Abstract
Background
Epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) such as gefitinib can provide better efficacy and prolonged progression free survival (PFS) than cytotoxic chemotherapy for metastatic lung non-squamous cell carcinoma harboring susceptible EGFR mutations when used as first-line therapy. Cytotoxic chemotherapy is regarded as being the standard therapy to overcome acquired resistance to an initial EGFR TKI. However, there is currently no consensus on how best to treat patients who develop resistance to both an initial EGFR TKI and chemotherapy.
Methods
We enrolled stage IV lung adenocarcinoma patients with an EGFR mutation and who had developed acquired resistance to gefitinib and cytotoxic chemotherapy from two university-affiliated hospitals in Taiwan from June 2011 to December 2014. Basic demographic data, included Eastern Cooperative Oncology Group (ECOG) performance status were collected, and the response rate, progression-free survival (PFS) and overall survival (OS) were analyzed.
Result
Two hundred and nine patients with mutated EGFR and who took gefitinib as the first-line therapy were identified in the study period, of whom 86 received second-line cytotoxic chemotherapy, and 60 who received third-line therapy were eligible for this study. The patients who received cytotoxic chemotherapy had a significantly higher disease control rate than those who received erlotinib (73% vs. 46%, p = 0.0363), however there were no significant differences in PFS (2.9 months vs. 3.1 months, p = 0.9049) and OS (8.9 months vs. 7.9 months, p = 0.4956). Platinum- or pemetrexed-based chemotherapy provided similar PFS and OS as others did. The only significant poor prognostic factors for OS were old age (≥65 years) (HR = 5.97 [2.65–13.44], p < 0.0001) and poor performance status (ECOG ≥2) (HR = 5.84 [2.61–13.09], p < 0.0001).
Conclusion
Retreatment with an EGFR TKI is not inferior to cytotoxic chemotherapy when used as salvage therapy for patients with adenocarcinoma with an EGFR mutation, especially if a third-generation EGFR TKI is not available, or if the reason for resistance is unknown or is not related to the T790M mutation. Old age and poor ECOG score were both poor prognostic factors in the salvage therapy.
Keywords: Epidermal growth factor receptor, Gefitinib, Acquired resistance, Chemotherapy, Erlotinib
Background
Lung cancer continues to be the leading cause of death among patients with malignant tumors worldwide. Several large scaled studies showed epidermal growth factor receptor (EGFR) - tyrosine kinase inhibitors (TKIs) were associated with a good response rate approximating 70% as well as a progression-free survival (PFS) rate of 9-13 months in patients with lung cancer harboring EGFR activating mutations [1–5]. However, the development of acquired resistance to the first-line EGFR-TKI treatment is inevitable and most of these patients needed subsequent salvage therapy [6, 7].
Because approximately half of the acquired resistance comes from T790M mutation, some new drugs were designed to conquer this resistance [8–12]. However, these new drugs were not available worldwide, included in Taiwan. In addition, not all patients could be proved to have T790M mutation because re-biopsy was usually not available [13]. Furthermore, up to 50% of the acquired resistance are not related to T790M mutation and are still unresolvable [6, 7]. In clinical practice, cytotoxic chemotherapy was considered to be the better treatment than another subsequent EGFR-TKI when resistance developed. In our prior report, we had demonstrated pemetrexed-based platinum chemotherapy maybe the most optimal second-line cytotoxic chemotherapy and it could prolong the PFS and overall survival (OS) [14]. Moreover, the development of acquired resistance to the second-line therapy is still inevitable. Generally speaking, cytotoxic chemotherapy is the most common choice to treat acquired resistance. Recently, Becker et al. demonstrated that retreatment with EGFR-TKI was an option for patients with NSCLC who were initially benefited from previous EGFR-TKI treatment [15]. Several small-scale studies and case reports on retreatment with the same or another EGFR-TKI have been published, however the results have been inconsistent [16–24]. Till now, no study was design to compare the efficacy of readministered EGFR-TKI or cytotoxic chemotherapy as the third-line in mutated NSCLC patients.
We therefore conduct a retrospective cohort study in two university-affiliated hospitals in Taiwan and try to explore the most optimal third-line treatment to these patients with stage IV lung adenocarcinoma initially harboring susceptible EGFR mutation and who finally developed acquired resistance to the front-line therapy in real world.
Methods
Patient identification
Patients with stage IV lung adenocarcinoma was diagnosed and treated between June 2011 and December 2014 in two Kaohsiung Medical University affiliated hospitals (Kaohsiung Medical University Hospitals and Kaohsiung Municipal Ta-Tung Hospital) in Taiwan were identified and followed until June 2015. The diagnosis of lung cancer was confirmed pathologically according to World Health Organization pathology classification, and tumor staging was made by a special committee including clinical pulmonologists, medical oncologists, chest surgeons, radiologists, pathologists and radiation oncologists according to the seventh American Joint Committee on Cancer staging system. Patients were included if they: (1) had adequate tumor specimens for EGFR mutation examination and had susceptible EGFR mutation; (2) were treated with gefitinib as the first line and subsequently received cytotoxic chemotherapy as the second-line treatment; (3) received EGFR-TKI or cytotoxic chemotherapy as the third-line treatment.
Baseline clinical characteristics were determined by retrospective chart review, including age at diagnosis, gender, Eastern Cooperative Oncology Group (ECOG) performance status at the beginning of the first-line gefitinib treatment and at the start of the second- and third-line treatment, smoking history and tumor histology. Mutations in the EGFR gene were analyzed using an EGFR RGQ kit (Qiagen,UK) which utilized amplification refractory mutation specific (ARMS) PCR polymerase chain reactions and Scorpion technologies for detection and/or direct sequencing as our previous report. The initial treatment response was classified based on serial imaging studies using the revised Response Evaluation Criteria in Solid Tumors (RECIST 1.1) criteria. The third-line cytotoxic chemotherapy included docetaxel, pemetrexed, vinorelbin, gemcitabine and with or without platinum derivatives (cisplatin or carboplatin).
The progression-free survival (PFS3) and overall survival (OS3) on the third-line treatment were defined as the durations from the start of the third-line treatment to the date of disease progression on imaging exam and the date of death, respectively.
The Institutional Review Board (IRB) of Kaohsiung Medical University Hospital (KMUH) approved this study (KMUHIRB-E(II)-20150162). Considering the retrospective nature of the study, we could not obtain patients consent for use of clinical data. IRB of KMUH waived the need for written informed consent from the patients. In addition, patient records were anonymous and de-identified prior to the analyses.
Statistical analysis
Categorical variables and continuous variables were compared using the χ 2 test and the Student’s t-test, respectively. Survival times were estimated using the Kaplan-Meier method, with differences between the groups compared using the log-rank test. Cox regression analyses were used to determine the predicting factors for PFS3 and OS3. All statistical analyses were performed using SAS software (version 9.3 for Windows, SAS Institute Inc., Cary, NC, USA). Statistical significance was set at a two-sided p value of less than 0.05.
Result
Patient characteristics
During the study period, a total of 209 patients with stage IV lung adenocarcinoma harboring susceptible EGFR mutation who had received gefitinib as the first-line therapy were enrolled, and 86 of them had received cytotoxic chemotherapy as their second-line treatment. From these patients, 60 of them received a third-line treatment, including 29 (48%), 1 (2%), and 30 (50%) patients received erlotinib, gefitinib, and cytotoxic chemotherapy as their third-line treatment, respectively (Table 1). One patient received both erlotinib and bevacizumab and one patient received gefitinib as the third-line treatment were excluded for our subsequent analyses, because the main objective of this study was to compare the outcomes of using erlotinib alone and those of using cytotoxic chemotherapy as the third-line treatment. As summarized in Table 2, there were no significant differences in the baseline clinical characteristics between the patients receiving cytotoxic chemotherapy and those receiving erlotinib as their third-line treatment.
Table 1.
Regimens used as the third-line treatment
| Regimen | All patients | Study cohort |
|---|---|---|
| Erlotinib | 29 (48%)a | 28 (48%) |
| Gefitinib | 1 (2%) | |
| Chemotherapy without platinum: | ||
| Pemetrexed | 1 (2%) | 1 (2%) |
| Gemcitabine | 7 (12%) | 7 (12%) |
| Vinorelbine | 7 (12%) | 7 (12%) |
| Taxanes | 5 (8%) | 5 (9%) |
| Platinum-based doublet: | ||
| Pemetrexed + Platinum | 4 (7%) | 4 (7%) |
| Gemcitabine + Platinum | 1 (2%) | 1 (2%) |
| Vinorelbine + Platinum | 2 (3%) | 2 (3%) |
| Taxanes + Platinum | 3 (5%) | 3 (5%) |
| Total | 60 | 58 |
Data are presented as n (%)
aIncluding one patient receiving both bevacizumab and erlotinib
Table 2.
Clinical characteristics and treatment response of the study cohort
| Variables | All patients | Chemotherapy | Erlotinib | P value |
|---|---|---|---|---|
| N (%) | 58 | 30 | 28 | |
| Age (year) -mean ± SD | 60.8 ± 10.8 | 59.2 ± 11 | 62.6 ± 10.4 | 0.2228 |
| Age -n (%) | 0.6431 | |||
| <65 years old | 39 (67%) | 21 (70%) | 18 (64%) | |
| ≥65 years old | 19 (33%) | 9 (30%) | 10 (36%) | |
| Sex-n (%) | 0.1949 | |||
| Female | 38 (66%) | 22 (73%) | 16 (57%) | |
| Male | 20 (34%) | 8 (27%) | 12 (43%) | |
| Smoking history-n (%) | 0.4214 | |||
| Never smoker | 45 (78%) | 22 (73%) | 23 (82%) | |
| Ever smoker | 13 (22%) | 8 (27%) | 5 (18%) | |
| TTF-1 staining-n (%) | 0.3411 | |||
| Negative | 0 (0%) | 0 (0%) | 0 (0%) | |
| Positive | 52 (90%) | 28 (93%) | 24 (86%) | |
| Not performed | 6 (10%) | 2 (7%) | 4 (14%) | |
| EGFR gene mutation site-n (%) | 0.6671 | |||
| Exon18 | 3 (5%) | 1 (3%) | 2 (7%) | |
| Exon19 | 30 (52%) | 16 (53%) | 14 (50%) | |
| Exon19 + Exon21 | 1 (2%) | 0 (0%) | 1 (4%) | |
| Exon21 | 24 (41%) | 13 (43%) | 11 (39%) | |
| Metastatic sites on initial diagnosis-n (%) | 0.3083 | |||
| ≤1 | 23 (40%) | 10 (33%) | 13 (46%) | |
| ≥2 | 35 (60%) | 20 (67%) | 15 (54%) | |
| Performance status while starting gefitinib-n (%) | 0.1307 | |||
| ECOG ≤1 | 48 (83%) | 27 (90%) | 21 (75%) | |
| ECOG ≥2 | 10 (17%) | 3 (10%) | 7 (25%) | |
| Progression-free survival of gefitinib (month) -median (IQR) | 9.4 (6.2-13.8) | 9.8 (6.1-12.5) | 9.1 (7.6-16.1) | 0.3466 |
| Performance status while starting the second-line treatment-n (%) | 0.8848 | |||
| ECOG ≤1 | 43 (74%) | 22 (73%) | 21 (75%) | |
| ECOG ≥2 | 15 (26%) | 8 (27%) | 7 (25%) | |
| Progression-free survival of the second-line treatment (month) -median (IQR) | 4.1 (2.7-6.1) | 3.5 (2.0-4.7) | 5.0 (2.9-7.0) | 0.1836 |
| Performance status while starting the third-line treatment-n (%) | 0.0606 | |||
| ECOG ≤1 | 26 (45%) | 17 (57%) | 9 (32%) | |
| ECOG ≥2 | 32 (55%) | 13 (43%) | 19 (68%) | |
| Initial treatment response to the third-line treatment-n (%) | 0.0842 | |||
| Partial response | 1 (2%) | 1 (3%) | 0 (0%) | |
| Stable disease | 34 (59%) | 21 (70%) | 13 (46%) | |
| Progressive disease | 23 (40%) | 8 (27%) | 15 (54%) | |
| Initial disease control rate with the third-line treatment (%) | 59% | 73% | 46% | 0.0363 |
Similar outcomes while using either chemotherapy or erlotinib as the third-line treatment
While patients with poorer performance status while starting the third-line treatment tended to choose erlotinib as their third-line treatment (p = 0.0606), patients using cytotoxic chemotherapy as the third-line treatment seemed having a significantly higher initial disease control rate than those using erlotinib (73% vs. 46%, p = 0.0363) (Table 2). Patients receiving chemotherapy and those receiving erlotinib had similar PFS3 (median PFS3: 2.9 vs. 3.1 months, log-rank p = 0.8945) and OS3 (median OS3: 8.9 vs. 7.9 months, log-rank p = 0.4956) (Fig. 1a, b). On multivariable Cox regression analysis controlling for sex, age, and the performance status, the use of erlotinib as the third-line treatment was not a significant predicting factor for PFS3 (HR = 0.79 [0.43–1.44], p = 0.4341) or OS3 (HR = 0.82 [0.41–1.64], p = 0.5706) (Table 4). The only significant poor prognostic factor for PFS3 was the poorer performance status (ECOG ≥2) while starting the third-line treatment (HR = 2.21 [1.20–4.07], p = 0.0109). The only significant poor prognostic factors for OS3 were elder age (≥65) (HR = 5.97 [2.65–13.44], p < 0.0001) and the poorer performance status (ECOG ≥2) while starting the third-line treatment (HR = 5.84 [2.61–13.09], p < 0.0001).
Fig. 1.
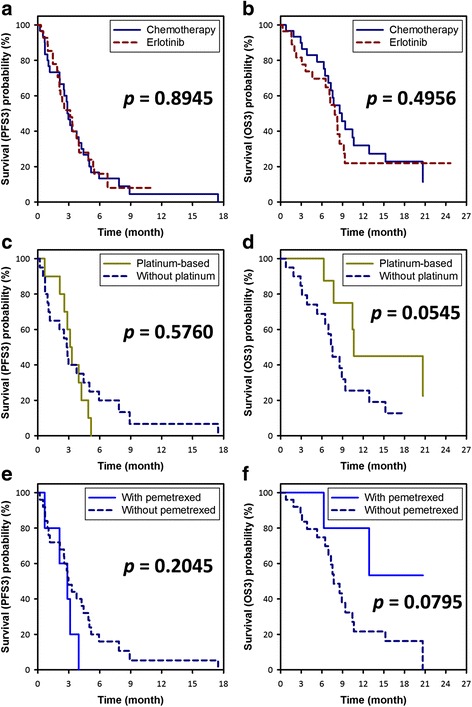
Kaplan-Meier curves of progression-free survival (PFS3; A,C,E) and overall survival (OS3; B,D,F) with the third-line treatment. a, b Analyses of the whole study cohort showed that patients receiving chemotherapy and those receiving erlotinib had similar PFS3 (MST of PFS3: 2.9 vs. 3.1 months, log-rank p = 0.8945) and OS3 (MST of OS3: 8.9 vs. 7.9 months, log-rank p = 0.4956). c, d Analyses of the patients receiving chemotherapy showed that patients receiving platinum-based doublet had a similar PFS3 and a trend for better OS3 as compared with those receiving chemotherapy without platinum (MST of PFS3: 3.2 vs. 2.8 months, log-rank p = 0.5760; MST of OS3: 10.6 vs. 7.5 months, log-rank p = 0.0545). e, f Analyses of the patients receiving chemotherapy showed that patients receiving pemetrexed had a similar PFS3 and a trend for better OS3 as compared with those receiving chemotherapy without pemetrexed (MST of PFS3: 2.9 vs. 3.0 months, log-rank p = 0.2045; MST of OS3: undefined vs. 7.7 months, log-rank p = 0.0795). Abbreviation: MST = median survival time
Table 4.
Cox regression analyses for the factors predicting progression-free survival (PFS3) and overall survival (OS3) with the third-line treatment
| Clinical features | Progression-free survival with the third-line treatment (PFS3) | Overall survival with the third-line treatment (OS3) | ||
|---|---|---|---|---|
| Univariate analysis | Multivariable analysis | Univariate analysis | Multivariable analysis | |
| All study cohort | ||||
| Sex (male vs. female) | 0.80 [0.44 - 1.45] | 0.94 [0.50 - 1.79] | 0.61 [0.29 - 1.28] | 0.70 [0.32 - 1.51] |
| Age (≥65 vs. <65 years old) | 0.80 [0.44 - 1.46] | 0.78 [0.43 - 1.42] | 3.09 [1.52 - 6.28] | 5.97 [2.65 - 13.44] |
| Performance status while starting the third-line treatment (ECOG ≥2 vs. ≤1) | 2.08 [1.18 - 3.66] | 2.21 [1.20 - 4.07] | 3.30 [1.64 - 6.64] | 5.84 [2.61 - 13.09] |
| The third-line treatment (erlotinib vs. cytotoxic chemotherapy) | 0.96 [0.55 - 1.68] | 0.79 [0.43 - 1.44] | 1.25 [0.65 - 2.40] | 0.82 [0.41 - 1.64] |
| Patients receiving cytotoxic chemotherapy as the third-line treatment | ||||
| Sex (male vs. female) | 1.01 [0.44 - 2.34] | 1.57 [0.51 - 4.81] | 0.93 [0.33 - 2.59] | 1.21 [0.35 - 4.19] |
| Age (≥65 vs. <65 years old) | 0.57 [0.24 - 1.36] | 0.62 [0.22 - 1.71] | 3.10 [1.11 - 8.69] | 4.79 [1.40 - 16.41] |
| Performance status while starting the third-line treatment (ECOG ≥2 vs. ≤1) | 2.00 [0.93 - 4.31] | 2.88 [1.12 - 7.36] | 6.62 [2.35 - 18.64] | 19.78 [4.39 - 89.03] |
| The third-line treatment (platinum-based doublet vs. chemotherapy without platinum) | 1.26 [0.56 - 2.84] | 0.97 [0.38 - 2.46] | 0.35 [0.12 - 1.07] | 1.12 [0.31 - 4.07] |
| The third-line treatment (with pemetrexed vs. without pemetrexed) | 1.91 [0.69 - 5.27] | 2.34 [0.70 - 7.84] | 0.29 [0.07 - 1.26] | 0.14 [0.02 - 0.89] |
Outcomes of patients using chemotherapy as the third-line treatment
We further analyzed the outcomes of patients using cytotoxic chemotherapy as their third-line treatment (Table 3). Patients receiving platinum-based doublet, as compared with those receiving chemotherapy without platinum, had a similar PFS3 (median PFS3: 3.2 vs. 2.8 months, log-rank p = 0.5760) and a trend for better OS3 (median OS3: 10.6 vs. 7.5 months, log-rank p = 0.0545) (Fig. 1c, d). Patients receiving pemetrexed, as compared with those receiving chemotherapy without pemetrexed, had a similar PFS3 (median PFS3: 2.9 vs. 3.0 months, log-rank p = 0.2045) and a trend for better OS3 (median OS3: undefined vs. 7.7 months, log-rank p = 0.0795) (Fig. 1e, f). On multivariable Cox regression analysis, the only significant poor prognostic factor for PFS3 was the poorer performance status (ECOG ≥2) while starting the third-line treatment (HR = 2.88 [1.12–7.36], p = 0.0278), while the use of platinum-based doublet (p = 0.9513) or pemetrexed-based regimen (p = 0.1673) was not a significant predicting factor for PFS3 (Table 4). In terms of OS3, elder age (≥65) (HR = 4.79 [1.40–16.41], p = 0.0126) and poorer performance status (ECOG ≥2) while starting the third-line treatment (HR = 19.78 [4.39–89.03], p = 0.0001) significantly predicted poorer OS3, while the use of pemetrexed-based regimen as the third-line treatment significantly predicted better OS3 (HR = 0.14 [0.02–0.89], p = 0.0366).
Table 3.
Clinical characteristics and treatment response of all patients receiving cytotoxic chemotherapy as the third-line treatment
| Variables | Chemotherapy without platinum | Platinum-based doublet | P value | Without pemetrexed | With pemetrexed | P value |
|---|---|---|---|---|---|---|
| N (%) | 20 | 10 | 25 | 5 | ||
| Age (year) -mean ± SD | 59.2 ± 12.3 | 59 ± 8.3 | 0.9665 | 59.8 ± 11.4 | 56.1 ± 9.1 | 0.4982 |
| Age -n (%) | 0.0910 | 0.1088 | ||||
| <65 years old | 12 (60%) | 9 (90%) | 16 (64%) | 5 (100%) | ||
| ≥65 years old | 8 (40%) | 1 (10%) | 9 (36%) | 0 (0%) | ||
| Sex-n (%) | 0.1444 | 0.1396 | ||||
| Female | 13 (65%) | 9 (90%) | 17 (68%) | 5 (100%) | ||
| Male | 7 (35%) | 1 (10%) | 8 (32%) | 0 (0%) | ||
| Smoking history-n (%) | 0.1444 | 0.7119 | ||||
| Never smoker | 13 (65%) | 9 (90%) | 18 (72%) | 4 (80%) | ||
| Ever smoker | 7 (35%) | 1 (10%) | 7 (28%) | 1 (20%) | ||
| TTF-1 staining-n (%) | 0.6048 | 0.5127 | ||||
| Negative | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Positive | 19 (95%) | 9 (90%) | 23 (92%) | 5 (100%) | ||
| Not performed | 1 (5%) | 1 (10%) | 2 (8%) | 0 (0%) | ||
| EGFR gene mutation site-n (%) | 0.0225 | 0.1909 | ||||
| Exon18 | 0 (0%) | 1 (10%) | 1 (4%) | 0 (0%) | ||
| Exon19 | 14 (70%) | 2 (20%) | 15 (60%) | 1 (20%) | ||
| Exon21 | 6 (30%) | 7 (70%) | 9 (36%) | 4 (80%) | ||
| Metastatic sites on initial diagnosis-n (%) | 0.5839 | 0.1659 | ||||
| ≤1 | 6 (30%) | 4 (40%) | 7 (28%) | 3 (60%) | ||
| ≥2 | 14 (70%) | 6 (60%) | 18 (72%) | 2 (40%) | ||
| Performance status while starting gefitinib-n (%) | 0.1967 | 0.4142 | ||||
| ECOG ≤1 | 17 (85%) | 10 (100%) | 23 (92%) | 4 (80%) | ||
| ECOG ≥2 | 3 (15%) | 0 (0%) | 2 (8%) | 1 (20%) | ||
| Progression-free survival of gefitinib (month) -median (IQR) | 9.3 (6.1-12.3) | 10.5 (7.5-15.3) | 0.5919 | 8.6 (6.1-11.4) | 14.7 (12.5-15.4) | 0.0144 |
| Performance status while starting the second-line treatment-n (%) | 0.5593 | 0.7119 | ||||
| ECOG ≤1 | 14 (70%) | 8 (80%) | 18 (72%) | 4 (80%) | ||
| ECOG ≥2 | 6 (30%) | 2 (20%) | 7 (28%) | 1 (20%) | ||
| Progression-free survival of the second-line treatment (month) -median (IQR) | 4.2 (2.3-6.2) | 2.5 (1.0-4.0) | 0.0105 | 4.0 (2.4-6.0) | 2.1 (1.0-2.2) | 0.1359 |
| Performance status while starting the third-line treatment-n (%) | 0.2974 | 0.8691 | ||||
| ECOG ≤1 | 10 (50%) | 7 (70%) | 14 (56%) | 3 (60%) | ||
| ECOG ≥2 | 10 (50%) | 3 (30%) | 11 (44%) | 2 (40%) | ||
| Initial treatment response to the third-line treatment-n (%) | 0.1513 | 0.0353 | ||||
| Partial response | 0 (0%) | 1 (10%) | 0 (0%) | 1 (20%) | ||
| Stable disease | 13 (65%) | 8 (80%) | 17 (68%) | 4 (80%) | ||
| Progressive disease | 7 (35%) | 1 (10%) | 8 (32%) | 0 (0%) | ||
| Initial disease control rate with the third-line treatment (%) | 65% | 90% | 0.1444 | 68% | 100% | 0.1396 |
Discussion
Our study was designed to determine the treatment strategies in patients who initially harbored EGFR mutation and developed acquired resistance to the first-line Gefitinib and second-line cytotoxic chemotherapy. We found that re-treated with EGFR-TKIs were not inferior to traditional cytotoxic chemotherapy if they were selected as the third-line therapy in initial EGFR-mutated adenocarcinoma patients. In addition, platinum doublet chemotherapy is not superior to non-platinum-based chemotherapy. Finally, no statistic profit in PFS was observed between different cytotoxic chemotherapeutic agents.
Gefitinib had been permitted by NIH bureau in Taiwan to treat patients with lung adenocarcinoma with susceptible EGFR mutation as the first-line therapy based on several phase III studies since June 2011 [1–4]. Despite gefitinib showed good efficacy and longer PFS than cytotoxic chemotherapy, acquired resistance to EGFR-TKI treatment always occurred, and these patients needed subsequent cytotoxic chemotherapy as the second-line therapy. Pemetrexed based platinum chemotherapy was considered to be a promising therapy in our previous report. [14] However, the standard therapy of the third-line therapy in such patients with initially mutated EGFR was still uncertain. Prior second cytotoxic chemotherapy always resulted in poorer performance status and adverse drug reaction of the cytotoxic chemotherapy also causes patients to be afraid of receiving further third-line cytotoxic chemotherapy. Some studies showed lung cancer cell lines regained susceptibility to EGFR-TKI after resting for a period, and this was called EGFR-TKI holiday [16, 25]. The TKI-holiday theory was proposed by Becker et al., who showed a high response rate (36%) and disease control rate (86%) in patients who received a second EGFR-TKI after a median interval of about 9.5 (3–36) months [15]. They concluded that retreatment with EGFR-TKI was an option for patients with NSCLC who initially benefitted from previous EGFR-TKI treatment and then experienced recurrence after standard cytotoxic chemotherapy. Unlike traditional cytotoxic chemotherapy, EGFR-TKIs induced minimal hematological or non-hematological adverse drugs reaction and were considered to be well-tolerated drugs, especially for the elderly and patients with poorer performance status. Therefore, some physicians proposed that re-administration of 1 st or 2 nd generation EGFR TKI to conquer acquire resistance in patients with lung non-squamous cell carcinoma initially harboring EGFR mutation. Since retreatment with an EGFR-TKI may have a good disease control rate, several case reports and case series have been published [16, 17, 20–24, 26]. Our previous report showed female ever smoker had a poorer prognosis in patients who were retreated with EGFR-TKI in Taiwan [27]. Furthermore, the responses to the re-administered EGFR-TKI as the third-line therapy in initially mutated EGFR patients have been inconsistent [16–24] and cytotoxic chemotherapy is still the most common salvage therapy to those having acquired resistance to second-line chemotherapy. The current cohort study indicated that EGFR-TKI could provide similar efficacy as traditional cytotoxic chemotherapy does.
We had proposed that pemetrexed-based platinum chemotherapy may conquer acquired resistance to the first-line EGFR-TKI and it was regarded as the most optimal choice for second-line therapy [14]. The median PFS was as high as 6.4 months and it was significantly longer than other platinum-based chemotherapy such as gemcitabine, vinorelbine, docetaxel. However, efficacy of cytotoxic chemotherapy is still uncertain. In all NSCLC patients, included initially EGFR mutated or non-mutated patients, Sun et al. reported that pemetrexed is the optimal drug with good efficacy and a tolerable toxicity if it was used as the third-line therapy, the median PFS was 2.83 months with 22% response rate [28]. Chang et al. documented a similar report that when pemetrexed was as the third- or 4th –line, it provided PFS of 3.2 months, OS of 11.6 months, and the response rate of 16.3% [29]. Chen et al. showed the response rate was 10% and PFS was 2.6 months when using pemetrexed as third-line treatment, and the median survivals were 13.4 months for pemetrexed in third-line salvage therapy [30]. However, the role of pemetrexed as the third- or later-line therapy in EGFR mutated patients is still challenging.
In EGFR mutated patients, Shukuya et al. firstly showed a subgroup analysis of NSCLC patients who initially harbored EGFR mutation, and received single agent chemotherapy with docetaxel or pemetrexed as the 3rd line therapy [31]. The response rate was 17.6% and PFS was 113 days. In our report, pemetrexed-based therapy could not show better PFS, but it was observed to contribute to a higher OS than based on based on our multivariate Cox regression model analysis though the sample size is relatively small.
There were several limitations in this study. First, our study is a retrospective study and sample size is too small. However, this is the first cohort study in the mutated adenocarcinoma patients and we demonstrated the real world data in Taiwan. Second, no re-biopsy specimens were collected and the accurate molecular mechanisms are unknown. Third, erlotinib was the re-administered EGFR-TKI in this study based on the Taiwan NIH policy, which covers erlotinib as the 3rd line treatment for NSCLC when patients with EGFR mutations exhibit resistance to initial treatment with gefitinib and cytotoxic chemotherapy. To date, third-generation EGFR TKIs such as osimertinib (AZD 9291) which has been approved to overcome the T790M mutation is not permitted in Taiwan NIH and in many countries.
Conclusion
In conclusion, we pointed out that re-administration of EGFR-TKI and cytotoxic chemotherapy shared the similar clinical efficacy in third-line therapy in patients who had lung adenocarcinoma initially harbored susceptible EGFR mutation, if the 3rd EGFR TKI is not available, unknown resistance mechanism or not related to T790M mutation. All cytotoxic chemotherapy had similar outcome though pemetrexed seems to have better OS. Large-scaled randomized controlled trial is necessary to confirm our findings.
Acknowledgement
The grant was supported by KMUH97-7G60 in Kaohsiung Medical University Hospital and kmtth-104-021 in Kaohsiung Municipal Ta-Tung Hospital and Taiwan.
Funding
The entire study was funded by grants from KMUH97-7G60 in Kaohsiung Medical University Hospital and kmtth-104-021 in Kaohsiung Municipal Ta-Tung Hospital.
Availability of data and materials
The raw data are available with the corresponding author, and any researcher interested to gain access to the raw data can address his/her request to the corresponding author at the contact information mentioned in the manuscript.
Authors’ contribution
CJY and JYH drafted the manuscript. MJT did data collection, critical review and analysis. KLW, TCL, SHC and JYL participated data collection and data review. MSH checked and corrected the data and as a consultant. IWC and JSH contributed to study design, planning and data analysis, and revising the manuscript. All authors have read and approved the final version of the manuscript.
Competing interests
The authors declare that they have no competing interest.
Ethics approval and consent to participate
The Institutional Review Board (IRB) of Kaohsiung Medical University Hospital (KMUH) approved this study (KMUHIRB-E(II)-20150162). Considering the retrospective nature of the study, we could not obtain patients consent for use of clinical data. IRB of KMUH waived the need for written informed consent from the patients. In addition, patient records were anonymous and de-identified prior to the analyses.
Abbreviations
- EGFR
Epidermal growth factor receptor
- OS
Overall survival
- PFS
Progression free survival
- TKIs
Tyrosine kinase inhibitors
Contributor Information
Chih-Jen Yang, Email: cheya@cc.kmu.edu.tw.
Jen-Yu Hung, Email: jenyuhung@gmail.com.
Ming-Ju Tsai, Email: 960216kmuh@gmail.com.
Kuan-Li Wu, Email: wuyuehfeng@gmail.com.
Ta-Chih Liu, Email: d730093@cc.kmu.edu.tw.
Shah-Hwa Chou, Email: shhwch@cc.kmu.edu.tw.
Jui-Ying Lee, Email: rockwell@kmu.edu.tw.
Jui-Sheng Hsu, Phone: +886-7-3208159, Email: jshsu@kmu.edu.tw.
Ming-Shyan Huang, Email: shyang@kmu.edu.tw.
Inn-Wen Chong, Phone: +886-7-3208159, Email: chong@kmu.edu.tw, Email: chong@cc.kmu.edu.tw.
References
- 1.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE, Meyerson M. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 2.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, Porta R, Cobo M, Garrido P, Longo F, Moran T, Insa A, de Marinis F, Corre R, Bover I, Illiano A, Dansin E, De Castro J, Milella M, Reguart N, Altavilla G, Jimenez U, Provencio M, Moreno MA, Terrasa J, Munoz-Langa J, Valdivia J, Isla D, Domine M, Molinier O, Mazieres J, Baize N, Garcia-Campelo R, Robinet G, Rodriguez-Abreu D, Lopez-Vivanco G, Gebbia V, Ferrera-Delgado L, Bombaron P, Bernabe R, Bearz A, Artal A, Cortesi E, Rolfo C, Sanchez-Ronco M, Drozdowskyj A, Queralt C, de Aguirre I, Ramirez JL, Sanchez JJ, Molina MA, Taron M, Paz-Ares L. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 3.Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, Asami K, Katakami N, Takada M, Yoshioka H, Shibata K, Kudoh S, Shimizu E, Saito H, Toyooka S, Nakagawa K, Fukuoka M. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 4.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, Nishiwaki Y, Ohe Y, Yang JJ, Chewaskulyong B, Jiang H, Duffield EL, Watkins CL, Armour AA, Fukuoka M. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 5.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, Yoshimori K, Harada T, Ogura T, Ando M, Miyazawa H, Tanaka T, Saijo Y, Hagiwara K, Morita S, Nukiwa T. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 6.Tan CS, Gilligan D, Pacey S. Treatment approaches for EGFR-inhibitor-resistant patients with non-small-cell lung cancer. Lancet Oncol. 2015;16:e447–e459. doi: 10.1016/S1470-2045(15)00246-6. [DOI] [PubMed] [Google Scholar]
- 7.Oxnard GR, Arcila ME, Chmielecki J, Ladanyi M, Miller VA, Pao W. New strategies in overcoming acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in lung cancer. Clin Cancer Res. 2011;17:5530–5537. doi: 10.1158/1078-0432.CCR-10-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Planchard D, Loriot Y, Andre F, Gobert A, Auger N, Lacroix L, Soria JC. EGFR-independent mechanisms of acquired resistance to AZD9291 in EGFR T790M-positive NSCLC patients. Ann Oncol. 2015;26:2073–2078. doi: 10.1093/annonc/mdv319. [DOI] [PubMed] [Google Scholar]
- 9.Yver A. Osimertinib (AZD9291) - a science-driven, collaborative approach to rapid drug design and development. Ann Oncol. 2016;27(6):1167–1170. doi: 10.1093/annonc/mdw129. [DOI] [PubMed] [Google Scholar]
- 10.Chuang JC, Salahudeen AA, Wakelee HA: Rociletinib, a third generation EGFR tyrosine kinase inhibitor: current data and future directions. Expert Opin Pharmacother. 2016;17:989–93. [DOI] [PubMed]
- 11.Janne PA, Yang JC, Kim DW, Planchard D, Ohe Y, Ramalingam SS, Ahn MJ, Kim SW, Su WC, Horn L, Haggstrom D, Felip E, Kim JH, Frewer P, Cantarini M, Brown KH, Dickinson PA, Ghiorghiu S, Ranson M. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015;372:1689–1699. doi: 10.1056/NEJMoa1411817. [DOI] [PubMed] [Google Scholar]
- 12.Sequist LV, Soria JC, Goldman JW, Wakelee HA, Gadgeel SM, Varga A, Papadimitrakopoulou V, Solomon BJ, Oxnard GR, Dziadziuszko R, Aisner DL, Doebele RC, Galasso C, Garon EB, Heist RS, Logan J, Neal JW, Mendenhall MA, Nichols S, Piotrowska Z, Wozniak AJ, Raponi M, Karlovich CA, Jaw-Tsai S, Isaacson J, Despain D, Matheny SL, Rolfe L, Allen AR, Camidge DR. Rociletinib in EGFR-mutated non-small-cell lung cancer. N Engl J Med. 2015;372:1700–1709. doi: 10.1056/NEJMoa1413654. [DOI] [PubMed] [Google Scholar]
- 13.Jiang T, Ren S, Zhou C. Role of circulating-tumor DNA analysis in non-small cell lung cancer. Lung Cancer. 2015;90:128–134. doi: 10.1016/j.lungcan.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Yang CJ, Tsai MJ, Hung JY, Liu TC, Chou SH, Lee JY, Hsu JS, Tsai YM, Huang MS, Chong IW. Pemetrexed had significantly better clinical efficacy in patients with stage IV lung adenocarcinoma with susceptible EGFR mutations receiving platinum-based chemotherapy after developing resistance to the first-line gefitinib treatment. Onco Targets Ther. 2016;9:1579–1587. doi: 10.2147/OTT.S100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becker A, Crombag L, Heideman DA, Thunnissen FB, van Wijk AW, Postmus PE, Smit EF. Retreatment with erlotinib: Regain of TKI sensitivity following a drug holiday for patients with NSCLC who initially responded to EGFR-TKI treatment. Eur J Cancer. 2011;47:2603–2606. doi: 10.1016/j.ejca.2011.06.046. [DOI] [PubMed] [Google Scholar]
- 16.Song T, Yu W, Wu SX. Subsequent treatment choices for patients with acquired resistance to EGFR-TKIs in non-small cell lung cancer: restore after a drug holiday or switch to another EGFR-TKI? Asian Pac J Cancer Prev. 2014;15:205–213. doi: 10.7314/APJCP.2014.15.1.205. [DOI] [PubMed] [Google Scholar]
- 17.Hata A, Katakami N, Yoshioka H, Fujita S, Kunimasa K, Nanjo S, Otsuka K, Kaji R, Tomii K, Iwasaku M, Nishiyama A, Hayashi H, Morita S, Ishida T. Erlotinib after gefitinib failure in relapsed non-small cell lung cancer: clinical benefit with optimal patient selection. Lung Cancer. 2011;74:268–273. doi: 10.1016/j.lungcan.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Zhao ZR, Li W, Long H. Readministration of EGFR tyrosine kinase inhibitor in non-small cell lung cancer patients after initial failure, what affects its efficacy? Sci Rep. 2014;4:5996. doi: 10.1038/srep05996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao ZR, Wang JF, Lin YB, Wang F, Fu S, Zhang SL, Su XD, Jiang L, Zhang YG, Shao JY, Long H. Mutation abundance affects the efficacy of EGFR tyrosine kinase inhibitor readministration in non-small-cell lung cancer with acquired resistance. Med Oncol. 2014;31:810. doi: 10.1007/s12032-013-0810-6. [DOI] [PubMed] [Google Scholar]
- 20.Chang JW, Chou CL, Huang SF, Wang HM, Hsieh JJ, Hsu T, Cheung YC. Erlotinib response of EGFR-mutant gefitinib-resistant non-small-cell lung cancer. Lung Cancer. 2007;58:414–417. doi: 10.1016/j.lungcan.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 21.Garfield DH. Modern treatment of lung cancer: case 2. Response to erlotinib after failure of gefitinib in a patient with advanced non-small-cell lung carcinoma. J Clin Oncol. 2005;23:7738–7740. doi: 10.1200/JCO.2005.02.4471. [DOI] [PubMed] [Google Scholar]
- 22.Wong AS, Soong R, Seah SB, Lim SW, Chuah KL, Nga ME, Chin TM, Soo RA. Evidence for disease control with erlotinib after gefitinib failure in typical gefitinib-sensitive Asian patients with non-small cell lung cancer. J Thorac Oncol. 2008;3:400–404. doi: 10.1097/JTO.0b013e318168c801. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe S, Tanaka J, Ota T, Kondo R, Tanaka H, Kagamu H, Ichikawa K, Koshio J, Baba J, Miyabayashi T, Narita I, Yoshizawa H. Clinical responses to EGFR-tyrosine kinase inhibitor retreatment in non-small cell lung cancer patients who benefited from prior effective gefitinib therapy: a retrospective analysis. BMC Cancer. 2011;11:1. doi: 10.1186/1471-2407-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee DH, Kim SW, Suh C, Yoon DH, Yi EJ, Lee JS. Phase II study of erlotinib as a salvage treatment for non-small-cell lung cancer patients after failure of gefitinib treatment. Ann Oncol. 2008;19:2039–2042. doi: 10.1093/annonc/mdn423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma SV, Lee DY, Li B, Quinlan MP, Takahashi F, Maheswaran S, McDermott U, Azizian N, Zou L, Fischbach MA, Wong KK, Brandstetter K, Wittner B, Ramaswamy S, Classon M, Settleman J. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141:69–80. doi: 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viswanathan A, Pillot G, Govindan R. Lack of response to erlotinib after progression on gefitinib in patients with advanced non-small cell lung cancer. Lung Cancer. 2005;50:417–418. doi: 10.1016/j.lungcan.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Yang CJ, Tsai MJ, Hung JY, Tsai YM, Lee JY, Chou SH, Liu TC, Shen MC, Huang MS, Chong IW. Poorer prognosis in Taiwanese female ever smokers with stage IV lung adenocarcinoma who were readministered a tyrosine kinase inhibitor. Onco Targets Ther. 2016;9:1511–1518. doi: 10.2147/OTT.S100169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun JM, Lee KW, Kim JH, Kim YJ, Yoon HI, Lee JH, Lee CT, Lee JS. Efficacy and toxicity of pemetrexed as a third-line treatment for non-small cell lung cancer. Jpn J Clin Oncol. 2009;39:27–32. doi: 10.1093/jjco/hyn118. [DOI] [PubMed] [Google Scholar]
- 29.Chang MH, Ahn JS, Lee J, Kim KH, Park YH, Han J, Ahn MJ, Park K. The efficacy of pemetrexed as a third- or fourth-line therapy and the significance of thymidylate synthase expression in patients with advanced non-small cell lung cancer. Lung Cancer. 2010;69:323–329. doi: 10.1016/j.lungcan.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Chen YM, Shih JF, Fan WC, Wu CH, Chou KT, Tsai CM, Lee YC, Perng RP, Whang-Peng J. Third-line or fourth-line chemotherapy in non-small-cell lung cancer patients with relatively good performance status. J Chin Med Assoc. 2011;74:209–214. doi: 10.1016/j.jcma.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Shukuya T, Ko R, Mori K, Kato M, Yagishita S, Kanemaru R, Honma Y, Shibayama R, Koyama R, Shimada N, Takahashi K. Prognostic factors in non-small cell lung cancer patients who are recommended to receive single-agent chemotherapy (docetaxel or pemetrexed) as a second- or third-line chemotherapy: in the era of oncogenic drivers and molecular-targeted agents. Cancer Chemother Pharmacol. 2015;76:771–776. doi: 10.1007/s00280-015-2843-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data are available with the corresponding author, and any researcher interested to gain access to the raw data can address his/her request to the corresponding author at the contact information mentioned in the manuscript.


