Abstract
Background
Plasma concentration of retinol is an accepted indicator to assess the vitamin A (retinol) status in cattle. However, the determination of vitamin A requires a time consuming multi-step procedure, which needs specific equipment to perform extraction, centrifugation or saponification prior to high-performance liquid chromatography (HPLC).
Methods
The concentrations of retinol in whole blood (n = 10), plasma (n = 132) and serum (n = 61) were measured by a new rapid cow-side test (iCheck™ FLUORO) and compared with those by HPLC in two independent laboratories in Germany (DE) and Japan (JP).
Results
Retinol concentrations in plasma ranged from 0.033 to 0.532 mg/L, and in serum from 0.043 to 0.360 mg/L (HPLC method). No significant differences in retinol levels were observed between the new rapid cow-side test and HPLC performed in different laboratories (HPLC vs. iCheck™ FLUORO: 0.320 ± 0.047 mg/L vs. 0.333 ± 0.044 mg/L, and 0.240 ± 0.096 mg/L vs. 0.241 ± 0.069 mg/L, lab DE and lab JP, respectively). A similar comparability was observed when whole blood was used (HPLC vs. iCheck™ FLUORO: 0.353 ± 0.084 mg/L vs. 0.341 ± 0.064 mg/L). Results showed a good agreement between both methods based on correlation coefficients of r2 = 0.87 (P < 0.001) and Bland-Altman blots revealed no significant bias for all comparison.
Conclusions
With the new rapid cow-side test (iCheck™ FLUORO) retinol concentrations in cattle can be reliably assessed within a few minutes and directly in the barn using even whole blood without the necessity of prior centrifugation. The ease of the application of the new rapid cow-side test and its portability can improve the diagnostic of vitamin A status and will help to control vitamin A supplementation in specific vitamin A feeding regimes such as used to optimize health status in calves or meat marbling in Japanese Black cattle.
Electronic supplementary material
The online version of this article (doi:10.1186/s12917-017-1042-3) contains supplementary material, which is available to authorized users.
Keywords: Cattle, Vitamin A, Biomarker, Blood, Method comparison, Cow-side assay
Background
Vitamin A (retinol) is an essential micronutrient not only to ascertain vision but also to modulate growth and development [1–4]. Cattle as well as other herbivorous animals are unable to synthesize retinol de novo and they have to obtain their vitamin A primarily from dietary β-carotene, which is converted into retinol within the enterocytes [5]. Independently from provitamin A activity, β-carotene can be absorbed intact in the gut and is considered to have positive effects on reproductive performance and immune response in cattle [6]. All green forages are rich in β-carotene and thus provide a high vitamin A value mainly during pasture conditions [7]. Low concentrations of vitamin A in blood resulting from dietary β-carotene restriction or lacking in vitamin A supplementation and are related to an increased risk of disease [8]. Neonate calves are particularly prone to develop symptoms of vitamin A deficiency, because they have low blood vitamin A concentrations and insufficient hepatic vitamin A stores at birth [9]. Low hepatic vitamin A storage is associated with stillborn and postnatal calf losses as a consequence of an insufficient vitamin A intake of the mother cows especially during non-grazing conditions [10]. In addition, cases of dermatopathy associated with hypovitaminosis A were recently described in juvenile Angus calves, which could be successfully treated with parenteral vitamin A supplementation [11]. Thus, the measurement of blood retinol concentrations in mother cows as well as in neonate and juvenile calves is a prerequisite for evaluation of their health status.
Low vitamin A plasma concentrations are generally found in Japanese Black cattle and are associated with an increased intramuscular or marbling adipose deposition without influencing other adipose depots [12–15]. Thus, to optimize the desired intramuscular fat marbling that increases the carcass value, Japanese Black fattening cattle are provided with a high concentrate diet deficient in vitamin A or β-carotene. It was suggested that optimal marbling scores are obtained when animals are kept very close to health deteriorating low vitamin A plasma concentrations in a range of 100–150 μg/L (40–50 IU/dl) over a period of usually 12 month during the fattening period [16]. However, in Japanese Black cattle low vitamin A plasma concentrations have been reported to be associated with an impaired immune function [17] and increased morbidity and mortality [18]. Therefore, blood vitamin A levels in Japanese Black cattle needs to be carefully monitored and nutritionally controlled. Additional reduced vitamin A plasma concentrations are also observed as a consequence of the acute phase reaction e.g. during inflammation or around parturition [19–21]. Thus, due to the restrict feeding management, the induced vitamin A deficiency in Japanese Black cattle can cause a serious health problem if not monitored and nutritionally controlled properly.
Under practical management conditions, vitamin A deficiency is difficult to diagnose and marginal deficiency may exist without obvious deficiency symptoms. Measurement of retinol in blood plasma is an appropriate marker to assess vitamin A status but it is currently a time consuming and cost-intensive multi-step procedure. Specific equipment is needed to perform extraction and centrifugation or saponification. Vitamin A is finally measured by colorimetric methods [22] or high performance liquid chromatography (HPLC) [23]. All preanalytic steps and the final analyses have to be performed in qualified analytical laboratories by highly trained personal. Alternatively suggested methods such as thin layer chromatography are similar complicated and not very reliable [24]. So far, however, no method has been described which is also able to measure vitamin A directly at cow-side in the barn or even determining vitamin A directly from whole blood prior to centrifugation.
This study presents data for a new, fast, easy to perform and laboratory-independent test to measure retinol in whole blood, plasma or serum. Results are compared with those obtained by HPLC as the reference method at two independent laboratories. The new assay is based on a separation and extraction principal published for the determination of β-carotene in blood plasma [25] as well as human and cow milk [26, 27].
Methods
The comparison was performed at two independent laboratory sites at the University of Potsdam, Germany (lab DE) and at the Obihiro University, Japan (lab JP).
Animals and sampling
Lab DE
A total of 132 blood samples were collected from dairy cows (n = 40) and bulls (n = 92) into EDTA-evacuated tubes (Saarstedt, Nümbrecht, Germany) and immediately separated by centrifugation (1500 × g; 10 min; 4 °C). The plasma was frozen at −80 °C and analyzed for their vitamin A concentrations with both methods (HPLC and iCheck™ FLUORO) within three months. In addition, 10 blood samples were taken from dairy cattle and collected into EDTA-evacuated tubes (Saarstedt) and analyzed freshly for their vitamin A content within 24 h after acquisition both in whole blood and in plasma after removal of the erythrocytes by centrifugation (1500 × g; 10 min; 4 °C). All samples were obtained from institutional farms (Research Station Frankenforst, Faculty of Agriculture, University of Bonn, Koenigswinter, Germany and Verein Ostfriesischer Stammviehzuechter, Leer, Germany).
Lab JP
A total of 61 blood samples from Holstein dairy cows (n = 29) and Japanese Black cattle (n = 32) were obtained by caudal venipuncture using non-heparinized and silicone-coated 9-mL tubes (Venoject, Autosep, Gel and Clot. Act., VP-AS109K; Terumo Corporation, Japan). Samples were collected from the institutional farm of the Obihiro University of Agriculture and Veterinary Medicine, Japan and Akiyama Farm Animal Clinic, Japan. To obtain serum, blood samples were coagulated for 15 min at 38 °C in an incubator. All tubes were centrifuged at 2000 × g for 20 min at 4 °C, and serum samples were kept at −30 °C until analysis within three months.
Vitamin A determination by HPLC
HPLC lab DE
A modified gradient reverse-phase HPLC system (Shimadzu, Duisburg, Germany) was used [28]. Briefly, vitamin A as retinol or retinyl esters was extracted from plasma and thereafter separated on a reverse-phase column (ReproSil 70 C18 column, 200 × 3.0 mm; inside diameter 5 μm; Dr. Maisch GmbH, Ammerbuch, Germany). The solvent system consisted of solvent A with methanol and solvent B with ethyl acetate at a flow rate of 0.5 mL/min (pump LC 20-AD, Shimadzu). Retinol was identified based on retention time in comparison to an external standard (Sigma, Munich, Germany) by use of a photodiode array detector (PDA SPDM-20A, Shimadzu). Vitamin A was quantified by measuring the absorption at 325 nm.
HPLC lab JP
The concentrations of retinol in serum were determined by HPLC, as described previously [29]. Extraction efficiency was 96%. Intra- and inter-assay CVs averaged 2.1% and 3.3%, respectively.
Vitamin A determination by a portable fluorometer
The novel cow-side test for vitamin A consists of an extraction unit that contains all necessary chemicals for extraction and separation and a portable fluorometer (iCheck™ FLUORO, BioAnalyt GmbH, Teltow, Germany). The combination of these two components enables to extract vitamin A from whole blood without prior separation of plasma in a single step at cow-side. The analysis of whole blood, blood plasma or serum with the new and innovative assay system was done as recommended by the manufacturer. A total volume of 500 μl of whole blood, plasma or serum was injected with an included syringe into the extraction and measuring vials. Thereafter it was shaken intensively for 10 s and then let settle for 5 min until complete phase separation. Finally, the vials were inserted into the fluorometer and measured. The quantification of vitamin A in whole blood, plasma or serum is based on the specific autofluorescent characteristics of excitation and emission wavelengths. Since that autofluorescence is based on the retinol moiety, any form of vitamin A is included in the quantification. Results were recorded as μg/L whole blood, plasma or serum.
Statistical analyses
The data were analysed using SPSS version 23.0 software (SPSS, Munich, Germany). The results obtained by HPLC and the new fluorometric method were judged for method acceptability as suggested for clinical laboratories [30]. Associations between the results were determined with Pearson correlation method. Mean results obtained by HPLC and fluorometer were compared with a paired t test. The difference of results obtained from HPLC and fluorometer were analyzed by one sample t test. A Bland-Altman bias plot [31] was done to determine the analytical accuracy of the two analytical methods. The analytical detection limit was defined as proposed in the concept of ‘functional sensitivity’. This is defined as the lowest concentration of an assay that can be measured with an inter-assay CV of 20% [32]. Values of P < 0.05 were assigned as statistically significant.
Results and discussion
The concentrations of retinol in plasma or serum as determined by HPLC and the new method ranged from 0.033 mg/L to 0.532 mg/L for plasma in Lab DE and from 0.043 mg/L to 0.360 mg/L for serum in Lab JP (Additional file 1: Table S1). No significant differences were observed between both methods at two different laboratories for the average values obtained (HPLC vs. new method: 0.320 ± 0.047 mg/L vs. 0.333 ± 0.044. mg/L; mean ± SD in Lab DE and 0.240 ± 0.096 mg/L vs. 0.241 ± 0.069 mg/L; mean ± SD in Lab JP).
The analytical advantage of the new innovative test method is to measure retinol from whole blood directly. This avoids the time consuming and limiting preanalytical step of centrifugation to obtain plasma or serum. In consequence, the new assay can be directly used in the barn. When the subset of 10 whole blood samples were assayed for retinol by the new test method and compared to plasma levels assessed by HPLC in Lab DE, a similar good agreement was found as for the use of plasma (HPLC plasma: 0.353 ± 0.084 mg/L vs. new test plasma: 0.340 ± 0.063 mg/L vs. new test whole blood 0.341 ± 0.064 mg/L; mean ± SD; see Additional file 2: Table S2). There were significantly correlations between concentrations of retinol measured in whole blood by the new method and retinol measured in plasma by HPLC (r2 = 0.84; P < 0.001) as well as retinol measured in plasma by the new method (r2 = 0.87; P < 0.001). The average packed cell volume of the 10 whole blood samples in this study was 31.7 ± 2.7% (mean ± SD).
The comparison between plasma or serum samples assayed with the two different methods in Lab DE (plasma) and Lab JP (serum) indicated a strong correlation between methods (Fig. 1a, b). Results show that the novel cow-side test for vitamin A correlated very well with HPLC analysis (r2 = 0.876 and 0.870, Lab DE and Lab JP, respectively, both P < 0.001). The calculated differences in retinol concentrations obtained by HPLC and fluorometer were not significantly different (P-values >0.05 for both Lab DE and Lab JP) indicating an acceptable level of agreement between the two assay methods. Based on the Bland-Altman plot (Fig. 1c, d) no systematic error and a good level of agreement occurred between the two methods and only 4% of the differences in measured values felt outside the 95% acceptability limits.
Fig. 1.
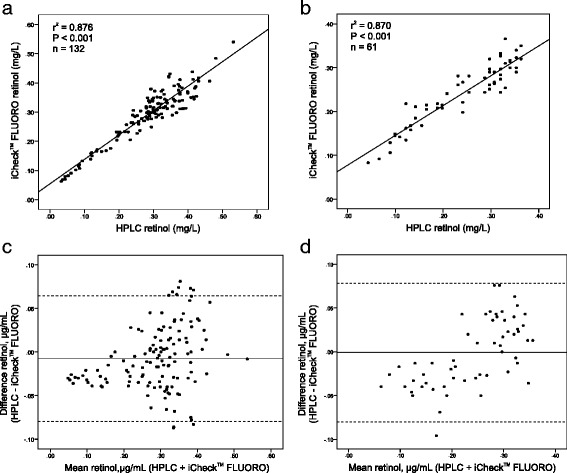
Correlation analyses demonstrate close association between plasma retinol as measured by HPLC and iCheck™ fluorometer in lab Germany (a) and lab Japan (b). Bland-Altman plot showing the mean difference (solid line) and the 95% confidence interval (dotted lines) of the retinol values in blood plasma obtained by HPLC and iCheck™ fluorometer in lab Germany (c) and lab Japan (d)
Based on multiple determinations of selected samples the intra-assay and inter-assay precision were calculated for each method (Lab DE). In average, the coefficient of variance (CV) was in an order of magnitude generally accepted for average analysis of this kind. The CV was slightly higher in the HPLC method compared to the new method (5.3% vs. 2.3%, respectively).
Conclusions
The comparison of the new cow-side test (iCheck™ FLUORO) to measure vitamin A in cattle whole blood, blood plasma or serum showed a very good agreement, reliability and accuracy with the cumbersome, time-intensive and expensive HPLC analytical technique. The assay allows the determination of vitamin A directly in whole blood within 5 min even at cow-side at the barn without a further sample preparation. Because all chemicals are sealed in the vial, the staff is not directly exposed to potentially hazardous organic chemicals. Additionally, the limited volume of organic solvent necessary is positive with regard to environmentally and ecologically critical waste. The novel test realizes important aspects of a cow-side assay: it is sensitive, specific and rapid. Finally, the test delivers clear end-point results which can help to optimize nutritional interventions in cattle.
Additional files
Comparison of retinol concentrations in plasma (n = 132, obtained from Lab Germany) and serum (n = 61, obtained from Lab Japan) measured by HPLC and iCheck™ FLUORO (PDF 16 kb).
Concentrations of retinol in plasma (n = 10) measured by HPLC and iCheck™ FLUORO and whole blood measured by iCheck™ FLUORO (PDF 7 kb).
Acknowledgments
The authors are grateful to the technicians of the Department of Physiology and Pathophysiology, University of Potsdam for their excellent technical assistance, constant helpfulness, and overall kindness. The authors thank Dr. K. Akiyama (Akiyama Farm Animal Clinic, Japan) for supporting our research. Blood samples were made available by the Research Station Frankenforst, Faculty of Agriculture, University of Bonn, Koenigswinter, Germany. We thank the staff and Dr. Johanna F.L. Heinz, Institute of Animal Science, University of Bonn for their support. The authors thank VOST (Verein Ostfriesischer Stammviehzuechter) for providing blood samples of bulls.
Funding
The authors received no financial support for authorship except from the free supply of consumables for the new test by BioAnalyt GmbH. This study was supported by a Grant-in-Aid for Scientific Research (CK) and the Global COE program from the Japan Society for the Promotion of Science, and the Foerderverein Biotechnologieforschung e.V. (FBF), Bonn, Germany. We acknowledge the support of the Deutsche Forschungsgemeinschaft and Open Access Publishing Fund of University of Potsdam.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its additional information files.
Authors’ contributions
JR carried out HPLC analysis, analyzed the data, performed statistical analysis and participated in drafting the manuscript. HS, CK, NH, XK participated in study design and sample acquisition and contributed to the drafting of the manuscript. AM, FJS participated in its design and coordination and contributed to draft the manuscript. All authors read and approved the manuscript.
Authors’ information
Not applicable.
Competing interests
Florian J. Schweigert is shareholder of BioAnalyt. Other authors declare no potential conflict of interest with respect to the authorship and publication of this article.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Lab DE: The study was done according to the German Animal Welfare Law (released on 05/18/2006, last changes on 12/03/2015) and the animal care and use protocol was approved by the Animal Welfare Committee (Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen, Germany, AZ 8.87–51.05.20.10.258).
Lab JP: All experimental procedures complied with the Guidelines for the care and use of agricultural animals of Obihiro University, and were approved by the Committee on the Ethics of Animal Experiments (approval numbers: #20–13; #20–128 and #21–14). All samples from Holstein cows were obtained from cattle kept at an institutional farm. Therefore, owner consent to participate the study was not required. Blood samples from Japanese Black cattle were supplied from the owner of Akiyama farm, who is a veterinarian.
Abbreviations
- EDTA
Ethylenediaminetetraacetic acid
- HPLC
High-performance liquid chromatography
- iCheck™ FLUORO
Portable fluorometer for quantitative vitamin A determination
- Lab DE
Laboratory located in Germany
- Lab JP
Laboratory located in Japan
Contributor Information
Jens Raila, Email: jens.raila@uni-potsdam.de.
Chiho Kawashima, Email: kawasima@obihiro.ac.jp.
Helga Sauerwein, Email: sauerwein@uni-bonn.de.
Nadine Hülsmann, Email: nadine-frische@gmx.de.
Christoph Knorr, Email: cknorr@gwdg.de.
Akio Myamoto, Email: akiomiya@obihiro.ac.jp.
Florian J. Schweigert, Email: florian.schweigert@uni-potsdam.de
References
- 1.Ikeda S, Kitagawa M, Imai H, Yamada M. The roles of vitamin A for cytoplasmic maturation of bovine oocytes. J Reprod Dev. 2005;51:23–35. doi: 10.1262/jrd.51.23. [DOI] [PubMed] [Google Scholar]
- 2.Clagett-Dame M, Knutson D. Vitamin A in reproduction and development. Nutrients. 2011;3:385–428. doi: 10.3390/nu3040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blomhoff R, Blomhoff HK. Overview of retinoid metabolism and function. J Neurobiol. 2006;66:606–630. doi: 10.1002/neu.20242. [DOI] [PubMed] [Google Scholar]
- 4.Gomez E, Caamano JN, Rodriguez A, De Frutos C, Facal N, Diez C. Bovine early embryonic development and vitamin A. Reprod Domest Anim. 2006;41(Suppl 2):63–71. doi: 10.1111/j.1439-0531.2006.00770.x. [DOI] [PubMed] [Google Scholar]
- 5.von Lintig J. Provitamin A metabolism and functions in mammalian biology. Am J Clin Nutr. 2012;96:1234S–1244S. doi: 10.3945/ajcn.112.034629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawashima C, Matsui M, Shimizu T, Kida K, Miyamoto A. Nutritional factors that regulate ovulation of the dominant follicle during the first follicular wave postpartum in high-producing dairy cows. J Reprod Dev. 2012;58:10–16. doi: 10.1262/jrd.11-139N. [DOI] [PubMed] [Google Scholar]
- 7.Elgersma A, Soegaard K, Jensen SK. Fatty acids, alpha-tocopherol, beta-carotene, and lutein contents in forage legumes, forbs, and a grass-clover mixture. J Agric Food Chem. 2013;61:11913–11920. doi: 10.1021/jf403195v. [DOI] [PubMed] [Google Scholar]
- 8.Weiss WP. Requirements of fat-soluble vitamins for dairy cows: a review. J Dairy Sci. 1998;81:2493–2501. doi: 10.3168/jds.S0022-0302(98)70141-9. [DOI] [PubMed] [Google Scholar]
- 9.Gallina AM, Helmboldt CF, Frier HI, Nielsen SW, Eaton HD. Bone growth in the hypovitaminotic A calf. J Nutr. 1970;100:129–141. doi: 10.1093/jn/100.1.129. [DOI] [PubMed] [Google Scholar]
- 10.Waldner CL, Blakley B. Evaluating micronutrient concentrations in liver samples from abortions, stillbirths, and neonatal and postnatal losses in beef calves. J Vet Diagn Invest. 2014;26:376–89. doi: 10.1177/1040638714526597. [DOI] [PubMed] [Google Scholar]
- 11.Baldwin TJ, Rood KA, Kelly EJ, Hall JO. Dermatopathy in juvenile Angus cattle due to vitamin A deficiency. J Vet Diagn Invest. 2012;24:763–6. doi: 10.1177/1040638712445767. [DOI] [PubMed] [Google Scholar]
- 12.Adachi K, Katsura N, Nomura Y, Arikawa A, Hidaka M, Onimaru T. Serum vitamin A and vitamin E in Japanese black fattening cattle in Miyazaki prefecture as determined by automatic column-switching high performance liquid chromatography. J Vet Med Sci. 1996;58:461–464. doi: 10.1292/jvms.58.461. [DOI] [PubMed] [Google Scholar]
- 13.Kato Y, Ito M, Hirooka H. Genetic parameters of serum vitamin A and total cholesterol concentrations and the genetic relationships with carcass traits in an F1 cross between Japanese Black sires and Holstein dams. J Anim Sci. 2011;89:951–958. doi: 10.2527/jas.2010-2872. [DOI] [PubMed] [Google Scholar]
- 14.Oka A, Maruo Y, Miki T, Yamasaki T, Saito T. Influence of vitamin A on the quality of beef from the Tajima strain of Japanese Black cattle. Meat Sci. 1998;48:159–167. doi: 10.1016/S0309-1740(97)00086-7. [DOI] [PubMed] [Google Scholar]
- 15.Pickworth CL, Loerch SC, Fluharty FL. Effects of timing and duration of dietary vitamin A reduction on carcass quality of finishing beef cattle. J Anim Sci. 2012;90:2677–2691. doi: 10.2527/jas.2011-4756. [DOI] [PubMed] [Google Scholar]
- 16.Adachi K, Kawano H, Tsuno K, Nomura Y, Yamamoto N, Arikawa A, Tsuji A, Adachi M, Onimaru T, Ohwada K. Relationship between serum biochemical values and marbling scores in Japanese Black steers. J Vet Med Sci. 1999;61:961–964. doi: 10.1292/jvms.61.961. [DOI] [PubMed] [Google Scholar]
- 17.Yano H, Ohtsuka H, Miyazawa M, Abiko S, Ando T, Watanabe D, Matsuda K, Kawamura S, Arai T, Morris S. Relationship between immune function and serum vitamin A in Japanese black beef cattle. J Vet Med Sci. 2009;71:199–202. doi: 10.1292/jvms.71.199. [DOI] [PubMed] [Google Scholar]
- 18.Adachi K, Kawano H, Tsuno K, Nomura Y, Katsura N, Arikawa A, Tsuji A, Onimaru T. Values of the serum components in Japanese black beef steers at farms with high productivity and low frequencies of disease and death in Miyazaki Prefecture. J Vet Med Sci. 1997;59:873–877. doi: 10.1292/jvms.59.873. [DOI] [PubMed] [Google Scholar]
- 19.Trevisi E, Amadori M, Bakudila AM, Bertoni G. Metabolic changes in dairy cows induced by oral, low-dose interferon-alpha treatment. J Anim Sci. 2009;87:3020–3029. doi: 10.2527/jas.2008-1178. [DOI] [PubMed] [Google Scholar]
- 20.Rezamand P, Hoagland TA, Moyes KM, Silbart LK, Andrew SM. Energy status, lipid-soluble vitamins, and acute phase proteins in periparturient Holstein and Jersey dairy cows with or without subclinical mastitis. J Dairy Sci. 2007;90:5097–5107. doi: 10.3168/jds.2007-0035. [DOI] [PubMed] [Google Scholar]
- 21.Kawashima C, Nagashima S, Sawada K, Schweigert FJ, Miyamoto A, Kida K. Effect of beta-carotene supply during close-up dry period on the onset of first postpartum luteal activity in dairy cows. Reprod Domest Anim. 2010;45:282–287. doi: 10.1111/j.1439-0531.2009.01558.x. [DOI] [PubMed] [Google Scholar]
- 22.Katsoulos PD, Roubies N, Panousis N, Karatzanos P, Karatzias H. Long-term fluctuations and effect of age on serum concentrations of certain fat-soluble vitamins in dairy cows. Vet Clin Pathol. 2005;34:362–367. doi: 10.1111/j.1939-165X.2005.tb00062.x. [DOI] [PubMed] [Google Scholar]
- 23.Schweigert FJ, Zucker H. Concentrations of vitamin A, beta-carotene and vitamin E in individual bovine follicles of different quality. J Reprod Fertil. 1988;82:575–579. doi: 10.1530/jrf.0.0820575. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki J, Katoh N. A simple and cheap methods for measuring serum vitamin A in cattle using only a spectrophotometer. Nihon Juigaku Zasshi. 1990;52:1281–1283. doi: 10.1292/jvms1939.52.1281. [DOI] [PubMed] [Google Scholar]
- 25.Raila J, Enjalbert F, Mothes R, Hurtienne A, Schweigert FJ. Validation of a new point-of-care assay for determination of beta-carotene concentration in bovine whole blood and plasma. Vet Clin Pathol. 2012;41:119–122. doi: 10.1111/j.1939-165X.2012.00400.x. [DOI] [PubMed] [Google Scholar]
- 26.Laillou A, Renaud C, Berger J, Moench-Pfanner R, Fontan L, Avallone S. Assessment of a portable device to quantify vitamin A in fortified foods (flour, sugar, and milk) for quality control. Food Nutr Bull. 2014;35:449–457. doi: 10.1177/156482651403500407. [DOI] [PubMed] [Google Scholar]
- 27.Engle-Stone R, Haskell MJ, La Frano MR, Ndjebayi AO, Nankap M, Brown KH. Comparison of breast milk vitamin A concentration measured in fresh milk by a rapid field assay (the iCheck FLUORO) with standard measurement of stored milk by HPLC. Eur J Clin Nutr. 2014;68:938–940. doi: 10.1038/ejcn.2014.63. [DOI] [PubMed] [Google Scholar]
- 28.Schweigert FJ, Steinhagen B, Raila J, Siemann A, Peet D, Buscher U. Concentrations of carotenoids, retinol and alpha-tocopherol in plasma and follicular fluid of women undergoing IVF. Hum Reprod. 2003;18:1259–1264. doi: 10.1093/humrep/deg249. [DOI] [PubMed] [Google Scholar]
- 29.De Ruyter MG, De Leenheer AP. Determination of serum retinol (vitamin A) by high-speed liquid chromatography. Clin Chem. 1976;22:1593–1595. [PubMed] [Google Scholar]
- 30.Jensen AL, Kjelgaard-Hansen M. Method comparison in the clinical laboratory. Vet Clin Pathol. 2006;35:276–286. doi: 10.1111/j.1939-165X.2006.tb00131.x. [DOI] [PubMed] [Google Scholar]
- 31.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. doi: 10.1016/S0140-6736(86)90837-8. [DOI] [PubMed] [Google Scholar]
- 32.Spencer CA, Takeuchi M, Kazarosyan M, MacKenzie F, Beckett GJ, Wilkinson E. Interlaboratory/intermethod differences in functional sensitivity of immunometric assays of thyrotropin (TSH) and impact on reliability of measurement of subnormal concentrations of TSH. Clin Chem. 1995;41:367–374. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of retinol concentrations in plasma (n = 132, obtained from Lab Germany) and serum (n = 61, obtained from Lab Japan) measured by HPLC and iCheck™ FLUORO (PDF 16 kb).
Concentrations of retinol in plasma (n = 10) measured by HPLC and iCheck™ FLUORO and whole blood measured by iCheck™ FLUORO (PDF 7 kb).
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its additional information files.


