diagnostic test capable of discriminating infected from vaccinated animals (the so-called “DIVA” test) would be required to allow BCG vaccination to run alongside the current testing and slaughter control strategies. To this end, we have modified the IFN-γ release assay by replacing the tuberculin reagents with two peptide cocktails that have been shown to induce responses in blood from M. bovis-infected cattle but not from BCG vaccinates (5, 6). These previous studies evaluated the sensitivity of the DIVA IFN-γ blood test (DIT) by using samples from nonvaccinated naturally infected “field reactor” cattle. Given that the target population for this test would be BCG-vaccinated animals that failed to be protected from M. bovis infection, we repeated this assessment but in the target population of BCG-vaccinated and -infected cattle. To this end, we utilized several BCG vaccine-M. bovis challenge studies to assess the DIT performance in BCG-vaccinated cattle subsequently experimentally infected with M. bovis and in which infection was confirmed postmortem by analysis of pathology and mycobacterial culture. In addition, we explored different scenarios under which a DIT may be implemented alongside BCG vaccination: e.g., as an additional post-SICCT test to resolve potential false-positive skin test results (serial testing scenario) or as a direct replacement of the SICCT test (standalone testing scenario).
RESULTS
In order to provide an estimate for the relative sensitivity of the DIT, we combined the results of several BCG vaccine-M. bovis challenge studies (Table 1) considering two different testing scenarios. First, we evaluated the performance of the DIT when used serially to the skin test in order to resolve a positive SICCT result. In this scenario, a SICCT skin test would remain the primary diagnostic test, with the DIVA blood test then being applied to skin-test-positive animals to differentiate M. bovis-infected from false-positive animals. To this end, test results from 89 BCG-vaccinated M. bovis-infected cattle (with confirmed infection) that had tested positive in the SICCT skin test (tested after the animals had been experimentally infected with M. bovis) were analyzed. As summarized in Table 2, 86 out of 89 animals gave a positive result in the DIT, resulting in a relative test sensitivity of 96.6% (95% confidence interval [CI], 90.5% to 99.3%). This compared favorably to the standard tuberculin readout of bovine tuberculin (PPDB) minus avian tuberculin (PPDA) (B-A), where 83 out of 89 animals tested positive (relative test sensitivity, 93.3% [95% CI, 85.9% to 97.5%]). In a second scenario, we considered the possibility of the DIT as an alternative to the SICCT skin test as a primary diagnostic tool and evaluated its performance as a standalone test. To this end, test results from 73 BCG-vaccinated M. bovis-infected cattle (with confirmed infection) were analyzed. Of these animals, 53 gave a positive result in the DIT, resulting in a relative test sensitivity of 72.6% (95% CI, 60.9% to 82.4%), which was significantly lower (P < 0.0001) than that estimated under the serial testing scenario (Table 2). Lower test sensitivity was also observed for the B-A readout (87.7%) than under the serial test scenario, although this did not reach statistical significance.
TABLE 1.
Summary of the number of animals used from each vaccine trial
| Study | No. of animals used for: |
Reference | |||||
|---|---|---|---|---|---|---|---|
| DIT sensitivity analysisa |
Skin test boosting effects on DIT resultsb |
||||||
| Serial testing | Standalone testing | Infected |
Noninfected |
||||
| BCG vaccinated | Nonvaccinated | BCG vaccinated | Nonvaccinated | ||||
| 1 | 24 | 0 | 0 | 0 | 0 | 0 | 3 |
| 2 | 21 | 26 | 26 | 8 | 0 | 0 | 20 |
| 3 | 30 | 31 | 31 | 14 | 0 | 0 | 21 |
| 4 | 3 | 3 | 3 | 5 | 0 | 0 | Materials and Methods |
| 5 | 11 | 13 | 13 | 8 | 11 | 11 | Materials and Methods |
| 6 | 0 | 0 | 0 | 0 | 19 | 0 | Materials and Methods |
TABLE 2.
Relative sensitivities of the DIT for BCG-vaccinated M. bovis-infected animals under different testing scenarios
| Scenario | Testa | No. positive/total | % sensitivity (95% CI) |
|---|---|---|---|
| Serial testing | B-A | 83/89 | 93.3 (85.9–97.5) |
| DIT | 86/89 | 96.6 (90.5–99.3) | |
| Standalone testing | B-A | 64/73 | 87.7 (77.9–94.2) |
| DIT | 53/73 | 72.6 (60.9–82.4)b |
For B-A, the test is positive if the ΔOD for PPDB − PPDA is >0.1. For DIT, the test is positive if the ΔOD for ESAT-6–CFP-10 − nil and/or Rv3615c − nil is >0.1.
P < 0.0001 by Fisher's exact test (compared to the serial testing scenario).
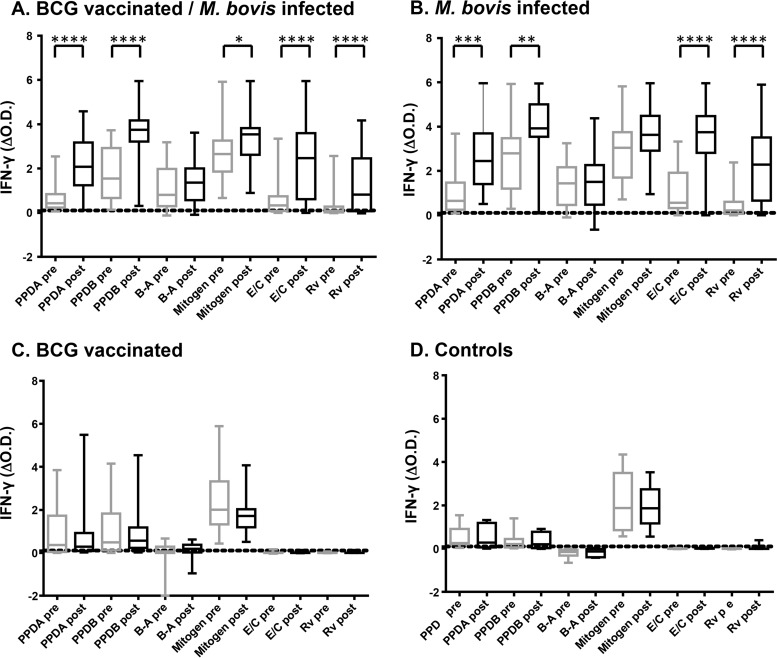
Given that a greater proportion of infected animals tested positive by the DIT when performed after animals had received the SICCT skin test, we hypothesized that the tuberculin reagents used in the skin test may be boosting a systemic T cell response to the DIVA antigens. To test this hypothesis, we directly compared IFN-γ responses in samples taken from the same animal on the day of the skin test and 5 to 8 days later. For this analysis, IFN-γ responses in a total of 73 BCG-vaccinated M. bovis-infected animals (with confirmed infection) were analyzed (Table 1); the results are summarized in Fig. 1A. Strikingly, significantly elevated levels of IFN-γ production in response to stimulation with either tuberculin (PPDA or PPDB) or DIVA peptide cocktail reagents (ESAT-6–CFP-10 or Rv3615c) were observed in blood samples collected post-SICCT skin testing from BCG-vaccinated M. bovis-infected cattle (Fig. 1A). Given that a comparative readout of PPDB − PPDA is used to determine tuberculin test results and that increased responses to both PPDB and PPDA were observed, SICCT skin testing overall had a limited and nonsignificant effect on the tuberculin B-A readout used to interpret test results as positive or negative (Fig. 1A), with 64/73 and 68/73 animals testing positive at pre- and post-skin test time points, respectively (Table 3). In contrast, SICCT skin testing had a more dramatic effect on the DIT readout, where the number of test positives significantly increased from 53/73 to 67/73 (Table 3 [P < 0.01]). Similar results were observed in nonvaccinated M. bovis-infected animals, where IFN-γ responses to tuberculin and DIVA reagents were significantly elevated when evaluated post-skin testing (Fig. 1B). Again, skin testing had no significant impact on the number of test-positive responses detected using B-A to interpret test results (33/35 and 30/35 at pre- and post-skin test time points, respectively [Table 3]). In contrast to BCG-vaccinated M. bovis-infected animals, the elevated levels of IFN-γ seen to the DIVA reagents post-skin testing had no significant impact on the number of DIT-positive responses, with 32/35 and 34/35 positive responses detected at pre- and post-skin test time points, respectively (Table 3). In these nonvaccinated M. bovis-infected animals, the level of IFN-γ induced by the ESAT-6–CFP-10 peptide cocktail, when analyzed prior to skin testing, was sufficient to indicate a positive DIT result in all but 3 animals tested. Of the remaining 3 animals, the boost in the ESAT-6–CFP-10-specific IFN-γ response seen after skin testing was sufficient to identify 2 additional animals as DIT positive.
FIG 1.
SICCT boosts IFN-γ responses to DIVA blood test reagents. The panels show antigen-induced IFN-γ production in blood samples obtained from (A) 73 BCG-vaccinated M. bovis-infected, (B) 35 M. bovis-infected, and (C) 30 BCG-vaccinated animals and (D) 11 nonvaccinated noninfected controls both on the day of (pre) and 1 week after (post) SICCT skin testing. Boxes represent the 25th, 50th, and 75th percentiles, while whiskers represent minimum and maximum values. E/C and Rv represent the ESAT-6–CFP-10 and Rv3615c peptide cocktails, respectively, while the horizontal dotted line represents the 0.1 cutoff value. ΔOD values for the stated stimuli were calculated by subtracting the OD for the nil antigen control. ****, P < 0.0001; ***, P < 0.001; **, P < 0.01; *, P < 0.5 (Friedman test with Dunn's multiple comparison test).
TABLE 3.
Effect of SICCT skin testing on DIT results
| Animal group | Testa | No. positive/total | % sensitivity (95% CI) | % specificity (95% CI) |
|---|---|---|---|---|
| BCG vaccinated M. bovis infected | Pre-skin test B-A | 64/73 | 87.7 (77.9–94.2) | NA |
| Post-skin test B-A | 68/73 | 93.2 (84.7–97.7) | NA | |
| Pre-skin test DIT | 53/73 | 72.6 (60.9–82.4) | NA | |
| Post-skin test DIT | 67/73b | 91.8 (83.0–96.9) | NA | |
| Nonvaccinated M. bovis infected | Pre-skin test B-A | 33/35 | 94.3 (80.8–99.3) | NA |
| Post-skin test B-A | 30/35 | 85.7 (69.7–95.2) | NA | |
| Pre-skin test DIT | 32/35 | 91.4 (76.9–98.2) | NA | |
| Post-skin test DIT | 34/35 | 97.1 (85.1–99.9) | NA | |
| BCG vaccinated noninfected | Pre-skin test B-A | 14/30 | NAc | 53.3 (34.3–71.7) |
| Post-skin test B-A | 19/30 | NA | 36.7 (19.9–56.1) | |
| Pre-skin test DIT | 1/30 | NA | 96.7 (82.8–99.9) | |
| Post-skin test DIT | 2/30 | NA | 93.3 (77.9–99.2) | |
| Nonvaccinated noninfected | Pre-skin test B-A | 0/11 | NA | 100 (71.5–100) |
| Post-skin test B-A | 0/11 | NA | 100 (71.5–100) | |
| Pre-skin test DIT | 0/11 | NA | 100 (71.5–100) | |
| Post-skin test DIT | 1/11 | NA | 90.9 (58.7–99.8) |
For B-A, the test is positive if the ΔOD for PPDB − PPDA is >0.1. For DIT, the test is positive if the ΔOD for either ESAT-6–CFP-10 − nil or Rv3615c − nil is >0.1.
P < 0.01 by Fisher's exact test compared to pre-skin test DIVA.
NA, not applicable.
In order to assess the impact of SICCT testing on the specificity of the DIT, we performed a similar analysis of BCG-vaccinated noninfected animals (Fig. 1C). In contrast to infected animals, no overall significant elevation in the magnitude of the IFN-γ response to any individual test reagent was observed post-skin testing. Of the 30 BCG-vaccinated noninfected animals, 14 and 19 tested positive with the B-A readout pre- and post-skin test, respectively (Table 3), although this increase in test positives was not statistically significant. In contrast, only 1/30 BCG-vaccinated noninfected animals tested positive by the DIT pre-skin test, which increased only slightly to 2 test positives post-skin test (Table 3). Finally, the effect of SICCT testing on blood responses in non-BCG-vaccinated noninfected controls was assessed. Similar to BCG vaccinates, no overall significant elevation in the magnitude of the IFN-γ response to any individual test reagent was observed post-skin testing (Fig. 1D). No animals tested positive with the B-A readout at either pre- or post-skin test time points, while only one animal tested positive to the DIVA readout, which occurred post-SICCT skin testing (Table 3).
Having shown that a recent SICCT skin test subsequently increases the IFN-γ response to the DIVA blood test reagents in M. bovis-infected animals, we were interested in the duration of this effect. The DIT was performed on 14 animals from study 5 with confirmed M. bovis infection (9 BCG-vaccinated M. bovis-infected and 5 non-BCG-vaccinated M. bovis-infected animals) at days 0, 8, and 14 post-skin testing (Fig. 2). Compared to pre-skin test data (day 0), significantly increased levels of IFN-γ in response to either of the DIVA peptide cocktail reagents (ESAT-6–CFP-10 or Rv3615c) were observed at day 8 post-skin test. For ESAT-6–CFP-10 responses, these levels remained significantly elevated at day 14 post-skin test. Although overall IFN-γ responses to the Rv3615c peptide cocktail were no longer significantly elevated at day 14 post-skin test, a greater proportion of the animals tested positive to Rv3615c at this time point than at day 0 (9/14 positive at day 14 compared to 5/14 positive at day 0), although this increase in test positives did not reach statistical significance.
FIG 2.
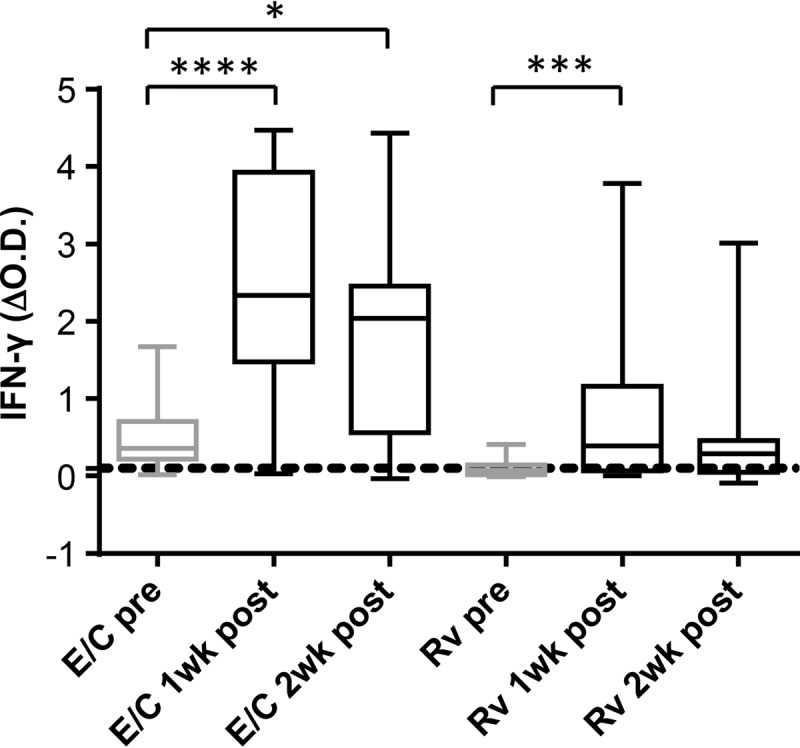
SICCT-boosted ESAT-6–CFP-10-specific IFN-γ responses persist for at least 14 days. Antigen-induced IFN-γ production in blood samples obtained from animals in study 5 with confirmed M. bovis infection (9 BCG-vaccinated M. bovis-infected and 5 non-BCG-vaccinated M. bovis-infected animals) on the day of (pre) and 8 days (1wk post) and 14 days (2wk post) after SICCT skin testing. Boxes represent the 25th, 50th, and 75th percentiles, while whiskers represent minimum and maximum values. E/C and Rv represent the ESAT-6–CFP-10 and Rv3615c peptide cocktails, respectively, while the horizontal dotted line represents the 0.1 cutoff value. ΔOD values for the stated stimuli were calculated by subtracting the OD for the nil antigen control. ****, P < 0.0001; ***, P < 0.001; *, P < 0.5 (Friedman test with Dunn's multiple comparison test).
DISCUSSION
Previous studies from our group exploring the performance of the DIT estimated a relative test sensitivity of 91% in cattle naturally infected with M. bovis (5). In agreement, our data from nonvaccinated experimentally infected cattle (Table 3, pre-skin test DIVA) show a similar level of test sensitivity. However, we appreciated that the target population for assessing the sensitivity of the DIT would be BCG-vaccinated animals that failed to be fully protected from M. bovis infection. Although not complete in all animals, BCG vaccination does provide protection from bovine TB following M. bovis challenge, as evidenced by a reduction in (i) the absolute numbers of tuberculous lesions, (ii) the overall lesion score, and (iii) the number of viable bacteria recovered (7–11). It has been shown that ESAT-6-induced IFN-γ production in experimentally infected cattle correlates positively with the degree of disease-induced pathology (12), suggesting that factors that result in limiting the degree of disease progression may impact the performance of diagnostic tests utilizing this antigen. This is important as ESAT-6 is a component of the DIT. When assessed with the pre-skin test, our results do indeed demonstrate a lower relative sensitivity of the DIT in M. bovis-infected cattle that had previously been vaccinated with BCG (72.6% [Table 3]). However, when test performance was reassessed in the same animals 1 week post-SICCT testing, the relative sensitivity of the DIT was significantly increased from 72.6% to 91.8%, a level similar to that seen in nonvaccinated M. bovis-infected animals. We speculate that although M. bovis infection drives a T cell population that produces IFN-γ in response to ESAT-6 stimulation, prior BCG vaccination reduces the level of disease pathology and mycobacterial burden to an extent that these T cell responses in some infected animals are below the limit of detection for this ex vivo DIVA blood test. However, a recent skin test with bovine tuberculin has the potential to boost these responses in vivo (potentially by increasing the frequency of ESAT-6-specific T cells), which are then more frequently detected in the ex vivo DIT.
The primary objective of this study was to determine the relative sensitivity of the DIT in disclosing BCG-vaccinated animals that failed to be protected from subsequent M. bovis challenge. To this end, we used the presence of visible lesions and/or M. bovis culture positivity to confirm infection. However, we also identified 16 BCG-vaccinated animals that exhibited no visible lesions or culture positivity following M. bovis challenge. This is a difficult group to precisely characterize. They may consist of animals that were “fully” protected by BCG vaccination. Alternatively, although protected, it is possible that there is low-level infection that is below the level of detection using the current “gold standard” tools available (e.g., the presence of visible lesions or culture positivity) which is capable of maintaining an M. bovis-specific cellular immune response. In support of this, 4 of the 16 animals (25%) tested positive in the DIT when assessed prior to SICCT skin testing, which increased to 5 (31%) when tested 1 week post-skin testing. Furthermore, we speculate that the remaining DIT-negative animals represent those in which BCG vaccination fully protected from M. bovis challenge.
Administration of the SICCT skin test resulted in the boosting of systemic mycobacterium-specific IFN-γ responses, which under some circumstances (e.g., responses to the ESAT-6–CFP-10 peptide cocktail) persisted at least 14 days post-skin testing. Previous published reports on the effect of tuberculin skin testing on in vitro IFN-γ responses are contradictory (13), highlighting the potential influence of several factors, including the nature of M. bovis infection (e.g., natural versus experimental infection), the route of skin test administration (e.g., caudal fold of the tail versus the neck region), blood storage (e.g., 8 versus 24 h), the diagnostic reagents used in either the skin test or the IFN-γ test (e.g., tuberculin versus defined antigens), and the readout used to evaluate responses (e.g., measurement of IFN-γ levels per se versus scoring of test-positive/negative responses). In our study, M. bovis-infected animals showed significant increases in both PPDA- and PPDB-induced IFN-γ levels 1 week after SICCT skin testing, suggesting that antigens present in the tuberculin reagents used in the skin test were reactivating mycobacterium-specific T cells primed earlier following infection with M. bovis. Given that this boosting effect was also observed in BCG-vaccinated M. bovis-infected animals, it is also possible that the skin test was reactivating T cells primed by the initial BCG vaccination. However, this seems unlikely as we saw no significant boosting effect in noninfected BCG-vaccinated animals (Fig. 1C). Furthermore, elevated IFN-γ responses to the ESAT-6–CFP-10 and Rv3615c peptide cocktails were observed in M. bovis-infected animals. As BCG vaccination does not prime an IFN-γ response to these reagents (5, 6), these results support the interpretation that SICCT skin testing was reactivating T cells primed by M. bovis infection. In addition, a small but significant elevated mitogen-induced IFN-γ response was observed in the BCG-vaccinated M. bovis-infected group. As skin testing did not induce an increase in the unstimulated control samples for these animals (data not shown), there was no evidence that the assays performed after skin testing had higher overall optical density (OD) values. Thus, we speculate that SICCT skin testing may also induce a state of “hyperresponsiveness” to stimulation in these animals. However, this needs to be taken with some caution as this phenomenon was not observed for the other animal groups studied.
Although the SICCT test boosted PPDA- and PPDB-induced IFN-γ responses in M. bovis-infected animals, this had no significant impact on overall test positivity rates as these reagents are used as a comparative readout (i.e., B-A readout). In contrast, significantly more M. bovis-infected animals tested positive to the DIT when evaluated post-skin testing. This was due to the fact that the SICCT test boosted peptide-specific IFN-γ responses but had no impact on the nil antigen control used in the quantification of the DIT (data not shown). Importantly, the SICCT test did not significantly boost IFN-γ levels to the DIVA reagents in noninfected animals, demonstrating that the specificity of these reagents would be maintained if used post-skin testing in either BCG vaccinates or nonvaccinated controls.
The serial testing scenario described in this article provides an attractive approach to DIVA testing alongside BCG vaccination for the following reason: although BCG vaccination sensitized cattle to the tuberculin-based SICCT skin test, where 80% of vaccinated but uninfected cattle tested positive 6 months after vaccination, the proportion of false-positive responses declined dramatically to approximately 10% at 9 months postvaccination (14). Therefore, if used at a suitable period of time after BCG vaccination, the standard SICCT skin test could still be used as the primary diagnostic test, with an additional DIT only being applied to the skin test positives (which theoretically should be comprised of fewer false-positive animals), thus reducing the number and cost of additional tests required. One caveat, however, is that the initial disclosing SICCT skin test is not 100% sensitive. Indeed, SICCT test sensitivities in different countries and settings are reported to range from 75% to 95% (15, 16), with a recent study estimating the relative SICCT test sensitivity in Great Britain as 85% (95% CI, 78% to 91%) or 81% (95% CI, 70% to 89%) for the severe or standard interpretation, respectively (17). Thus, in the serial testing scenario, it is possible that truly M. bovis-infected animals may be missed by the initial skin test and not subjected to the DIT. However, as our data highlight, the relative sensitivity of the DIT is significantly lower when used as a standalone test, and to fully maximize its sensitivity to detect infected animals, blood samples should be taken after a recent skin test, a situation that has also been suggested for serology testing for bovine TB (18, 19). Importantly, the data presented herein demonstrate that this would have minimal impact on the specificity of the DIT.
MATERIALS AND METHODS
Animals. (i) BCG-vaccinated M. bovis-infected animals.
Data for assessing the performance of the DIT under different testing scenarios were collected from several independent BCG vaccine-M. bovis challenge studies (summarized in Table 1). Details of the vaccination and infection protocols for studies 1 (3), 2 (20), and 3 (21) are as previously described. For study 4, male Holstein Friesian calves were vaccinated with BCG Danish SSI subcutaneously (s.c.) (equivalent to 5 human doses) at approximately 6 weeks of age. Eleven weeks after vaccination, all animals were experimentally infected with 9 × 103 CFU of M. bovis AF2122/97 administered endobronchially via an endoscope. For study 5, male Holstein Friesian and female Aberdeen Angus Cross or Limousin Cross calves were vaccinated with BCG Danish SSI s.c. (equivalent to 5 human doses) at approximately 6 weeks of age. Fifty-two weeks after the initial vaccination, some animals were revaccinated with either an equivalent dose of BCG or with 2 × 109 PFU of an adenovirus construct containing Mycobacterium tuberculosis Ag85A. One hundred four weeks after the initial vaccination, all animals were experimentally infected with 1.0 × 104 to 1.5 × 104 CFU of M. bovis AF2122/97 administered endobronchially via an endoscope.
The tuberculin skin test was performed 10 to 12 weeks postinfection. With the exception of study 1, blood sampling for the Bovigam assay was performed just prior to performing the skin test for evaluation of the DIT in the standalone testing scenario. Blood sampling was also performed 5 to 8 days post-skin testing in studies 1 to 5 for evaluation of the DIT in the serial testing scenario and again in some animals from study 5 at day 14 post-skin testing.
In all vaccine-challenge studies (studies 1 to 5), postmortems were performed to determine the level of pathology and M. bovis burden. For the serial testing scenario, only data from SICCT skin-test-positive animals with M. bovis infection (confirmed via pathology and/or culture) were included in the sensitivity analysis, whereas for the standalone testing scenario, data from all animals demonstrating M. bovis infection (irrespective of the SICCT skin test result) were used in the sensitivity analysis.
(ii) Non-BCG-vaccinated M. bovis-infected animals.
Data were collected from the non-BCG-vaccinated experimentally M. bovis-infected controls from studies 2 to 5 (Table 1). Postmortem, tuberculin skin testing, and blood sampling for the Bovigam assay were performed as described above.
(iii) BCG-vaccinated noninfected animals.
Animals were from Great Britain (study 5) and Spain (study 6), and all were more than 6 months of age when tested. The animals included both male and female cattle and a mix of breeds (Holstein, Holstein Friesian, Aberdeen Angus Cross, and Norwegian Red Cross). In total, samples were obtained from 30 animals vaccinated with BCG Danish SSI s.c. (equivalent to 5 human doses). These animals were sourced from herds officially bovine TB free and without recent history of TB. Blood samples for the Bovigam assay were taken at the time of tuberculin skin testing and again 7 days later.
(iv) Non-BCG-vaccinated noninfected animals.
Data were collected from the non-BCG-vaccinated noninfected controls from study 5 (Table 1). These animals were sourced from Great Britain herds officially bovine TB free and without recent history of TB and included both male and female cattle from different breeds (Holstein Friesian Cross and Aberdeen Angus Cross breeds). Blood samples for the Bovigam assay were taken at the time of tuberculin skin testing and again 7 days later.
(v) Ethics statement.
Animal procedures for studies in the United Kingdom, New Zealand, and Spain were approved by the following ethics committees, respectively: (i) Animal and Plant Health Agency (APHA) Animal Welfare and Ethical Review Body, (ii) AgResearch Grasslands Animal Ethics Committee (permit no. 12413), and (iii) Ethical Committee of the Autonomous Region of Madrid (reference no. 10/397359.9/11).
Tuberculin skin test.
Animals were inoculated intradermally at separate sites on the side of the neck with 0.1-ml volumes containing either 2,500 IU of avian tuberculin (PPDA) or 3,000 IU of bovine tuberculin (PPDB) (both Prionics, Switzerland). The skin fold thickness was measured with calipers prior to and 72 h after inoculation of the PPDs. Positive responses to the SICCT test were recorded if the Δ skin thickness for PPDB – PPDA was >4 mm (standard OIE [Office International des Epizooties] and U.K. test interpretation).
Bovigam assay.
Heparinized blood samples (250 μl) in duplicate wells of a 96-well plate or 1.5-ml blood samples in 24-well plates were stimulated for 20 to 24 h at 37°C in 5% CO2 with the following: (i) PPDB (either Prionics [300-U/ml final concentration] or APHA, Weybridge [10-μg/ml final concentration; used in study 1 only]), (ii) PPDA (either Prionics [250-U/ml final concentration] or APHA [10-μg/ml final concentration; used in study 1 only]), (iii) ESAT-6–CFP-10 peptide cocktail (Pepceuticals, U.K.; 5-μg/ml/peptide final concentration), (iv) Rv3615c peptide cocktail (Pepceuticals; 5-μg/ml/peptide final concentration), (v) a positive mitogen control of pokeweed mitogen (Sigma, U.K.; 10-μg/ml final concentration, used in studies 2 to 5) or staphylococcal enterotoxin B (Sigma; 1-μg/ml final concentration, used in studies 1 and 6), and (vi) a negative control (nil) of RPMI 1640 alone (Sigma). All cultures were performed within 24 h of drawing the blood sample. Tissue culture plates were centrifuged (300 × g for 10 min) to pellet cells, cell-free supernatants were harvested, and the IFN-γ content was measured using the Bovigam enzyme-linked immunosorbent assay (ELISA) kit (Prionics) following the manufacturer's instructions. The Multiskan Ascent (Thermo Labsystems) plate reader was used to measure absorbance values at a wavelength of 450 nm. A positive response to the tuberculin reagents was recorded if the ΔOD for PPDB – PPDA is >0.1, while a positive response to the DIT was recorded if the ΔOD for either ESAT-6–CFP-10 – nil or Rv3615c – nil is >0.1. All animals tested showed a positive IFN-γ response to the mitogen control stimulation (OD > 0.45).
Statistical analysis.
Statistical analysis was performed using GraphPad Prism 5 software (GraphPad Software, Inc.). Comparison of DIT sensitivities was performed using the Fisher exact test, while comparison of IFN-γ levels was performed using the Friedman test (repeated measures nonparametric analysis of variance [ANOVA]) with Dunn's multiple comparison test.
ACKNOWLEDGMENTS
This study was funded by the Department for Environment, Food and Rural affairs (DEFRA; project codes SE3224, SE3227, SE3237, SE3266, and SE3268), U.K.
We are indebted to the staff of the Animal Service Unit for their dedication to the welfare of the animals housed at APHA, Weybridge, and to the Mycobacteria Unit of VISAVET and Alberto Diez (MAEVA-SERVET S.L.) for technical assistance. APHA hold 3 patents for the use of Rv3615c in diagnostic tests for bovine tuberculosis (WO/2009/060184, WO/2011/135369, and WO/2012/010875).
REFERENCES
- 1.Waters WR, Palmer MV, Buddle BM, Vordermeier HM. 2012. Bovine tuberculosis vaccine research: historical perspectives and recent advances. Vaccine 30:2611–2622. doi: 10.1016/j.vaccine.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 2.Buddle BM, Hewinson RG, Vordermeier HM, Wedlock DN. 2013. Subcutaneous administration of a 10-fold-lower dose of a commercial human tuberculosis vaccine, Mycobacterium bovis bacillus Calmette-Guerin Danish, induced levels of protection against bovine tuberculosis and responses in the tuberculin intradermal test similar to those induced by a standard cattle dose. Clin Vaccine Immunol 20:1559–1562. doi: 10.1128/CVI.00435-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dean G, Whelan A, Clifford D, Salguero FJ, Xing Z, Gilbert S, McShane H, Hewinson RG, Vordermeier M, Villarreal-Ramos B. 2014. Comparison of the immunogenicity and protection against bovine tuberculosis following immunization by BCG-priming and boosting with adenovirus or protein based vaccines. Vaccine 32:1304–1310. doi: 10.1016/j.vaccine.2013.11.045. [DOI] [PubMed] [Google Scholar]
- 4.Thom ML, McAulay M, Vordermeier HM, Clifford D, Hewinson RG, Villarreal-Ramos B, Hope JC. 2012. Duration of immunity against Mycobacterium bovis following neonatal vaccination with bacillus Calmette-Guerin Danish: significant protection against infection at 12, but not 24, months. Clin Vaccine Immunol 19:1254–1260. doi: 10.1128/CVI.00301-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sidders B, Pirson C, Hogarth PJ, Hewinson RG, Stoker NG, Vordermeier HM, Ewer K. 2008. Screening of highly expressed mycobacterial genes identifies Rv3615c as a useful differential diagnostic antigen for the Mycobacterium tuberculosis complex. Infect Immun 76:3932–3939. doi: 10.1128/IAI.00150-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vordermeier HM, Whelan A, Cockle PJ, Farrant L, Palmer N, Hewinson RG. 2001. Use of synthetic peptides derived from the antigens ESAT-6 and CFP-10 for differential diagnosis of bovine tuberculosis in cattle. Clin Diagn Lab Immunol 8:571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buddle BM, de Lisle GW, Pfeffer A, Aldwell FE. 1995. Immunological responses and protection against Mycobacterium bovis in calves vaccinated with a low dose of BCG. Vaccine 13:1123–1130. doi: 10.1016/0264-410X(94)00055-R. [DOI] [PubMed] [Google Scholar]
- 8.Buddle BM, Wedlock DN, Parlane NA, Corner LA, De Lisle GW, Skinner MA. 2003. Revaccination of neonatal calves with Mycobacterium bovis BCG reduces the level of protection against bovine tuberculosis induced by a single vaccination. Infect Immun 71:6411–6419. doi: 10.1128/IAI.71.11.6411-6419.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hope JC, Thom ML, McAulay M, Mead E, Vordermeier HM, Clifford D, Hewinson RG, Villarreal-Ramos B. 2011. Identification of surrogates and correlates of protection in protective immunity against Mycobacterium bovis infection induced in neonatal calves by vaccination with M. bovis BCG Pasteur and M. bovis BCG Danish. Clin Vaccine Immunol 18:373–379. doi: 10.1128/CVI.00543-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hope JC, Thom ML, Villarreal-Ramos B, Vordermeier HM, Hewinson RG, Howard CJ. 2005. Vaccination of neonatal calves with Mycobacterium bovis BCG induces protection against intranasal challenge with virulent M. bovis. Clin Exp Immunol 139:48–56. doi: 10.1111/j.1365-2249.2005.02668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wedlock DN, Denis M, Vordermeier HM, Hewinson RG, Buddle BM. 2007. Vaccination of cattle with Danish and Pasteur strains of Mycobacterium bovis BCG induce different levels of IFNgamma post-vaccination, but induce similar levels of protection against bovine tuberculosis. Vet Immunol Immunopathol 118:50–58. doi: 10.1016/j.vetimm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Vordermeier HM, Chambers MA, Cockle PJ, Whelan AO, Simmons J, Hewinson RG. 2002. Correlation of ESAT-6-specific gamma interferon production with pathology in cattle following Mycobacterium bovis BCG vaccination against experimental bovine tuberculosis. Infect Immun 70:3026–3032. doi: 10.1128/IAI.70.6.3026-3032.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schiller I, Vordermeier HM, Waters WR, Whelan AO, Coad M, Gormley E, Buddle BM, Palmer M, Thacker T, McNair J, Welsh M, Hewinson RG, Oesch B. 2010. Bovine tuberculosis: effect of the tuberculin skin test on in vitro interferon gamma responses. Vet Immunol Immunopathol 136:1–11. doi: 10.1016/j.vetimm.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Whelan AO, Coad M, Upadhyay BL, Clifford DJ, Hewinson RG, Vordermeier HM. 2011. Lack of correlation between BCG-induced tuberculin skin test sensitisation and protective immunity in cattle. Vaccine 29:5453–5458. doi: 10.1016/j.vaccine.2011.05.057. [DOI] [PubMed] [Google Scholar]
- 15.de la Rua-Domenech R, Goodchild AT, Vordermeier HM, Hewinson RG, Christiansen KH, Clifton-Hadley RS. 2006. Ante mortem diagnosis of tuberculosis in cattle: a review of the tuberculin tests, gamma-interferon assay and other ancillary diagnostic techniques. Res Vet Sci 81:190–210. doi: 10.1016/j.rvsc.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Monaghan ML, Doherty ML, Collins JD, Kazda JF, Quinn PJ. 1994. The tuberculin test. Vet Microbiol 40:111–124. doi: 10.1016/0378-1135(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 17.Karolemeas K, de la Rua-Domenech R, Cooper R, Goodchild AV, Clifton-Hadley RS, Conlan AJ, Mitchell AP, Hewinson RG, Donnelly CA, Wood JL, McKinley TJ. 2012. Estimation of the relative sensitivity of the comparative tuberculin skin test in tuberculous cattle herds subjected to depopulation. PLoS One 7:e43217. doi: 10.1371/journal.pone.0043217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casal C, Diez-Guerrier A, Alvarez J, Rodriguez-Campos S, Mateos A, Linscott R, Martel E, Lawrence JC, Whelan C, Clarke J, O'Brien A, Dominguez L, Aranaz A. 2014. Strategic use of serology for the diagnosis of bovine tuberculosis after intradermal skin testing. Vet Microbiol 170:342–351. doi: 10.1016/j.vetmic.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 19.Lyashchenko K, Whelan AO, Greenwald R, Pollock JM, Andersen P, Hewinson RG, Vordermeier HM. 2004. Association of tuberculin-boosted antibody responses with pathology and cell-mediated immunity in cattle vaccinated with Mycobacterium bovis BCG and infected with M. bovis. Infect Immun 72:2462–2467. doi: 10.1128/IAI.72.5.2462-2467.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dean GS, Clifford D, Whelan AO, Tchilian EZ, Beverley PC, Salguero FJ, Xing Z, Vordermeier HM, Villarreal-Ramos B. 2015. Protection induced by simultaneous subcutaneous and endobronchial vaccination with BCG/BCG and BCG/adenovirus expressing antigen 85A against Mycobacterium bovis in cattle. PLoS One 10:e0142270. doi: 10.1371/journal.pone.0142270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parlane NA, Shu D, Subharat S, Wedlock DN, Rehm BH, de Lisle GW, Buddle BM. 2014. Revaccination of cattle with bacille Calmette-Guerin two years after first vaccination when immunity has waned, boosted protection against challenge with Mycobacterium bovis. PLoS One 9:e106519. doi: 10.1371/journal.pone.0106519. [DOI] [PMC free article] [PubMed] [Google Scholar]