Abstract
In this study, we aimed to investigate the influence of ACTN3 R577X gene polymorphism on muscle damage responses in athletes competing in an ultra-endurance race. Twenty moderate to well-trained ultra-runners who had entered in an official 37.1 km adventure race (22.1 km mountain biking, 10.9 km trekking, 4.1 km water trekking, 30 m rope course, and orienteering) volunteered for the study. Blood samples were collected for genotyping and analysis of muscle protein levels before and after the race. Percentage changes (pre- to post-race) of serum myoglobin [XX = 5,377% vs. RX/RR = 1,666%; P = 0.005, effect size (ES) = 1.73], creatine kinase (XX = 836.5% vs. RX/RR = 455%; P = 0.04, ES = 1.29), lactate dehydrogenase (XX = 82% vs. RX/RR = 65%; P = 0.002, ES = 1.61), and aspartate aminotransferase (XX = 148% vs. RX/RR = 75%; P = 0.02, ES = 1.77) were significantly greater for XX than RX/RR genotypes. ES analysis confirmed a large magnitude of muscle damage in XX genotype ultra-runners. Therefore, athletes with the ACTN3 577XX genotype experienced more muscle damage after an adventure race. This suggests that ultra-runners with alpha-actinin-3 deficiency may be more susceptible to rhabdomyolysis and associated health complications during ultra-endurance competitions.
Keywords: Muscle damage, Ultra-endurance, Adventure race, Genetics, ACTN3
INTRODUCTION
Adventure racing (AR) is an ultra-endurance sport that is growing in popularity. The competitions vary from 6 h to 10 days and comprise multiple outdoor sports disciplines, including mountain biking, trekking, kayaking, and roped ascent/descent. The races are held in natural environments and involve map reading and navigation. Races are thus fairly demanding and impose substantial physiological stress on athletes [1–4]. Here it is hypothesized that genetic polymorphism can influence the magnitude of muscle damage in ultra-runners after an AR competition.
It is well documented that ultra-endurance practice results in pronounced muscle damage [5–12], possibly as a result of mechanical disruption of the fibre, disturbances in calcium homeostasis and inflammatory processes [13, 14]. Muscle protein serum level is a common measure of muscle damage [15, 16]. Some investigations report increased muscle protein serum levels during AR events [3, 4, 17].
In most cases, muscle damage is unrelated to serious health complications. However, excessive muscle damage can cause the release of significant amounts of intracellular components into the blood stream, leading to rhabdomyolysis. Dramatic elevations in blood myoglobin levels can cause renal impairment, mainly in conditions of heat stress and dehydration [15, 16, 18].
Previous studies have demonstrated that ACTN3 R577X (rs1815739) gene polymorphism (more specifically, the XX genotype) influences the magnitude of muscle damage after eccentric exercise. Higher muscle protein serum level and higher pain scores were observed in polymorphism carriers than noncarriers after an eccentric training session [19, 20]. Consistent with this, Seto et al. [21] observed increased force deficits in ACTN3 knockout mouse muscles subjected to eccentric contractions, mimicking the XX genotype in humans.
The ACTN3 gene encodes for the alpha-actinin-3 protein, and a common stop codon polymorphism in this gene (C→T transition at position 1747 in exon 16; SNP ID: rs1815739; 11q13-q14) causes a complete deficiency of the protein in XX homozygotes, which is present in approximately 20% of the population worldwide. This deficiency does not cause muscle disease, but results in differences in skeletal muscle function, including lower muscle strength [22, 23].
Alpha-actinin-3 is one of the major components of the Z-disk in type II muscle fibres and is responsible for crosslink and anchor actin filaments. Therefore, as Z-disks are the structures most vulnerable to muscle damage, it is plausible that alpha-actinin-3 deficiency can interfere with the structural integrity of sarcomeres and muscle cells during muscle contractions. In line with this, changes in composition and interaction of Z-disk proteins observed in ACTN3 knockout muscles provide a possible explanation for the greater susceptibility to muscle damage in XX individuals [21, 24].
Based on the above statements, we hypothesized that ultra-runners with alpha-actinin-3 deficiency can experience more muscle damage during an ultra-endurance competition. Therefore, this study aimed to investigate the influence of ACTN3 R577X (rs1815739) gene polymorphism on muscle damage responses in athletes competing in an official AR competition.
MATERIALS AND METHODS
Participants
Twenty athletes from ten teams (5 females and 15 males) were studied during participation in an official AR. Volunteers were moderately to well trained, experienced in long distance sports, had a minimum of two years of AR experience, reported an absence of musculoskeletal injury, and completed the race without or with minimal navigation errors. This study was approved by the local research ethics committee (no. 95/2015). All participants were informed of the research procedures and purposes of the investigation and gave their written consent prior to participation.
Race description
This study was conducted during the fourth race of the 2015 Haka Race Series, during the second weekend of November, in the city of Passa Quatro (Minas Gerais, Brazil). The AR started at 9:00 am and covered a total of 37.1 km, consisting of 22.1 km of mountain biking, 10.9 km of trekking, 4.1 km of water trekking, and a 30 m rope course, performed in a variety of terrains (off-road trails, watercourses, coast, mountains, and paths) by orienteering. Sixty-six athletes, including our volunteers, completed this competition, and their race time ranged from 6 h and 5 min to 12 h and 30 min. The weather was clear, with temperatures ranging between 22°C and 31°C on race day.
Haka Race is an annual series of four adventure races included in the Brazilian Ranking of ARs. Competitions range from 35 to 50 km, and athletes of both sexes may participate as individuals (solo) or in teams (pairs or quartets). The route is only revealed the day before or the day of the start when the participants receive the topographic map of the region and the race book with the geographic coordination of each mandatory stop between start and finish. The team must pass all mandatory stops to cross the finish line as an official finisher. Failure to fulfil the competition requirements results in penalties and elimination.
Research design
Volunteers were evaluated before and after the race. Pre-race measurements were performed in the afternoon before the start of the race at 4 h postprandial, whereas post-race evaluation occurred immediately after the race. Athletes completed a questionnaire on basic demographic and characteristics of training, answered the International Physical Activity Questionnaire (IPAQ), and underwent body composition evaluation before the race. Blood samples were collected before and after the race. Additionally, athletes were asked to rate their level of perceived exertion after the competition. The race time was recorded by official race reports.
International Physical Activity Questionnaire
The short version of the IPAQ was explained to volunteers, and one researcher remained near to them during completion, in order to be available to answer any questions. The questionnaire aimed to determine the physical activity level of athletes, calculated assuming metabolic equivalents of 3.3, 4.0, and 8.0 METs for low, moderate, and intense activities, respectively. Moderate activity level represents achieving a minimum of at least 600 MET-min/week, while high activity level represents achieving a minimum of at least 3,000 MET-min/week [25].
Body composition
Volunteers fulfilled a pre-test protocol for body composition evaluation, including: a) a 4-h postprandial condition, b) not drinking water 3 h before the test, c) not using medication that interferes with body water homeostasis 48 h before the test, d) abstaining from vigorous physical exercise 24 h before the test, e) going to the bathroom at least 30 min before the test, and f) not wearing metal accessories during the test. All evaluations were conducted on athletes without shoes/socks and wearing light clothes. Height was measured using a calibrated stadiometer (Filizzola, Brazil) and body composition was estimated using bioelectrical impedance analysis (BIA) (Inbody 120; Biospace Co., Seoul, Korea).
Blood sampling
Each time, 9 mL of whole blood was obtained from an antecubital vein by a certified nurse and aliquoted into individual vacutainers containing a coagulation enhancer and splitting gel (5 mL) or EDTA (4 mL) (Vacuette, Greiner Bio-One). Five-millilitre blood samples were immediately centrifuged (3000 rpm for 10 minutes) (Baby I - 206 BL - Fanem) and serum aliquoted into Eppendorf tubes. Then, all tubes were stored and shipped on dry ice. Specimens were placed in a -80°C ultralow freezer at the laboratory until analysis.
Genotyping
Genomic DNA was isolated from the buffy coat of centrifuged whole blood, previously collected in EDTA tubes, using the QIAamp DNA Blood Mini Kit (Qiagen) according to the manufacturer’s instructions. Genotyping of ACTN3 R577X (rs1815739) polymorphism was conducted using a TaqMan SNP Genotyping Assay (Assay ID: C___590093_1_; Applied Biosystems, Foster City, CA, USA), and the reaction was performed in an Applied Biosystems 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The genotyping mixture (total of 20 μL) contained 10 μl of TaqMan Universal Master Mix, 1 μl of assay mix, 2 μL of genomic DNA (10 ng/μL) and 7 μL of ultra-pure water for each reaction. The results were analyzed using TaqMan Genotyper Software (Applied Biosystems, Foster City, CA, USA). The genotyping success rate was 100%, and no discordant genotypes were observed in duplicate samples tested.
Measurements of muscle damage proteins
Serum myoglobin (Mb) levels were measured via chemiluminescent microparticle immunoassay (Abbott–Architect STAT Myoglobin kit; Architect system – Abbott Diagnostics, IL, USA). The reference range for serum Mb using this method is values lower than 155 ng/mL. Serum creatine kinase (CK), lactate dehydrogenase (LDH), and aspartate aminotransferase (AST) concentrations were measured using a semi-automated clinical analyzer (BioClin kits; BioClin 100 analyzer, Belo Horizonte, Brazil). The reference range for serum CK, LDH, and AST using this method is 24–195 U/L, 200–480 U/L, and 10–39 U/L, respectively.
Perception exertion
The rating of perceived exertion was measured on the RPE-Borg Scale, which ranges from 6 to 20, where six means “no exertion at all,” and 20 means “maximal exertion” [26]. The volunteers were instructed to report effort experienced during the competition, indicating a value in the Borg Scale, immediately after the race.
Statistical analysis
The Hardy-Weinberg equilibrium was evaluated by the χ2 test. Normal distribution and homogeneity of the data were verified by the Shapiro-Wilk test and Bartlett’s test, respectively. Then, the Mann-Whitney U-test was used to compare the data between XX and RX/RR genotypes (recessive model). Effect size (ES) was also calculated to determine the magnitude of the difference between comparisons. The threshold adopted was: trivial (0–0.19), small (0.20–0.49), medium (0.50–0.79), and large (0.80 and greater). Statistical significance was set at P < 0.05. Data were expressed as the median and 25th–75th interquartile range. Statistical procedures were carried out using GraphPad Prism 6 and GPOWER 3.1 software.
RESULTS
The prevalence of the different ACTN3 genotypes was RR 35%, RX 45%, and XX 20%. This corresponds to the R and X allele frequencies of 57.5 and 42.5%, respectively, and is in Hardy-Weinberg equilibrium (χ2 = 0.12; P = 0.72). Physical characteristics, body composition, and training status of athletes were similar (P > 0.05) between XX and RX/RR genotypes, as presented in Table I. Moreover, there was no difference in sex distribution between genotypes, with an identical frequency of females (25%) in XX and RX/RR carriers. The R and X alleles frequencies were also similar between females (60% and 40%, respectively) and males (57 and 43%, respectively).
TABLE 1.
Physical characteristics, body composition and training status in XX and RX/RR genotypes.
| XX (n=4) | RX/RR (n=16) | P value | |
|---|---|---|---|
| Age (years) | 44.0 (36.2 – 45.0) | 37.5 (31.5 – 42.7) | 0.15 |
| Height (cm) | 170.0 (159.8 – 178.0) | 171.5 (166.8 – 176.8) | 0.66 |
| Body weight (kg) | 70.4 (58.9 – 82.3) | 71.6 (64.5 – 80.0) | 0.81 |
| Body mass index (kg/m2) | 24.3 (23.0 – 25.9) | 25.1 (22.8 – 26.3) | 0.89 |
| Fat free mass (kg) | 33.1 (25.5 – 37.9) | 35.0 (30.8 – 38.2) | 0.73 |
| Fat mass (kg) | 12.8 (11.4 – 16.6) | 11.8 (8.5 – 15.6) | 0.58 |
| Fat mass (%) | 20.1 (15.8 – 22.2) | 17.8 (12.0– 21.7) | 0.53 |
| AR experience (years) | 3.5 (2.0 – 5.0) | 5.0 (2.0 – 8.0) | 0.39 |
| IPAQ (METs) | 4,310 (3,978 – 5,774) | 3,172 (2,072 – 4,288) | 0.12 |
| Running volume (km/week) | 10 (6 – 50) | 15 (10 – 24) | 0.81 |
| Bike volume (km/week) | 50.0 (30.0 – 100.0) | 55.0 (28.5 – 71.2) | 0.88 |
Note: AR=Adventure race; IPAQ= International Physical Activity Questionnaire. Data are expressed as Median (25th – 75th inter-quartile).
Regarding adventure race performance, race time was similar (P = 0.33) between genotype groups: 9 h and 50 min (9 h and 25 min–10 h and 14 min) and 9 h and 16 min (8 h and 54 min–10 h and 2 min) for XX and RX/RR carriers, respectively. Furthermore, perceived exertion was also similar (P = 0.99) between genotype groups during the race: 13.50 (9.75–16.50) and 13.00 (12.00–15.00) for XX and RX/RR genotypes, respectively.
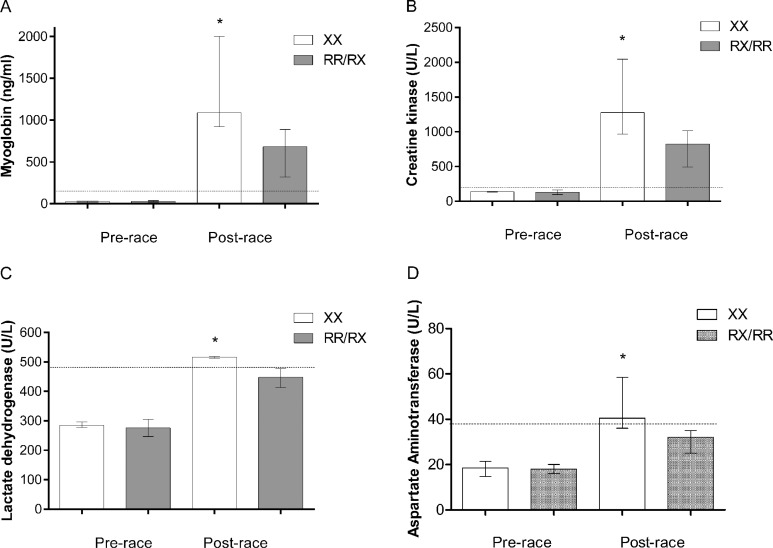
Serum concentrations of Mb, CK, LDH, and AST were similar (P > 0.05) for the two genotype groups before the race, and within the reference range. However, XX genotype presented significantly higher median values after the race for Mb (XX = 1089.0 vs. RX/RR= 681.9 ng/mL; P=0.007, ES= 1.44), CK (XX = 1,278.0 vs. RX/RR = 824.5 U/L; P= 0.01, ES=1.32), LDH (XX = 516.5 vs. RX/RR = 448.0 U/L; P= 0.02, ES= 0.64), and AST levels (XX= 40.5 vs. RX/RR = 32 U/L; P = 0.02, ES = 1.09) compared to RX/RR genotypes, as illustrated in Figure 1.
FIG. 1.
Serum concentrations of (A) myoglobin; (B) creatine kinase, (C) lactate dehydrogenase and (D) aspartate aminotransferase of athletes with XX and RX/RR genotypes for ACTN3 gene before and after the race, expressed as the median and 25th–75th interquartile range. Dotted lines delimit the upper value of the reference range of the studied variables. *Significant difference (P<0.05) compared to RR/RX genotypes after the race.
Percentage changes (pre- to post-race) of serum Mb (XX = 5,377% vs. RX/RR = 1,666%; P = 0.005, ES = 1.73), CK (XX = 836.5% vs. RX/RR = 455%; P = 0.04, ES = 1.29), LDH (XX = 82% vs. RX/RR = 65%; P = 0.002, ES=1.61), and AST (XX = 148% vs. RX/RR=75%; P = 0.02, ES = 1.77) were also significantly greater for XX than RX/RR genotypes.
DISCUSSION
This pilot study aimed to investigate the influence of ACTN3 R577X (rs1815739) gene polymorphism on muscle damage responses in athletes competing in an official AR competition. Our main findings indicate that XX genotype athletes had higher serum Mb, CK, LDH, and AST levels. The hypothesis that ultra-runners with XX genotype are more susceptible to muscle damage during an ultra-endurance competition is thus accepted, making a unique contribution to the literature.
The structural differences in ACTN3-XX fibres seem to be the mechanism of this function. The existing literature suggests that alpha-actinin-3 deficiency causes up-regulation of and variation in the interaction of a diverse network of Z-line proteins such as α-actinin-2, myotilin, desmin, αβ-crystallin, γ-filamin and ZASP in the ACTN3 knockout muscles. Changes in overall protein composition of Z-line proteins thus interfere with the elastic properties of muscle fibres, augmenting susceptibility to contraction-induced muscle damage in XX individuals [21, 24].
Ultra-runners with XX genotype were similar in physical characteristics, training status, and race performance to RX/RR carriers, which are among the factors that could also impact on the magnitude of exercise-induced muscle damage [15, 16]. Thus, our main findings were not influenced by these aspects. Furthermore, despite evidence that XX genotype can be advantageous for prolonged exercise [27–29], our results of race time and perceived exertion are consistent with some studies that found similar performance between athletes with alpha-actinin-3 deficiency and their counterparts during ultra-endurance competitions (i.e., races performed for duration longer than four hours) [30, 31].
Changes in serum Mb, CK, LDH, and AST were 3.2, 1.8, 1.3, and 1.9 times higher for XX genotype than RX/RR genotypes after an adventure sprint race (approximately 9.5 h of race time), and ES analysis confirmed a large magnitude of muscle damage in XX genotype ultra-runners in the present investigation. Participants with alpha-actinin-3 deficiency had augmented Mb levels, three-to-five times higher than those in 20 ultra-runners (three females; 17 males) after an adventure expedition race (approximately 55 h of race time) [4].
The results presented here expand on a limited number of previous investigations showing that individuals with XX genotype may be more susceptible to muscle damage during exercise. Pimenta et al. [19] concur, reporting that soccer athletes with XX genotype have higher levels of serum CK activity and alpha-actin levels than RR carriers, after a training session with intermittent circuit exercises at maximal intensity. Moreover, Vincent et al. [20] reported that serum CK activity tended to be higher in XX genotype than RR genotype individuals, after an eccentric exercise bout composed of multiple series of knee extensions. Corroborating these findings, in a study of knockout mice for alpha-actinin-3 protein, Seto et al. [21] confirmed that mutant mice exhibited greater force deficits after eccentric contraction, suggesting higher susceptibility to muscle damage.
Other studies report contradictory results and no association effect. Venckunas et al. [32] found that RR genotype individuals had a greater voluntary force decrement and slower recovery than XX genotype, after a drop jump exercise session (40 cm x 50 repetitions), with no difference for plasma CK activity. Clarkson et al. [18] found no association between ACTN3 R577X (rs1815739) gene polymorphism and increases in CK and Mb levels, after 50 maximal eccentric contractions of the elbow flexor muscles. These contradictory results may be explained as a result of the heterogeneity of the population studies and the training status, since neither study investigated well-trained individuals [18, 32]. Other gene polymorphisms have also been associated with muscle damage [33–35], and the interaction effect between two or more gene polymorphisms on muscle damage susceptibility can better clarify this response.
CONCLUSIONS
In conclusion, the initial results of this pilot study strongly suggest pronounced muscle damage in athletes with ACTN3 577XX genotype competing in an AR. This supports the concept that an important gene variation of ACTN3 locus contributes to susceptibility to exercise-induced muscle damage. Although further research is required in larger population groups, the findings presented here are novel and clinically relevant. It suggests that ultra-runners with alpha-actinin-3 deficiency may be more susceptible to rhabdomyolysis and associated health complications during ultra-endurance competitions. Furthermore, polymorphism carriers may also require a longer time for recovery than noncarriers after a race.
Acknowledgements
We would like to thank volunteers, staffs and Haka Race director for their essential contribution to this study. This work was supported by the Fundo de Apoio ao Ensino, à Pesquisa e Extensão (FAEPEX - UNICAMP) (no.1152/14) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (PNPD20131967).
Conflict of interests
The authors declared no conflict of interests regarding the publication of this manuscript.
REFERENCES
- 1.Levada-Pires AC, Fonseca CE, Hatanaka E, Alba-Loureiro T, D’Angelo A, Velhote FB, et al. The effect of an adventure race on lymphocyte and neutrophil death. Eur J Appl Physiol. 2010;109:447–53. doi: 10.1007/s00421-010-1363-4. [DOI] [PubMed] [Google Scholar]
- 2.Lucas SJ, Anglem N, Roberts WS, Anson JG, Palmer CD, Walker RJ, et al. Intensity and physiological strain of competitive ultra-endurance exercise in humans. J Sports Sci. 2008;26:477–89. doi: 10.1080/02640410701552872. [DOI] [PubMed] [Google Scholar]
- 3.Tossige-Gomes R, Ottone VO, Oliveira PN, Viana DJ, Araujo TL, Gripp FJ, et al. Leukocytosis, muscle damage and increased lymphocyte proliferative response after an adventure sprint race. Braz J Med Biol Res. 2014;47:492–8. doi: 10.1590/1414-431X20143187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wichardt E, Mattsson CM, Ekblom B, Henriksson-Larsen K. Rhabdomyolysis/myoglobinemia and NSAID during 48 h ultra-endurance exercise (adventure racing) Eur J Appl Physiol. 2011;111:1541–4. doi: 10.1007/s00421-010-1774-2. [DOI] [PubMed] [Google Scholar]
- 5.Baur DA, Bach CW, Hyder WJ, Ormsbee MJ. Fluid retention, muscle damage, and altered body composition at the Ultraman triathlon. Eur J Appl Physiol. 2016;116:447–58. doi: 10.1007/s00421-015-3291-9. [DOI] [PubMed] [Google Scholar]
- 6.Del Coso J, Gonzalez-Millan C, Salinero JJ, Abian-Vicen J, Soriano L, Garde S, et al. Muscle damage and its relationship with muscle fatigue during a half-iron triathlon. PLoS One. 2012;7:e43280. doi: 10.1371/journal.pone.0043280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nieman DC, Dumke CL, Henson DA, McAnulty SR, Gross SJ, Lind RH. Muscle damage is linked to cytokine changes following a 160-km race. Brain Behav Immun. 2005;19:398–403. doi: 10.1016/j.bbi.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Nieman DC, Henson DA, Dumke CL, Oley K, McAnulty SR, Davis JM, et al. Ibuprofen use, endotoxemia, inflammation, and plasma cytokines during ultramarathon competition. Brain Behav Immun. 2006;20:578–84. doi: 10.1016/j.bbi.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Skenderi KP, Kavouras SA, Anastasiou CA, Yiannakouris N, Matalas AL. Exertional Rhabdomyolysis during a 246-km continuous running race. Med Sci Sports Exerc. 2006;38:1054–7. doi: 10.1249/01.mss.0000222831.35897.5f. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki K, Peake J, Nosaka K, Okutsu M, Abbiss CR, Surriano R, et al. Changes in markers of muscle damage, inflammation and HSP70 after an Ironman Triathlon race. Eur J Appl Physiol. 2006;98:525–34. doi: 10.1007/s00421-006-0296-4. [DOI] [PubMed] [Google Scholar]
- 11.Waskiewicz Z, Klapcinska B, Sadowska-Krepa E, Czuba M, Kempa K, Kimsa E, et al. Acute metabolic responses to a 24-h ultra-marathon race in male amateur runners. Eur J Appl Physiol. 2012;112:1679–88. doi: 10.1007/s00421-011-2135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Son HJ, Lee YH, Chae JH, Kim CK. Creatine kinase isoenzyme activity during and after an ultra-distance (200 km) run. Biol Sport. 2015;32:357–61. doi: 10.5604/20831862.1163384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarkson PM, Hubal MJ. Exercise-induced muscle damage in humans. Am J Phys Med Rehabil. 2002;81:S52–69. doi: 10.1097/00002060-200211001-00007. [DOI] [PubMed] [Google Scholar]
- 14.Clarkson PM, Sayers SP. Etiology of exercise-induced muscle damage. Can J Appl Physiol. 1999;24:234–48. doi: 10.1139/h99-020. [DOI] [PubMed] [Google Scholar]
- 15.Banfi G, Colombini A, Lombardi G, Lubkowska A. Metabolic markers in sports medicine. Adv Clin Chem. 2012;56:1–54. doi: 10.1016/b978-0-12-394317-0.00015-7. [DOI] [PubMed] [Google Scholar]
- 16.Brancaccio P, Lippi G, Maffulli N. Biochemical markers of muscular damage. Clin Chem Lab Med. 2010;48:757–67. doi: 10.1515/CCLM.2010.179. [DOI] [PubMed] [Google Scholar]
- 17.Borgenvik M, Nordin M, Mikael Mattsson C, Enqvist JK, Blomstrand E, Ekblom B. Alterations in amino acid concentrations in the plasma and muscle in human subjects during 24 h of simulated adventure racing. Eur J Appl Physiol. 2012;112:3679–88. doi: 10.1007/s00421-012-2350-8. [DOI] [PubMed] [Google Scholar]
- 18.Clarkson PM, Hoffman EP, Zambraski E, Gordish-Dressman H, Kearns A, Hubal M, et al. ACTN3 and MLCK genotype associations with exertional muscle damage. J Appl Physiol (1985). 2005;99:564–9. doi: 10.1152/japplphysiol.00130.2005. [DOI] [PubMed] [Google Scholar]
- 19.Pimenta EM, Coelho DB, Cruz IR, Morandi RF, Veneroso CE, de Azambuja Pussieldi G, et al. The ACTN3 genotype in soccer players in response to acute eccentric training. Eur J Appl Physiol. 2012;112:1495–503. doi: 10.1007/s00421-011-2109-7. [DOI] [PubMed] [Google Scholar]
- 20.Vincent B, Windelinckx A, Nielens H, Ramaekers M, Van Leemputte M, Hespel P, et al. Protective role of alpha-actinin-3 in the response to an acute eccentric exercise bout. J Appl Physiol (1985). 2010;109:564–73. doi: 10.1152/japplphysiol.01007.2009. [DOI] [PubMed] [Google Scholar]
- 21.Seto JT, Lek M, Quinlan KG, Houweling PJ, Zheng XF, Garton F, et al. Deficiency of alpha-actinin-3 is associated with increased susceptibility to contraction-induced damage and skeletal muscle remodeling. Hum Mol Genet. 2011;20:2914–27. doi: 10.1093/hmg/ddr196. [DOI] [PubMed] [Google Scholar]
- 22.Clarkson PM, Devaney JM, Gordish-Dressman H, Thompson PD, Hubal MJ, Urso M, et al. ACTN3 genotype is associated with increases in muscle strength in response to resistance training in women. J Appl Physiol (1985). 2005;99:154–63. doi: 10.1152/japplphysiol.01139.2004. [DOI] [PubMed] [Google Scholar]
- 23.North KN, Yang N, Wattanasirichaigoon D, Mills M, Easteal S, Beggs AH. A common nonsense mutation results in alpha-actinin-3 deficiency in the general population. Nat Genet. 1999;21:353–4. doi: 10.1038/7675. [DOI] [PubMed] [Google Scholar]
- 24.Lee FXZ, Houweling PJ, North KN, Quinlan KGR. How does α-actinin-3 deficiency alter muscle function? Mechanistic insights into ACTN3, the ‘gene for speed’. Biochimica et Biophysica Acta. 2016;1863:686–93. doi: 10.1016/j.bbamcr.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 25.Fogelholm M, Malmberg J, Suni J, Santtila M, Kyrolainen H, Mantysaari M, et al. International Physical Activity Questionnaire: Validity against fitness. Med Sci Sports Exerc. 2006;38:753–60. doi: 10.1249/01.mss.0000194075.16960.20. [DOI] [PubMed] [Google Scholar]
- 26.Borg G. Borg’s Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics; 1998. [Google Scholar]
- 27.Ahmetov II, Fedotovskaya ON. Current Progress in Sports Genomics. Adv Clin Chem. 2015;70:247–314. doi: 10.1016/bs.acc.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Pasqua LA, Bueno S, Artioli GG, Lancha AH, Jr, Matsuda M, Marquezini MV, et al. Influence of ACTN3 R577X polymorphism on ventilatory thresholds related to endurance performance. J Sports Sci. 2016;34:163–70. doi: 10.1080/02640414.2015.1040823. [DOI] [PubMed] [Google Scholar]
- 29.Holdys J, Krysciak J, Stanislawski D, Gronek P. Polymorphism of the alpha-actn3 gene in individuals practising different sports disciplines. Biol Sport. 2011;28:101–6. [Google Scholar]
- 30.Grealy R, Herruer J, Smith CL, Hiller D, Haseler LJ, Griffiths LR. Evaluation of a 7-Gene Genetic Profile for Athletic Endurance Phenotype in Ironman Championship Triathletes. PLoS One. 2015;10:e0145171. doi: 10.1371/journal.pone.0145171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grealy R, Smith CL, Chen T, Hiller D, Haseler LJ, Griffiths LR. The genetics of endurance: frequency of the ACTN3 R577X variant in Ironman World Championship athletes. J Sci Med Sport. 2013;16:365–71. doi: 10.1016/j.jsams.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 32.Venckunas T, Skurvydas A, Brazaitis M, Kamandulis S, Snieckus A, Moran CN. Human alpha-actinin-3 genotype association with exercise-induced muscle damage and the repeated-bout effect. Appl Physiol Nutr Metab. 2012;37:1038–46. doi: 10.1139/h2012-087. [DOI] [PubMed] [Google Scholar]
- 33.Ahmetov II, Naumov VA, Donnikov AE, Maciejewska-Karlowska A, Kostryukova ES, Larin AK, et al. SOD2 gene polymorphism and muscle damage markers in elite athletes. Free Radic Res. 2014;48:948–55. doi: 10.3109/10715762.2014.928410. [DOI] [PubMed] [Google Scholar]
- 34.Hubal MJ, Devaney JM, Hoffman EP, Zambraski EJ, Gordish-Dressman H, Kearns AK, et al. CCL2 and CCR2 polymorphisms are associated with markers of exercise-induced skeletal muscle damage. J Appl Physiol (1985). 2010;108:1651–8. doi: 10.1152/japplphysiol.00361.2009. [DOI] [PubMed] [Google Scholar]
- 35.Yamin C, Duarte JA, Oliveira JM, Amir O, Sagiv M, Eynon N, et al. IL6 (-174) and TNFA (-308) promoter polymorphisms are associated with systemic creatine kinase response to eccentric exercise. Eur J Appl Physiol. 2008;104:579–86. doi: 10.1007/s00421-008-0728-4. [DOI] [PubMed] [Google Scholar]