Abstract
The aim of this study was to analyse the effectiveness of new haematology parameters related to reticulocytes and mature red blood cells to differentiate pre latent and latent iron deficiency. The study included 219 female athletes (aged 15-20 years) representing volleyball, handball, cycling, canoeing, cross-country skiing, swimming and judo. To assess iron status the concentration of ferritin, soluble transferrin receptor (sTfR), iron and total iron binding capacity (TIBC) were determined in serum. In addition to blood morphology, the mean cellular haemoglobin content in erythrocytes (CH) and reticulocytes (CHr), mean cellular haemoglobin concentration in reticulocytes (CHCMr), the percentage of erythrocytes (HYPOm) and reticulocytes (HYPOr) with decreased cellular haemoglobin concentration, the percentage of erythrocytes (LowCHm) and reticulocytes (LowCHr) with decreased cellular haemoglobin content, and percentage of erythrocytes with decreased volume (MICROm) were determined. Subjects with ferritin <30 ng/ml were classified as having stage I (pre-latent) iron deficiency (ID). The second stage (latent ID) was diagnosed when low ferritin was accompanied by elevated sTfR and/or elevated TIBC values. The frequency of ID (without anaemia symptoms) was high, amounting to 60% (stage I in 45%, stage II in 15% of subjects). In subjects with stage I ID significant changes in haematological variables concerned mainly reticulocytes: CHCMr (p<.001), CHr (p<.05), LowCHr (p<.05), HYPOr (p<.001) in comparison to normal iron stores. In athletes with latent ID, there were also significant changes (p<.001) in many indices of mature red blood cells, i.e. haemoglobin concentration (Hb), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC), CH, %LowCHm, as well as %MICROm (p<.01) in relation to the group without iron deficiency. The main finding of this study was that the diminished or exhausted iron stores had already caused changes in reticulocytes, and intensified iron deficiency (stage II) increased changes in both reticulocytes’ and erythrocytes’ hypochromia indices, while microcythaemia symptoms appeared later. This suggests that the markers of hypochromia relating especially to reticulocytes are useful for diagnosis of early ID in athletes with absence of an acute phase reaction.
Keywords: Female athletes, Iron deficiency, Hypochromia markers, Erythrocytes, Reticulocytes, Ferritin, sTfR, TIBC, Reticulocyte and erythrocyte indices, Athletes
INTRODUCTION
Iron deficiency is still common, especially among females of reproductive age [1]. Female athletes are considered to be at greater risk of compromised iron status than their inactive counterparts [2, 3, 4]. Iron status is the result of the balance between the rate of erythropoiesis and the size of the iron stores [5]. Negative iron balance in female athletes might be due to insufficient dietary iron intake [6], menstrual blood losses [7], increased iron losses via haemolysis, sweating, gastrointestinal bleeding, urinary blood losses [8] and increase of hepcidin concentration due to exercise induced by acute inflammation [9].
The gold standard for diagnosis of ID is bone marrow aspiration. However, this method is rarely used, and less invasive blood iron status indices, such as ferritin (Ferr), soluble transferrin receptor (sTfR), total iron binding capacity (TIBC) and iron concentration are used instead. Additionally, for screening iron deficiency anaemia, mature red blood cell indices are widely used. Despite the availability of many indicators of iron status, some biochemical parameters such as Ferr, TIBC, iron and hepcidin may change under the acute phase response [10] which makes assessment of iron status difficult. Problems with ID detection may also be encountered in athletes since physical exertion alone induces inflammatory states and affects some indicators of iron metabolism [11, 12, 13]. Blood haemoglobin concentration and other mature erythrocyte indices are a measure of iron deficiency anaemia, but due to the long lifespan of mature erythrocytes (120 days), the detection of latent and overt iron deficiency may be delayed. Additionally, the substantial changes in plasma volume often observed in athletes [14, 15] do not facilitate the proper evaluation of haematological status, falsely indicating for example iron deficiency anaemia.
So far, no consensus has been reached on the best definition of ID. Therefore the search is ongoing for new markers that could be more reliable in assessing iron status.
In recent years, the development of haematology analyzers has allowed for the implementation of new indicators of mature red blood cells and reticulocytes. Automated reticulocyte analysis by flow cytometry provides both the number of reticulocytes as well as several indices of them that can be helpful in the diagnosis of iron status [16]. These indicators are good in the detection of ID because reticulocytes have a short normal life span. They persist in the circulation for only 24-48 hours; therefore the haemoglobin in reticulocytes is a good early marker of iron deficiency [5, 17, 18]. The amount of haemoglobin in reticulocytes is expressed by mean cellular haemoglobin content in reticulocytes (CHr), which estimates the actual availability of iron to the erythron. Imbalance between the erythroid marrow iron requirement and the actual iron supply results in reduction of the CHr value, which leads to an increase in the percentage of immature red blood cells with reduced cellular haemoglobin concentration (%HYPOr) and with decreased mean cellular haemoglobin content in them (%LowCHr) [5]. Progression in development of iron deficiency induces similar changes in respective mature erythrocyte indices of hypochromia, i.e. in mean cellular haemoglobin (CH) and percentage of erythrocytes with reduced cellular haemoglobin concentration (%HYPO) and with decreased mean cellular haemoglobin content in erythrocytes (%LowCH).
Current results show that especially CHr and percentage of hypochromic erythrocytes together with biochemical markers are able to distinguish iron deficiency anaemia from anaemia of chronic diseases [19, 20]. It seems that these markers may also be useful in detection of iron deficiency in the two first stages, i.e. pre-latent and latent iron deficiency. The last study of Urrechaga et al. [18] indicated that markers of hypochromia are reliable tests for quick detection of subclinical iron deficiency in non anaemic premenopausal women. So far, there are only a few studies using reticulocyte and erythrocyte indicators of hypochromia in assessing iron status [3] and detection of early stages of iron deficiency in athletes [21, 22].
The aim of this study was to analyse the effectiveness of new haematology parameters related to reticulocytes as well as mature red blood cells to differentiate pre-latent and latent iron deficiency.
MATERIALS AND METHODS
Subjects
Two hundred and twenty-four young female athletes (aged 15-20 years) representing volleyball (n=49), handball (n=24), cycling (n=54), canoeing (n=24), cross-country skiing (n=15), swimming (n=28), midle and long distance running (n=16) and judo (n=14) participated in the study in the years 2014-2015. All subjects underwent a medical examination upon entering the study and were in various phases of the training cycle. The subjects were not interviewed regarding iron intake in the diet or iron supplementation. The results of 219 athletes who were found healthy and in whom we did not observe any symptoms of an acute phase response (subjects with normal values of C-reactive protein concentration (CRP), erythrocyte sedimentation rate (ESR) and white blood cell count (WBC)) were included in the statistical analysis. The study was performed according to the Declaration of Helsinki and approved by the local ethics committee. Written informed consent was obtained from participants or their parents if the subject was under 18 years of age. Basic data concerning subjects are presented in Table 1.
TABLE 1.
Basic characteristics, haematological and iron status indices in the whole group of studied female athletes (n=219)
| Variable | Mean value, SD and (range) | Normal range | ||
|---|---|---|---|---|
| Age | (years) | 16.9 ± 1.8 | (13.7-20) | - |
| Body mass | (kg) | 61.7 ± 9.3 | (42-104) | - |
| Height | (cm) | 170.7 ±7.5 | (153-190) | - |
| Athletic experience | (h/week) | 6.0 ± 2.4 | (2-15) | - |
| Ferritin | (ng/ml) | 27.1 ± 15.5 | (5-104) | 30-120 |
| sTfR | (μg/ml) | 5.46 ± 1.69 | (2.9-12.2) | 2.9-8.3 |
| TIBC | (μg/dl) | 337.3 ± 37.7 | (250-449) | 250-390 |
| Iron | (μg/dl) | 89.0 ± 39.9 | (22-257) | 37-165 |
| RBC | (x106/μl) | 4.7 ± 0.29 | (4.0-5.4) | 4.2-5.4 |
| HCT | (%) | 40.7± 2.3 | (36-47) | 37-47 |
| Hb | (g/dl) | 137.6 ± 8.4 | (116-163) | 120-160 |
| MCH | (pg) | 29.3 ± 1.45 | (23-32) | 26-32 |
| MCHC | (g/L) | 337.7 ± 11.6 | (303-381) | 330-370 |
| MCV | (fL) | 86.8 ± 3.55 | (77-95) | 81-99 |
| CH | (pg) | 29.1 ± 1.38 | (24-32) | - |
| RDW | (%) | 13.1 ± 0.59 | (12.0-15.8) | 11.5-14.5 |
| HYPOm | (%) | 1.20 ± 2.1 | (0.04-13.4) | - |
| LowCHm | (%) | 29.0 ± 13.4 | (9.0-89) | - |
| MICROm | (%) | 0.72 ± 0.61 | (0.1-5.70 | - |
| Reticulocytes | (%) | 1.51 ± 0.42 | (0.5-2.6) | 0.5-2.5 |
| Reticulocytes | (103/μl) | 71.0 ± 20.6 | (25-132) | 22-139 |
| MCVr | (fl) | 100.9 ± 3.2 | (90-109) | 101-119 |
| CHCMr | (g/dL) | 30.7 ± 1.23 | (27-34) | 33-37 |
| CHr | (pg) | 30.87 ± 1.49 | (25-34) | 27-32 |
| LowCHr | (%) | 13.4 ± 11.1 | (1.4-74) | - |
| HYPOr | (%) | 16.1 ± 13.3 | (1.7-68) | - |
| Body Iron | (mg/kg) | 3.89 ± 2.69 | (-4.0-11.4) | - |
| CRP | (ng/dL) | 0.20 ± 0.58 | (0.0-4.4) | <5 |
| ESR | (mm/h) | 4.16 ± 2.53 | (1-13) | <15 |
| WBC | (x103/μl) | 4.06 ± 1.39 | (3.2-10.3) | 4.5-10.4 |
Note: Hb – haemoglobin concentration in erythrocytes, HCT – haematocrit, RBC – red blood cell count, MCHC – mean corpuscular haemoglobin concentration in erythrocytes, MCV – mean corpuscular volume of erythrocytes, MCH – mean corpuscular haemoglobin in erythrocytes, CH – mean cellular haemoglobin content in erythrocytes, %HYPOm – percentage of red blood cells with decreased cellular haemoglobin concentration, %LowCHm – percentage of red blood cells with decreased mean cellular haemoglobin content in erythrocytes, %MICROm – percentage of erythrocytes with decreased volume, RDW – red cell distribution width, CHr – mean cellular haemoglobin in reticulocytes, #RET – absolute number of reticulocytes, %RET – absolute reticulocyte count as a percentage, CHCMr – mean cellular haemoglobin concentration of reticulocytes, MCVr – mean corpuscular volume of reticulocytes, %HYPOr – percentage of reticulocytes with decreased cellular haemoglobin concentration, %LowCHr – percentage of reticulocytes with decreased mean cellular haemoglobin content in reticulocytes, WBC – white blood cell count, ESR – erythrocyte sedimentation rate after one hour, CRP – C-reactive protein concentration, sTfR – soluble transferrin receptor concentration, TIBC – total iron binding capacity value.
Blood analysis
To assess iron status the blood was withdrawn from the antecubital vein in the morning (between 8 and 9 a.m.) in the preprandial state, after overnight fasting. To eliminate any residual effect of physical movement and ensure the data collected reflect a time resting baseline, sample collection started after 10-15 min rest in a seated position.
In whole blood the following measurements were performed in mature erythrocytes using the ADVIA 120 haematology system (Siemens Healthcare, Germany): haemoglobin concentration (Hb), haematocrit (HCT), red blood cell count (RBC), mean corpuscular haemoglobin concentration (MCHC), mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), mean cellular haemoglobin content (CH), percentage of erythrocytes with decreased cellular haemoglobin concentration (%HYPOm), percentage of erythrocytes with decreased cellular haemoglobin content (%LowCHm), percentage of erythrocytes with decreased volume (%MICROm), and red cell volume distribution width (RDW). In reticulocytes the following parameters were measured: mean cellular haemoglobin content (CHr), reticulocyte count expressed as an absolute number (#RET) and as a percentage of absolute value (%RET), mean cellular haemoglobin concentration (CHCMr), mean corpuscular volume (MCVr), percentage of reticulocytes with decreased cellular haemoglobin concentration (%HYPOr), and percentage of reticulocyte population with decreased cellular haemoglobin content (%LowCHr). The analysis were done up to 2 hours after blood collection. Within-run precision of the haematological parameters, expressed as coefficient of variations (CV), obtained from 20 repetitions of the same blood sample was as follows: Hb 1.26%; HCT 1.01%; RBC 0.93%; MCHC 0.88%; MCV 0.15%; MCH 0.89%; CH 0.19; RDW 0.98%; %HYPOm 13.8%; %LowCHm 1.72%; %MICROm 6.68%; CHr 0.31%; #RET 5.05%; %RET 5.31%; CHCMr 0.62%; MCVr 0.69%; %HYPOr 21.4% and %LowCHr 21.4%.
In blood serum the following assays were conducted: soluble transferrin receptor (sTfR) concentration by using immunoenzymatic commercial kits (Ramco, USA); ferritin concentration and iron concentration by using an immunoenzymatic method (Pentra, USA), and total iron binding capacity (TIBC) by using a colorimetric method (BioMaxima, Poland). Inter-assay variability for those indices did not exceed 6.0, 7.5, 4.3 and 3.9% respectively.
Additionally, to assess the acute phase reaction white blood cell count (WBC) (ADVIA 120 haematology system, Siemens Healthcare, Germany), erythrocyte sedimentation rate (ESR) after one hour in whole blood, and C-reactive protein concentration (CRP) in serum (immunoturbidimetric method, Pentra, USA) were determined. CRP reagent used in the study has covered wide range of linearity containing both normal (1.0 – 5.0mg/L) and inflammatory response ranges (5.0 – 160.0 mg/L). Reproducibility of the test performance (between-run precision) did not exceed 4.3%.
All analyses were done in a laboratory with an implemented quality system.
On the basis of results of ferritin and sTfR concentrations, body iron stores were calculated, using a special algorithm, which was developed by Cook et al. [23] exclusively for sTfR determined by the Ramco method.
The definition of each stage of ID was based on ferritin, sTfR, TIBC and 3 basic morphological indices, i.e. Hb, RBC and HCT. Subjects in whom the value of ferritin was lower than 30 ng/ml were classified as being pre-latent iron deficient (stage I of ID). The athletes with low ferritin concentration (<30 ng/ml) and increased sTfR (>8.3 μg/ml) and/or TIBC (>390 μg/dL) were classified as subjects with latent iron deficiency (stage II of ID). Due to low specificity and wide diurnal variation of iron concentration, this indicator as well as transferrin saturation were not taken into account as a criterion of iron deficiency [5, 24].
Iron deficiency anaemia was diagnosed when low ferritin concentration and increased values of sTfR and TIBC were accompanied by low values of Hb (<12 g/L), HCT (<37%) and RBC (<4.2 x109/L).
Statistical analysis
The distributions of raw data in the case of ferritin, sTfR and iron concentrations as well as #RET and RDW values differed from normal (Kolmogorov-Smirnov test p<0.20); therefore the non parametric Kruskal-Wallis ANOVA with multiple comparisons was used to test differences between groups of athletes with two stages of iron deficiency and athletes with normal iron status. The frequency of iron deficiency between studied sports disciplines was analyzed by the chi-square test. Statistical analysis was performed using STATISTICA (data analysis software system), version 12. Results were considered significant if the two-tailed p-value was less than 0.05.
RESULTS
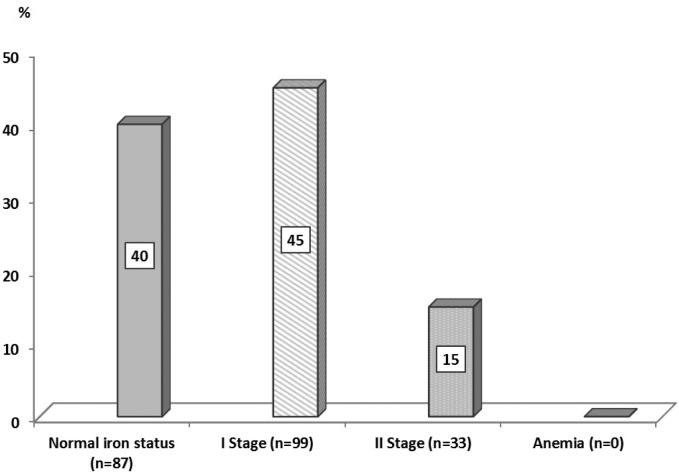
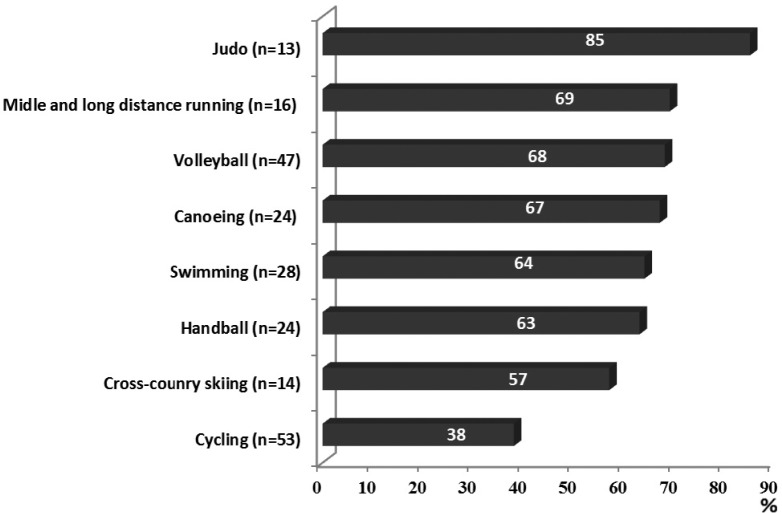
The frequency of iron deficiency was high: 60%. Identified deficiencies were exclusively latent, since in no case of iron-deficiency anaemia was detected. The first stage, i.e. pre-latent iron deficiency, was observed in 45%, and stage II (latent iron deficiency) in 15% of female athletes (Figure 1). The incidence of iron deficiency differed in various sport disciplines (χ2 = 17.03; p <0.05). The highest frequency of iron deficiency was observed in judo (85%), whereas the lowest was observed in cycling (38%) (Figure 2).
FIG. 1.
Percentage of female athletes with and without iron deficiency.
FIG. 2.
Frequencies of iron deficiencies in the studied sport disciplines.
Note: chi-square=17.03; p<0.05.
The iron status and haematological indices in the three groups of female athletes i.e. with stage I and II and without iron deficiency are presented in Table 2. Since the ferritin concentration was used as a primary and first indicator in classifying iron deficiency, its mean values were automatically significantly lower in both groups with iron deficiency. Mean values of ferritin also differed between the two groups with iron deficiency: it was significantly lower (0.01) in athletes with stage II iron deficiency.
TABLE 2.
Haematological and iron metabolism indices (mean±SD) in subgroups with normal iron status and with iron deficiency.
| Variable | Normal iron stores | Iron deficiency | Kruskal-Wallis test | ||
|---|---|---|---|---|---|
| (n=87) | I stage (n=99) | II stage (n=33) | |||
| Ferritin | (ng/ml) | 41.8 ± 13.6 | 19.0 ± 5.5*** | 12.5 ± 5.3***^^ | H= 166.899; p<.001 |
| sTfR | (μg/ml) | 4.7 ± 1.16 | 5.2 ± 1.1** | 8.00 ± 2.1***^^^ | H= 62.849; p<.001 |
| TIBC | (μg/dl) | 319 ± 30.8 | 338 ± 30.4** | 383 ± 35.0***^^^ | H= 59.601; p<.001 |
| Iron | (μg/dl) | 97.3 ± 41.8 | 87.7 ± 37.2 | 70.1 ± 37.7** | H=11.3018; p=.0035 |
| RBC | (x106/μl) | 4.7 ± 0.30 | 4.7 ± 0.28 | 4.74 ± 0.30 | H= 0.821; p=.6634 |
| HCT | (%) | 40.9 ± 2.5 | 40.7 ± 2.1 | 40.5 ± 2.4 | H= 1.266; p=.5310 |
| Hb | (g/dl) | 139.2 ± 8.7 | 137.8 ± 7.4 | 132.7 ± 9.0***^^ | H= 14.279; p<.001 |
| MCH | (pg) | 29.7 ± 1.06 | 29.4 ± 1.32 | 28.1 ± 1.97***^^^ | H= 20.865; p<.001 |
| MCHC | (g/L) | 340.2 ± 11.7 | 338.9 ± 9.9 | 327.9 ± 11.0***^^^ | H= 24.547; p<.001 |
| MCV | (fL) | 87.2 ± 3.3 | 86.9 ± 3.3 | 85.5 ± 4.6 | H= 5.219; p=.0736 |
| CH | (pg) | 29.6 ± 1.03 | 29.2 ± 1.20 | 27.8 ± 1.86***^^^ | H= 24.685 p<.001 |
| RDW | (%) | 12.8 ± 0.43 | 13.1 ± 0.52** | 13.6 ± 0.78***^^ | H= 31.997; p<.001 |
| HYPOm | (%) | 0.55 ± 0.68 | 0.90 ± 1.03 ** | 3.82 ± 3.94***^^^ | H= 39.583; p<.001 |
| LowCHm | (%) | 24.5 ± 8.9 | 28.3 ± 11.4 | 42.6 ± 19.3***^^^ | H= 25.072; p<.001 |
| MICROm | (%) | 0.63 ± 0.41 | 0.64 ± 0.4 | 1.22 ± 1.13**^^ | H= 12.902; p=.0016 |
| Reticulocytes | (%) | 1.46 ± 0.41 | 1.53 ± 0.40 | 1.60 ± 0.37 | H= 3.782; p=.1509 |
| Reticulocytes | (109/L) | 78.4 ± 19.7 | 71.5 ± 21.1 | 76.6 ± 20.6 | H= 4.046; p=.1323 |
| MCVr | (fl) | 101.0 ± 3.2 | 101.2 ± 3.0 | 100.1 ± 3.72 | H= 2.045; p=.3597 |
| CHCMr | (g/dL) | 31.3 ± 0.99 | 30.6 ± 0.98*** | 29.4 ± 1.39***^^^ | H= 43.378; p<.001 |
| CHr | (pg) | 31.4 ± 1.07 | 30.9 ± 1.28* | 29.3 ±1.86***^^^ | H= 34.803; p<.001 |
| LowCHr | (%) | 9.2 ± 5.1 | 12.5 ± 8.0* | 27.2 ± 18.2***^^^ | H= 36.645; p<.001 |
| HYPOr | (%) | 10.1 ± 6.8 | 15.1 ± 8.8*** | 34.7 ± 20.0***^^^ | H= 43.537; p<.001 |
| Body Iron | (mg/kg) | 6.33 ± 1.32 | 3.08 ± 1.50*** | -0.07 ± 1.85***^^^ | H= 160.157; p<.001 |
Note: *Significantly different from the respective value in group with normal iron status.
p<0.05
p<0.01
p<0.001
Significantly different between stage I and II iron deficiency.
p<0.01
p<0.001.
Hb – haemoglobin concentration in erythrocytes, HCT – haematocrit, RBC – red blood cell count, MCHC – mean corpuscular haemoglobin concentration in erythrocytes, MCV mean corpuscular volume of erythrocytes, MCH – mean corpuscular haemoglobin in erythrocytes, CH – mean cellular haemoglobin content in erythrocytes, %HYPOm – percentage of red blood cells with decreased cellular haemoglobin concentration, %LowCHm – percentage of red blood cells with decreased mean cellular haemoglobin content in erythrocytes, %MICROm – percentage of erythrocytes with decreased volume, RDW – red cell distribution width, #RET – absolute number of reticulocytes, %RET – absolute reticulocyte count as a percentage, CHr – mean cellular haemoglobin in reticulocytes, CHCMr – mean cellular haemoglobin concentration in reticulocytes, MCVr – mean corpuscular volume of reticulocytes, %HYPOr – percentage of reticulocytes with decreased cellular haemoglobin concentration, %LowCHr – percentage of reticulocytes with decreased mean cellular haemoglobin content in reticulocytes, sTfR – soluble transferrin receptor concentration, TIBC – total iron binding capacity value.
In comparison to athletes with normal iron status the subjects with stage I iron deficiency had a significantly higher sTfR concentration (p<0.01) and TIBC values (p<0.01), although both indices remained within reference values. Additionally, in athletes with stage I ID a significantly lower value of body iron (p<0.001) was observed. With regards to haematological variables these two groups differed significantly mainly in reticulocyte parameters. The group with low ferritin concentration had significantly lower values of CHCMr (p<0.001) and CHr (p<0.05), as well as higher percentages of HYPOr (p<0.001) and LowCHr (p<0.05). Within the mature erythrocyte markers both groups differed in %HYPOm (p<0.05) and RDW values (p<0.001) only (Table 2).
In the athletes with stage II ID, differences in indices of iron status were more profound in the case of TIBC value (p<0.001) and sTfR concentration (p<0.001), body iron (p<0.001), and additionally iron concentration (p<0.01) compared to the group with normal iron status. Furthermore, the two groups differed significantly in reticulocyte markers as well as many indices of mature red blood cells. Athletes with stage II iron deficiency had significantly lower values of CHCMr (p<0.001), CHr (p<0.001), Hb (p<0.001), MCH (p<0.001), MCHC (p<0.001) and CH (p<0.001), and higher values of %LowCHr (p<0.001), %HYPOr (p<0.001), %LowCHm (p<0.001), %HYPOm (p<0.001), %MICROm (p<0.01) and RDW (p<0.001) (Table 2).
Differences between the two groups with iron deficiency (stage I and II) concerned both haematological and iron status indices and were very similar to those observed between groups with normal iron status and stage II ID (Table 2).
DISCUSSION
Iron is a micronutrient involved in numerous biologic processes, many of which are vital for not only health but also athletic performance [25, 26, 27]. On the other hand, iron deficiency is the most common deficiency in the world, and some studies indicate that in athletes iron deficiency occurs more frequently than in the general population [27, 28, 29]. For these reasons early detection of iron deficiency in athletes is of great importance.
The incidence of iron deficiency observed in this study was high (60%) – the highest reported so far in Polish female athletes. In our earlier studies the incidence of latent iron deficiency in young female athletes at the same age ranged from 26% in the 1990s [7] to 57% in 2009 [30]. It is worth noting that in the present study iron status was assessed only in healthy athletes without symptoms of infection or inflammation, which minimized false negative results. Moreover, our results agree with other studies. Similarly high rates of iron deficiency over 50% were observed in adolescent Swedish [29] and young German [31] athletes practising different sports as well as in elite Swedish female soccer players aged 16-28 years [32]. Relatively high incidence of iron deficiency (from 50 to 89%) was confirmed by results in highly active American women [4] and in Canadian junior elite soccer players [33], although it should be emphasized that the frequency of iron deficiency varied in accordance with applied criteria, most of all the value of ferritin. In this study the highest frequency of iron deficiency observed in female judoists could be the result of the periodic dietary restrictions due to the necessity for weight reduction.
In healthy persons, serum ferritin concentration is a marker of iron stores, and low levels of this parameter unequivocally indicate decreased or depleted iron stores. Due to the wide inter-individual variation of serum ferritin in females (10-120 ng/ml – according to the Pentra method) the lower limit is not well established for identification of iron deficiency [1]. Ferritin levels lower than 12 ng/ml are generally considered as a marker of depleted iron stores (absolute iron deficiency), but in athletes very often higher values from 16 to 35 ng/ml are used to detect iron deficiency [4, 7, 25, 31, 32, 33]. The use of values of ferritin higher than 12 ng/ml as a criterion of absolute iron deficiency is reasonable, because a classical study found that signs of iron-deficient erythropoiesis in premenopausal women started already at ferritin concentrations ranging from 25 to 40 ng/ml [34]. There are also results indicating a lack of iron in the bone marrow at a ferritin concentration of 30 ng/ml [35, 36]. Mast et al. [35] showed that application of a critical cut-off value of 30 ng/ml for diagnosing iron deficiency resulted in improved sensitivity and specificity of ferritin to 92% and 98%, respectively, and only one of the results was falsely positive, when this diagnostic limit was adopted. Many reports concerning not only athletes applied a ferritin level of 30 to 35 ng/mL as the common denominator for considering ferritin as low and thus initiating iron supplementation [5, 18, 31, 36, 37, 38]. It seems reasonable, because in the present study in athletes with stage II ID (with elevated values of TIBC and/or sTfR) serum ferritin concentration was in the range 5-28 ng/ml, among which in 13 subjects (45%) it exceeded 12 ng/ml, and in 3 athletes it was higher than 20 ng/ml. Similar results were observed in the Di Santolo et al. study [2], in which about half of female athletes with elevated sTfR had serum ferritin above 12 ng/ml. For all these reasons, in the present study the value of ferritin of 30 ng/ml was applied as the lower limit for assessing iron status. Furthermore, we wanted to characterize each stage of iron deficiency in terms of hypochromia indices, using milder, but often used in athletes, criteria of iron deficiency.
Reticulocytes are the youngest red blood cells which are released from the bone marrow into circulating blood. They mature for 1–3 days within the bone marrow and circulate for 1–2 days before becoming mature erythrocytes. This short period of residence in the blood means that indices of reticulocytes better reflect the current iron supply to the bone marrow for red blood cell production than for mature erythrocytes [17]. Among the indices of immature red blood cells, reticulocyte haemoglobin content (CHr) is the most frequently used as an early marker for diagnosis of iron deficiency in adults [35] and children [17]. Other indicators of reticulocytes as well as erythrocytes such as percentage of hypochromic cells (%LowCH, and %HYPO) and mean cellular haemoglobin concentration in reticulocytes (CHCMr) are studied less frequently, but they are very important since they indicate haemoglobinization of mature and immature red blood cells [5].
Significantly lower values of indicators concern haemoglobin content in reticulocytes (lower values of CHr and CHCMr, higher %LowCHr and %HYPOr) and significantly higher values of two parameters of mature erythrocytes (RDW, and %HYPO) in athletes with pre-latent iron deficiency indicate that adverse changes in erythropoiesis begin already when iron stores are exhausted or strongly reduced. Moreover, quantitative assessment of body iron (expressed in milligrams per kg body mass) indicates that the mean value of this indicator in athletes with stage I iron deficiency was significantly lower (3.08 ± 1.50mg/kg) than that observed in athletes with normal iron status (6.33 ± 1.32 mg/kg). It is worth noting that body iron in the athletes without iron deficiency was even higher than that observed in healthy US women aged 20-45 years (5.5± 3.35 mg/kg) [23]. All these results confirm that use of a ferritin value of 30 ng/ml as a criterion of iron deficiency was correct.
Significant differences in many haematological indicators between athletes with stage II ID and the group without iron deficiency as well as between both groups with iron deficit clearly indicate that magnitude and range of adverse changes in athletes with stage II ID are larger and concern additionally many parameters of mature erythrocytes, i.e. Hb, MCH, MCHC, CH and %LowCHm. Moreover, the mean value of body iron in groups with stage II iron deficiency was near zero, and in more than half of subjects within this group was below zero, which indicates tissue iron deficiency [23]. These results clearly demonstrate that stage II iron deficiency causes visible disturbances in erythropoiesis, which markedly increase the risk of iron deficiency anaemia. It is interesting that in iron deficient athletes these changes did not include either the number or the volume of reticulocytes and RBC count, even in stage II iron deficiency, although a significantly higher percentage of microcytic erythrocytes (%MICROm) was observed in the group with stage II iron deficiency. Mean cellular haemoglobin content in reticulocytes (CHr) and erythrocytes (CH) is a product of corpuscular volume of reticulocytes (MCVr) and erythrocytes (MCV) and cellular haemoglobin concentration, i.e. CHCMr and MCHC, respectively [5, 35]. The lack of changes in volumes of both reticulocytes and erythrocytes in deficient athletes indicates that the decrease in cellular haemoglobin concentration (CHCMr and MCHC, respectively) had the main impact on lower values of CHr and CH in athletes with latent ID, and additionally CHr in the group with pre-latent ID. Also, the significantly higher percentage of HYPOr and LowCHr values in both iron deficiency groups demonstrate that along with deterioration of iron status, the percentage of reticulocytes with decreased haemoglobin content leaving bone marrow increases. These results indicate that early changes caused by iron deficiency are related mainly to qualitative disturbances in haemoglobin production leading to hypochromia, while quantitative changes concerning the number as well as volume of reticulocytes occur later. This deterioration especially in haemoglobinization of reticulocytes suggests that CHr and the proportion of hypochromic immature red cells (%HYPOr and %LowCHr) are direct, sensitive and early indicators of iron deficient erythropoiesis, although so far exact and generally accepted threshold values for these indices have not been established [5]. The development of iron deficiency is a process, and it is difficult to identify when synthesis of haemoglobin start to be impaired, and these new indicators especially of reticulocytes help to determine this moment. The present results indicate that in female athletes the first disturbances in erythropoiesis due to iron deficiency begin when only ferritin concentration is decreased.
The lack of response of the hypochromia indices during acute phase reaction increases their usefulness in detecting disturbances of iron turnover in erythron. It may suggests their role as usefull pre-screening markers of disorders related to iron deficiency, when for example ferritin or another markers of iron status are unavailable. It is also worth to note that in cost/benefit terms, monitoring of hypochromia markers is economically beneficial due to relatively low cost of determination, therefore it seems reasonable to include hypochromia indices to assess iron status, only if the necessary equipment is available.
CONCLUSIONS
The main finding of this study was that the diminished or exhausted iron stores have already caused changes in reticulocytes, and intensification of iron deficiency (stage II) increases changes in both reticulocyte and erythrocyte hypochromia indices. These results indicate that the markers of hypochromia relating especially to reticulocytes are useful for diagnosis of early stages of iron deficiency in athletes with absence of an acute phase reaction.
The analysis of these new indicators in conjunction with routine morphological measurements and standard iron parameters allows for better assessment of iron status and more accurate and earlier diagnosis of the degree of iron deficiency. It is important, because despite the lack of anaemia, among studied female athletes, the frequency of latent iron deficiencies (stage I and II) was very high. Moreover, the relatively high percentage of female athletes with stage II iron deficiency with more pronounced changes in haematological indices indicates that competitive young female athletes should be regularly monitored, to prevent the development of iron-deficiency anaemia.
Acknowledgements
The study was supported by the Ministry of Sport and Tourism of the Republic of Poland (2014/0066/0223/SubB/DSW/DZK/JD, and 2015/0015/0223/SubB/DSW).
Conflict of interests
There are no conflicts of interest for any author
REFERENCES
- 1.Alaunyte I, Stojceska V, Plunkett A. Iron and the female athletes: a review of dietary treatment methods for improving iron status and exercise performance. J Int Soc Sports Nutr. 2015;6:12–38. doi: 10.1186/s12970-015-0099-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Santolo M, Stel G, Banfi G, Gonano F, Cauci S. Anemia and iron status in young fertile non-professional female athletes. Eur J Appl Physiol. 2008;102:703–709. doi: 10.1007/s00421-007-0647-9. [DOI] [PubMed] [Google Scholar]
- 3.Milic R, Martynovic J, Dopsaj M, Dopsaj V. Haematological and iron-related parameters in male and female athletes according to different metabolic energy demands. Eur J Appl Physiol. 2011;111:449–458. doi: 10.1007/s00421-010-1656-7. [DOI] [PubMed] [Google Scholar]
- 4.Woolf K, Thomas MMS, Hahn N, Vaughan LA, Carlson AG, Hinton P. Iron status in highly active and sedentary young women. Int J Sport Nutr Exerc Metab. 2009;19:519–535. doi: 10.1123/ijsnem.19.5.519. [DOI] [PubMed] [Google Scholar]
- 5.Urrcheaga E, Borque L, Escanero JF. New laboratory parameters for the assessment of iron status and erythropoiesis. In: Berhard Leon V., editor. Advances in Medicine and Biology. 1. Vol. 47. New York: Nova Science Publishers; 2012. pp. 185–207. [Google Scholar]
- 6.Hinton PS, Sanford TC, Davidson MM, Yakushko OF, Beck NC. Nutrient intake and dietary behviors of male and female collegiate athletes. Int J Sport Nutr Exerc Metab. 2004;14:389–405. doi: 10.1123/ijsnem.14.4.389. [DOI] [PubMed] [Google Scholar]
- 7.Malczewska J, Raczynski G, Stupnicki R. Iron status in female endurance athletes and in non-athletes. Int J Sport Nutr Exerc Metab. 2000;109:260–276. doi: 10.1123/ijsnem.10.3.260. [DOI] [PubMed] [Google Scholar]
- 8.Hinton P. Iron and the endurance athletes. Appl Physiol Nutr Metab. 2014;39:1012–1018. doi: 10.1139/apnm-2014-0147. [DOI] [PubMed] [Google Scholar]
- 9.Peeling P. Exercise as a mediator of hepcidin activity in athletes. Eur J Appl Physiol. 2010;110:877–883. doi: 10.1007/s00421-010-1594-4. [DOI] [PubMed] [Google Scholar]
- 10.Shin DH, Kim HS, Park MJ, Suh IB, Shin KS. Utility of Access Soluble Transferrin Receptor (sTfR) and sTfR/log Ferritin Index in Diagnosing Iron Deficiency Anemia. Ann Clin Lab Sci. 2015;45:396–402. [PubMed] [Google Scholar]
- 11.Malczewska J, Błach W, Stupnicki R. The effects of physical exercise on the concentration of ferritin and transferrin receptor in plasma of female judoists. Int J Sports Med. 2000;21:175–179. doi: 10.1055/s-2000-299. [DOI] [PubMed] [Google Scholar]
- 12.Souglis AG, Bogdanis GC, Giannopoulou I, Papadopoulos Ch, Apostolidis NG. Comparison of inflammatory responses and muscle damage indices following a soccer, basketball, volleyball and handball game at an elite competitive level. Res Sports Med. 2015;23:59–72. doi: 10.1080/15438627.2014.975814. [DOI] [PubMed] [Google Scholar]
- 13.Souglis AG, Papapanagiotou A, Bogdanis GC, Travlos AK, Apostolidis NG, Geladas ND. Comparison of inflammatory responses to a soccer match between elite male and female players. J Strength Cond Res. 2015;29:1227–1233. doi: 10.1519/JSC.0000000000000767. [DOI] [PubMed] [Google Scholar]
- 14.Gore CJ, Morkeberg J, Shmidt W, Garvican LA, Fellman N. Plasma volume shift during multiday racing. Clin Chem Lab Med. 2013;51:e107–9. doi: 10.1515/cclm-2012-0728. [DOI] [PubMed] [Google Scholar]
- 15.Voss SC, Alsayrafi M, Bourdon PC, Klodt F, Nonis D, Hopkins WG, Schumacher YO. Variability of serum markers of erythropoiesis during 6 days of racing in highly trained cyclists. Int J Sport Med. 2014;35:89–94. doi: 10.1055/s-0033-1345177. [DOI] [PubMed] [Google Scholar]
- 16.Wollmann M, Gerzson BM, Schwert V, Figuera RW, de Oliveira Ritzel G. Reticulocyte maturity indices in iron deficiency anemia. Rev Bras Hematol Hemoter. 2014;36:25–28. doi: 10.5581/1516-8484.20140009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lorenz L, Arand J, Buchner K, Wacker-Gussmann A, Peter A, Poets Ch, Franz AR. Reticulocyte hemoglobin content as a marker of iron deficiency. Arch Dis Child Fetal Neonatal Ed. 2015;100:F198–F202. doi: 10.1136/archdischild-2014-306076. [DOI] [PubMed] [Google Scholar]
- 18.Urrechaga E, Borque L, Escanero J. Clinical value of hypochromia markers in the detection of latent iron deficiency in nonanemic premenopausal women. J Clin Lab Anal. 2016;30:623–627. doi: 10.1002/jcla.21912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodnough LT, Nemeh E, Ganz T. Detection, evaluation and management of iron-restricted erythropoiesis. Blood. 2010;116:4754–4761. doi: 10.1182/blood-2010-05-286260. [DOI] [PubMed] [Google Scholar]
- 20.Torino ABB, Gilberti MFP, daCosta E, deLima GAF, Grotto HZW. Evaluation of erythrocyte and reticulocyte parameters as indicative of iron deficiency in patient with anemia of chronic disease. Rev Bras Hematol Hemoter. 2015;37:77–81. doi: 10.1016/j.bjhh.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Auersperger I, Škof B, Leskošek B, Knap B, Jerin A, Lainscak M. Exercise-induced changes in iron status and hepcidin response in female runners. PloS One. 2013;8:e58090. doi: 10.1371/journal.pone.0058090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voss SC, Varamenti E, Elzain Elgingo M, Bourdon PC. New parameters and reference values for monitoring iron status in Midldle Eastern adolescent male athletes. J Sports Med Phys Fitness. 2014;54:179–85. [PubMed] [Google Scholar]
- 23.Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood. 2003;101:3359–3364. doi: 10.1182/blood-2002-10-3071. [DOI] [PubMed] [Google Scholar]
- 24.Coyne D. Iron indices: what do the really mean? Kidney Int. 2006;(Suppl):S4–8. doi: 10.1038/sj.ki.5000404. [DOI] [PubMed] [Google Scholar]
- 25.Della-Valle DM, Haas JD. Impact of iron depletion without anemia on performance in trained endurance athletes at the beginning of a training season: a study of female collegiate rowers. Int J Sport Nutr Exerc Metab. 2011;21:501–506. doi: 10.1123/ijsnem.21.6.501. [DOI] [PubMed] [Google Scholar]
- 26.Hinton PS, Sinclair LM. Iron supplementation maintains ventilator threshold and improves energetic sufficiency in iron-deficient nonanemic athletes. Eur J Clin Nutr. 2007;61:30–39. doi: 10.1038/sj.ejcn.1602479. [DOI] [PubMed] [Google Scholar]
- 27.Rodenberg ER, Gustafson S. Iron as an ergogenic aid: ironclad evidence? Curr. Sports Med Rep. 2007;6:258–264. [PubMed] [Google Scholar]
- 28.Dubnov G, Constantini NW. Prevalence of iron depletion and anemia in top-level basketball players. Int J Sport Nutr Exerc Metab. 2004;14:30–37. doi: 10.1123/ijsnem.14.1.30. [DOI] [PubMed] [Google Scholar]
- 29.Sandström G, Börjesson M, Rödjer S. Iron Deficiency in Adolescent Female Athletes. Clin J Sport Med. 2012;22:495–500. doi: 10.1097/JSM.0b013e3182639522. [DOI] [PubMed] [Google Scholar]
- 30.Malczewska-Lenczowska J, Szczepańska B, Śliwińska J. The frequency of iron deficiency in adolescent athletes. Polish J Sport Med. 2010;26(suppl.1):11. [Google Scholar]
- 31.Koehler K, Braun H, Achtzehn S, Hildebrand U, Predel H-G, Master J, Schänzer W. Iron status in elite young athletes: gender-dependent influences of diet and exercise. Eur J Appl Physiol. 2012;112:513–523. doi: 10.1007/s00421-011-2002-4. [DOI] [PubMed] [Google Scholar]
- 32.Landahl G, Adolfsson P, Borjesson M, Mannheimer C, Rodjer S. Iron deficiency and anemia: a common problem in female elite soccer players. Int J Sport Nutr Exerc Metab. 2005;15:689–694. doi: 10.1123/ijsnem.15.6.689. [DOI] [PubMed] [Google Scholar]
- 33.Gibson JG, Stuart-Hill L, Martin S, Gaul C. Nutrition status of junior elite canadian female soccer players. Int J Sport Nutr Exerc Metab. 2011;21:507–514. doi: 10.1123/ijsnem.21.6.507. [DOI] [PubMed] [Google Scholar]
- 34.Hallberg L, Bengtsson C, Lapidus L, Lindstedt G, Lundberg P-A, Hultén L. Screening for iron deficiency: an analysis based on bone-marrow examinations and serum ferritin determinations in a population sample of women. Br J Haematol. 1993;84:787–98. doi: 10.1111/j.1365-2141.1993.tb03225.x. [DOI] [PubMed] [Google Scholar]
- 35.Mast AE, Binder MA, Lu Q, Flax S, Dietzen DJ. Clinical utility of reticulocyte hemoglobin content in the diagnosis of iron deficiency. Blood. 2002;99:1489–1491. doi: 10.1182/blood.v99.4.1489. [DOI] [PubMed] [Google Scholar]
- 36.North M, Dallalio G, Donath AS, Melink R, Means RT. Serum transferrin receptor levels in patients undergoing evaluation of iron stores: correlation with other parameters and observed versus predicted results. Clin Lab Haem. 1997;19:93–97. doi: 10.1046/j.1365-2257.1997.00041.x. [DOI] [PubMed] [Google Scholar]
- 37.Fallon KE. Utility of hematological and iron-related screening in elite athletes. Clin Sport Med. 2004;14:145–152. doi: 10.1097/00042752-200405000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Nielsen P, Nachtigall D. Iron supplementation in athletes. Sports Med. 1998;26:207–216. doi: 10.2165/00007256-199826040-00001. [DOI] [PubMed] [Google Scholar]