Abstract
Glioblastomas (GBM) are very difficult to treat and their aggressiveness is one of the main reasons for this as well as for the frequent recurrences. MicroRNAs post-transcriptionally regulate their target genes through interaction between their seed sequence and 3′UTR of the target mRNAs. We previously reported that miR-296-3p is regulated by neurofibromatosis 2 (NF2) and enhances the invasiveness of GBM cells via SOCS2/STAT3. In this study, we investigated whether miR-296-5p, which originates from the same precursor miRNA as miR-296-3p, can increase the invasiveness of GBM cells. It was observed that miR-296-5p potentiated the invasion of various GBM cells including LN229, T98G, and U87MG. Through bioinformatics approaches, two genes were identified as miR-296-5p targets: caspase-8 (CASP8) and nerve growth factor receptor (NGFR). From results obtained from Ago2 immunoprecipitation and luciferase assays, we found that miR-296-5p downregulates CASP8 and NGFR through direct interaction between seed sequence of the miRNA and 3′UTR of the target mRNA. Knockdown of CASP8 or NGFR also increased the invasive ability of GBM cells, indicating that CASP8 and NGFR are involved in potentiation of invasiveness by miR-296-5p. Consistent with our findings, CASP8 was downregulated in brain metastatic lung cancer cells, which have a high level of miR-296-5p, compared to parental cells, suggesting that miR-296-5p may be generally associated with the acquisition of invasiveness. Collectively, our results implicate miR-296-5p as a potential cause of invasiveness in cancer and suggest it as a promising therapeutic target for GBM.
Keywords: caspase-8, glioblastoma, invasiveness, miR-296-5p, nerve growth factor receptor
INTRODUCTION
Glioblastoma multiforme (GBM) is the most aggressive and lethal type of brain tumor in adults (Lee et al., 2014; Omuro and DeAngelis, 2013; Thomas et al., 2014). Due to the extremely high invasiveness, approximately 70% of GBM patients suffer from recurrence. Moreover, the invasive ability of GBM cells renders them incurable by current treatment options like surgery, radiotherapy, and chemotherapy. For these reasons, a better understanding of the molecular mechanisms underlying the acquisition of invasiveness will be of help in developing new therapeutic strategies for GBM treatment.
MicroRNAs (miRNA) are small non-coding RNAs with approximately 20~23 nucleotides. They are transcribed by RNA polymerase II and then cleaved sequentially by Drosha and Dicer to form miRNA. They are important in post-transcriptional gene regulation as they can suppress the expression of their target gene through complementary base pairing between their seed sequence and 3′UTR of the target mRNA.
Caspase-8 (CASP8) is a cysteine protease, which triggers apoptotic cell death in response to receptor-mediated extra-cellular stimuli (Lee et al., 2015; Oberst and Green, 2011). CASP8 is known to play a critical role in the processes of extrinsic apoptosis, which is mediated by the activation of death receptors such as those belonging to the tumor necrosis receptor (TNF) family (Wilson et al., 2009). Loss of CASP8 in neuroblastomas is associated with increased meta-static potential (Stupack et al., 2006). CASP8 is generally downregulated in metastatic cells in vivo compared to that in parental cells. Nerve growth factor receptor (NGFR), which is also termed as p75NTR, is a glycosylated trans-membrane receptor without a cytoplasmic tyrosine kinase domain. It is known to play important roles in cell survival, migration, and invasion through interaction with several ligands and co-receptors (Barker, 2004; Reichardt, 2006). NGFR functions as a tumor suppressor by negatively regulating cell proliferation and metastatic potential including migratory and invasive abilities.
In a previous study, we demonstrated that knockdown of neurofibromatosis 2 (NF2) leads to nuclear localization and the transactivation of YAP by inducing its dephosphorylation (Lee et al., 2016). Nuclear YAP transcriptionally increases the expression of miR-296-3p, which potentiates the invasiveness of GBM cells via the miR-296-3p/SOCS2/STAT3 axis. During the process of miRNA biogenesis, miR-296-5p is transcribed concomitant with miR-296-3p. In this study, we investigated the effect of miR-296-5p, which is a partner miRNA to miR-296-3p, on the invasiveness of GBM cells. We found that miR-296-5p activates the invasive activity of GBM cells (LN229, T98G, and U87MG) by suppressing the expression of CASP8 and NGFR. In conclusion, our results indicate that both strands of mir-296, miR-296-3p and miR-296-5p, are closely associated with invasiveness and are promising therapeutic targets for GBM.
MATERIALS AND METHODS
Cell culture and transfection
Human GBM cell lines (LN229, T98G, and U87MG) were maintained in Dulbecco’s modified Eagle medium (DMEM, Hyclone, GE Healthcare, UK) supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic-antimycotic solution (GIBCO-BRL, Thermo Fisher Scientific, USA). All GBM cells were authenticated through short tandem repeat profiling. For overexpression of miR-296-5p, cells were transfected with control (Con) miRNA or miRNA-296-5p mimic (pre-miR-296-5p, GenePharma, Shanghai, China) using Lipofectamine2000 (Invitrogen, Thermo Scientific) according to the manufacturer’s protocol. For knockdown experiments, cells were transfected with control (CTRL) siRNA or indicated siRNAs. SiRNAs for CASP8 (GCUGCUCUUCCGAAUUAAUUU) and NGFR (CGAGCACAUAGACUCCUUU) were synthesized in Bioneer (Korea).
Evaluation of the invasiveness of GBM cells
The invasiveness of GBM cells was assessed using a BD Bio-coat™ Matrigel invasion chamber (BD Bioscience, USA). Briefly, equal number of cells in serum-free media was added into the upper and lower chambers. Cellular invasion was triggered by the addition of complete medium containing 10% FBS into the lower chamber. After incubation for 24 h, invaded cells were fixed by methanol and then stained with 0.1% hematoxylin and eosin. Invasiveness was determined by counting the number of invaded cells from more than twelve fields.
Western blot analysis
For western blot analysis, cells were lysed in RIPA buffer containing a protease and phosphatase inhibitors (Roche, Switzerland). Equal amounts of protein were separated by SDS-polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes (Millipore, USA). The membranes were blocked and then incubated with the indicated primary antibody and the appropriate secondary antibody. Bands were visualized using the enhanced chemoluminescence solution (Amersham, UK). Antibodies used for CASP8 (#9746) and NGFR/p75NTR (sc-6188) were from Cell Signaling Technology and Santa Cruz Biotechnology, respectively. GAPDH (ab8245, Abcam) was used as loading control.
RNA isolation and RT-qPCR analysis
Total RNA was isolated using TRIzol reagent (Ambion, USA) according to the manufacturer’s instructions. After cDNA was synthesized using the SuperScript III First-Strand Synthesis System (Invitrogen, USA), the level of mRNA was quantified by reverse transcription quantitative-PCR (RT-qPCR) (ABI Prism 7600) using Power SYBR® Green PCR Master Mix (Applied Biosystems, USA). RT-qPCR was performed using the following primers: CASP8, forward: CATCCAGTCACTTTGCCAGA, reverse: GCATCTGTTTCCCC ATGTTT; NGFR, forward: CGACAACCTCATCCCTGTCT, reverse: GCTGTTCCACCTCTTGAAGG. The level of GAPDH mRNA was used for normalization (forward: TGCACCACC AACTGCTTAGC, reverse: GGCATGGACTGTGGTCATGAG). To determine the levels of primary and mature miRNAs, RT-qPCR was performed using primary miR-296-specific primers (forward: TGCCTAATTCAGAGGGTTGG, reverse: CTCCACTCCTGGCACACAG) and mature miRNA-296-5p-specific TaqMan primer (MIMAT0000690, Applied Biosystems, USA), respectively.
Ago2 immunoprecipitation (IP)
To examine whether miR-296-5p directly binds to CASP8 and NGFR mRNA, IP was performed using an Ago2-specific antibody (Sigma-Aldrich, USA). Cytoplasmic lysates were incubated with Ago2 antibody-coated Dynabeads® Protein G (10004D, Invitrogen). Beads were washed with PEB buffer more than five times, and RNA in the Ago2-IP materials was then isolated. The enrichment of CASP8 and NGFR mRNA in Ago2-IP was determined by RT-qPCR.
Luciferase reporter assay
To investigate whether downregulation of CASP8 and NGFR by miR-296-5p is caused by the direct interaction between the miRNA seed sequence and the 3′UTR of their target mRNA, pmirGLO dual-luciferase vectors (E133A, Promega) containing the 3′UTR of CASP8 and NGFR mRNA were constructed: pmirGLO-CASP8 3′UTR and pmirGLO-NGFR 3′UTR, respectively. We prepared two individual vectors containing wild-type or mutated seed region sequences of the miR-296-5p-binding site in the 3′UTR of CASP8 and NGFR mRNAs. Cells were transfected with control or pre-miR-296-5p and plated into the 24-well plate. After 24 h, cells were transfected with either wild-type or mutant-type luciferase vector. Effect of the miR-296-5p on luciferase expression was assessed using a Dual-GLO™ Luciferase Assay System (E2940, Promega).
RESULTS
Invasive ability of GBM cells is potentiated by miR-296-5p
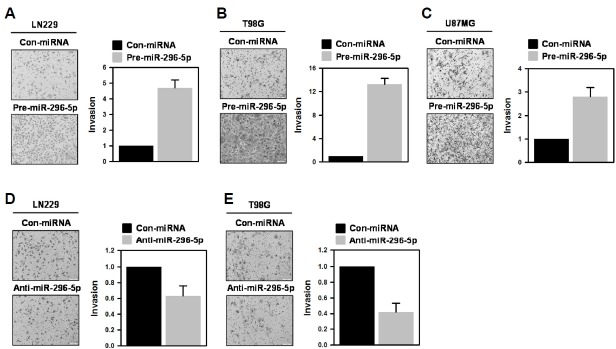
Previously, we reported that NF2 induces the phosphorylation of YAP and thus inhibits its transactivation by hindering nuclear localization (Lee et al., 2016). It was also found that knockdown of NF2 increases the expression of miR-296-3p, which augments the invasiveness of GBM T98G cells via SOCS2/STAT3 axis. In this report, we further investigated whether miR-296-5p, which is co-expressed with miR-296-3p, induces similar effects on the invasive activity of GBM cells. First, we examined the invasive ability of miR-296-5p-overexpressed GBM cells. LN229, T98G, and U87MG cells were transfected with control miRNA or pre-miR-296-5p and invasive activity was assessed using Transwell invasion assay. Overexpression of miR-296-5p enhanced the invasiveness of all tested cells. The number of invaded cells was increased by about 4.8-fold (Fig. 1A), 13.2-fold (Fig. 1B), and 2.8-fold (Fig. 1C) in LN229, T98G, and U87MG cells, respectively. Based on the effect of miR-296-5p on their invasiveness, LN229 and T98G cells were chosen for further experiments. From these results, we found that miR-296-5p, similar to its partner miR-296-3p, has the potential to control the invasiveness of GBM cells.
Fig. 1.
miRNA-296-5p enhances the invasiveness of GBM cells.
To investigate the effect of miR-296-5p on invasiveness, three GBM cells (LN229 (A), T98G (B), and U87MG(C)) were transfected with control miRNA or pre-miR-296-5p. After 48 h, the invasive activity was determined by counting the number of invaded cells from more than twelve fields. All experiments were performed more than three times and data are represented as mean ± S.D.
CASP8 and NGFR are targets of miR-296-5p
To investigate the molecular mechanism by which miR-296-5p potentiates the invasive ability of GBM cells, we investigated the target genes of miR-296-5p. For this, we used several databases for miRNA target prediction including TargetScan and miRanda. We identified two putative miR-296-5p target genes: CASP8 and NGFR. Predicted binding sites for miR-296-5p in the 3′UTRs of CASP8 and NGFR mRNAs are shown in Fig. 2A and 2B, respectively. Next, we checked the effect of miR-296-5p on the expression of CASP8 and NGFR in LN229 (Fig. 2C and 2D) and T98G (Figs. 2E and 2F) cells. For overexpression, a miR-296-5p-mimic was introduced into these cells. The protein and mRNA expression of CASP8 and NGFR was downregulated by miR-296-5p in both cells. Based on these results, CASP8 and NGFR can be considered novel targets of miR-296-5p.
Fig. 2.
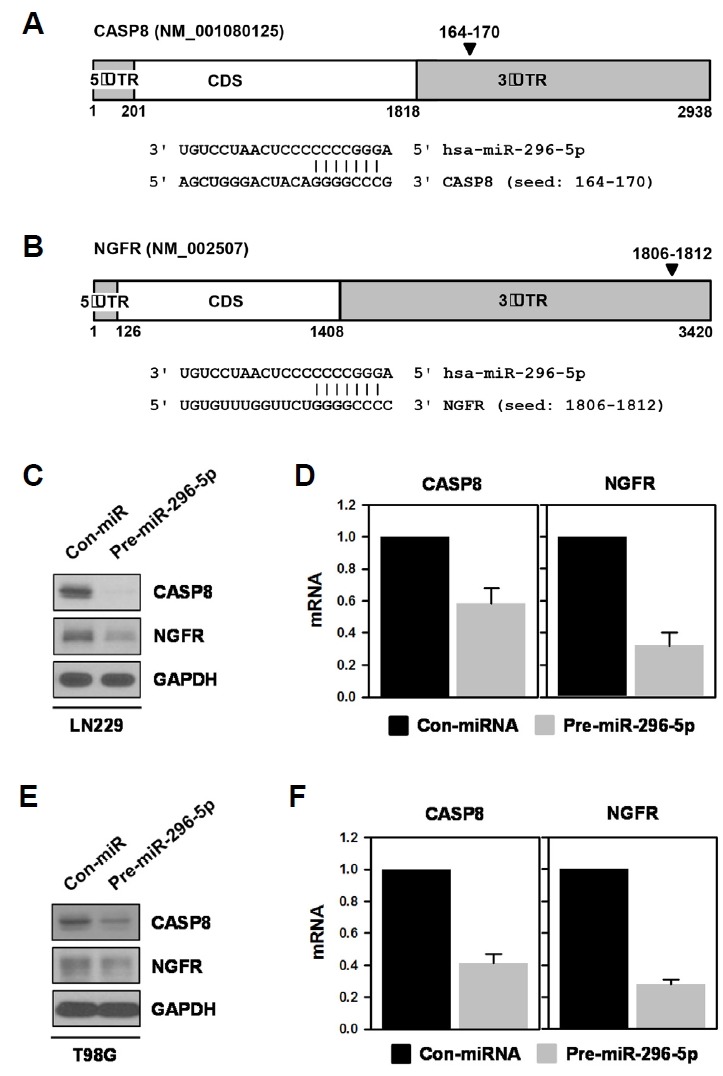
CASP8 and NGFR are targets of miR-296-5p.
(A, B) A bioinformatics search using several prediction databases identified CASP8 and NGFR as putative targets of miR-296-5p. Schematics showing miR-296-5p-binding site in the 3′UTR of CASP8 (A) and NGFR mRNA (B). (C, D) LN229 cells were transfected with control miRNA or pre-miR-296-5p. After 48 h, whole-cell lysates were prepared and the expression levels of CASP8 and NGFR were determined by Western blot analysis (C). The levels of CASP8 and NGFR mRNA were also assessed by RT-qPCR (D). (E, F) T98G cells were transfected as described above. The protein and mRNA levels of CASP8 and NGFR were examined by Western blot (E) and RT-qPCR analysis (F), respectively. All experiments were performed more than three times and data are represented as mean ± S.D.
miR-296-5p directly interacts to the 3’UTR of CASP8 and NGFR mRNA
To verify the direct interaction of miR-296-5p and the 3′UTR of CASP8 and NGFR mRNAs, an IP assay using Ago2-specific antibody was performed. The LN229 cells were first transfected with control miRNA or pre-miR-296-5p; cytoplasmic lysates were then prepared and used for Ago2-IP assay. The level of CASP8 and NGFR mRNA in the IP materials was assessed by RT-qPCR analysis. As shown in Fig. 3A, miR-296-5p increased the level of Ago2-bound CASP8 mRNA in both GBM cells. Similarly, the enrichment of NGFR mRNA in Ago2- IP was also increased by miR-296-5p (Fig. 3B). These results suggest that miR-296-5p-containing RISC complex binds to the 3′UTR of CASP8 and NGFR mRNA, which is responsible for downregulation of CASP8 and NGFR. Direct interaction between miR-296-5 and target mRNA was further verified using chimeric luciferase vectors. We manufactured two luciferase vectors containing wild-type and mutated sequences of the miR-296-5p-binding site from the CASP8 and NGFR mRNAs (Figs. 3C and 3D). In both the luciferase vectors harboring miR-296-5p-binding sequences, miR-296-5p suppressed the luciferase activity. Conversely, disruption of the direct interaction between the seed sequence of the miRNA and the 3′UTR region of its target mRNA by point mutations abolished the miR-296-5p-mediated downregulation of luciferase expression, indicating that this direct interaction is responsible for the downregulation of CASP8 and NGFR by miR-296-5p.
Fig. 3.
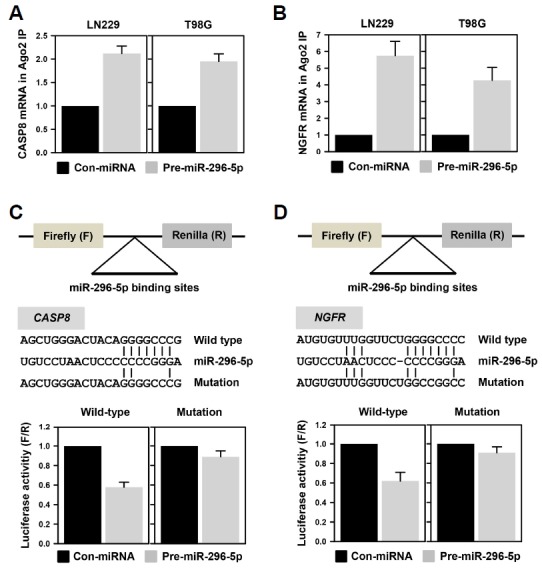
miR-296-5p directly interacts with the 3′UTR of CASP8 and NGFR mRNAs.
(A, B) To verify whether miR-296-5p-containing RISC can bind to the 3′UTR of CASP8 (A) and NGFR (B) mRNAs, LN229 and T98G cells were transfected with control miRNA or pre-miR-296-5p. Cytoplasmic lysates of transfected cells were immunoprecipitated with Ago2 antibody. The levels of CASP8 and NGFR mRNAs in IP materials were determined by RT-qPCR analysis. (C, D) To verify the direct interaction between seed sequence of miRNA and 3′UTR of target mRNA, the luciferase vectors containing wild-type and mutated sequences of miR-296-5p-binding site were constructed [(C), CASP8 mRNA; (D), NGFR mRNA]. To disrupt interaction between miR-NA and target 3′UTR, four nucleotides in seed region were mutated. The expression of luciferase was assessed by measuring its activity using a Dual-GLO™ Luciferase Assay System. All experiments were performed more than three times and data are represented as mean ± S.D.
Downregulation of CASP8 and NGFR is involved in miR-296-5p-mediated invasiveness
From the above results, we found that miR-296-5p increased the invasiveness of GBM cells and suppressed the expression of CASP8 and NGFR. Next, we investigated whether downregulation of CASP8 and NGFR is necessary for the enhancement of GBM invasion by miR-296-5p. LN229 and T98G cells were transfected with CASP8- or NGFR-specific siRNA and invasive activity was determined by Transwell invasion assay. In both cells, knockdown of CASP8 increased the number of invaded cells (Figs. 4A and 4B). NGFR-silenced cells also showed higher invasiveness as compared to control cells (Figs. 4C and 4D). Based on these results, we concluded that miR-296-5p regulates the invasive ability of GBM cells through post-transcriptional regulation of CASP8 and NGFR.
Fig. 4.
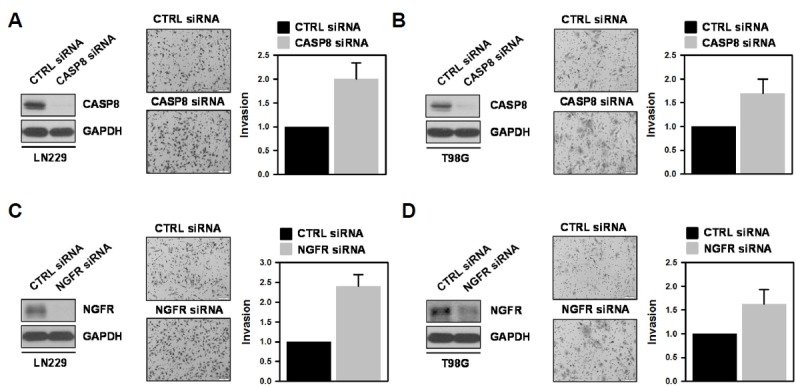
Knockdown of CASP8 or NGFR increases the invasiveness of GBM cells.
(A, C) LN229 cells were transfected with CASP8 (A) or NGFR (C) siRNAs. After 48 h, the level of CASP8 or NGFR was examined by western blot analysis, and invasiveness was assessed by Transwell invasion assay. Invaded cells were photographed under the microscope and counted from more than twelve fields. (B, D) T98G cells were transfected and invasive activity of transfected cells was assessed as described above. All experiments were performed more than three times and data are represented as mean ± S.D.
miR-296-5p is involved in the acquisition of invasive property
In order to generalize our findings, we investigated the role of miR-296-5p in the invasiveness of lung adenocarcinoma cells using previously established brain metastatic PC14PE6/LvBr4 cells, which are generated from lung adenocarcinoma PC14PE6 cells through repeated intracardiac injection (Hwang et al., 2015; 2016). Since PC14PE6 cells barely express NGFR, we could only compare the expression level of CASP8. CASP8 protein and mRNA were found to be highly expressed in parental PC14PE6 cells compared to brain metastatic PC14PE6/LvBr4 cells (Figs. 5A and 5B, respectively). As expected, the level of miR-296-5p was higher in the brain metastatic PC14PE6/LvBr4 cells than in the parental PC14PE6 cells (Fig. 5C). Next, we tested whether miR-296-5p suppresses the expression of CASP8 and enhances invasive property in parental PC14PE6 cells. It was found that miR-296-5p decreased the expression level of CASP8 protein (Fig. 5D) and mRNA (Fig. 5E). Along with downregulation of CASP8, invasiveness was potentiated as well in miR-296-5p-overexpressed PC14PE6 cells (Fig. 5F). Collectively, our results demonstrate that miR-296-5p is closely associated with induction of invasiveness in GBM cells through downregulation of CASP8 and NGFR and is a promising therapeutic target for GBM.
Fig. 5.
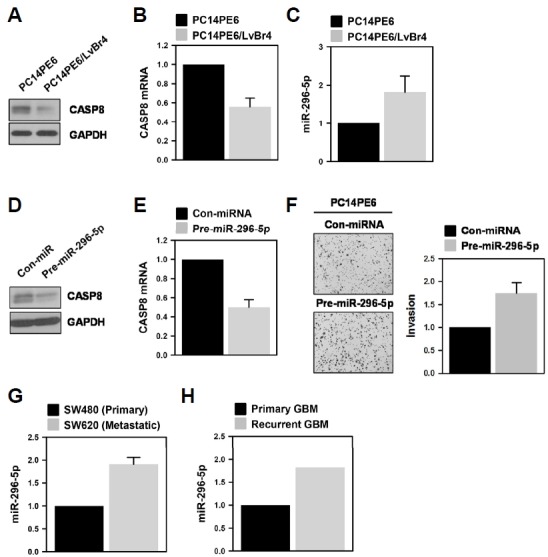
miR-296-5p is involved in the invasive property of brain metastatic lung cancer cells.
(A–C) The expression level of CASP8 protein (A) and mRNA (B) in PC14PE6 lung adenocarcinoma and its brain metastatic derivative (PC14PE6/LvBr4) was determined by Western blot and RT-qPCR analysis, respectively. The level of miR-296-5p was assessed by RT-qPCR using miR-296-5p-specific TaqMan primer. All experiments were performed more than three times and data are represented as mean ± S.D. (D–F) Parental PC14PE6 cells were transfected with control miRNA or pre-miR-296-3p. The expression levels of CASP8 protein (D) and mRNA (E) were determined by Western blot and RT-qPCR analysis, respectively. The effect of miR-296-5p on the invasiveness of PC14PE6 cells was assessed by Transwell invasion assay (F). Invaded cells were stained and photographed under microscope. The invasive activity was determined by counting the number of invaded cells from more than twelve fields. All experiments were performed more than three times and data are represented as mean ± S.D.
DISCUSSION
GBM is a highly prevalent and incurable type of brain cancer, classified as the most aggressive and severe type (Ohgaki and Kleihues, 2007). Many individual genes have been implicated in the induction of invasiveness in glioma and shown to be strongly associated with the poor prognosis of patients (Freije et al., 2004; Liang et al., 2005). In this regard, their expression should be tightly controlled in the cancer cells. miRNAs are important in post-transcriptional gene regulation as they can downregulate the expression of target genes through complementary binding of miRNA within RISC to the 3′UTR of the target mRNA, which results in mRNA degradation or translational suppression (Winter and Diederichs, 2011). Through comparison of the miRNA expression profiles of GBM samples and normal tissues, several miRNAs have been found to play critical roles in the progression of GBM. As an oncogenic miRNA, miR-21 inhibits apoptotic processes, promotes invasive property, and activates several receptor-mediated cellular signaling pathways (Moore and Zhang, 2010).
In a previous report, we demonstrated that miR-296-3p is upregulated by NF2 knockdown and dramatically increases the invasiveness of GBM cells (Lee et al., 2016). During miR-NA biogenesis, primary miRNA is transcribed by RNA polymerase II and sequentially cleaved by a nuclease. Thus, two strands of mature miRNA are produced from one precursor miRNA. In this study, we investigated the effect of miR-296-5p, a partner miRNA of miR-296-3p, on the invasiveness of GBM cells. Similar to results we obtained with miR-296-3p, invasive ability was potentiated by miR-296-5p. By directly binding to the 3′UTR of the target mRNA, miR-296-5p suppressed the expression of CASP8 and NGFR. Moreover, knockdown of CASP8 or NGFR increased the invasiveness of GBM cells, indicating that these proteins are involved in the potentiation of invasiveness by miR-296-5p.
CASP8 is a component of the programmed cell death machinery and is known to be a tumor suppressor. It plays an important role in death receptor-mediated cell death. CASP8 is frequently deleted or silenced mainly through the methylation of its promoter region. Loss of CASP8 is observed in diverse types of cancer including small cell lung carcinoma, retinoblastoma, and medulloblastoma (Hopkins-Donaldson et al., 2003; Pingoud-Meier et al., 2003; Teitz et al., 2001). Interestingly, CASP8 selectively influences the apoptosis of invading cells at the tumor margin rather than in the primary tumor (Stupack et al., 2006). Through interaction with un-ligated integrin, CASP8 can trigger the apoptotic process. It was reported that loss of CASP8 renders cancer cells refractory to integrin-mediated cell death and re-introduction of CASP8 sensitizes neuroblastoma cells to drug-induced apoptosis. In addition to roles in extrinsic cell death, deficiency of CASP8 confers aggressiveness to neuroblastoma and is closely associated with poor prognosis (Pingoud-Meier et al., 2003; Teitz et al., 2001). It has also been reported that mutations in CASP8 promote cellular migration and invasion and confers resistance to death receptor-mediated apoptosis in head and neck cancer (Li et al., 2014). Thus, CASP8 has been identified as a metastasis suppressor.
NGFR is a multifunctional cell surface receptor and belongs to the TNF receptor family. Interestingly, NGFR plays different roles depending on the cell context. In brain tumor and melanomas, it functions as an oncogene (tumor-promoting) (Marchetti et al., 2004; Menter et al., 1994). In contrast, NGFR impairs tumorigenesis and functions as a tumor suppressor in bladder, stomach, and liver cancers (Jin et al., 2007; Quann et al., 2007; Sachs et al., 2007; Tabassum et al., 2003). NGFR is highly expressed in invasive glioma cells and contributes to their high invasiveness (Johnston et al., 2007; Wang et al., 2008). Intramembrane proteolysis of NGFR is required for invasion in glioma cells (Wang et al., 2008).
In this study, we observed that miR-296-5p is able to activate the invasive activity of GBM cells (LN229, T98G, and U87MG) through downregulation of CASP8 and NGFR. In accordance with previous reports, it was found that CASP8 and NGFR function as tumor suppressor in GBM. Previously we reported that nuclear localization of YAP is responsible for the transcription of primary miR-296 and thus regulates the biosynthesis of miR-296-3p and -5p as well. Based on our previous and present data, both strands of precursor mir-296 (miR-296-3p and -5p) are main players in the acquisition of invasive ability by activating STAT3 pathway and by targeting CASP8 and NGFR, respectively, which suggests that mir-296 is a promising target for GBM treatment
ACKNOWLEDGMENTS
This research was supported by a grant from the Mid-career Research Program through the National Research Foundation (NRF) funded by the Ministry of Science, ICT, and Future Planning, Republic of Korea (2014R1A2A1A11053130).
REFERENCES
- Barker P.A. p75NTR is positively promiscuous: novel partners and new insights. Neuron. 2004;42:529–533. doi: 10.1016/j.neuron.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Freije W.A., Castro-Vargas F.E., Fang Z., Horvath S., Cloughesy T., Liau L.M., Mischel P.S., Nelson S.F. Gene expression profiling of gliomas strongly predicts survival. Cancer Res. 2004;64:6503–6510. doi: 10.1158/0008-5472.CAN-04-0452. [DOI] [PubMed] [Google Scholar]
- Hopkins-Donaldson S., Ziegler A., Kurtz S., Bigosch C., Kandioler D., Ludwig C., Zangemeister-Wittke U., Stahel R. Silencing of death receptor and caspase-8 expression in small cell lung carcinoma cell lines and tumors by DNA methylation. Cell Death Differ. 2003;10:356–364. doi: 10.1038/sj.cdd.4401157. [DOI] [PubMed] [Google Scholar]
- Hwang S.J., Lee H.W., Kim H.R., Song H.J., Lee D.H., Lee H., Shin C.H., Joung J.G., Kim D.H., Joo K.M., et al. Overexpression of microRNA-95-3p suppresses brain metastasis of lung adenocarcinoma through downregulation of cyclin D1. Oncotarget. 2015;6:20434–20448. doi: 10.18632/oncotarget.3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S.J., Lee H.W., Kim H.R., Lee H., Shin C.H., Yun S.I., Lee D.H., Kim D.H., Kim K.K., Joo K.M., et al. Ubiquitin-specific protease 4 controls metastatic potential through beta-catenin stabilization in brain metastatic lung adenocarcinoma. Sci Rep. 2016;6:21596. doi: 10.1038/srep21596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H., Pan Y., Zhao L., Zhai H., Li X., Sun L., He L., Chen Y., Hong L., Du Y., et al. p75 neurotrophin receptor suppresses the proliferation of human gastric cancer cells. Neoplasia. 2007;9:471–478. doi: 10.1593/neo.07175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston A.L., Lun X., Rahn J.J., Liacini A., Wang L., Hamilton M.G., Parney I.F., Hempstead B.L., Robbins S.M., Forsyth P.A., et al. The p75 neurotrophin receptor is a central regulator of glioma invasion. PLoS Biol. 2007;5:e212. doi: 10.1371/journal.pbio.0050212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W.S., Kwon J., Yun D.H., Lee Y.N., Woo E.Y., Park M.J., Lee J.S., Han Y.H., Bae I.H. Specificity protein 1 expression contributes to Bcl-w-induced aggressiveness in glioblastoma multiforme. Mol Cells. 2014;37:17–23. doi: 10.14348/molcells.2014.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Park S., Kang Y.S., Park S. EphA receptors form a complex with caspase-8 to induce apoptotic cell death. Mol Cells. 2015;38:349–355. doi: 10.14348/molcells.2015.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Hwang S.J., Kim H.R., Shin C.H., Choi K.H., Joung J.G., Kim H.H. Neurofibromatosis 2 (NF2) controls the invasiveness of glioblastoma through YAP-dependent expression of CYR61/CCN1 and miR-296-3p. Biochim Biophys Acta. 2016;1859:599–611. doi: 10.1016/j.bbagrm.2016.02.010. [DOI] [PubMed] [Google Scholar]
- Li C., Egloff A.M., Sen M., Grandis J.R., Johnson D.E. Caspase-8 mutations in head and neck cancer confer resistance to death receptor-mediated apoptosis and enhance migration, invasion, and tumor growth. Mol Oncol. 2014;8:1220–1230. doi: 10.1016/j.molonc.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Diehn M., Watson N., Bollen A.W., Aldape K.D., Nicholas M.K., Lamborn K.R., Berger M.S., Botstein D., Brown P.O., et al. Gene expression profiling reveals molecularly and clinically distinct subtypes of glioblastoma multiforme. Proc Natl Acad Sci USA. 2005;102:5814–5819. doi: 10.1073/pnas.0402870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti D., Aucoin R., Blust J., Murry B., Greiter-Wilke A. p75 neurotrophin receptor functions as a survival receptor in brain-metastatic melanoma cells. J Cell Biochem. 2004;91:206–215. doi: 10.1002/jcb.10649. [DOI] [PubMed] [Google Scholar]
- Menter D.G., Herrmann J.L., Marchetti D., Nicolson G.L. Involvement of neurotrophins and growth factors in brain metastasis formation. Invasion Metastasis. 1994;14:372–384. [PubMed] [Google Scholar]
- Moore L.M., Zhang W. Targeting miR-21 in glioma: a small RNA with big potential. Exp Opin Ther Targets. 2010;14:1247–1257. doi: 10.1517/14728222.2010.527334. [DOI] [PubMed] [Google Scholar]
- Oberst A., Green D.R. It cuts both ways: reconciling the dual roles of caspase 8 in cell death and survival. Nat Rev Mol Cell Biol. 2011;12:757–763. doi: 10.1038/nrm3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgaki H., Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol. 2007;170:1445–1453. doi: 10.2353/ajpath.2007.070011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omuro A., DeAngelis L.M. Glioblastoma and other malignant gliomas: a clinical review. Jama. 2013;310:1842–1850. doi: 10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- Pingoud-Meier C., Lang D., Janss A.J., Rorke L.B., Phillips P.C., Shalaby T., Grotzer M.A. Loss of caspase-8 protein expression correlates with unfavorable survival outcome in childhood medulloblastoma. Clin Cancer Res. 2003;9:6401–6409. [PubMed] [Google Scholar]
- Quann E.J., Khwaja F., Djakiew D. The p38 MAPK pathway mediates aryl propionic acid induced messenger rna stability of p75 NTR in prostate cancer cells. Cancer Res. 2007;67:11402–11410. doi: 10.1158/0008-5472.CAN-07-1792. [DOI] [PubMed] [Google Scholar]
- Reichardt L.F. Neurotrophin-regulated signalling pathways. Philosophical transactions of the Royal Society of London. Series B, Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs B.D., Baillie G.S., McCall J.R., Passino M.A., Schachtrup C., Wallace D.A., Dunlop A.J., MacKenzie K.F., Klussmann E., Lynch M.J., et al. p75 neurotrophin receptor regulates tissue fibrosis through inhibition of plasminogen activation via a PDE4/cAMP/PKA pathway. J Cell Biol. 2007;177:1119–1132. doi: 10.1083/jcb.200701040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupack D.G., Teitz T., Potter M.D., Mikolon D., Houghton P.J., Kidd V.J., Lahti J.M., Cheresh D.A. Potentiation of neuroblastoma metastasis by loss of caspase-8. Nature. 2006;439:95–99. doi: 10.1038/nature04323. [DOI] [PubMed] [Google Scholar]
- Tabassum A., Khwaja F., Djakiew D. The p75(NTR) tumor suppressor induces caspase-mediated apoptosis in bladder tumor cells. Int J Cancer. 2003;105:47–52. doi: 10.1002/ijc.11038. [DOI] [PubMed] [Google Scholar]
- Teitz T., Lahti J.M., Kidd V.J. Aggressive childhood neuroblastomas do not express caspase-8: an important component of programmed cell death. J Mol Med. 2001;79:428–436. doi: 10.1007/s001090100233. [DOI] [PubMed] [Google Scholar]
- Thomas A.A., Brennan C.W., DeAngelis L.M., Omuro A.M. Emerging therapies for glioblastoma. JAMA Neurol. 2014;71:1437–1444. doi: 10.1001/jamaneurol.2014.1701. [DOI] [PubMed] [Google Scholar]
- Wang L., Rahn J.J., Lun X., Sun B., Kelly J.J., Weiss S., Robbins S.M., Forsyth P.A., Senger D.L. Gamma-secretase represents a therapeutic target for the treatment of invasive glioma mediated by the p75 neurotrophin receptor. PLoS Biol. 2008;6:e289. doi: 10.1371/journal.pbio.0060289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson N.S., Dixit V., Ashkenazi A. Death receptor signal transducers: nodes of coordination in immune signaling networks. Nat Immunol. 2009;10:348–355. doi: 10.1038/ni.1714. [DOI] [PubMed] [Google Scholar]
- Winter J., Diederichs S. MicroRNA biogenesis and cancer. Method Mol Biol. 2011;676:3–22. doi: 10.1007/978-1-60761-863-8_1. [DOI] [PubMed] [Google Scholar]