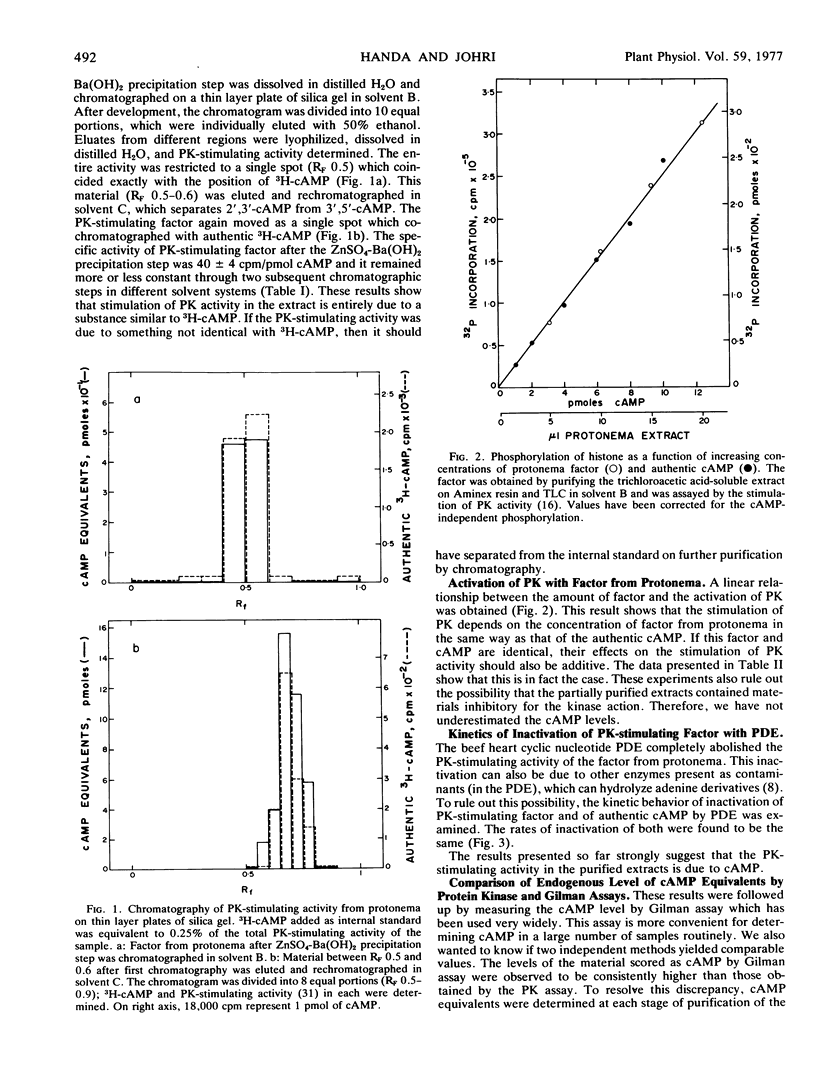
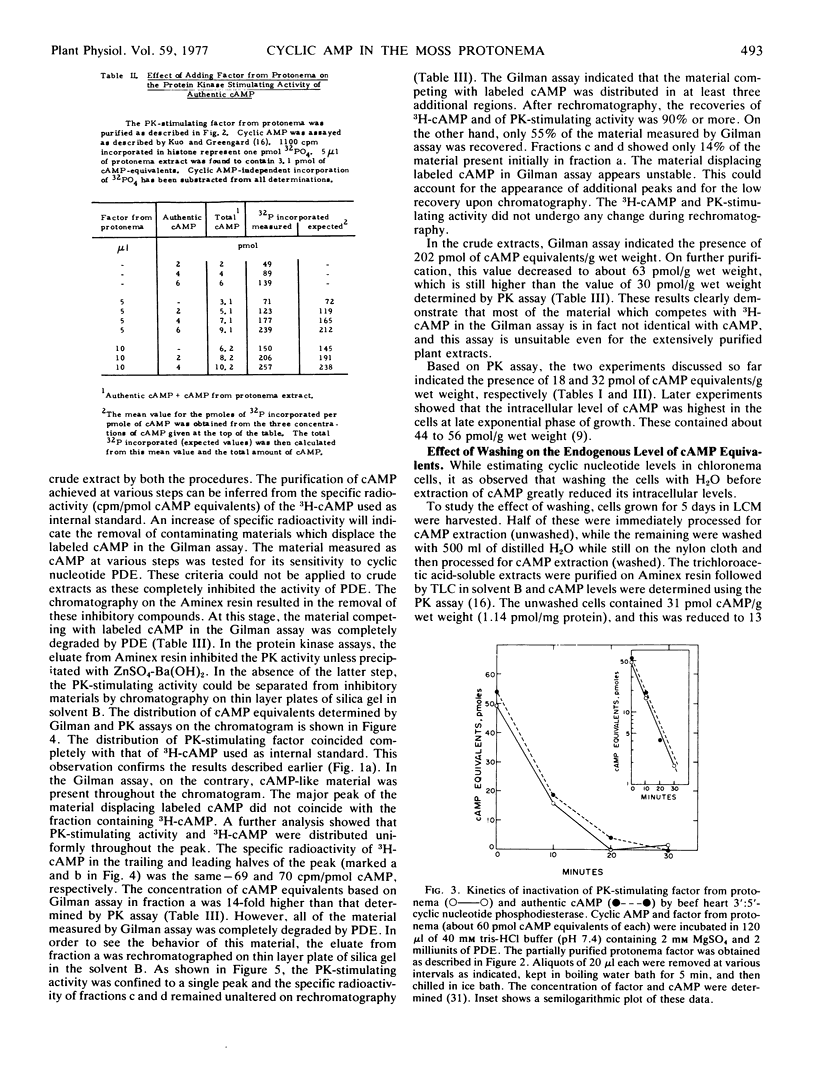
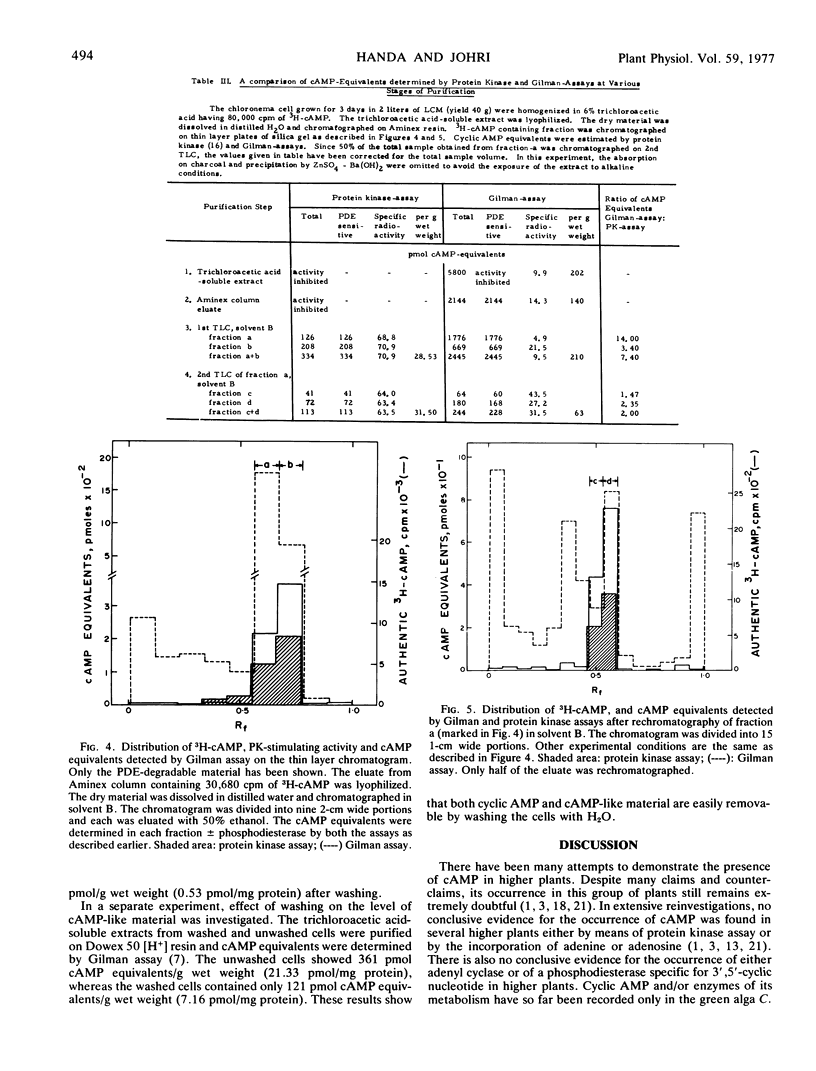
Abstract
From the protonema of the moss Funaria hygrometrica (L.) Sibth, a factor indistinguishable from cyclic adenosine 3′:5′-monophosphate (cAMP) has been isolated. The factor stimulated the activity of protein kinase from rabbit skeletal muscle and co-chromatographed with authentic cAMP in two solvent systems. Its ability to stimulate protein kinase activity was completely abolished by 3′:5′-cyclic nucleotide phosphodiesterase, the rate of inactivation being similar to that of authentic cAMP. Based on these properties, this factor is identified as 3′,5′-cAMP. Cyclic AMP could be readily removed from the cells and washing the cells with water reduced the endogenous level of cAMP by 2- to 3-fold. A comparison of cAMP levels by protein kinase and Gilman assays was made. The intracellular levels determined by protein kinase assay were about 7-fold lower than the values obtained by Gilman assay. This discrepancy was due to the presence of unidentified compounds which were completely degraded by 3′:5′-cyclic nucleotide phosphodiesterase. Although these displaced labeled cAMP in the Gilman assay, they did not stimulate the protein kinase activity. The protonema may contain cyclic nucleotides other than cAMP; these will not be detected in the protein kinase assay due to the specificity of this reaction. The crude extracts were found to be unsuitable for assaying cAMP by either method.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amrhein N., Filner P. Adenosine 3':5'-Cyclic Monophosphate in Chlamydomonas reinhardtii: Isolation and Characterization. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1099–1103. doi: 10.1073/pnas.70.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer U., Amrhein N. Cyclic nucleotide phosphodiesterase of Chlamydomonas reinhardtii. Biochim Biophys Acta. 1974 Apr 25;341(2):412–420. doi: 10.1016/0005-2744(74)90234-4. [DOI] [PubMed] [Google Scholar]
- Flawiá M. M., Torres H. N. Adenylate cyclase activity in Neurospora crassa. I. General properties. J Biol Chem. 1972 Nov 10;247(21):6873–6879. [PubMed] [Google Scholar]
- Gilman A. G. Protein binding assays for cyclic nucleotides. Adv Cyclic Nucleotide Res. 1972;2:9–24. [PubMed] [Google Scholar]
- Goldberg N. D., Larner J., Sasko H., O'Toole A. G. Enzymic analysis of cyclic 3', 5'-AMP in mammalian tissues and urine. Anal Biochem. 1969 Apr 4;28(1):523–544. doi: 10.1016/0003-2697(69)90208-5. [DOI] [PubMed] [Google Scholar]
- Jacobson A., Lodish H. F. Genetic control of development of the cellular slime mold, Dictyostelium discoideum. Annu Rev Genet. 1975;9:145–185. doi: 10.1146/annurev.ge.09.120175.001045. [DOI] [PubMed] [Google Scholar]
- Keirns J. J., Carritt B., Freeman J., Eisenstadt J. M., Bitensky M. W. Adenosine 3',5' cyclic monophosphate in Euglena gracilis. Life Sci. 1973 Aug 16;13(4):287–302. doi: 10.1016/0024-3205(73)90220-8. [DOI] [PubMed] [Google Scholar]
- Krishna G., Weiss B., Brodie B. B. A simple, sensitive method for the assay of adenyl cyclase. J Pharmacol Exp Ther. 1968 Oct;163(2):379–385. [PubMed] [Google Scholar]
- Kuo J. F., Greengard P. An assay method for cyclic AMP and cyclic GMP based upon their abilities to activate cyclic AMP-dependent and cyclic GMP-dependent protein kinases. Adv Cyclic Nucleotide Res. 1972;2:41–50. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin P. P. Cyclic nucleotides in higher plants? Adv Cyclic Nucleotide Res. 1974;4(0):439–460. [PubMed] [Google Scholar]
- Niles R. M., Mount M. S. Failure to Detect Cyclic 3', 5'-Adenosine Monophosphate in Healthy and Crown Gall Tumorous Tissues of Vicia faba. Plant Physiol. 1974 Sep;54(3):372–373. doi: 10.1104/pp.54.3.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ownby J. D., Ross C. W. Studies on the presence of adenosine cyclic 3':5'-monophosphate in oat coleoptiles. Plant Physiol. 1975 Feb;55(2):346–351. doi: 10.1104/pp.55.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickenberg H. V. Cyclic AMP in prokaryotes. Annu Rev Microbiol. 1974;28(0):353–369. doi: 10.1146/annurev.mi.28.100174.002033. [DOI] [PubMed] [Google Scholar]
- Schendel P. F., Wells R. D. The synthesis and purification of (gamma-32P)-adenosine triphosphate with high specific activity. J Biol Chem. 1973 Dec 10;248(23):8319–8321. [PubMed] [Google Scholar]
- Scott W. A., Solomon B. Cyclic 3',5'-AMP phosphodiesterase of Neurospora crassa. Biochem Biophys Res Commun. 1973 Aug 6;53(3):1024–1030. doi: 10.1016/0006-291x(73)90194-0. [DOI] [PubMed] [Google Scholar]
- Silverman P. M., Epstein P. M. Cyclic nucleotide metabolism coupled to cytodifferentiation of Blastocladiella emersonii. Proc Natl Acad Sci U S A. 1975 Feb;72(2):442–446. doi: 10.1073/pnas.72.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sy J., Richter D. Content of cyclic 3',5'-adenosine monophosphate and adenylyl cyclase in yeast at various growth conditions. Biochemistry. 1972 Jul 18;11(15):2788–2791. doi: 10.1021/bi00765a009. [DOI] [PubMed] [Google Scholar]
- Uno I., Yamaguchi M., Ishikawa T. The effect of light on fruiting body formation and adenosine 3':5'-cyclic monophosphate metabolism in Coprinus macrorhizus. Proc Natl Acad Sci U S A. 1974 Feb;71(2):479–483. doi: 10.1073/pnas.71.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wastila W. B., Stull J. T., Mayer S. E., Walsh D. A. Measurement of cyclic 3',5'-denosine monophosphate by the activation of skeletal muscle protein kinase. J Biol Chem. 1971 Apr 10;246(7):1996–2003. [PubMed] [Google Scholar]


