Abstract
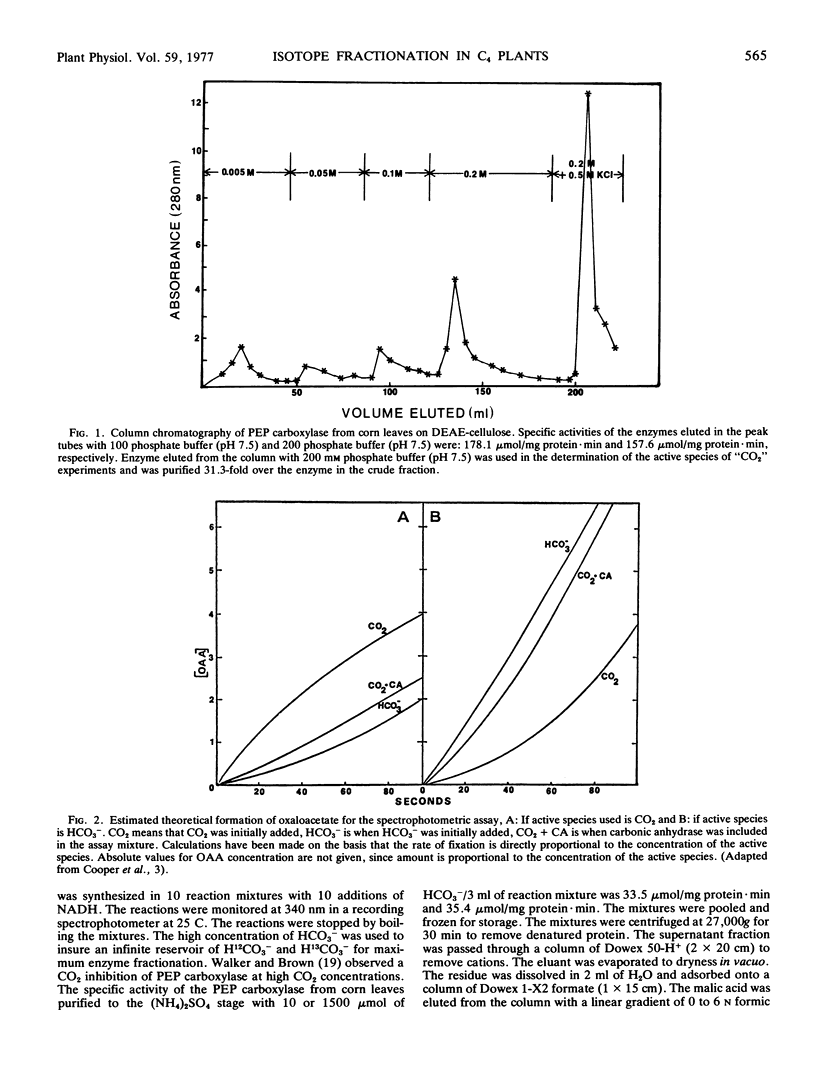
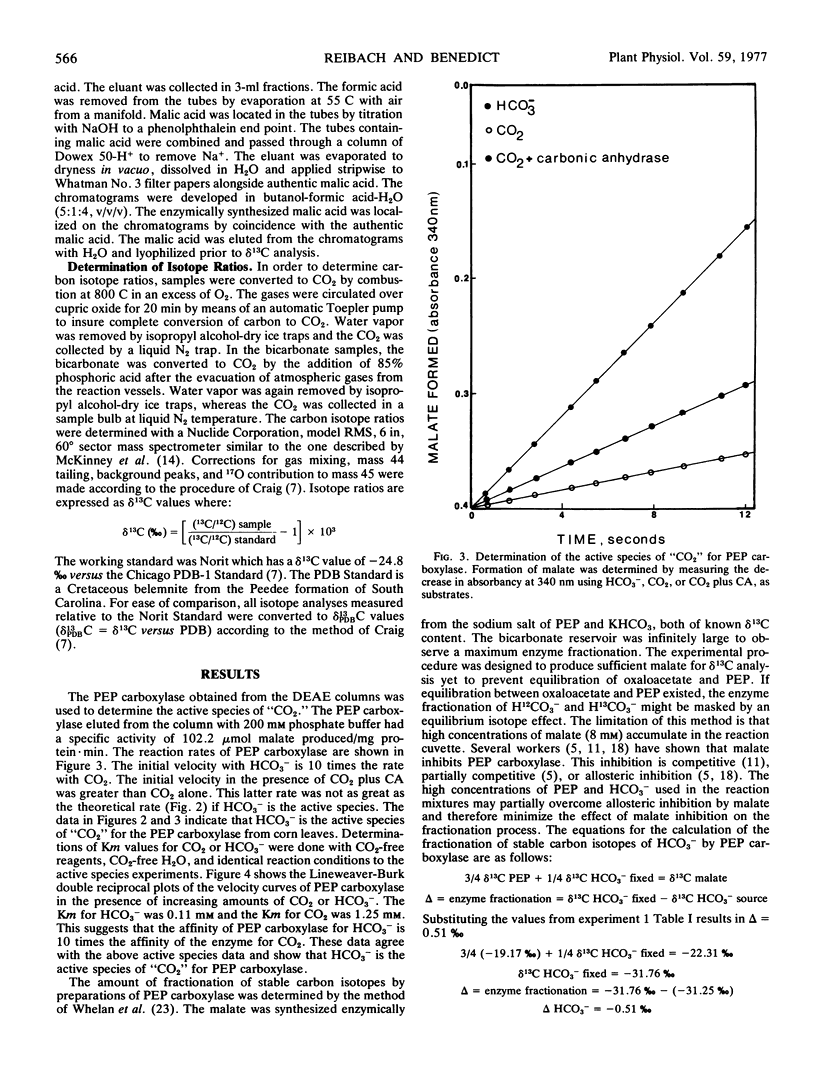
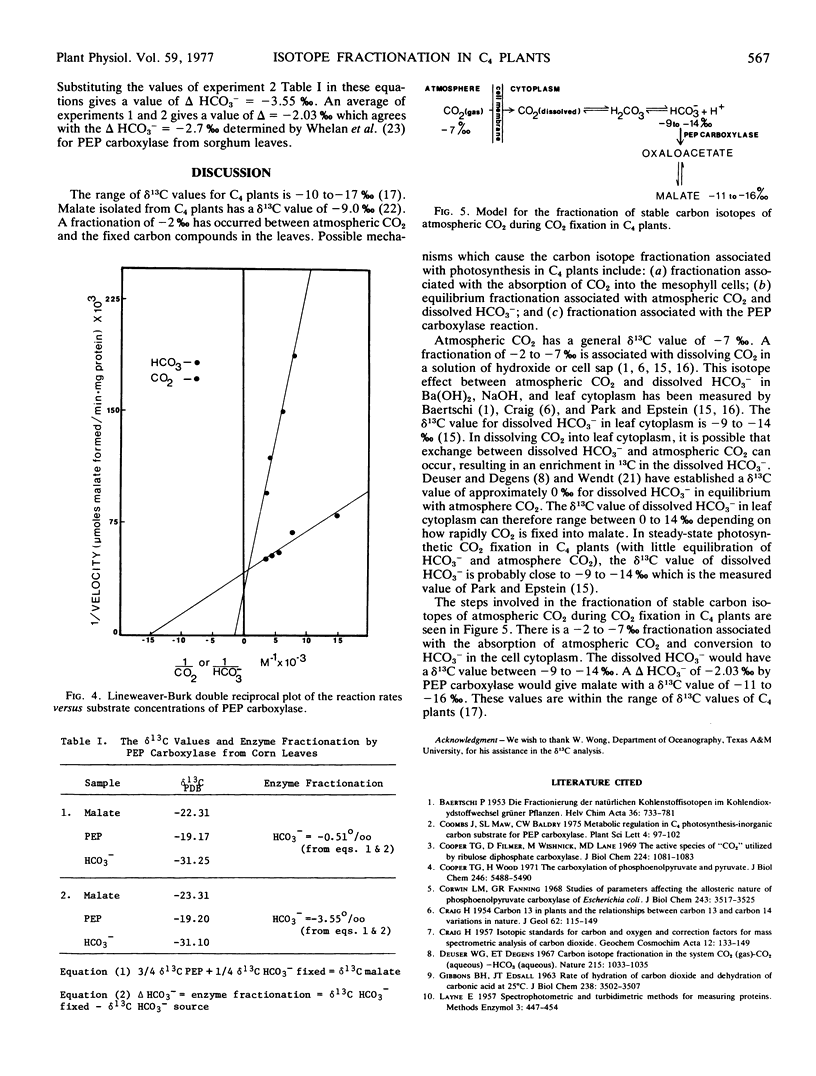
The active species of “CO2” and the amount of fractionation of stable carbon isotopes have been determined for a partially purified preparation of phosphoenolpyruvate (PEP) carboxylase (EC 4.1.1.31) from corn (Zea mays) leaves. The rates of the enzyme reactions, using substrate amounts of HCO3−, CO2 or CO2 plus carbonic anhydrase, show that HCO3− is the active species of “CO2” utilized by PEP carboxylase. The Km values for CO2 and HCO3− are 1.25 mm and 0.11 mm, respectively, which further suggest the preferential utilization of HCO3− by PEP carboxylase. The amount of fractionation of stable carbon isotopes by PEP carboxylase from an infinite pool of H12CO3− and H13CO3− was −2.03‰. This enzyme fractionation (δ), together with the fractionation associated with absorption of CO2 into plant cells and the equilibrium fractionation associated with atmospheric CO2 and dissolved HCO3− are discussed in relation to the fractionation of stable carbon isotopes of atmospheric CO2 during photosynthesis in C4 plants.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooper T. G., Filmer D. The active species of "CO2" utilized by ribulose diphosphate carboxylase. J Biol Chem. 1969 Feb 10;244(3):1081–1083. [PubMed] [Google Scholar]
- Cooper T. G., Wood H. G. The carboxylation of phosphoenolpyruvate and pyruvate. II. The active species of "CO2" utilized by phosphoenlpyruvate carboxylase and pyruvate carboxylase. J Biol Chem. 1971 Sep 10;246(17):5488–5490. [PubMed] [Google Scholar]
- Corwin L. M., Fanning G. R. Studies of parameters affecting the allosteric nature of phosphoenolpyruvate carboxylase of Escherichia coli. J Biol Chem. 1968 Jun 25;243(12):3517–3525. [PubMed] [Google Scholar]
- GIBBONS B. H., EDSALL J. T. RATE OF HYDRATION OF CARBON DIOXIDE AND DEHYDRATION OF CARBONIC ACID AT 25 DEGREES. J Biol Chem. 1963 Oct;238:3502–3507. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maruyama H., Easterday R. L., Chang H. C., Lane M. D. The enzymatic carboxylation of phosphoenolpyruvate. I. Purification and properties of phosphoenolpyruvate carboxylase. J Biol Chem. 1966 May 25;241(10):2405–2412. [PubMed] [Google Scholar]
- McKINNEY C. R., McCREA J. M., EPSTEIN S., ALLEN H. A., UREY H. C. Improvements in mass spectrometers for the measurement of small differences in isotope abundance ratios. Rev Sci Instrum. 1950 Aug;21(8):724–730. doi: 10.1063/1.1745698. [DOI] [PubMed] [Google Scholar]
- Park R., Epstein S. Metabolic fractionation of C & C in plants. Plant Physiol. 1961 Mar;36(2):133–138. doi: 10.1104/pp.36.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting I. P., Osmond C. B. Photosynthetic phosphoenolpyruvate carboxylases: characteristics of alloenzymes from leaves of c(3) and c(1) plants. Plant Physiol. 1973 Mar;51(3):439–447. doi: 10.1104/pp.51.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan T., Sackett W. M., Benedict C. R. Carbon isotope discrimination in a plant possessing the C4 dicarboxylic acid pathway. Biochem Biophys Res Commun. 1970 Dec 9;41(5):1205–1210. doi: 10.1016/0006-291x(70)90214-7. [DOI] [PubMed] [Google Scholar]
- Whelan T., Sackett W. M. Enzymatic fractionation of carbon isotopes by phosphoenolpyruvate carboxylase from c(4) plants. Plant Physiol. 1973 Jun;51(6):1051–1054. doi: 10.1104/pp.51.6.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]


