Abstract
MicroRNAs (miRNAs) are short non-coding RNAs that regulate genes posttranscriptionally. Past studies have reported that miR-210 is up-regulated in many cancers including cervical cancer, and plays a pleiotropic role in carcinogenesis. However, its role in regulating response towards anti-cancer agents has not been fully elucidated. We have previously reported that the natural compound 1’S-1’-acetoxychavicol acetate (ACA) is able to induce cytotoxicity in various cancer cells including cervical cancer cells. Hence, this study aims to investigate the mechanistic role of miR-210 in regulating response towards ACA in cervical cancer cells. In the present study, we found that ACA down-regulated miR-210 expression in cervical cancer cells, and suppression of miR-210 expression enhanced sensitivity towards ACA by inhibiting cell proliferation and promoting apoptosis. Western blot analysis showed increased expression of mothers against decapentaplegic homolog 4 (SMAD4), which was predicted as a target of miR-210 by target prediction programs, following treatment with ACA. Luciferase reporter assay confirmed that miR-210 binds to sequences in 3′UTR of SMAD4. Furthermore, decreased in SMAD4 protein expression was observed when miR-210 was overexpressed. Conversely, SMAD4 protein expression increased when miR-210 expression was suppressed. Lastly, we demonstrated that overexpression of SMAD4 augmented the anti-proliferative and apoptosis-inducing effects of ACA. Taken together, our results demonstrated that down-regulation of miR-210 conferred sensitivity towards ACA in cervical cancer cells by targeting SMAD4. These findings suggest that combination of miRNAs and natural compounds could provide new strategies in treating cervical cancer.
Keywords: apoptosis, cervical cancer, microRNA, natural compound, SMAD4
INTRODUCTION
Cervical cancer is one of the leading malignancies affecting women worldwide. In 2012, an estimated 528,000 new cases were diagnosed and 266,000 deaths were reported (Torre et al., 2015). Chemotherapy is often used alongside surgery and radiotherapy to improve the overall response and survival in cancer patients. Despite this, clinical outcomes remained dismal, especially in patients with recurrent or advanced cancer due to drug resistance and toxicities (Crafton and Salani, 2016; Gottesman, 2002). Hence, there is an on-going effort to develop effective targeted therapies for cervical cancer.
The use of naturally occurring compounds is promising because they are able to target multiple signaling pathways, besides having lower toxicities compared to some conventional chemotherapy agents (Millimouno et al., 2014). Some examples of the plant-derived natural compounds currently used in chemotherapy are paclitaxel, topotecan, vinblastine and vincristine (Cragg and Newman, 2005). The 1’S-1’-acetoxychavicol acetate (ACA) is a natural compound isolated from the wild ginger, Alpinia conchigera. We have previously reported that ACA is able to induce comparable level of cytotoxicity to cisplatin when used as stand-alone, and potentiates the effects of cisplatin when used in combination on various cancer cell lines including cervical cancer (Awang et al., 2010; Phuah et al., 2013).
MicroRNAs (miRNAs) are highly conserved short non-coding RNAs which regulate gene expression by binding to its target sequences in messenger RNAs (mRNAs) to induce mRNA degradation or suppress translation (Lagos-Quintana et al., 2001). Since one miRNA can target many genes and one gene can be targeted by different miRNAs (Wu et al., 2010), they are able to regulate various cellular processes such as cell death (Othman et al., 2013), metastasis (Ho et al., 2014), proliferation (Liang et al., 2015) and chemosensitivity (Kim et al., 2015). Studies have shown that natural compounds such as curcumin and resveratrol can modify miRNA expression, and changes in the expression of these miRNAs can subsequently affect their anti-cancer activities (Phuah and Nagoor, 2014).
The up-regulation of miR-210, which is associated with poorer prognosis, have been reported in various cancers such as acute myeloid leukemia (Tang et al., 2015), breast (Camps et al., 2008), pancreatic (Greither et al., 2010) and cervical cancer (Rao et al., 2012). Over the years, a growing body of evidence has revealed the pleiotropic roles of miR-210 in cancer initiation and progression by targeting genes involved in regulating cell proliferation, apoptosis, cell cycle, angiogenesis, metastasis and DNA repair (Dang and Myers, 2015; Qin et al., 2014). However, the mechanistic role of miR-210 in regulating sensitivity towards anti-cancer agents has not been fully elucidated.
In this study, we found that miR-210 is down-regulated following treatment with ACA in both cervical cancer cell lines. Functional studies carried out indicated that suppression of miR-210 enhanced sensitivity towards ACA by decreasing cell proliferation and promoting apoptosis. The protein expression of SMAD4, which was identified as a putative target of miR-210 by miRNA target prediction programs, was found to be up-regulated following treatment with ACA. The direct binding of miR-210 to SMAD4 was subsequently confirmed using luciferase reporter assay. We also demonstrated that overexpression of miR-210 reduced SMAD4 protein expression while inhibition of miR-210 increased SMAD4 protein expression. Lastly, we showed that ectopic expression of SMAD4 augmented the anti-proliferative and apoptosis-inducing effects of ACA.
MATERIALS AND METHODS
Cell culture and ACA
The human cervical cancer cells Ca Ski and SiHa obtained from American Type Cell Culture (ATCC) were cultured in RPMI-1640 (Hylcone, USA) and DMEM (Hyclone, USA) containing 10.0% (v/v) fetal bovine serum (FBS) (Hyclone, USA), and maintained at 37.0°C and 5.0% CO2 in humidified atmosphere. The ACA was provided by the Centre for Natural Product Research and Drug Discovery (CENAR), Department of Chemistry, University of Malaya, Malaysia.
Transient transfection
miRNA transfection
Cells were plated at density of 3.0 × 105 cells and allowed to attach overnight. Cells were then transfected with 100 nM of miRIDIAN microRNA human hsa-miR-210 mimic or miRIDIAN microRNA human hsa-miR-210 hairpin inhibitor (Thermo Fisher Scientific, USA), complexed with DharmaFECT 1 Transfection Reagent (Thermo Fisher Scientific, USA) for 24 h according to the manufacturer’s protocol. miRIDIAN microRNA Mimic Negative Control #1 and miRIDIAN microRNA Hairpin Inhibitor Negative Control #1 (Thermo Fisher Scientific, USA) were used as negative controls.
Plasmid transfection
Cells were plated at density of 1.5 × 105 cells and allowed to attach overnight. Cells were then transfected with 50 ng of pCMV6-SMAD4 (Origene Technologies, USA) complexed with DharmaFECT 1 Transfection Reagent (Thermo Fisher Scientific, USA) for 12 h according to manufacturer’s protocol. The empty vector pCMV6 (Origene Technologies, USA) was used as negative control.
Quantitative real-time RT-PCR (RT-qPCR)
The total RNA was extracted using miRNeasy Mini Kit (Qiagen, Germany) and cDNA was synthesized with TaqMan® MicroRNA Reverse Transcription Kit (Applied Biosystems, USA) using Veriti 96-Well Thermal Cycler (Applied Biosystems, USA) according to the manufacturer’s protocol. The miRNA expression level was determined with TaqMan® MicroRNA Assays (Applied Biosystems, USA) according to the manufacturer’s protocol using Bio-Rad CFX96™ Real-Time PCR Detection System (Bio-Rad Laboratories, USA) and analyzed with Bio-Rad CFX Manager v2.1 (Bio-Rad Laboratories, USA). The U6 small nuclear RNA was used as an internal control to normalize RNA input. Fold changes were calculated using the 2−ΔΔCt method (Livak and Schmittgen, 2001), and presented as normalized fold expression.
MTT cell viability assay
Transfected cells were treated with ACA for 12 h (plasmid transfection) or 48 h (miRNA transfection). Following incubation, 30.0 μl of 3-(4,5-dimethylthiazol-2-γl)-2,5-diphenyl-tetrazoliumbromide (MTT) reagent (5.0 mg/ml) was added into each well and incubated in the dark at 37°C for 1 h. Media containing excess MTT reagent was then removed and formazan crystals were dissolved with 200.0 μl dimethyl sulfoxide. Results were obtained by measuring the absorbance at 570 nm using microtiter plate reader (Tecan, Switzerland).
Annexin V/PI assay
Transfected cells were treated with ACA (20 μM for Ca Ski and 30 μM for SiHa) for 12 h (plasmid transfection) or 48 h (miRNA transfection). Apoptotic cells were detected using BD Pharmingen™ Annexin V-FITC Apoptosis Detection Kit (BD Biosciences, USA) according to the manufacturer’s protocol. Results were obtained using BD FACSCanto II flow cytometer (BD Biosciences, USA) and analyzed with BD FACSDiva software (BD Biosciences, USA).
Caspase 3/7 assay
Transfected cells were treated with ACA (20 μM for Ca Ski and 30 μM for SiHa) for 6 h (plasmid transfection) or 12 h (miRNA transfection). The Caspase-Glo® 3/7 Assay (Promega, USA) was used to measure caspase-3 and -7 activities using GLOMAX®-Multi Jr (Promega, USA) according to the manufacturer’s protocol.
Vector construction
The total RNA extracted was first converted into cDNA using RevertAid First Strand cDNA (Thermo Fisher Scientific, USA). The 3′UTR regions of SMAD4 containing the predicted binding site were then amplified from the cDNA and inserted into the pmirGLO Dual-Luciferase miRNA Target Expression Vector (Promega, USA) to construct the luciferase reporter constructs. Site-directed mutations in the predicted binding site for SMAD4 were perfomed using the QuikChange Multi Site-Directed Mutagenesis Kit (Stratagene, USA) according to the manufacturer’s protocol. The sequences of the constructs were verified by sequencing.
Dual-luciferase assay
Cells were co-transfected with 40 ng of 3′UTR reporter constructs containing wild-type or mutated binding sites and 100 nM of miR-210 mimic or mimic negative control using DharmaFECT 1 Transfection Reagent (Thermo Fisher Scientific, USA). The firefly and Renilla luciferase activities were measured 48 h post-transfection with Dual-Glo® Luciferase Assay System (Promega, USA) according to the manufacturer’s protocol using GLOMAX®-Multi Jr (Promega, USA). The firefly luciferase activity was normalized to Renilla activity, which was used as an internal control.
Western blot
Proteins were extracted using RIPA buffer containing 1× Halt Protease and Phosphatase Inhibitor Cocktail (Thermo Fisher Scientific, USA) and quantified using BCA Protein Assay Kit (Pierce, USA) according to manufacturer’s protocol. Total cell lysates were fractionated by 12% sodium dodecyl sulfate-polyacrylamide (SDS-PAGE) gel electrophoresis and transferred onto nitrocellulose membranes. Membranes were blocked for 1 h with 5% (w/v) non-fat milk followed by overnight incubation at 4°C with primary antibodies against SMAD4 (1:1000; Cell Signaling Technology, USA) and GAPDH (1:1000; Cell Signaling Technology, USA). Detection was carried out using secondary IgG HRP-linked antibody and anti-biotin HRP-linked antibody (1:1000; Cell Signaling Technology, USA). Bands were visualized using Western-Bright Quantum (Advansta, USA) on Fusion FX7 system (Vilber Lourmat, France) and quantified using the ImageJ Analyst software (NIH, USA), with band intensities normalized to GAPDH, which was used as loading control.
Statistical analysis
All experiments were carried out in triplicates and presented as mean values ± standard error mean. Student’s t-test was used to determine the statistical significance of results, whereby value of P < 0.05 was considered as statistically significant.
RESULTS
ACA down-regulates mir-210 expression and suppression of miR-210 confers sensitivity towards ACA
To study the effects of ACA on miR-210 expression, the expression of miR-210 in cells treated with ACA was determined using RT-qPCR. Figure 1A indicated that miR-210 expression is down-regulated following treatment with ACA in both Ca Ski and SiHa cells. To study the function of miR-210, we transfected miR-210 hairpin inhibitor and hairpin inhibitor negative control into both cervical cancer cell lines. Results from RT-qPCR showed that transfection with miR-210 hairpin inhibitor successfully suppressed miR-210 expression level when compared to hairpin inhibitor negative control (Fig. 1B). Next, the effects of ACA on the transfected cells were analyzed using MTT cell viability assay. As shown in Fig. 1C, cells transfected with miR-210 hairpin inhibitor were more sensitive towards ACA, indicating that down-regulation of miR-210 conferred sensitivity towards ACA. However, no significant changes in sensitivity towards ACA were observed when miR-210 was overexpressed (data not shown).
Fig. 1.
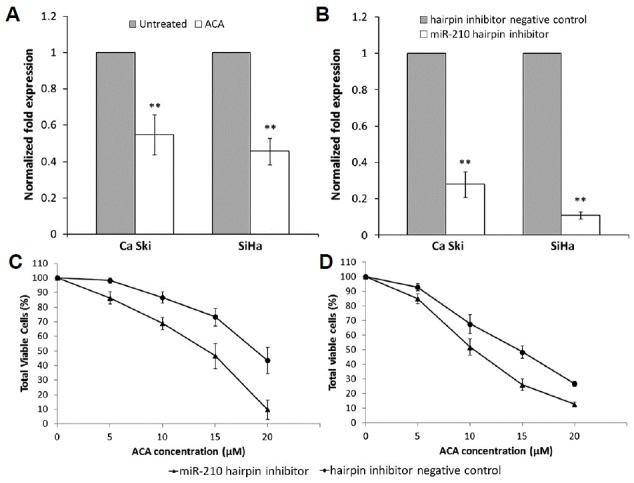
ACA down-regulates miR-210 expression and suppression of miR-210 confers sensitivity towards ACA.
(A) Expression level of miR-210 as measured by RT-qPCR following treatment with ACA (B) Expression level of miR-210 as measured by RT-qPCR after transfection with miR-210 hairpin inhibitor. (C, D) Dose-response curves on Ca Ski (C) and SiHa (D) cells transfected with miR-210 hairpin inhibitor followed by treatment with ACA. **P < 0.05.
Suppression of miR-210 increases ACA-induced apoptosis
To determine if the effects from combinatorial treatment with miR-210 hairpin inhibitor and ACA were modulated by apoptosis, Annexin V/PI and Caspase 3/7 assays were utilized. Transfection with miR-210 hairpin inhibitor markedly increased the apoptotic cells following exposure to ACA (Fig. 2A). Correspondingly, Fig. 2B showed that suppression of miR-210 induced higher Caspase 3/7 activity in ACA-treated cells. Taken together, these results showed that suppression of miR-210 promoted ACA-induced apoptosis. No significant differences were observed when miR-210 was overex-pressed in the cells (data not shown).
Fig. 2.
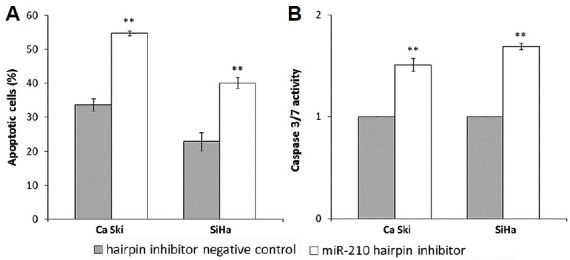
Suppression of miR-210 increases ACA-induced apoptosis.
(A) Apoptosis effects on cells transfected with miR-210 hairpin inhibitor followed by exposure to ACA. (B) Caspase 3/7 activity on cells transfected with miR-210 hairpin inhibitor followed by exposure to ACA. **P < 0.05.
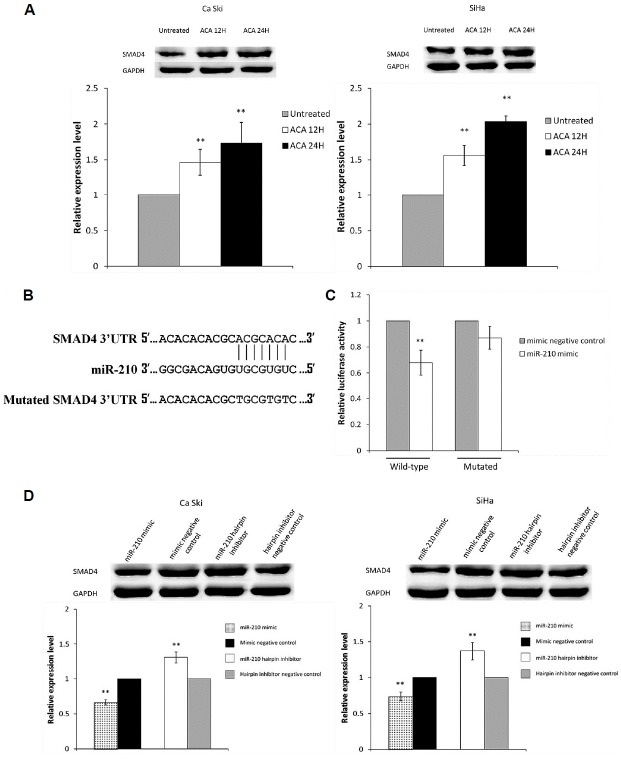
miR-210 directly targets SMAD4
To identify the potential targets of miR-210, miRNA target prediction programs were used. Both TargetScan v7.1 and miRanda predicted SMAD4 as a putative target of miR-210. Western blot was carried out to study the effects of ACA on SMAD4 protein expression, and results showed that SMAD4 is up-regulated following treatment with ACA (Fig. 3A). To confirm that miR-210 directly targets the 3′UTR of SMAD4, luciferase reporter vector containing wild-type or mutated binding site for SMAD4 (Fig. 3B) were constructed. Figure 3C showed that miR-210 overexpression significantly reduced luciferase activity when co-transfected with vector containing wild-type binding site but not in vector containing mutated binding site, confirming that SMAD4 is direct target of miR-210. To assess if miR-210 can regulate SMAD4 protein expression, western blots were performed. Results showed that overexpression of miR-210 reduced SMAD4 protein level, while inhibition of miR-210 increased SMAD4 protein level (Fig. 3D).
Fig. 3.
miR-210 directly targets SMAD4.
(A) SMAD4 protein expression following treatment with ACA. (B) Predicted binding site between miR-210 and SMAD4 3′UTR and sequence of mutated SMAD4 3′UTR. (C) Luciferase activity for cells co-transfected with wild-type or mutated SMAD4 3′UTR and miR-210 mimic or mimic negative control. (D) SMAD4 protein expression following transfection with miR-210 mimic, mimic negative control, miR-210 hairpin inhibitor or hairpin inhibitor negative control. **P < 0.05.
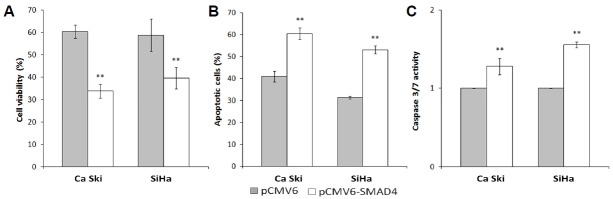
Overexpression of SMAD4 augments anti-proliferative and apoptosis-inducing effects of ACA
To assess the role of SMAD4 in regulating response towards ACA, SMAD4 was transiently overexpressed using pCMV6-XL5 vector containing SMAD4 sequence (pCMV6-SMAD4) while empty vector lacking SMAD4 sequence (pCMV6) was used as negative control. To evaluate the anti-proliferative effects of ACA on the transfected cells, MTT cell viability assay was carried out. Results showed a significant reduction in the cell viability when SMAD4 is overexpressed (Fig. 4A). To determine if SMAD4 overexpression augments ACA-induced apoptosis, transfected cells treated with ACA were analyzed using Annexin/PI assay. As seen in Fig. 4B, a significant increase in the number of apoptotic cells was observed when SMAD4 is overexpressed. To verify this, the activation of Caspase 3/7 was assayed. Similarly, a marked increase in Caspase 3/7 activity was observed in SMAD4-overexpressing cells treated with ACA (Fig. 4C). These results demonstrated that overexpression of SMAD4 augmented the anti-proliferative and apoptosis-inducing effects of ACA.
Fig. 4.
Overexpression of SMAD4 augments anti-proliferative and apoptosis-inducing effects of ACA.
(A) Cell viability assay on SMAD4-overexpressing cells treated with ACA. (B) Apoptosis effects on SMAD4-overexpressing cells treated with ACA. (C) Caspase 3/7 activity on SMAD4-overexpressing cells treated with ACA. **P < 0.05.
DISCUSSION
The use of cisplatin-based regimens has long been reported to be most active in treating cervical cancer despite median overall survival being only around 1 year (Monk et al., 2009). However, the addition of targeted therapy such as bevacizumab, a humanized monoclonal antibody against vascular endothelial growth factor A (VEGF-A), has greatly improved the overall survival in advanced or recurrent cervical cancer compared to the use of chemotherapy alone. Subsequently, US Food and Drug Administration approved the use of bevacizumab in persistent, recurrent or metastatic cervical cancer in combination with chemotherapy in 2014 (Crafton and Salani, 2016). This advancement has spurred interests to identify new combinations to improve efficacies in chemotherapy.
The miR-210 is frequently up-regulated in many cancer types, including cervical cancer (Rao et al., 2012). In cervical cancer patients, miR-210 was also found to be expressed at higher levels in HPV-positive patients than HPV-negative patients (Lou et al., 2016). Since miR-210 is overexpressed in HPV-positive Ca Ski and SiHa cells compared to normal cervical tissues and HPV-negative cervical cancer cell line (C-33a) (Martinez et al., 2008), these two cell lines were selected to be used for this study.
Previous studies have reported that down-regulation of miR-210 in combination with radiotherapy enhanced anti-tumor effects on human hepatoma cell lines and xenograft (Yang et al., 2012; 2013). Beside this, an association between miR-210 plasma levels and trastuzumab resistance in patients with HER2-positive breast cancer was also observed (Jung et al., 2012). These studies revealed the potential role for miR-210 in cancer therapies. In the present study, we demonstrated that down-regulation of miR-210 enhanced sensitivity towards ACA by augmenting ACA-induced apoptosis, indicating that miR-210 plays a role in regulating response towards anti-cancer agents.
In addition, we have also identified SMAD4 as a direct target of miR-210. Loss or reduction in SMAD4 function has been reported in many cancers such as breast (Liu et al., 2015), colorectal (Miyaki et al., 1999) and cervical cancer (Baldus et al., 2005; Kloth et al., 2008). Additionally, reduced SMAD4 expression was also observed in HPV-positive tumors compared to HPV-negative tumors (Baez et al., 2005; Kloth et al., 2008). Contrastingly, SMAD4 was reported to be oncogenic in other cancers such as hepatocellular carcinoma (Hernanda et al., 2015), suggesting that its role might be cell-context-dependent. Past studies have shown that ectopic expression of SMAD4 induced apoptosis on SiHa cells (Lee et al., 2001), and reduced cervical cancer growth in nude mice (Klein-Scory et al., 2007), indicating a tumor suppressive role for SMAD4 in cervical cancer. Different groups have reported a positive correlation between SMAD4 expression and sensitivity towards cetuximab in head and neck squamous cell carcinoma (Cheng et al., 2015) as well as 5-fluorouracil in breast (Yu et al., 2013) and colorectal cancer (Papageorgis et al., 2011; Zhang et al., 2014; 2016). In this study, we showed that overexpression of SMAD4 in cervical cancer cells augmented anti-proliferative and apoptosis-inducing effects of ACA, indicating that SMAD4 plays a role in regulating response towards anti-cancer agents.
In summary, our results demonstrated that down-regulation of miR-210 enhanced sensitivity towards ACA in cervical cancer cells by regulating SMAD4. These findings suggest that combination of miRNAs and natural compounds could provide new strategies in treating cervical cancer.
ACKNOWLEDGEMENTS
This study was supported by the University of Malaya Research Grant (UMRG) (RP001B-13BIO) and RU015-2016.
REFERENCES
- Awang K., Azmi M.N., Aun L.I., Aziz A.N., Ibrahim H., Nagoor N.H. The apoptotic effect of 1’s-1’-acetoxychavicol acetate from Alpinia conchigera on human cancer cells. Molecules. 2010;15:8048–8059. doi: 10.3390/molecules15118048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baez A., Cantor A., Fonseca S., Marcos-Martinez M., Mathews L.A., Muro-Cacho C.A., Munoz-Antonia T. Differences in Smad4 expression in human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck squamous cell carcinoma. Clin Cancer Res. 2005;11:3191–3197. doi: 10.1158/1078-0432.CCR-04-1299. [DOI] [PubMed] [Google Scholar]
- Baldus S.E., Schwarz E., Lohrey C., Zapatka M., Landsberg S., Hahn S.A., Schmidt D., Dienes H.P., Schmiegel W.H., Schwarte-Waldhoff I. Smad4 deficiency in cervical carcinoma cells. Oncogene. 2005;24:810–819. doi: 10.1038/sj.onc.1208235. [DOI] [PubMed] [Google Scholar]
- Camps C., Buffa F.M., Colella S., Moore J., Sotiriou C., Sheldon H., Harris A.L., Gleadle J.M., Ragoussis J. hsa-miR-210 Is induced by hypoxia and is an independent prognostic factor in breast cancer. Clin Cancer Res. 2008;14:1340–1348. doi: 10.1158/1078-0432.CCR-07-1755. [DOI] [PubMed] [Google Scholar]
- Cheng H., Fertig E.J., Ozawa H., Hatakeyama H., Howard J.D., Perez J., Considine M., Thakar M., Ranaweera R., Krigsfeld G., et al. Decreased SMAD4 expression is associated with induction of epithelial-to-mesenchymal transition and cetuximab resistance in head and neck squamous cell carcinoma. Cancer Biol Ther. 2015;16:1252–1258. doi: 10.1080/15384047.2015.1056418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crafton S.M., Salani R. Beyond chemotherapy: an overview and review of targeted therapy in cervical cancer. Clin Ther. 2016;38:449–458. doi: 10.1016/j.clinthera.2016.02.007. [DOI] [PubMed] [Google Scholar]
- Cragg G.M., Newman D.J. Plants as a source of anti-cancer agents. J Ethnopharmacol. 2005;100:72–79. doi: 10.1016/j.jep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Dang K., Myers K.A. The role of hypoxia-induced miR-210 in cancer progression. Int J Mol Sci. 2015;16:6353–6372. doi: 10.3390/ijms16036353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman M.M. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- Greither T., Grochola L.F., Udelnow A., Lautenschlager C., Wurl P., Taubert H. Elevated expression of microRNAs 155, 203, 210 and 222 in pancreatic tumors is associated with poorer survival. Int J Cancer. 2010;126:73–80. doi: 10.1002/ijc.24687. [DOI] [PubMed] [Google Scholar]
- Hernanda P.Y., Chen K., Das A.M., Sideras K., Wang W., Li J., Cao W., Bots S.J., Kodach L.L., de Man R.A., et al. SMAD4 exerts a tumor-promoting role in hepatocellular carcinoma. Oncogene. 2015;34:5055–5068. doi: 10.1038/onc.2014.425. [DOI] [PubMed] [Google Scholar]
- Ho C.S., Yap S.H., Phuah N.H., In L.L., Hasima N. MicroRNAs associated with tumour migration, invasion and angiogenic properties in A549 and SK-Lu1 human lung adenocarcinoma cells. Lung Cancer. 2014;83:154–162. doi: 10.1016/j.lungcan.2013.11.024. [DOI] [PubMed] [Google Scholar]
- Jung E.J., Santarpia L., Kim J., Esteva F.J., Moretti E., Buzdar A.U., Di Leo A., Le X.F., Bast R.C., Jr, Park S.T., et al. Plasma microRNA 210 levels correlate with sensitivity to trastuzumab and tumor presence in breast cancer patients. Cancer. 2012;118:2603–2614. doi: 10.1002/cncr.26565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Kim H., Park D., Jeoung D. miR-335 targets SIAH2 and confers sensitivity to anti-cancer drugs by increasing the expression of HDAC3. Mol Cells. 2015;38:562–572. doi: 10.14348/molcells.2015.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Scory S., Zapatka M., Eilert-Micus C., Hoppe S., Schwarz E., Schmiegel W., Hahn S.A., Schwarte-Waldhoff I. High-level inducible Smad4-reexpression in the cervical cancer cell line C4-II is associated with a gene expression profile that predicts a preferential role of Smad4 in extracellular matrix composition. BMC Cancer. 2007;7:209. doi: 10.1186/1471-2407-7-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloth J.N., Kenter G.G., Spijker H.S., Uljee S., Corver W.E., Jordanova E.S., Fleuren G.J., Gorter A. Expression of Smad2 and Smad4 in cervical cancer: absent nuclear Smad4 expression correlates with poor survival. Mod Pathol. 2008;21:866–875. doi: 10.1038/modpathol.2008.62. [DOI] [PubMed] [Google Scholar]
- Lagos-Quintana M., Rauhut R., Lendeckel W., Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- Lee S., Cho Y.S., Shim C., Kim J., Choi J., Oh S., Kim J., Zhang W., Lee J. Aberrant expression of Smad4 results in resistance against the growth-inhibitory effect of transforming growth factor-beta in the SiHa human cervical carcinoma cell line. Int J Cancer. 2001;94:500–507. doi: 10.1002/ijc.1494. [DOI] [PubMed] [Google Scholar]
- Liang Z., Li S., Xu X., Xu X., Wang X., Wu J., Zhu Y., Hu Z., Lin Y., Mao Y., et al. MicroRNA-576-3p inhibits proliferation in bladder cancer cells by targeting cyclin D1. Mol Cells. 2015;38:130–137. doi: 10.14348/molcells.2015.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Yu C., Shi Y., Jiang J., Liu Y. SMAD4 expression in breast ductal carcinoma correlates with prognosis. Oncol Lett. 2015;10:1709–1715. doi: 10.3892/ol.2015.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lou M., Jia Y., Duan Z., Wu J., Luo M., Wang Y. Correlation between human papillomavirus and microRNA-210 in hypoxia-associated human cervical cancer. Int J Clin Exp Pathol. 2016;9:1148–1157. [Google Scholar]
- Martinez I., Gardiner A.S., Board K.F., Monzon F.A., Edwards R.P., Khan S.A. Human papillomavirus type 16 reduces the expression of microRNA-218 in cervical carcinoma cells. Oncogene. 2008;27:2575–2582. doi: 10.1038/sj.onc.1210919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millimouno F.M., Dong J., Yang L., Li J., Li X. Targeting apoptosis pathways in cancer and perspectives with natural compounds from mother nature. Cancer Prev Res. 2014;7:1081–1107. doi: 10.1158/1940-6207.CAPR-14-0136. [DOI] [PubMed] [Google Scholar]
- Miyaki M., Iijima T., Konishi M., Sakai K., Ishii A., Yasuno M., Hishima T., Koike M., Shitara N., Iwama T., et al. Higher frequency of Smad4 gene mutation in human colorectal cancer with distant metastasis. Oncogene. 1999;18:3098–3103. doi: 10.1038/sj.onc.1202642. [DOI] [PubMed] [Google Scholar]
- Monk B.J., Sill M.W., McMeekin D.S., Cohn D.E., Ramondetta L.M., Boardman C.H., Benda J., Cella D. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2009;27:4649–4655. doi: 10.1200/JCO.2009.21.8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Othman N., In L.L., Harikrishna J.A., Hasima N. Bcl-xL silencing induces alterations in hsa-miR-608 expression and subsequent cell death in A549 and SK-LU1 human lung adenocarcinoma cells. PLoS One. 2013;8:e81735. doi: 10.1371/journal.pone.0081735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papageorgis P., Cheng K., Ozturk S., Gong Y., Lambert A.W., Abdolmaleky H.M., Zhou J.R., Thiagalingam S. Smad4 inactivation promotes malignancy and drug resistance of colon cancer. Cancer Res. 2011;71:998–1008. doi: 10.1158/0008-5472.CAN-09-3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phuah N.H., Nagoor N.H. Regulation of microRNAs by natural agents: new strategies in cancer therapies. Biomed Res Int. 2014;2014:804510. doi: 10.1155/2014/804510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phuah N.H., In L.L., Azmi M.N., Ibrahim H., Awang K., Nagoor N.H. Alterations of microRNA expression patterns in human cervical carcinoma cells (Ca Ski) toward 1’S-1’-acetoxychavicol acetate and cisplatin. Reprod Sci. 2013;20:567–578. doi: 10.1177/1933719112459220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Q., Furong W., Baosheng L. Multiple functions of hypoxia-regulated miR-210 in cancer. J Exp Clin Cancer Res. 2014;33:50. doi: 10.1186/1756-9966-33-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao Q., Shen Q., Zhou H., Peng Y., Li J., Lin Z. Aberrant microRNA expression in human cervical carcinomas. Med Oncol. 2012;29:1242–1248. doi: 10.1007/s12032-011-9830-2. [DOI] [PubMed] [Google Scholar]
- Tang X., Chen L., Yan X., Li Y., Xiong Y., Zhou X. Overexpression of miR-210 is associated with poor prognosis of acute myeloid leukemia. Med Sci Monit. 2015;21:3427–3433. doi: 10.12659/MSM.894812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- Wu S., Huang S., Ding J., Zhao Y., Liang L., Liu T., Zhan R., He X. Multiple microRNAs modulate p21Cip1/Waf1 expression by directly targeting its 3′ untranslated region. Oncogene. 2010;29:2302–2308. doi: 10.1038/onc.2010.34. [DOI] [PubMed] [Google Scholar]
- Yang W., Sun T., Cao J., Liu F., Tian Y., Zhu W. Downregulation of miR-210 expression inhibits proliferation, induces apoptosis and enhances radiosensitivity in hypoxic human hepatoma cells in vitro. Exp Cell Res. 2012;318:944–954. doi: 10.1016/j.yexcr.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Yang W., Wei J., Sun T., Liu F. Effects of knockdown of miR-210 in combination with ionizing radiation on human hepatoma xenograft in nude mice. Radiat Oncol. 2013;8: 102. doi: 10.1186/1748-717X-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S.L., Lee D.C., Son J.W., Park C.G., Lee H.Y., Kang J. Histone deacetylase 4 mediates SMAD family member 4 deacetylation and induces 5-fluorouracil resistance in breast cancer cells. Oncol Rep. 2013;30:1293–1300. doi: 10.3892/or.2013.2578. [DOI] [PubMed] [Google Scholar]
- Zhang B., Zhang B., Chen X., Bae S., Singh K., Washington M.K., Datta P.K. Loss of Smad4 in colorectal cancer induces resistance to 5-fluorouracil through activating Akt pathway. Br J Cancer. 2014;110:946–957. doi: 10.1038/bjc.2013.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Leng C., Wu C., Zhang Z., Dou L., Luo X., Zhang B., Chen X. Smad4 sensitizes colorectal cancer to 5-fluorouracil through cell cycle arrest by inhibiting the PI3K/Akt/CDC2/survivin cascade. Oncol Rep. 2016;35:1807–1815. doi: 10.3892/or.2015.4479. [DOI] [PubMed] [Google Scholar]