 The activation mode of a rotaxane-based organocatalyst with both secondary amine and squaramide catalytic units can be switched with acid or base, affording different products from a mixture of three building blocks.
The activation mode of a rotaxane-based organocatalyst with both secondary amine and squaramide catalytic units can be switched with acid or base, affording different products from a mixture of three building blocks.
Abstract
The activation mode of a rotaxane-based organocatalyst with both secondary amine and squaramide catalytic units can be switched with acid or base. The macrocycle blocks whichever of the catalytic sites it is positioned over. The switchable rotaxane catalyst generates different products from a mixture of three building blocks according to the location of the macrocyclic ring in the rotaxane.
Introduction
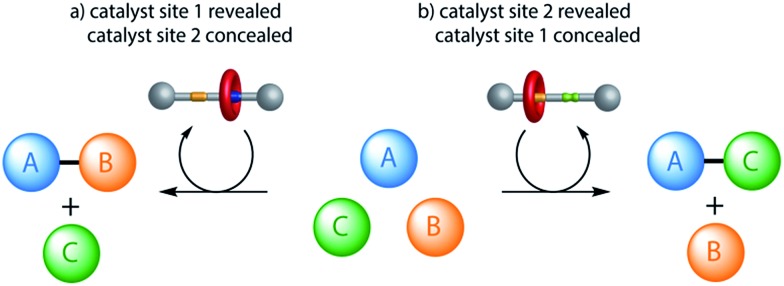
Synthetic catalysts have previously been developed where a stimulus can be used to turn the catalytic activity ‘on’ or ‘off’1,2 or to change the stereochemical outcome of a reaction.3 Here we report on an artificial system that can switch between two different modes of organocatalysis,4 each promoting a different chemical transformation. The result is a molecular catalyst that can be used to produce different reaction outcomes from a mixture of building blocks (Fig. 1).
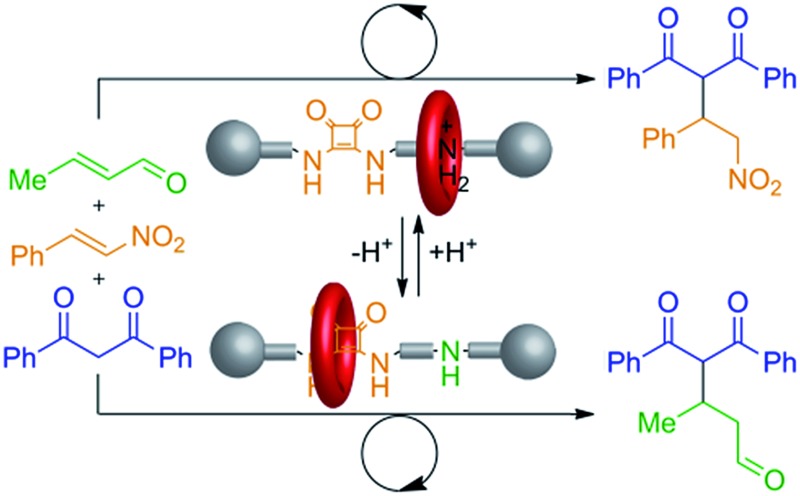
Fig. 1. Different products from a mixture of building blocks using a rotaxane catalyst switchable between two different active sites (e.g. 1/1-H+·CF3CO2 –, Fig. 2). Alternative reactions are promoted (involving particular functional groups on different building blocks) according to which active site of the catalyst is revealed (e.g. Fig. 4).
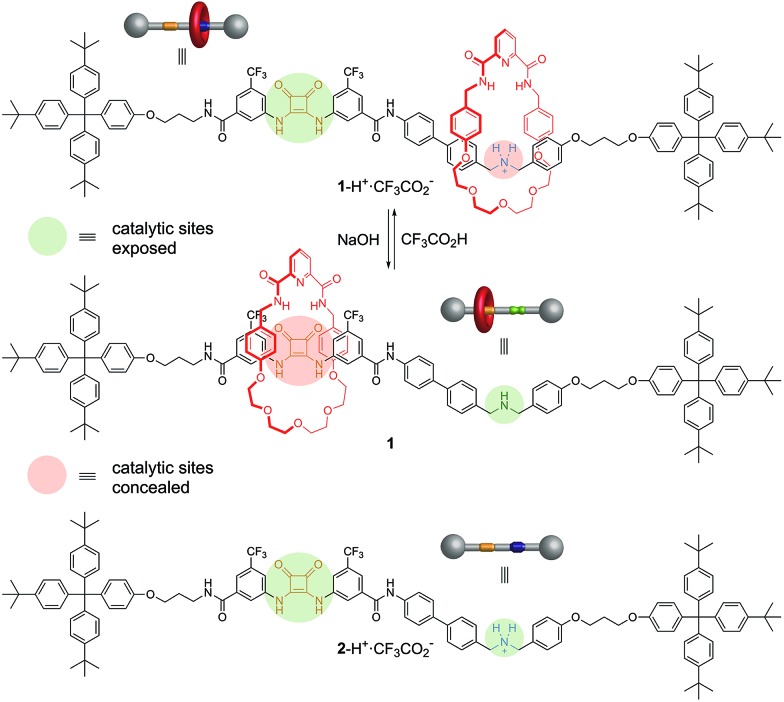
The switchable catalyst employed is a [2]rotaxane in which the position of the macrocycle can be changed5 to block one or other of two organocatalytic active sites.2,6 The rotaxane (1/1-H+·CF3CO2 –, Fig. 2) features a thread bearing dibenzylamine/dibenzylammonium and squaramide units as the catalytic centres. The activities of the organocatalytic sites are based on different activation mechanisms: the secondary amine/ammonium unit is able2 to promote iminium7 (and potentially enamine8 and trienamine9) catalysis while squaramide-catalyzed reactions proceed through the activation of electrophiles by hydrogen bonding.10 The macrocycle of the rotaxane contains a pyridyl-2,6-dicarboxyamide unit that can bind effectively to the squaramide residue, and a crown ether-like region that has a very high affinity for secondary ammonium ions but not for non-protonated amines.11 A rigid spacer was introduced between the two active sites on the thread to prevent folding. Accordingly, when the rotaxane is protonated (1-H+·CF3CO2 –) the macrocycle should preferentially encapsulate the dibenzylammonium group, masking it from being available for catalysis (iminium catalysis ‘off’) while leaving the squaramide site accessible (hydrogen bond catalysis ‘on’). In the neutral form of the rotaxane (1) the squaramide should be the preferred binding site for the macrocycle, concealing it and making it unavailable for catalysis (hydrogen bond catalysis ‘off’) whilst leaving the secondary amine exposed (iminium catalysis ‘on’).12
Fig. 2. Acid–base control of the position of the macrocycle in rotaxane 1 (iminium catalysis ‘on’; hydrogen bond catalysis ‘off’)/1-H+·CF3CO2 – (iminium catalysis ‘off’; hydrogen bond catalysis ‘on’) and the structure of the corresponding thread 2-H+·CF3CO2 – (both iminium catalysis and hydrogen bond catalysis ‘on’).
Results and discussion
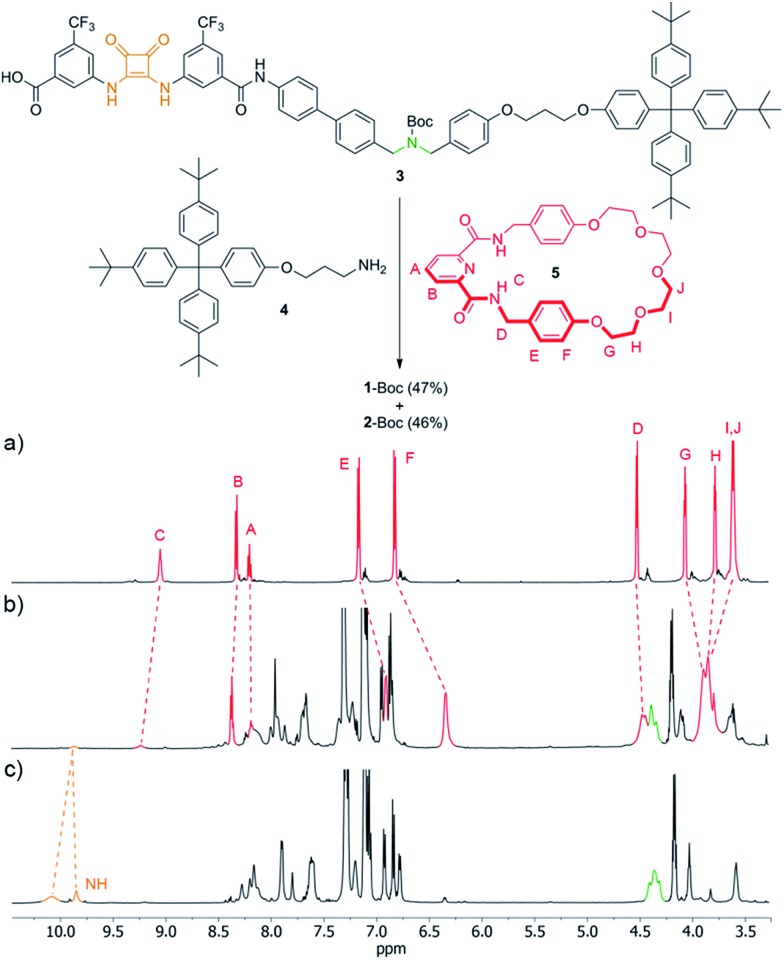
The synthesis of 1 utilized the intended pyridinedicarboxamide-squaramide recognition motif to promote the threading of a suitable squaramide derivative, 3, through the cavity of macrocycle 5, covalently capturing the interlocked structure through amide bond formation with bulky ‘stopper’ 4 (Fig. 3, see ESI† for details). [2]Rotaxane 1-Boc was isolated in 47% yield along with the non-interlocked thread (2-Boc, 46%).
Fig. 3. Hydrogen bond mediated assembly of [2]rotaxane 1-Boc and thread 2-Boc. Reagents and conditions: PyBroP, iPr2NEt, CH2Cl2 : THF : CH3CN (60 : 35 : 5), RT, 20 h. 1H NMR spectra (600 MHz, d 6-acetone, 293 K): (a) macrocycle 5; (b) [2]rotaxane 1-Boc; (c) thread 2-Boc.
The 1H NMR spectra (Fig. 3a–c) of the macrocycle (5), thread (2-Boc) and rotaxane (1-Boc) confirms the threaded architecture of 1-Boc with the macrocycle residing around the squaramide unit. The downfield shift of the HC amide protons in the rotaxane compared to the parent macrocycle (ΔδHC = 0.19 ppm) and the shifts of the protons on the central region of the polyether chain (ΔδHH = 0.08 ppm; ΔδHI,J = 0.25 ppm) indicate hydrogen bonding between the macrocycle and both sides, hydrogen bond donors and acceptors, of the thread squaramide unit. Protons of the phenyl rings of the macrocycle are shifted upfield in the rotaxane (ΔδHE = –0.26 ppm; ΔδHF = –0.48 ppm) due to shielding by the ring currents of the squaramide ring and aryl substituents.
Deprotection of the dibenzylamine moiety using trifluoroacetic acid afforded rotaxane 1-H+·CF3CO2 – (see ESI† for details). A solution of 1-H+·CF3CO2 – in CH2Cl2 was washed with NaOH(aq) (2 M) to produce 1, 1H NMR spectroscopy confirming the change of position of the macrocycle (see ESI†). Addition of CF3CO2H (1.4 equiv.) to 1 in CH2Cl2 smoothly regenerated 1-H+·CF3CO2 – (see ESI†).
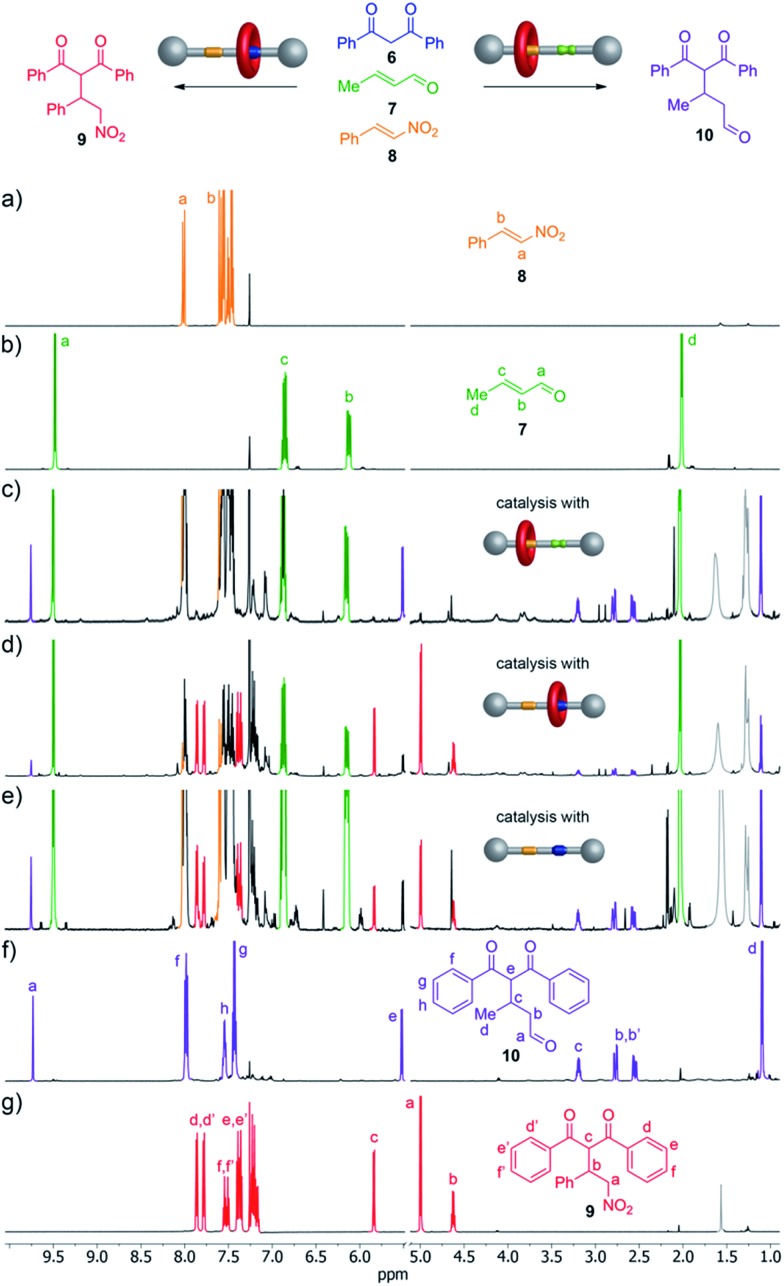
We investigated the ability of the rotaxane and the thread to perform organocatalytic reactions in both their protonated (1-H+·CF3CO2 –; 2-H+·CF3CO2 –) and unprotonated (1) states. Secondary amines can promote the Michael addition of 1,3-dicarbonyl nucleophiles to α,β-unsaturated aldehydes via iminium catalysis.13 When using a nitroalkene instead of the unsaturated aldehyde a similar Michael addition can occur if the electrophile is activated by hydrogen bond catalysts such as (thio)urea or squaramide derivatives.14 Accordingly, we reasoned that the rotaxane might be able to catalyse the Michael addition of 1,3-diphenylpropane-1,3-dione (6) selectively to either crotonaldehyde (7) or trans-β-nitrostyrene (8) according to which type of organocatalytic group was exposed on the thread.
A mixture of 6 (0.5 M), 7 and 8 in a 1 : 2 : 1 ratio, 10 mol% NaOAc15 and 5 mol% of the potential catalyst (1, 1-H+·CF3CO2 – or 2-H+·CF3CO2 –) was stirred in CH2Cl2 at room temperature (Fig. 4, top). Rotaxane 1 (secondary amine exposed) catalyzed the Michael addition of 6 to crotonaldehyde (7) to give 10 (40% conversion after 72 h) with high selectivity (only a trace of 9, the addition product to trans-β-nitrostyrene, present in the reaction mixture as evidenced by 1H NMR spectroscopy, Fig. 4c). Use of the protonated form of the rotaxane, 1-H+·CF3CO2 –, (squaramide exposed) resulted in the formation of 9 with a conversion of 75% after 18 h with only a few percent of 10 present in the reaction mixture (Fig. 4d).
Fig. 4. The Michael addition of 6 to crotonaldehyde (7) or trans-β-nitrostyrene (8) using rotaxanes 1, 1-H+·CF3CO2 – or thread 2-H+·CF3CO2 – as catalysts. Conditions: 5 mol% catalyst, 10 mol% NaOAc, 0.5 M 6 (1 equiv.), 7 (2 equiv), 8 (1 equiv.), RT, 18 h (1-H+·CF3CO2 –) or 72 h (1 or 2-H+·CF3CO2 –). 1H NMR spectra (600 MHz, CDCl3, 293 K): (a) trans-β-nitrostyrene (8); (b) crotonaldehyde (7); (c) reaction mixture of 6, 7 and 8 after 72 h in the presence of 1; (d) reaction mixture of 6, 7 and 8 after 18 h in the presence of 1-H+·CF3CO2 –; (e) reaction mixture of 6, 7 and 8 after 72 h in the presence of 2-H+·CF3CO2 –; (f) 10; (g) 9.
In contrast to the selectivity found with both forms of the rotaxane catalyst, when the thread 2-H+·CF3CO2 – was employed as the catalyst (both organocatalytic sites exposed) 9 and 10 were formed in a close-to-1 : 1 ratio (15% conversion after 72 h, Fig. 4e).
Conclusions
A rotaxane with two different organocatalytic sites, a squaramide unit and a dibenzylamine group, separated by a rigid spacer, has been demonstrated to promote Michael addition reactions through either iminium ion or hydrogen-bond-activated catalysis. The system can be switched between the two activation modes through acid–base-mediated control of the position of the rotaxane macrocycle to conceal one site on the thread and reveal the other. The switchable organocatalyst was used to promote the Michael addition of 1,3-diphenylpropan-1,3-dione (6) to either crotonaldehyde (7) or trans-β-nitrostyrene (8) according to the catalyst state, with modest conversions (40–75%) and good selectivity in both modes.
The ability to select which components of a mixture react together, affording different product outcomes from a common set of building blocks, is a promising use of artificial molecular machines in chemical synthesis.16
Acknowledgments
This research was funded by the EPSRC. We are grateful to the following organizations for postdoctoral fellowships: Fundacja na Rzecz Nauki Polskiej (to B.L.), Fonds Spécial de Recherche – Fédération Wallonie-Bruxelles and Wallonie-Bruxelles International (to G.D.B.), and the European Union 7th Framework Marie Curie Intra-European Fellowship Programme (to V.B.).
Footnotes
References
- For examples of catalysts that can be switched ‘on’ and ‘off’ by a specific stimulus, see: Würthner F., Rebek Jr J., Angew. Chem., Int. Ed. Engl., 1995, 34 , 446 Yoon H. J., Kuwabara J., Kim J.-H., Mirkin C. A., Science, 2010, 330 , 66, Sohtome Y., Tanaka S., Takada K., Yamaguchi T., Nagasawa K., Angew. Chem., Int. Ed., 2010, 49 , 9254, Berná J., Alajarín M., Orenes R.-A., J. Am. Chem. Soc., 2010, 132 , 10741, Zirngast M., Pump E., Leitgeb A., Albering J. H., Slugovc C., Chem. Commun., 2011, 47 , 2261, Berryman O. B., Sather A. C., Lledó A., Rebek Jr J., Angew. Chem., Int. Ed., 2011, 50 , 9400, Lüning U., Angew. Chem., Int. Ed., 2012, 51 , 8163, Neilson B. M., Bielawski C. W., J. Am. Chem. Soc., 2012, 134 , 12693, Schmittel M., De S., Pramanik S., Angew. Chem., Int. Ed., 2012, 51 , 3832, Schmittel M., Pramanik S., De S., Chem. Commun., 2012, 48 , 11730, Viehmann P., Hecht S., Beilstein J. Org. Chem., 2012, 8 , 1825, Wilson D., Branda N. R., Angew. Chem., Int. Ed., 2012, 51 , 5431, Neilson B. M., Bielawski C. W., Chem. Commun., 2013, 49 , 5453, Neilson B. M., Bielawski C. W., Organometallics, 2013, 32 , 3121, Neilson B. M., Bielawski C. W., ACS Catal., 2013, 3 , 1874, Osorio-Planes L., Rodríguez-Escrich C., Pericás M. A., Org. Lett., 2014, 16 , 1704, McGuirk C. M., Stern C. L., Mirkin C. A., J. Am. Chem. Soc., 2014, 136 , 4689 . [Google Scholar]
- For rotaxane-based secondary amine organocatalysts whose efficacy can be turned ‘on’ and ‘off’ by acid–base switching of the position of the macrocycle, see: Blanco V., Carlone A., Hänni K. D., Leigh D. A., Lewandowski B., Angew. Chem., Int. Ed., 2012, 51 , 5166 Blanco V., Leigh D. A., Marcos V., Morales-Serna J. A., Nussbaumer A. L., J. Am. Chem. Soc., 2014, 136 , 4905, Blanco V., Leigh D. A., Lewandowska U., Lewandowski B., Marcos V., J. Am. Chem. Soc., 2014, 136 , 15775 . [Google Scholar]
- Wang J., Feringa B. L., Mortezaei S., Catarineu N. R., Canary J. W., Vlatkovic M., Bernardi L., Otten E., Feringa B. L. Science. J. Am. Chem. Soc. Chem. Commun. 2011;2012;2014;33113450:1429. 8054, 7773. [Google Scholar]
- Lelais G., MacMillan D. W. C., Erkkilä A., Majander I., Pihko P. M., Melchiorre P., Marigo M., Carlone A., Bartoli G., Bertelsen S., Jørgensen K. A. Aldrichimica Acta. Chem. Rev. Angew. Chem., Int. Ed. Chem. Soc. Rev. 2006;2007;2008;2009;391074738:79. 5416, 6138, 2178. [Google Scholar]
- Kay E. R., Leigh D. A., Zerbetto F., Angew. Chem., Int. Ed., 2007, 46 , 72 , For examples of rotaxane-based molecular machines performing other useful tasks, see: Collier C. P., Wong E. W., Belohradský M., Raymo F. M., Stoddart J. F., Kuekes P. J., Williams R. S., Heath J. R., Science, 1999, 285 , 391, Pérez E. M., Dryden D. T. F., Leigh D. A., Teobaldi G., Zerbetto F., J. Am. Chem. Soc., 2004, 126 , 12210, Wang Q.-C., Qu D.-H., Ren J., Chen K., Tian H., Angew. Chem., Int. Ed., 2004, 43 , 2661, Leigh D. A., Morales M. Á. F., Pérez E. M., Wong J. K. Y., Saiz C. G., Slawin A. M. Z., Carmichael A. J., Haddleton D. M., Brouwer A. M., Buma W. J., Wurpel G. W. H., León S., Zerbetto F., Angew. Chem., Int. Ed., 2005, 44 , 3062, Berná J., Leigh D. A., Lubomska M., Mendoza S. M., Pérez E. M., Rudolf P., Teobaldi G., Zerbetto F., Nat. Mater., 2005, 4 , 704, Nguyen T. D., Tseng H.-R., Celestre P. C., Flood A. H., Liu Y., Stoddart J. F., Zink J. I., Proc. Natl. Acad. Sci. U. S. A., 2005, 102 , 10029, Huang Y.-L., Hung W.-C., Lai C.-C., Liu Y.-H., Peng S.-M., Chiu S.-H., Angew. Chem., Int. Ed., 2007, 46 , 6629, Zhou W., Li J., He X., Li C., Lv J., Li Y., Wang S., Liu H., Zhu D., Chem.–Eur. J., 2008, 14 , 754, Fernandes A., Viterisi A., Coutrot F., Potok S., Leigh D. A., Aucagne V., Papot S., Angew. Chem., Int. Ed., 2009, 48 , 6443, Gassensmith J. J., Matthys S., Lee J.-J., Wojcik A., Kamat P., Smith B. D., Chem.–Eur. J., 2010, 16 , 2916, Lussis P., Svaldo-Lanero T., Bertocco A., Fustin C.-A., Leigh D. A., Duwez A.-S., Nat. Nanotechnol., 2011, 6 , 553, Leontiev A. V., Jemmett C. A., Beer P. D., Chem.–Eur. J., 2011, 17 , 816, Serpell C. J., Chall R., Thompson A. L., Beer P. D., Dalton Trans., 2011, 40 , 12052, Fernandes A., Viterisi A., Aucagne V., Leigh D. A., Papot S., Chem. Commun., 2012, 48 , 2083, Avellini T., Li H., Coskun A., Barin G., Trabolsi A., Basuray A. N., Dey S. K., Credi A., Silvi S., Stoddart J. F., Venturi M., Angew. Chem., Int. Ed., 2012, 51 , 1611 . [Google Scholar]
- For rotaxanes incorporating catalytic centers, see: Thordarson P., Bijsterveld E. J., Rowan A. E., Nolte R. J., Nature, 2003, 424 , 915 Tachibana Y., Kihara N., Takata T., J. Am. Chem. Soc., 2004, 126 , 3438, Hattori G., Hori T., Miyake Y., Nishibayashi Y., J. Am. Chem. Soc., 2007, 129 , 12930, Li Y., Feng Y., He Y.-M., Chen F., Pan J., Fan Q.-H., Tetrahedron Lett., 2008, 49 , 2878, Miyagawa N., Watanabe M., Matsuyama T., Koyama Y., Moriuchi T., Hirao T., Furusho Y., Takata T., Chem. Commun., 2010, 46 , 1920, Suzaki Y., Shimada K., Chihara E., Saito T., Tsuchido Y., Osakada K., Org. Lett., 2011, 13 , 3774, Leigh D. A., Marcos V., Wilson M. R., ACS Catal., 2014, 4 , 4490 .12931181 [Google Scholar]
- Tsogoeva S. B., Almasi D., Alonso D. A., Nájera C., Vicario J. L., Badía D., Carrillo L., Erkkilä A., Majander I., Pihko P. M., Shimizu M., Hachiya I., Mizota I. Eur. J. Org. Chem. Tetrahedron: Asymmetry. Synthesis. Chem. Rev. Chem. Commun. 2007;2007;2007;2007;2009;18107:1701. 299, 2065, 5416, 874. [Google Scholar]
- Notz W., Tanaka F., Barbas III C. F., Mukherjee S., Yang J. W., Hoffmann S., List B., Guillena G., Nájera C., Ramon D. J., Kano T., Maruoka K., Deng D., Kumar S., Wang H., Desmarchelier A., Coeffard V., Moreau X., Greck C. Acc. Chem. Res. Chem. Rev. Tetrahedron: Asymmetry. Chem. Sci. Chem. Commun. Tetrahedron. 2004;2007;2007;2013;2014;2014;371071845070:580. 5471, 2249, 907, 4272, 2491. [Google Scholar]
- Jiang H., Albrecht Ł., Jørgensen K. A., Kumar I., Ramaraju P., Mir N. A., Jurberg I. D., Chatterjee I., Tanert R., Melchiorre P. Chem. Sci. Org. Biomol. Chem. Chem. Commun. 2013;2013;2013;41149:2287. 709, 4869. [Google Scholar]
- Malerich J. P., Hagihara K., Rawal V. H., Zhu Y., Malerich J. P., Rawal V. H., Alemán J., Parra A., Jiang H., Jørgensen K. A. J. Am. Chem. Soc. Angew. Chem., Int. Ed. Chem.–Eur. J. 2008;2010;2011;1304917:14416. 153, 6890. doi: 10.1021/ja805693p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh D. A., Thomson A. R., Org. Lett., 2006, 8 , 5377 , For other host–guest complexes and rotaxanes that employ this or similar motifs, see: Huang L., Hung W.-C., Lai C.-C., Liu Y.-H., Peng S.-M., Chiu S.-H., Angew. Chem., Int. Ed., 2007, 46 , 6629, Hsueh S.-Y., Kuo C.-T., Lu T.-W., Lai C.-C., Liu Y.-H., Hsu H.-F., Peng S.-M., Chen C.-h., Chiu S.-H., Angew. Chem., Int. Ed., 2010, 49 , 9170, Liu L., Liu Y., Liu P., Wu J., Guan Y., Hu X., Lin C., Yang Y., Sun X., Ma J., Wang L., Chem. Sci., 2013, 4 , 1701 .17078722 [Google Scholar]
- The acid–base switching of the position of a related macrocycle between dibenzylamine/ammonium motifs and amide-based functional groups in a rotaxane has previously been described (ref. 11)
- Brandau S., Landa A., Franzén J., Marigo M., Jørgensen K. A., Carlone A., Marigo M., North C., Landa A., Jørgensen K. A., Carlone A., Cabrera S., Marigo M., Jørgensen K. A., Lathrop S. P., Rovis T. Angew. Chem., Int. Ed. Chem. Commun. Angew. Chem., Int. Ed. J. Am. Chem. Soc. 2006;2006;2007;2009;4546131:4305. 4928, 1101, 13628. [Google Scholar]
- Okino T., Hoashi Y., Furukawa T., Xu X., Takemoto Y., Wang J., Li H., Duan W., Zu L., Wang W., Connon S. J., Zhang Z.-H., Dong X.-Q., Chen D., Wang C.-J., Zhang Z., Schreiner P. R., Storer R. I., Aciro C., Jones L. H. J. Am. Chem. Soc. Org. Lett. Chem.–Eur. J. Chem.–Eur. J. Chem. Soc. Rev. Chem. Soc. Rev. 2005;2005;2006;2008;2009;2011;127715143840:119. 4713, 5418, 8780, 1187, 2330. [Google Scholar]
- Sodium acetate is used as a base to activate the nucleophile
- Lewandowski B., De Bo G., Ward J. W., Papmeyer M., Kuschel S., Aldegunde M. J., Gramlich P. M. E., Heckmann D., Goldup S. M., D'Souza D. M., Fernandes A. E., Leigh D. A., De Bo G., Kuschel S., Leigh D. A., Lewandowski B., Papmeyer M., Ward J. W. Science. J. Am. Chem. Soc. 2013;2014;339136:189. 5811. doi: 10.1126/science.1229753. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.