Abstract
Necrotizing enterocolitis (NEC) is a devastating disease of prematurity with significant morbidity and mortality. Immaturity of intestinal host defenses predisposes the premature infant gut to injury. An abnormal bacterial colonization pattern with a deficiency of commensal bacteria may lead to a further breakdown of these host defense mechanisms, predisposing the infant to NEC. The presence of probiotic and commensal bacteria within the gut has been shown to mature the intestinal defense system through a variety of mechanisms. We have shown that commensal and probiotic bacteria can promote intestinal host defenses by reducing apoptotic signaling, blocking inflammatory signaling, and maturing barrier function in immature intestinal epithelia. Future studies aimed at elucidating the mechanisms by which probiotic and commensal bacteria exert their effects will be critical to developing effective preventive therapies for NEC.
Keywords: LGG; Lactobacillus rhamnosus GG, Probiotics, Microbiota, Commensal bacteria, Inflammation, Apoptosis, Tight junctions, ROS; Reactive oxygen species, Innate immune system, Intestinal epithelial cell, IL-10
1. Introduction
The extremely preterm neonate faces many challenges following early birth. Their fragile gastrointestinal (GI) systems are in a critical state of development and are forced to adapt quickly to extrauterine life. This complex organ provides a large interface with the external environment. When the GI system functions properly, it not only facilitates optimal nutrition, but also plays a unique role in host defense. A breakdown in this essential function poses a significant threat to the health of the premature infant. More specifically, immaturity of intestinal host defenses may predispose infants to necrotizing enterocolitis (NEC). Despite advances in the medical care of premature infants, NEC rates remain unchanged [1-3], and it continues to be one of the most devastating and unpredictable diseases of prematurity. Unfortunate patients who contract this disease are at high risk for adverse neurodevelopmental outcomes [4,5]. The etiology of NEC has not been fully elucidated, but it is likely multifactorial, involving immaturity of intestinal host defenses and abnormal bacterial colonization [6-9].
Colonization of the initially sterile intestinal ecosystem occurs postnatally as dietary and environmental changes occur. Complete colonization resembling that of an adult is reached by two years of age and, remarkably, contains up to 1 × 1014 colony-forming units (CFUs) [10]. Appropriate colonization with commensal bacteria is important for intestinal function and development [11-13] and may play a central role in the postnatal maturation of intestinal host defenses [14]. Neonates who are born prematurely have an abnormal intestinal microbial composition [13,15,16], which may predispose them to a failure of postnatal evolution of critical innate defenses and lead to NEC. As further evidence for the importance of commensal bacteria, preterm infants with altered intestinal flora due to prolonged antibiotic therapy are more likely to develop NEC [17,18]. Other factors that influence proper colonization include maternal exposure to antibiotics, mode of delivery, human breast milk feedings, and the hospital environment. Preterm infants are more likely to be delivered by cesarean section and experience delayed enteral feeding, which make them less likely to acquire commensal flora perinatally from passage through the birth canal or from human milk feedings. This may lead to decreased colonization of beneficial probiotic bacteria, including species of Bifidobacterium, Lactobacillus and Bacteroides [19,20]. The hospital environment, with its preponderance of pathogenic organisms [21], also negatively affects the intestinal colonization of beneficial commensal bacteria.
Studies have shown commensal bacteria regulate many intestinal defenses including barrier function, mucin and IgA secretion, inflammation, and homeostatic processes such as proliferation and apoptosis [22-26]. We believe that immature intestinal host defenses play a critical role in the pathogenesis of neonatal intestinal inflammatory diseases, including NEC, and commensal bacteria (or their products) can promote the maturation of these host defenses. Understanding this process is crucial to developing potential therapeutic (prebiotic, probiotic or postbiotic) interventions to better treat or prevent these devastating diseases. Specific areas of host defense that can be promoted by commensal and probiotic bacteria include intestinal epithelial cell proliferation and apoptosis, innate immune regulation, and epithelial barrier function.
2. Murine model of postnatal intestinal development
Epidemiologic studies indicate that incidence and postnatal age of NEC onset is inversely proportional to gestational age [9,27-30]. Thus, infants born earlier not only have a higher NEC incidence but also develop NEC at a later postnatal age, with a developmental window of susceptibility at 30-32 weeks [9,27-30]. Susceptibility to NEC is likely due to developmental immaturity in intestinal host defenses such as exaggerated apoptotic and inflammatory responses [7,31] and decreased barrier function. In order to better understand developmental differences in intestinal host defenses that may play a role in NEC, our laboratory has successfully modeled premature intestinal epithelia in 0-3 week old preweaned mice. Rodents are altricial species and thus their intestines are functionally immature at birth [32]. Premature human intestines and preweaned murine intestines are similar in that mucosal immunity and GALT are maturing postnatally [14,33]. The neonatal murine intestinal epithelial architecture and barrier function are relatively immature at birth compared to the neonatal human intestine and continues to mature over the first three weeks of life [14,34]. Rodent intestines in the 2nd week of life are thought to represent the maturity of early 3rd trimester human intestines, and thus, we and others have successfully used murine intestines in the 2nd week of life to model premature human intestines [14,32,35-48].
Using this murine model of postnatal intestinal development, we have characterized the ontogeny of key intestinal host defenses. Specifically, we have shown that intestinal epithelial apoptotic [49,50] and proinflammatory [51] responses peak at 2 weeks in the murine gut at a time when intestinal epithelial barrier function remains immature [52]. This developmental period of dysregulated intestinal host defenses may resemble the developmental period of peak susceptibility to NEC seen in premature infants. Thus the 2-week-old murine intestine may be an ideal model for the preterm human intestine. Further, we have successfully employed this novel murine model of postnatal intestinal development to investigate the mechanisms by which commensal and probiotic bacteria promote regulation and maturation of intestinal host defenses.
3. Commensal and probiotic bacteria regulate apoptotic responses in immature intestinal epithelia
Apoptosis, when regulated correctly, is generally regarded as a protective process for the host and results in a balance between cell proliferation and cell death. It allows damaged cells to be removed in response to injurious stimuli (microbial, hypoxic, or chemical) [23] without further impairment of the surrounding tissue, but problems may arise when apoptotic activity is excessive or aberrant. There is histopathologic evidence in humans that apoptosis plays a role in the early events of the development of NEC [53,54], and, in animal models of NEC, epithelial apoptosis precedes gross bowel necrosis [55]. We have shown that immature intestinal epithelial cells exhibit exaggerated responses to apoptotic stimuli [49-51]. Commensal bacteria have been shown to reduce the incidence of NEC [56]. Therefore, we investigated whether commensal Escherichia coli could regulate apoptotic signaling in the developing gut.
Commensal strains of E. coli, which are obtained from the maternal GI tract, populate the intestines of term newborns very early in life [11,12,57]. Mirpuri et al. showed that a previously known, anti-inflammatory [22,58] commensal strain of E. coli isolated from healthy human colon reduces epithelial apoptosis in a murine model of developing intestine [50]. Ontogeny studies demonstrated that immature murine intestinal epithelia were most susceptible to inducible apoptosis at 2 weeks of postnatal age. However, when we fed neonatal mice with E. coli prior to inducing apoptosis, we found that intestinal epithelia in both the small intestine and colon showed a 50% reduction in induced apoptosis. E. coli mitigated apoptotic responses via IFN-αA-dependent upregulation of the antiapoptotic protein, GBP-1.
We have shown that probiotic bacteria can also regulate intestinal epithelial apoptotic responses. Probiotics are defined as ‘living micro-organisms, which upon ingestion in sufficient numbers, exert health benefits beyond basic nutrition’ [59]. These beneficial bacteria can foster a normal intestinal colonization pattern in addition to directly supporting optimal functioning of intestinal epithelial cells. Experimental NEC in animal models has shown a reduction in both the severity [60] and incidence [61,62] of NEC after receiving probiotics. There has been much enthusiasm surrounding similar results in human trials, with a reduced incidence of NEC following probiotic administration [56,63]. However, this excitement has been attenuated by the concern over their safety and little knowledge about the optimal species, dosing and duration of treatment to use. There have been reports of sepsis following probiotic therapy in immunocompromised children [64-66], which has raised the question of possible harm for similarly immunocompromised extremely preterm infants [67]. Specific bacteria that have been studied in clinical trials include species of Lactobacillus and Bifidobacterium, and Streptococcus thermophilus [68-75]. Lactobacillus rhamnosus GG (LGG) is of particular interest as it has been shown to be effective in preventing cytokine-induced apoptosis in adult intestinal epithelial cells [26,76] and is one of the most effective commensal species in mitigating inflammatory responses [22,58]. Our laboratory has recently reported that LGG can reduce apoptotic signaling in immature intestinal epithelia via upregulation of genes involved in cellular proliferation and migration and mitogen activated protein kinase (MAPK) pathways known to be important for growth, differentiation, and cytoprotection [49].
4. Probiotic bacteria regulate inflammatory and anti-inflammatory signaling in immature intestinal epithelia
In addition to regulating apopotic signaling, probiotic bacteria can regulate inflammatory signaling. We have recently shown that the probiotic LGG can regulate inflammation both by blocking inflammatory signaling via NF-κB [51] and by upregulating anti-inflammatory signaling via IL-10 [42]. Inflammation defends against harmful pathogens by alerting and directing cells of the innate immune system to a site of injury. The vast majority of the time this initial response works appropriately and guards the host from further damage, but uncontrolled inflammation may lead to acute or chronic inflammatory diseases. With a lack of commensal bacteria that have the ability to control inflammation [77], the premature intestinal environment is predisposed to exaggerated inflammatory responses, possibly leading to NEC [8,78]. In animal NEC models, the expression of known pro-inflammatory cytokines TNF-α [79-82], MIP-2 [83], and IL-6 [84,85] have been shown to be increased. Activation of the IL-10 pathway negatively regulates the expression of these cytokines [86-92], and there has been speculation that IL-10-dependent suppression of inflammatory mediators may be a final common pathway protecting the developing intestine from uncontrolled inflammation and NEC [42]. Indeed, IL-10 deficient mice develop spontaneous colitis [93] and are more susceptible to NEC [94]. Also, mutations in genes encoding IL-10 receptor subunit proteins were found in multiple infants who had severe, early onset enterocolitis [95].
Using our murine model of premature intestines, we recently reported that intestinal epithelial inflammatory responses peak at 2 weeks in the neonatal mouse. Further, we demonstrated that probiotic bacteria (LGG) could block intestinal epithelial inflammatory signaling via NF-κB by inducing homeostatic ROS signaling. Probiotic and commensal bacteria can induce intestinal epithelial ROS production which in turn can regulate homeostatic processes via inactive oxidation of key regulatory enzymes. We demonstrated that in immature intestinal epithelia, LGG can induce physiologic ROS expression which in turn prevents NF-κB activation through oxidative inactivation of its regulatory enzyme, Ubc12. Physiologic ROS signaling is distinct from oxidative stress (defined as an imbalance in the oxidation–reduction pathways leading to excessive ROS generation) and has been implicated in many disease processes [96-99]. Diseases specific to prematurity such as retinopathy of prematurity, chronic lung disease, intraventricular hemorrhage and NEC have all been linked to oxidative stress, but attempts at administering antioxidants to premature infants have led to disappointing results [100-102]. This is likely because physiologic ROS signaling regulates many homeostatic processes [99], and thus, the global suppression of that signaling may lead to undesirable effects. Expounding on the mechanisms by which commensal bacteria or probiotics induce innate immune responses via intestinal epithelial ROS regulation could be key to developing targeted preventive therapies for intestinal inflammatory diseases in neonates and children.
We also recently discovered that LGG can regulate anti-inflammatory pathways in the developing murine gut. Following the administration of LGG, the colonic expression of MIP-2 and TNF-α were decreased and the colonic expression of the IL-10R2 receptor subunit was induced [42]. We also demonstrated that the reduction in pro-inflammatory cytokine expression was dependent on the IL-10 receptor. These results suggest that the anti-inflammatory effects of LGG are mediated through induction of the IL-10R2 receptor subunit. Future studies in this area will focus on cell-specific sources of IL-10 and IL-10 receptors.
5. Commensal and probiotic bacteria improve tight junction-dependent epithelial barrier function in the developing intestine
Proper functioning of the epithelial barrier is imperative for intestinal health, and the breakdown of this critical defense has been implicated in the pathogenesis of multiple intestinal inflammatory diseases, including idiopathic inflammatory bowel disease (IBD) [103,104], infectious enteritis, and NEC [7,105-107]. Early postnatal maturation of the epithelium leads to more selective permeability in both premature neonates [108,109] and neonatal animal models [110-112]. As an essential component of barrier function, tight junctions (TJs) regulate paracellular permeability and maintain separation of tissue compartments by sealing the intercellular space [113,114]. Studies have implicated abnormal TJ protein expression in the development of an impaired barrier function in both IBD [115] and rodent models of NEC [116,117]. The presence of commensal bacteria has been shown to maintain and improve barrier function through interactions with toll-like receptors (TLRs) [118] and by the up-regulation of beneficial genes [23]. The abnormal microbial colonization patterns and lack of normal commensal bacteria in premature neonates can further compromise their intestinal barrier, which may lead to intestinal inflammation and injury secondary to the systemic entry of toxin from the gut lumen [108,119-121].
Using our murine model of intestinal development, we characterized intestinal barrier function and TJ expression in the immature gut [52]. We demonstrated that intestinal barrier function (as measured by gut permeability) matures between 2 and 3 weeks of life. Claudin 3 was the most upregulated TJ protein during this developmental period. Further, we determined that both commensal and probiotic bacteria were able to induce maturation of both intestinal barrier function and Claudin 3 expression, likely via toll-like receptor signaling. Finally, we were able to demonstrate for the first time that both live and heat-killed probiotic bacteria (LGG) could accelerate maturation of intestinal barrier function and Claudin 3 expression in vivo. These studies provide evidence for and a potential mechanism by which postbiotics may be used to mature key host defenses in order to prevent NEC in the premature gut.
6. Summary
Commensal bacteria foster a healthy intestinal microbiome, and these resident bacteria are vital in protecting the host from devastating intestinal diseases, including NEC. Infants born prematurely have low bacterial diversity due to their immaturity, hospital environment, and necessary medical care. Premature intestines also have immature host defenses which may lead to increased apoptotic signaling, increased inflammatory signaling, and reduced gut barrier function. Prebiotic, probiotic, or postbiotic therapy may be able to restore essential strains of commensal flora necessary for maturation of these intestinal host defenses in the preterm gut. Lactobacillus species specifically appear to be a commensal that is lacking in preterm infants and is also more susceptible to eradication in response to antibiotic treatment and stress [11]. We have shown that Lactobacillus species can augment host intestinal defenses by promoting cytoprotective gene expression and anti-inflammatory signaling, blocking inflammatory signaling, and improving gut barrier function (Fig. 1). Further exploration of the mechanisms by which probiotics and commensal bacteria exert their effects will hopefully result in effective therapies for the premature neonate that will decrease the incidence and severity of NEC.
Fig. 1.
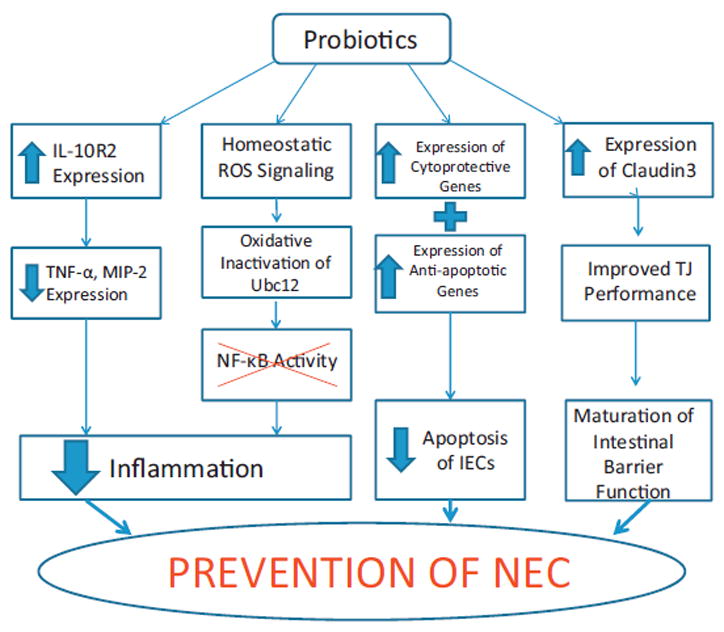
Overview of mechanisms by which probiotics exert their effects, leading to a decreased incidence of NEC.
Abbreviations
- IFN
interferon
- NF-κB
nuclear factor kappa B
- IL
interleukin
- TNF-α
tumor necrosis factor alpha
- MIP
macrophage inflammatory protein
- ROS
reactive oxygen species
- LGG
Lactobacillus rhamnosus GG
References
- 1.Guillet R, Stoll BJ, Cotten CM, Gantz M, McDonald S, Poole WK, Phelps DL H. National Institute of Child, N. Human Development Neonatal Research. Association of H2-blocker therapy and higher incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2006;117:e137–e142. doi: 10.1542/peds.2005-1543. [DOI] [PubMed] [Google Scholar]
- 2.Horbar JD, Badger GJ, Carpenter JH, Fanaroff AA, Kilpatrick S, LaCorte M, Phibbs R, Soll RF N. Members of the Vermont Oxford. Trends in mortality and morbidity for very low birth weight infants, 1991–1999. Pediatrics. 2002;110:143–151. doi: 10.1542/peds.110.1.143. [DOI] [PubMed] [Google Scholar]
- 3.Lemons JA, Bauer CR, Oh W, Korones SB, Papile LA, Stoll BJ, Verter J, Temprosa M, Wright LL, Ehrenkranz RA, Fanaroff AA, Stark A, Carlo W, Tyson JE, Donovan EF, Shankaran S, Stevenson DK. Very low birth weight outcomes of the National Institute of Child health and human development neonatal research network, January 1995 through December 1996. NICHD Neonatal Research Network. Pediatrics. 2001;107:E1. doi: 10.1542/peds.107.1.e1. [DOI] [PubMed] [Google Scholar]
- 4.Hintz SR, Kendrick DE, Stoll BJ, Vohr BR, Fanaroff AA, Donovan EF, Poole WK, Blakely ML, Wright L, Higgins R NNR Network. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics. 2005;115:696–703. doi: 10.1542/peds.2004-0569. [DOI] [PubMed] [Google Scholar]
- 5.Shah TA, Meinzen-Derr J, Gratton T, Steichen J, Donovan EF, Yolton K, Alexander B, Narendran V, Schibler KR. Hospital and neurodevelopmental outcomes of extremely low-birth-weight infants with necrotizing enterocolitis and spontaneous intestinal perforation. Journal of Perinatology: Official Journal of the California Perinatal Association. 2012;32:552–558. doi: 10.1038/jp.2011.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai D, Walker WA. Role of bacterial colonization in neonatal necrotizing enterocolitis and its prevention. Zhonghua Min Guo xiao er ke yi xue hui za zhi [Journal]. Zhonghua Minguo xiao er ke yi xue hui. 1998;39:357–365. [PubMed] [Google Scholar]
- 7.Grave GD, Nelson SA, Walker WA, Moss RL, Dvorak B, Hamilton FA, Higgins R, Raju TN. New therapies and preventive approaches for necrotizing enterocolitis: report of a research planning workshop. Pediatric Research. 2007;62:510–514. doi: 10.1203/PDR.0b013e318142580a. [DOI] [PubMed] [Google Scholar]
- 8.Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet. 2006;368:1271–1283. doi: 10.1016/S0140-6736(06)69525-1. [DOI] [PubMed] [Google Scholar]
- 9.Neu J. Neonatal necrotizing enterocolitis: an update. Acta Paediatrica. 2005;94:100–105. doi: 10.1111/j.1651-2227.2005.tb02163.x. [DOI] [PubMed] [Google Scholar]
- 10.Berg RD. The indigenous gastrointestinal microflora. Trends in Microbiology. 1996;4:430–435. doi: 10.1016/0966-842x(96)10057-3. [DOI] [PubMed] [Google Scholar]
- 11.Mshvildadze M, Neu J, Mai V. Intestinal microbiota development in the premature neonate: establishment of a lasting commensal relationship? Nutrition Reviews. 2008;66:658–663. doi: 10.1111/j.1753-4887.2008.00119.x. [DOI] [PubMed] [Google Scholar]
- 12.Hooper LV. Bacterial contributions to mammalian gut development. Trends in Microbiology. 2004;12:129–134. doi: 10.1016/j.tim.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Morowitz MJ, Poroyko V, Caplan M, Alverdy J, Liu DC. Redefining the role of intestinal microbes in the pathogenesis of necrotizing enterocolitis. Pediatrics. 2010;125:777–785. doi: 10.1542/peds.2009-3149. [DOI] [PubMed] [Google Scholar]
- 14.McCracken VJ, Lorenz RG. The gastrointestinal ecosystem: a precarious alliance among epithelium, immunity and microbiota. Cellular Microbiology. 2001;3:1–11. doi: 10.1046/j.1462-5822.2001.00090.x. [DOI] [PubMed] [Google Scholar]
- 15.Mshvildadze M, Neu J. The infant intestinal microbiome: friend or foe? Early Human Development. 2010;86(Suppl. 1):67–71. doi: 10.1016/j.earlhumdev.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mshvildadze M, Neu J, Shuster J, Theriaque D, Li N, Mai V. Intestinal microbial ecology in premature infants assessed with non-culture-based techniques. The Journal of Pediatrics. 2010;156:20–25. doi: 10.1016/j.jpeds.2009.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agarwal R, Sharma N, Chaudhry R, Deorari A, Paul VK, Gewolb IH, Panigrahi P. Effects of oral Lactobacillus GG on enteric microflora in low-birth-weight neonates. Journal of Pediatric Gastroenterology and Nutrition. 2003;36:397–402. doi: 10.1097/00005176-200303000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Cotten CM, Taylor S, Stoll B, Goldberg RN, Hansen NI, Sanchez PJ, Ambalavanan N, Benjamin DK, Jr N.N.R. Network. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123:58–66. doi: 10.1542/peds.2007-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noverr MC, Huffnagle GB. Does the microbiota regulate immune responses outside the gut? Trends in Microbiology. 2004;12:562–568. doi: 10.1016/j.tim.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Sudo N, Yu XN, Aiba Y, Oyama N, Sonoda J, Koga Y, Kubo C. An oral introduction of intestinal bacteria prevents the development of a long-term Th2-skewed immunological memory induced by neonatal antibiotic treatment in mice. Clinical and Experimental Allergy: Journal of the British Society for Allergy and Clinical Immunology. 2002;32:1112–1116. doi: 10.1046/j.1365-2222.2002.01430.x. [DOI] [PubMed] [Google Scholar]
- 21.Goldmann DA, Leclair J, Macone A. Bacterial colonization of neonates admitted to an intensive care environment. The Journal of Pediatrics. 1978;93:288–293. doi: 10.1016/s0022-3476(78)80523-x. [DOI] [PubMed] [Google Scholar]
- 22.Collier-Hyams LS, Sloane V, Batten BC, Neish AS. Cutting edge: bacterial modulation of epithelial signaling via changes in neddylation of cullin-1. Journal of Immunology. 2005;175:4194–4198. doi: 10.4049/jimmunol.175.7.4194. [DOI] [PubMed] [Google Scholar]
- 23.Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host–microbial relationships in the intestine. Science. 2001;291:881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 24.Kelly D, Campbell JI, King TP, Grant G, Jansson EA, Coutts AG, Pettersson S, Conway S. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-gamma and RelA. Nature Immunology. 2004;5:104–112. doi: 10.1038/ni1018. [DOI] [PubMed] [Google Scholar]
- 25.Tao Y, Drabik KA, Waypa TS, Musch MW, Alverdy JC, Schneewind O, Chang EB, Petrof EO. Soluble factors from Lactobacillus GG activate MAPKs and induce cytoprotective heat shock proteins in intestinal epithelial cells. American Journal of Physiology Cell Physiology. 2006;290:C1018–C1030. doi: 10.1152/ajpcell.00131.2005. [DOI] [PubMed] [Google Scholar]
- 26.Yan F, Polk DB. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. The Journal of Biological Chemistry. 2002;277:50959–50965. doi: 10.1074/jbc.M207050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guthrie SO, Gordon PV, Thomas V, Thorp JA, Peabody J, Clark RH. Necrotizing enterocolitis among neonates in the United States. Journal of Perinatology: Official Journal of the California Perinatal Association. 2003;23:278–285. doi: 10.1038/sj.jp.7210892. [DOI] [PubMed] [Google Scholar]
- 28.Llanos AR, Moss ME, Pinzon MC, Dye T, Sinkin RA, Kendig JW. Epidemiology of neonatal necrotising enterocolitis: a population-based study. Paediatric and Perinatal Epidemiology. 2002;16:342–349. doi: 10.1046/j.1365-3016.2002.00445.x. [DOI] [PubMed] [Google Scholar]
- 29.Stoll BJ, Kanto WP, Jr, Glass RI, Nahmias AJ, Brann AW., Jr Epidemiology of necrotizing enterocolitis: a case control study. The Journal of Pediatrics. 1980;96:447–451. doi: 10.1016/s0022-3476(80)80696-2. [DOI] [PubMed] [Google Scholar]
- 30.Stoll BJ. Epidemiology of necrotizing enterocolitis. Clinics in Perinatology. 1994;21:205–218. doi: 10.1016/S0095-5108(18)30341-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neu J, Walker WA. Necrotizing enterocolitis. The New England Journal of Medicine. 2011;364:255–264. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henning SJ. Development of the gastrointestinal tract. The Proceedings of the Nutrition Society. 1986;45:39–44. doi: 10.1079/pns19860033. [DOI] [PubMed] [Google Scholar]
- 33.Bauer E, Williams BA, Smidt H, Verstegen MW, Mosenthin R. Influence of the gastrointestinal microbiota on development of the immune system in young animals. Current Issues in Intestinal Microbiology. 2006;7:35–51. [PubMed] [Google Scholar]
- 34.McElroy SJ, Weitkamp JH. Innate immunity in the small intestine of the preterm infant. NeoReviews. 2011;12:e517–e526. doi: 10.1542/neo.12-9-e517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clarke RM, Hardy RN. An analysis of the mechanism of cessation of uptake of macromolecular substances by the intestine of the young rat (‘closure’) The Journal of Physiology. 1969;204:127–134. doi: 10.1113/jphysiol.1969.sp008903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Claud EC, Lu L, Anton PM, Savidge T, Walker WA, Cherayil BJ. Developmentally regulated IkappaB expression in intestinal epithelium and susceptibility to flagellin-induced inflammation. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:7404–7408. doi: 10.1073/pnas.0401710101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simon TC, Gordon JI. Intestinal epithelial cell differentiation: new insights from mice, flies and nematodes. Current Opinion in Genetics & Development. 1995;5:577–586. doi: 10.1016/0959-437x(95)80026-3. [DOI] [PubMed] [Google Scholar]
- 38.Henning SJ. Postnatal development: coordination of feeding, digestion, and metabolism. The American Journal of Physiology. 1981;241:G199–G214. doi: 10.1152/ajpgi.1981.241.3.G199. [DOI] [PubMed] [Google Scholar]
- 39.Carlile AE, Beck F. Maturation of the ileal epithelium in the young rat. Journal of Anatomy. 1983;137(Pt. 2):357–369. [PMC free article] [PubMed] [Google Scholar]
- 40.MohanKumar K, Kaza N, Jagadeeswaran R, Garzon SA, Bansal A, Kurundkar AR, Namachivayam K, Remon JI, Bandepalli CR, Feng X, Weitkamp JH, Maheshwari A. Gut mucosal injury in neonates is marked by macrophage infiltration in contrast to pleomorphic infiltrates in adult: evidence from an animal model. American Journal of Physiology Gastrointestinal and Liver Physiology. 2012;303:G93–G102. doi: 10.1152/ajpgi.00016.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pacha J. Development of intestinal transport function in mammals. Physiological Reviews. 2000;80:1633–1667. doi: 10.1152/physrev.2000.80.4.1633. [DOI] [PubMed] [Google Scholar]
- 42.Mirpuri J, Sotnikov I, Myers L, Denning TL, Yarovinsky F, Parkos CA, Denning PW, Louis NA. Lactobacillus rhamnosus (LGG) regulates IL-10 signaling in the developing murine colon through upregulation of the IL-10R2 receptor subunit. PLoS ONE. 2012;7:e51955. doi: 10.1371/journal.pone.0051955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sodhi CP, Neal MD, Siggers R, Sho S, Ma C, Branca MF, Prindle T, Jr, Russo AM, Afrazi A, Good M, Brower-Sinning R, Firek B, Morowitz MJ, Ozolek JA, Gittes GK, Billiar TR, Hackam DJ. Intestinal epithelial Toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology. 2012;143:708–718. e701–705. doi: 10.1053/j.gastro.2012.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sodhi CP, Shi XH, Richardson WM, Grant ZS, Shapiro RA, Prindle T, Jr, Branca M, Russo A, Gribar SC, Ma C, Hackam DJ. Toll-like receptor-4 inhibits enterocyte proliferation via impaired beta-catenin signaling in necrotizing enterocolitis. Gastroenterology. 2010;138:185–196. doi: 10.1053/j.gastro.2009.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walthall K, Cappon GD, Hurtt ME, Zoetis T. Postnatal development of the gastrointestinal system: a species comparison. Birth defects research Part B, Developmental and Reproductive Toxicology. 2005;74:132–156. doi: 10.1002/bdrb.20040. [DOI] [PubMed] [Google Scholar]
- 46.Maheshwari A, Kelly DR, Nicola T, Ambalavanan N, Jain SK, Murphy-Ullrich J, Athar M, Shimamura M, Bhandari V, Aprahamian C, Dimmitt RA, Serra R, Ohls RK. TGF-beta2 suppresses macrophage cytokine production and mucosal inflammatory responses in the developing intestine. Gastroenterology. 2011;140:242–253. doi: 10.1053/j.gastro.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang C, Sherman MP, Prince LS, Bader D, Weitkamp JH, Slaughter JC, McElroy SJ. Paneth cell ablation in the presence of Klebsiella pneumoniae induces necrotizing enterocolitis (NEC)-like injury in the small intestine of immature mice. Disease Models & Mechanisms. 2012;5:522–532. doi: 10.1242/dmm.009001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mollen KP, Gribar SC, Anand RJ, Kaczorowski DJ, Kohler JW, Branca MF, Dubowski TD, Sodhi CP, Hackam DJ. Increased expression and internalization of the endotoxin coreceptor CD14 in enterocytes occur as an early event in the development of experimental necrotizing enterocolitis. Journal of Pediatric Surgery. 2008;43:1175–1181. doi: 10.1016/j.jpedsurg.2008.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin PW, Nasr TR, Berardinelli AJ, Kumar A, Neish AS. The probiotic Lactobacillus GG may augment intestinal host defense by regulating apoptosis and promoting cytoprotective responses in the developing murine gut. Pediatric Research. 2008;64:511–516. doi: 10.1203/PDR.0b013e3181827c0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mirpuri J, Brazil JC, Berardinelli AJ, Nasr TR, Cooper K, Schnoor M, Lin PW, Parkos CA, Louis NA. Commensal Escherichia coli reduces epithelial apoptosis through IFN-alphaA-mediated induction of guanylate binding protein-1 in human and murine models of developing intestine. Journal of Immunology. 2010;184:7186–7195. doi: 10.4049/jimmunol.0903116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin PW, Myers LE, Ray L, Song SC, Nasr TR, Berardinelli AJ, Kundu K, Murthy N, Hansen JM, Neish AS. Lactobacillus rhamnosus blocks inflammatory signaling in vivo via reactive oxygen species generation. Free Radical Biology & Medicine. 2009;47:1205–1211. doi: 10.1016/j.freeradbiomed.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patel RM, Myers LS, Kurundkar AR, Maheshwari A, Nusrat A, Lin PW. Probiotic bacteria induce maturation of intestinal claudin 3 expression and barrier function. The American Journal of Pathology. 2012;180:626–635. doi: 10.1016/j.ajpath.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ford H, Watkins S, Reblock K, Rowe M. The role of inflammatory cytokines and nitric oxide in the pathogenesis of necrotizing enterocolitis. Journal of Pediatric Surgery. 1997;32:275–282. doi: 10.1016/s0022-3468(97)90194-9. [DOI] [PubMed] [Google Scholar]
- 54.Santulli TV, Schullinger JN, Heird WC, Gongaware RD, Wigger J, Barlow B, Blanc WA, Berdon WE. Acute necrotizing enterocolitis in infancy: a review of 64 cases. Pediatrics. 1975;55:376–387. [PubMed] [Google Scholar]
- 55.Jilling T, Lu J, Jackson M, Caplan MS. Intestinal epithelial apoptosis initiates gross bowel necrosis in an experimental rat model of neonatal necrotizing enterocolitis. Pediatric Research. 2004;55:622–629. doi: 10.1203/01.PDR.0000113463.70435.74. [DOI] [PubMed] [Google Scholar]
- 56.Alfaleh K, Anabrees J, Bassler D, Al-Kharfi T. Probiotics for prevention of necrotizing enterocolitis in preterm infants. The Cochrane Database of Systematic Reviews. 2011;(3) doi: 10.1002/14651858.CD005496.pub3. CD005496. [DOI] [PubMed] [Google Scholar]
- 57.Conroy ME, Shi HN, Walker WA. The long-term health effects of neonatal microbial flora. Current Opinion in Allergy and Clinical Immunology. 2009;9:197–201. doi: 10.1097/ACI.0b013e32832b3f1d. [DOI] [PubMed] [Google Scholar]
- 58.Kumar A, Wu H, Collier-Hyams LS, Hansen JM, Li T, Yamoah K, Pan ZQ, Jones DP, Neish AS. Commensal bacteria modulate cullin-dependent signaling via generation of reactive oxygen species. The EMBO Journal. 2007;26:4457–4466. doi: 10.1038/sj.emboj.7601867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bourlioux P, Koletzko B, Guarner F, Braesco V. The intestine and its microflora are partners for the protection of the host: report on the Danone Symposium “The Intelligent Intestine,” held in Paris, June 14, 2002. The American Journal of Clinical Nutrition. 2003;78:675–683. doi: 10.1093/ajcn/78.4.675. [DOI] [PubMed] [Google Scholar]
- 60.Akisu M, Baka M, Yalaz M, Huseyinov A, Kultursay N. Supplementation with Saccharomyces boulardii ameliorates hypoxia/reoxygenation-induced necrotizing enterocolitis in young mice. European Journal of Pediatric Surgery: Official Journal of Austrian Association of Pediatric Surgery. [et al] = Zeitschrift fur Kinderchirurgie. 2003;13:319–323. doi: 10.1055/s-2003-43580. [DOI] [PubMed] [Google Scholar]
- 61.Butel MJ, Roland N, Hibert A, Popot F, Favre A, Tessedre AC, Bensaada M, Rimbault A, Szylit O. Clostridial pathogenicity in experimental necrotising enterocolitis in gnotobiotic quails and protective role of bifidobacteria. Journal of Medical Microbiology. 1998;47:391–399. doi: 10.1099/00222615-47-5-391. [DOI] [PubMed] [Google Scholar]
- 62.Caplan MS, Miller-Catchpole R, Kaup S, Russell T, Lickerman M, Amer M, Xiao Y, Thomson R., Jr Bifidobacterial supplementation reduces the incidence of necrotizing enterocolitis in a neonatal rat model. Gastroenterology. 1999;117:577–583. doi: 10.1016/s0016-5085(99)70450-6. [DOI] [PubMed] [Google Scholar]
- 63.Wang Q, Dong J, Zhu Y. Probiotic supplement reduces risk of necrotizing enterocolitis and mortality in preterm very low-birth-weight infants: an updated meta-analysis of 20 randomized, controlled trials. Journal of Pediatric Surgery. 2012;47:241–248. doi: 10.1016/j.jpedsurg.2011.09.064. [DOI] [PubMed] [Google Scholar]
- 64.Kunz AN, Fairchok MP, Noel JM. Lactobacillus sepsis associated with probiotic therapy. Pediatrics. 2005;116:517. doi: 10.1542/peds.2005-0475. [DOI] [PubMed] [Google Scholar]
- 65.Land MH, Rouster-Stevens K, Woods CR, Cannon ML, Cnota J, Shetty AK. Lactobacillus sepsis associated with probiotic therapy. Pediatrics. 2005;115:178–181. doi: 10.1542/peds.2004-2137. [DOI] [PubMed] [Google Scholar]
- 66.Patel RM, Lin PW. Developmental biology of gut-probiotic interaction. Gut Microbes. 2010;1:186–195. doi: 10.4161/gmic.1.3.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bell EF. Preventing necrotizing enterocolitis: what works and how safe? Pediatrics. 2005;115:173–174. doi: 10.1542/peds.2004-2360. [DOI] [PubMed] [Google Scholar]
- 68.Bin-Nun A, Bromiker R, Wilschanski M, Kaplan M, Rudensky B, Caplan M, Hammerman C. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. The Journal of Pediatrics. 2005;147:192–196. doi: 10.1016/j.jpeds.2005.03.054. [DOI] [PubMed] [Google Scholar]
- 69.Dani C, Biadaioli R, Bertini G, Martelli E, Rubaltelli FF. Probiotics feeding in prevention of urinary tract infection, bacterial sepsis and necrotizing enterocolitis in preterm infants. A prospective double-blind study. Biology of the Neonate. 2002;82:103–108. doi: 10.1159/000063096. [DOI] [PubMed] [Google Scholar]
- 70.Hoyos AB. Reduced incidence of necrotizing enterocolitis associated with enteral administration of Lactobacillus acidophilus and Bifidobacterium infantis to neonates in an intensive care unit. International Journal Of Infectious Diseases: Official Publication of the International Society for Infectious Diseases. 1999;3:197–202. doi: 10.1016/s1201-9712(99)90024-3. [DOI] [PubMed] [Google Scholar]
- 71.Lin HC, Su BH, Chen AC, Lin TW, Tsai CH, Yeh TF, Oh W. Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2005;115:1–4. doi: 10.1542/peds.2004-1463. [DOI] [PubMed] [Google Scholar]
- 72.Braga TD, da Silva GA, de Lira PI, de Carvalho Lima M. Efficacy of Bifidobacterium breve and Lactobacillus casei oral supplementation on necrotizing enterocolitis in very-low-birth-weight preterm infants: a double-blind, randomized, controlled trial. The American Journal of Clinical Nutrition. 2011;93:81–86. doi: 10.3945/ajcn.2010.29799. [DOI] [PubMed] [Google Scholar]
- 73.Mihatsch WA, Vossbeck S, Eikmanns B, Hoegel J, Pohlandt F. Effect of Bifidobacterium lactis on the incidence of nosocomial infections in very-low-birth-weight infants: a randomized controlled trial. Neonatology. 2010;98:156–163. doi: 10.1159/000280291. [DOI] [PubMed] [Google Scholar]
- 74.Sari FN, Dizdar EA, Oguz S, Erdeve O, Uras N, Dilmen U. Oral probiotics: Lactobacillus sporogenes for prevention of necrotizing enterocolitis in very low-birth weight infants: a randomized, controlled trial. European Journal of Clinical Nutrition. 2011;65:434–439. doi: 10.1038/ejcn.2010.278. [DOI] [PubMed] [Google Scholar]
- 75.Underwood MA, Salzman NH, Bennett SH, Barman M, Mills DA, Marcobal A, Tancredi DJ, Bevins CL, Sherman MP. A randomized placebo-controlled comparison of 2 prebiotic/probiotic combinations in preterm infants: impact on weight gain, intestinal microbiota, and fecal short-chain fatty acids. Journal of Pediatric Gastroenterology and Nutrition. 2009;48:216–225. doi: 10.1097/MPG.0b013e31818de195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology. 2007;132:562–575. doi: 10.1053/j.gastro.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Neish AS, Gewirtz AT, Zeng H, Young AN, Hobert ME, Karmali V, Rao AS, Madara JL. Prokaryotic regulation of epithelial responses by inhibition of IkappaB-alpha ubiquitination. Science. 2000;289:1560–1563. doi: 10.1126/science.289.5484.1560. [DOI] [PubMed] [Google Scholar]
- 78.Lin PW, Nasr TR, Stoll BJ. Necrotizing enterocolitis: recent scientific advances in pathophysiology and prevention. Seminars in Perinatology. 2008;32:70–82. doi: 10.1053/j.semperi.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 79.Halpern MD, Clark JA, Saunders TA, Doelle SM, Hosseini DM, Stagner AM, Dvorak B. Reduction of experimental necrotizing enterocolitis with anti-TNF-alpha. American Journal of Physiology Gastrointestinal and Liver Physiology. 2006;290:G757–G764. doi: 10.1152/ajpgi.00408.2005. [DOI] [PubMed] [Google Scholar]
- 80.Huang L, Tan X, Crawford SE, Hsueh W. Platelet-activating factor and endotoxin induce tumour necrosis factor gene expression in rat intestine and liver. Immunology. 1994;83:65–69. [PMC free article] [PubMed] [Google Scholar]
- 81.Sun X, Caplan MS, Hsueh W. Tumour necrosis factor and endotoxin synergistically activate intestinal phospholipase A2 in mice. Role of endogenous platelet activating factor and effect of exogenous platelet activating factor. Gut. 1994;35:215–219. doi: 10.1136/gut.35.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tan X, Hsueh W, Gonzalez-Crussi F. Cellular localization of tumor necrosis factor (TNF)-alpha transcripts in normal bowel and in necrotizing enterocolitis. TNF gene expression by Paneth cells, intestinal eosinophils, and macrophages. The American Journal of Pathology. 1993;142:1858–1865. [PMC free article] [PubMed] [Google Scholar]
- 83.Han XB, Liu X, Hsueh W, De Plaen IG. Macrophage inflammatory protein-2 mediates the bowel injury induced by platelet-activating factor. American Journal of Physiology Gastrointestinal and Liver Physiology. 2004;287:G1220–G1226. doi: 10.1152/ajpgi.00231.2004. [DOI] [PubMed] [Google Scholar]
- 84.Harris MC, Costarino AT, Jr, Sullivan JS, Dulkerian S, McCawley L, Corcoran L, Butler S, Kilpatrick L. Cytokine elevations in critically ill infants with sepsis and necrotizing enterocolitis. The Journal of Pediatrics. 1994;124:105–111. doi: 10.1016/s0022-3476(94)70264-0. [DOI] [PubMed] [Google Scholar]
- 85.Morecroft JA, Spitz L, Hamilton PA, Holmes SJ. Plasma cytokine levels in necrotizing enterocolitis. Acta Paediatrica. 1994:396. 18–20. doi: 10.1111/j.1651-2227.1994.tb13235.x. [DOI] [PubMed] [Google Scholar]
- 86.Aste-Amezaga M, Ma X, Sartori A, Trinchieri G. Molecular mechanisms of the induction of IL-12 and its inhibition by IL-10. Journal of Immunology. 1998;160:5936–5944. [PubMed] [Google Scholar]
- 87.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. The Journal of Experimental Medicine. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kasama T, Strieter RM, Lukacs NW, Lincoln PM, Burdick MD, Kunkel SL. Interleukin-10 expression and chemokine regulation during the evolution of murine type II collagen-induced arthritis. The Journal of Clinical Investigation. 1995;95:2868–2876. doi: 10.1172/JCI117993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kopydlowski KM, Salkowski CA, Cody MJ, van Rooijen N, Major J, Hamilton TA, Vogel SN. Regulation of macrophage chemokine expression by lipopolysaccharide in vitro and in vivo. Journal of Immunology. 1999;163:1537–1544. [PubMed] [Google Scholar]
- 90.Rajasingh J, Bord E, Luedemann C, Asai J, Hamada H, Thorne T, Qin G, Goukassian D, Zhu Y, Losordo DW, Kishore R. IL-10-induced TNF-alpha mRNA destabilization is mediated via IL-10 suppression of p38 MAP kinase activation and inhibition of HuR expression. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology. 2006;20:2112–2114. doi: 10.1096/fj.06-6084fje. [DOI] [PubMed] [Google Scholar]
- 91.Smallie T, Ricchetti G, Horwood NJ, Feldmann M, Clark AR, Williams LM. IL-10 inhibits transcription elongation of the human TNF gene in primary macrophages. The Journal of Experimental Medicine. 2010;207:2081–2088. doi: 10.1084/jem.20100414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Standiford TJ, Strieter RM, Lukacs NW, Kunkel SL. Neutralization of IL-10 increases lethality in endotoxemia. Cooperative effects of macrophage inflammatory protein-2 and tumor necrosis factor. Journal of Immunology. 1995;155:2222–2229. [PubMed] [Google Scholar]
- 93.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 94.Emami CN, Chokshi N, Wang J, Hunter C, Guner Y, Goth K, Wang L, Grishin A, Ford HR. Role of interleukin-10 in the pathogenesis of necrotizing enterocolitis. American Journal of Surgery. 2012;203:428–435. doi: 10.1016/j.amjsurg.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schaffer AA, Noyan F, Perro M, Diestelhorst J, Allroth A, Murugan D, Hatscher N, Pfeifer D, Sykora KW, Sauer M, Kreipe H, Lacher M, Nustede R, Woellner C, Baumann U, Salzer U, Koletzko S, Shah N, Segal AW, Sauerbrey A, Buderus S, Snapper SB, Grimbacher B, Klein C. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. The New England Journal of Medicine. 2009;361:2033–2045. doi: 10.1056/NEJMoa0907206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jones DP. Redefining oxidative stress. Antioxidants & Redox Signaling. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 97.Lambeth JD. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radical Biology & Medicine. 2007;43:332–347. doi: 10.1016/j.freeradbiomed.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Terada LS. Specificity in reactive oxidant signaling: think globally, act locally. The Journal of Cell Biology. 2006;174:615–623. doi: 10.1083/jcb.200605036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Veal EA, Day AM, Morgan BA. Hydrogen peroxide sensing and signaling. Molecular Cell. 2007;26:1–14. doi: 10.1016/j.molcel.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 100.Brion LP, Bell EF, Raghuveer TS. Vitamin E supplementation for prevention of morbidity and mortality in preterm infants. The Cochrane Database of Systematic Reviews. 2003;(4) doi: 10.1002/14651858.CD003665. CD003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Darlow BA, Austin NC. Selenium supplementation to prevent short-term morbidity in preterm neonates. The Cochrane Database of Systematic Reviews. 2003;(4) doi: 10.1002/14651858.CD003312. CD003312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Suresh GK, Davis JM, Soll RF. Superoxide dismutase for preventing chronic lung disease in mechanically ventilated preterm infants. The Cochrane Database of Systematic Reviews. 2001;(1) doi: 10.1002/14651858.CD001968. CD001968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Clayburgh DR, Shen L, Turner JR. A porous defense: the leaky epithelial barrier in intestinal disease, Laboratory Investigation. A Journal of Technical Methods and Pathology. 2004;84:282–291. doi: 10.1038/labinvest.3700050. [DOI] [PubMed] [Google Scholar]
- 104.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 105.Alfaleh K, Bassler D. Probiotics for prevention of necrotizing enterocolitis in preterm infants. The Cochrane Database of Systematic Reviews. 2008;(1) doi: 10.1002/14651858.CD005496.pub2. CD005496. [DOI] [PubMed] [Google Scholar]
- 106.Gordon PV, Swanson JR, Attridge JT, Clark R. Emerging trends in acquired neonatal intestinal disease: is it time to abandon Bell’s criteria? Journal of Perinatology: Official Journal of the California Perinatal Association. 2007;27:661–671. doi: 10.1038/sj.jp.7211782. [DOI] [PubMed] [Google Scholar]
- 107.Henry MC, Moss RL. Neonatal necrotizing enterocolitis. Seminars in Pediatric Surgery. 2008;17:98–109. [Google Scholar]
- 108.van Elburg RM, Fetter WP, Bunkers CM, Heymans HS. Intestinal permeability in relation to birth weight and gestational and postnatal age, Archives of Disease in Childhood. Fetal and Neonatal Edition. 2003;88:F52–F55. doi: 10.1136/fn.88.1.F52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Weaver LT, Laker MF, Nelson R. Intestinal permeability in the newborn. Archives of Disease in Childhood. 1984;59:236–241. doi: 10.1136/adc.59.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Foulkes EC, Mort TL, Buncher CR. Intestinal cadmium permeability in mature and immature rats, Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine. 1991;197:477–481. doi: 10.3181/00379727-197-43285. [DOI] [PubMed] [Google Scholar]
- 111.Lecce JG, Broughton CW. Cessation of uptake of macromolecules by neonatal guinea pig, hamster and rabbit intestinal epithelium (closure) and transport into blood. The Journal of Nutrition. 1973;103:744–750. doi: 10.1093/jn/103.5.744. [DOI] [PubMed] [Google Scholar]
- 112.Urao M, Okuyama H, Drongowski RA, Teitelbaum DH, Coran AG. Intestinal permeability to small- and large-molecular-weight substances in the newborn rabbit. Journal of Pediatric Surgery. 1997;32:1424–1428. doi: 10.1016/s0022-3468(97)90553-4. [DOI] [PubMed] [Google Scholar]
- 113.Anderson JM, Van Itallie CM. Tight junctions. Current Biology. 2008;18:R941–R943. doi: 10.1016/j.cub.2008.07.083. [DOI] [PubMed] [Google Scholar]
- 114.Balda MS, Fallon MB, Van Itallie CM, Anderson JM. Structure, regulation, and pathophysiology of tight junctions in the gastrointestinal tract. The Yale Journal of Biology and Medicine. 1992;65:725–735. discussion 737–740. [PMC free article] [PubMed] [Google Scholar]
- 115.Gassler N, Rohr C, Schneider A, Kartenbeck J, Bach A, Obermuller N, Otto HF, Autschbach F. Inflammatory bowel disease is associated with changes of enterocytic junctions. American Journal of Physiology Gastrointestinal and Liver Physiology. 2001;281:G216–G228. doi: 10.1152/ajpgi.2001.281.1.G216. [DOI] [PubMed] [Google Scholar]
- 116.Clark JA, Doelle SM, Halpern MD, Saunders TA, Holubec H, Dvorak K, Boitano SA, Dvorak B. Intestinal barrier failure during experimental necrotizing enterocolitis: protective effect of EGF treatment, American Journal of Physiology. Gastrointestinal and Liver Physiology. 2006;291:G938–G949. doi: 10.1152/ajpgi.00090.2006. [DOI] [PubMed] [Google Scholar]
- 117.Shiou SR, Yu Y, Chen S, Ciancio MJ, Petrof EO, Sun J, Claud EC. Erythropoietin protects intestinal epithelial barrier function and lowers the incidence of experimental neonatal necrotizing enterocolitis. The Journal of Biological Chemistry. 2011;286:12123–12132. doi: 10.1074/jbc.M110.154625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 119.Laukoetter MG, Bruewer M, Nusrat A. Regulation of the intestinal epithelial barrier by the apical junctional complex. Current Opinion in Gastroenterology. 2006;22:85–89. doi: 10.1097/01.mog.0000203864.48255.4f. [DOI] [PubMed] [Google Scholar]
- 120.Piena-Spoel M, Albers MJ, ten Kate J, Tibboel D. Intestinal permeability in newborns with necrotizing enterocolitis and controls: does the sugar absorption test provide guidelines for the time to (re-) introduce enteral nutrition? Journal of Pediatric Surgery. 2001;36:587–592. doi: 10.1053/jpsu.2001.22288. [DOI] [PubMed] [Google Scholar]
- 121.Weaver LT, Laker MF, Nelson R, Lucas A. Milk feeding and changes in intestinal permeability and morphology in the newborn. Journal of Pediatric Gastroenterology and Nutrition. 1987;6:351–358. doi: 10.1097/00005176-198705000-00008. [DOI] [PubMed] [Google Scholar]


