Abstract
Pseudoxanthoma elasticum (PXE) is a genetic metabolic disease with autosomal recessive inheritance caused by mutations in the ABCC6 gene. The lack of functional ABCC6 protein leads to ectopic mineralization that is most apparent in the elastic tissues of the skin, eyes and blood vessels. The clinical prevalence of PXE has been estimated at between 1 per 100,000 and 1 per 25,000, with slight female predominance. The first clinical sign of PXE is almost always small yellow papules on the nape and sides of the neck and in flexural areas. The papules coalesce, and the skin becomes loose and wrinkled. The mid-dermal elastic fibers are short, fragmented, clumped and calcified. Dystrophic calcification of Bruch’s membrane, revealed by angioid streaks, may trigger choroidal neovascularization and, ultimately, loss of central vision and blindness in late-stage disease. Lesions in small and medium-sized artery walls may result in intermittent claudication and peripheral artery disease. Cardiac complications (myocardial infarction, angina pectoris) are thought to be relatively rare but merit thorough investigation. Ischemic strokes have been reported. PXE is a metabolic disease in which circulating levels of an anti-mineralization factor are low. There is good evidence to suggest that the factor is inorganic pyrophosphate (PPi), and that the circulating low levels of PPi and decreased PPi/Pi ratio result from the lack of ATP release by hepatocytes harboring the mutant ABCC6 protein. However, the substrate(s) bound, transported or modulated by the ABCC6 protein remain unknown. More than 300 sequence variants of the ABCC6 gene have been identified. There is no cure for PXE; the main symptomatic treatments are vascular endothelial growth factor inhibitor therapy (for ophthalmic manifestations), lifestyle, lipid-lowering and dietary measures (for reducing vascular risk factors), and vascular surgery (for severe cardiovascular manifestations). Future treatment options may include gene therapy/editing and pharmacologic chaperone therapy.
Keywords: Pseudoxanthoma elasticum, Metabolic disease, Ectopic mineralization, Skin, Angioid streak, Choroidal neovascularization, Peripheral arterial disease, ABCC6
Background
Disease name and synonyms
Pseudoxanthoma elasticum (PXE); OMIM #264800
Grönblad-Strandberg syndrome
ICD-10: Q82.8; ORPHA #758
Definition
The term “pseudoxanthoma elasticum” was coined by the French dermatologist Ferdinand-Jean Darier in 1896 [1], by reference to the yellowish tone of skin features (seen in true cases of xanthoma) and the lax aspect of the skin at flexural surfaces. Darier also observed abnormal histological features of the skin. However, skin plaques in what was probably PXE were first described by Rigal in 1881 [2]. The link between retinal angioid streaks and skin features in PXE was reported by Grönblad and by Strandberg in 1929 [3, 4], and PXE is occasionally referred to as Grönblad-Strandberg syndrome. PXE is a genetic disease with autosomal recessive inheritance in which dystrophic calcification (i.e. the abnormal accumulation of calcium/phosphate complexes) leads to cutaneous, ocular, cardiovascular and other manifestations [5, 6]. Most of the published evidence suggests that PXE is a metabolic disease, with decreased plasma pyrophosphate (PPi) levels being one of the strongest candidates for pathophysiology [7–10]. The effects of calcification are most apparent in the elastic tissues in the skin, eyes and blood vessels [11]. The deposits in PXE consist of calcium hydrogen phosphate, calcium hydroxyapatite and, to a lesser extent, iron precipitates [12, 13].
Epidemiology
The clinical prevalence of PXE has been estimated at between 1 per 100,000 and 1 per 25,000 of the general population, with slight female predominance [14, 15]. However, there are few data on allelic frequencies.
Clinical description
Cutaneous manifestations
The first clinical sign of PXE, with onset typically in childhood or adolescence [16] tends to be the characteristic skin changes (small yellow papules with diameter of up to 10 mm) on the nape and sides of the neck and in flexural areas (such as the axillae, the antecubital fossae, and periumbilical, inguinal and popliteal areas) [17] (Figs. 1 and 2). The oral, vaginal and rectal mucosae may also be affected. The papules are initially isolated or found in patches but coalesce into reticulated plaques as the disease progresses, giving a cobblestone aspect to the skin. The skin subsequently becomes loose and wrinkled, albeit not to the extent seen in cutis laxa [16]. It has been suggested that the presence of horizontal and oblique mental (chin) creases before the age of 30 years is specific for PXE [18]. Histological features of PXE may be found in the absence of overt skin lesions in patients with angioid streaks and macroscopically normal skin [19]. In rare cases, patients with genetically confirmed PXE may have histologically normal skin [20].
Fig. 1.

Characteristic cutaneous feature of PXE: yellow papules on the nape of the neck give the skin a peau d’orange aspect
Fig. 2.

Characteristic advanced cutaneous feature of PXE: involvement of axillary flexural folds
Electron microscopy of the skin reveals bulky, sometimes needle-like mineral deposits that disrupt and break elastic fibers (particularly in the mid-dermis) [13, 21, 22] (Fig. 3). Collagen irregular fibrils have been reported in the skin, myocardium and pericardium [23]. It has been reported that areas of clinically normal skin in PXE patient also contain damaged elastic fibers; it remains to be seen whether this change is an early marker for PXE [21].
Fig. 3.
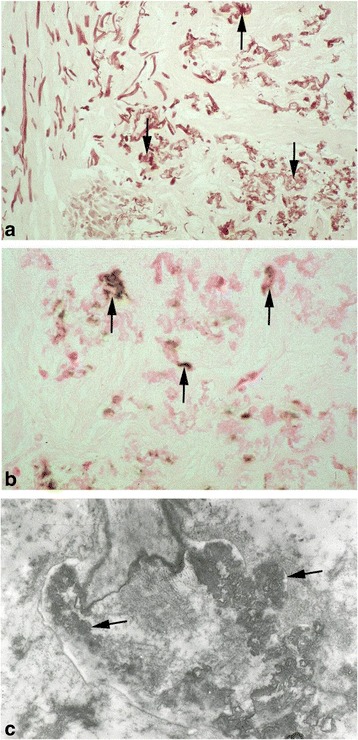
Characteristic histological features of PXE in skin biopsies. a Orcein staining: the elastic fibers of the dermis are fragmented and thickened. b Von Kossa staining: calcification of elastic fibers. c Viewed under the electron microscope, the elastic fibers’ morphology is abnormal
Ophthalmological manifestations
The ophthalmological manifestations of PXE are the most serious, since they can lead to blindness in late-stage disease. The characteristic ocular feature of PXE is the presence of angioid streaks in the retina [24] (Fig. 4). The streaks are variable in color (red/brown/grey) and reflect lesions in Bruch’s membrane - the innermost, elastic layer of the choroid. They can be observed several years after the onset of skin changes. The term “angioid” derives from the streaks’ aspect when viewed in fundoscopy, and these lesions are not vessels per se. The angioid streaks may become symptomatic when they approach the fovea of the macula. As the disease progresses, the calcification of Bruch’s membrane may trigger choroidal neovascularization. New subretinal vessels grow through the lesions in Bruch’s membrane, coating the posterior pole of the retina and eventually leading to hemorrhage, scarring, loss of central vision and thus blindness (Fig. 4) if not treated [24–26]. However, angioid streaks are not pathognomic for PXE because they may be present in diseases such as sickle cell disease, thalassemia and, more rarely, Ehlers-Danlos syndrome [24, 27]. It has been reported that angioid streaks are often preceded by drusen-like retinal peau d’orange changes in the temporal part of the macular region [28]. The peau d’orange sign was observed in 96% of patients with skin signs of PXE [16]. “Comet tail”, “punched-out” and “paired wing” lesions have also been described in PXE patients, and it has been suggested that the comet lesions are pathognomonic for PXE [28]. In a study of 107 PXE patients, visual impairment was associated with a major degradation in vision-related quality of life measured with the Impact of Vision Impairment questionnaire [29].
Fig. 4.
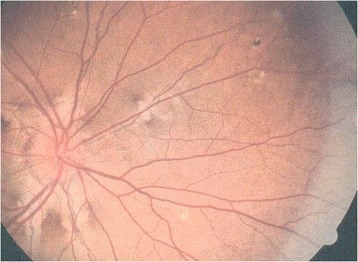
Characteristic ophthalmological feature of PXE: angioid streaks on the fundus
Vascular and systemic manifestations
Vascular signs (with the exception of claudication) usually become apparent years after the onset of skin and ocular changes. Patients with PXE have an elevated risk of vascular disease because the media and intima of blood vessels (mainly small and medium-sized arteries) are also affected by the dystrophic calcium/phosphate (Pi) mineralization of connective tissue that characterizes this metabolic disease [30]. The primary clinical expression of the arterial wall mineralization is intermittent claudication in both lower and upper limbs and peripheral artery disease [31, 32]. Involvement of the vascular wall (particularly in distal vessels) may lower the success of surgical procedures and should prompt the pre-operative assessment of all candidate vessels [33, 34]. Aneurysms [35, 36], stroke [31, 36], transient ischemic attack [36, 37], stenosis of medium-sized arteries such as the radial and carotid arteries [38, 39], and stenosis of the aorta [39] have also been reported. PXE has been described as a unique monogenic model of peripheral artery disease in which arterial wall remodeling is associated with an abnormally low ankle-brachial index (i.e. the ratio of the systolic blood pressure at the dorsalis pedis or posterior tibial artery to the highest systolic blood pressure of the left or right brachial artery), independently of cardiovascular risk factors [32, 40]. In contrast, cardiac complications (myocardial infarction, angina pectoris, etc.) are thought to be relatively rare but, when present, merit thorough investigation [41].
The frequency of ischemic stroke (although not clearly established) appears to be higher than in the general population [42], with a value of 15% in a cohort of 38 PXE patients [31] and 7% in another cohort of 100 patients, giving a relative risk of 3.6 versus the general population [36]. Carotid rete mirabile has been reported in association with PXE [37, 42].
It is noteworthy that in a study of 107 PXE patients, it was found that cardiovascular complications of the disease had relatively little impact on health-related quality of life, using the 36-item Short Form Health Survey [29].
Furthermore, it has also been suggested that heterozygous carriers of ABCC6 mutations (estimated frequency in the general population: up to 1 in 80) have an increased risk of cardiovascular calcification and premature coronary artery disease [15, 43].
Around 15% of PXE patients will experience hemorrhage of the gastrointestinal or urinary tract (especially the stomach), compared with around 0.1% in the general population [16, 25, 44].
Calcification of the kidneys, breasts, pancreas, testicles, liver and spleen has variously been observed in patients with PXE. With the possible exception of the kidneys, this calcification is not thought to have a major clinical impact [28].
PXE may have an impact on some aspects of lung function. In a functional study of 35 PXE patients, 11 had a significantly low carbon monoxide diffusing capacity [45]; this was interpreted as a possible preclinical state for interstitial lung disease.
Etiology
Genetics and molecular biology
PXE is a genetic disease with autosomal recessive inheritance. PXE-causing mutations in the ABCC6 gene on chromosome 16 were discovered in 2000 [46–51]. The ABCC6 gene consists of 31 exons, coding for a protein of 1503 amino acids (molecular weight: 165 kDa).
In the literature, there are 48 ABC (“adenosine triphosphate (ATP) binding cassette”) genes, divided into seven subfamilies (A to G). The ABCC subfamily includes 12 genes, including ABCC6 and ABCC7 (the latter also being known as CFTR – the mutated gene in cystic fibrosis), and a pseudogene (ABCC13). For reasons of structural homology, the protein encoded by the ABCC6 gene has been included in the multidrug resistance protein subfamily, some members of which export organic ions derived from exogenous sources (such as cancer drug metabolites) [52, 53]. Hence, in some older publications, ABCC6 is referred to as MRP6. The ABCC4, ABCC5, ABCC11 and ABCC12 proteins contain two membrane-spanning domains interspersed with two nucleotide-binding domains. The sulf onylurea receptor units SUR1 and SUR2 (encoded by ABCC8 and ABCC9) also have four domains, whereas the ABCC1, ABCC2, ABCC3, ABCC6 and ABCC10 proteins have an additional N-terminus domain. A three-dimensional model of the ABCC6 protein has been proposed by homology with the high-resolution structures of other ABC proteins [54]. However, in the absence of experimental confirmation by X-ray crystallography or high-resolution nuclear magnetic resonance, the accuracy of this model can be questioned.
ABCC6 gene expression is regulated in a tissue-specific manner [55]. It has been suggested that in addition to the proximal promoter, a primate-specific sequence (+629/+688) in the first intron of the human ABCC6 gene has a tissue-specific role [56]. The finding that the master regulator hepatocyte nuclear factor 4 α (HNF4α) binds to a highly conserved site (−209/−145) within the promoter may account for the predominant expression of ABCC6 in the liver [57].
Whether or how endogenous or exogenous substrates are transported by ABCC6 has not been well characterized. Even though ABCC6 had been included in the MRP family by homology, the molecular mechanism by which ABCC6 could transport drugs or their metabolites has not been defined. Hence, ABCC6 is unlikely to be involved in clinical multidrug resistance [53]. According to the results of in vitro experiments with membrane vesicles containing ABCC6 transfected into Chinese hamster ovary cells, the transfected cells were not notably resistant to etoposide, teniposide, doxorubicin, daunorubicin, actinomycin D or cisplatin [58].
As in any autosomal recessive disease, it is generally accepted that heterozygous carriers of a mutation in one ABCC6 allele do not develop PXE [59, 60]. However, some heterozygotes seem to display clinical and histopathological features of PXE [61–63]. The observation of abnormally mineralized skin areas in a woman with a p.R1141X mutation in ABCC6 and a p.V255M mutation in GGCX (coding for gamma-glutamyl carboxylase) [64] has prompted the consideration of a forme fruste of PXE (OMIM #177850). When considering PXE-like manifestations in heterozygotes, it is possible that an unrecognized mutation affects the second supposedly wild-type allele and thus still corresponds to recessive inheritance [31]. However, as noted above, it has been suggested that heterozygotes for ABCC6 mutations have an elevated risk of cardiovascular calcification [15].
Pathophysiology
While the genetic nature of the disease is well recognized, the pathophysiological mechanism of PXE has yet to be fully understood. It has been reported that although ATP secretion from the liver is ABCC6-dependent, ATP itself is not transported by ABCC6. However, the ABCC6-dependent secretion of ATP is the main source of pyrophosphate (PPi) in the circulation [9, 10]. Plasma PPi levels in Abcc6 (−/−) mice are around 40% of those found in wild-type mice, and the plasma PPi/Pi ratio is low in PXE patients [9, 10]. Hence, on the basis of experiments in HEK293 cells overexpressing either human or rat ABCC6 and in vivo experiments in Abcc6 (−/−) mice, PPi has been proposed as the candidate circulating factor involved in PXE metabolic disease [9, 65].
Although ABCC6 is expressed primarily in the liver, the kidneys and the intestine in healthy subjects, the damage in PXE patients occurs most obviously at remote sites. Two main hypotheses can be considered. Firstly, the cell-based hypothesis holds that a lack of functional ABCC6 protein at peripheral sites leads to ectopic mineralization [66]. Although cultured fibroblasts taken from PXE patients’ dermis display biochemical and genetic abnormalities [66, 67], the cell-based hypothesis is weakened by the fact that ABCC6 mRNA is expressed at only low to moderate levels in tissues outside the liver in healthy controls [47] [68].
The second, predominant paradigm for PXE is that of a systemic, metabolic disease in which the lack of production or release of one or more circulating factors from the liver (where ABCC6 is usually most strongly expressed) leads to ectopic mineralization. One variant of this metabolic hypothesis holds that the circulating factor usually suppresses or controls mineralization. Hence, in the absence of functional ABCC6 protein, the lack of these circulating factors leads to systemic, dystrophic mineralization throughout the body, including the skin, eyes and arteries. In a striking experimental proof of the metabolic disease hypothesis in the Abcc6-deficient (Abcc6 (−/−)) mouse model, the absence of functional abcc6 protein in the mutant was complemented by parabiotic heterogenetic pairing (surgical joining of the circulation with that of a wild-type mouse). The pairing stopped the connective tissue mineralization in the abcc6 (−/−) mouse – supposedly through the reintroduction of one or more critical anti-mineralization factors present in the wild-type mouse blood in sufficient quantity [69].
As mentioned above, PPi has been convincingly proposed as the candidate anti-mineralization circulating factor in PXE [9, 65]. High Pi levels have been mentioned as a calcification factor in PXE, on the basis of dietary supplementation experiments in the abcc6 (−/−) mouse model [70]. However, PXE patients have a normal parathyroid hormone status, and a placebo-controlled clinical trial of an orally administered sevelamer hydrochloride phosphate binder failed to demonstrate a significant effect on elastic fiber calcification and clinical lesions in PXE [71]. However, the latter results may have been biased by the presence of magnesium stearate in the excipient. If Pi does have a role in PXE pathophysiology, it has been proposed it would rather be exerted through the decreased PPi/Pi ratio [9, 10].
Other molecules with a suggested role in PXE are the anti-mineralization proteins matrix Gla-protein (MGP) and fetuin-A, with a suggested link to chronic kidney disease (CKD). Serum levels of MGP and fetuin-A are moderately low in PXE patients [72] and abnormally low in patients with CKD [73]. The MGP knockout mouse shows spontaneous calcification of arteries and cartilage [74]. Interestingly, a murine model of CKD displayed low levels of Abcc6 protein but normal Abcc6 mRNA levels – suggesting a post-transcriptional or post-translational deficiency [75].
On the basis of animal model experiments, it has also been hypothesized that low vitamin K export from the liver would decrease the gamma-carboxylation of anti-mineralization proteins [76, 77]. Furthermore, MGP is not carboxylated in the elastic fibers of PXE patients [78], and PXE-like calcification of elastic fibers is observed in patients with mutation in the GGCX gene [78]. However, the failure of supplementation trials in murine models of PXE weakens the vitamin K hypothesis [79–81].
Adenosine is another candidate for the circulating factor in PXE, in view of the similarities between PXE and the disease known as “arterial calcification due to deficiency of CD73” (ACDC, in which extracellular adenosine monophosphate cannot be converted to adenosine) [82, 83]. Indeed, patients with ACDC and CD73-deficient mice develop dystrophic calcification, [84, 85]. However, this hypothesis is weakened by the lack of adenosine transport by ABCC6 in in vitro experiments [86].
It has also been suggested that oxidative stress is a pathophysiologic factor in PXE because (i) some PXE patients display biochemical signs of oxidative stress [87], (ii) some patients with β-thalassemia or sickle cell anemia – both conditions in which systemic free radical levels are elevated - can display PXE-like manifestations [88–91], and (iii) oxidative stress inhibits expression of ABCC6 gene in human cell lines. In the mouse, there is one report suggesting that abcc6 protein localizes to the mitochondria-associated membrane [92]. However, studies of frozen mouse and human liver sections and primary hepatocytes have clearly demonstrated that the main cellular location of ABCC6 protein is the basolateral plasma membrane [93].
Lastly, on the basis of microarray gene expression analyses of wild-type, Abcc6 deficient and Abcc6-transgenic mice [94, 95], it was postulated that the failure of mutant 6 to export one or more substrates from hepatocytes induces changes in the regulation and expression of genes encoding or modulating systemic anti-mineralization factors (the “hepatic intoxication” hypothesis). However, the differences in gene expression were small and were not significant after correction for multiple testing [94], and the changes in the liver’s metabolic profile did not appear to be reflected in the plasma profile [95]. Furthermore, liver function in general is not perturbed in patients in patients with PXE.
Most of the detailed experimental data on the pathophysiology of PXE comes from Abcc6-deficient models in the zebrafish [77, 96, 97] and in the mouse [98–102]. The zebrafish model is a useful tool for testing potential therapies, such as premature termination codon read-through [103]. However, this model’s experimental value is limited by the fact that the fetus dies about a week after fertilization [97]. In the mouse, all the Abcc6 −/− models develop dystrophic mineralization, with deposits in skin, retina and arteries that resembles the features of PXE in humans. For example, arterial calcium accumulation is 1.5- to 2-fold higher in Abcc6 −/− knock-out mice than in wild-type mice [104]. A study of Abcc6-deficient mice highlighted the activation of the bone morphogenic protein 2 (BMP2)-SMAD-RUNX2 signaling pathway – a critical mediator of vascular calcification [105].
Genotype-phenotype correlations are generally weak [61]. It has been suggested that the nonsense mutation p. Arg1141* might predispose patients to cardiovascular disease, independently of hyperlipidemia [43, 62, 63, 106, 107] and that the ABCC6 p. Arg1268Gln polymorphism [50] is associated with the early onset of the disease’s characteristic angioid streaks [108, 109]. ABCC6 mutations have also been occasionally linked to a lethal disorder known as generalized arterial calcification of infancy (GACI; OMIM 173335) associated with mutations in the ENPP1 gene coding for the ectonucleotide pyrophosphatase/phosphodiesterase-1 regulator of bone mineralization [110]. Death occurs in utero or in the first few months of life. Mutations in ENPP1 on chromosome 6q23 have been found in the majority of patients with GACI [111].
Diagnosis
Clinical criteria
There are no widely accepted and applied international guidelines for the clinical and genetic diagnosis of PXE. Historically (and notably before the discovery of the ABCC6 gene’s causal role in PXE), patients were screened for three major criteria and two minor criteria [112]. The three major criteria were (i) characteristic skin involvement with yellow cobblestone lesions in flexural locations, (ii) characteristic histopathologic features of the lesional skin, with elastic tissue or von Kossa stains, and (iii) characteristic ocular disease, with angioid streaks, peau d’orange lesions or maculopathy in adults older than 20 years of age. The two minor criteria were characteristic histopathologic features of non-lesional skin and a history of PXE in first-degree relatives. However, this historical classification does not always fit well with molecular data on ABCC6 [60].
A new classification was proposed in 2010 (Table 1) [28]. It comprises a semi-standardized work-up: (i) examination of the skin by a dermatologist or specialist physician familiar with PXE, (ii) hematoxylin–eosin, Verhoeff–van Gieson (elastin) and von Kossa (calcium) staining of a skin biopsy from an affected lesion (Fig. 3) or, if not applicable, a biopsy from the lateral side of the neck, (iii) fundoscopy of the posterior pole of both eyes by an experienced ophthalmologist (checking for peau d’orange, angioid streaks, macular degeneration, comets, and wing signs), and optional fluorescein or indocyanine green angiography and fundus autofluorescence (for angioid streaks) [28]. In practice, the presence of characteristic yellow cobblestone skin lesions alone will usually prompt screening for ABCC6 mutations.
Table 1.
Revised diagnostic criteria for PXE (adapted from [28])
| Major diagnostic criteria |
| 1. Skin a. Yellowish papules and/or plaques on the lateral side of the neck and/or flexural areas of the body; or b. Increase of morphologically altered elastin with fragmentation, clumping and calcification of elastic fibers in a skin biopsy taken from clinically affected skin |
| 2. Eye a. Peau d’orange of the retina; or b. One or more angioid streaks (ASs), each at least as long as one disk diameter. When in doubt, fluorescein or indocyanine green angiography of the fundus is needed for confirmation. |
| 3. Genetics a. A pathogenic mutation of both alleles of the ABCC6 gene; or b. A first-degree relative (parent, sib, child) who meets independently the diagnostic criteria for definitive PXE |
| Minor diagnostic criteria |
| 1. Eye a. One AS shorter than one disk diameter; or b. One or more ‘comets’ in the retina; or c. One or more ‘wing signs’ in the retina |
| 2. Genetics a. A pathogenic mutation of one allele of the ABCC6 gene |
| Requirements for the diagnosis of PXE |
| a. Definitive diagnosis The presence of two (or more) major criteria not belonging to the same (skin, eye, genetic) category |
| b. Probable diagnosis The presence of two major eye or two major skin criteria, or The presence of one major criterion and one or more minor criteria not belonging to the same category as the major criterion |
| c. Possible diagnosis The presence of a single major criterion, or The presence of one or more minor criteria |
Sickle cell anemia, beta-thalassemia, and PXE-like phenotype with cutis laxa and multiple coagulation factor deficiency should be excluded, if mutational analysis of ABCC6 is negative or not available. Signs and symptoms in PXE may arise with increasing age. If a patient is <30 years a probable or possible diagnosis of PXE should be considered provisional and dermatologic and ophthalmologic examinations should be repeated after 5 years
Laboratory diagnosis
Biochemical diagnosis
There are no specific or generally informative biochemical assays for PXE. Hemoglobin profiling and vitamin-K-dependent coagulation factor assays may be used to rule out sickle cell disease, beta thalassemia and multiple coagulation factor deficiency [28].
Molecular biology
As mentioned above, patients will be screened for ABCC6 mutations unless the clinical findings are unambiguous. More than 300 unique DNA sequence variants of the ABCC6 gene (mostly missense mutations) have been identified to date [https://www.ncbi.nlm.nih.gov/clinvar/?term=ABCC6[gene]]. Around 90% of patients with clinical PXE will have a mutation in both alleles.
The mutational profile varies from one ethnic group to another [113]. For example, the p.Arg1141* (p.R1141X) mutation is common in European populations [113], less common in Northern American populations [114] and was absent in a group of 22 Chinese patients (in whom 15 previously unreported mutations were detected) [115]. The del23-29 mutation is common in northern Europe and the northern Mediterranean region, whereas the p.Gly1321Ser mutation is prevalent in North America but rare in Europe [114]. The p.Arg1138Trp missense mutation may be a marker for French descent (since it is found in France and in French-speaking Canada), whereas the 2542delG frameshift mutation occurs predominantly in Japanese patients [113]. In contrast, the prevalence of p.Gln378* and p.Arg1339Cys mutations appear to be similar worldwide, suggesting recurrent mutational events. Overall, disease-causing missense mutations appear to be concentrated at domain–domain interfaces, with a 4.25-fold higher mutation rate [54]. Copy number variations in the two ABCC6 pseudogenes ABCC6Ψ1 and ABCC6Ψ2 [116, 117] have been found to be more common in PXE patients than in controls, although the clinical significance of this, if any, is unclear [118, 119].
Non-disease-causing polymorphisms have been identified; interestingly, an individual who was homozygous for an ABCC6 p.Arg1268Gln polymorphism did not have symptoms of PXE, and the Gln1268 (Q1268) allele had a frequency of 0.19 in healthy controls [50].
Histology
Light microscopy
Elastin is stained with Verhoeff–van Gieson reagent, and calcium deposits are revealed with Von Kossa staining [11, 17] (Fig. 3). The mid-dermal elastic fibers are short, fragmented, clumped and calcified. These characteristics are strongly suggestive of PXE but not pathognomic. Elastic fiber clumping and calcification are only present in clinically affected skin in mutated ABCC6 homozygotes or compound heterozygotes [28]. Splitting, thickening, coiling, calcification and flower-like deformation of skin collagen fibers are observed in some but not all PXE patients [16] and so are not thought to be clinically relevant.
As in the skin, histochemical assessment of Bruch’s membrane also reveals calcium deposits [12]. Similarly, elastic fibers become mineralized and disrupted in blood vessel walls, the myocardium and the pericardium [23]. Arterial vessels are most strongly affected, although fragmentation of elastic fibers in the vena cava has also been reported [23].
Differential diagnosis
Dermatological and connective tissue diseases
Intense solar elastosis of the nape of the neck in elderly people can mimic the macroscopic aspect of PXE skin features [120]. PXE-like macroscopic skin lesions are also observed after chronic D-penicillamine therapy [121] and in “acquired PXE” (perforating calcific elastosis, a non-inherited skin disease mainly affecting the peri-umbilical region in multiparous women) [122]. Some of the features of PXE can arise in rare dermatological disease such as late-onset focal dermal elastosis [123], papillary dermal elastolysis [124], mid-dermal elastolysis [125] and PXE-like skin manifestations with retinitis pigmentosa [78]. As mentioned above, angioid streaks can very occasionally be observed in Ehlers-Danlos syndrome. All these differential diagnoses can be ruled out by genetic testing for ABCC6 mutations.
β-thalassemia and sickle cell anemia
As mentioned above, skin manifestations resembling those seen in PXE and (in some cases) angioid streaks have been observed in individuals with β-thalassemia and sickle cell disease who clearly lack ABCC6 gene mutations [89, 90]. Hence, angioid streaks are not pathognomonic for PXE. Thalassemic patients with PXE-like skin lesions also manifest PXE-like vessel alterations that progress with time [126]. Interesting, progressive, liver-specific down-regulation of abcc6 was found in a murine model of β-thalassemia [127].
Body skin hyperlaxity due to vitamin K dependent coagulation factor deficiency
Body skin hyperlaxity due to vitamin K-dependent coagulation factor deficiency is an autosomal recessive disorder caused by mutations in either the GGCX or VKORC1 gene [128, 129]. Although the disorder is not associated with ABCC6 gene mutations, patients may display skin manifestations similar to those observed in PXE and cutis laxa [130]. In PXE with diffuse skin folds, screening for GGCX mutations can be considered. However, the disease progression is quite different, with the development of leathery lesions [131].
Management
Management of cutaneous manifestations
Although aesthetic concerns can prompt some patients to seek treatment for nuchal and axillary symptoms of the disease [132–134], surgery for these non-life-threatening symptoms should be implemented with caution [17].
The suggested role of oxidative stress in PXE has prompted an ad hoc attempt at antioxidant therapy with daily doses of tocopherol acetate and ascorbic acid in one patient [63]. The skin lesions had regressed at 12 months but had started to progress again at 18 months. Furthermore, administration of an antioxidant diet in the Abcc6 −/− mouse model had no effect on mineralization [135].
Management of ophthalmologic manifestations
Intravitreal treatment with vascular endothelial growth factor (VEGF) inhibitors (such as bevacizumab) has rapidly become an effective treatment for stopping choroidal neovascularization – often the most critical symptom of PXE [136–138]. Accordingly, physical treatments such as photodynamic therapy have become less extensively used. Contact sports should be avoided, due to the risk of retinal hemorrhage.
Management of vascular and systemic manifestations
The current treatment approach for slowing or limiting the cardiovascular manifestations of PXE is based on the reduction of cardiovascular risk factors through lifestyle changes (smoking cessation, weight loss, daily walking, moderate physical exercise, etc.). In terms of drug treatment, a survey of 1,747 patients with PXE (reported on in a study of atorvastatin administration in a murine model of PXE) suggested that a third were taking or had taken cholesterol-lowering agents [139]. Acetylsalicylic acid is typically contraindicated in PXE, due to the increased likelihood of bleeding from a diseased retinal neovasculature [140]. In particular, patients with gastrointestinal hemorrhage should avoid nonsteroidal anti-inflammatory drugs and antiplatelet agents [15]. However, this risk must be balanced against the potential benefits in the prevention of thrombophilia.
In the event of arterial stenosis, standard surgical bypass or percutaneous angioplasty can be performed [31, 32]. Weakness of the vascular wall (particularly in distal vessels) may alter the choice of vessels for surgical grafts and should prompt the pre-operative assessment of all candidate vessels. For example, use of the saphenous vein may be preferable to the highly patent internal mammary artery, which can also be affected, for coronary bypass [33, 34].
The various pathophysiological hypotheses for PXE (involving putative circulating pro- or anti-mineralization factors) have prompted researchers to test the effects of dietary supplementation in animal models and humans. Magnesium supplementation improved some disease indicators in the Abcc6 (−/−) mouse [141, 142]. Twice-daily magnesium oxide supplementation has been tested in PXE patients in a 2-year clinical trial (ClinicalTrials.gov NCT01525875). However, the results had not been published at the time of writing. It has also been suggested that a high calcium intake in early life correlates with severity of PXE, although it is not known whether a low-calcium diet in infancy would be feasible with a view to restricting ectopic mineralization. In experiments with Abcc6 −/− and Enpp1 asj mice, the administration of high oral doses or lower subcutaneous doses of bisphosphonates or etidronate prevented ectopic mineralization [143, 144].
Lastly, it has been postulated that protein conformation modifiers may allow synthesis of a functional, full-length ABCC6 protein. In in vitro experiments with polarized MDCKII cells [145], wild-type ABCC6 protein localized to the basolateral plasma membrane. The drug sodium 4-phenylbutyrate (approved as a treatment for urea cycle disorders) [146] restored the plasma membrane localization of four “mistargeted” ABCC6 mutants (p.Arg1114Pro, p.Ser1121Trp, p.Gln1347His, p.Arg1314Trp) in vitro and in vivo in the mouse liver [145, 147]. Encouragingly, treatment with sodium 4-phenylbutyrate also reduced dystrophic calcification in the Abcc6 −/− mouse [148]. However, the small number of mutants tested means that this approach must be further characterized and studied.
Gene therapy
PXE is a candidate for gene therapy. Given that mutant ABCC6 heterozygotes have few or no features of PXE, the presence of one healthy allele or moderate expression should be enough to relieve the symptoms of the disease. Since ABCC6 is most strongly expressed in the healthy liver, targeting a transgene to this organ is logical. Novel technologies and delivery options for liver-directed gene therapy are being developed [149, 150]. In the rodent, efficient gene transfer to the liver can be conveniently obtained by tail vein injection of viral and non-viral vector systems [149, 151]. Plasmid-based gene therapy has been tested in the Abcc6 −/− murine model of PXE [152]. A cDNA encoding human ABCC6 was subcloned into a non-viral, liver-specific expression vector carrying the mouse albumin promoter and a fetoprotein enhancer. The vector was delivered by a single tail vein injection of 3-month-old Abcc6 −/− mice. Functional human ABCC6 protein was transiently expressed in 13% of the animal’s hepatocytes, on average. Expression was associated with significantly less intense calcification 3 to 7 days after induced cardiac cryoinjury [152].
However, several shortcomings of the gene therapy approach will need to be overcome [151]. As required for all gene therapies, it will be essential to check that delivery of an ABCC6 transgene is safe and does not induce severe immune reactions or insertional oncogenesis [153].
Genetic counselling
PXE is transmitted according to a Mendelian autosomal recessive inheritance, with a 25% risk of recurrence in siblings.
Although calcification of the placenta and low birth weight have been reported, the risk of pregnancy is not elevated for both the fetus and the mother, and there is no reason to contraindicate pregnancy. Since inheritance is autosomal recessive, children conceived by a PXE patient and a non-affected individual will not be affected – except in cases of endogamy or genetic isolates in which pseudodominance has been reported [154].
Prenatal diagnosis
In theory, the discovery of causal mutations in ABCC6 has rendered prenatal testing and preimplantation genetic diagnosis possible [49]. However, given that PXE is not life-threatening, the ethical justification for prenatal diagnosis is subject to debate.
Unresolved questions and perspectives
The recently proposed “metabolic hypothesis” for PXE [7–9] has opened up some interesting opportunities for mechanistic and therapeutic research. An appropriate PPi/Pi ratio is critical for prevention of ectopic mineralization under homeostatic conditions and the most prominent candidate for the dystrophic calcification observed in PXE is decreased PPi/Pi ratio. Hence, there is a clear need for robust, double-blind, placebo-controlled clinical trials of dietary treatments, anti-mineralization agents, anti-osteoclastic drugs, vitamin K, etidronate, anti-oxidants and pharmacologic chaperones [145, 147, 155, 156] in PXE patients, with support from disease advocacy organizations [157, 158].
Genotype-phenotype correlations must be better defined. Next-generation sequencing, bioinformatics and the various "omics" technologies are now being used to study regulation and expression of ABCC6, and to search for possible disease modifier genes [66, 84, 154, 159, 160].
While heterozygous carriers of autosomal recessive diseases are typically considered to be healthy, several publications have emphasized the potential association between heterozygosity for the p.R1141X ABCC6 mutation and a variety of more common conditions, such as coronary artery disease [43]. Further research on digenism and/or putative modifier genes would be of value [154, 159, 160].
Liver-directed gene therapy/editing may become a treatment option in the future if stable, liver-specific expression is ensured, ABCC6-modified hepatocytes have a growth advantage, and any potential safety concerns have been addressed.
Conclusions
PXE is now a well-characterized, autosomal recessive, metabolic, genetic disease of ectopic mineralization that affects the skin, eye and blood vessels. Although not life-threatening, PXE is associated with a risk of blindness, decreased quality of life and peripheral vascular compromise. There is no cure for PXE and patients should be monitored on a regular basis (clinical examinations, exploration of the vascular tree with MR angiography and ultrasound, fundus examination of the posterior pole of both eyes). Behavioral and lifestyle factors include moderate exercise and the avoidance of trauma to the eyes. If quality of life is significantly impaired by skin manifestations, plastic surgery can be considered. Some precautions should be taken prior to vascular surgery. Although the exact pathophysiological mechanisms underlying the metabolic disease have yet to be identified, the suggested role of PPi as the circulating anti-mineralization factor should open up opportunities for the clinical development and validation of disease-modifying treatments.
Acknowledgements
The author thanks the patients and their families for continuous support.
Funding
“Plan National Maladies Rare”, from the French Ministry of Health.
Availability of data and materials
Not applicable (no datasets were generated or analyzed during the current study).
Authors’ contributions
DPG conceived, designed and wrote the article.
Competing interests
The author declares that he has no competing interests.
Consent for publication
Not applicable (no individual person’s data in any form).
Ethics approval and consent to participate
Not applicable (review article, not involving human participants, human data or human tissue).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ACDC
Arterial calcification due to deficiency of CD73
- BMP2
Bone morphogenic protein 2
- CKD
Chronic kidney disease
- GACI
Generalized arterial calcification of infancy
- GGCX
Gamma-glutamyl carboxylase
- HNF4α
Hepatocyte nuclear factor 4 alpha
- MGP
Matrix Gla-protein
- MRP
Multidrug resistance protein
- PPi
Pyrophosphate
- PXE
Pseudoxanthoma elasticum
- VEGF
Vascular endothelia growth factor
References
- 1.Darier J. Pseudoxanthoma elasticum. Monatshefte Prakt Derm. 1896;23:609–17. [Google Scholar]
- 2.Rigal D. Observation pour servir à l’histoire de la cheloide diffuse xanthelasmique. Ann Dermatol Syph (Paris) 1881;2:491–501. [Google Scholar]
- 3.Gronblad E. Angioid streaks - pseudoxanthoma elasticum. Acta Ophthal. 1929;7:329. doi: 10.1111/j.1755-3768.1929.tb07934.x. [DOI] [Google Scholar]
- 4.Strandberg J. Pseudoxanthoma elasticum. Z Haut Geschlechtskr. 1929;31:689. [Google Scholar]
- 5.Le Saux O, Martin L, Aherrahrou Z, Leftheriotis G, Varadi A, Brampton CN. The molecular and physiological roles of ABCC6: more than meets the eye. Front Genet. 2012;3:289. doi: 10.3389/fgene.2012.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Q, Jiang Q, Uitto J. Ectopic mineralization disorders of the extracellular matrix of connective tissue: molecular genetics and pathomechanisms of aberrant calcification. Matrix Biol. 2014;33:23–8. doi: 10.1016/j.matbio.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang QU, Uitto J. Pseudoxanthoma elasticum: a metabolic disease? J Invest Dermatol. 2006;126:1440–1. doi: 10.1038/sj.jid.5700267. [DOI] [PubMed] [Google Scholar]
- 8.Jiang QEM, Dibra F, Wang K, Uitto J. Pseudoxanthoma elasticum is a metabolic disease. J Invest Dermatol. 2009;129:348–54. doi: 10.1038/jid.2008.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jansen RS, Duijst S, Mahakena S, Sommer D, Szeri F, Varadi A, et al. ABCC6-mediated ATP secretion by the liver is the main source of the mineralization inhibitor inorganic pyrophosphate in the systemic circulation-brief report. Arterioscler Thromb Vasc Biol. 2014;34:1985–9. doi: 10.1161/ATVBAHA.114.304017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jansen RS, Kucukosmanoglu A, de Haas M, Sapthu S, Otero JA, Hegman IE, et al. ABCC6 prevents ectopic mineralization seen in pseudoxanthoma elasticum by inducing cellular nucleotide release. Proc Natl Acad Sci U S A. 2013;110:20206–11. doi: 10.1073/pnas.1319582110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosen MJ, Lamoen A, De Paepe A, Vanakker OM. Histopathology of pseudoxanthoma elasticum and related disorders: histological hallmarks and diagnostic clues. Scientifica (Cairo) 2012;2012:598262. doi: 10.6064/2012/598262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen O. Bruch’s membrane in pseudoxanthoma elasticum. Histochemical, ultrastructural and x-ray microanalytical study of the membrane and angioid streak areas. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1977;203:311–20. doi: 10.1007/BF00409836. [DOI] [PubMed] [Google Scholar]
- 13.Walker ER, Frederickson RG, Mayes MD. The mineralization of elastic fibers and alterations of extracellular matrix in pseudoxanthoma elasticum. Ultrastructure, immunocytochemistry, and X-ray analysis. Arch Dermatol. 1989;125:70–6. doi: 10.1001/archderm.1989.01670130072009. [DOI] [PubMed] [Google Scholar]
- 14.Struk B, Neldner KH, Rao VS, St Jean P, Lindpaintner K. Mapping of both autosomal recessive and dominant variants of pseudoxanthoma elasticum to chromosome 16p13.1. Hum Mol Genet. 1997;6:1823–8. doi: 10.1093/hmg/6.11.1823. [DOI] [PubMed] [Google Scholar]
- 15.Chassaing N, Martin L, Calvas P, Le Bert M, Hovnanian A. Pseudoxanthoma elasticum: a clinical, pathophysiological and genetic update including 11 novel ABCC6 mutations. J Med Genet. 2005;42:881–92. doi: 10.1136/jmg.2004.030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neldner KH. Pseudoxanthoma elasticum. Clin Dermatol. 1988;6:1–159. doi: 10.1016/0738-081X(88)90003-X. [DOI] [PubMed] [Google Scholar]
- 17.Marconi B, Bobyr I, Campanati A, Molinelli E, Consales V, Brisigotti V, et al. Pseudoxanthoma elasticum and skin: Clinical manifestations, histopathology, pathomechanism, perspectives of treatment. Intractable Rare Dis Res. 2015;4:113–22. doi: 10.5582/irdr.2015.01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lebwohl M, Lebwohl E, Bercovitch L. Prominent mental (chin) crease: a new sign of pseudoxanthoma elasticum. J Am Acad Dermatol. 2003;48:620–2. doi: 10.1067/mjd.2003.195. [DOI] [PubMed] [Google Scholar]
- 19.Brown SJ, Talks SJ, Needham SJ, Taylor AE. Pseudoxanthoma elasticum: biopsy of clinically normal skin in the investigation of patients with angioid streaks. Br J Dermatol. 2007;157:748–51. doi: 10.1111/j.1365-2133.2007.08076.x. [DOI] [PubMed] [Google Scholar]
- 20.Van Loey S, Leys A. Pseudoxanthoma elasticum confirmed by genetic analysis but not by skin biopsy: a case report and review of the literature. Bull Soc Belge Ophtalmol. 2013;322:83–7. [PubMed] [Google Scholar]
- 21.Lebwohl M, Schwartz E, Lemlich G, Lovelace O, Shaikh-Bahai F, Fleischmajer R. Abnormalities of connective tissue components in lesional and non-lesional tissue of patients with pseudoxanthoma elasticum. Arch Dermatol Res. 1993;285:121–6. doi: 10.1007/BF01112912. [DOI] [PubMed] [Google Scholar]
- 22.Contri MB, Boraldi F, Taparelli F, De Paepe A, Ronchetti IP. Matrix proteins with high affinity for calcium ions are associated with mineralization within the elastic fibers of pseudoxanthoma elasticum dermis. Am J Pathol. 1996;148:569–77. [PMC free article] [PubMed] [Google Scholar]
- 23.Gheduzzi D, Sammarco R, Quaglino D, Bercovitch L, Terry S, Taylor W, et al. Extracutaneous ultrastructural alterations in pseudoxanthoma elasticum. Ultrastruct Pathol. 2003;27:375–84. doi: 10.1080/01913120390248584. [DOI] [PubMed] [Google Scholar]
- 24.Georgalas I, Tservakis I, Papaconstaninou D, Kardara M, Koutsandrea C, Ladas I. Pseudoxanthoma elasticum, ocular manifestations, complications and treatment. Clin Exp Optom. 2011;94:169–80. doi: 10.1111/j.1444-0938.2010.00559.x. [DOI] [PubMed] [Google Scholar]
- 25.Hu X, Plomp AS, van Soest S, Wijnholds J, de Jong PT, Bergen AA. Pseudoxanthoma elasticum: a clinical, histopathological, and molecular update. Surv Ophthalmol. 2003;48:424–38. doi: 10.1016/S0039-6257(03)00053-5. [DOI] [PubMed] [Google Scholar]
- 26.Orssaud C, Roche O, Dufier JL, Germain DP. Visual Impairment in Pseudoxanthoma Elasticum: A Survey of 40 Patients. Ophthalmic Genet. 2015;36:327–32. doi: 10.3109/13816810.2014.886268. [DOI] [PubMed] [Google Scholar]
- 27.Georgalas I, Papaconstantinou D, Koutsandrea C, Kalantzis G, Karagiannis D, Georgopoulos G, et al. Angioid streaks, clinical course, complications, and current therapeutic management. Ther Clin Risk Manag. 2009;5:81–9. [PMC free article] [PubMed] [Google Scholar]
- 28.Plomp AS, Toonstra J, Bergen AA, van Dijk MR, de Jong PT. Proposal for updating the pseudoxanthoma elasticum classification system and a review of the clinical findings. Am J Med Genet A. 2010;152A:1049–58. doi: 10.1002/ajmg.a.33329. [DOI] [PubMed] [Google Scholar]
- 29.Finger RP, Fenwick E, Marella M, Charbel Issa P, Scholl HP, Holz FG, et al. The relative impact of vision impairment and cardiovascular disease on quality of life: the example of pseudoxanthoma elasticum. Health Qual Life Outcomes. 2011;9:113. doi: 10.1186/1477-7525-9-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leftheriotis G, Omarjee L, Le Saux O, Henrion D, Abraham P, Prunier F, et al. The vascular phenotype in Pseudoxanthoma elasticum and related disorders: contribution of a genetic disease to the understanding of vascular calcification. Front Genet. 2013;4:4. doi: 10.3389/fgene.2013.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vanakker OM, Leroy BP, Coucke P, Bercovitch LG, Uitto J, Viljoen D, et al. Novel clinico-molecular insights in pseudoxanthoma elasticum provide an efficient molecular screening method and a comprehensive diagnostic flowchart. Hum Mutat. 2008;29:205. doi: 10.1002/humu.9514. [DOI] [PubMed] [Google Scholar]
- 32.Leftheriotis G, Abraham P, Le Corre Y, Le Saux O, Henrion D, Ducluzeau PH, et al. Relationship between ankle brachial index and arterial remodeling in pseudoxanthoma elasticum. J Vasc Surg. 2011;54:1390–4. doi: 10.1016/j.jvs.2011.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iliopoulos J, Manganas C, Jepson N, Newman DC. Pseudoxanthoma elasticum: is the left internal mammary artery a suitable conduit for coronary artery bypass grafting? Ann Thorac Surg. 2002;73:652–3. doi: 10.1016/S0003-4975(01)03011-9. [DOI] [PubMed] [Google Scholar]
- 34.Lodge AJ, Dodd LG, Lowe JE. Arterial conduits should be evaluated preoperatively in coronary artery bypass patients with pseudoxanthoma elasticum. Tex Heart Inst J. 2005;32:576–8. [PMC free article] [PubMed] [Google Scholar]
- 35.Zimmo L, Rudarakanchana N, Thompson M, Hamady MS, Cheshire NJ, Bicknell CD. Renal artery aneurysm formation secondary to pseudoxanthoma elasticum. J Vasc Surg. 2013;57:842–4. doi: 10.1016/j.jvs.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 36.van den Berg JS, Hennekam RC, Cruysberg JR, Steijlen PM, Swart J, Tijmes N, et al. Prevalence of symptomatic intracranial aneurysm and ischaemic stroke in pseudoxanthoma elasticum. Cerebrovasc Dis. 2000;10:315–9. doi: 10.1159/000016076. [DOI] [PubMed] [Google Scholar]
- 37.Vasseur M, Carsin-Nicol B, Ebran JM, Willoteaux S, Martin L, Leftheriotis G, et al. Carotid rete mirabile and pseudoxanthoma elasticum: an accidental association? Eur J Vasc Endovasc Surg. 2011;42:292–4. doi: 10.1016/j.ejvs.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 38.Boutouyrie P, Germain DP, Tropeano AI, Laloux B, Carenzi F, Zidi M, et al. Compressibility of the carotid artery in patients with pseudoxanthoma elasticum. Hypertension. 2001;38:1181–4. doi: 10.1161/hy1101.096108. [DOI] [PubMed] [Google Scholar]
- 39.Germain DP, Boutouyrie P, Laloux B, Laurent S. Arterial remodeling and stiffness in patients with pseudoxanthoma elasticum. Arterioscler Thromb Vasc Biol. 2003;23:836–41. doi: 10.1161/01.ATV.0000067428.19031.28. [DOI] [PubMed] [Google Scholar]
- 40.Leftheriotis G, Kauffenstein G, Hamel JF, Abraham P, Le Saux O, Willoteaux S, et al. The contribution of arterial calcification to peripheral arterial disease in pseudoxanthoma elasticum .PLoS One. 2014;9:e96003. doi: 10.1371/journal.pone.0096003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Debette S, Germain DP. Neurologic manifestations of inherited disorders of connective tissue. Handb Clin Neurol. 2014;119:565–76. doi: 10.1016/B978-0-7020-4086-3.00037-0. [DOI] [PubMed] [Google Scholar]
- 42.Karam C, Soulat G, Germain DP, Lacombe P, Dubourg O. Coronary CT angiography for chest pain in pseudoxanthoma elasticum and cardiac intervention management. J Cardiovasc Comput Tomogr. 2015;9:238–41. doi: 10.1016/j.jcct.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Trip MD, Smulders YM, Wegman JJ, Hu X, Boer JM, ten Brink JB, et al. Frequent mutation in the ABCC6 gene (R1141X) is associated with a strong increase in the prevalence of coronary artery disease. Circulation. 2002;106:773–5. doi: 10.1161/01.CIR.0000028420.27813.C0. [DOI] [PubMed] [Google Scholar]
- 44.Goral V, Demir D, Tuzun Y, Keklikci U, Buyukbayram H, Bayan K, et al. Pseudoxantoma elasticum, as a repetitive upper gastrointestinal hemorrhage cause in a pregnant woman. World J Gastroenterol. 2007;13:3897–9. doi: 10.3748/wjg.v13.i28.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pingel S, Passon SG, Pausewang KS, Blatzheim AK, Pizarro C, Tuleta I, et al. Pseudoxanthoma elasticum - also a lung disease? the respiratory affection of patients with Pseudoxanthoma elasticum. PLoS One. 2016;11:e0162337. doi: 10.1371/journal.pone.0162337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perdu J, Germain DP. Identification of novel polymorphisms in the pM5 and MRP1 (ABCC1) genes at locus 16p13.1 and exclusion of both genes as responsible for pseudoxanthoma elasticum. Hum Mutat. 2001;17:74–5. doi: 10.1002/1098-1004(2001)17:1<74::AID-HUMU14>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 47.Bergen AA, Plomp AS, Schuurman EJ, Terry S, Breuning M, Dauwerse H, et al. Mutations in ABCC6 cause pseudoxanthoma elasticum. Nat Genet. 2000;25:228–31. doi: 10.1038/76109. [DOI] [PubMed] [Google Scholar]
- 48.Le Saux O, Urban Z, Tschuch C, Csiszar K, Bacchelli B, Quaglino D, et al. Mutations in a gene encoding an ABC transporter cause pseudoxanthoma elasticum. Nat Genet. 2000;25:223–7. doi: 10.1038/76102. [DOI] [PubMed] [Google Scholar]
- 49.Ringpfeil F, Lebwohl MG, Christiano AM, Uitto J. Pseudoxanthoma elasticum: mutations in the MRP6 gene encoding a transmembrane ATP-binding cassette (ABC) transporter. Proc Natl Acad Sci U S A. 2000;97:6001–6. doi: 10.1073/pnas.100041297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Germain DP, Perdu J, Remones V, Jeunemaitre X. Homozygosity for the R1268Q mutation in MRP6, the pseudoxanthoma elasticum gene, is not disease-causing. Biochem Biophys Res Commun. 2000;274:297–301. doi: 10.1006/bbrc.2000.3101. [DOI] [PubMed] [Google Scholar]
- 51.Struk B, Cai L, Zach S, Ji W, Chung J, Lumsden A, et al. Mutations of the gene encoding the transmembrane transporter protein ABC-C6 cause pseudoxanthoma elasticum. J Mol Med (Berl) 2000;78:282–6. doi: 10.1007/s001090000114. [DOI] [PubMed] [Google Scholar]
- 52.Slot AJ, Molinski SV, Cole SP. Mammalian multidrug-resistance proteins (MRPs) Essays Biochem. 2011;50:179–207. doi: 10.1042/bse0500179. [DOI] [PubMed] [Google Scholar]
- 53.Chen ZS, Tiwari AK. Multidrug resistance proteins (MRPs/ABCCs) in cancer chemotherapy and genetic diseases. FEBS J. 2011;278:3226–45. doi: 10.1111/j.1742-4658.2011.08235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fulop K, Barna L, Symmons O, Zavodszky P, Varadi A. Clustering of disease-causing mutations on the domain-domain interfaces of ABCC6. Biochem Biophys Res Commun. 2009;379:706–9. doi: 10.1016/j.bbrc.2008.12.142. [DOI] [PubMed] [Google Scholar]
- 55.Aranyi T, Bacquet C, de Boussac H, Ratajewski M, Pomozi V, Fulop K, et al. Transcriptional regulation of the ABCC6 gene and the background of impaired function of missense disease-causing mutations. Front Genet. 2013;4:27. doi: 10.3389/fgene.2013.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ratajewski M, de Boussac H, Sachrajda I, Bacquet C, Kovacs T, Varadi A, et al. ABCC6 expression is regulated by CCAAT/enhancer-binding protein activating a primate-specific sequence located in the first intron of the gene. J Invest Dermatol. 2012;132:2709–17. doi: 10.1038/jid.2012.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Boussac H, Ratajewski M, Sachrajda I, Koblos G, Tordai A, Pulaski L, et al. The ERK1/2-hepatocyte nuclear factor 4alpha axis regulates human ABCC6 gene expression in hepatocytes. J Biol Chem. 2010;285:22800–8. doi: 10.1074/jbc.M110.105593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Belinsky MG, Chen ZS, Shchaveleva I, Zeng H, Kruh GD. Characterization of the drug resistance and transport properties of multidrug resistance protein 6 (MRP6, ABCC6) Cancer Res. 2002;62:6172–7. [PubMed] [Google Scholar]
- 59.Miksch S, Lumsden A, Guenther UP, Foernzler D, Christen-Zach S, Daugherty C, et al. Molecular genetics of pseudoxanthoma elasticum: type and frequency of mutations in ABCC6. Hum Mutat. 2005;26:235–48. doi: 10.1002/humu.20206. [DOI] [PubMed] [Google Scholar]
- 60.Christen-Zach S, Huber M, Struk B, Lindpaintner K, Munier F, Panizzon RG, et al. Pseudoxanthoma elasticum: evaluation of diagnostic criteria based on molecular data. Br J Dermatol. 2006;155:89–93. doi: 10.1111/j.1365-2133.2006.07278.x. [DOI] [PubMed] [Google Scholar]
- 61.Pfendner EG, Vanakker OM, Terry SF, Vourthis S, McAndrew PE, McClain MR, et al. Mutation detection in the ABCC6 gene and genotype-phenotype analysis in a large international case series affected by pseudoxanthoma elasticum. J Med Genet. 2007;44:621–8. doi: 10.1136/jmg.2007.051094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hornstrup LS, Tybjaerg-Hansen A, Haase CL, Nordestgaard BG, Sillesen H, Grande P, et al. Heterozygosity for R1141X in ABCC6 and risk of ischemic vascular disease. Circ Cardiovasc Genet. 2011;4:534–41. doi: 10.1161/CIRCGENETICS.110.958801. [DOI] [PubMed] [Google Scholar]
- 63.Akoglu G, Li Q, Gokoz O, Gazyagci AS, Uitto J. Clinical and histopathological characteristics of a family with R1141X mutation of pseudoxanthoma elasticum - presymptomatic testing and lack of carrier phenotypes. Int J Dermatol. 2014;53:692–8. doi: 10.1111/ijd.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li Q, Grange DK, Armstrong NL, Whelan AJ, Hurley MY, Rishavy MA, et al. Mutations in the GGCX and ABCC6 genes in a family with pseudoxanthoma elasticum-like phenotypes. J Invest Dermatol. 2009;129:553–63. doi: 10.1038/jid.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Q, Aranyi T, Varadi A, Terry SF, Uitto J. Research progress in pseudoxanthoma elasticum and related ectopic mineralization disorders. J Invest Dermatol. 2016;136:550–6. doi: 10.1016/j.jid.2015.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuzaj P, Kuhn J, Michalek RD, Karoly ED, Faust I, Dabisch-Ruthe M, et al. Large-scaled metabolic profiling of human dermal fibroblasts derived from pseudoxanthoma elasticum patients and healthy controls. PLoS One. 2014;9:e108336. doi: 10.1371/journal.pone.0108336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hendig D, Langmann T, Kocken S, Zarbock R, Szliska C, Schmitz G, et al. Gene expression profiling of ABC transporters in dermal fibroblasts of pseudoxanthoma elasticum patients identifies new candidates involved in PXE pathogenesis. Lab Invest. 2008;88:1303–15. doi: 10.1038/labinvest.2008.96. [DOI] [PubMed] [Google Scholar]
- 68.Kool M, van der Linden M, de Haas M, Baas F, Borst P. Expression of human MRP6, a homologue of the multidrug resistance protein gene MRP1, in tissues and cancer cells. Cancer Res. 1999;59:175–82. [PubMed] [Google Scholar]
- 69.Jiang Q, Oldenburg R, Otsuru S, Grand-Pierre AE, Horwitz EM, Uitto J. Parabiotic heterogenetic pairing of Abcc6−/−/Rag1−/− mice and their wild-type counterparts halts ectopic mineralization in a murine model of pseudoxanthoma elasticum. Am J Pathol. 2010;176:1855–62. doi: 10.2353/ajpath.2010.090983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li Q, Kingman J, Uitto J. Mineral content of the maternal diet influences ectopic mineralization in offspring of Abcc6(−/−) mice. Cell Cycle. 2015;14:3184–9. doi: 10.1080/15384101.2015.1068473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yoo JY, Blum RR, Singer GK, Stern DK, Emanuel PO, Fuchs W, et al. A randomized controlled trial of oral phosphate binders in the treatment of pseudoxanthoma elasticum. J Am Acad Dermatol. 2011;65:341–8. doi: 10.1016/j.jaad.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 72.Hendig D, Schulz V, Arndt M, Szliska C, Kleesiek K, Gotting C. Role of serum fetuin-A, a major inhibitor of systemic calcification, in pseudoxanthoma elasticum. Clin Chem. 2006;52:227–34. doi: 10.1373/clinchem.2005.059253. [DOI] [PubMed] [Google Scholar]
- 73.Suliman ME, Garcia-Lopez E, Anderstam B, Lindholm B, Stenvinkel P. Vascular calcification inhibitors in relation to cardiovascular disease with special emphasis on fetuin-A in chronic kidney disease. Adv Clin Chem. 2008;46:217–62. doi: 10.1016/S0065-2423(08)00406-X. [DOI] [PubMed] [Google Scholar]
- 74.Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 75.Lau WL, Liu S, Vaziri ND. Chronic kidney disease results in deficiency of ABCC6, the novel inhibitor of vascular calcification. Am J Nephrol. 2014;40:51–5. doi: 10.1159/000365014. [DOI] [PubMed] [Google Scholar]
- 76.Schurgers LJ, Uitto J, Reutelingsperger CP. Vitamin K-dependent carboxylation of matrix Gla-protein: a crucial switch to control ectopic mineralization. Trends Mol Med. 2013;19:217–26. doi: 10.1016/j.molmed.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 77.Mackay EW, Apschner A, Schulte-Merker S. Vitamin K reduces hypermineralisation in zebrafish models of PXE and GACI. Development. 2015;142:1095–101. doi: 10.1242/dev.113811. [DOI] [PubMed] [Google Scholar]
- 78.Kariminejad A, Bozorgmehr B, Najafi A, Khoshaeen A, Ghalandari M, Najmabadi H, et al. Retinitis pigmentosa, cutis laxa, and pseudoxanthoma elasticum-like skin manifestations associated with GGCX mutations. J Invest Dermatol. 2014;134:2331–8. doi: 10.1038/jid.2014.191. [DOI] [PubMed] [Google Scholar]
- 79.Jiang Q, Li Q, Grand-Pierre AE, Schurgers LJ, Uitto J. Administration of vitamin K does not counteract the ectopic mineralization of connective tissues in Abcc6 (−/−) mice, a model for pseudoxanthoma elasticum. Cell Cycle. 2011;10:701–7. doi: 10.4161/cc.10.4.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brampton C, Yamaguchi Y, Vanakker O, Van Laer L, Chen LH, Thakore M, et al. Vitamin K does not prevent soft tissue mineralization in a mouse model of pseudoxanthoma elasticum. Cell Cycle. 2011;10:1810–20. doi: 10.4161/cc.10.11.15681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gorgels TG, Waarsing JH, Herfs M, Versteeg D, Schoensiegel F, Sato T, et al. Vitamin K supplementation increases vitamin K tissue levels but fails to counteract ectopic calcification in a mouse model for pseudoxanthoma elasticum. J Mol Med (Berl) 2011;89:1125–35. doi: 10.1007/s00109-011-0782-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.St Hilaire C, Ziegler SG, Markello TC, Brusco A, Groden C, Gill F, et al. NT5E mutations and arterial calcifications. N Engl J Med. 2011;364:432–42. doi: 10.1056/NEJMoa0912923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Markello TC, Pak LK, St Hilaire C, Dorward H, Ziegler SG, Chen MY, et al. Vascular pathology of medial arterial calcifications in NT5E deficiency: implications for the role of adenosine in pseudoxanthoma elasticum. Mol Genet Metab. 2011;103:44–50. doi: 10.1016/j.ymgme.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.De Vilder EY, Vanakker OM. From variome to phenome: Pathogenesis, diagnosis and management of ectopic mineralization disorders. World J Clin Cases. 2015;3:556–74. doi: 10.12998/wjcc.v3.i7.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li Q, Price TP, Sundberg JP, Uitto J. Juxta-articular joint-capsule mineralization in CD73 deficient mice: similarities to patients with NT5E mutations. Cell Cycle. 2014;13:2609–15. doi: 10.4161/15384101.2014.943567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Szabo Z, Varadi A, Li Q, Uitto J. ABCC6 does not transport adenosine - relevance to pathomechanism of pseudoxanthoma elasticum. Mol Genet Metab. 2011;104:421. doi: 10.1016/j.ymgme.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 87.Garcia-Fernandez MI, Gheduzzi D, Boraldi F, Paolinelli CD, Sanchez P, Valdivielso P, et al. Parameters of oxidative stress are present in the circulation of PXE patients. Biochim Biophys Acta. 2008;1782:474–81. doi: 10.1016/j.bbadis.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 88.Voskou S, Aslan M, Fanis P, Phylactides M, Kleanthous M. Oxidative stress in beta-thalassaemia and sickle cell disease. Redox Biol. 2015;6:226–39. doi: 10.1016/j.redox.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baccarani-Contri M, Bacchelli B, Boraldi F, Quaglino D, Taparelli F, Carnevali E, et al. Characterization of pseudoxanthoma elasticum-like lesions in the skin of patients with beta-thalassemia. J Am Acad Dermatol. 2001;44:33–9. doi: 10.1067/mjd.2001.110045. [DOI] [PubMed] [Google Scholar]
- 90.Hamlin N, Beck K, Bacchelli B, Cianciulli P, Pasquali-Ronchetti I, Le Saux O. Acquired Pseudoxanthoma elasticum-like syndrome in beta-thalassaemia patients. Br J Haematol. 2003;122:852–4. doi: 10.1046/j.1365-2141.2003.04484.x. [DOI] [PubMed] [Google Scholar]
- 91.Fabbri E, Forni GL, Guerrini G, Borgna-Pignatti C. Pseudoxanthoma-elasticum-like syndrome and thalassemia: an update. Dermatol Online J. 2009;15:7. [PubMed] [Google Scholar]
- 92.Martin LJ, Lau E, Singh H, Vergnes L, Tarling EJ, Mehrabian M, et al. ABCC6 localizes to the mitochondria-associated membrane. Circ Res. 2012;111:516–20. doi: 10.1161/CIRCRESAHA.112.276667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pomozi V, Le Saux O, Brampton C, Apana A, Ilias A, Szeri F, et al. ABCC6 is a basolateral plasma membrane protein. Circ Res. 2013;112:e148–51. doi: 10.1161/CIRCRESAHA.111.300194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rasmussen MS, M.; Moestrup, SK. Is classical pseudoxanthoma elasticum a consequence of hepatic ‘intoxication’ due to ABCC6 substrate accumulation in the liver? Expert Rev Endocr Metabol. 2013;8:37–46. [DOI] [PubMed]
- 95.Rasmussen MR, Nielsen KL, Laursen MR, Nielsen CB, Svendsen P, Dimke H, et al. Untargeted Metabolomics Analysis of ABCC6-Deficient Mice Discloses an Altered Metabolic Liver Profile. J Proteome Res. 2016;15:4591–600. doi: 10.1021/acs.jproteome.6b00669. [DOI] [PubMed] [Google Scholar]
- 96.Li Q, Frank M, Thisse CI, Thisse BV, Uitto J. Zebrafish: a model system to study heritable skin diseases. J Invest Dermatol. 2011;131:565–71. doi: 10.1038/jid.2010.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li Q, Sadowski S, Frank M, Chai C, Varadi A, Ho SY, et al. The abcc6a gene expression is required for normal zebrafish development. J Invest Dermatol. 2010;130:2561–8. doi: 10.1038/jid.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li Q, Guo H, Chou DW, Berndt A, Sundberg JP, Uitto J. Mouse models for pseudoxanthoma elasticum: genetic and dietary modulation of the ectopic mineralization phenotypes. PLoS One. 2014;9:e89268. doi: 10.1371/journal.pone.0089268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gorgels TG, Hu X, Scheffer GL, van der Wal AC, Toonstra J, de Jong PT, et al. Disruption of Abcc6 in the mouse: novel insight in the pathogenesis of pseudoxanthoma elasticum. Hum Mol Genet. 2005;14:1763–73. doi: 10.1093/hmg/ddi183. [DOI] [PubMed] [Google Scholar]
- 100.Klement JF, Matsuzaki Y, Jiang QJ, Terlizzi J, Choi HY, Fujimoto N, et al. Targeted ablation of the abcc6 gene results in ectopic mineralization of connective tissues. Mol Cell Biol. 2005;25:8299–310. doi: 10.1128/MCB.25.18.8299-8310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Heston WE, Vlahakis G. Mammary tumors, plaques, and hyperplastic alveolar nodules in various combinations of mouse inbred strains and the different lines of the mammary tumor virus. Int J Cancer. 1971;7:141–8. doi: 10.1002/ijc.2910070116. [DOI] [PubMed] [Google Scholar]
- 102.Aherrahrou Z, Doehring LC, Ehlers EM, Liptau H, Depping R, Linsel-Nitschke P, et al. An alternative splice variant in Abcc6, the gene causing dystrophic calcification, leads to protein deficiency in C3H/He mice. J Biol Chem. 2008;283:7608–15. doi: 10.1074/jbc.M708290200. [DOI] [PubMed] [Google Scholar]
- 103.Zhou Y, Jiang Q, Takahagi S, Shao C, Uitto J. Premature termination codon read-through in the ABCC6 gene: potential treatment for pseudoxanthoma elasticum. J Invest Dermatol. 2013;133:2672–7. doi: 10.1038/jid.2013.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kauffenstein G, Pizard A, Le Corre Y, Vessieres E, Grimaud L, Toutain B, et al. Disseminated arterial calcification and enhanced myogenic response are associated with abcc6 deficiency in a mouse model of pseudoxanthoma elasticum. Arterioscler Thromb Vasc Biol. 2014;34:1045–56. doi: 10.1161/ATVBAHA.113.302943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hosen MJ, Coucke PJ, Le Saux O, De Paepe A, Vanakker OM. Perturbation of specific pro-mineralizing signalling pathways in human and murine pseudoxanthoma elasticum. Orphanet J Rare Dis. 2014;9:66. doi: 10.1186/1750-1172-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Koblos G, Andrikovics H, Prohaszka Z, Tordai A, Varadi A, Aranyi T. The R1141X loss-of-function mutation of the ABCC6 gene is a strong genetic risk factor for coronary artery disease. Genet Test Mol Biomarkers. 2010;14:75–8. doi: 10.1089/gtmb.2009.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pisciotta L, Tarugi P, Borrini C, Bellocchio A, Fresa R, Guerra D, et al. Pseudoxanthoma elasticum and familial hypercholesterolemia: a deleterious combination of cardiovascular risk factors. Atherosclerosis. 2010;210:173–6. doi: 10.1016/j.atherosclerosis.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 108.Sato N, Nakayama T, Mizutani Y, Yuzawa M. Novel mutations of ABCC6 gene in Japanese patients with Angioid streaks. Biochem Biophys Res Commun. 2009;380:548–53. doi: 10.1016/j.bbrc.2009.01.117. [DOI] [PubMed] [Google Scholar]
- 109.Li Q, Sadowski S, Uitto J. Angioid streaks in Pseudoxanthoma Elasticum: role of the p.R1268Q mutation in the ABCC6 gene. J Invest Dermatol. 2011;131:782–5. doi: 10.1038/jid.2010.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nitschke Y, Baujat G, Botschen U, Wittkampf T, du Moulin M, Stella J, et al. Generalized arterial calcification of infancy and pseudoxanthoma elasticum can be caused by mutations in either ENPP1 or ABCC6. Am J Hum Genet. 2012;90:25–39. doi: 10.1016/j.ajhg.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kalal IG, Seetha D, Panda A, Nitschke Y, Rutsch F. Molecular diagnosis of generalized arterial calcification of infancy (GACI) J Cardiovasc Dis Res. 2012;3:150–4. doi: 10.4103/0975-3583.95373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lebwohl M, Neldner K, Pope FM, De Paepe A, Christiano AM, Boyd CD, et al. Classification of pseudoxanthoma elasticum: report of a consensus conference. J Am Acad Dermatol. 1994;30:103–7. doi: 10.1016/S0190-9622(08)81894-4. [DOI] [PubMed] [Google Scholar]
- 113.Larusso J, Ringpfeil F, Uitto J. Pseudoxanthoma elasticum: a streamlined, ethnicity-based mutation detection strategy. Clin Transl Sci. 2010;3:295–8. doi: 10.1111/j.1752-8062.2010.00243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Le Saux O, Beck K, Sachsinger C, Silvestri C, Treiber C, Goring HH, et al. A spectrum of ABCC6 mutations is responsible for pseudoxanthoma elasticum. Am J Hum Genet. 2001;69:749–64. doi: 10.1086/323704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jin L, Jiang Q, Wu Z, Shao C, Zhou Y, Yang L, et al. Genetic heterogeneity of pseudoxanthoma elasticum: the Chinese signature profile of ABCC6 and ENPP1 mutations. J Invest Dermatol. 2015;135:1294–302. doi: 10.1038/jid.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Germain DP. Pseudoxanthoma elasticum: evidence for the existence of a pseudogene highly homologous to the ABCC6 gene. J Med Genet. 2001;38:457–61. doi: 10.1136/jmg.38.7.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pulkkinen L, Nakano A, Ringpfeil F, Uitto J. Identification of ABCC6 pseudogenes on human chromosome 16p: implications for mutation detection in pseudoxanthoma elasticum. Hum Genet. 2001;109:356–65. doi: 10.1007/s004390100582. [DOI] [PubMed] [Google Scholar]
- 118.Kringen MK, Stormo C, Grimholt RM, Berg JP, Piehler AP. Copy number variations of the ATP-binding cassette transporter ABCC6 gene and its pseudogenes. BMC Res Notes. 2012;5:425. doi: 10.1186/1756-0500-5-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kringen MK, Stormo C, Berg JP, Terry SF, Vocke CM, Rizvi S, et al. Copy number variation in the ATP-binding cassette transporter ABCC6 gene and ABCC6 pseudogenes in patients with pseudoxanthoma elasticum. Mol Genet Genomic Med. 2015;3:233–7. doi: 10.1002/mgg3.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lewis KG, Bercovitch L, Dill SW, Robinson-Bostom L. Acquired disorders of elastic tissue: part I. Increased elastic tissue and solar elastotic syndromes. J Am Acad Dermatol. 2004;51:1–21. doi: 10.1016/j.jaad.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 121.Ibanez-Samaniego L, Ochoa-Palominos A, Catalina-Rodriguez MV, Salcedo-Plaza M, Clemente-Ricote G. Penicillamine induced pseudo-pseudoxanthoma elasticum in a patient with Wilson's disease, which role plays the hepatologist? Rev Esp Enferm Dig. 2015;107:190–1. [PubMed] [Google Scholar]
- 122.Kazakis AM, Parish WR. Periumbilical perforating pseudoxanthoma elasticum. J Am Acad Dermatol. 1988;19:384–8. doi: 10.1016/S0190-9622(88)70183-8. [DOI] [PubMed] [Google Scholar]
- 123.Wang AR, Fonder MA, Telang GH, Bercovitch L, Robinson-Bostom L. Late-onset focal dermal elastosis: an uncommon mimicker of pseudoxanthoma elasticum. J Cutan Pathol. 2012;39:957–61. doi: 10.1111/j.1600-0560.2012.01979.x. [DOI] [PubMed] [Google Scholar]
- 124.Val-Bernal JF, Gonzalez-Vela MC, Leon-Castillo A, Armesto S. Papillary dermal elastosis. Am J Dermatopathol. 2017;39:150–2. doi: 10.1097/DAD.0000000000000653. [DOI] [PubMed] [Google Scholar]
- 125.Gambichler T. Mid-dermal elastolysis revisited. Arch Dermatol Res. 2010;302:85–93. doi: 10.1007/s00403-009-1004-0. [DOI] [PubMed] [Google Scholar]
- 126.Cianciulli P, Sorrentino F, Maffei L, Amadori S, Cappabianca MP, Foglietta E, et al. Cardiovascular involvement in thalassaemic patients with pseudoxanthoma elasticum-like skin lesions: a long-term follow-up study. Eur J Clin Invest. 2002;32:700–6. doi: 10.1046/j.1365-2362.2002.01032.x. [DOI] [PubMed] [Google Scholar]
- 127.Martin L, Douet V, VanWart CM, Heller MB, Le Saux O. A mouse model of beta-thalassemia shows a liver-specific down-regulation of Abcc6 expression. Am J Pathol. 2011;178:774–83. doi: 10.1016/j.ajpath.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Weston BW, Monahan PE. Familial deficiency of vitamin K-dependent clotting factors. Haemophilia. 2008;14:1209–13. doi: 10.1111/j.1365-2516.2008.01853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Brenner B, Kuperman AA, Watzka M, Oldenburg J. Vitamin K-dependent coagulation factors deficiency. Semin Thromb Hemost. 2009;35:439–46. doi: 10.1055/s-0029-1225766. [DOI] [PubMed] [Google Scholar]
- 130.Li Q, Schurgers LJ, Smith AC, Tsokos M, Uitto J, Cowen EW. Co-existent pseudoxanthoma elasticum and vitamin K-dependent coagulation factor deficiency: compound heterozygosity for mutations in the GGCX gene. Am J Pathol. 2009;174:534–40. doi: 10.2353/ajpath.2009.080865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vanakker OM, Martin L, Gheduzzi D, Leroy BP, Loeys BL, Guerci VI, et al. Pseudoxanthoma elasticum-like phenotype with cutis laxa and multiple coagulation factor deficiency represents a separate genetic entity. J Invest Dermatol. 2007;127:581–7. doi: 10.1038/sj.jid.5700610. [DOI] [PubMed] [Google Scholar]
- 132.Viljoen DL, Bloch C, Beighton P. Plastic surgery in pseudoxanthoma elasticum: experience in nine patients. Plast Reconstr Surg. 1990;85:233–8. doi: 10.1097/00006534-199002000-00011. [DOI] [PubMed] [Google Scholar]
- 133.Ng AB, O'Sullivan ST, Sharpe DT. Plastic surgery and pseudoxanthoma elasticum. Br J Plast Surg. 1999;52:594–6. doi: 10.1054/bjps.1999.3139. [DOI] [PubMed] [Google Scholar]
- 134.Marwah M, Godse K, Patil S, Nadkarni N, Gautam M. Surgical correction of pseudoxanthoma elasticum. J Cutan Aesthet Surg. 2012;5:212–3. doi: 10.4103/0974-2077.101390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li Q, Jiang Q, Uitto J. Pseudoxanthoma elasticum: oxidative stress and antioxidant diet in a mouse model (Abcc6−/−) J Invest Dermatol. 2008;128:1160–4. doi: 10.1038/sj.jid.5701145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Verbraak FD. Antivascular endothelial growth factor treatment in pseudoxanthoma elasticum patients. Dev Ophthalmol. 2010;46:96–106. doi: 10.1159/000320012. [DOI] [PubMed] [Google Scholar]
- 137.Neri P, Salvolini S, Mariotti C, Mercanti L, Celani S, Giovannini A. Long-term control of choroidal neovascularisation secondary to angioid streaks treated with intravitreal bevacizumab (Avastin) Br J Ophthalmol. 2009;93:155–8. doi: 10.1136/bjo.2008.145896. [DOI] [PubMed] [Google Scholar]
- 138.Myung JS, Bhatnagar P, Spaide RF, Klancnik JM, Jr, Cooney MJ, Yannuzzi LA, et al. Long-term outcomes of intravitreal antivascular endothelial growth factor therapy for the management of choroidal neovascularization in pseudoxanthoma elasticum. Retina. 2010;30:748–55. doi: 10.1097/IAE.0b013e3181c596b1. [DOI] [PubMed] [Google Scholar]
- 139.Guo H, Li Q, Chou DW, Uitto J. Atorvastatin counteracts aberrant soft tissue mineralization in a mouse model of pseudoxanthoma elasticum (Abcc6(−)/(−)) J Mol Med (Berl) 2013;91:1177–84. doi: 10.1007/s00109-013-1066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liew G, Mitchell P, Wong TY, Rochtchina E, Wang JJ. The association of aspirin use with age-related macular degeneration. JAMA Intern Med. 2013;173:258–64. doi: 10.1001/jamainternmed.2013.1583. [DOI] [PubMed] [Google Scholar]
- 141.Li Q, Larusso J, Grand-Pierre AE, Uitto J. Magnesium carbonate-containing phosphate binder prevents connective tissue mineralization in Abcc6(−/−) mice-potential for treatment of pseudoxanthoma elasticum. Clin Transl Sci. 2009;2:398–404. doi: 10.1111/j.1752-8062.2009.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li Q, Uitto J. The mineralization phenotype in Abcc6 (−/−) mice is affected by Ggcx gene deficiency and genetic background--a model for pseudoxanthoma elasticum. J Mol Med (Berl) 2010;88:173–81. doi: 10.1007/s00109-009-0522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li Q, Sundberg JP, Levine MA, Terry SF, Uitto J. The effects of bisphosphonates on ectopic soft tissue mineralization caused by mutations in the ABCC6 gene. Cell Cycle. 2015;14:1082–9. doi: 10.1080/15384101.2015.1007809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li Q, Kingman J, Sundberg JP, Levine MA, Uitto J. Dual effects of bisphosphonates on ectopic skin and vascular soft tissue mineralization versus bone microarchitecture in a mouse model of generalized arterial calcification of infancy. J Invest Dermatol. 2016;136:275–83. doi: 10.1038/JID.2015.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pomozi V, Brampton C, Fulop K, Chen LH, Apana A, Li Q, et al. Analysis of pseudoxanthoma elasticum-causing missense mutants of ABCC6 in vivo; pharmacological correction of the mislocalized proteins. J Invest Dermatol. 2014;134:946–53. doi: 10.1038/jid.2013.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kolb PS, Ayaub EA, Zhou W, Yum V, Dickhout JG, Ask K. The therapeutic effects of 4-phenylbutyric acid in maintaining proteostasis. Int J Biochem Cell Biol. 2015;61:45–52. doi: 10.1016/j.biocel.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 147.Le Saux O, Fulop K, Yamaguchi Y, Ilias A, Szabo Z, Brampton CN, et al. Expression and in vivo rescue of human ABCC6 disease-causing mutants in mouse liver. PLoS One. 2011;6:e24738. doi: 10.1371/journal.pone.0024738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pomozi V, Brampton C, Szeri F, Dedinszki D, Kozak E, van de Wetering K, et al. Functional rescue of ABCC6 deficiency by 4-phenylbutyrate therapy reduces dystrophic calcification in Abcc6−/− mice. J Invest Dermatol. 2017;137:595–602. doi: 10.1016/j.jid.2016.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Aravalli RN, Steer CJ. Gene editing technology as an approach to the treatment of liver diseases. Expert Opin Biol Ther. 2016;16:595–608. doi: 10.1517/14712598.2016.1158808. [DOI] [PubMed] [Google Scholar]
- 150.Chuah MK, Petrus I, De Bleser P, Le Guiner C, Gernoux G, Adjali O, et al. Liver-specific transcriptional modules identified by genome-wide in silico analysis enable efficient gene therapy in mice and non-human primates. Mol Ther. 2014;22:1605–13. doi: 10.1038/mt.2014.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yokoo T, Kamimura K, Abe H, Kobayashi Y, Kanefuji T, Ogawa K, et al. Liver-targeted hydrodynamic gene therapy: Recent advances in the technique. World J Gastroenterol. 2016;22:8862–8. doi: 10.3748/wjg.v22.i40.8862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Brampton C, Aherrahrou Z, Chen LH, Martin L, Bergen AA, Gorgels TG, et al. The level of hepatic ABCC6 expression determines the severity of calcification after cardiac injury. Am J Pathol. 2014;184:159–70. doi: 10.1016/j.ajpath.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rothe M, Schambach A, Biasco L. Safety of gene therapy: new insights to a puzzling case. Curr Gene Ther. 2014;14:429–36. doi: 10.2174/1566523214666140918110905. [DOI] [PubMed] [Google Scholar]
- 154.Germain DP, Jurca-Simina IE. Principles of Human Genetics. In: Burlina AP, editor. Textbook of Treatable Neurometabolic Diseases. New York: Springer; 2017. In press.
- 155.Germain DP, Fan JQ. Pharmacological chaperone therapy by active-site-specific chaperones in Fabry disease: in vitro and preclinical studies. Int J Clin Pharmacol Ther. 2009;47:S111–7. doi: 10.5414/CPP47111. [DOI] [PubMed] [Google Scholar]
- 156.Germain DP, Hughes DA, Nicholls K, Bichet DG, Giugliani R, Wilcox WR, et al. Treatment of Fabry’s Disease with the Pharmacologic Chaperone Migalastat. N Engl J Med. 2016;375:545–55. doi: 10.1056/NEJMoa1510198. [DOI] [PubMed] [Google Scholar]
- 157.Terry SF. Disease advocacy organizations catalyze translational research. Front Genet. 2013;4:101. doi: 10.3389/fgene.2013.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Uitto J, Li Q, van de Wetering K, Váradi A, Terry SF. Insights into Pathomechanisms and Treatment Development in Heritable Ectopic Mineralization Disorders: Summary of the PXE International Biennial Research Symposium-2016. J Invest Dermatol. 2017;137:790–5. doi: 10.1016/j.jid.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hosen MJ, Van Nieuwerburgh F, Steyaert W, Deforce D, Martin L, Leftheriotis G, et al. Efficiency of exome sequencing for the molecular diagnosis of pseudoxanthoma elasticum. J Invest Dermatol. 2015;135:992–8. doi: 10.1038/jid.2014.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hendig D, Knabbe C, Gotting C. New insights into the pathogenesis of pseudoxanthoma elasticum and related soft tissue calcification disorders by identifying genetic interactions and modifiers. Front Genet. 2013;4:114. doi: 10.3389/fgene.2013.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable (no datasets were generated or analyzed during the current study).


