Abstract
Background
Mac-2 Binding Protein Glycosylation isomer (M2BPGi) is a novel serological glyco-biomarker for staging liver fibrosis. Here, we aimed to evaluate the efficiency of serum M2BPGi in identifying liver fibrosis stages in Chinese patients with chronic hepatitis C infection.
Methods
Serum M2BPGi levels were evaluated in 680 patients with chronic hepatitis C and 164 healthy controls who underwent the Fibro Scan® test of liver fibrosis. The diagnostic accuracy of serum M2BPGi values was compared to that of other fibrosis markers, including Fibro Scan®, the aspartate transaminase to platelet ratio index (APRI), the fibrosis index based on four factors (FIB4), and the gamma-glutamyltranspeptidase to platelet ratio (GPR).
Results
Among the chronic hepatitis C patients, the median serum M2BPGi level increased with increasing fibrosis score as follows: 0.88 (≤F2), 1.70 (F2/F3), and 5.68 (cirrhosis). M2BPGi concentrations could also distinguish between healthy controls (0.38 ± 0.24) and hepatitis C patients (1.57 ± 2.28). After adjusting for potential confounders, M2BPGi was the most significant factor associated with the liver stiffness measurement (effect size = 0.275, P < 0.001). The optimum cutoff values of serum M2BPGi for patients with F2 and F4 were 0.945 and 1.355, respectively. The area under the curve of serum M2BPGi for prediction of significant fibrosis (F ≥ 4) using was comparable to that of APRI (0.892 vs. 0.873), while it was superior to that of other alternative markers, including FIB4 (0.818) and GPR (0.851). Compared with other non-invasive markers, M2BPGi had the greatest specificity for diagnosing cirrhosis and cirrhosis in hepatitis C patients.
Conclusions
Our results suggest that the level of serum M2BPGi would be a simple and reliable diagnostic tool for identifying liver fibrosis stage in Chinese patients with chronic hepatitis.
Keywords: Chronic hepatitis C, Liver fibrosis, Mac-2 Binding Protein Glycosylation isomer, Cirrhosis
Background
Hepatitis C virus (HCV) is a major cause of liver disease worldwide. HCV establishes persistent infection that often results in chronic hepatitis followed by liver fibrosis, cirrhosis, from which hepatocellular carcinoma arises. The stage of chronic liver disease is mainly evaluated based on the degree of liver fibrosis. Therefore, assessment of the degree of liver fibrosis is important in clinical practice. Liver biopsy is the gold standard method for evaluating the degree of liver fibrosis [1]. However, the invasiveness nature of liver biopsy makes it un-practical approach, especially for patients who require follow-up [2].
Recently, glycoprotein-based biomarkers (glyco-biomarker) have been emerged as novel disease biomarkers. Mac-2 Binding Protein Glycosylation isomer (M2BPGi), which is also named hyperglycosylated Wisteria floribunda agglutinin-positive Mac-2 binding protein (WFA+ -M2BP) is a new serological glyco-biomarker that has been recently developed for predicting the stage of liver fibrosis [3, 4]. This technical approach may be applicable to the development pipeline for a wide variety of glyco diagnostic tools.
Developed in France by Echosens, transient elastography (Fibro Scan®) is a novel, noninvasive technique that is used to assess the degree of liver fibrosis. The Vibration Controlled Transient Elastography™ system (VCTE™) implemented on the Fibro Scan®[5] is the most validated and commonly used elastography method worldwide and has been approved in China by the China Food and Drug Administration [6, 7]. Data suggest VCTE™ is reliable in diagnosing cirrhosis in patients with chronic liver disease [8], liver fibrosis in autoimmune hepatitis [9] advanced fibrosis in patients with alcoholic liver disease [10], significant fibrosis in patients with chronic hepatitis C [11], and in biliary diseases [12]. In recent years, the liver stiffness measurement (LSM) which is resulting from transient elastography has been applied to predict liver fibrosis in many hospitals in China [13, 14].
This study aimed to (1) identify optimal serum M2BPGi cut-off values for evaluating the stage of liver fibrosis in Chinese patients with chronic hepatitis C, (2) identify the factors independently associated with M2BPGi, and (3) compare the diagnostic value of M2BPGi versus the fibrosis biomarkers aspartate transaminase to platelet ratio index (APRI), fibrosis index based on four factors (FIB-4), and gamma-glutamyltrans peptidase to platelet ratio (GPR).
Methods
Patients
We retrospectively reviewed 1001 hepatitis C patients and 164 healthy controls who underwent Fibro Scan® testing and color Doppler ultrasound at The First Hospital of Jilin University (Changchun, China). Of these, 321 patients who had fatty liver, which might influence the value of the Fibro Scan®, were excluded from the study. The study was approved by the Ethics Committee at the First Hospital of Jilin University, Changchun, China and the written informed consent was obtained from each patient enrolled in this study.
LSM
Fibro Scan® (Echosens, France) was performed by skillful operator to assess LSM value. Ten LSM values were recoreded and the median value calculated by the statistics analyze system was called as the final score. According to Tsochatzis [15] et al, the liver stiffness cut-offs were staged on a scale of 0–4 according to Fibro Scan® given as 7.6 (range 5.1–10.1), 10.9 (8.0–15.4), and 15.3 (11.9–26.5) kPa for stages F2, F3, and F4 in hepatitis C patients respectively.
Direct measurement of serum M2BPGi
Serum M2BPGi levels were quantified by a lectin-antibody sandwich immunoassay using HISCL-5000 immunoanalyzer (Sysmex Co., Hyogo, Japan) [4, 16]. M2BPGi values conjugated to WFA were indexed with scored values using the following equation: Cutoff index = ([M2BPGi] sample − [M2BPGi] NC)/([M2BPGi] PC −[M2BPGi] NC), where [M2BPGi] sample is the M2BPGi level in the serum sample, PC is the positive control, and NC is negative control. The positive control was supplied as a calibration solution preliminarily standardized to yield a cutoff value of 1.0.
Surrogate serum markers
On the same day that Fibro Scan® examination was performed, blood specimens were obtained and subjected to hematological and biochemical examinations. Hyaluronic acid level, type 4 collagen (CIV) level, laminin (LN) level, platelet count, aspartate aminotransferase (AST) level and alanine aminotransferase (ALT) level were determined in the same laboratory. APRI, FIB-4, and GPR scores were calculated based on the following formulae:
GPR = GGT/platelet count, where GGT is γ-glutamyltransferase.
Statistical analysis
Differences between continuous variables of paired samples were analyzed using Mann-Whitney U test. Receiver operating characteristic (ROC) curve and area under the curve (AUCs) were used to determine the optimal liver stiffness cutoffs for fibrosis staging. The optimal liver stiffness cutoffs were determined based on the optimal sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). Statistical analysis of the differences between the AUCs was based on the theory of generalized U-statistics. The P <0.05 was considered significant.
Results
Baseline clinical characteristics of patients
The baseline clinical characteristics of enrolled patients are shown in Table 1. The median age was 54.9 years with a male predominance (68.4%) in the hepatitis C group, while the median age was 56.5 years with a female predominance (54.7%) in the control group. Two hundred ninety-one patients were treated with interferon (IFN) + ribavirin (RBV). The median (interquartile range, IQR) values for the body mass index (BMI), APRI, FIB-4 index, AST/ALT ratio, and GPR were 23.4 (21.5–25.5), 0.48 (0.32–1.01), 2.81 (1.87–4.65), 1.44 (1.07–2.69), and 0.18 (0.09–0.47), respectively, in the hepatitis C-infected group. The fibrosis stage was < F2 (LSM < 7.6) for 437 cases (64.3%), F2 or F3 (7.6 ≤ LSM < 15.3) for 175 cases (25.7%), and F4 (LSM > 15.3) for 68 cases (10.0%). BMI, APRI, FIB-4 index, AST/ALT ratio, GPR, and M2BPGi level differed significantly between hepatitis C patients and healthy controls.
Table 1.
Patients’ clinical characteristics and laboratory data
| Features | Cases (n = 680) | Healthy (n = 164) | P value |
|---|---|---|---|
| Male/Female, n | 465/215 | 76/88 | |
| Age (years) | 54.9 ± 11.0 | 56.8 ± 14.8 | 0.02 |
| IFN therapy N (%) | 291 (42.8%) | 0 (0.00%) | |
| Habitual alcohol intake N (%) | 275 (40.4%) | ||
| Body mass index | 23.6 ± 3.1 | 24.0 ± 3.1 | 0.114 |
| Platelet count (×109/l) | 166.7 ± 74.8 | 228.3 ± 45.1 | <0.001 |
| AST (15–46 IU/l) | 49.2 ± 39.9 | 26.4 ± 10.2 | <0.001 |
| ALT (0–40 IU/l) | 37.7 ± 43.8 | 23.7 ± 15.1 | 0.011 |
| GGT (12–73 IU/l) | 63.7 ± 105.3 | 27.2 ± 27.7 | <0.001 |
| AFP (0–6.2 IU/l) | 7.46 ± 33.9 | ||
| HCV genotype N (%) | |||
| 1b | 126 (18.5%) | ||
| 2a | 94 (13.8%) | ||
| 1b/2a | 2 (0.3%) | ||
| Unknown | 459 (67.4%) | ||
| Fibrosis markers | |||
| APRI | 1.06 ± 2.72 | 0.30 ± 0.13 | <0.001 |
| FIB4 | 4.84 ± 12.05 | 1.33 ± 0.55 | <0.001 |
| AST/ALT ratio | 2.62 ± 2.80 | 1.45 ± 1.43 | <0.001 |
| GPR | 0.58 ± 1.21 | 0.12 ± 0.12 | <0.001 |
| M2BPGi | 1.57 ± 2.28 | 0.38 ± 0.23 | <0.001 |
| Fibrosis stage (0 ~ 1/2 ~ 3/4) | 437/175/68 | 155/9/0 | |
Correlation between M2BPGi and LSM in patients with HCV infection
Among chronic hepatitis C patients, the median level of serum M2BPGi was positively associated with fibrosis stage as 0.88 (<F2), 1.70 (F2/F3), and 5.68 (F4). As the median serum M2BPGi level was 0.38 in healthy controls, there was also a significant difference between healthy controls and hepatitis C patients (P < 0.001). Figure 1a shows a box-plot of liver fibrosis stage (LSM) vs. M2BPGi level. Moreover, significant differences were noted for M2BPGi level among the fibrosis stages. The differences were significant (P < 0.001) between healthy controls and chronic hepatitis C patients and between F2/3 and F4 stages (P < 0.001). Using Spearman rank correlation analysis, a positive correlation between LSM and M2BPGi level (rho = 0.504, P < 0.001) was observed (Fig. 1b).
Fig. 1.
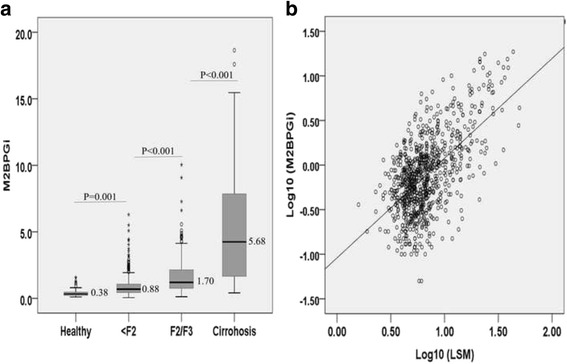
a The serum Wisteria floribunda agglutinin-positive Mac-2 binding protein (M2BPGi) values for each fibrosis stage. The top and bottom of each box represent the first and third quartiles, respectively, with the height of the box representing the interquartile range, covering 50% of the values. The line across each box represents the median. The whiskers show the highest and lowest values. b Correlation of log10 (M2BPGi) values with log10 (LSM)
Variables associated with LSM obtained by Fibro Scan®
Correlation between LSM and baseline demographic (age and gender), hematological and biochemical characteristics (platelet count, total protein, albumin, ALT, AST, alkaline phosphatase, γ-glutamyltransferase, bilirubin, FIB-4 index, APRI, AST/ALT ratio, and GPR) was also examined (Table 2). LSM was significantly correlated with ALT (rho = 403, P < 0.001), AST (rho = 0.499, P < 0.001), alkaline phosphatase (ALP; rho = 0.309, P < 0.001), GGT (rho = 0.432, P < 0.001), total bilirubin (rho = 0.274, P < 0.001), FIB-4 (rho = 0.412, P < 0.001), APRI (rho = 0.536, P < 0.001), and all histological results and was inversely correlated with platelet count (rho = -0.386, P < 0.001) and AST/ALT ratio (rho = -0.141, P < 0.001). Gender, age, cigarette smoking, platelet count, total protein, albumin, liver function markers (AST, ALT, ALP, GGT, TB, HA, LN, α-fetoprotein) and M2BPGi level were associated with LSM in univariate analysis. Subsequently these factors were entered in logistic regression multivariable analysis. This analysis identified platelet count (effect size = -0.07, P ≤ 0.05),total protein level (effect size = -0.08, P ≤ 0.05), ALP (effect size = 0.116, P ≤ 0.05), GGT (effect size = 0.13, P < 0.001), total bilirubin (effect size = 0.111, P = 0.001), HA (effect size = 0.137, P < 0.001), CIV (effect size = 0.099, P ≤ 0.05), AFP (effect size = 0.069, P ≤ 0.05), and M2BPGi (effect size = 0.275, P < 0.001) levels as independent factors that were significantly associated with LSM (Table 2). Notably, serum level of M2BPGi was the most significant factor associated with LSM.
Table 2.
Variables associated with the LSM according to liner regression analyses
| Characteristic | Correlation analysis | liner regression analysis | ||
|---|---|---|---|---|
| rho | P value | Effect size | P value | |
| Age | 0.188 | <0.001 | 0.015 | 0.606 |
| Gender | −0.099 | 0.01 | −0.026 | 0.404 |
| BMI | 0.038 | 0.318 | - | - |
| Laboratory findings | ||||
| Platelet count | −0.386 | <0.001 | −0.07 | 0.02 |
| Total protein | 0.194 | <0.001 | −0.08 | 0.032 |
| Albumin | −0.18 | <0.001 | −0.071 | 0.073 |
| ALT | 0.403 | <0.001 | 0.017 | 0.732 |
| AST | 0.499 | <0.001 | 0.008 | 0.893 |
| ALP | 0.309 | <0.001 | 0.116 | 0.001 |
| GGT | 0.432 | <0.001 | 0.13 | <0.001 |
| Total Billirubin | 0.274 | <0.001 | 0.111 | 0.001 |
| HA | 0.338 | <0.001 | 0.137 | <0.001 |
| LN | 0.234 | <0.001 | 0.03 | 0.38 |
| CIV | 0.34 | <0.001 | 0.099 | 0.003 |
| AFP | 0.379 | <0.001 | 0.069 | 0.033 |
| Fibrosis markers | ||||
| FIB-4 indext | 0.412 | <0.001 | - | - |
| APRI | 0.536 | <0.001 | - | - |
| AST/ALT ratio | −0.141 | <0.001 | - | - |
| GPR | 0.487 | <0.001 | - | - |
| M2BPGi | 0.504 | <0.001 | 0.275 | <0.001 |
Comparison of AUCs and cut-off values for fibrosis markers
ROC analyses were carried out to evaluate the diagnostic accuracy of serum M2BPGi, FIB-4 index, APRI, and GPR for fibrosis stage in chronic hepatitis C. The calculated AUC, optimal cut-off value, sensitivity, specificity, PPV, and NPV for each fibrosis stage are listed in Table 3. The AUCs were 0.774 and 0.892 for ≥ F2 and F4, respectively. The optimal cut-off values predicted fibrosis stages ≥ F2 and F4 were 0.945 and 1.355, respectively.
Table 3.
Diagnostic performance of M2BPGi in 680 patients with chronic hepatitis C
| Fibrosis stage | Cutoff | AUC | Sensitivity | Specificity | P value | |
|---|---|---|---|---|---|---|
| ≥F2 | M2BPGi | 0.945 | 0.774 (0.736, 0.812) | 0.745 | 0.694 | Reference |
| APRI | 0.635 | 0.787 (0.750, 0.824) | 0.708 | 0.799 | >0.05 | |
| FIB4 | 3.145 | 0.702 (0.661, 0.743) | 0.650 | 0.686 | <0.001 | |
| GPR | 0.221 | 0.764 (0.726, 0.801) | 0.704 | 0.716 | >0.05 | |
| ≥F4 | M2BPGi | 1.355 | 0.892 (0.851, 0.933) | 0.868 | 0.786 | Reference |
| (Cirrhosis) | APRI | 0.885 | 0.873 (0.835, 0.910) | 0.838 | 0.783 | >0.05 |
| FIB4 | 4.395 | 0.818 (0.768, 0.868) | 0.750 | 0.775 | <0.05 | |
| GPR | 0.291 | 0.851 (0.808, 0.894) | 0.882 | 0.691 | >0.05 |
The ROC curves for M2BPGi, FIB-4 index, APRI, and GPR for predicting severe fibrosis (≥F2) and cirrhosis (F4) are shown in Fig. 2a–b. The AUC of M2BPGi for predicting significant fibrosis (≥F2) was significantly greater than those for the FIB-4 index (AUC = 0.702, P < 0.001), and similar to those for GPR (AUC = 0.764, P > 0.05) and APRI (AUC = 0.787, P > 0.05). For estimating cirrhosis, the AUC for M2BPGi level (AUC = 0.892) was superior to those for the FIB-4 (AUC = 0.818, P < 0.05), but the differences were not significant and were comparable to that or the APRI (AUC = 0.873, P = 0.598) and GPR (AUC = 0.851, P = 0.352). Overall, serum M2BPGi showed high reliability for the diagnosis of significant fibrosis and cirrhosis.
Fig. 2.

The diagnostic capabilities of the M2BPGi values for assessing the stage of liver fibrosis. The AUCs for serum M2BPGi in diagnosing liver fibrosis were as follows: a 0.774 for stage ≥ F2; b 0.892 for stage ≥ F4
Discussion
Early detection and intervention are critical for successes in disease curability. In chronic hepatitis C infection, it is quite critical to screen the patients for the status of liver fibrosis because those with advance fibrosis are at greater risk of developing severe complications [17–19]. Although a liver biopsy is the gold standard for staging of liver fibrosis, its invasive nature restricts its application in the clinic, especially in follow up cases. Therefore, the field of non-invasive approaches for liver staging has been recently evolved [20–23]. In the present study, we found that the serum M2BPGi levels as a simple and reliable glyco-biomarker diagnostic tool for staging of liver fibrosis in chronic hepatitis C patients (Fig. 2). The glycan-based immunoassay was previously developed as a simple system for automatically detecting unique fibrosis-related glyco-alterations [24–27]. To the best of our knowledge, the present study is the first study to evaluate the efficiency of serum M2BPGi in prediction of liver fibrosis stage in a large population of Chinese patients with HCV infection.
This study clearly demonstrated that the M2BPGi level in chronic hepatitis C patients increased with the progression of liver fibrosis stage (Fig. 2). This result came in a good agreement with previous studies [28–30]. Moreover, M2BPGi concentrations could also distinguish between healthy controls and hepatitis C patients, and this result suggests that alterations in serum M2BPGi may forecast liver disease. We also showed that M2BPGi level is an independent factor significantly associated with liver fibrosis in hepatitis C patients. Several other variables have been proposed to contribute to liver fibrosis, such as platelet count, total protein, ALP, GGT, total billirubin, HA, CIV, and AFP in our study (Table 2). Interestingly, among nine variables, including M2BPGi level, platelet count, total protein, ALP, GGT, total billirubin, HA, CIV, and AFP, M2BPGi level was the most significant serum marker associated with LSM.
Studies have showed that several risk scores such as the APRI, FIB-4, and GPR, which are calculated using the above variables, appeared to be a good surrogate marker for predicting fibrosis stages. Additionally, our results also showed that the M2BPGi level was strongly associated with other noninvasive fibrosis markers. As the AUCs of previously reported fibrosis markers (APRI, FIB-4, and GPR) increased by the progression of liver fibrosis stage, the AUC of M2BPGi increased accordingly as well. It worth to note that serum level of M2BPGi showed higher efficiency in predicting liver fibrosis grade F ≥ 2 than other surrogate markers, such as the FIB-4 and GDR (Table 3). Taken together, this suggests that the M2BPGi assay would be a good serological marker for predicting and differentiating liver fibrosis stags, especially the early and significant fibrosis stages.
On the other hand, M2BPGi level was also efficient in predicting cirrhosis (LSM > 15.3) with an AUC of 0.892 at a cut-off of 1.355, which is consistent with previous studies. In a study that included 106 Japanese patients with hepatitis C, cut-off values of M2BP for four fibrosis stages (F1–F4) ranged from 1.0 to 2.64 [31]. In another Japanese study performed on 137 primary biliary cirrhosis patients, performances were better than ours, with AUCs for significant fibrosis, severe fibrosis, and cirrhosis of 0.979, 0.933, and 0.965, respectively, and the cut off values for M2BPGi in fibrosis stages > F1, >F2, >F3, and > F4 were 0.7, 1.0, 1.4, and 2.0, respectively [14]. In our study, M2BPGi did not perform well for classifying patients at the extremes of significant and severe fibrosis (F2–F3). The reason may be coursed that Fibro Scan® is less accurate for detecting significant fibrosis (F2–F3) compared with Castera L [32].
The range of the M2BPGi serum thresholds observed in chronic hepatitis C patientswas different from those described in primary biliary cirrhosis (PBC) or non-alcoholic fatty liver disease [14, 33], typeIautoimmune hepatitis [34], and hepatitis B (Matsumoto and Umemura, personal communications). This difference might be contributed to the difference in patients’ ethnicity or due to the fact that the majority of our cohort was asymptomatic and exhibited mild fibrosis.
There are two main strengths of this study. First, relatively large sample size (n = 680) with well characterized baseline clinical characteristics. Second, in this study, we excluded patients who had fatty liver disease, which may have affected the Fibro Scan® value. On the other hand, the present study also has several limitations. First, we estimated the fibrosis stage using LSM and APRI or FIB4 in this study, while liver biopsy was not considered which could be a source of bias.
Conclusions
Our study suggests that the level of serum M2BPGi would be a simple and reliable diagnostic tool for identifying liver fibrosis stage in Chinese patients with chronic hepatitis.
Acknowledgement
We thank Sysmex Company who provide M2BPGi testing reagent in our study.
Funding
This work was sponsored by the National Science and Technology Major Project (2014ZX10002002), the National Basic Research Program of China (973 Program) (2015CB554304), the National Natural Science Foundation of China under Grant nos. 81373057, 81301472 and 81301415, the Jilin Province Science and Technology Development Plan (20160520158JH), the Chinese foundation for hepatitis prevention and control--WBE liver fibrosis foundation (CFHPC20131053) and the Sysmex Company.
Availability of data and material
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Authors’ contributions
HX, WK, XC, XW, RW and XG performed the laboratory testing for HCV, HBV, HIV, and M2BPGi in this study. HW, LQ & YQ conducted Fibro Scan® test and collected information on demographic variables (age, gender, and race). LL conducted Fibro Scan® test and performed ultrasound examination. HX performed statistical analyses and drafted the manuscript. JN and YP participated in the design of the study and applied for the funding. All authors have read and approved the final manuscript.
Competing interest
Sysmex Company provided reagents and fund which were paid for sample management and test in this study. Sysmex Company did not involve in designing, samples collection, sample test, data analysis and paper writing in this study. We also declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study was approved by the Ethics Committee at the First Hospital of Jilin University, Changchun, China. Written informed consent was obtained from all patients who participated.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ALT
Alanine aminotransferase
- anti-HBc
Antibody to hepatitis B core antigen
- anti-HBsAg
Antibody to HBsAg
- APRI
Aspartate transaminase to platelet ratio index
- AST
aspartate aminotransferase
- CI
Confidence interval
- CIV
Type 4 collagen
- ELISA
Enzyme-linked immunosorbent assay
- FIB4
Fibrosis index based on four factors
- GPR
Gamma-glutamyltranspeptidase to platelet ratio
- HA
Hyaluronic acid
- HBsAg
Hepatitis B surface antigen
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- IQR
Inter-quartile range
- LN
Laminin
- LSM
Liver stiffness measurement
- M2BPGi
Mac-2 Binding Protein Glycosylation isomer
- OR
Odds ratio
- PBC
Primary biliary cirrhosis
- RNA
Ribonucleic acid
- VCTE
Vibration Controlled Transient Elastography™ system
- WFA + -M2BP
Wisteria floribunda agglutinin-positive human Mac-2-binding AFP (α-fetoprotein)
Contributor Information
Hongqin Xu, Email: hongqinxu11@163.com.
Wenli Kong, Email: 857469157@qq.com.
Lei Liu, Email: 934931521@qq.com.
Xiumei Chi, Email: xiumeichi@hotmail.com.
Xiaomei Wang, Email: xiaomei_wang2010@163.com.
Ruihong Wu, Email: wuruihong1984@163.com.
Xiuzhu Gao, Email: Leona.1985@163.com.
Huan Wang, Email: huanwang2009@163.com.
Limei Qu, Email: qulimei82@163.com.
Yue Qi, Email: qq9377@sina.com.
Yu Pan, Email: yupan20000@163.com.
Junqi Niu, Email: junqiniu@aliyun.com.
References
- 1.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344(7):495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 2.Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38(6):1449–57. doi: 10.1016/j.hep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 3.Yamada N, Sanada Y, Tashiro M, Hirata Y, Okada N, Ihara Y, et al. Serum Mac-2 binding protein glycosylation isomer predicts grade F4 liver fibrosis in patients with biliary atresia. J Gastroenterol. 2017;52(2):245–52. [DOI] [PubMed]
- 4.Kuno A, Ikehara Y, Tanaka Y, Ito K, Matsuda A, Sekiya S, et al. A serum "sweet-doughnut" protein facilitates fibrosis evaluation and therapy assessment in patients with viral hepatitis. Sci Rep. 2013;3:1065. doi: 10.1038/srep01065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29(12):1705–13. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Bonder A, Afdhal N. Utilization of FibroScan in clinical practice. Curr Gastroenterol Rep. 2014;16(2):372. doi: 10.1007/s11894-014-0372-6. [DOI] [PubMed] [Google Scholar]
- 7.Tapper EB, Castera L, Afdhal NH. FibroScan (vibration-controlled transient elastography): where does it stand in the United States practice. Clin Gastroenterol Hepatol. 2015;13(1):27–36. doi: 10.1016/j.cgh.2014.04.039. [DOI] [PubMed] [Google Scholar]
- 8.Ganne-Carrie N, Ziol M, de Ledinghen V, Douvin C, Marcellin P, Castera L, et al. Accuracy of liver stiffness measurement for the diagnosis of cirrhosis in patients with chronic liver diseases. Hepatology. 2006;44(6):1511–7. doi: 10.1002/hep.21420. [DOI] [PubMed] [Google Scholar]
- 9.Anastasiou OE, Büchter M, Baba HA, Korth J, Canbay A, Gerken G, Kahraman A. Performance and utility of transient elastography and non-invasive markers of liver fibrosis in patients with autoimmune hepatitis: a single centre experience. Hepat Mon. 2016;16(11):e40737. doi: 10.5812/hepatmon.40737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen-Khac E, Chatelain D, Tramier B, Decrombecque C, Robert B, Joly JP, et al. Assessment of asymptomatic liver fibrosis in alcoholic patients using fibroscan: prospective comparison with seven non-invasive laboratory tests. Aliment Pharmacol Ther. 2008;28(10):1188–98. doi: 10.1111/j.1365-2036.2008.03831.x. [DOI] [PubMed] [Google Scholar]
- 11.Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, et al. Non-invasive assessment of liver fibrosis by stiffness measurement: a prospective multicentre study in patients with chronic hepatitis C. Hepatology. 2005;41:48–54. doi: 10.1002/hep.20506. [DOI] [PubMed] [Google Scholar]
- 12.Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51(2):454–62. doi: 10.1002/hep.23312. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, Wang L, Wang L, Li G, Huang A, Yin P, et al. Liver stiffness measurement, better than APRI, Fibro index, Fib-4, and NBI gastroscopy, predicts portal hypertension in patients with cirrhosis. Cell Biochem Biophys. 2015;71(2):865–73. doi: 10.1007/s12013-014-0275-z. [DOI] [PubMed] [Google Scholar]
- 14.Shen QL, Chen YJ, Wang ZM, Zhang TC, Pang WB, Shu J, et al. Assessment of liver fibrosis by Fibroscan as compared to liver biopsy in biliary atresia. World J Gastroenterol. 2015;21(22):6931–6. doi: 10.3748/wjg.v21.i22.6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsochatzis EA, Gurusamy KS, Ntaoula S, Cholongitas E, Davidson BR, Burroughs AK. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J Hepatol. 2011;54(4):650–9. doi: 10.1016/j.jhep.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 16.Umemura T, Joshita S, Sekiguchi T, Usami Y, Shibata S, Kimura T, et al. Serum Wisteria floribunda agglutinin-positive mac-2-binding protein level predicts liver fibrosis and prognosis in primary biliary cirrhosis. Am J Gastroenterol. 2015;110(6):857–64. doi: 10.1038/ajg.2015.118. [DOI] [PubMed] [Google Scholar]
- 17.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population based cohort study. Gastroenterology. 1992;129:113–21. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Yasui K, Hashimoto E, Komorizono Y, Koike K, Arii S, Imai Y, et al. Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2011;9:428–33. doi: 10.1016/j.cgh.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 19.Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–54. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 20.Yoneda M, Mawatari H, Fujita K, Yonemitsu K, Kato S, Takahashi H, et al. Type IV collagen 7 s domain is an independent clinical marker of the severity of fibrosis in patients with nonalcoholic steatohepatitis before the cirrhotic stage. J Gastroenterol. 2007;42:375–81. doi: 10.1007/s00535-007-2014-3. [DOI] [PubMed] [Google Scholar]
- 21.Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology. 2007;46(1):32–6. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 22.Guha IN, Parkes J, Roderick P, Chattopadhyay D, Cross R, Harris S, et al. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology. 2008;47:455–60. doi: 10.1002/hep.21984. [DOI] [PubMed] [Google Scholar]
- 23.Ochi H, Hirooka M, Koizumi Y, Miyake T, Tokumoto Y, Soga Y, et al. Real-time tissue elastography for evaluation of hepatic fibrosis and portal hypertension in nonalcoholic fatty liver diseases. Hepatology. 2012;56:1271–8. doi: 10.1002/hep.25756. [DOI] [PubMed] [Google Scholar]
- 24.Ito K, Kuno A, Ikehara Y, Sugiyama M, Saito H, Aoki Y, et al. LecT-Hepa, a glyco-marker derived from multiple lectins, as a predictor of liver fibrosis in chronic hepatitis C patients. Hepatology. 2012;56:1448–56. doi: 10.1002/hep.25815. [DOI] [PubMed] [Google Scholar]
- 25.Kuno A, Sato T, Shimazaki H, Unno S, Saitou K, Kiyohara K, et al. Reconstruction of a robust glycodiagnostic agent supported by multiple lectin-assisted glycan profiling. Proteomics Clin Appl. 2013;7(9-10):642–7. doi: 10.1002/prca.201300010. [DOI] [PubMed] [Google Scholar]
- 26.Kuno A, Ikehara Y, Tanaka Y, Angata T, Unno S, Sogabe M, et al. Multilectin assay for detecting fibrosis-specific glyco-alteration by means of lectin microarray. Clin Chem. 2011;57(1):48–56. [DOI] [PubMed]
- 27.Kuno A, Ikehara Y, Tanaka Y, Saito K, Ito K, Tsuruno C, et al. LecT-Hepa: a triplex lectin-antibody sandwich immunoassay for estimating the progression dynamics of liver fibrosis assisted by a bedside clinical chemistry analyzer and an automated pretreatment machine. Clin Chim Acta. 2011;412:1767–72. [DOI] [PubMed]
- 28.Ura K, Furusyo N, Ogawa E, Hayashi T, Mukae H, Shimizu M, et al. Serum WFA+ -M2BP is a non-invasive liver fibrosis marker that can predict the efficacy of direct-acting anti-viral-based triple therapy for chronic hepatitis C. Aliment Pharmacol Ther. 2015;43:114–24. doi: 10.1111/apt.13431. [DOI] [PubMed] [Google Scholar]
- 29.Toyoda H, Kumada T, Tada T, Kaneoka Y, Maeda A, Korenaga M, et al. Serum WFA+ -M2BP levels as a prognostic factor in patients with early hepatocellular carcinoma undergoing curative resection. Liver Int. 2015;36:293–301. doi: 10.1111/liv.12907. [DOI] [PubMed] [Google Scholar]
- 30.Yamasaki K, Tateyama M, Abiru S, Komori A, Nagaoka S, Saeki A, et al. Elevated serum levels of Wisteria floribunda agglutinin-positive human Mac-2 binding protein predict the development of hepatocellular carcinoma in hepatitis C patients. Hepatology. 2014;60(5):1563–70. doi: 10.1002/hep.27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toshima T, Shirabe K, Ikegami T, Yoshizumi T, Kuno A, Togayachi A, et al. A novel serum marker, glycosylated Wisteria floribunda agglutinin-positive Mac-2 binding protein (WFA (+)-M2BP), for assessing liver fibrosis. J Gastroenterol. 2015;50(1):76–84. doi: 10.1007/s00535-014-0946-y. [DOI] [PubMed] [Google Scholar]
- 32.Castera L. Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology. 2012;142:1293–302. doi: 10.1053/j.gastro.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 33.Abe M, Miyake T, Kuno A, Imai Y, Sawai Y, Hino K, et al. Association between Wisteria floribunda agglutinin-positive Mac-2 binding protein and the fibrosis stage of non-alcoholic fatty liver disease. J Gastroenterol. 2015;50(7):776–84. doi: 10.1007/s00535-014-1007-2. [DOI] [PubMed] [Google Scholar]
- 34.Nishikawa H, Enomoto H, Iwata Y, Hasegawa K, Nakano C, Takata R, et al. Clinical significance of serum Wisteria floribunda agglutinin positive Mac-2-binding protein level and high-sensitivity C-reactive protein concentration in autoimmune hepatitis. Hepatol Res. 2016;46(7):613–21. doi: 10.1111/hepr.12596. [DOI] [PubMed] [Google Scholar]


