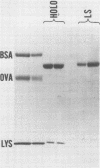
Abstract
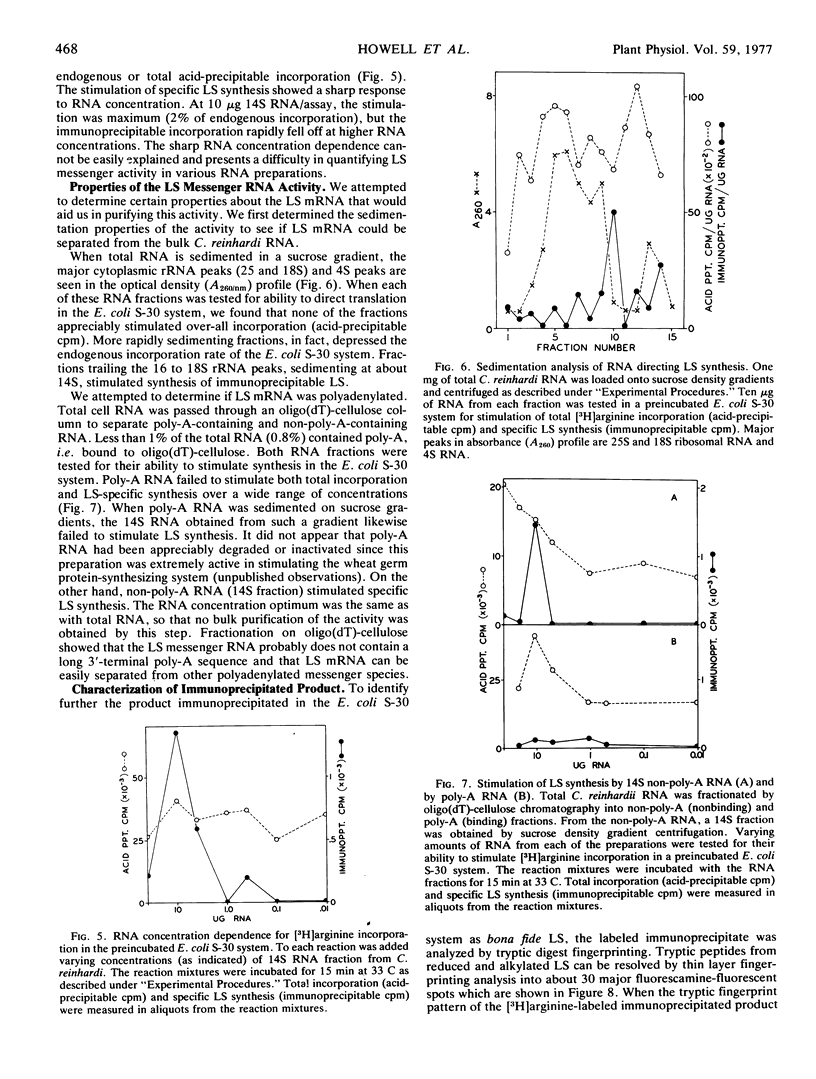
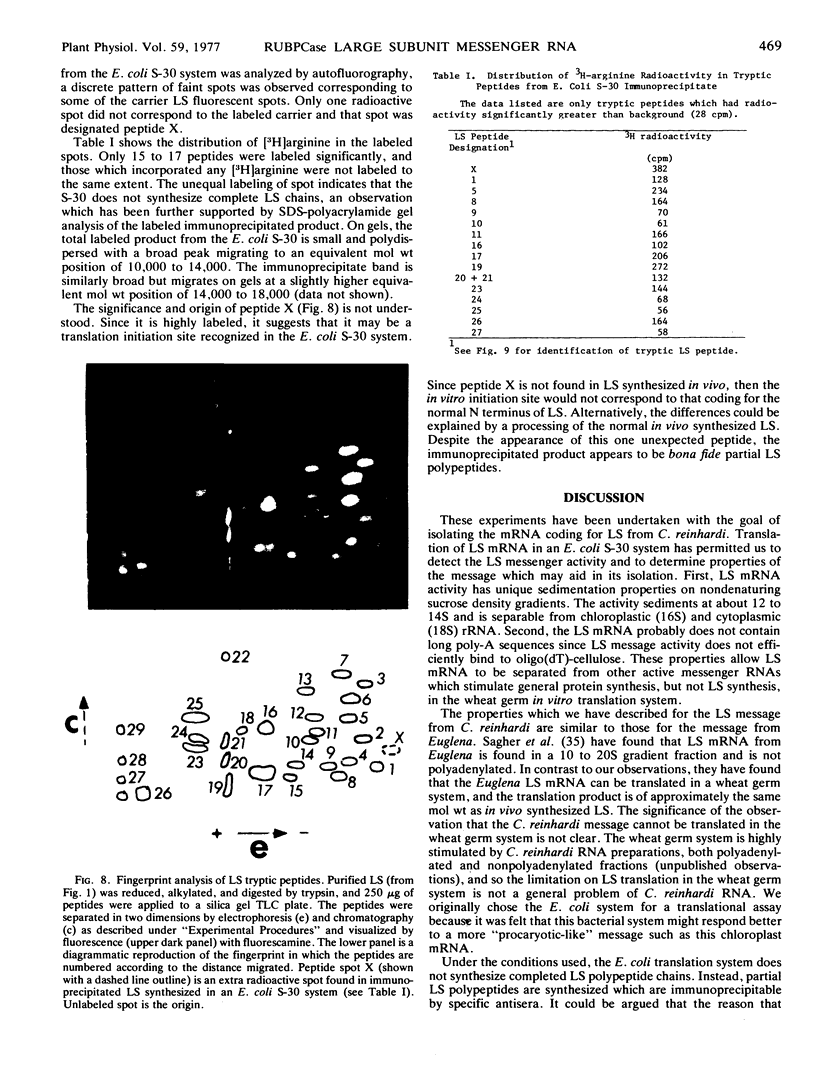
Properties of the mRNA coding for the large subunit of ribulose-1,5-bisphosphate carboxylase from Chlamydomonas reinhardi were determined. Large subunit synthesis, directed by RNA from partially purified whole cell extracts, was detected by specific immunoprecipitation of polypeptide products synthesized in a heterologous translation system derived from Escherichia coli. Large subunit synthesis showed sharp RNA concentration dependence in an E. coli translation system, and at optimal RNA concentrations, immunoprecipitable large subunit synthesis accounted for 2% of the total incorporation. Large subunit messenger activity sedimented at 12 to 14S on nondenaturing sucrose gradients and did not bind to oligo(dT)-cellulose suggesting the mRNA is not polyadenylated. The immunoprecipitable products translated in vitro are not complete polypeptide chains, but are smaller peptides identifiable as large subunit fragments by tryptic fingerprint analysis. No immunoprecipitable product was obtained when similar RNA fractions were tested in a wheat germ translation system.
Full text
PDF

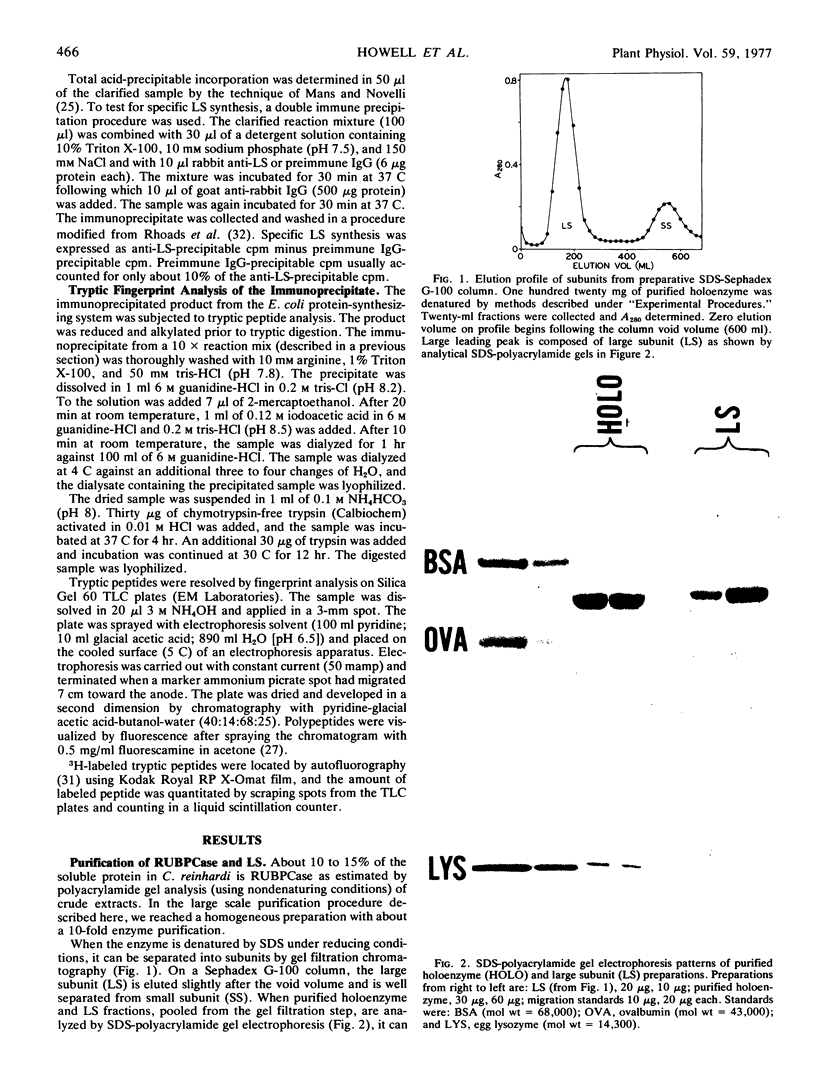
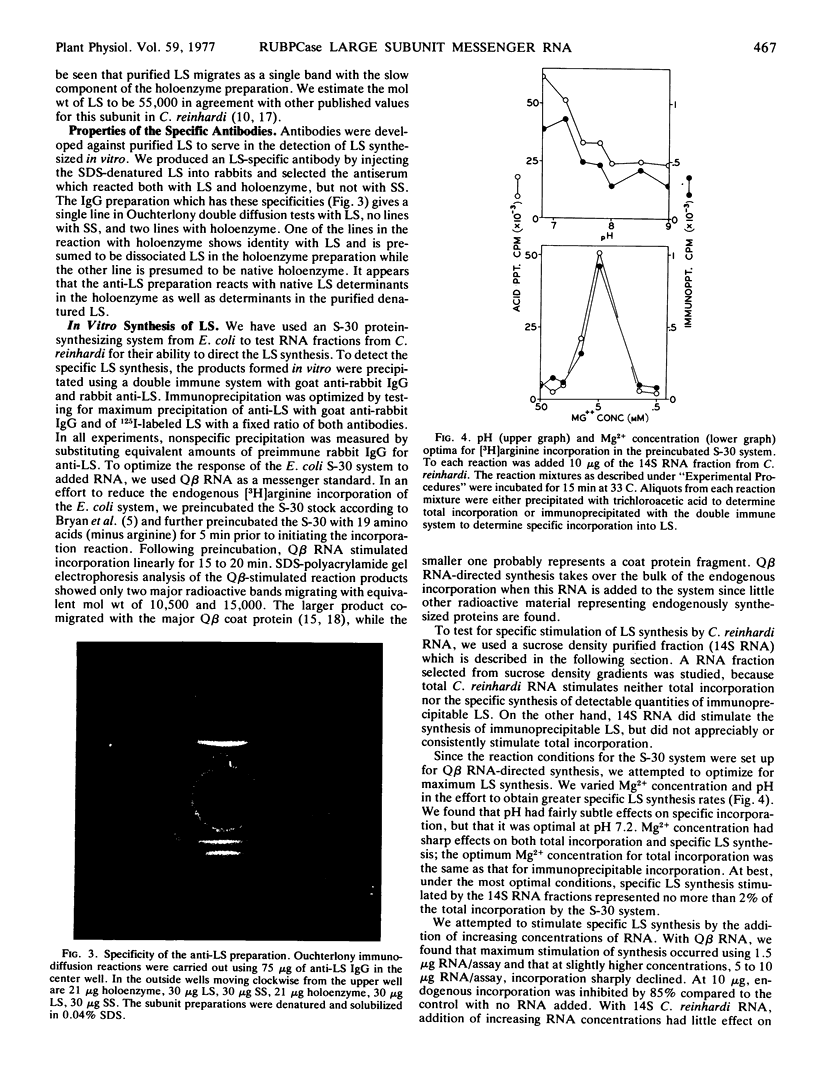

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T. S., Eisenberg D., Eiserling F. A., Weissman L. The structure of form I crystals of D-ribulose-1,5-diphosphate carboxylase. J Mol Biol. 1975 Feb 5;91(4):391–399. doi: 10.1016/0022-2836(75)90267-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair G. E., Ellis R. J. Protein synthesis in chloroplasts. I. Light-driven synthesis of the large subunit of fraction I protein by isolated pea chloroplasts. Biochim Biophys Acta. 1973 Aug 24;319(2):223–234. doi: 10.1016/0005-2787(73)90013-0. [DOI] [PubMed] [Google Scholar]
- Blair G. E., Ellis R. J. Protein synthesis in chloroplasts. I. Light-driven synthesis of the large subunit of fraction I protein by isolated pea chloroplasts. Biochim Biophys Acta. 1973 Aug 24;319(2):223–234. doi: 10.1016/0005-2787(73)90013-0. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bryan R. N., Sugiura M., Hayashi M. DNA- dependent RNA-directed protein synthesis in vitro. I. Extent of genome transcription. Proc Natl Acad Sci U S A. 1969 Feb;62(2):483–489. doi: 10.1073/pnas.62.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattolico R. A., Jones R. F. Isolation of stable ribosomal RNA from whole cells of Chlamydomonas reinhardtii. Biochim Biophys Acta. 1972 May 10;269(2):259–264. doi: 10.1016/0005-2787(72)90435-2. [DOI] [PubMed] [Google Scholar]
- Chan P. H., Wildman S. G. Chloroplast DNA codes for the primary structure of the large subunit of fraction I protein. Biochim Biophys Acta. 1972 Sep 14;277(3):677–680. doi: 10.1016/0005-2787(72)90115-3. [DOI] [PubMed] [Google Scholar]
- Gelvin S., Howell S. H. Identification and Precipitation of the Polyribosomes in Chlamydomonas reinhardi Involved in the Synthesis of the Large Subunit of d-ribulose-1,5-bisphosphate Carboxylase. Plant Physiol. 1977 Mar;59(3):471–477. doi: 10.1104/pp.59.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givan A. L., Criddle R. S. Ribulosediphosphate carboxylase from Chlamydomonas reinhardi: purification, properties and its mode of synthesis in the cell. Arch Biochem Biophys. 1972 Mar;149(1):153–163. doi: 10.1016/0003-9861(72)90309-8. [DOI] [PubMed] [Google Scholar]
- Gooding L. R., Roy H., Jagendorf A. T. Immunological identification of nascent subunits of wheat ribulose diphosphate carboxylase on ribosomes of both chloroplast and cytoplasmic origin. Arch Biochem Biophys. 1973 Nov;159(1):324–335. doi: 10.1016/0003-9861(73)90458-x. [DOI] [PubMed] [Google Scholar]
- Gray J. C., Kekwick R. G. The synthesis of the small subunit of ribulose 1,5-bisphosphate carboxylase in the french bean Phaseolus vulgaris. Eur J Biochem. 1974 May 15;44(2):491–500. doi: 10.1111/j.1432-1033.1974.tb03507.x. [DOI] [PubMed] [Google Scholar]
- Gray J. C., Kerwick R. G. An immunological investigation of the structure and function of ribulose 1,5-bisphosphate carboxylase. Eur J Biochem. 1974 May 15;44(2):481–489. doi: 10.1111/j.1432-1033.1974.tb03506.x. [DOI] [PubMed] [Google Scholar]
- Hartley M. R., Wheeler A., Ellis R. J. Protein synthesis in chloroplasts. V. Translation of messenger RNA for the large subunit of fraction I protein in a heterologous cell-free system. J Mol Biol. 1975 Jan 5;91(1):67–77. doi: 10.1016/0022-2836(75)90372-1. [DOI] [PubMed] [Google Scholar]
- Horiuchi K., Webster R. E., Matsuhashi S. Gene products of bacteriophage Q beta. Virology. 1971 Aug;45(2):429–439. doi: 10.1016/0042-6822(71)90343-6. [DOI] [PubMed] [Google Scholar]
- Kawashima N., Wildman S. G. Studies on fraction I protein. IV. Mode of inheritance of primary structure in relation to whether chloroplast or nuclear DNA contains the code for a chloroplast protein. Biochim Biophys Acta. 1972 Feb 23;262(1):42–49. doi: 10.1016/0005-2787(72)90217-1. [DOI] [PubMed] [Google Scholar]
- Kung S. Tobacco fraction 1 protein: a unique genetic marker. Science. 1976 Feb 6;191(4226):429–434. doi: 10.1126/science.1108201. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- McFadden B. A. Autotrophic CO2 assimilation and the evolution of ribulose diphosphate carboxylase. Bacteriol Rev. 1973 Sep;37(3):289–319. doi: 10.1128/br.37.3.289-319.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez E., Lai C. Y. Reaction of peptides with fluorescamine on paper after chromatography or electrophoresis. Anal Biochem. 1975 May 12;65(1-2):281–292. doi: 10.1016/0003-2697(75)90511-4. [DOI] [PubMed] [Google Scholar]
- RABIN B. R., TROWN P. W. INHIBITION OF CARBOXYDISMUTASE BY IODOACETAMIDE. Proc Natl Acad Sci U S A. 1964 Mar;51:497–501. doi: 10.1073/pnas.51.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randerath K. An evaluation of film detection methods for weak beta-emitters, particularly tritium. Anal Biochem. 1970 Mar;34:188–205. doi: 10.1016/0003-2697(70)90100-4. [DOI] [PubMed] [Google Scholar]
- Rhoads R. E., McKnight G. S., Schimke R. T. Quantitative measurement of ovalbumin messenger ribonucleic acid activity. Localization in polysomes, induction by estrogen, and effect of actinomycin D. J Biol Chem. 1973 Mar 25;248(6):2031–2039. [PubMed] [Google Scholar]
- Roy H., Patterson R., Jagendorf A. T. Identification of the small subunit of ribulose 1,5-bisphosphate carboxylase as a product of wheat leaf cytoplasmic ribosomes. Arch Biochem Biophys. 1976 Jan;172(1):64–73. doi: 10.1016/0003-9861(76)90048-5. [DOI] [PubMed] [Google Scholar]
- Rutner A. C., Lane M. D. Nonidentical subunits of ribulose diphosphate carboxylase. Biochem Biophys Res Commun. 1967 Aug 23;28(4):531–537. doi: 10.1016/0006-291x(67)90346-4. [DOI] [PubMed] [Google Scholar]
- Sagher D., Grosfeld H., Edelman M. Large subunit ribulosebisphosphate carboxylase messenger RNA from Euglena chloroplasts. Proc Natl Acad Sci U S A. 1976 Mar;73(3):722–726. doi: 10.1073/pnas.73.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueoka N., Chiang K. S., Kates J. R. Deoxyribonucleic acid replication in meiosis of Chlamydomonas reinhardi. I. Isotopic transfer experiments with a strain producing eight zoospores. J Mol Biol. 1967 Apr 14;25(1):47–66. doi: 10.1016/0022-2836(67)90278-1. [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Akazawa T. Structure and function of chloroplast proteins. I. Subunit structure of wheat-fraction-I protein. J Biochem. 1967 Oct;62(4):474–482. doi: 10.1093/oxfordjournals.jbchem.a128691. [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Ito T., Akazawa T. Subunit structure of ribulose 1,5-diphosphate carboxylase from Chlorella ellipsoidea. Biochemistry. 1971 Aug 31;10(18):3406–3411. doi: 10.1021/bi00794a014. [DOI] [PubMed] [Google Scholar]
- Weber K., Kuter D. J. Reversible denaturation of enzymes by sodium dodecyl sulfate. J Biol Chem. 1971 Jul 25;246(14):4504–4509. [PubMed] [Google Scholar]