Abstract
Mood disorders (MDs) are chronic, recurrent mental diseases that affect millions of individuals worldwide. Although the biogenic amine model has provided some clinical utility, a need remains to better understand the interrelated mechanisms that contribute to neuroplasticity deficits in MDs and the means by which various therapeutics mitigate them. Of those therapeutics being investigated, physical activity (PA) has shown clear and consistent promise. Accordingly, the aims of this review are to (1) explicate key modulators, processes, and interactions that impinge upon multiple susceptibility points to effectuate neuroplasticity deficits in MDs; (2) explore the putative mechanisms by which PA mitigates these features; (3) review protocols used to induce the positive effects of PA in MDs; and (4) highlight implications for clinicians and researchers.
1. Introduction
Major depressive disorder (MDD) and bipolar disorder (BP) are chronic mood disorders (MDs) that adversely affect over 400 million persons worldwide [1]. Pathognomonic features of MDD include the persistence of one or more episodes of sadness or anhedonia in a two-week period, together with a range of cognitive and somatic symptoms (e.g., changes in appetite, sleep patterns, energy level, concentration, or physical activity and feelings of worthlessness and guilt) [2]. In BP, persons exhibit similar symptoms in the depressive phase but alternate to euphoric states during the manic phase—a state characterized by excessive activity and libido and grandiose thinking [2]. Recent attention has focused on the inability of extant treatment approaches to induce remission of symptoms in a significant number of affected persons [3, 4], prompting the diversification of efforts to derive more effective treatment strategies.
Fortunately, convergent evidence demonstrates that physical activity (PA) confers neuroplastic effects [5, 6] and may serve as an effective intervention for MDs [7–12]. Physical exercise is a subcategory of PA that connotes purposeful, planned, and structured endeavors undertaken to improve skill or physical fitness level [12]. PA alters the progression of MD neuropathology by optimizing the levels of neurotransmitters [13], neurotrophic factors [13, 14], beta-endorphins [15], cortisol [16, 17], and muscle-derived protein (peroxisome proliferator-activated receptor gamma coactivator 1-α [PGC-1α]) [18]. Moreover, regular PA optimizes processes involved in neurogenesis [19, 20], immune function [21, 22], stress regulation [23, 24], antioxidant defense [25, 26], circadian rhythms [27–29], epigenetic modifications [30, 31], and the maintenance of telomere length [32–35].
Via these complex and interrelated mechanisms, PA may reduce the risk for MDs [36–38], the degree of symptoms [10, 39], the incidence of relapse [40, 41], and caregiver burden [42]. This evidence, along with its relatively low-risk profile and ease of implementation [43], has led to the incorporation of PA into basic clinical management protocols for MDs [44, 45]. Because it is important that clinicians and scientists understand the means by which PA can alter pathophysiological substrates, from both a self- and patient-education perspective, the aims of this review are to (1) elucidate key substrates implicated in MD pathobiology, (2) explore the mechanisms by which PA can mitigate them, (3) examine protocols used to effectuate the positive effects of PA in MDs, and (4) highlight implications for clinicians and scientists.
2. The Neurobiology of Major Depressive and Bipolar Disorders
Recent decades have noted dramatic progress in the neurobiological understanding and treatment of psychiatric conditions. On the one hand, psychiatrists have refined diagnostic categories based on clinical symptoms [46, 47]. On the other hand, neuroscientists have derived evidence of transdiagnostic biomarkers across psychiatric conditions [48–50], including those that modulate neuroplasticity substrates in MDs [51–54]. Early biologic theories focused upon neurotransmitters, particularly the biogenic amines [55]. Subsequent advances in technology heralded new abilities to characterize general and distinctive cellular and molecular mechanisms, genetic contributions, and structural correlates of psychiatric disease [56, 57]. Although a complete description of these advances is beyond the scope of this article, a cursory review of MD neurobiology will be presented before focusing on neuroplasticity substrates. The reader is referred to the following excellent reviews for a more comprehensive presentation on the neurobiology of MDD [3, 58–60] and BP [61–64].
2.1. Major Depressive Disorder
Neuroimaging studies of depression and analysis of surgical lesions (that induce or reverse depressive symptoms) have revealed mood circuits [65, 66]. Implicated in these circuits are several brain structures and regions, including the dorsal prefrontal cortex, ventral prefrontal cortex, anterior cingulate gyrus, amygdala, hippocampus, striatum, and thalamus [67–69]. Drevets et al. have emphasized the pathophysiological processes and dysfunction of multiple pathways that adversely affect mood circuits and structures [66, 70], including those related to genetic, epigenetic, and environmental factors. Results from a twin study suggest that the heritability of MDD is 38% [71]. Preclinical studies implicating epigenetic mechanisms have shown that maternal behavior alters the function of stress-related genes [72] and that antidepressants alter the regulation of DNA [73, 74]. Other studies have shown that depletion in neurotransmitter levels contributes to depressive symptoms [55, 75]: slow-acting neurotransmitters (e.g., dopamine, serotonin, and norepinephrine) appear to interact with signaling proteins found inside the cell membrane in a way that allows the receiving cells to process signals from glutamate and γ-aminobutyric acid (GABA). Accordingly, therapeutic agents for MDs were derived to increase monoamine transmission acutely, either by inhibiting neuronal reuptake or by inhibiting degradation in the synaptic cleft. While this strategy has demonstrated some utility in the alleviation of symptoms, the fact that monoamine depletion fails to produce depressive symptoms in healthy individuals [76] or worsen depressive symptoms in persons with MDD [77, 78] induced a more comprehensive search for mechanisms. Subsequent work has implicated general disruption in neurogenesis [79], trophic factor level and function [80], antioxidant defense [81], hypothalamic-pituitary-adrenal (HPA) axis function [82], immune regulation [83], neuroplasticity [84], and circadian rhythms [85, 86], changes that collectively contribute to neuronal network alterations [84, 87]. Interestingly, the patterns of disruption to neurogenesis, immune system function, and antioxidant defense in MDD are similar in many respects to the patterns of disruption that are seen in BP. Also notable is evidence that has suggested a “kindling process” wherein depressive episodes are triggered more readily over time [88] and the number of prior episodes is a better predictor of future episodes than life stress is [89].
2.2. Bipolar Disorder
The complex pathophysiology of BP is undoubtedly mediated by genetic and epigenetic factors acting in concert with environmental stressors [90] to effectuate functional and structural abnormalities in the interconnected limbic, striatal, and fronto-cortical neurotransmitter neuronal circuits [91] and in plasticity substrates [51–54]. A robust genetic basis for BP has been derived from familial and identical twin studies: concordance rates for BP among identical twins typically range from 40 to 70%, with the estimated heritability reaching as high as 90% [92]. Notwithstanding, genome-wide studies have failed to detect single-gene contributions, supporting the premise that BP is a polygenic condition [92]. Strikingly, while some distinctions in genetic risks exist between BP and other psychiatric conditions, a high degree of genetic overlap has been reported among BP, MDD, and schizophrenia [48, 93]. Putative interactions between genes and life stresses are thought to effectuate disruptions in homeostatic and neuroplastic mechanisms [94, 95] and increase the severity of symptoms [95]. Parallel work has demonstrated disruption of glucocorticoid signaling [96], neurogenesis [97], immune-inflammatory imbalance [98], and antioxidant defense [99], changes that contribute to increased loss of volume in brain regions vital for mood regulation and cognitive function [65]. The identification and understanding of mechanistic pathways common to BP and MDD offer an opportunity to deploy novel lifestyle interventions such as PA to target these disorders in an integrated fashion.
3. Neuroplasticity
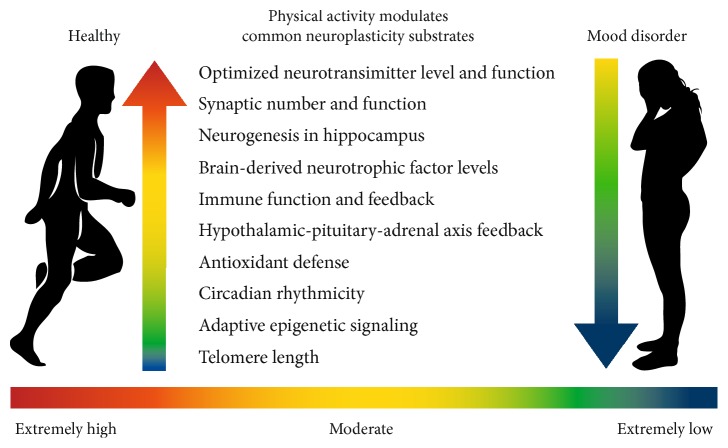
The ability of the central nervous system to continuously adapt to challenges is accomplished by neuroplasticity, a process whereinhttps://en.wikipedia.org/wiki/Neuronneurons change and reorganize to meet the demands of the environment [100]. Neuroplasticity is dependent upon stimulus-induced synaptic activity and membrane depolarization which, in turn, induce receptor trafficking, the activation of a multiplicity of genes, and the release of neurotransmitters. The secondary messengers effectuate downstream changes in the brain that permit its resculpting. Emotional and cognitive learning, neural homeostasis, and adjustments in behavior rely on biological correlates of neural plasticity for adaptation [53, 101]. Plastic changes in the brain can be maladaptive wherein a net loss of function occurs [102], a situation that reifies in MDs [103, 104]. On the other hand, brain plasticity can be adaptive when a gain of function occurs [105]. That is, PA can modulate common neuroplasticity substrates in the brain (as described in Figure 1) and then cognitive stimulation (e.g., cognitive behavioral therapy) can increase the likelihood of behavioral change in MDs [106].
Figure 1.
Physical activity modulates common neuroplasticity substrates in the brain. Here, the effects of various levels of PA are illustrated for the person who is healthy and the person with a MD.
4. Measurement of Voluntary Physical Activity in Humans
Voluntary PA refers to locomotor activity that is not directly required for survival or motivated by an external factor (such as searching for food, shelter, or mates; interacting with competitors; or avoiding predators) [107]. Human voluntary PA occurs in a multiplicity of ways and varies tremendously in both intensity and duration, both of which modulate its physiological consequences. Several indirect and direct assessment methods have been used to investigate the effects of voluntary PA in humans—including retrospective questionnaires, surveys, activity logs, motion sensors, heart rate monitors, calorimetry, and direct observation [108]—with different methods possessing unique strengths and weaknesses. The majority of early studies used self-reports of PA, particularly given their ease of administration, cost effectiveness, positive acceptance, and lack of intrusiveness on personal habits [109, 110]. Yet, while the administration of questionnaires at the population level has proved to be a feasible method of assessment, some evidence suggests that they are the least valid and reliable measure [107, 110–112]. In contrast, direct measures of voluntary PA assess energy expenditure [113] or actual movement [114] and are less susceptible to response and recall biases [113, 115, 116]. Notwithstanding, large-scale studies using direct measures have not been feasible in the general population [117]. Additionally, it seems plausible that long-term monitoring of PA with direct measures would sacrifice face validity by increasing intrusiveness and burden on participants [107, 116]. The inverse relationship between the validity and feasibility of assessment methods [107] has prompted recent investigations to use direct measures and standardized interventions in smaller populations. Studies of this type are vital because direct measures provide a means to examine a cause and effect relationship between PA and neuroplasticity substrates. Accordingly, several investigations that aim to determine the neurobiological, psychological, and physiological effects of PA on humans are systematically reviewed in Table 1. Analysis of these investigations reveals that PA generally produces antidepressant effects and improves cognitive function, enhances quality of life, improves sleep, optimizes brain-derived neurotrophic factor (BDNF) levels and function in the hippocampus, and enhances fitness measures.
Table 1.
Clinical trials of physical activity in persons with mood disorders. To determine the effects of PA on the brain in humans affected by MDs, a computer search of MEDLINE using the terms “mood disorder,” “physical activity,” and “exercise” was used to produce a list of interventional studies. Then, manual searches of key references were performed to identify additional studies. Articles met inclusion criteria if they were peer-reviewed interventional studies in persons diagnosed with MDD or BP. Articles were excluded if they were reviews, case reports, conference abstracts, expert opinions, or clinical studies of adolescents. Duplicate articles and those not available in English language were excluded also. Based on this search and subsequent screening, 37 articles that spanned from 1987 to 2016 were identified. Whereas extensive variations existed in the studies with regard to age, sex, degree of symptoms, phase of disease, and setting, 97% of RCTs (31 out of 32) that measured behavioral outcomes reported positive associations between PA and recovery from depressive symptoms [40, 41, 267, 316, 463–489] by utilizing training ranges of 100–250 min per week for a duration of 2–6 months [40, 41, 316, 463–468, 472–474, 476–485, 487, 488]. One report achieved relief of depressive symptoms following 60 minutes of PA for a duration of 5 weeks [489], whereas another study reported that participants obtained relief following PA 30 min/day for a duration of 1 week [470]. The modalities used in the programs varied, but most of the programs deployed some form of aerobic activity as a core component [40, 41, 96, 464–483, 485–488, 490, 491]. Notably, the one study that failed to find an association between PA and depressive symptoms used a relaxation group as a control [492], a fact that may be problematic given preliminary evidence that stress reduction activities reduce cortisol abnormalities and, in turn, may mitigate depressive symptoms [489]. The remaining studies reported that PA reduced sleep problems [382, 383, 487]; normalized BDNF levels in some studies [267, 268], but failed to do so in others [490]; and reduced cortisol levels [489]. Nevertheless, extant RCTs are still few and leave many questions unresolved.
| References | Sample | Modality | Frequency & duration of PA | Assessment |
|---|---|---|---|---|
| [463] | Mean age of 75 y/o with MDD (n = 121) | Sertraline only; sertraline + supervised nonprogressive PA (<70% peak heart rate); sertraline + supervised progressive aerobic activity (60% peak heart rate) |
60 min/session 3 d/wk for 24 wks | Reduced depressive symptoms on HAM-D and CGI in all groups, but earlier and higher remission rates in exercise groups at 4, 8, and 12 wks |
| [464] | 50 y/o or greater with MDD (n = 156) | Aerobic exercise (70–85% max HR); aerobic exercise (70–85% max HR) + standard medication; or standard medication only | Supervised 45 min sessions 3 d/wk × 16 wks | Reduced depressive symptoms on BDI and HAM-D in all groups, but response was quicker in medication-only group |
| [465] | 19–78 y/o with depressive symptoms (n = 112) |
Aerobic exercise outside during daylight hours (60% max HR) + prompts to take a specific vitamin regimen or control | 20 min per session 5 d/wk × 8 wks | Reduced depressive symptoms in both groups, but more so in exercise group; specifically, ↓ depressive symptoms on CES-D in exercise group; ↓ anger and tension on POMS in exercise group; ↑ vitality in exercise group |
| [466] | 18–65 y/o with MDD (n = 62) | Add-on aerobic exercise × 10 wks; add-on basic body awareness therapy × 10 wks; or single consult for advice on PA + care as usual | 55–60 min session 2 d/wk × 10 wks; group basic body awareness therapy 2 d/wk × 60 min; or advice on PA on one occasion | Reduced depressive symptoms on MADRS in all groups (−10.3 in aerobic PA, −5.8 in body awareness, and −4.6 in advice only group); ↑ cardiovascular fitness gains in aerobic exercise group; ↓ self-rated depression symptoms in PA and basic body awareness groups |
| [41] | 50 y/o or greater with MDD (n = 133) | Aerobic activity (70–85% max HR); aerobic activity (70–85% max HR) + sertraline; or sertraline only | Supervised 45 min sessions 3 d/wk × 16 wks then follow-up 24 wks after study conclusion | Reduced depressive symptoms on HAM-D; ↑ rate of partial or full recovery from depressive symptoms on HAM-D in exercise group; and ↓ rate of relapse for MDD in exercise group |
| [316] | 18–20 y/o with mild to moderate depression (n = 28) | Exercise regimen or usual daily activities | 50 min sessions 5 d/wk × 8 weeks for each regimen | Exercise regimen reduced depressive symptoms on CES-D; ↓ cortisol; and ↓ urinary secretion of epinephrine |
| [467] | 20–64 y/o with MDD (n = 82) | Aerobic exercise + care as usual or care as usual only | Progressive exercise 45–60 min per session 3 d/wk × 8 wks | Combination of exercise + fluoxetine group exhibited greater reduction in depressive symptoms on BDI and ICD-10 than fluoxetine alone |
| [468] | 18–35 y/o with MDD or minor depression (n = 40) | Aerobic (80% max HR); strength training (50–60% max HR); or control |
Supervised sessions 4 d/wk × 8 wks | Reduced depressive symptoms on BDI and HAM-D in both exercise groups following intervention and at 12 mo follow-up |
| [469] | 20–45 y/o with diagnosis of MDD (n = 80) | 4 aerobic exercise treatment groups that varied according to intensity: low dose (7.5 kcal/kg/wk for 3 or 5 d/wk × 12 wks); high dose (17.5 kcal/kg/wk for 3 or 5 d/wk × 12 wks); or control | Supervised aerobic activity × 12 wks | Reduced depressive symptoms on HAM-D for high-dose aerobic exercise (17.5 kcal/kg/wk 3–5 d/wk) |
| [470] | 20–53 y/o with MDD (n = 38), somatization syndrome (n = 26), or healthy controls (n = 47) |
Aerobic exercise or control | 30 min/d for 1 wk or reduced PA for 1 wk | Reduced depressive symptoms on BDI 2 following 1 wk of exercise in persons with MDD, but not other groups; ↑ monocytes in healthy controls, but not in persons with MDD or somatization syndrome |
| [471] | 18–65 y/o with MDD and sedentary lifestyle and with residual cognitive or attention impairments following tx with SSRIs for 8–12 wks (n = 39) | High-dose aerobic exercise (target of either 16 KKW—the equivalent to walking 4 mph × 210 min/wk) or low-dose aerobic control (4 KKW—the equivalent to walking 3.0 mph for 75 min/wk) | Initial supervision during sessions then transition to home-based program × 12 wks | Reduced depressive symptoms in both groups on IDS-C, but greater effect in high-dose exercise group; high dose PA ↑ spatial working memory and both groups ↑ cognitive function (psychomotor speed and executive function) |
| [472] | 60 y/o or greater women who were overweight or moderately depressed (n = 106) | Add-on supervised aerobic exercise + strengthening activities or usual care | Supervised 50 min session 3 d/wk × 24 wks | Reduced depressive symptoms and anxiety on GDS, STAI, and EQ-5D in intervention group; ↓ BMI in intervention group |
| [473] | 40 y/o or greater with diagnosis of MDD (n = 102) | Supervised aerobic exercises (70–85% of max HR); sertraline; or placebo | 45 min session 3 d/wk × 16 wks | Reduced depressive symptoms in both groups on HAM-D and BDI along with higher remission rates compared to placebo; ↔ between groups in verbal memory, verbal fluency, or working memory |
| [40] | Mean age of 51 y/o with MDD and sedentary (n = 202) | Supervised aerobic exercise (70–80% of max HR); home-based exercise; sertraline; or placebo | 45 min session 3 d/wk × 16 wks | At 12 mo follow-up, exercisers who reported 180 min/wk exhibited reduced depressive symptoms on HAM-D scores and a ↓ risk for relapse in comparison with persons who reported 0 min of exercise |
| [474] | 18 y/o or greater with MDD (n = 42) | Structured group exercise (50% max HR) or usual care |
45 min session 3 d/wk × 6 wks | Reduced depressive symptoms on MADRS and BDI-2 in both groups, but ↑ response (> 50% decrease of symptoms on MADRS) in exercise group; ↓ diastolic blood pressure in exercise group; ↓ waist circumference in exercise group; ↑ HDL in exercise group; ↑ cardiorespiratory capacity in exercise group |
| [475] | 75 y/o or greater with depressive symptoms (n = 193) | Individualized; home-based exercise program (i.e., balance, strength, and aerobic activity); or control | 52 wks | Reduced depressive symptoms on GDS and ↑ mental health-related quality of life in both groups, but no difference between groups |
| [476] | 18 y/o or greater with depressive symptoms (n = 23) | Low-frequency aerobic exercise (within target HR); high-frequency aerobic exercise; or high-frequency aerobic exercise + group team building intervention | 1 aerobic activity 30 min session 1 d/wk × 8 wks; 30 min session 3–5 d/wk × 8 wks; 30 min session 3–5 d/wk + group team building × 8 wks | Persons in high-frequency aerobic groups exhibited reduced depressive symptoms on BDI-2, but team-building intervention ↔ depressive symptoms |
| [477] | 22–63 y/o with depressive symptoms (n = 80) |
Aerobics + bright light or aerobics + normal light | Individualized aerobic training 2-3 d/wk × 8 wks | At 8 wks, reduced depressive symptoms on HAM-D and ATYP in both groups, but greater effect in aerobics + bright light group; ↑ in vitality on RAND in both groups, but more so in bright light group |
| [478] | 26–63 y/o with depressive symptoms (n = 98) |
Aerobics + bright light; aerobics + normal light; or stretching in bright light | Supervised sessions 2 d/wk × 8 wks | Reduced depressive symptoms on HAM-D in both aerobic groups; reduced depressive symptoms on SIGH-SAD-SR in aerobic + bright light group; ↔ in serum lipid levels or BMI in any group |
| [485] | 31–52 y/o with dysthymia and MDD (n = 99) | Add-on aerobic exercise (70% max HR); nonaerobic exercise; or usual care | Supervised 60 min sessions 3 d/wk × 8 wks | Reduced depressive symptoms on BDI in both exercise groups; ↑ VO2 max in aerobic exercise group |
| [479] | 21–70 y/o or greater with MDD or BD (n = 75) | Chronotherapeutic intervention (consisting of wake therapy, bright light therapy, sleep phase advance, and sleep time stabilization) or individualized aerobic exercise plan | 30 min sessions 5 d/wk × 29 wks | Reduced depressive symptoms on HAM-D in both groups, but even greater response in chronotherapy group—at 9 wks remission rate was 45% for chronotherapy group versus 23% for PA group and at 29 wks remission was 62% for chronotherapy group versus 38% for PA group |
| [480] | 53 y/o or greater with mood disorder who were poor responders to antidepressant meds (n = 86) |
Add-on exercise (aerobic, strengthening, and stretching) or health education talks | Supervised activity for 60 min session 2 d/wk × 10 wks | Reduced depressive symptoms on HAM-D in both groups, but response more positive in exercise group |
| [487] | 65 y/o or greater with and without depressive symptoms who are sedentary (n = 451) | Aerobic exercise (60 to 80% max HR) or progressive strength training (50–75% 1 rep max) | Supervised training 60 min session 3 d/wk × 10/wks | Reduced depressive symptoms on GDS in both strength training and aerobic exercise groups; ↑ plasma BDNF in strength training group |
| [481] | 60 y/o or greater with osteoarthritis of knee and depressive symptoms (n = 438) |
Aerobic exercise (50–70% max HR); strength training; or health education | Supervised walking 60 min session 3 d/wk then home-based aerobic activity × 15 mo or supervised progressive strength training 60 min session 3 d/wk × 3 mo + home-based continuation of training × 15 mo | Reduced depressive symptoms on CES-D in aerobic exercise group; ↔ depressive symptoms on CES-D in strength training group; both aerobic and strength training ↓ pain, ↓ self-reported disability, and ↑ walking speed |
| [486] | 50 y/o or greater with MDD (n = 200) | Add-on aerobic home-based program (target of 150 min per wk) and strength training + usual care or usual care only | Exercise 3 d/wk for strength training for all major muscle groups + 30 min session aerobic activity 5 d/wk × 12 wks | Reduced depressive symptoms on MADRS in both groups at 12-, 26-, and 52-week follow-up assessments |
| [489] | 18–65 y/o with MDD (n = 60) | Add-on yoga to quetiapine fumarate or escitalopram or no yoga | Supervised 60 session 1 d/wk × 5 wks | Reduced depressive symptoms on HAM-D; trend towards ↓ cortisol secretion in both groups |
| [482] | 18–60 y/o with MDD (n = 26) | Add-on aerobic exercise at patient selected intensity + usual care or usual care only | 16.5 kcal/kg/wk × 3 d/wk | Reduced depressive symptoms on HAM-D and QoL measure in psychological domain |
| [483] | 18–60 y/o severely depressed inpatients with MDD (n = 50) |
Add-on aerobic PA (with goal of 15.5 kcal/kg/wk) + usual care or usual care only |
Supervised session 3 d/wk (mean length 23.36 days ± 9 days) | Reduced depressive symptoms on HAM-D and ↑ quality of life (World Health Organization Quality of Life Assessment Instrument-Brief version (WHOQOL-BREF) during second wk of treatment and at discharge |
| [484] | 69–73 y/o with MDD, minor depressive symptoms, or dysthymia (n = 32) |
Progressive resistance training (3 sets of 8 repetitions of 80% 1 rep max) × 10 wks + unsupervised exercise or health education |
Supervised 45 min sessions 3 d/wk × 10 wks followed by unsupervised resistance training 2-3 d/wk × 10 wks | Reduced depressive symptoms in exercise group on BDI at 20 wks and 26 mo follow-up; ↑ morale on measures of aging on the Philadelphia Geriatric Morale Scale |
| [488] | 18–55 y/o with MDD (n = 57) | Add-on aerobic exercise (60–85% VO2 max) + sertraline or sertraline only |
Supervised sessions 4 d/wk × 4 wks | Reduced depressive symptoms on HAM-D in both groups, but response occurred with lower dosage in exercisers; ↑ VO2 max in exercisers |
| [492] | 18–55 y/o with MDD who were medicated and unmedicated and received psychotherapy (n = 165) | Strength training (2 or 3 trials of 12 reps at 50% max and increasing to 8 reps of 75% max); aerobic exercise (70% max heart rate); or control (stretching and relaxation groups (n = 55 for each) | Supervised training 90 min per session 2 d/wk × 16 wks | ↔ in depressive symptoms between three groups on HAM-D at 4 mo and 12 mo; ↔ in cognitive symptoms between the three groups at 4 mo and 12 mo |
| [268] | 50 y/o or greater with remitted MDD (n = 35) | Modified incremental walking protocol | Supervised single 30 min exercise bout | ↑ BDNF towards levels comparable to healthy controls |
| [267] | 22 y/o or greater with MDD (n = 18) | Progressive exercise until 125 beats per minute | Supervised single aerobic exercise bout | ↑ BDNF |
| [383] | 18–70 y/o with nonremitted MDD (n = 126) | Augmentation of SSRI with 16 kilocalories per kilogram of body weight per wk × 12 wks (equivalent to 150 min per wk at moderate intensity) or 4 kilocalories per kilogram of body weight per wk × 12 wks |
Sensor monitored and partially supervised × 12 wks | ↓ in hypersomnia on IDS-C, a change that was correlated with ↓ BDNF and ↓ IL-1β; lower baseline levels of IL-1β predicted greater improvements in insomnia |
| [490] | 18–60 y/o with MDD (n = 79) | Aerobic exercise (80% aerobic capacity) or control | Supervised 45 min sessions 3 d/wk × 3 mo | ↔ hippocampal volume; BDNF; VEGF; or IGF-1 in exercise group |
| [382] | 18–70 y/o with nonremitted MDD (n = 122) | Augmentation of SSRI with 16 kilocalories per kilogram of body weight per wk × 12 wks (equivalent to 150 min per wk at moderate intensity) or 4 kilocalories per kilogram of body weight per wk × 12 wks | Sensor monitored and partially supervised × 12 wks | ↓ insomnia as measured on IDS-C in both groups |
| [491] | 18–60 y/o with MDD (n = 53) versus healthy controls (n = 58) | Aerobic exercise (80% max heart rate) or control | Supervised sessions 45 min session 3 d/wk × 3 mo | ↓ at-rest levels of copeptin in participants with high exercise compliance |
ATYP: Atypical Depression Symptoms Addendum to Hamilton Depression Rating Scale; BAI: Beck Anxiety Inventory; BDI: Beck Depression Inventory; CES-D: Center for Epidemiologic Studies Depression; GWB: General Well-Being Schedule; GDS: Geriatric Depression Scale; HAM-D: Hamilton Depression Rating Scale; ICD-10-D: International Classification of Diseases-Depression; IDS-SR: Inventory of Depressive Symptomatology-Self Reported; POMS: Profile of Mood States; GCPS: Graded Chronic Pain Scale; CGI: Global Improvement of Depression; MADRS: Montgomery and Asberg Depression Rating Scale; QoL: quality of life; QALY: quality-adjusted life years using EuroQol (EQ-5D); RAND: RAND 36-Item Health Survey; SIGH-SAD-SR: Seasonal Affective Disorders Version Self-Rating Format; STAI: State-Trait Anxiety Inventory; WHOQOL-BREF: World Health Organization Quality of Life Assessment Instrument-Brief version.
5. Measurement of Voluntary Physical Activity in Animal Models
Multiple animal studies have been conducted to ascertain the effects of PA on brain structure and function. Specifically, investigators have deployed a voluntary wheel-running model in rodents to simulate PA in humans [118, 119], an avenue that provides unfettered hypothesis-driven discovery. Bolstering support for the model is evidence that (1) voluntary wheel running is a self-rewarding behavior that allows rodents to choose how much to run while avoiding the stress of forced running or investigator handling [107, 120, 121], (2) rodents show a conditioned preference to the place associated with wheel running [122] and can perform an instrumental reaction to garner access [123], (3) age-related decrements in PA occur in both rodents and humans [124, 125], (4) both running wheel access in rodents and voluntary PA in humans induce changes in brain reward systems [107], and (5) voluntary wheel running and voluntary PA occur in low-energy expenditure contexts such as laboratory housing and industrialized Western society [107, 126].
The historical preference for voluntary wheel running as opposed to forced treadmill running has derived from the notion that forced running on motorized treadmills may cause the release of stress-linked hormones, which could negate neuroplastic mechanisms. Bolstering this idea are preclinical and clinical studies that have shown that restriction of control during activity is deleterious [127–129], whereas the ability to exert control is beneficial for neurobiology and mental health [130]. Recently expanding on this notion, Greenwood and colleagues [131] sought to investigate the effects of forced and voluntary PA on emotional resilience. To do so, they designed a set of experiments with five exercise conditions: sedentary controls, forced treadmill training with motion initiated by foot shock, forced running on motorized wheels designed to approximate rats' voluntary running behavior, voluntary wheel running, and voluntary wheel running in an environment matched to the forced-wheel group. Then, all animals were exposed to stress-inducing conditions (restrained in Plexiglas tubes and exposed to uncontrollable tail shock), and their escape and fear responses were monitored. Interestingly, both forced and voluntary PA on running wheels prevented the behavioral consequences of stress, but forced treadmill training with foot shocks did not. The fact that wheel runners whose activity was forcibly started and stopped in naturalistic patterns (a model that mimics circuit training) also exhibited increased stress resistance [131] suggests that the ability of PA to regulate emotion and stress resilience may be modulated by the pattern of aerobic activity as long as (1) intensity, duration, and distance thresholds are sufficient; (2) the pattern of activity is naturalistic; and (3) the degree of stress is minimized [132].
While the study of affective symptoms in rodents is difficult, well-developed behavioral assays exist, as thoughtfully characterized in a review by Holmes [133]. From these studies, it has been shown that running reverses stress-related deficits and mitigates depressive and manic behavioral outcomes. Also, most of these studies report that PA increases neurogenesis in the hippocampus, reduces the length of corticosteroid exposure following stress, improves circadian rhythmicity, increases synaptic plasticity gene activity, and increases hippocampal BDNF in models of depression. A summary of preclinical studies that investigated the effects of voluntary wheel and motorized treadmill running on neurobiological, psychological, and physiological outcomes in rodents is systematically presented in Table 2.
Table 2.
Preclinical studies of physical activity in rodent models of mood disorder. To determine the effects of PA on the brain of rodents, a computer search of MEDLINE using the terms “voluntary wheel running,” “rodents,” “depression,” and “mania” was used to produce a list of studies. Then, manual searches of key references were performed to identify additional studies. Articles met inclusion criteria if they were peer-reviewed and performed in rodent models of depression or bipolar disorder. Articles were excluded if they were reviews, conference abstracts, or expert opinions. Duplicate articles and those not available in English language were excluded also. Based on this search and subsequent screening, a total of 28 articles that spanned from 2001 to 2016 were identified. Of those studies, 20 examined the effects of PA on behavioral outcomes. Strikingly, PA mitigated adverse outcomes in 95% (19 out of 20) of the studies that utilized behavioral measures, including those that examined depressive [493–508], anxiety [493, 496], and social-[495] and fear-avoidant behaviors [131, 509]. Another study reported that exercise mitigated manic-like behavior [250]. The remaining studies generally reported that exercise mitigated cognitive impairments [131, 499, 509], optimized the glucocorticoid response [500, 501, 509, 510] and neurotransmitter levels [497, 498, 511], optimized BDNF in the hippocampus [251, 493, 499, 500, 504, 505, 512, 513], increased neurogenesis [251, 502, 505, 506, 513], enhanced sleep [250, 514], and increased synaptic markers [508].
| References | MD model | Age (wks) | Treatment modality | Duration (wks) | Measure | Outcome |
|---|---|---|---|---|---|---|
| [496] | Wistar rats exposed to chronic unpredictable stress | 8 | Voluntary wheel running | 4 | Sucrose preference, elevated plus-maze, elevated T-maze, and forced-swim test |
Reduced depressive-like symptoms and ↓ anxiety symptoms in stressed rats |
| [499] | C57BL/6 mice exposed to chronic uncontrollable stress |
8-9 | Voluntary wheel running | 4 | Forced swim, tail suspension, water maze, and BDNF |
Reduced depressive-like symptoms, ↑ spatial memory, ↑ mature BDNF |
| [493] | C57BL/6 mice exposed to chronic uncontrollable stress |
“Adult” status, but actual age not specified | Voluntary wheel running | 3-4 | Learned helplessness, forced swim, tail suspension, elevated plus maze, and BDNF |
Reduced depressive-like symptoms and ↓ anxiety symptoms in wheel runners, ↑ hippocampal BDNF mRNA |
| [500] | Sprague-Dawley rats exposed to chronic uncontrollable stress | Not specified | Voluntary wheel running | 4 wks | Sucrose preference test, open field test, Morris water maze, corticoste rone, BDNF RNA, and glucocorticoid receptor RNA |
Reduced depressive-like symptoms, ↑ hippocampal BDNF RNA following stress, ↓ corticosterone following stress, ↑ glucocorticoid RNA receptor following stress |
| [501] | Sprague-Dawley rats exposed to chronic uncontrollable stress | “Adult” status, but actual age not specified | Voluntary wheel running | 4 wks voluntary wheel running prior to stress followed by 4 wks voluntary wheel running after stress | IgM antibodies, IgG2a antibodies, and distance ran |
Voluntary wheel running reduced stress-induced depressive-like symptoms and ↓ stress- induced alterations in immune function |
| [503] | Fischer F344 rats exposed to chronic uncontrollable stress | “Adult” status, but actual age not specified | Voluntary wheel running | 2 and 6 | Freezing behavior and shuttle-box escape learning | 6 wks running after exposure to uncontrollable stress reduced depressive-like symptoms and anxiety symptoms in runners |
| [515] | Fischer F344 rats exposed to chronic uncontrollable stress | 8 | Voluntary wheel running | 5 | Social exploration, shock-elicited freezing, escape behavior, corticosterone, and body weight |
Runners exhibited ↓ body weight, ↓ anxiety and reduced depressive-like symptoms, and ↓ corticosterone response to stress |
| [505] | C57BL/6 mice exposed to chronic uncontrollable stress | 8 | Stressed animals with no treatment, a dietary supplement (antioxidants), voluntary wheel running, or both dietary supplement & free access to running wheel |
4 | Neurogenesis, hippocampal BDNF mRNA, VGF serum, and saccharin preference |
Combination of diet and exercise (but neither alone) reduced depressive-like symptoms, ↑ neurogenesis in dentate gyrus in diet + exercise group, ↑ BDNF mRNA in hippocampus of exercise + diet group, and trend towards ↑ VGF in exercise + dietary supplement group only |
| [506] | ICR mice exposed to chronic uncontrollable stress | 8 | Treadmill running 60 min, treadmill running + SU1498 [a VGF receptor (Flk-1) inhibitor], and control |
2 | Open-field test, forced swim test, open-field test, BrdU, Ki67, and CD31 immunohistochemistry | Treadmill group exhibited ↑ hippocampal neurogenesis in dentate gyrus and ↓ anxiety and reduced depressive-like symptoms in a manner that is dependent on VEGF-Flk-1 signaling |
| [498] | Swiss mice | Age not specified | Voluntary wheel running | 3 | Forced swim, tail suspension, and open-field test |
Reduced depressive-like symptoms, an effect that appeared related to availability of bioamines |
| [507] | Diurnal sand rats housed in either short photoperiod (SP) (5 hr light/19 hr dark) or neutral light (12 light/12 dark) | 24 | Voluntary wheel running | 3 | Elevated plus-maze, forced-swim test, and social interaction | ↓ disruptions in activity rhythms of SP/exercise animals, ↓ anxiety and reduced depressive- like symptoms for SP/running wheel group, ↑ number and duration of social interaction for SP/running wheel group |
| [504] | C57BL/6J mice | 6-7 | Voluntary wheel running | 4 | VGF protein, BDNF protein, plasticity genes (Egr2, Grb2, ornithine decarboxylase-1, synapsin-1, and synCAM), forced-swim, and tail-suspension tests |
Reduced depressive- like symptoms, ↑ in VGF protein and ↑ in BDNF protein in hippocampus, altered synaptic plasticity gene profile activity |
| [502] | Flinders Sensitive Line (FSL) rat | 22 | Administered escitalopram, escitalopram + voluntary wheel running, vehicle diet, or vehicle diet + voluntary wheel running |
4 | BrdU immunohistochemistry and forced-swim test | ↑ in hippocampal neurogenesis in escitalopram, escitalopram + wheel running, and wheel running only, reduced depressive-like symptoms in wheel runners and escitalopram + wheel running groups |
| [497] | SwHi rats and SwLo rats | 6–14 | Voluntary wheel running | 3 | Forced-swim test | Reduced depressive-like symptoms in SwLo wheel runners with concomitant ↑ galanin mRNA in locus coeruleus |
| [508] | Sprague-Dawley rats | “Adult” status, but actual age not specified | Voluntary wheel running wheel during CORT administration; voluntary wheel running wheel prior to CORT administration; voluntary wheel running prior to and concurrent with CORT administration |
2 | Forced-swim test, sucrose- preference test, BrdU immunohistochemistry, synaptophysin, and BDNF |
Reduced depressive-like symptoms in animals that ran prior to and concurrent with CORT administration, ↑ neurogenesis and cell survival in animals that ran prior to and concurrent with CORT administration, ↔ BDNF or IGF-1 in animals running prior to and concurrent to CORT administration, ↑ synaptophysin proteins in animals running prior to and concurrent to CORT administration |
| [131] | Fischer 344 rats exposed to chronic uncontrollable stress | “Adult” status, but actual age not specified | Treadmills, motorized running wheels, or voluntary wheel running | 6 | Shock-elicited freezing and shuttle-box escape |
Both forced and voluntary wheel running reduced fear conditioning and ↓ cognitive impairments |
| [515] | Fischer 344 rats exposed to chronic uncontrollable stress | “Adult” status, but actual age not specified | Voluntary wheel running | 6 | Shuttle-box escape | Running reduced fear conditioning and cognitive learning, ↓ 5-HT2CR mRNA in basolateral amygdala and dorsal striatum |
| [495] | C57BL/6 mice exposed to chronic social defeat stress | 10 | Voluntary wheel running | <1 | Social-interaction test, open-field test, monoamines |
Reduced social avoidance in those that ran 2 hours after stress, ↔ anxiety levels, ↔ in depressive symptoms |
| [250] | Myshkin mice (Myk/+) | 6–12 | Administered melatonin or voluntary wheel running |
6 | Open-field test, elevated plus-maze, light–dark box, accelerating rotarod, EEG and EMG recordings during sleep, and BDNF protein |
Both melatonin and PA reduced manic behaviors, melatonin ↑ sleep duration, ↔ in hippocampal BDNF mRNA following exercise |
| [516] | C57BL/6 mice exposed to lipopolysacharide | 16 and 88 | Voluntary wheel running | 4 for young mice and 10 for aged mice | Tail-suspension, sucrose preference, TNF-a, IL-1B, IL-6, and IFN-γ |
↔ in depressive- like behavior in presence of ↑ TNF-a, IL-1B, IL-6, and IFN-γ |
| [511] | Fischer 344 rats exposed to acute uncontrollable stress | “Adult” status, but actual age not specified | Voluntary wheel running | 6-7 | Serotonin and dopamine in striatum | Running prevented stress-induced elevation of extracellular serotonin and potentiated dopamine concentration in the dorsal striatum |
| [494] | Outbred Hsd:ICR mice stressed by wheel removal | 7–9 wks | Voluntary wheel running | 6 days | Forced swim, tail- suspension, and behavioral despair |
High CORT males deprived access to running wheel showed ↑ depressive symptoms |
| [510] | C57BL/6 mice exposed to chronic uncontrollable stress | 6 | Voluntary wheel running | 4 | Corticosterone and adrenal weight | More rapid corticosterone response but returned to baseline more quickly in wheel runners, ↑ adrenal weight |
| [513] | C57BL/6 mice exposed to chronic uncontrollable stress | 8 | Voluntary wheel running | 3–6 | Corticosterone, BDNF, neurogenesis, open-field, elevated O-maze, dark- light box, forced swim, and learned helplessness |
↑ hippocampal BDNF, ↑ neurogenesis, ↑ cortico-sterone metabolites, ↑ anxiety-like behavior in novel and aversive environments |
| [512] | Fischer F344 rats exposed to chronic uncontrollable stress | “Adult” status, but actual age not specified | Voluntary wheel running | 3 and 6 | Shuttle-box escape and conditioned fear | 6 wks of running ↑ hippocampal BDNF mRNA and protein following stress |
| [251] | C57Bl/10 mice | 8 | Voluntary wheel running, fluoxetine, or combination of fluoxetine and voluntary wheel running |
3 | BDNF, IGF-1, and BrdU immunohistochemistry |
↑ in hippocampal BDNF in fluoxetine group only, and ↑ in neurogenesis with fluoxetine group only |
| [517] | TrkBhGFAP and TrkB Nestin mice that were conditionally ablated for the gene encoding TrkB, the high affinity receptor for BDNF, in a regional and cell-type-specific manner | Postnatal day 15 and “adult” status specified, but not actual age | Voluntary wheel running | 6 | Dark light test; open-field test; forced swim test, BrdU incorporating cells | neural Progenitor cell deletion of trkB, both in embryos and in the adult, causes ↓ hippocampal neurogenesis and ↓ behavioral improvements following chronic antidepressant administration or wheel running |
| [514] | F344 rats exposed to chronic uncontrollable stress | 8 | Voluntary wheel running prior to stress exposure | 6 | Sleep and temperature rhythms |
↑ entrainment of sleep/wake behavior and ↓ disruption of diurnal rhythms of sleep and temperature following stress |
PA and MDs in preclinical studies. BDNF: brain-derived neurotrophic factor; CORT: corticosterone; CUS: chronic unpredictable stress; SP: short photoperiod; STAT3: signal transducer and activator of transcription pathway 3; tx: treatment; VEGF: vascular endothelial growth factor.
Evidence that impairments in neural plasticity are implicated in MDs [103] and that PA modulates common neuroplasticity substrates (neurotransmitters, synaptic number and function, neurogenesis, BDNF, inflammation, stress reactivity, antioxidant defense, circadian rhythm, epigenetic modifications, and alteration of telomere length) in MDD and BP has led to numerous attempts to harness neuroplasticity to promote healing and recovery [134], particularly as it applies to psychiatric disease [135, 136]. Fortunately, convergent evidence suggests that long-term PA is positively correlated with positive neurobiological, affective, and cognitive outcomes, as reviewed below.
6. Neurotransmitter Levels and Function and Physical Activity
A pathognomonic feature of MDD [55, 137] and BP [137–139] is aberrant neurotransmitter level and function, a situation that can adversely affect synaptic plasticity. It has been suggested that depressive symptoms are caused in part by a deficiency in neurotransmitter levels (serotonin, norepinephrine, and dopamine), whereas manic symptoms are caused by excess levels, a notion premised on the study of psychological and cellular actions of several psychotropic agents [140]. Both types of fluctuation are problematic given the inverted U-shaped function of neurotransmitters: moderate levels are required for optimal emotional and cognitive function [141]. Fortunately, PA can optimize the synthesis, metabolism, and release of serotonin [142–146], norepinephrine [143, 147, 148], dopamine [143, 149, 150], and glutamate [151–153].
In the serotonergic system, the ability of PA to restore depleted neurotransmitter levels in the cerebral cortex, hypothalamus, brainstem, and hippocampus [143, 154] occurs via three primary mechanisms. PA increases the relative proportion of free tryptophan peripherally [155], a condition which favors influx across the blood-brain barrier [156]. Also, PA modulates the activity of tryptophan hydroxylase [157, 158] and inactivates indoleamine 2,3-dioxygenase and tryptophan 2,3-dioxygenase, which are rate-limiting enzymes that, following stress, shunt metabolism of tryptophan towards the kynurenine pathway in lieu of the serotonin pathway [156, 159, 160].
In the noradrenergic system, PA's neuroprotective effects stem from an adaptive response [145] wherein upregulated galanin expression hyperpolarizes noradrenergic neurons and thereby inhibits excessive norepinephrine release from the locus coeruleus [161–164]. Moreover, PA increases the conversion of cortisol to its inactivated form (cortisone) [165] to dampen an individual's reactivity to stress [166–168].
In the dopaminergic system, long-term PA optimizes dopamine metabolism [149, 169] via putative mechanisms that modulate tyrosine hydroxylase activity [170], rate of dopaminergic turnover [171], and calcium levels [172, 173]. The optimization of dopamine levels is important because dopamine levels modulate motivation and reward behavior [174]. That is, deficiencies in dopamine have been related to depression [175, 176], whereas excess dopamine levels have been related to mania [98].
Recently, considerable work has focused on the putative role of glutamate and the N-methyl-D-aspartate receptor (NMDAR) in the pathophysiology of MDs. Physiologic modulation of glutamate is imperative given its central role in synaptic strength and plasticity [177–179], yet alterations in plasma, serum, and cerebrospinal fluid have been reported in MDs [180]. Notably, PA enhances glutamate turnover and prevents excitotoxicity [151, 181] by improving calcium regulation [182]. The former mechanisms reciprocally interact with corticosteroid signaling and neural plasticity processes. Conversely, PA can mitigate glutamate hypofunctioning [183]. Maddock and colleagues [152] demonstrated that long-term PA increases glutamate in the anterior cingulate cortex, a significant finding given that glutamate contributes to the production of glutathione, a pervasive antioxidant in the central nervous system. Finally, PA increases the expression of NR2A and NR2B glutamatergic receptors in the hippocampus, receptors that are associated with neurogenesis and synaptic plasticity [184, 185].
Altogether, these studies suggest that PA modulates the underlying pathobiology of MDD [186] and BP [187] by altering the levels of key neurotransmitters that regulate emotional and cognitive health. In turn, the modulation of these neurotransmitters promotes the maintenance, repair, and survival of neurons and induces changes in molecular and cellular plasticity [188–190], effects similar to those exerted by antidepressants and antipsychotics.
7. Synaptic Number and Function and Physical Activity
As fundamental sites of communication between a neuron and its partner cell, synapses play an important role in emotion and cognition. Synapses exhibit plasticity, wherein synaptic function and structure are modified in response to activity and factors in the cellular milieu. Long-term potentiation is one form of functional synaptic plasticity, wherein synaptic connections between synapses are strengthened following activity, a process that is fundamental to learning and memory [191]. Long-term depression is another form of functional plasticity, one that is associated with the process of forgetting, where a set of synapses display a reduced capacity to elicit a response in one another [192]. Working in concert, long-term potentiation and long-term depression regulate homeostatic plasticity and the function of neuronal circuits [193]. Structural plasticity refers to changes in the 3-dimensional structure of neurons and their connections.
Convergent evidence suggests that changes in structural and functional plasticity at the synapse are relevant to MDs [194–198] and can adversely affect emotional [199] and cognitive function [200]. Indeed, loss of synapses is a common characteristic of MDD [201, 202], resulting in disconnection and loss of function in key brain regions [80, 203, 204] such as the association cortices and hippocampal region [205]. Rodent models of BP have revealed concentrated levels of ankryin-G at the synapse (which is vital for AMPAR-mediated synaptic transmission and maintenance of spine morphology), an intriguing finding given that this gene is robustly associated with the disorder [206].
The lack of noninvasive methods for the study of synaptic function precludes direct examination in humans in vivo, prompting the use of proxy measures. Accordingly, neuroimaging studies have revealed smaller hippocampal volume in persons with MDD and BP [194–198, 207]. Moreover, the study of the hippocampus in persons with MDs has revealed that volumes are inversely associated with symptom severity and duration, but positively associated with treatment outcomes [196, 208–210].
Fascinatingly, studies have shown that PA mitigates deficits in synaptic plasticity in the hippocampus. It has been shown that for aging adults, long-term exercise counters age-related decrements in hippocampus size and protects against memory impairment [211–214]. Another study has demonstrated that healthy people who participated in long-term PA (e.g., aerobic exercise 3 times per week, 30 minutes per session, for 12 weeks) exhibited increased hippocampal volume [214], a finding that could be attributed to an increase in the number of synapses, their projections, or a combination of both. Erickson and colleagues [212] demonstrated that 1 year of aerobic exercise of moderate intensity improved memory and hippocampal volume in healthy older adults, effectively reversing the age-related loss of volume by 1-2 years. Extending these studies, Makizako and colleagues [20] demonstrated that hippocampal volume was the link between moderate PA and memory augmentation in people with mild cognitive impairment and that greater durations of moderate PA resulted in increased hippocampal volume and improved memory. Also, preclinical work has demonstrated that aerobic exercise reverses age-related decrements in long-term potentiation in the dentate gyrus region of the hippocampus [215, 216] and increases spine density in the entorhinal cortex and CA1 region [217]. Moreover, voluntary wheel running by rodents for 3 weeks changes the level of several gene transcripts known to be associated with synaptic structure and plasticity, indicating that PA elicits different gene expression profiles relevant for brain function [31]. Together, these studies suggest that PA promotes structural and functional plasticity in key regions of the brain that are adversely affected by MDs and, thereby, may be used to promote functional connectivity in persons with MDs [215, 216, 218].
8. Neurogenesis and Physical Activity
Neurogenesis in the adult mammalian brain is a form of experience-dependent plasticity wherein stem cells within distinct regions of the brain give rise to new neurons [219, 220] that then migrate to the dentate gyrus of the hippocampus to become integrated into circuits important for learning, memory, and emotional regulation [5, 6, 221, 222]. The 20,000,000 neurons generated over the course of a lifetime replace dead or dying neurons [219] and, in turn, enhance functional capacity to modify neural circuitry in an environmentally dependent manner [223] as both intrinsic and extrinsic factors alter the rate of neurogenesis [223, 224]. Such is relevant for persons with MDD [79] and BP [97] because the elevations in glucocorticoid levels that frequently accompany MDs reduce neurogenesis rates and effectuate volumetric decrements in the hippocampus [225]. Conversely, pharmacological blockade of glucocorticoid receptors [226] blocks decrements in hippocampal size, as can antidepressant [227] or lithium [207] administration.
Also, preclinical and clinical work suggest that rates of neurogenesis can be optimized with exercise. Voluntary wheel running in rodents potently induces neurogenesis in the dentate gyrus, changes that result from increased proliferation and differentiation of neurons [228–230]. The newly born neurons can then integrate into the hippocampal architecture, a process that takes 4–8 weeks [231]. Interestingly, the newly integrated hippocampal neurons exhibit a lower excitability threshold and enhanced neuroplastic capabilities [223]. The latter fact suggests that PA not only mitigates volumetric decrements but also may contribute to neuroplastic changes that enable the reversal of MD-related emotional and cognitive deficits. Indeed, enhanced neurogenesis in animal models has been positively correlated with improvements in learning and memory [19, 232]. A clinical study has demonstrated that regular aerobic exercise of moderate intensity (as measured by an accelerometer worn on the hip for 2 weeks) was associated with increased hippocampal volume in older adults, findings positively associated with the encoding of new memories [233]. Thus, PA-induced hippocampal neurogenesis offers considerable hope for exploiting newly born cells and their heightened plasticity to reestablish hippocampal brain circuits that have been damaged as a result of the neuroprogression of MDs [224, 228–230, 234, 235], particularly when paired with environmental enrichment (e.g., cognitive behavioral therapy).
9. BDNF and Physical Activity
Neurotrophins—vital proteins in the brain—contribute to the survival, growth, and maintenance of neurons [218, 236] and participate in a variety of learning and memory functions [237]. BDNF, one of the most widely distributed neurotrophins in the brain, plays a vital role in the maintenance of neurons that underlie emotion and cognition, including those adversely affected in MDs [238, 239]. The neuronal atrophy and dysfunction incurred during the course of MDs effectuate disruptions in neurotrophic support, particularly BDNF. A bevy of BDNF-related abnormalities have been associated with MDs. It has been shown that (1) serum levels of BDNF are reduced in persons with BP [240, 241] and MDD [242, 243]; (2) reductions of BDNF occur in the hippocampus in MDD and BP [244]; (3) serum levels of BDNF normalize in response to several treatments (e.g., antidepressants in MDD [242, 245], mood stabilizers and antipsychotics in BP [246–248], electroconvulsive therapy in MDD and BP [249], and PA in models of BP [250] and depression [251]); (4) polymorphisms in the BDNF gene are associated with MDD [252, 253] and BP [254, 255]; and (5) mature BDNF plays a critical role in brain plasticity and is intimately involved in cognitive and mood-related behaviors [236]. Accordingly, BDNF is generally regarded as a putative biomarker for BP [239, 256] and MDD [239, 257].
Recognition of the aforementioned facts, along with the fact that BDNF is highly inducible by PA, has piqued the interest of multiple laboratories in recent years [6]. Accordingly, it has been shown that PA robustly upregulates the expression of BDNF in the hippocampus of rodents [258–261], changes that endure for days [261]. Moreover, recent work has demonstrated that peroxisome proliferator-activated gamma receptor coactivator 1-alpha (PGC-1α) and FNDC5, muscle-derived proteins that increase following endurance exercise, regulate BDNF expression in the brain in the hippocampus of mice [262]. Such is relevant for stress-induced depression given the interaction with neuroinflammatory and neuroplasticity pathways [263, 264] via alterations in tryptophan degradation [264, 265] and 5-HT1A receptor activation [266]. Thus, PA may protect against stress-induced depression by altering kynurenine metabolism [18] and, thereby, modulating BDNF levels.
Notably, PA-induced increases in BDNF have been recapitulated in unmedicated patients with MDD [267], elderly persons with remitted depression [268], and women with BP [269]. Moreover, parallel clinical studies in elderly adults have linked acute aerobic PA (until heart rate reached 85% of max capacity) with alterations in frontal cognitive functions [270], changes that may be particularly beneficial for the domains of attention, processing speed, and memory [271].
Together, these results suggest that PA may effectuate central neuroplastic adaptations via optimization of BDNF levels in persons with MDD and BP. The ability of PA to enhance BDNF release and function in the synapse, promote dendritic spine integrity, and concomitantly activate other cellular pathways [80, 179, 203, 272] is a cornerstone for neuroplastic processes that are necessary to repair and reorganize circuits damaged during the course of MDs.
10. Inflammation, Immune Function, and Physical Activity
The dramatic release of inflammatory cytokines following chronic mental or physical stress is a well-known harbinger of MDD [83, 273] and BP [98, 274] through mechanisms involving neuroplasticity, cell resilience, and neuronal survival [275, 276]. It has been shown that persons with MDD exhibit elevated levels of inflammatory cytokines including C-reactive protein (CRP), interleukin- (IL-) 1, IL-6, and tumor necrosis factor-α (TNF-α) [277, 278]. Yet, the most robust evidence for the role of inflammation in the causation of MDD derives from evidence that chronic cytokine immunotherapy induces depression in a significant number of patients [279, 280]. This trend is also seen in BP. Persons who were in the depressive phase exhibited increased IL-2, IL-6, IL-8, CRP, and TNF-α [274, 276]. Persons with BP in the manic phase exhibited increased proinflammatory markers (CRP, IL-2, IL-4, IL-6, and TNF-α) [274, 276], and mood symptoms have been positively correlated with IL-6 and IL-2 [276]. Fortunately, administration of cyclooxygenase-2 inhibitors and antagonists of TNF-α inhibits inflammatory markers in persons with MDD and BP [281–284]. Moreover, chronic lithium treatment (over 3 months) in euthymic persons with BP resulted in lower levels of peripheral blood lymphocytes secreting IL-2, IL-6, IL-10, and IFN-γ [285]. Another study has demonstrated that inflammatory factors were associated with cognitive performance in euthymic persons with BP. That is, TNF-α was associated with intrusions on the California Verbal Learning Test, IL-8 was associated with repetitions, and IFN-γ was negatively correlated with recollection deficits [286]. Whether the relationship of the immune response with MDD and BP is primary or secondary has yet to be determined. Nevertheless, the suggestion that PA may play an anti-inflammatory role and mitigate pathobiology in the brain warrants close consideration.
Notably, clinical studies demonstrate that PA attenuates the inflammatory process and provides a more resilient stress response [21, 22, 287, 288]. A randomized controlled trial (RCT) in healthy aging adults demonstrated that those who participated in progressive aerobic activity (15 minutes increasing to 40 minutes) twice per week for a 6-month duration exhibited a significant improvement in immune system function [287]. Similar results were found in a study of elderly women undergoing aerobic exercise (60 minutes per session, 3 times per week, for 16 weeks) [288]. Another study of fitness level (as denoted by heart rate) was associated with IL-6 and TNF-α response following stress [22], which is important because cytokines may adversely alter glucocorticoid receptor function to contribute to an excessive inflammatory response in MDs [289]. Finally, a study in healthy men demonstrated that PA directed monocytes towards anti-inflammatory pathways [290], a mechanism that likely supports plasticity in the brain [291]. Together, this evidence makes it seem plausible that PA induces an adaptive immune response and mitigates an exaggerated inflammatory response that can be deleterious to brain plasticity.
Admittedly, findings from human studies relating PA and immune function have varied, a situation that likely reflects unaccounted-for influences. Nevertheless, current exercise guidelines issued by the American College of Sports Medicine and the Surgeon General suggest that moderate exercise (150 minutes per week at 40% to 60% of aerobic capacity) can be deployed to induce positive immune health [292]. This notion is reaffirmed by a consensus statement drafted by international experts in the field of exercise immunology that states moderate levels of regular exercise optimize immune function [21].
11. HPA Function and Physical Activity
The HPA axis is an adaptive mechanism designed to respond to stress. Chronic stress is associated with hyperactivity of the HPA axis and increased levels of glucocorticoids [293, 294], even in the absence of external stressors. Several lines of evidence have implicated stress-related hyperactivity and dysregulation of the HPA axis with MDD [295, 296] and BP [62, 96]. Persons with MDD exhibit excessive HPA activity as measured by increased CRH in cerebrospinal fluid; increased cortisol in plasma, urine, and cerebrospinal fluid [297]; and increased rates of nonsuppression following administration of the synthetic glucocorticoid, dexamethasone (known as the dexamethasone suppression test) [298–300]. Persons with BP exhibited increased levels of ACTH and cortisol (basal and postdexamethasone), but not of CRH [96]. Euthymic persons with BP exhibited a flatter diurnal slope of cortisol secretion than healthy persons. Moreover, persons with a history of many episodes exhibited higher cortisol levels, reduced cortisol reactivity to daily stress, and a flatter diurnal slope than persons with fewer episodes [301]. Persons with psychotic and nonpsychotic major depression exhibited distinct patterns of HPA axis reactivity—a combination of depressive and psychotic symptoms induced a greater nadir in evening cortisol than depressive symptoms in isolation [302]. Investigation of a rodent model of BP reports stress-induced hypersecretion of glucocorticoids [303]. The relentless dysregulation of glucocorticoids in MDs can effectuate neuronal atrophy secondary to changes in neurochemistry, neuronal excitability, resilience, and plasticity of the hippocampus [196, 304–306]. In turn, these changes contribute to neurotoxicity [294] and can promote neuroprogression in MDD [307] and BP [308]. Also noteworthy is the fact that cytokines disrupt neuroendocrine function (e.g., IL-1, TNF-α, and interferon-α) by inhibiting glucocorticoid receptor signaling [275, 309] as reflected by decrements in glucocorticoid translocation and activation of glucocorticoid receptor-inducible enzymes [309, 310].
Although acute PA sharply increases levels of cortisol, regular PA mitigates an overactive stress response [23, 311]. Following stimulation, the hypothalamus secretes CRH, which then induces the release of ACTH from the pituitary gland. In turn, ACTH interacts with the adrenal gland to initiate the release of cortisol in humans and corticosterone in some animals (e.g., rodents, amphibians, reptiles, and birds) [312]. Within this context, acute exercise functions as a stressor, but regular exercise initiates neuroprotective effects. Bolstering the latter notion is evidence that long-term training reduces the response to both physical exercise [312] and other forms of stressor challenge [24], effects that may stem from altered density and efficiency of mineralocorticoid receptors, lower levels of circulating cortisol, and inhibition of cortisol synthesis [24, 312]. The ability of PA to attenuate HPA dysregulation is especially important for preventing hippocampal atrophy [313–315] and reversing cognitive deficits in aging populations [20, 212] and those with affective disorders [316], as hippocampal neurons persistently exposed to elevated glucocorticoids retract their dendrites and exhibit fewer dendritic spines [317]. Fortunately, the degree of dendritic branching in hippocampal neurons and the overall number of dendritic spines increase when animals are exposed to voluntary wheel running [217, 318, 319], alterations that enhance neuroplasticity and that mimic antidepressant actions. Translating these findings to humans at the behavioral level, it has been shown that 8 weeks of exercise improved depressive symptoms and levels of 24-hour urinary cortisol [316]. Another study demonstrated that 12 weeks of high-intensity aerobic exercise enhanced mood and optimized responsiveness of the HPA to the dexamethasone responsiveness test in persons experiencing chronic pain [320]. Altogether, these studies suggest that PA may mitigate HPA dysregulation in persons with MDD or BP, a notion that requires a further study.
12. Antioxidant Defense and Physical Activity
Oxidative stress is an imbalance between antioxidants and reactive oxygen species (ROS) (e.g., superoxide, hydrogen peroxide, and hydroxyl radical) [321], a problematic situation in the brain given high metabolic demands and low antioxidant capacity [322]. Oxidative stress is particularly germane to the topic of MDs given alterations in cerebral metabolic rates [323], ROS-induced lipid peroxidation, and antioxidant enzyme activity in BP [81, 324, 325] and MDD [99, 326, 327]. Moreover, oxidative changes in the milieu interfere with the stability of genomic DNA in the brain in MDs [328], changes that are correlated with severity of depressive and manic symptoms [329] and frequency of manic episodes [330].
Notably, aerobic exercise appears to increase adaptability to ROS-induced lipid peroxidation and decrease overall levels of ROS [25, 331]. These mechanisms stem in part from the ability of PA to increase antioxidant gene expression (e.g., superoxide dismutases and glutathione peroxidase) and, thereby, antioxidant enzymatic housekeeping activities in the brain [332, 333]. Together, these studies suggest that long-term exercise may optimize the enzymatic antioxidant system and mitigate oxidative damage. Such is imperative for persons with MDs given that the kinase proteins that induce structural and functional changes in synapses require specific redox environments and that synaptic activity can be modulated via ROS levels [26].
13. Circadian Rhythmicity and Physical Activity
Physiological processes such as feeding behavior, motor activity, hormonal secretion, and autonomic nervous function exhibit naturally occurring rhythms that are referred to as circadian rhythmicity [334]. Central to the control of circadian rhythmicity is the suprachiasmatic nucleus (SCN), a structure located in the anterior hypothalamus and composed of neurons that regulate different body functions according to rhythms that vary with the 24-hour night/day light cycle [335]. The SCN's rhythm is endogenously generated but can be synchronized to the environment (in a process referred to as entrainment) by capturing exogenous and endogenous cues that are referred to as zeitgebers. Common zeitgebers include PA, light, temperature, and food. For instance, light exposure decreases the production of melatonin (a hormone that controls sleep and wakefulness). Conversely, darkness effectuates increases in melatonin secretion, particularly two hours prior to bedtime, a change that increases the propensity for sleep [336]. Then, melatonin levels continue to increase, peak in the middle of the night, and finally decline towards the beginning of day [337]. Thereby, melatonin levels signal to the SCN the time of day. In turn, the SCN can interpret the information and use it to regulate other clocks in the brain and periphery.
Another SCN pathway involves cortisol. Cortisol levels typically peak after waking and then wane during the night, a fluctuation that can adjust the peripheral clocks in almost all organs of the body [337]. Importantly, cortisol is unable to reach the SCN. Therefore, abatement of stress provides a critical window for the SCN to resynchronize peripheral clocks [338]. Poststress resynchronization is important because desynchronization is linked to psychiatric illness [339, 340]. Indeed, reductions in nocturnal melatonin with concomitant increases in nocturnal ACTH and cortisol make it difficult to maintain sleep [341]. In turn, insufficient quantity and quality of sleep engenders disruptions in multiple regulatory systems, particularly the metabolic, immune, and cardiovascular systems [342, 343].
Multiple lines of evidence have implicated circadian disturbances and MDs. Genome-wide association studies have linked polymorphisms in core circadian genes with MDD [344, 345] and BP [345, 346]. Sleep disturbances have been documented in persons with MDD [85] and BP during the manic, depressive, and euthymic states [86, 347]. An estimated 70% of persons with MDD have reported problems with transitioning to sleep, frequent awakenings, and nonrestorative sleep [348]. For persons with BP, poor sleep quality, nighttime awakenings, and inadequate sleep are predictive factors for conversion [346]. During manic episodes, 69–99% of patients exhibited a decreased need for sleep, whereas 23–78% of persons experiencing depression exhibited hypersomnia [349]. Insomnia has been reported during the euthymic phase [349]. Some evidence suggests that persons with BP exhibit lower levels of melatonin during the euthymic, depressed, and manic phases [350]. Additionally, persons with BP exposed to a light source at night exhibit a greater suppression of melatonin synthesis than healthy controls [351], an effect that is mitigated by administration of lithium carbonate and sodium valproate [352, 353]. Similarly, a decrease in serum melatonin has been reported in persons with MDD [354]. Finally, persons with BP exhibit elevated night cortisol during the manic and depressive episodes [355, 356], an effect that can be mitigated therapeutically by administration of cortisol antagonists [357].
Given that SCN disturbances are associated with chronic stress [358] and disease [359], that persons with MDD and BP exhibit sleep disturbances and altered SCN function [360], and that gene expression patterns that regulate neuroplasticity vary with the sleep/wake cycle [361, 362], it is logical to surmise that persons with MDs exhibit circadian-related deficits in neuroplasticity [363]. Fortunately, evidence suggests that zeitgebers like PA [364], light exposure [365], social contacts, and the scheduling of rest and activity [366] can modulate rhythmic abnormalities by altering body temperature, gene expression, or the activity of several brain regions that project to the SCN (e.g., raphe nuclei and pineal gland) [367].
Supporting this notion is evidence that regular PA induces neurochemical changes that qualitatively and quantitatively improve sleep across patient populations [27–29, 368–372]. One RCT in sedentary adults with insomnia demonstrated that increasing PA to the level recommended in public health guidelines (≥150 min of moderate- to vigorous-intensity PA per week) improved sleep quality [28], whereas other studies demonstrated that adults who fail to achieve sufficient levels of PA incur sleep problems [372–375]. Other work showed that 3 months of fitness training in the middle of the day improved the consolidation of the sleep/wake cycle in older men [376]. Another study demonstrated that late-afternoon exercise improved measures of cognitive abilities in older adults [29]. Parallel investigations demonstrated that exercise induced phase delays in humans [377, 378] and accelerated re-entrainment of an acutely shifted sleep-wake cycle [379, 380]. Also, it has been demonstrated that PA between noon and evening can phase-advance melatonin rhythms [381]. In patients with nonremitted MDD, a 12-week RCT showed that PA augmentation effectuated improvements in self-reported sleep quality [382]. Later work revealed that PA-induced reductions in hypersomnia were positively correlated with reductions in BDNF and IL-1β, a trend that was not present in those with insomnia, suggesting differential biomarker associations for hypersomnia and insomnia [383]. Finally, preclinical work in a rodent model of BP demonstrated that both melatonin and voluntary wheel running were effective at reducing mania-related behaviorhttps://www.ncbi.nlm.nih.gov/pubmed/?term=Kirshenbaum%20GS%5BAuthor%5D&cauthor=true&cauthor_uid=24342563 [250].
While the practical implications of the aforementioned studies have yet to be clarified fully, they suggest that sleep abnormalities in MDs can be reduced with long-term PA [384–386]. Efforts to reduce sleep deficits are warranted given the morbidity associated with insomnia and the deleterious effects of sedatives. Moreover, adequate sleep is essential for plasticity processes: reactivation of regions used during the day must occur at night to consolidate memories and promote cognitive, emotional, and motor recovery [387–389].
14. Adaptive Epigenetic Signaling and Physical Activity
Epigenetic mechanisms have been implicated in the neurobiology of MDs [390–396]. Epigenetics refers to functional alterations in chromatin structure (e.g., DNA methylation, histone methylation, and histone acetylation) that induce modifications in gene expression, not changes in DNA sequence per se [397, 398]. That is, the chromatin structure that is determined by acetylation and methylation patterns can relax the spacing between nucleosomes to permit an increase or decrease in gene transcription, respectively [399]. Neurons utilize dynamic epigenetic modifications to regulate gene expression in an experience-dependent manner [400]. Thus, it has been suggested that neuronal plasticity is controlled in part by chromatin-remodeling processes [401], and emergent evidence has implicated chromatin modification in stress-induced illness, memory impairments, depression, and different phases of BP [402–406]. Basic support for epigenetic mechanisms in the pathobiology of MDD stems from evidence of discordance among monozygotic twins [407] along with evidence that environmental factors (e.g., early adversity) can increase the risk for depression [408, 409]. Similarly, a study of monozygotic twins discordant for BP revealed that four regions of the genome have significant alterations in methylation patterns, differences that may contribute to altered dopamine transmission and neuroendocrine function [410]. While the means by which these mechanisms impinge on MD pathobiology are not understood fully, it has become increasingly clear that these adaptations mediate long-lasting changes in vulnerability and resilience, factors relevant for MDs.
Fortunately, endogenous biochemical signals derived from peripheral cues such as PA appear to alter gene expression epigenetically [30, 411, 412] and induce physiological responses throughout the body. Thereby, PA may mitigate stress-induced changes in gene expression in persons with MDD and BP. Recent work in young and aged rats showed that two weeks of treadmill exercise induced age-dependent changes on epigenetic parameters in the hippocampus [413]. Tsankova and colleagues demonstrated that repeated stress increased histone H3K27 methylation in the hippocampus and suppressed the BDNF gene promoter region, an effect that was reversible with imipramine administration [414] or PA [415]. Recently, Januar and colleagues [416] reported similar results. They found that persons with depression at baseline, as well those with chronic late-life depression, demonstrated higher BDNF methylation levels, an effect that was influenced by the presence of three single-nucleotide polymorphisms (rs6265, rs7103411, and rs908867) [416]. Lindholm and colleagues [30] demonstrated that long-term, unilateral exercise (45 minutes, 4 times per week × 3 months) induced more than 5000 methylation changes in regulatory enhancement regions that alter gene expression, providing direct evidence that the regulation and maintenance of exercise-training adaptation is associated with epigenetic changes. In addition to preclinical data demonstrating that a multiplicity of genes that regulate synaptic structure and plasticity are altered by PA [31], the aforementioned studies make it seem plausible that epigenetic changes are responsible for a significant portion of MD-related pathobiological changes and suggest that PA is a strong candidate for reversing symptoms, particularly by promoting stable changes in BDNF gene expression. Moreover, given that chromatin modifications are associated with stress-induced illness, memory impairments, depression, and different phases of BP [393, 394, 402–406, 415, 417, 418], it seems likely that epigenetic profiles in the peripheral blood, particularly in BDNF, could be used as a biomarker for those who are susceptible to MDs, as well as for tracking therapeutic response following PA interventions in persons with MDs [30]. The latter notion is based on evidence that BDNF is robustly upregulated centrally and peripherally [419–422] following acute and long-term exercise [423, 424] and that plasma BDNF levels are linked to alterations in brain BDNF levels [270, 425], synaptic plasticity, and learning ability [271].
15. Telomere Length and Physical Activity
Recent evidence suggests a correlation between telomere length and MD pathobiology. Telomeres—the tiny, protective caps found on the terminals of DNA strands—protect DNA from damage during the processes of cell division and replication. Telomeres naturally shorten and fray with cellular aging, a hallmark process that is accelerated by adverse health and lifestyle circumstances [426]. Although the specific mechanisms underlying telomere shortening remain unknown, recent evidence has intimated that telomere shortening in MDs may be attributed to oxidative stress and inflammation, processes that can induce telomeric DNA damage and accelerate aging by 10 years [99, 427–429]. Moreover, it has been shown that telomere maintenance is critical for stem cell function in the context of persistent neuronal turnover and that adult stem cells exhibit high levels of telomerase (an enzyme that extends the telomere sequences after cell division) [430–432], making it seem likely that hippocampal neurogenesis depends on telomere dynamics [433].
Shorter telomere length has been reported in persons with remitted MDD and current MDD, findings that correlate with the severity and duration of symptoms [434] andhttp://www.ncbi.nlm.nih.gov/pubmed/24217256mayhttp://www.ncbi.nlm.nih.gov/pubmed/24217256relatehttp://www.ncbi.nlm.nih.gov/pubmed/24217256tohttp://www.ncbi.nlm.nih.gov/pubmed/24217256ahttp://www.ncbi.nlm.nih.gov/pubmed/24217256person'shttp://www.ncbi.nlm.nih.gov/pubmed/24217256overallhttp://www.ncbi.nlm.nih.gov/pubmed/24217256 state of resiliency [435]. In postmortem samples, shortened telomere length was observed across brain regions, including the hippocampus in persons with MDD [436]. Also, telomere shortening has been reported in persons with BP [437], and the length was inversely correlated with the number of depressive episodes and response to lithium [438]. Strikingly, recent findings show that lithium increases the expression of the gene that encodes telomerase in human neural progenitor cells [439], intimating that the accelerated aging processes that occur in BP may be mitigated through the neuroprotective effects of lithium.
Similarly, recent clinical studies suggest that PA has protective effects on telomeres [32]. In general, persons who report more PA have significantly longer telomere length [440, 441]. Loprinzi and colleagues [33] reported a clear dose-dependent relationship between the degree of PA engagement and shorter leukocyte telomere lengths: persons who participated in a single type of PA were 3% less likely to have very short telomeres compared to a sedentary individual, whereas persons who reported that they engaged in 4 types of PA were 59% less likely to have very short telomeres. Werner and colleagues [442] reported that peripheral blood leukocytes from professional endurance athletes exhibited increased telomerase activity and reduced expression of cell-cycle inhibitors compared with those from untrained individuals. Preclinical evidence suggests that PA has a protective effect on telomere length in neurons in brain regions associated with depression [34, 35].
Together, this emerging evidence suggests that PA may attenuate MD-related disease and provide a means to protect telomeres from accelerated aging. The fact that telomere maintenance is critical for stem cell function in the context of persistent turnover [430–432] makes it seem likely that hippocampal neurogenesis depends on telomere dynamics and that PA may positively affect these processes, a notion that awaits further investigation.
16. Implications for Translation, Unresolved Issues, and Future Directions
Finding an effective treatment for MD-related impairments and symptoms remains an unmet goal. Notwithstanding, the use of animal models and human research have resulted in considerable progress toward understanding common neuroplasticity substrates that are implicated in MDD and BP, along with the identification of new targets and biomarkers for therapeutic intervention. Presented here is a broad assessment of biomedical evidence intimating that PA can optimize neuroplasticity substrates and processes across brain regions so that neuronal networks responsible for emotional and cognitive regulation can reestablish their connectivity to better meet environmental and biological demands in a use-dependent manner [84, 399, 443–445]. Consistent with the delayed effects of PA, this process would take weeks to induce network changes of sufficient magnitude to effectuate downstream changes in mood and behavior. Another key thrust of this notion is that the interplay between these neuroplasticity substrates is important for appropriate network function, not isolated factors per se. Studies of these interrelated processes are imperative given evidence that interventions applied earlier in the course of disease are more likely to achieve disease modification, whereas those applied later may have a significant but more limited effect [446, 447].
While the beneficial nature of PA for persons with MDD has been clearly established [10, 448], there is a relative dearth of evidence for persons with BP. Cooney and colleagues conducted a meta-analysis of high-quality RCTs published up to March 2013 to determine whether there is enough evidence to support the deployment of PA in clinical populations with depression [10]. Thirty-nine studies with a total of 2326 participants were included in the review. The authors reported that exercise produced effects comparable to treatment with antidepressants or psychotherapy. Another meta-analytic study reported that aerobic exercise moderately reduced the signs of depression, with populations over 60 years of age deriving the greatest effect [448]. A more recent meta-analytic review attempted to determine optimal parameters for using exercise to treat depression (e.g., frequency, intensity, duration, and type of exercise). They noted that all five RCTs meeting inclusion criteria were aerobic in nature (walking on treadmill or outdoors, cycling on a stationary bike, or training on an elliptical machine) [449]. Moreover, positive evidence was found that aerobic exercise of moderate intensity, undertaken 3 times weekly for a minimum of 9 weeks, was successful in treating depression [449]. Separate clinical studies in persons with BP suggest that exercise, in combination with mood stabilizers, improves outcomes [450, 451], and routine exercise is a common wellness strategy practiced by persons with high-functioning BP [452]. For instance, one preliminary trial noted a trend towards significance for persons with BP in an inpatient psychiatric treatment unit that participated voluntarily in a walking group during their admission [451]. Another preliminary trial in persons with BP found the addition of 100 minutes of weekly exercise to baseline activities, in conjunction with nutrition and wellness activities, effectuated improvements in quality of life, depressive symptoms, and weight loss [453]. A meta-analysis of six studies reported the feasibility and benefit of PA in persons with BP, particularly noting decrements in symptoms of depression, anxiety, and stress [454].
Given that it is generally held that positive, supportive relationships have a beneficial effect on the maintenance of psychological health [455], further consideration of the influence of social interactions during group exercise is warranted, particularly in aging individuals. Group activities such as exercise may help retirees deal more effectively with the loss of social relationships and influence in the workplace by providing an opportunity to meet and socialize with others [456]; disproving stereotypes; and promoting feelings of autonomy, relatedness to others, and competence [457]. In turn, positive social relationships may facilitate the adherence to leisure-time PA. A large prospective cohort study of middle-aged adults found that adults who experienced high levels of emotional support were more likely to adhere to recommended levels of leisure-time PA at follow-up in comparison to those with lower levels of social support [458]. Alternatively, social participation and engagement may reflect an index of behavioral plasticity [459, 460] wherein social engagement helps aging persons to compensate for changes in neural systems involved in emotional and cognitive functioning, a notion that awaits future investigation.
Clearly, large-scale, multiple-site clinical investigations that study the relationship between exercise and MDs are needed. The degree to which polymorphisms (common variations in gene sequence) determine an individual's response to PA is largely unknown. Future work that combines the study of genetic background (e.g., polymorphisms in BDNF), postexercise serum BDNF, and affective and cognitive measures with neuroimaging studies of MD-related circuits could be used to determine the “dose” of PA requisite to mitigate structural and functional changes in the brain across patient populations. Moreover, strategies for overcoming the core symptoms of depression (e.g., loss of interest, motivation, and energy; low self-worth feelings and self-confidence; psychosomatic complaints; and comorbid health problems) and mania need to be clearly delineated and articulated so that exercise can be personalized.
In summary, the data presented here suggest that moderate PA—a target that is practical, well tolerated, and likely to optimize exercise adherence—can be used to improve the neurobiological impairments and behavioral symptoms associated with MDD and BP. Because of this, PA should be advocated to reduce symptoms, prevent relapse, and mitigate residual symptoms vis-a-vis the promotion of good health habits [461]. The success of prevention campaigns will require significant changes in philosophy and approach. Evidence suggests that 50% or less of mental health professionals recommended exercise for depression, and less than a third of those felt confident in making individualized recommendations [364, 462]. Ultimately, it is hoped that a more consolidated treatment view and research approach will translate into novel therapeutic avenues of preventive and curative value for persons with MDD and BP.
Abbreviations
- ACTH:
Adrenocorticotropic hormone
- BDNF:
Brain-derived neurotrophic factor
- BP:
Bipolar disorder
- CRH:
Corticotropin-releasing hormone
- FNDC5:
Fibronectin type III domain-containing protein 5
- GABA:
Gamma aminobutyric acid
- HPA:
Hypothalamic-pituitary-adrenal axis
- IGF-1:
Insulin-like growth factor 1
- IL-1:
Interleukin-1
- IL-6:
Interleukin-6
- MDD:
Major depressive disorder
- MDs:
Mood disorders
- NMDAR:
N-Methyl-D-aspartate receptor
- PA:
Physical activity
- PGC-1α:
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- RCT:
Randomized controlled trial
- ROS:
Reactive oxygen species
- SCN:
Suprachiasmatic nucleus
- TNF-α:
Tumor necrosis factor-α
- VEGF:
Vascular endothelial growth factor.
Conflicts of Interest
The author declares no competing interests.
References
- 1.World Health Organization. Mental Disorders Fact Sheet. 2016. [DOI] [PubMed]
- 2.Association AP. Diagnostic and Statistical Manual of Mental Disorders. 5th. Washington, DC, USA: American Psychiatric Pub; 2013. [Google Scholar]
- 3.Duman R. S. Neurobiology of stress, depression, and rapid acting antidepressants: remodeling synaptic connections. Depression and Anxiety. 2014;31(4):291–296. doi: 10.1002/da.22227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kupfer D. J. The increasing medical burden in bipolar disorder. JAMA. 2005;293(20):2528–2530. doi: 10.1001/jama.293.20.2528. [DOI] [PubMed] [Google Scholar]
- 5.Phillips C., Baktir M. A., Das D., Lin B., Salehi A. The link between physical activity and cognitive dysfunction in Alzheimer disease. Physical Therapy. 2015;95(7):1046–1060. doi: 10.2522/ptj.20140212. [DOI] [PubMed] [Google Scholar]
- 6.Phillips C., Baktir M. A., Srivatsan M., Salehi A. Neuroprotective effects of physical activity on the brain: a closer look at trophic factor signaling. Frontiers in Cellular Neuroscience. 2014;8:p. 170. doi: 10.3389/fncel.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kvam S., Kleppe C. L., Nordhus I. H., Hovland A. Exercise as a treatment for depression: a meta-analysis. Journal of Affective Disorders. 2016;202:67–86. doi: 10.1016/j.jad.2016.03.063. [DOI] [PubMed] [Google Scholar]
- 8.Schuch F. B., Vancampfort D., Rosenbaum S., Richards J., Ward P. B., Stubbs B. Exercise improves physical and psychological quality of life in people with depression: a meta-analysis including the evaluation of control group response. Psychiatry Research. 2016;241:47–54. doi: 10.1016/j.psychres.2016.04.054. [DOI] [PubMed] [Google Scholar]
- 9.Stanton R., Happell B., Hayman M., Reaburn P. Exercise interventions for the treatment of affective disorders - research to practice. Frontiers in Psychiatry. 2014;5:p. 46. doi: 10.3389/fpsyt.2014.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooney G. M., Dwan K., Greig C. A., et al. Exercise for depression. Cochrane Database of Systematic Reviews. 2013;(9, article CD004366) doi: 10.1002/14651858.CD004366.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melo M. C., Daher E. F., Albuquerque S. G., de Bruin V. M. Exercise in bipolar patients: a systematic review. Journal of Affective Disorders. 2016;198:32–38. doi: 10.1007/s12017-009-8079-9. [DOI] [PubMed] [Google Scholar]
- 12.Bherer L., Erickson K. I., Liu-Ambrose T. Physical exercise and brain functions in older adults. Journal of Aging Research. 2013;2013:p. 2. doi: 10.1155/2013/197326.197326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winter B., Breitenstein C., Mooren F. C., et al. High impact running improves learning. Neurobiology of Learning and Memory. 2007;87(4):597–609. doi: 10.1016/j.nlm.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Russo-Neustadt A. A., Alejandre H., Garcia C., Ivy A. S., Chen M. J. Hippocampal brain-derived neurotrophic factor expression following treatment with reboxetine, citalopram, and physical exercise. Neuropsychopharmacology. 2004;29(12):2189–2199. doi: 10.1038/sj.npp.1300514. [DOI] [PubMed] [Google Scholar]
- 15.Schwarz L., Kindermann W. Beta-endorphin, catecholamines, and cortisol during exhaustive endurance exercise. International Journal of Sports Medicine. 1989;10(5):324–328. doi: 10.1055/s-2007-1024922. [DOI] [PubMed] [Google Scholar]
- 16.Traustadottir T., Bosch P. R., Cantu T., Matt K. S. Hypothalamic-pituitary-adrenal axis response and recovery from high-intensity exercise in women: effects of aging and fitness. The Journal of Clinical Endocrinology and Metabolism. 2004;89(7):3248–3254. doi: 10.1210/jc.2003-031713. [DOI] [PubMed] [Google Scholar]
- 17.Mastorakos G., Pavlatou M., Diamanti-Kandarakis E., Chrousos G. P. Exercise and the stress system. Hormones (Athens, Greece) 2005;4(2):73–89. [PubMed] [Google Scholar]
- 18.Agudelo L. Z., Femenia T., Orhan F., et al. Skeletal muscle PGC-1alpha1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell. 2014;159(1):33–45. doi: 10.1016/j.cell.2014.07.051. [DOI] [PubMed] [Google Scholar]
- 19.Mustroph M. L., Chen S., Desai S. C., Cay E. B., DeYoung E. K., Rhodes J. S. Aerobic exercise is the critical variable in an enriched environment that increases hippocampal neurogenesis and water maze learning in male C57BL/6J mice. Neuroscience. 2012;219:62–71. doi: 10.1016/j.neuroscience.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makizako H., Liu-Ambrose T., Shimada H., et al. Moderate-intensity physical activity, hippocampal volume, and memory in older adults with mild cognitive impairment. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2015;70(4):480–486. doi: 10.1093/gerona/glu136. [DOI] [PubMed] [Google Scholar]
- 21.Walsh N. P., Gleeson M., Pyne D. B., et al. Position statement. Part two: maintaining immune health. Exercise Immunology Review. 2011;17:64–103. [PubMed] [Google Scholar]
- 22.Hamer M., Steptoe A. Association between physical fitness, parasympathetic control, and proinflammatory responses to mental stress. Psychosomatic Medicine. 2007;69(7):660–666. doi: 10.1097/PSY.0b013e318148c4c0. [DOI] [PubMed] [Google Scholar]
- 23.Pedersen B. K., Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scandinavian Journal of Medicine & Science in Sports. 2006;16(Supplement 1):3–63. doi: 10.1111/j.1600-0838.2006.00520.x. [DOI] [PubMed] [Google Scholar]
- 24.Webb H. E., Rosalky D. S., Tangsilsat S. E., McLeod K. A., Acevedo E. O., Wax B. Aerobic fitness affects cortisol responses to concurrent challenges. Medicine and Science in Sports and Exercise. 2013;45(2):379–386. doi: 10.1249/MSS.0b013e318270b381. [DOI] [PubMed] [Google Scholar]
- 25.Radak Z., Chung H. Y., Goto S. Exercise and hormesis: oxidative stress-related adaptation for successful aging. Biogerontology. 2005;6(1):71–75. doi: 10.1007/s10522-004-7386-7. [DOI] [PubMed] [Google Scholar]
- 26.Beckhauser T. F., Francis-Oliveira J., De Pasquale R. Reactive oxygen species: physiological and physiopathological effects on synaptic plasticity. Journal of Experimental Neuroscience. 2016;10(Supplement 1):23–48. doi: 10.4137/JEN.S39887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen H. M., Wu Y. C., Tsai C. M., Tzeng J. I., Lin C. C. Relationships of circadian rhythms and physical activity with objective sleep parameters in lung cancer patients. Cancer Nursing. 2015;38(3):215–223. doi: 10.1097/NCC.0000000000000163. [DOI] [PubMed] [Google Scholar]
- 28.Singh N. A., Clements K. M., Fiatarone M. A. A randomized controlled trial of the effect of exercise on sleep. Sleep. 1997;20(2):95–101. doi: 10.1093/sleep/20.2.95. [DOI] [PubMed] [Google Scholar]
- 29.Benloucif S., Orbeta L., Ortiz R., et al. Morning or evening activity improves neuropsychological performance and subjective sleep quality in older adults. Sleep. 2004;27(8):1542–1551. doi: 10.1093/sleep/27.8.1542. [DOI] [PubMed] [Google Scholar]
- 30.Lindholm M. E., Marabita F., Gomez-Cabrero D., et al. An integrative analysis reveals coordinated reprogramming of the epigenome and the transcriptome in human skeletal muscle after training. Epigenetics. 2014;9(12):1557–1569. doi: 10.4161/15592294.2014.982445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tong L., Shen H., Perreau V. M., Balazs R., Cotman C. W. Effects of exercise on gene-expression profile in the rat hippocampus. Neurobiology of Disease. 2001;8(6):1046–1056. doi: 10.1006/nbdi.2001.0427. [DOI] [PubMed] [Google Scholar]
- 32.Simpson R. J., Cosgrove C., Chee M. M., et al. Senescent phenotypes and telomere lengths of peripheral blood T-cells mobilized by acute exercise in humans. Exercise Immunology Review. 2010;16:40–55. [PubMed] [Google Scholar]
- 33.Loprinzi P. D., Loenneke J. P., Blackburn E. H. Movement-based behaviors and leukocyte telomere length among US adults. Medicine and Science in Sports and Exercise. 2015;47(11):2347–2352. doi: 10.1249/MSS.0000000000000695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolf S. A., Melnik A., Kempermann G. Physical exercise increases adult neurogenesis and telomerase activity, and improves behavioral deficits in a mouse model of schizophrenia. Brain, Behavior, and Immunity. 2011;25(5):971–980. doi: 10.1016/j.bbi.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 35.Botha M., Grace L., Bugarith K., et al. The impact of voluntary exercise on relative telomere length in a rat model of developmental stress. BMC Research Notes. 2012;5(1):p. 697. doi: 10.1186/1756-0500-5-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haarasilta L. M., Marttunen M. J., Kaprio J. A., Aro H. M. Correlates of depression in a representative nationwide sample of adolescents (15–19 years) and young adults (20–24 years) European Journal of Public Health. 2004;14(3):280–285. doi: 10.1093/eurpub/14.3.280. [DOI] [PubMed] [Google Scholar]
- 37.Abu-Omar K., Rutten A., Lehtinen V. Mental health and physical activity in the European Union. Sozial- und Präventivmedizin. 2004;49(5):301–309. doi: 10.1007/s00038-004-3109-8. [DOI] [PubMed] [Google Scholar]
- 38.Motl R. W., Birnbaum A. S., Kubik M. Y., Dishman R. K. Naturally occurring changes in physical activity are inversely related to depressive symptoms during early adolescence. Psychosomatic Medicine. 2004;66(3):336–342. doi: 10.1097/01.psy.0000126205.35683.0a. [DOI] [PubMed] [Google Scholar]
- 39.Pemberton R., Fuller Tyszkiewicz M. D. Factors contributing to depressive mood states in everyday life: a systematic review. Journal of Affective Disorders. 2016;200:103–110. doi: 10.1016/j.jad.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 40.Hoffman B. M., Babyak M. A., Craighead W. E., et al. Exercise and pharmacotherapy in patients with major depression: one-year follow-up of the SMILE study. Psychosomatic Medicine. 2011;73(2):127–133. doi: 10.1097/PSY.0b013e31820433a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Babyak M., Blumenthal J. A., Herman S., et al. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosomatic Medicine. 2000;62(5):633–638. doi: 10.1097/00006842-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Christofoletti G., Oliani M. M., Bucken-Gobbi L. T., Gobbi S., Beinotti F., Stella F. Physical activity attenuates neuropsychiatric disturbances and caregiver burden in patients with dementia. Clinics (São Paulo, Brazil) 2011;66(4):613–618. doi: 10.1590/S1807-59322011000400015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barbour K. A., Edenfield T. M., Blumenthal J. A. Exercise as a treatment for depression and other psychiatric disorders: a review. Journal of Cardiopulmonary Rehabilitation and Prevention. 2007;27(6):359–367. doi: 10.1097/01.HCR.0000300262.69645.95. [DOI] [PubMed] [Google Scholar]
- 44.Gelenberg A. J., Freeman M. P., Markowitz J. C., et al. Psychiatrists TRANZCo. National Guideline Clearinghouse. 3rd. Washington, DC: American Psychological Association; 2010. Practice guidelines for the treatment of patients with major depressive disorder. [DOI] [Google Scholar]
- 45.Ranjbar E., Memari A. H., Hafizi S., Shayestehfar M., Mirfazeli F. S., Eshghi M. A. Depression and exercise: a clinical review and management guideline. Asian Journal of Sports Medicine. 2015;6(2, article e24055) doi: 10.5812/asjsm.6(2)2015.24055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fischer B. A. A review of American psychiatry through its diagnoses: the history and development of the Diagnostic and Statistical Manual of Mental Disorders. The Journal of Nervous and Mental Disease. 2012;200(12):1022–1030. doi: 10.1097/NMD.0b013e318275cf19. [DOI] [PubMed] [Google Scholar]
- 47.Spitzer R. L., Endicott J., Robins E. Research diagnostic criteria: rationale and reliability. Archives of General Psychiatry. 1978;35(6):773–782. doi: 10.1001/archpsyc.1978.01770300115013. [DOI] [PubMed] [Google Scholar]
- 48.Cross-Disorder Group of the Psychiatric Genomics C. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381(9875):1371–1379. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wittenborn A. K., Rahmandad H., Rick J., Hosseinichimeh N. Depression as a systemic syndrome: mapping the feedback loops of major depressive disorder. Psychological Medicine. 2016;46(3):551–562. doi: 10.1017/S0033291715002044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goodkind M., Eickhoff S. B., Oathes D. J., et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72(4):305–315. doi: 10.1001/jamapsychiatry.2014.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Du J., Quiroz J. A., Gray N. A., Szabo S. T., Zarate C. A., Jr., Manji H. K. Regulation of cellular plasticity and resilience by mood stabilizers: the role of AMPA receptor trafficking. Dialogues in Clinical Neuroscience. 2004;6(2):143–155. doi: 10.31887/DCNS.2004.6.2/jdu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zarate C. A., Jr., Du J., Quiroz J., et al. Regulation of cellular plasticity cascades in the pathophysiology and treatment of mood disorders: role of the glutamatergic system. Annals of the New York Academy of Sciences. 2003;1003(1):273–291. doi: 10.1196/annals.1300.017. [DOI] [PubMed] [Google Scholar]
- 53.Sale A., Berardi N., Maffei L. Environment and brain plasticity: towards an endogenous pharmacotherapy. Physiological Reviews. 2014;94(1):189–234. doi: 10.1152/physrev.00036.2012. [DOI] [PubMed] [Google Scholar]
- 54.Manji H. K., Moore G. J., Rajkowska G., Chen G. Neuroplasticity and cellular resilience in mood disorders. Molecular Psychiatry. 2000;5(6):578–593. doi: 10.1038/sj.mp.4000811. [DOI] [PubMed] [Google Scholar]
- 55.Hirschfeld R. M. History and evolution of the monoamine hypothesis of depression. The Journal of Clinical Psychiatry. 2000;61(Supplement 6):4–6. [PubMed] [Google Scholar]
- 56.Weintraub B. Molecular Endocrinology: Basic Concepts and Clinical Correlations. New York, NY, USA: Raven Press; 1995. [Google Scholar]
- 57.Cuellar A. K., Johnson S. L., Winters R. Distinctions between bipolar and unipolar depression. Clinical Psychology Review. 2005;25(3):307–339. doi: 10.1016/j.cpr.2004.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weisenbach S. L., Kumar A. Current understanding of the neurobiology and longitudinal course of geriatric depression. Current Psychiatry Reports. 2014;16(9):p. 463. doi: 10.1007/s11920-014-0463-y. [DOI] [PubMed] [Google Scholar]
- 59.Villanueva R. Neurobiology of major depressive disorder. Neural Plasticity. 2013;2013:p. 7. doi: 10.1155/2013/873278.873278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chirita A. L., Gheorman V., Bondari D., Rogoveanu I. Current understanding of the neurobiology of major depressive disorder. Romanian Journal of Morphology and Embryology. 2015;56(Supplement 2):651–658. [PubMed] [Google Scholar]
- 61.Harrison P. J. Molecular neurobiological clues to the pathogenesis of bipolar disorder. Current Opinion in Neurobiology. 2016;36:1–6. doi: 10.1016/j.conb.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muneer A. The neurobiology of bipolar disorder: an integrated approach. Chonnam Medical Journal. 2016;52(1):18–37. doi: 10.4068/cmj.2016.52.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maletic V., Raison C. Integrated neurobiology of bipolar disorder. Frontiers in Psychiatry. 2014;5:p. 98. doi: 10.3389/fpsyt.2014.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Manji H. K., Quiroz J. A., Payne J. L., et al. The underlying neurobiology of bipolar disorder. World Psychiatry. 2003;2(3):136–146. [PMC free article] [PubMed] [Google Scholar]
- 65.Masdeu J. C. Neuroimaging in psychiatric disorders. Neurotherapeutics. 2011;8(1):93–102. doi: 10.1007/s13311-010-0006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Price J. L., Drevets W. C. Neural circuits underlying the pathophysiology of mood disorders. Trends in Cognitive Sciences. 2012;16(1):61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 67.Nagafusa Y., Okamoto N., Sakamoto K., et al. Assessment of cerebral blood flow findings using 99mTc-ECD single-photon emission computed tomography in patients diagnosed with major depressive disorder. Journal of Affective Disorders. 2012;140(3):296–299. doi: 10.1016/j.jad.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 68.Kito S., Hasegawa T., Koga Y. Cerebral blood flow ratio of the dorsolateral prefrontal cortex to the ventromedial prefrontal cortex as a potential predictor of treatment response to transcranial magnetic stimulation in depression. Brain Stimulation. 2012;5(4):547–553. doi: 10.1016/j.brs.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 69.Willeumier K., Taylor D. V., Amen D. G. Decreased cerebral blood flow in the limbic and prefrontal cortex using SPECT imaging in a cohort of completed suicides. Translational Psychiatry. 2011;1(8, article e28) doi: 10.1038/tp.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Drevets W. C., Savitz J., Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectrums. 2008;13(8):663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kendler K. S., Gatz M., Gardner C. O., Pedersen N. L. A Swedish national twin study of lifetime major depression. The American Journal of Psychiatry. 2006;163(1):109–114. doi: 10.1176/appi.ajp.163.1.109. [DOI] [PubMed] [Google Scholar]
- 72.Weaver I. C., Cervoni N., Champagne F. A., et al. Epigenetic programming by maternal behavior. Nature Neuroscience. 2004;7(8):847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 73.Massart R., Mongeau R., Lanfumey L. Beyond the monoaminergic hypothesis: neuroplasticity and epigenetic changes in a transgenic mouse model of depression. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2012;367(1601):2485–2494. doi: 10.1098/rstb.2012.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baudry A., Mouillet-Richard S., Schneider B., Launay J. M., Kellermann O. miR-16 targets the serotonin transporter: a new facet for adaptive responses to antidepressants. Science. 2010;329(5998):1537–1541. doi: 10.1126/science.1193692. [DOI] [PubMed] [Google Scholar]
- 75.Delgado P. L. Depression: the case for a monoamine deficiency. The Journal of Clinical Psychiatry. 2000;61(Supplement 6):7–11. [PubMed] [Google Scholar]
- 76.Salomon R. M., Miller H. L., Krystal J. H., Heninger G. R., Charney D. S. Lack of behavioral effects of monoamine depletion in healthy subjects. Biological Psychiatry. 1997;41(1):58–64. doi: 10.1016/0006-3223(95)00670-2. [DOI] [PubMed] [Google Scholar]
- 77.Delgado P. L., Price L. H., Miller H. L., et al. Serotonin and the neurobiology of depression. Effects of tryptophan depletion in drug-free depressed patients. Archives of General Psychiatry. 1994;51(11):865–874. doi: 10.1001/archpsyc.1994.03950110025005. [DOI] [PubMed] [Google Scholar]
- 78.Berman R. M., Sanacora G., Anand A., et al. Monoamine depletion in unmedicated depressed subjects. Biological Psychiatry. 2002;51(6):469–473. doi: 10.1016/S0006-3223(01)01285-9. [DOI] [PubMed] [Google Scholar]
- 79.Yuan T. F., Paes F., Arias-Carrion O., Ferreira Rocha N. B., de Sa Filho A. S., Machado S. Neural mechanisms of exercise: anti-depression, neurogenesis, and serotonin signaling. CNS & Neurological Disorders Drug Targets. 2015;14(10):1307–1311. doi: 10.2174/1871527315666151111124402. [DOI] [PubMed] [Google Scholar]
- 80.Duman R. S., Monteggia L. M. A neurotrophic model for stress-related mood disorders. Biological Psychiatry. 2006;59(12):1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 81.Brown N. C., Andreazza A. C., Young L. T. An updated meta-analysis of oxidative stress markers in bipolar disorder. Psychiatry Research. 2014;218(1-2):61–68. doi: 10.1016/j.psychres.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 82.Pariante C. M., Lightman S. L. The HPA axis in major depression: classical theories and new developments. Trends in Neurosciences. 2008;31(9):464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 83.Hashmi A. M., Butt Z., Umair M. Is depression an inflammatory condition? A review of available evidence. The Journal of the Pakistan Medical Association. 2013;63(7):899–906. [PubMed] [Google Scholar]
- 84.Castren E. Is mood chemistry? Nature Reviews. Neuroscience. 2005;6(3):241–246. doi: 10.1038/nrn1629. [DOI] [PubMed] [Google Scholar]
- 85.Tsuno N., Besset A., Ritchie K. Sleep and depression. The Journal of Clinical Psychiatry. 2005;66(10):1254–1269. doi: 10.4088/JCP.v66n1008. [DOI] [PubMed] [Google Scholar]
- 86.Milhiet V., Boudebesse C., Bellivier F., et al. Circadian abnormalities as markers of susceptibility in bipolar disorders. Frontiers in Bioscience (Scholar Edition) 2014;6:120–137. doi: 10.2741/s419. [DOI] [PubMed] [Google Scholar]
- 87.Treadway M. T., Pizzagalli D. A. Imaging the pathophysiology of major depressive disorder - from localist models to circuit-based analysis. Biology of Mood & Anxiety Disorders. 2014;4(1):p. 5. doi: 10.1186/2045-5380-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Post R. M., Rubinow D. R., Ballenger J. C. Conditioning and sensitisation in the longitudinal course of affective illness. The British Journal of Psychiatry. 1986;149(2):191–201. doi: 10.1192/bjp.149.2.191. [DOI] [PubMed] [Google Scholar]
- 89.Kendler K. S., Thornton L. M., Gardner C. O. Stressful life events and previous episodes in the etiology of major depression in women: an evaluation of the “kindling” hypothesis. The American Journal of Psychiatry. 2000;157(8):1243–1251. doi: 10.1176/appi.ajp.157.8.1243. [DOI] [PubMed] [Google Scholar]
- 90.Pregelj P. Gene environment interactions in bipolar disorder. Psychiatria Danubina. 2011;23(Supplement 1):S91–S93. [PubMed] [Google Scholar]
- 91.Bowden C. L. Towards an integrated biological model of bipolar disorder. In: Young L. T., Joffe R. T., editors. Bipolar Disorder: Biological Bodels and Their Clinical Application. New York, NY, USA: Dekker; 1997. pp. 235–254. [Google Scholar]
- 92.Craddock N., Sklar P. Genetics of bipolar disorder. Lancet. 2013;381(9878):1654–1662. doi: 10.1016/S0140-6736(13)60855-7. [DOI] [PubMed] [Google Scholar]
- 93.McMahon F. J., Akula N., Schulze T. G., et al. Meta-analysis of genome-wide association data identifies a risk locus for major mood disorders on 3p21.1. Nature Genetics. 2010;42(2):128–131. doi: 10.1038/ng.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Horesh N., Iancu I. A comparison of life events in patients with unipolar disorder or bipolar disorder and controls. Comprehensive Psychiatry. 2010;51(2):157–164. doi: 10.1016/j.comppsych.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 95.Johnson S. L. Life events in bipolar disorder: towards more specific models. Clinical Psychology Review. 2005;25(8):1008–1027. doi: 10.1016/j.cpr.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Belvederi Murri M., Prestia D., Mondelli V., et al. The HPA axis in bipolar disorder: systematic review and meta-analysis. Psychoneuroendocrinology. 2016;63:327–342. doi: 10.1016/j.psyneuen.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 97.Sylvia L. G., Ametrano R. M., Nierenberg A. A. Exercise treatment for bipolar disorder: potential mechanisms of action mediated through increased neurogenesis and decreased allostatic load. Psychotherapy and Psychosomatics. 2010;79(2):87–96. doi: 10.1159/000270916. [DOI] [PubMed] [Google Scholar]
- 98.Berk M., Kapczinski F., Andreazza A. C., et al. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neuroscience and Biobehavioral Reviews. 2011;35(3):804–817. doi: 10.1016/j.neubiorev.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 99.Scapagnini G., Davinelli S., Drago F., De Lorenzo A., Oriani G. Antioxidants as antidepressants: fact or fiction? CNS Drugs. 2012;26(6):477–490. doi: 10.2165/11633190-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 100.Malenka R. C., Nestler E. J., Hyman S. E. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience. 2nd. New York, NY, USA: McGraw-Hill Medical; 2009. [Google Scholar]
- 101.Schloesser R. J., Chen G., Manji H. K. Neurogenesis and neuroenhancement in the pathophysiology and treatment of bipolar disorder. International Review of Neurobiology. 2007;77:143–178. doi: 10.1016/S0074-7742(06)77005-2. [DOI] [PubMed] [Google Scholar]
- 102.Nudo R. J. Recovery after damage to motor cortical areas. Current Opinion in Neurobiology. 1999;9(6):740–747. doi: 10.1016/S0959-4388(99)00027-6. [DOI] [PubMed] [Google Scholar]
- 103.Pantazopoulos H., Berretta S. In sickness and in health: perineuronal nets and synaptic plasticity in psychiatric disorders. Neural Plasticity. 2016;2016:p. 23. doi: 10.1155/2016/9847696.9847696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Krystal J. H., Tolin D. F., Sanacora G., et al. Neuroplasticity as a target for the pharmacotherapy of anxiety disorders, mood disorders, and schizophrenia. Drug Discovery Today. 2009;14(13-14):690–697. doi: 10.1016/j.drudis.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cohen L. G., Celnik P., Pascual-Leone A., et al. Functional relevance of cross-modal plasticity in blind humans. Nature. 1997;389(6647):180–183. doi: 10.1038/38278. [DOI] [PubMed] [Google Scholar]
- 106.Kempermann G., Fabel K., Ehninger D., et al. Why and how physical activity promotes experience-induced brain plasticity. Frontiers in Neuroscience. 2010;4:p. 189. doi: 10.3389/fnins.2010.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Garland T., Jr., Schutz H., Chappell M. A., et al. The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: human and rodent perspectives. The Journal of Experimental Biology. 2011;214(Part 2):206–229. doi: 10.1242/jeb.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Westerterp K. R. Assessment of physical activity: a critical appraisal. European Journal of Applied Physiology. 2009;105(6):823–828. doi: 10.1007/s00421-009-1000-2. [DOI] [PubMed] [Google Scholar]
- 109.Kowalski K., Rhodes R., Naylor P. J., Tuokko H., MacDonald S. Direct and indirect measurement of physical activity in older adults: a systematic review of the literature. International Journal of Behavioral Nutrition and Physical Activity. 2012;9(1):p. 148. doi: 10.1186/1479-5868-9-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dishman R. K. Gene-physical activity interactions in the etiology of obesity: behavioral considerations. Obesity (Silver Spring) 2008;16(Supplement 3):S60–S65. doi: 10.1038/oby.2008.520. [DOI] [PubMed] [Google Scholar]
- 111.Shephard R. J. Limits to the measurement of habitual physical activity by questionnaires. British Journal of Sports Medicine. 2003;37(3):197–206. doi: 10.1136/bjsm.37.3.197. discussion 06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.de Vilhena e Santos D. M., Katzmarzyk P. T., Seabra A. F., Maia J. A. Genetics of physical activity and physical inactivity in humans. Behavior Genetics. 2012;42(4):559–578. doi: 10.1007/s10519-012-9534-1. [DOI] [PubMed] [Google Scholar]
- 113.Wilcox S., Ainsworth B. E. The measurement of physical activity. In: Shumaker S. A., Ockene J., Riekert K. A., editors. The Handbook of Health Behavior Change. New York, NY, USA: Springer Publishing Company, LLC; 2009. pp. 327–346. [Google Scholar]
- 114.Dinger M. K., Oman R. F., Taylor E. L., Vesely S. K., Able J. Stability and convergent validity of the Physical Activity Scale for the Elderly (PASE) The Journal of Sports Medicine and Physical Fitness. 2004;44(2):186–192. [PubMed] [Google Scholar]
- 115.Prince S. A., Adamo K. B., Hamel M. E., Hardt J., Connor Gorber S., Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. International Journal of Behavioral Nutrition and Physical Activity. 2008;5(1):p. 56. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Adamo K. B., Prince S. A., Tricco A. C., Connor-Gorber S., Tremblay M. A comparison of indirect versus direct measures for assessing physical activity in the pediatric population: a systematic review. International Journal of Pediatric Obesity. 2009;4(1):2–27. doi: 10.1080/17477160802315010. [DOI] [PubMed] [Google Scholar]
- 117.Joosen A. M., Gielen M., Vlietinck R., Westerterp K. R. Genetic analysis of physical activity in twins. The American Journal of Clinical Nutrition. 2005;82(6):1253–1259. doi: 10.1093/ajcn/82.6.1253. [DOI] [PubMed] [Google Scholar]
- 118.Rezende E. L., Gomes F. R., Chappell M. A., Garland T., Jr Running behavior and its energy cost in mice selectively bred for high voluntary locomotor activity. Physiological and Biochemical Zoology. 2009;82(6):662–679. doi: 10.1086/605917. [DOI] [PubMed] [Google Scholar]
- 119.Kelly S. A., Pomp D. Genetic determinants of voluntary exercise. Trends in Genetics. 2013;29(6):348–357. doi: 10.1016/j.tig.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Novak C. M., Burghardt P. R., Levine J. A. The use of a running wheel to measure activity in rodents: relationship to energy balance, general activity, and reward. Neuroscience and Biobehavioral Reviews. 2012;36(3):1001–1014. doi: 10.1016/j.neubiorev.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brene S., Bjornebekk A., Aberg E., Mathe A. A., Olson L., Werme M. Running is rewarding and antidepressive. Physiology & Behavior. 2007;92(1-2):136–140. doi: 10.1016/j.physbeh.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lett B. T., Grant V. L., Byrne M. J., Koh M. T. Pairings of a distinctive chamber with the aftereffect of wheel running produce conditioned place preference. Appetite. 2000;34(1):87–94. doi: 10.1006/appe.1999.0274. [DOI] [PubMed] [Google Scholar]
- 123.Belke T. W., Garland T., Jr. A brief opportunity to run does not function as a reinforcer for mice selected for high daily wheel-running rates. Journal of the Experimental Analysis of Behavior. 2007;88(2):199–213. doi: 10.1901/jeab.2007.62-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sallis J. F. Age-related decline in physical activity: a synthesis of human and animal studies. Medicine and Science in Sports and Exercise. 2000;32(9):1598–1600. doi: 10.1097/00005768-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 125.Bronikowski A. M., Morgan T. J., Garland T., Jr., Carter P. A. The evolution of aging and age-related physical decline in mice selectively bred for high voluntary exercise. Evolution. 2006;60(7):1494–1508. [PubMed] [Google Scholar]
- 126.Booth F. W., Chakravarthy M. V., Gordon S. E., Spangenburg E. E. Waging war on physical inactivity: using modern molecular ammunition against an ancient enemy. Journal of Applied Physiology (1985) 2002;93(1):3–30. doi: 10.1152/japplphysiol.00073.2002. [DOI] [PubMed] [Google Scholar]
- 127.Clubb R., Mason G. Animal welfare: captivity effects on wide-ranging carnivores. Nature. 2003;425(6957):473–474. doi: 10.1038/425473a. [DOI] [PubMed] [Google Scholar]
- 128.Chiba S., Numakawa T., Ninomiya M., Richards M. C., Wakabayashi C., Kunugi H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2012;39(1):112–119. doi: 10.1016/j.pnpbp.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 129.Sullivan M. W., Lewis M. Contextual determinants of anger and other negative expressions in young infants. Developmental Psychology. 2003;39(4):693–705. doi: 10.1037/0012-1649.39.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Leotti L. A., Iyengar S. S., Ochsner K. N. Born to choose: the origins and value of the need for control. Trends in Cognitive Sciences. 2010;14(10):457–463. doi: 10.1016/j.tics.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Greenwood B. N., Spence K. G., Crevling D. M., Clark P. J., Craig W. C., Fleshner M. Exercise-induced stress resistance is independent of exercise controllability and the medial prefrontal cortex. The European Journal of Neuroscience. 2013;37(3):469–478. doi: 10.1111/ejn.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Arida R. M., Scorza C. A., da Silva A. V., Scorza F. A., Cavalheiro E. A. Differential effects of spontaneous versus forced exercise in rats on the staining of parvalbumin-positive neurons in the hippocampal formation. Neuroscience Letters. 2004;364(3):135–138. doi: 10.1016/j.neulet.2004.03.086. [DOI] [PubMed] [Google Scholar]
- 133.Holmes P. V. Rodent models of depression: reexamining validity without anthropomorphic inference. Critical Reviews in Neurobiology. 2003;15(2):143–174. doi: 10.1615/CritRevNeurobiol.v15.i2.30. [DOI] [PubMed] [Google Scholar]
- 134.Kays J. L., Hurley R. A., Taber K. H. The dynamic brain: neuroplasticity and mental health. The Journal of Neuropsychiatry and Clinical Neurosciences. 2012;24(2):118–124. doi: 10.1176/appi.neuropsych.24.1.118. [DOI] [PubMed] [Google Scholar]
- 135.Cramer S. C., Sur M., Dobkin B. H., et al. Harnessing neuroplasticity for clinical applications. Brain. 2011;134(Part 6):1591–1609. doi: 10.1093/brain/awr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Knochel C., Oertel-Knochel V., O'Dwyer L., et al. Cognitive and behavioural effects of physical exercise in psychiatric patients. Progress in Neurobiology. 2012;96(1):46–68. doi: 10.1016/j.pneurobio.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 137.Ghasemi M., Phillips C., Trillo L., De Miguel Z., Das D., Salehi A. The role of NMDA receptors in the pathophysiology and treatment of mood disorders. Neuroscience and Biobehavioral Reviews. 2014;47:336–358. doi: 10.1016/j.neubiorev.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 138.Jukic M. M., Carrillo-Roa T., Bar M., et al. Abnormal development of monoaminergic neurons is implicated in mood fluctuations and bipolar disorder. Neuropsychopharmacology. 2015;40(4):839–848. doi: 10.1038/npp.2014.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Coque L., Mukherjee S., Cao J. L., et al. Specific role of VTA dopamine neuronal firing rates and morphology in the reversal of anxiety-related, but not depression-related behavior in the ClockDelta19 mouse model of mania. Neuropsychopharmacology. 2011;36(7):1478–1488. doi: 10.1038/npp.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Barchus J. D., Altemus M. Monoamine Hypotheses of Mood Disorders. 6th. Philadelphia, PA: Lippincott-Raven; 1999. [Google Scholar]
- 141.Robbins T. W., Arnsten A. F. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annual Review of Neuroscience. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dunn A. L., Dishman R. K. Exercise and the neurobiology of depression. Exercise and Sport Sciences Reviews. 1991;19(1):41–98. [PubMed] [Google Scholar]
- 143.Meeusen R., De Meirleir K. Exercise and brain neurotransmission. Sports Medicine. 1995;20(3):160–188. doi: 10.2165/00007256-199520030-00004. [DOI] [PubMed] [Google Scholar]
- 144.Greenwood B. N., Foley T. E., Day H. E., et al. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. The Journal of Neuroscience. 2003;23(7):2889–2898. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Greenwood B. N., Fleshner M. Exercise, learned helplessness, and the stress-resistant brain. Neuromolecular Medicine. 2008;10(2):81–98. doi: 10.1007/s12017-008-8029-y. [DOI] [PubMed] [Google Scholar]
- 146.Chaouloff F., Elghozi J. L., Guezennec Y., Laude D. Effects of conditioned running on plasma, liver and brain tryptophan and on brain 5-hydroxytryptamine metabolism of the rat. British Journal of Pharmacology. 1985;86(1):33–41. doi: 10.1111/j.1476-5381.1985.tb09432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dunn A. L., Reigle T. G., Youngstedt S. D., Armstrong R. B., Dishman R. K. Brain norepinephrine and metabolites after treadmill training and wheel running in rats. Medicine and Science in Sports and Exercise. 1996;28(2):204–209. doi: 10.1097/00005768-199602000-00008. [DOI] [PubMed] [Google Scholar]
- 148.Dishman R. K. Brain monoamines, exercise, and behavioral stress: animal models. Medicine and Science in Sports and Exercise. 1997;29(1):63–74. doi: 10.1097/00005768-199701000-00010. [DOI] [PubMed] [Google Scholar]
- 149.Chaouloff F., Laude D., Guezennec Y., Elghozi J. L. Motor activity increases tryptophan, 5-hydroxyindoleacetic acid, and homovanillic acid in ventricular cerebrospinal fluid of the conscious rat. Journal of Neurochemistry. 1986;46(4):1313–1316. doi: 10.1111/j.1471-4159.1986.tb00656.x. [DOI] [PubMed] [Google Scholar]
- 150.Bliss E. L., Ailion J. Relationship of stress and activity to brain dopamine and homovanillic acid. Life Sciences. 1971;10(20):1161–1169. doi: 10.1016/0024-3205(71)90276-1. [DOI] [PubMed] [Google Scholar]
- 151.Herbst E. A., Holloway G. P. Exercise increases mitochondrial glutamate oxidation in the mouse cerebral cortex. Applied Physiology, Nutrition, and Metabolism. 2016;41(7):799–801. doi: 10.1139/apnm-2016-0033. [DOI] [PubMed] [Google Scholar]
- 152.Maddock R. J., Casazza G. A., Fernandez D. H., Maddock M. I. Acute modulation of cortical glutamate and GABA content by physical activity. The Journal of Neuroscience. 2016;36(8):2449–2457. doi: 10.1523/JNEUROSCI.3455-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lynch W. J., Peterson A. B., Sanchez V., Abel J., Smith M. A. Exercise as a novel treatment for drug addiction: a neurobiological and stage-dependent hypothesis. Neuroscience and Biobehavioral Reviews. 2013;37(8):1622–1644. doi: 10.1016/j.neubiorev.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dey S., Singh R. H., Dey P. K. Exercise training: significance of regional alterations in serotonin metabolism of rat brain in relation to antidepressant effect of exercise. Physiology & Behavior. 1992;52(6):1095–1099. doi: 10.1016/0031-9384(92)90465-E. [DOI] [PubMed] [Google Scholar]
- 155.Chaouloff F. Physical exercise and brain monoamines: a review. Acta Physiologica Scandinavica. 1989;137(1):1–13. doi: 10.1111/j.1748-1716.1989.tb08715.x. [DOI] [PubMed] [Google Scholar]
- 156.Patrick R. P., Ames B. N. Vitamin D and the omega-3 fatty acids control serotonin synthesis and action, part 2: relevance for ADHD, bipolar disorder, schizophrenia, and impulsive behavior. The FASEB Journal. 2015;29(6):2207–2222. doi: 10.1096/fj.14-268342. [DOI] [PubMed] [Google Scholar]
- 157.Chaouloff F., Laude D., Merino D., Serrurrier B., Guezennec Y., Elghozi J. L. Amphetamine and alpha-methyl-p-tyrosine affect the exercise-induced imbalance between the availability of tryptophan and synthesis of serotonin in the brain of the rat. Neuropharmacology. 1987;26(8):1099–1106. doi: 10.1016/0028-3908(87)90254-1. [DOI] [PubMed] [Google Scholar]
- 158.Chaoloff F. The serotonin hypothesis. In: Morgan W. P., editor. Physical Activity and Mental Health. Talyor & Francis: Washington, D.C.; 2013. [Google Scholar]
- 159.Kiank C., Zeden J. P., Drude S., et al. Psychological stress-induced, IDO1-dependent tryptophan catabolism: implications on immunosuppression in mice and humans. PLoS One. 2010;5(7, article e11825) doi: 10.1371/journal.pone.0011825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ito Y., Yonekura R., Maruta K., et al. Tryptophan metabolism was accelerated by exercise in rat. Advances in Experimental Medicine and Biology. 2003;527:531–535. doi: 10.1007/978-1-4615-0135-0_61. [DOI] [PubMed] [Google Scholar]
- 161.Pieribone V. A., Xu Z. Q., Zhang X., Grillner S., Bartfai T., Hokfelt T. Galanin induces a hyperpolarization of norepinephrine-containing locus coeruleus neurons in the brainstem slice. Neuroscience. 1995;64(4):861–874. doi: 10.1016/0306-4522(94)00450-J. [DOI] [PubMed] [Google Scholar]
- 162.Seutin V., Verbanck P., Massotte L., Dresse A. Galanin decreases the activity of locus coeruleus neurons in vitro. European Journal of Pharmacology. 1989;164(2):373–376. doi: 10.1016/0014-2999(89)90481-0. [DOI] [PubMed] [Google Scholar]
- 163.Murray P. S., Groves J. L., Pettett B. J., et al. Locus coeruleus galanin expression is enhanced after exercise in rats selectively bred for high capacity for aerobic activity. Peptides. 2010;31(12):2264–2268. doi: 10.1016/j.peptides.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Reiss J. I., Dishman R. K., Boyd H. E., Robinson J. K., Holmes P. V. Chronic activity wheel running reduces the severity of kainic acid-induced seizures in the rat: possible role of galanin. Brain Research. 2009;1266:54–63. doi: 10.1016/j.brainres.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 165.Gouarne C., Groussard C., Gratas-Delamarche A., Delamarche P., Duclos M. Overnight urinary cortisol and cortisone add new insights into adaptation to training. Medicine and Science in Sports and Exercise. 2005;37(7):1157–1167. doi: 10.1249/01.mss.0000170099.10038.3b. [DOI] [PubMed] [Google Scholar]
- 166.Traustadottir T., Bosch P. R., Matt K. S. The HPA axis response to stress in women: effects of aging and fitness. Psychoneuroendocrinology. 2005;30(4):392–402. doi: 10.1016/j.psyneuen.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 167.Rimmele U., Seiler R., Marti B., Wirtz P. H., Ehlert U., Heinrichs M. The level of physical activity affects adrenal and cardiovascular reactivity to psychosocial stress. Psychoneuroendocrinology. 2009;34(2):190–198. doi: 10.1016/j.psyneuen.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 168.von Haaren B., Ottenbacher J., Muenz J., Neumann R., Boes K., Ebner-Priemer U. Does a 20-week aerobic exercise training programme increase our capabilities to buffer real-life stressors? A randomized, controlled trial using ambulatory assessment. European Journal of Applied Physiology. 2016;116(2):383–394. doi: 10.1007/s00421-015-3284-8. [DOI] [PubMed] [Google Scholar]
- 169.de Castro J. M., Duncan G. Operantly conditioned running: effects on brain catecholamine concentrations and receptor densities in the rat. Pharmacology, Biochemistry, and Behavior. 1985;23(4):495–500. doi: 10.1016/0091-3057(85)90407-1. [DOI] [PubMed] [Google Scholar]
- 170.Greenwood B. N., Foley T. E., Le T. V., et al. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behavioural Brain Research. 2011;217(2):354–362. doi: 10.1016/j.bbr.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mathes W. F., Nehrenberg D. L., Gordon R., Hua K., Garland T., Jr., Pomp D. Dopaminergic dysregulation in mice selectively bred for excessive exercise or obesity. Behavioural Brain Research. 2010;210(2):155–163. doi: 10.1016/j.bbr.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Morris G., Berk M. The many roads to mitochondrial dysfunction in neuroimmune and neuropsychiatric disorders. BMC Medicine. 2015;13(1):p. 68. doi: 10.1186/s12916-015-0310-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Goffer Y., Xu D., Eberle S. E., et al. Calcium-permeable AMPA receptors in the nucleus accumbens regulate depression-like behaviors in the chronic neuropathic pain state. The Journal of Neuroscience. 2013;33(48):19034–19044. doi: 10.1523/JNEUROSCI.2454-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lammel S., Lim B. K., Malenka R. C. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology. 2014;76(Part B):351–359. doi: 10.1016/j.neuropharm.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Fibiger H. C. Dopaminergic-cholinergic interactions in the striatum. The Japanese Journal of Psychiatry and Neurology. 1991;45(2):p. 512. doi: 10.1111/j.1440-1819.1991.tb02536.x. [DOI] [PubMed] [Google Scholar]
- 176.Willner P., Muscat R., Phillips G. The role of dopamine in rewarded behavior: ability, insight, drive or incentive? Polish Journal of Pharmacology and Pharmacy. 1991;43(4):291–300. [PubMed] [Google Scholar]
- 177.Collingridge G. L., Bliss T. V. Memories of NMDA receptors and LTP. Trends in Neurosciences. 1995;18(2):54–56. doi: 10.1016/0166-2236(95)80016-U. [DOI] [PubMed] [Google Scholar]
- 178.Holtmaat A., Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nature Reviews Neuroscience. 2009;10(9):647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- 179.Kessels H. W., Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61(3):340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yildiz-Yesiloglu A., Ankerst D. P. Review of 1H magnetic resonance spectroscopy findings in major depressive disorder: a meta-analysis. Psychiatry Research. 2006;147(1):1–25. doi: 10.1016/j.pscychresns.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 181.Jia J., Hu Y. S., Wu Y., et al. Pre-ischemic treadmill training affects glutamate and gamma aminobutyric acid levels in the striatal dialysate of a rat model of cerebral ischemia. Life Sciences. 2009;84(15-16):505–511. doi: 10.1016/j.lfs.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 182.Sutoo D. E., Akiyama K. The mechanism by which exercise modifies brain function. Physiology & Behavior. 1996;60(1):177–181. doi: 10.1016/0031-9384(96)00011-X. [DOI] [PubMed] [Google Scholar]
- 183.Canevari L., Richter-Levin G., Bliss T. V. LTP in the dentate gyrus is associated with a persistent NMDA receptor-dependent enhancement of synaptosomal glutamate release. Brain Research. 1994;667(1):115–117. doi: 10.1016/0006-8993(94)91720-5. [DOI] [PubMed] [Google Scholar]
- 184.Lou S. J., Liu J. Y., Chang H., Chen P. J. Hippocampal neurogenesis and gene expression depend on exercise intensity in juvenile rats. Brain Research. 2008;1210:48–55. doi: 10.1016/j.brainres.2008.02.080. [DOI] [PubMed] [Google Scholar]
- 185.Vasuta C., Caunt C., James R., et al. Effects of exercise on NMDA receptor subunit contributions to bidirectional synaptic plasticity in the mouse dentate gyrus. Hippocampus. 2007;17(12):1201–1208. doi: 10.1002/hipo.20349. [DOI] [PubMed] [Google Scholar]
- 186.Pareja-Galeano H., Mayero S., Perales M., et al. Biological rationale for regular physical exercise as an effective intervention for the prevention and treatment of depressive disorders. Current Pharmaceutical Design. 2016;22(24):3764–3775. doi: 10.2174/1381612822666160322144537. [DOI] [PubMed] [Google Scholar]
- 187.Alsuwaidan M. T., Kucyi A., Law C. W., McIntyre R. S. Exercise and bipolar disorder: a review of neurobiological mediators. Neuromolecular Medicine. 2009;11(4):328–336. doi: 10.1007/s12017-009-8079-9. [DOI] [PubMed] [Google Scholar]
- 188.Garcia C., Chen M. J., Garza A. A., Cotman C. W., Russo-Neustadt A. The influence of specific noradrenergic and serotonergic lesions on the expression of hippocampal brain-derived neurotrophic factor transcripts following voluntary physical activity. Neuroscience. 2003;119(3):721–732. doi: 10.1016/S0306-4522(03)00192-1. [DOI] [PubMed] [Google Scholar]
- 189.Mattson M. P., Maudsley S., Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends in Neurosciences. 2004;27(10):589–594. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 190.Chen M. J., Nguyen T. V., Pike C. J., Russo-Neustadt A. A. Norepinephrine induces BDNF and activates the PI-3K and MAPK cascades in embryonic hippocampal neurons. Cellular Signalling. 2007;19(1):114–128. doi: 10.1016/j.cellsig.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 191.Bliss T. V., Collingridge G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 192.Bear M. F., Abraham W. C. Long-term depression in hippocampus. Annual Review of Neuroscience. 1996;19(1):437–462. doi: 10.1146/annurev.ne.19.030196.002253. [DOI] [PubMed] [Google Scholar]
- 193.Turrigiano G. G., Leslie K. R., Desai N. S., Rutherford L. C., Nelson S. B. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391(6670):892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- 194.McKinnon M. C., Yucel K., Nazarov A., MacQueen G. M. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. Journal of Psychiatry & Neuroscience. 2009;34(1):41–54. [PMC free article] [PubMed] [Google Scholar]
- 195.Kempton M. J., Salvador Z., Munafo M. R., et al. Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Archives of General Psychiatry. 2011;68(7):675–690. doi: 10.1001/archgenpsychiatry.2011.60. [DOI] [PubMed] [Google Scholar]
- 196.Otten M., Meeter M. Hippocampal structure and function in individuals with bipolar disorder: a systematic review. Journal of Affective Disorders. 2015;174:113–125. doi: 10.1016/j.jad.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 197.Strakowski S. M., Fleck D. E., Welge J., et al. fMRI brain activation changes following treatment of a first bipolar manic episode. Bipolar Disorders. 2016;18(6):490–501. doi: 10.1111/bdi.12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hafeman D. M., Chang K. D., Garrett A. S., Sanders E. M., Phillips M. L. Effects of medication on neuroimaging findings in bipolar disorder: an updated review. Bipolar Disorders. 2012;14(4):375–410. doi: 10.1111/j.1399-5618.2012.01023.x. [DOI] [PubMed] [Google Scholar]
- 199.Phillips M. L., Drevets W. C., Rauch S. L., Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biological Psychiatry. 2003;54(5):504–514. doi: 10.1016/S0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 200.Jarrard L. E. On the role of the hippocampus in learning and memory in the rat. Behavioral and Neural Biology. 1993;60(1):9–26. doi: 10.1016/0163-1047(93)90664-4. [DOI] [PubMed] [Google Scholar]
- 201.Bennett M. R. The prefrontal-limbic network in depression: a core pathology of synapse regression. Progress in Neurobiology. 2011;93(4):457–467. doi: 10.1016/j.pneurobio.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 202.Czeh B., Lucassen P. J. What causes the hippocampal volume decrease in depression? Are neurogenesis, glial changes and apoptosis implicated? European Archives of Psychiatry and Clinical Neuroscience. 2007;257(5):250–260. doi: 10.1007/s00406-007-0728-0. [DOI] [PubMed] [Google Scholar]
- 203.Krishnan V., Nestler E. J. The molecular neurobiology of depression. Nature. 2008;455(7215):894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Savitz J., Drevets W. C. Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neuroscience and Biobehavioral Reviews. 2009;33(5):699–771. doi: 10.1016/j.neubiorev.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Campbell S., Marriott M., Nahmias C., MacQueen G. M. Lower hippocampal volume in patients suffering from depression: a meta-analysis. The American Journal of Psychiatry. 2004;161(4):598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- 206.Smith K. R., Kopeikina K. J., Fawcett-Patel J. M., et al. Psychiatric risk factor ANK3/ankyrin-G nanodomains regulate the structure and function of glutamatergic synapses. Neuron. 2014;84(2):399–415. doi: 10.1016/j.neuron.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hajek T., Kopecek M., Hoschl C., Alda M. Smaller hippocampal volumes in patients with bipolar disorder are masked by exposure to lithium: a meta-analysis. Journal of Psychiatry & Neuroscience. 2012;37(5):333–343. doi: 10.1503/jpn.110143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Frodl T., Jager M., Smajstrlova I., et al. Effect of hippocampal and amygdala volumes on clinical outcomes in major depression: a 3-year prospective magnetic resonance imaging study. Journal of Psychiatry & Neuroscience. 2008;33(5):423–430. [PMC free article] [PubMed] [Google Scholar]
- 209.Malykhin N. V., Carter R., Seres P., Coupland N. J. Structural changes in the hippocampus in major depressive disorder: contributions of disease and treatment. Journal of Psychiatry & Neuroscience. 2010;35(5):337–343. doi: 10.1503/jpn.100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Simonetti A., Sani G., Dacquino C., et al. Hippocampal subfield volumes in short- and long-term lithium-treated patients with bipolar I disorder. Bipolar Disorders. 2016;18(4):352–362. doi: 10.1111/bdi.12394. [DOI] [PubMed] [Google Scholar]
- 211.Erickson K. I., Prakash R. S., Voss M. W., et al. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19(10):1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Erickson K. I., Voss M. W., Prakash R. S., et al. Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(7):3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Pereira A. C., Huddleston D. E., Brickman A. M., et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(13):5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Pajonk F. G., Wobrock T., Gruber O., et al. Hippocampal plasticity in response to exercise in schizophrenia. Archives of General Psychiatry. 2010;67(2):133–143. doi: 10.1001/archgenpsychiatry.2009.193. [DOI] [PubMed] [Google Scholar]
- 215.Voss M. W., Vivar C., Kramer A. F., van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends in Cognitive Sciences. 2013;17(10):525–544. doi: 10.1016/j.tics.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Christie B. R., Eadie B. D., Kannangara T. S., Robillard J. M., Shin J., Titterness A. K. Exercising our brains: how physical activity impacts synaptic plasticity in the dentate gyrus. Neuromolecular Medicine. 2008;10(2):47–58. doi: 10.1007/s12017-008-8033-2. [DOI] [PubMed] [Google Scholar]
- 217.Stranahan A. M., Khalil D., Gould E. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus. 2007;17(11):1017–1022. doi: 10.1002/hipo.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Cotman C. W., Berchtold N. C. Physical activity and the maintenance of cognition: learning from animal models. Alzheimers Dement. 2007;3(Supplement 2):S30–S37. doi: 10.1016/j.jalz.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 219.Nottebohm F. Why are some neurons replaced in adult brain? The Journal of Neuroscience. 2002;22(3):624–628. doi: 10.1523/JNEUROSCI.22-03-00624.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Nottebohm F. Neuronal replacement in adult brain. Brain Research Bulletin. 2002;57(6):737–749. doi: 10.1016/S0361-9230(02)00750-5. [DOI] [PubMed] [Google Scholar]
- 221.Christian K. M., Song H., Ming G. L. Functions and dysfunctions of adult hippocampal neurogenesis. Annual Review of Neuroscience. 2014;37:243–262. doi: 10.1146/annurev-neuro-071013-014134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Suh H., Deng W., Gage F. H. Signaling in adult neurogenesis. Annual Review of Cell and Developmental Biology. 2009;25:253–275. doi: 10.1146/annurev.cellbio.042308.113256. [DOI] [PubMed] [Google Scholar]
- 223.Schmidt-Hieber C., Jonas P., Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429(6988):184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- 224.Sahay A., Hen R. Adult hippocampal neurogenesis in depression. Nature Neuroscience. 2007;10(9):1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 225.Herbert J., Lucassen P. J. Depression as a risk factor for Alzheimer’s disease: genes, steroids, cytokines and neurogenesis - what do we need to know? Frontiers in Neuroendocrinology. 2016;41:153–171. doi: 10.1016/j.yfrne.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 226.Zalachoras I., Houtman R., Atucha E., et al. Differential targeting of brain stress circuits with a selective glucocorticoid receptor modulator. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(19):7910–7915. doi: 10.1073/pnas.1219411110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sheline Y. I., Gado M. H., Kraemer H. C. Untreated depression and hippocampal volume loss. The American Journal of Psychiatry. 2003;160(8):1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- 228.van Praag H., Kempermann G., Gage F. H. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neuroscience. 1999;2(3):266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 229.van Praag H., Shubert T., Zhao C., Gage F. H. Exercise enhances learning and hippocampal neurogenesis in aged mice. The Journal of Neuroscience. 2005;25(38):8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Uda M., Ishido M., Kami K., Masuhara M. Effects of chronic treadmill running on neurogenesis in the dentate gyrus of the hippocampus of adult rat. Brain Research. 2006;1104(1):64–72. doi: 10.1016/j.brainres.2006.05.066. [DOI] [PubMed] [Google Scholar]
- 231.Zhao C., Teng E. M., Summers R. G., Jr., Ming G. L., Gage F. H. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. The Journal of Neuroscience. 2006;26(1):3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Creer D. J., Romberg C., Saksida L. M., van Praag H., Bussey T. J. Running enhances spatial pattern separation in mice. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(5):2367–2372. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Coras R., Siebzehnrubl F. A., Pauli E., et al. Low proliferation and differentiation capacities of adult hippocampal stem cells correlate with memory dysfunction in humans. Brain. 2010;133(11):3359–3372. doi: 10.1093/brain/awq215. [DOI] [PubMed] [Google Scholar]
- 234.Drew M. R., Hen R. Adult hippocampal neurogenesis as target for the treatment of depression. CNS & Neurological Disorders Drug Targets. 2007;6(3):205–218. doi: 10.2174/187152707780619353. [DOI] [PubMed] [Google Scholar]
- 235.Eisch A. J., Petrik D. Depression and hippocampal neurogenesis: a road to remission? Science. 2012;338(6103):72–75. doi: 10.1126/science.1222941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Cowansage K. K., LeDoux J. E., Monfils M. H. Brain-derived neurotrophic factor: a dynamic gatekeeper of neural plasticity. Current Molecular Pharmacology. 2010;3(1):12–29. doi: 10.2174/1874467211003010012. [DOI] [PubMed] [Google Scholar]
- 237.Ahlskog J. E., Geda Y. E., Graff-Radford N. R., Petersen R. C. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clinic Proceedings. 2011;86(9):876–884. doi: 10.4065/mcp.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Autry A. E., Monteggia L. M. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacological Reviews. 2012;64(2):238–258. doi: 10.1124/pr.111.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Fernandes B. S., Berk M., Turck C. W., Steiner J., Goncalves C. A. Decreased peripheral brain-derived neurotrophic factor levels are a biomarker of disease activity in major psychiatric disorders: a comparative meta-analysis. Molecular Psychiatry. 2014;19(7):750–751. doi: 10.1038/mp.2013.172. [DOI] [PubMed] [Google Scholar]
- 240.Fernandes B. S., Molendijk M. L., Kohler C. A., et al. Peripheral brain-derived neurotrophic factor (BDNF) as a biomarker in bipolar disorder: a meta-analysis of 52 studies. BMC Medicine. 2015;13(1):p. 289. doi: 10.1186/s12916-015-0529-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Soares A. T., Andreazza A. C., Rej S., et al. Decreased brain-derived neurotrophic factor in older adults with bipolar disorder. The American Journal of Geriatric Psychiatry. 2016;24(8):596–601. doi: 10.1016/j.jagp.2016.02.052. [DOI] [PubMed] [Google Scholar]
- 242.Matrisciano F., Bonaccorso S., Ricciardi A., et al. Changes in BDNF serum levels in patients with major depression disorder (MDD) after 6 months treatment with sertraline, escitalopram, or venlafaxine. Journal of Psychiatric Research. 2009;43(3):247–254. doi: 10.1016/j.jpsychires.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Brunoni A. R., Lopes M., Fregni F. A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. The International Journal of Neuropsychopharmacology. 2008;11(8):1169–1180. doi: 10.1017/S1461145708009309. [DOI] [PubMed] [Google Scholar]
- 244.Dunham J. S., Deakin J. F., Miyajima F., Payton A., Toro C. T. Expression of hippocampal brain-derived neurotrophic factor and its receptors in Stanley consortium brains. Journal of Psychiatric Research. 2009;43(14):1175–1184. doi: 10.1016/j.jpsychires.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 245.Sen S., Duman R., Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biological Psychiatry. 2008;64(6):527–532. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Chang Y. C., Rapoport S. I., Rao J. S. Chronic administration of mood stabilizers upregulates BDNF and bcl-2 expression levels in rat frontal cortex. Neurochemical Research. 2009;34(3):536–541. doi: 10.1007/s11064-008-9817-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Yasuda S., Liang M. H., Marinova Z., Yahyavi A., Chuang D. M. The mood stabilizers lithium and valproate selectively activate the promoter IV of brain-derived neurotrophic factor in neurons. Molecular Psychiatry. 2009;14(1):51–59. doi: 10.1038/sj.mp.4002099. [DOI] [PubMed] [Google Scholar]
- 248.Grande I., Kapczinski F., Stertz L., et al. Peripheral brain-derived neurotrophic factor changes along treatment with extended release quetiapine during acute mood episodes: an open-label trial in drug-free patients with bipolar disorder. Journal of Psychiatric Research. 2012;46(11):1511–1514. doi: 10.1016/j.jpsychires.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 249.Brunoni A. R., Baeken C., Machado-Vieira R., Gattaz W. F., Vanderhasselt M. A. BDNF blood levels after electroconvulsive therapy in patients with mood disorders: a systematic review and meta-analysis. The World Journal of Biological Psychiatry. 2014;15(5):411–418. doi: 10.3109/15622975.2014.892633. [DOI] [PubMed] [Google Scholar]
- 250.Kirshenbaum G. S., Burgess C. R., Dery N., Fahnestock M., Peever J. H., Roder J. C. Attenuation of mania-like behavior in Na(+),K(+)-ATPase alpha3 mutant mice by prospective therapies for bipolar disorder: melatonin and exercise. Neuroscience. 2014;260:195–204. doi: 10.1016/j.neuroscience.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 251.Engesser-Cesar C., Anderson A. J., Cotman C. W. Wheel running and fluoxetine antidepressant treatment have differential effects in the hippocampus and the spinal cord. Neuroscience. 2007;144(3):1033–1044. doi: 10.1016/j.neuroscience.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 252.Legge R. M., Sendi S., Cole J. H., et al. Modulatory effects of brain-derived neurotrophic factor Val66Met polymorphism on prefrontal regions in major depressive disorder. The British Journal of Psychiatry. 2015;206(5):379–384. doi: 10.1192/bjp.bp.113.143529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Verhagen M., van der Meij A., van Deurzen P. A., et al. Meta-analysis of the BDNF Val66Met polymorphism in major depressive disorder: effects of gender and ethnicity. Molecular Psychiatry. 2010;15(3):260–271. doi: 10.1038/mp.2008.109. [DOI] [PubMed] [Google Scholar]
- 254.Neves-Pereira M., Mundo E., Muglia P., King N., Macciardi F., Kennedy J. L. The brain-derived neurotrophic factor gene confers susceptibility to bipolar disorder: evidence from a family-based association study. American Journal of Human Genetics. 2002;71(3):651–655. doi: 10.1086/342288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Matsuo K., Walss-Bass C., Nery F. G., et al. Neuronal correlates of brain-derived neurotrophic factor Val66Met polymorphism and morphometric abnormalities in bipolar disorder. Neuropsychopharmacology. 2009;34(8):1904–1913. doi: 10.1038/npp.2009.23. [DOI] [PubMed] [Google Scholar]
- 256.Frey B. N., Andreazza A. C., Houenou J., et al. Biomarkers in bipolar disorder: a positional paper from the International Society for Bipolar Disorders Biomarkers Task Force. The Australian and New Zealand Journal of Psychiatry. 2013;47(4):321–332. doi: 10.1177/0004867413478217. [DOI] [PubMed] [Google Scholar]
- 257.Zhao G., Zhang C., Chen J., et al. Ratio of mBDNF to proBDNF for differential diagnosis of major depressive disorder and bipolar depression. Molecular Neurobiology. 2016:1–10. doi: 10.1007/s12035-016-0098-6. [DOI] [PubMed] [Google Scholar]
- 258.Noble E. E., Mavanji V., Little M. R., Billington C. J., Kotz C. M., Wang C. Exercise reduces diet-induced cognitive decline and increases hippocampal brain-derived neurotrophic factor in CA3 neurons. Neurobiology of Learning and Memory. 2014;114:40–50. doi: 10.1016/j.nlm.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Ding Q., Ying Z., Gomez-Pinilla F. Exercise influences hippocampal plasticity by modulating brain-derived neurotrophic factor processing. Neuroscience. 2011;192:773–780. doi: 10.1016/j.neuroscience.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Russo-Neustadt A., Ha T., Ramirez R., Kesslak J. P. Physical activity-antidepressant treatment combination: impact on brain-derived neurotrophic factor and behavior in an animal model. Behavioural Brain Research. 2001;120(1):87–95. doi: 10.1016/S0166-4328(00)00364-8. [DOI] [PubMed] [Google Scholar]
- 261.Berchtold N. C., Chinn G., Chou M., Kesslak J. P., Cotman C. W. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133(3):853–861. doi: 10.1016/j.neuroscience.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 262.Wrann C. D., White J. P., Salogiannnis J., et al. Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell Metabolism. 2013;18(5):649–659. doi: 10.1016/j.cmet.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Yuen E. Y., Wei J., Liu W., Zhong P., Li X., Yan Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron. 2012;73(5):962–977. doi: 10.1016/j.neuron.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Liu W., Sheng H., Xu Y., Liu Y., Lu J., Ni X. Swimming exercise ameliorates depression-like behavior in chronically stressed rats: relevant to proinflammatory cytokines and IDO activation. Behavioural Brain Research. 2013;242:110–116. doi: 10.1016/j.bbr.2012.12.041. [DOI] [PubMed] [Google Scholar]
- 265.Gibney S. M., McGuinness B., Prendergast C., Harkin A., Connor T. J. Poly I:C-induced activation of the immune response is accompanied by depression and anxiety-like behaviours, kynurenine pathway activation and reduced BDNF expression. Brain, Behavior, and Immunity. 2013;28:170–181. doi: 10.1016/j.bbi.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 266.Yoshimura Y., Ishikawa C., Kasegai H., Masuda T., Yoshikawa M., Shiga T. Roles of 5-HT1A receptor in the expression of AMPA receptor and BDNF in developing mouse cortical neurons. Neuroscience Research. 2016;115:13–20. doi: 10.1016/j.neures.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 267.Gustafsson G., Lira C. M., Johansson J., et al. The acute response of plasma brain-derived neurotrophic factor as a result of exercise in major depressive disorder. Psychiatry Research. 2009;169(3):244–248. doi: 10.1016/j.psychres.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 268.Laske C., Banschbach S., Stransky E., et al. Exercise-induced normalization of decreased BDNF serum concentration in elderly women with remitted major depression. The International Journal of Neuropsychopharmacology. 2010;13(5):595–602. doi: 10.1017/S1461145709991234. [DOI] [PubMed] [Google Scholar]
- 269.Schuch F. B., da Silveira L. E., de Zeni T. C., et al. Effects of a single bout of maximal aerobic exercise on BDNF in bipolar disorder: a gender-based response. Psychiatry Research. 2015;229(1-2):57–62. doi: 10.1016/j.psychres.2015.07.072. [DOI] [PubMed] [Google Scholar]
- 270.Coelho F. G., Andrade L. P., Pedroso R. V., et al. Multimodal exercise intervention improves frontal cognitive functions and gait in Alzheimer's disease: a controlled trial. Geriatrics & Gerontology International. 2013;13(1):198–203. doi: 10.1111/j.1447-0594.2012.00887.x. [DOI] [PubMed] [Google Scholar]
- 271.Vaynman S., Gomez-Pinilla F. Revenge of the “sit”: how lifestyle impacts neuronal and cognitive health through molecular systems that interface energy metabolism with neuronal plasticity. Journal of Neuroscience Research. 2006;84(4):699–715. doi: 10.1002/jnr.20979. [DOI] [PubMed] [Google Scholar]
- 272.Lu Y., Christian K., Lu B. BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiology of Learning and Memory. 2008;89(3):312–323. doi: 10.1016/j.nlm.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Anisman H., Hayley S. Inflammatory factors contribute to depression and its comorbid conditions. Science Signaling. 2012;5(244, article pe45) doi: 10.1126/scisignal.2003579. [DOI] [PubMed] [Google Scholar]
- 274.Goldstein B. I., Kemp D. E., Soczynska J. K., McIntyre R. S. Inflammation and the phenomenology, pathophysiology, comorbidity, and treatment of bipolar disorder: a systematic review of the literature. The Journal of Clinical Psychiatry. 2009;70(8):1078–1090. doi: 10.4088/JCP.08r04505. [DOI] [PubMed] [Google Scholar]
- 275.Raison C. L., Capuron L., Miller A. H. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends in Immunology. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Brietzke E., Kauer-Sant'Anna M., Teixeira A. L., Kapczinski F. Abnormalities in serum chemokine levels in euthymic patients with bipolar disorder. Brain, Behavior, and Immunity. 2009;23(8):1079–1082. doi: 10.1016/j.bbi.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 277.Dowlati Y., Herrmann N., Swardfager W., et al. A meta-analysis of cytokines in major depression. Biological Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 278.Howren M. B., Lamkin D. M., Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosomatic Medicine. 2009;71(2):171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 279.Asnis G. M., De La Garza R., 2nd Interferon-induced depression in chronic hepatitis C: a review of its prevalence, risk factors, biology, and treatment approaches. Journal of Clinical Gastroenterology. 2006;40(4):322–335. doi: 10.1097/01.mcg.0000210099.36500.fe. [DOI] [PubMed] [Google Scholar]
- 280.Dieperink E., Willenbring M., Ho S. B. Neuropsychiatric symptoms associated with hepatitis C and interferon alpha: a review. The American Journal of Psychiatry. 2000;157(6):867–876. doi: 10.1176/appi.ajp.157.6.867. [DOI] [PubMed] [Google Scholar]
- 281.Na K. S., Lee K. J., Lee J. S., Cho Y. S., Jung H. Y. Efficacy of adjunctive celecoxib treatment for patients with major depressive disorder: a meta-analysis. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2014;48:79–85. doi: 10.1016/j.pnpbp.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 282.Soczynska J. K., Kennedy S. H., Goldstein B. I., Lachowski A., Woldeyohannes H. O., McIntyre R. S. The effect of tumor necrosis factor antagonists on mood and mental health-associated quality of life: novel hypothesis-driven treatments for bipolar depression? Neurotoxicology. 2009;30(4):497–521. doi: 10.1016/j.neuro.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 283.Menter A., Augustin M., Signorovitch J., et al. The effect of adalimumab on reducing depression symptoms in patients with moderate to severe psoriasis: a randomized clinical trial. Journal of the American Academy of Dermatology. 2010;62(5):812–818. doi: 10.1016/j.jaad.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 284.Muller N., Schwarz M. J., Dehning S., et al. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Molecular Psychiatry. 2006;11(7):680–684. doi: 10.1038/sj.mp.4001805. [DOI] [PubMed] [Google Scholar]
- 285.Boufidou F., Nikolaou C., Alevizos B., Liappas I. A., Christodoulou G. N. Cytokine production in bipolar affective disorder patients under lithium treatment. Journal of Affective Disorders. 2004;82(2):309–313. doi: 10.1016/j.jad.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 286.McIntyre R. S., Muzina D. J., Kemp D. E., et al. Bipolar disorder and suicide: research synthesis and clinical translation. Current Psychiatry Reports. 2008;10(1):66–72. doi: 10.1007/s11920-008-0012-7. [DOI] [PubMed] [Google Scholar]
- 287.Woods J. A., Ceddia M. A., Wolters B. W., Evans J. K., Lu Q., McAuley E. Effects of 6 months of moderate aerobic exercise training on immune function in the elderly. Mechanisms of Ageing and Development. 1999;109(1):1–19. doi: 10.1016/S0047-6374(99)00014-7. [DOI] [PubMed] [Google Scholar]
- 288.Crist D. M., Mackinnon L. T., Thompson R. F., Atterbom H. A., Egan P. A. Physical exercise increases natural cellular-mediated tumor cytotoxicity in elderly women. Gerontology. 1989;35(2-3):66–71. doi: 10.1159/000213001. [DOI] [PubMed] [Google Scholar]
- 289.Pace T. W., Hu F., Miller A. H. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain, Behavior, and Immunity. 2007;21(1):9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Radom-Aizik S., Zaldivar F. P., Jr., Haddad F., Cooper D. M. Impact of brief exercise on circulating monocyte gene and microRNA expression: implications for atherosclerotic vascular disease. Brain, Behavior, and Immunity. 2014;39:121–129. doi: 10.1016/j.bbi.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Ekdahl C. T., Claasen J. H., Bonde S., Kokaia Z., Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(23):13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.General OoS. The Surgeon General's Vision for a Healthy and Fit Nation. Rockville, MD, USA: Service UPH; 2010. [Google Scholar]
- 293.Lupien S. J., Buss C., Schramek T. E., Maheu F., Pruessner J. Hormetic influence of glucocorticoids on human memory. Nonlinearity in Biology, Toxicology, Medicine. 2005;3(1):23–56. doi: 10.2201/nonlin.003.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Sapolsky R. M., Uno H., Rebert C. S., Finch C. E. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. The Journal of Neuroscience. 1990;10(9):2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23(5):477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- 296.Steckler T., Holsboer F., Reul J. M. Glucocorticoids and depression. Baillière's Best Practice & Research. Clinical Endocrinology & Metabolism. 1999;13(4):597–614. doi: 10.1053/beem.1999.0046. [DOI] [PubMed] [Google Scholar]
- 297.Pariante C. M., Miller A. H. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biological Psychiatry. 2001;49(5):391–404. doi: 10.1016/S0006-3223(00)01088-X. [DOI] [PubMed] [Google Scholar]
- 298.Ising M., Kunzel H. E., Binder E. B., Nickel T., Modell S., Holsboer F. The combined dexamethasone/CRH test as a potential surrogate marker in depression. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2005;29(6):1085–1093. doi: 10.1016/j.pnpbp.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 299.Heuser I., Yassouridis A., Holsboer F. The combined dexamethasone/CRH test: a refined laboratory test for psychiatric disorders. Journal of Psychiatric Research. 1994;28(4):341–356. doi: 10.1016/0022-3956(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 300.Nemeroff C. B., Widerlov E., Bissette G., et al. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226(4680):1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- 301.Havermans R., Nicolson N. A., Berkhof J., deVries M. W. Patterns of salivary cortisol secretion and responses to daily events in patients with remitted bipolar disorder. Psychoneuroendocrinology. 2011;36(2):258–265. doi: 10.1016/j.psyneuen.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 302.Keller J., Flores B., Gomez R. G., et al. Cortisol circadian rhythm alterations in psychotic major depression. Biological Psychiatry. 2006;60(3):275–281. doi: 10.1016/j.biopsych.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 303.Zhou R., Lu Y., Han Y., et al. Mice heterozygous for cathepsin D deficiency exhibit mania-related behavior and stress-induced depression. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2015;63:110–118. doi: 10.1016/j.pnpbp.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 304.Marchand W. R., Valentina D. D., Jensen C. R. Neurobiology of mood disorders. Hospital Physician. 2005;43:17–26. [Google Scholar]
- 305.Manji H. K., Quiroz J. A., Sporn J., et al. Enhancing neuronal plasticity and cellular resilience to develop novel, improved therapeutics for difficult-to-treat depression. Biological Psychiatry. 2003;53(8):707–742. doi: 10.1016/S0006-3223(03)00117-3. [DOI] [PubMed] [Google Scholar]
- 306.Bremner J. D., Narayan M., Anderson E. R., Staib L. H., Miller H. L., Charney D. S. Hippocampal volume reduction in major depression. The American Journal of Psychiatry. 2000;157(1):115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- 307.Dedovic K., Ngiam J. The cortisol awakening response and major depression: examining the evidence. Neuropsychiatric Disease and Treatment. 2015;11:1181–1189. doi: 10.2147/NDT.S62289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Barbosa I. G., Bauer M. E., Machado-Vieira R., Teixeira A. L. Cytokines in bipolar disorder: paving the way for neuroprogression. Neural Plasticity. 2014;2014:p. 9. doi: 10.1155/2014/360481.360481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Miller A. H., Pariante C. M., Pearce B. D. Effects of cytokines on glucocorticoid receptor expression and function. Glucocorticoid resistance and relevance to depression. Advances in Experimental Medicine and Biology. 1999;461:107–116. doi: 10.1007/978-0-585-37970-8_7. [DOI] [PubMed] [Google Scholar]
- 310.Miller A. H., Pearce B. D., Ruzek M. C., Biron C. A. Interactions between the Hypothalamic-Pituitary-Adrenal Axis and Immune System during Viral Infection: Pathways for Environmental Effects of Disease Expression. New York, NY, USA: Oxford University Press; 2001. [Google Scholar]
- 311.Ploeger H. E., Takken T., de Greef M. H., Timmons B. W. The effects of acute and chronic exercise on inflammatory markers in children and adults with a chronic inflammatory disease: a systematic review. Exercise Immunology Review. 2009;15(1):6–41. [PubMed] [Google Scholar]
- 312.Stranahan A. M., Lee K., Mattson M. P. Central mechanisms of HPA axis regulation by voluntary exercise. Neuromolecular Medicine. 2008;10(2):118–127. doi: 10.1007/s12017-008-8027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Lupien S. J., de Leon M., de Santi S., et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nature Neuroscience. 1998;1(1):69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- 314.Apostolova L. G., Dutton R. A., Dinov I. D., et al. Conversion of mild cognitive impairment to Alzheimer disease predicted by hippocampal atrophy maps. Archives of Neurology. 2006;63(5):693–699. doi: 10.1001/archneur.63.5.693. [DOI] [PubMed] [Google Scholar]
- 315.Devanand D. P., Pradhaban G., Liu X., et al. Hippocampal and entorhinal atrophy in mild cognitive impairment: prediction of Alzheimer disease. Neurology. 2007;68(11):828–836. doi: 10.1212/01.wnl.0000256697.20968.d7. [DOI] [PubMed] [Google Scholar]
- 316.Nabkasorn C., Miyai N., Sootmongkol A., et al. Effects of physical exercise on depression, neuroendocrine stress hormones and physiological fitness in adolescent females with depressive symptoms. European Journal of Public Health. 2006;16(2):179–184. doi: 10.1093/eurpub/cki159. [DOI] [PubMed] [Google Scholar]
- 317.Woolley C. S., Gould E., McEwen B. S. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Research. 1990;531(1-2):225–231. doi: 10.1016/0006-8993(90)90778-A. [DOI] [PubMed] [Google Scholar]
- 318.Eadie B. D., Redila V. A., Christie B. R. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. The Journal of Comparative Neurology. 2005;486(1):39–47. doi: 10.1002/cne.20493. [DOI] [PubMed] [Google Scholar]
- 319.Redila V. A., Christie B. R. Exercise-induced changes in dendritic structure and complexity in the adult hippocampal dentate gyrus. Neuroscience. 2006;137(4):1299–1307. doi: 10.1016/j.neuroscience.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 320.Chatzitheodorou D., Mavromoustakos S., Milioti S. The effect of exercise on adrenocortical responsiveness of patients with chronic low back pain, controlled for psychological strain. Clinical Rehabilitation. 2008;22(4):319–328. doi: 10.1177/0269215507079858. [DOI] [PubMed] [Google Scholar]
- 321.Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biology. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Floyd R. A. Antioxidants, oxidative stress, and degenerative neurological disorders. Proceedings of the Society for Experimental Biology and Medicine. 1999;222(3):236–245. doi: 10.1046/j.1525-1373.1999.d01-140.x. [DOI] [PubMed] [Google Scholar]
- 323.Baxter L. R., Jr., Phelps M. E., Mazziotta J. C., et al. Cerebral metabolic rates for glucose in mood disorders. Studies with positron emission tomography and fluorodeoxyglucose F 18. Archives of General Psychiatry. 1985;42(5):441–447. doi: 10.1001/archpsyc.1985.01790280019002. [DOI] [PubMed] [Google Scholar]
- 324.Kuloglu M., Ustundag B., Atmaca M., Canatan H., Tezcan A. E., Cinkilinc N. Lipid peroxidation and antioxidant enzyme levels in patients with schizophrenia and bipolar disorder. Cell Biochemistry and Function. 2002;20(2):171–175. doi: 10.1002/cbf.940. [DOI] [PubMed] [Google Scholar]
- 325.Andreazza A. C., Kauer-Sant'anna M., Frey B. N., et al. Oxidative stress markers in bipolar disorder: a meta-analysis. Journal of Affective Disorders. 2008;111(2-3):135–144. doi: 10.1016/j.jad.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 326.Lopresti A. L., Maker G. L., Hood S. D., Drummond P. D. A review of peripheral biomarkers in major depression: the potential of inflammatory and oxidative stress biomarkers. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2014;48:102–111. doi: 10.1016/j.pnpbp.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 327.Maes M., Galecki P., Chang Y. S., Berk M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro) degenerative processes in that illness. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2011;35(3):676–692. doi: 10.1016/j.pnpbp.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 328.Raza M. U., Tufan T., Wang Y., Hill C., Zhu M. Y. DNA damage in major psychiatric diseases. Neurotoxicity Research. 2016;30(2):251–267. doi: 10.1007/s12640-016-9621-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Andreazza A. C., Frey B. N., Erdtmann B., et al. DNA damage in bipolar disorder. Psychiatry Research. 2007;153(1):27–32. doi: 10.1016/j.psychres.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 330.Soeiro-de-Souza M. G., Andreazza A. C., Carvalho A. F., Machado-Vieira R., Young L. T., Moreno R. A. Number of manic episodes is associated with elevated DNA oxidation in bipolar I disorder. The International Journal of Neuropsychopharmacology. 2013;16(7):1505–1512. doi: 10.1017/S1461145713000047. [DOI] [PubMed] [Google Scholar]
- 331.Friedland P. G., Petot R. P., Nunomura G. J., Castellani R. J., Kubat Z. Alzheimer as a disease of metabolic demand: benefits of physical and brain exercise. In: Radak Z., editor. Exercise and Diseases. Oxford: Meyer Sport; 2005. pp. 7–16. [Google Scholar]
- 332.Leeuwenburgh C., Heinecke J. W. Oxidative stress and antioxidants in exercise. Current Medicinal Chemistry. 2001;8(7):829–838. doi: 10.2174/0929867013372896. [DOI] [PubMed] [Google Scholar]
- 333.Radak Z., Chung H. Y., Goto S. Systemic adaptation to oxidative challenge induced by regular exercise. Free Radical Biology & Medicine. 2008;44(2):153–159. doi: 10.1016/j.freeradbiomed.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 334.Teo W., Newton M. J., McGuigan M. R. Circadian rhythms in exercise performance: implications for hormonal and muscular adaptation. Journal of Sports Science and Medicine. 2011;10(4):600–606. [PMC free article] [PubMed] [Google Scholar]
- 335.Klein D. C., Moore R. Y. Pineal N-acetyltransferase and hydroxyindole-O-methyltransferase: control by the retinohypothalamic tract and the suprachiasmatic nucleus. Brain Research. 1979;174(2):245–262. doi: 10.1016/0006-8993(79)90848-5. [DOI] [PubMed] [Google Scholar]
- 336.Murray G., Harvey A. Circadian rhythms and sleep in bipolar disorder. Bipolar Disorders. 2010;12(5):459–472. doi: 10.1111/j.1399-5618.2010.00843.x. [DOI] [PubMed] [Google Scholar]
- 337.Kalsbeek A., Perreau-Lenz S., Buijs R. M. A network of (autonomic) clock outputs. Chronobiology International. 2006;23(3):521–535. doi: 10.1080/07420520600651073. [DOI] [PubMed] [Google Scholar]
- 338.Nader N., Chrousos G. P., Kino T. Interactions of the circadian CLOCK system and the HPA axis. Trends in Endocrinology and Metabolism. 2010;21(5):277–286. doi: 10.1016/j.tem.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Mansour H. A., Wood J., Chowdari K. V., et al. Circadian phase variation in bipolar I disorder. Chronobiology International. 2005;22(3):571–584. doi: 10.1081/CBI-200062413. [DOI] [PubMed] [Google Scholar]
- 340.Wirz-Justice A. Chronobiology and mood disorders. Dialogues in Clinical Neuroscience. 2003;5(4):315–325. doi: 10.31887/DCNS.2003.5.4/awirzjustice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Kronfeld-Schor N., Einat H. Circadian rhythms and depression: human psychopathology and animal models. Neuropharmacology. 2012;62(1):101–114. doi: 10.1016/j.neuropharm.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 342.McEwen B. S. Sleep deprivation as a neurobiologic and physiologic stressor: allostasis and allostatic load. Metabolism. 2006;55(10) Supplement 2:S20–S23. doi: 10.1016/j.metabol.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 343.Redline S., Foody J. Sleep disturbances: time to join the top 10 potentially modifiable cardiovascular risk factors? Circulation. 2011;124(19):2049–2051. doi: 10.1161/CIRCULATIONAHA.111.062190. [DOI] [PubMed] [Google Scholar]
- 344.Hua P., Liu W., Chen D., et al. Cry1 and Tef gene polymorphisms are associated with major depressive disorder in the Chinese population. Journal of Affective Disorders. 2014;157:100–103. doi: 10.1016/j.jad.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Soria V., Martinez-Amoros E., Escaramis G., et al. Differential association of circadian genes with mood disorders: CRY1 and NPAS2 are associated with unipolar major depression and CLOCK and VIP with bipolar disorder. Neuropsychopharmacology. 2010;35(6):1279–1289. doi: 10.1038/npp.2009.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Melo M. C., Garcia R. F., Linhares Neto V. B., et al. Sleep and circadian alterations in people at risk for bipolar disorder: a systematic review. Journal of Psychiatric Research. 2016;83:211–219. doi: 10.1016/j.jpsychires.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 347.Milhiet V., Etain B., Boudebesse C., Bellivier F. Circadian biomarkers, circadian genes and bipolar disorders. Journal of Physiology, Paris. 2011;105(4–6):183–189. doi: 10.1016/j.jphysparis.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 348.Medina A. B., Lechuga D. A., Escandon O. S., Moctezuma J. V. Update of sleep alterations in depression. Sleep Science. 2014;7(3):165–169. doi: 10.1016/j.slsci.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Harvey A. G. Sleep and circadian rhythms in bipolar disorder: seeking synchrony, harmony, and regulation. The American Journal of Psychiatry. 2008;165(7):820–829. doi: 10.1176/appi.ajp.2008.08010098. [DOI] [PubMed] [Google Scholar]
- 350.Nurnberger J. I., Jr., Adkins S., Lahiri D. K., et al. Melatonin suppression by light in euthymic bipolar and unipolar patients. Archives of General Psychiatry. 2000;57(6):572–579. doi: 10.1001/archpsyc.57.6.572. [DOI] [PubMed] [Google Scholar]
- 351.Lewy A. J., Nurnberger J. I., Jr., Wehr T. A., et al. Supersensitivity to light: possible trait marker for manic-depressive illness. The American Journal of Psychiatry. 1985;142(6):725–727. doi: 10.1176/ajp.142.6.725. [DOI] [PubMed] [Google Scholar]
- 352.Hallam K. T., Olver J. S., Norman T. R. Effect of sodium valproate on nocturnal melatonin sensitivity to light in healthy volunteers. Neuropsychopharmacology. 2005;30(7):1400–1404. doi: 10.1038/sj.npp.1300739. [DOI] [PubMed] [Google Scholar]
- 353.Hallam K. T., Olver J. S., Horgan J. E., McGrath C., Norman T. R. Low doses of lithium carbonate reduce melatonin light sensitivity in healthy volunteers. The International Journal of Neuropsychopharmacology. 2005;8(2):255–259. doi: 10.1017/S1461145704004894. [DOI] [PubMed] [Google Scholar]
- 354.Bumb J. M., Enning F., Mueller J. K., et al. Differential melatonin alterations in cerebrospinal fluid and serum of patients with major depressive disorder and bipolar disorder. Comprehensive Psychiatry. 2016;68:34–39. doi: 10.1016/j.comppsych.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 355.Gallagher P., Watson S., Smith M. S., Young A. H., Ferrier I. N. Plasma cortisol-dehydroepiandrosterone (DHEA) ratios in schizophrenia and bipolar disorder. Schizophrenia Research. 2007;90(1–3):258–265. doi: 10.1016/j.schres.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 356.Linkowski P., Kerkhofs M., Van Onderbergen A., et al. The 24-hour profiles of cortisol, prolactin, and growth hormone secretion in mania. Archives of General Psychiatry. 1994;51(8):616–624. doi: 10.1001/archpsyc.1994.03950080028004. [DOI] [PubMed] [Google Scholar]
- 357.Young A. H. Antiglucocoticoid treatments for depression. The Australian and New Zealand Journal of Psychiatry. 2006;40(5):402–405. doi: 10.1080/j.1440-1614.2006.01813.x. [DOI] [PubMed] [Google Scholar]
- 358.Chrousos G. P. Stress and disorders of the stress system. Nature Reviews Endocrinology. 2009;5(7):374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- 359.Antle M. C., Smith V. M., Sterniczuk R., Yamakawa G. R., Rakai B. D. Physiological responses of the circadian clock to acute light exposure at night. Reviews in Endocrine & Metabolic Disorders. 2009;10(4):279–291. doi: 10.1007/s11154-009-9116-6. [DOI] [PubMed] [Google Scholar]
- 360.McCarthy M. J., Welsh D. K. Cellular circadian clocks in mood disorders. Journal of Biological Rhythms. 2012;27(5):339–352. doi: 10.1177/0748730412456367. [DOI] [PubMed] [Google Scholar]
- 361.Zhang R., Lahens N. F., Ballance H. I., Hughes M. E., Hogenesch J. B. A circadian gene expression atlas in mammals: implications for biology and medicine. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(45):16219–16224. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Cirelli C., Tononi G. Gene expression in the brain across the sleep-waking cycle. Brain Research. 2000;885(2):303–321. doi: 10.1016/S0006-8993(00)03008-0. [DOI] [PubMed] [Google Scholar]
- 363.Iyer R., Wang T. A., Gillette M. U. Circadian gating of neuronal functionality: a basis for iterative metaplasticity. Frontiers in Systems Neuroscience. 2014;8:p. 164. doi: 10.3389/fnsys.2014.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Burton N. W., Pakenham K. I., Brown W. J. Are psychologists willing and able to promote physical activity as part of psychological treatment? International Journal of Behavioral Medicine. 2010;17(4):287–297. doi: 10.1007/s12529-010-9087-8. [DOI] [PubMed] [Google Scholar]
- 365.Kim S. J., Benloucif S., Reid K. J., et al. Phase-shifting response to light in older adults. The Journal of Physiology. 2014;592(1):189–202. doi: 10.1113/jphysiol.2013.262899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Escames G., Ozturk G., Bano-Otalora B., et al. Exercise and melatonin in humans: reciprocal benefits. Journal of Pineal Research. 2012;52(1):1–11. doi: 10.1111/j.1600-079X.2011.00924.x. [DOI] [PubMed] [Google Scholar]
- 367.Swaab D. F. Suprachiasmatic nucleus (SCN) and pineal gland. In: Aminoff M. B., Swaab D. F., editors. Handbook of Clinical Neurology. Amsterdam, Netherlands: 2003. pp. 63–123. [DOI] [PubMed] [Google Scholar]
- 368.Naylor E., Penev P. D., Orbeta L., et al. Daily social and physical activity increases slow-wave sleep and daytime neuropsychological performance in the elderly. Sleep. 2000;23(1):87–95. [PubMed] [Google Scholar]
- 369.Kubitz K. A., Landers D. M., Petruzzello S. J., Han M. The effects of acute and chronic exercise on sleep. a meta-analytic review. Sports Medicine. 1996;21(4):277–291. doi: 10.2165/00007256-199621040-00004. [DOI] [PubMed] [Google Scholar]
- 370.Ablin J. N., Clauw D. J., Lyden A. K., et al. Effects of sleep restriction and exercise deprivation on somatic symptoms and mood in healthy adults. Clinical and Experimental Rheumatology. 2013;31(6) Supplement 79:S53–S59. [PubMed] [Google Scholar]
- 371.Baron K. G., Reid K. J., Zee P. C. Exercise to improve sleep in insomnia: exploration of the bidirectional effects. Journal of Clinical Sleep Medicine. 2013;9(8):819–824. doi: 10.5664/jcsm.2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Tanaka H., Taira K., Arakawa M., et al. Effects of short nap and exercise on elderly people having difficulty in sleeping. Psychiatry and Clinical Neurosciences. 2001;55(3):173–174. doi: 10.1046/j.1440-1819.2001.00813.x. [DOI] [PubMed] [Google Scholar]
- 373.Tanaka H., Taira K., Arakawa M., et al. Short naps and exercise improve sleep quality and mental health in the elderly. Psychiatry and Clinical Neurosciences. 2002;56(3):233–234. doi: 10.1046/j.1440-1819.2002.00995.x. [DOI] [PubMed] [Google Scholar]
- 374.Uezu E., Taira K., Tanaka H., et al. Survey of sleep-health and lifestyle of the elderly in Okinawa. Psychiatry and Clinical Neurosciences. 2000;54(3):311–3. doi: 10.1046/j.1440-1819.2000.00692.x. [DOI] [PubMed] [Google Scholar]
- 375.Foley D., Ancoli-Israel S., Britz P., Walsh J. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. Journal of Psychosomatic Research. 2004;56(5):497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 376.Van Someren E. J., Lijzenga C., Mirmiran M., Swaab D. F. Long-term fitness training improves the circadian rest-activity rhythm in healthy elderly males. Journal of Biological Rhythms. 1997;12(2):146–156. doi: 10.1177/074873049701200206. [DOI] [PubMed] [Google Scholar]
- 377.Buxton O. M., Frank S. A., L'Hermite-Baleriaux M., Leproult R., Turek F. W., Van Cauter E. Roles of intensity and duration of nocturnal exercise in causing phase delays of human circadian rhythms. The American Journal of Physiology. 1997;273(3) Part 1:E536–E542. doi: 10.1152/ajpendo.1997.273.3.E536. [DOI] [PubMed] [Google Scholar]
- 378.Baehr E. K., Eastman C. I., Revelle W., Olson S. H., Wolfe L. F., Zee P. C. Circadian phase-shifting effects of nocturnal exercise in older compared with young adults. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2003;284(6):R1542–R1550. doi: 10.1152/ajpregu.00761.2002. [DOI] [PubMed] [Google Scholar]
- 379.Yamanaka Y., Hashimoto S., Tanahashi Y., Nishide S. Y., Honma S., Honma K. Physical exercise accelerates reentrainment of human sleep-wake cycle but not of plasma melatonin rhythm to 8-h phase-advanced sleep schedule. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2010;298(3):R681–R691. doi: 10.1152/ajpregu.00345.2009. [DOI] [PubMed] [Google Scholar]
- 380.Miyazaki T., Hashimoto S., Masubuchi S., Honma S., Honma K. I. Phase-advance shifts of human circadian pacemaker are accelerated by daytime physical exercise. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2001;281(1):R197–R205. doi: 10.1152/ajpregu.2001.281.1.R197. [DOI] [PubMed] [Google Scholar]
- 381.Buxton O. M., Lee C. W., L'Hermite-Baleriaux M., Turek F. W., Van Cauter E. Exercise elicits phase shifts and acute alterations of melatonin that vary with circadian phase. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2003;284(3):R714–R724. doi: 10.1152/ajpregu.00355.2002. [DOI] [PubMed] [Google Scholar]
- 382.Rethorst C. D., Sunderajan P., Greer T. L., et al. Does exercise improve self-reported sleep quality in non-remitted major depressive disorder? Psychological Medicine. 2013;43(4):699–709. doi: 10.1017/S0033291712001675. [DOI] [PubMed] [Google Scholar]
- 383.Rethorst C. D., Greer T. L., Toups M. S., Bernstein I., Carmody T. J., Trivedi M. H. IL-1beta and BDNF are associated with improvement in hypersomnia but not insomnia following exercise in major depressive disorder. Translational Psychiatry. 2015;5(8, article e611) doi: 10.1038/tp.2015.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.McGlinchey E. L., Gershon A., Eidelman P., Kaplan K. A., Harvey A. G. Physical activity and sleep: day-to-day associations among individuals with and without bipolar disorder. Mental Health and Physical Activity. 2014;7(3):183–190. doi: 10.1016/j.mhpa.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Suto M., Murray G., Hale S., Amari E., Michalak E. E. What works for people with bipolar disorder? Tips from the experts. Journal of Affective Disorders. 2010;124(1-2):76–84. doi: 10.1016/j.jad.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 386.Proudfoot J., Whitton A., Parker G., Doran J., Manicavasagar V., Delmas K. Triggers of mania and depression in young adults with bipolar disorder. Journal of Affective Disorders. 2012;143(1–3):196–202. doi: 10.1016/j.jad.2012.05.052. [DOI] [PubMed] [Google Scholar]
- 387.Klinzing J. G., Rasch B., Born J., Diekelmann S. Sleep’s role in the reconsolidation of declarative memories. Neurobiology of Learning and Memory. 2016;136:166–173. doi: 10.1016/j.nlm.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 388.Walker M. P., Stickgold R. Sleep, memory, and plasticity. Annual Review of Psychology. 2006;57:139–166. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- 389.Tononi G., Cirelli C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014;81(1):12–34. doi: 10.1016/j.neuron.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Sabunciyan S., Aryee M. J., Irizarry R. A., et al. Genome-wide DNA methylation scan in major depressive disorder. PLoS One. 2012;7(4, article e34451) doi: 10.1371/journal.pone.0034451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Numata S., Ishii K., Tajima A., et al. Blood diagnostic biomarkers for major depressive disorder using multiplex DNA methylation profiles: discovery and validation. Epigenetics. 2015;10(2):135–141. doi: 10.1080/15592294.2014.1003743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Rao J. S., Keleshian V. L., Klein S., Rapoport S. I. Epigenetic modifications in frontal cortex from Alzheimer’s disease and bipolar disorder patients. Translational Psychiatry. 2012;2(7, article e132) doi: 10.1038/tp.2012.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Banigan M. G., Kao P. F., Kozubek J. A., et al. Differential expression of exosomal microRNAs in prefrontal cortices of schizophrenia and bipolar disorder patients. PLoS One. 2013;8(1, article e48814) doi: 10.1371/journal.pone.0048814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Gamazon E. R., Badner J. A., Cheng L., et al. Enrichment of cis-regulatory gene expression SNPs and methylation quantitative trait loci among bipolar disorder susceptibility variants. Molecular Psychiatry. 2013;18(3):340–346. doi: 10.1038/mp.2011.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Dempster E. L., Pidsley R., Schalkwyk L. C., et al. Disease-associated epigenetic changes in monozygotic twins discordant for schizophrenia and bipolar disorder. Human Molecular Genetics. 2011;20(24):4786–4796. doi: 10.1093/hmg/ddr416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Ikegame T., Bundo M., Murata Y., Kasai K., Kato T., Iwamoto K. DNA methylation of the BDNF gene and its relevance to psychiatric disorders. Journal of Human Genetics. 2013;58(7):434–438. doi: 10.1038/jhg.2013.65. [DOI] [PubMed] [Google Scholar]
- 397.Dudley K. J., Li X., Kobor M. S., Kippin T. E., Bredy T. W. Epigenetic mechanisms mediating vulnerability and resilience to psychiatric disorders. Neuroscience and Biobehavioral Reviews. 2011;35(7):1544–1551. doi: 10.1016/j.neubiorev.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 398.Vialou V., Feng J., Robison A. J., Nestler E. J. Epigenetic mechanisms of depression and antidepressant action. Annual Review of Pharmacology and Toxicology. 2013;53:59–87. doi: 10.1146/annurev-pharmtox-010611-134540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Duman R. S., Aghajanian G. K., Sanacora G., Krystal J. H. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nature Medicine. 2016;22(3):238–249. doi: 10.1038/nm.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Ma D. K., Jang M. H., Guo J. U., et al. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science. 2009;323(5917):1074–1077. doi: 10.1126/science.1166859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Borrelli E., Nestler E. J., Allis C. D., Sassone-Corsi P. Decoding the epigenetic language of neuronal plasticity. Neuron. 2008;60(6):961–974. doi: 10.1016/j.neuron.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Nestler E. J. Epigenetic mechanisms in psychiatry. Biological Psychiatry. 2009;65(3):189–190. doi: 10.1016/j.biopsych.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 403.Hobara T., Uchida S., Otsuki K., et al. Altered gene expression of histone deacetylases in mood disorder patients. Journal of Psychiatric Research. 2010;44(5):263–270. doi: 10.1016/j.jpsychires.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 404.Munkholm K., Vinberg M., Berk M., Kessing L. V. State-related alterations of gene expression in bipolar disorder: a systematic review. Bipolar Disorders. 2012;14(7):684–696. doi: 10.1111/bdi.12005. [DOI] [PubMed] [Google Scholar]
- 405.Sweatt J. D. Experience-dependent epigenetic modifications in the central nervous system. Biological Psychiatry. 2009;65(3):191–197. doi: 10.1016/j.biopsych.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Reul J. M., Chandramohan Y. Epigenetic mechanisms in stress-related memory formation. Psychoneuroendocrinology. 2007;32(Supplement 1):S21–S25. doi: 10.1016/j.psyneuen.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 407.Zhao J., Goldberg J., Bremner J. D., Vaccarino V. Association between promoter methylation of serotonin transporter gene and depressive symptoms: a monozygotic twin study. Psychosomatic Medicine. 2013;75(6):523–529. doi: 10.1097/PSY.0b013e3182924cf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Hammen C. Stress and depression. Annual Review of Clinical Psychology. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- 409.Anacker C., O'Donnell K. J., Meaney M. J. Early life adversity and the epigenetic programming of hypothalamic-pituitary-adrenal function. Dialogues in Clinical Neuroscience. 2014;16(3):321–333. doi: 10.31887/DCNS.2014.16.3/canacker. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Kuratomi G., Iwamoto K., Bundo M., et al. Aberrant DNA methylation associated with bipolar disorder identified from discordant monozygotic twins. Molecular Psychiatry. 2008;13(4):429–441. doi: 10.1038/sj.mp.4002001. [DOI] [PubMed] [Google Scholar]
- 411.Barres R., Yan J., Egan B., et al. Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metabolism. 2012;15(3):405–411. doi: 10.1016/j.cmet.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 412.McGee S. L., Hargreaves M. Histone modifications and exercise adaptations. Journal of Applied Physiology (1985) 2011;110(1):258–263. doi: 10.1152/japplphysiol.00979.2010. [DOI] [PubMed] [Google Scholar]
- 413.Elsner V. R., Lovatel G. A., Moyses F., et al. Exercise induces age-dependent changes on epigenetic parameters in rat hippocampus: a preliminary study. Experimental Gerontology. 2013;48(2):136–139. doi: 10.1016/j.exger.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Tsankova N. M., Berton O., Renthal W., Kumar A., Neve R. L., Nestler E. J. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nature Neuroscience. 2006;9(4):519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- 415.Gomez-Pinilla F., Zhuang Y., Feng J., Ying Z., Fan G. Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation. The European Journal of Neuroscience. 2011;33(3):383–390. doi: 10.1111/j.1460-9568.2010.07508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Januar V., Ancelin M. L., Ritchie K., Saffery R., Ryan J. BDNF promoter methylation and genetic variation in late-life depression. Translational Psychiatry. 2015;5(8, article e619) doi: 10.1038/tp.2015.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Sanchis-Gomar F., Garcia-Gimenez J. L., Perez-Quilis C., Gomez-Cabrera M. C., Pallardo F. V., Lippi G. Physical exercise as an epigenetic modulator: eustress, the “positive stress” as an effector of gene expression. Journal of Strength and Conditioning Research. 2012;26(12):3469–3472. doi: 10.1519/JSC.0b013e31825bb594. [DOI] [PubMed] [Google Scholar]
- 418.Niculescu A. B. Convergent functional genomics of psychiatric disorders. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics. 2013;162B(7):587–594. doi: 10.1002/ajmg.b.32163. [DOI] [PubMed] [Google Scholar]
- 419.Neeper S. A., Gomez-Pinilla F., Choi J., Cotman C. Exercise and brain neurotrophins. Nature. 1995;373(6510):p. 109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- 420.Tang S. W., Chu E., Hui T., Helmeste D., Law C. Influence of exercise on serum brain-derived neurotrophic factor concentrations in healthy human subjects. Neuroscience Letters. 2008;431(1):62–65. doi: 10.1016/j.neulet.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 421.Seifert T., Brassard P., Wissenberg M., et al. Endurance training enhances BDNF release from the human brain. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2010;298(2):R372–R377. doi: 10.1152/ajpregu.00525.2009. [DOI] [PubMed] [Google Scholar]
- 422.Rasmussen P., Brassard P., Adser H., et al. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Experimental Physiology. 2009;94(10):1062–1069. doi: 10.1113/expphysiol.2009.048512. [DOI] [PubMed] [Google Scholar]
- 423.Abel J. L., Rissman E. F. Running-induced epigenetic and gene expression changes in the adolescent brain. International Journal of Developmental Neuroscience. 2013;31(6):382–390. doi: 10.1016/j.ijdevneu.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Marlatt M. W., Potter M. C., Lucassen P. J., van Praag H. Running throughout middle-age improves memory function, hippocampal neurogenesis, and BDNF levels in female C57BL/6J mice. Developmental Neurobiology. 2012;72(6):943–952. doi: 10.1002/dneu.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Stranahan A. M., Lee K., Mattson M. P. Contributions of impaired hippocampal plasticity and neurodegeneration to age-related deficits in hormonal pulsatility. Ageing Research Reviews. 2008;7(3):164–176. doi: 10.1016/j.arr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Muezzinler A., Zaineddin A. K., Brenner H. A systematic review of leukocyte telomere length and age in adults. Ageing Research Reviews. 2013;12(2):509–519. doi: 10.1016/j.arr.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 427.Hovatta I., Juhila J., Donner J. Oxidative stress in anxiety and comorbid disorders. Neuroscience Research. 2010;68(4):261–275. doi: 10.1016/j.neures.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 428.Simon N. M., Smoller J. W., McNamara K. L., et al. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biological Psychiatry. 2006;60(5):432–5. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 429.Wolkowitz O. M., Mellon S. H., Epel E. S., et al. Leukocyte telomere length in major depression: correlations with chronicity, inflammation and oxidative stress—preliminary findings. PLoS One. 2011;6(3, article e17837) doi: 10.1371/journal.pone.0017837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Caporaso G. L., Lim D. A., Alvarez-Buylla A., Chao M. V. Telomerase activity in the subventricular zone of adult mice. Molecular and Cellular Neurosciences. 2003;23(4):693–702. doi: 10.1016/S1044-7431(03)00103-9. [DOI] [PubMed] [Google Scholar]
- 431.Flores I., Benetti R., Blasco M. A. Telomerase regulation and stem cell behaviour. Current Opinion in Cell Biology. 2006;18(3):254–260. doi: 10.1016/j.ceb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 432.Flores I., Canela A., Vera E., Tejera A., Cotsarelis G., Blasco M. A. The longest telomeres: a general signature of adult stem cell compartments. Genes & Development. 2008;22(5):654–667. doi: 10.1101/gad.451008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Ferron S., Mira H., Franco S., et al. Telomere shortening and chromosomal instability abrogates proliferation of adult but not embryonic neural stem cells. Development. 2004;131(16):4059–4070. doi: 10.1242/dev.01215. [DOI] [PubMed] [Google Scholar]
- 434.Verhoeven J. E., Revesz D., Epel E. S., Lin J., Wolkowitz O. M., Penninx B. W. Major depressive disorder and accelerated cellular aging: results from a large psychiatric cohort study. Molecular Psychiatry. 2014;19(8):895–901. doi: 10.1038/mp.2013.151. [DOI] [PubMed] [Google Scholar]
- 435.Puterman E., Epel E. S., Lin J., et al. Multisystem resiliency moderates the major depression-telomere length association: findings from the Heart and Soul Study. Brain, Behavior, and Immunity. 2013;33:65–73. doi: 10.1016/j.bbi.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Mamdani F., Rollins B., Morgan L., et al. Variable telomere length across post-mortem human brain regions and specific reduction in the hippocampus of major depressive disorder. Translational Psychiatry. 2015;5(9, article e636) doi: 10.1038/tp.2015.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Lima I. M., Barros A., Rosa D. V., et al. Analysis of telomere attrition in bipolar disorder. Journal of Affective Disorders. 2015;172:43–47. doi: 10.1016/j.jad.2014.09.043. [DOI] [PubMed] [Google Scholar]
- 438.Martinsson L., Wei Y., Xu D., et al. Long-term lithium treatment in bipolar disorder is associated with longer leukocyte telomeres. Translational Psychiatry. 2013;3(5, article e261) doi: 10.1038/tp.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Squassina A., Pisanu C., Corbett N., Alda M. Telomere length in bipolar disorder and lithium response. European Neuropsychopharmacology. 2015 doi: 10.1016/j.euroneuro.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 440.Cherkas L. F., Hunkin J. L., Kato B. S., et al. The association between physical activity in leisure time and leukocyte telomere length. Archives of Internal Medicine. 2008;168(2):154–158. doi: 10.1001/archinternmed.2007.39. [DOI] [PubMed] [Google Scholar]
- 441.Du M., Prescott J., Kraft P., et al. Physical activity, sedentary behavior, and leukocyte telomere length in women. American Journal of Epidemiology. 2012;175(5):414–422. doi: 10.1093/aje/kwr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Werner C., Furster T., Widmann T., et al. Physical exercise prevents cellular senescence in circulating leukocytes and in the vessel wall. Circulation. 2009;120(24):2438–2447. doi: 10.1161/CIRCULATIONAHA.109.861005. [DOI] [PubMed] [Google Scholar]
- 443.Ressler K. J., Mayberg H. S. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nature Neuroscience. 2007;10(9):1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Castren E. Neuronal network plasticity and recovery from depression. JAMA Psychiatry. 2013;70(9):983–9. doi: 10.1001/jamapsychiatry.2013.1. [DOI] [PubMed] [Google Scholar]
- 445.Price J. L., Drevets W. C. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35(1):192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Vavakova M., Durackova Z., Trebaticka J. Markers of oxidative stress and neuroprogression in depression disorder. Oxidative Medicine and Cellular Longevity. 2015;2015:p. 12. doi: 10.1155/2015/898393.898393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Kapczinski N. S., Mwangi B., Cassidy R. M., et al. Neuroprogression and illness trajectories in bipolar disorder. Expert Review of Neurotherapeutics. 2017;17(3):277–285. doi: 10.1080/14737175.2017.1240615. [DOI] [PubMed] [Google Scholar]
- 448.Silveira H., Moraes H., Oliveira N., Coutinho E. S., Laks J., Deslandes A. Physical exercise and clinically depressed patients: a systematic review and meta-analysis. Neuropsychobiology. 2013;67(2):61–68. doi: 10.1159/000345160. [DOI] [PubMed] [Google Scholar]
- 449.Stanton R., Reaburn P. Exercise and the treatment of depression: a review of the exercise program variables. Journal of Science and Medicine in Sport. 2014;17(2):177–182. doi: 10.1016/j.jsams.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 450.Kucyi A., Alsuwaidan M. T., Liauw S. S., McIntyre R. S. Aerobic physical exercise as a possible treatment for neurocognitive dysfunction in bipolar disorder. Postgraduate Medicine. 2010;122(6):107–116. doi: 10.3810/pgm.2010.11.2228. [DOI] [PubMed] [Google Scholar]
- 451.Ng F., Dodd S., Berk M. The effects of physical activity in the acute treatment of bipolar disorder: a pilot study. Journal of Affective Disorders. 2007;101(1–3):259–262. doi: 10.1016/j.jad.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 452.Murray G., Suto M., Hole R., Hale S., Amari E., Michalak E. E. Self-management strategies used by ‘high functioning’ individuals with bipolar disorder: from research to clinical practice. Clinical Psychology & Psychotherapy. 2011;18(2):95–109. doi: 10.1002/cpp.710. [DOI] [PubMed] [Google Scholar]
- 453.Sylvia L. G., Nierenberg A. A., Stange J. P., Peckham A. D., Deckersbach T. Development of an integrated psychosocial treatment to address the medical burden associated with bipolar disorder. Journal of Psychiatric Practice. 2011;17(3):224–232. doi: 10.1097/01.pra.0000398419.82362.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Wright K. A., Everson-Hock E. S., Taylor A. H. The effects of physical activity on physical and mental health among individuals with bipolar disorder: a systematic review. Mental Health and Physical Activity. 2009;2(2):86–94. doi: 10.1016/j.mhpa.2009.09.001. [DOI] [Google Scholar]
- 455.Ibarra-Rovillard M. S., Kuiper N. A. Social support and social negativity findings in depression: perceived responsiveness to basic psychological needs. Clinical Psychology Review. 2011;31(3):342–352. doi: 10.1016/j.cpr.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 456.Shepard R. J. Aging, Physical Activity, and Health. Champaign, IL: Human Kinetics; 1997. [Google Scholar]
- 457.Deci E. L., Ryan R. M. The “what” and “why” of goal pursuits: human needs and self-determination of behavior. Psychological Inquiry. 2000;11(4):227–268. doi: 10.1207/S15327965PLI1104_01. [DOI] [Google Scholar]
- 458.Kouvonen A., De Vogli R., Stafford M., et al. Social support and the likelihood of maintaining and improving levels of physical activity: the Whitehall II Study. European Journal of Public Health. 2012;22(4):514–518. doi: 10.1093/eurpub/ckr091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society. 2002;8(3):448–460. [PubMed] [Google Scholar]
- 460.Katzman R., Terry R., DeTeresa R., et al. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Annals of Neurology. 1988;23(2):138–144. doi: 10.1002/ana.410230206. [DOI] [PubMed] [Google Scholar]
- 461.Trivedi M. H., Greer T. L., Grannemann B. D., Chambliss H. O., Jordan A. N. Exercise as an augmentation strategy for treatment of major depression. Journal of Psychiatric Practice. 2006;12(4):205–213. doi: 10.1097/00131746-200607000-00002. [DOI] [PubMed] [Google Scholar]
- 462.Phongsavan P., Merom D., Bauman A., Wagner R. Mental illness and physical activity: therapists’ beliefs and practices. The Australian and New Zealand Journal of Psychiatry. 2007;41(5):458–459. doi: 10.1080/00048670701266862. [DOI] [PubMed] [Google Scholar]
- 463.Belvederi Murri M., Amore M., Menchetti M., et al. Physical exercise for late-life major depression. The British Journal of Psychiatry. 2015;207(3):235–242. doi: 10.1192/bjp.bp.114.150516. [DOI] [PubMed] [Google Scholar]
- 464.Blumenthal J. A., Babyak M. A., Moore K. A., et al. Effects of exercise training on older patients with major depression. Archives of Internal Medicine. 1999;159(19):2349–2356. doi: 10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- 465.Brown M. A., Goldstein-Shirley J., Robinson J., Casey S. The effects of a multi-modal intervention trial of light, exercise, and vitamins on women’s mood. Women & Health. 2001;34(3):93–112. doi: 10.1300/J013v34n03_06. [DOI] [PubMed] [Google Scholar]
- 466.Danielsson L., Papoulias I., Petersson E. L., Carlsson J., Waern M. Exercise or basic body awareness therapy as add-on treatment for major depression: a controlled study. Journal of Affective Disorders. 2014;168:98–106. doi: 10.1016/j.jad.2014.06.049. [DOI] [PubMed] [Google Scholar]
- 467.de la Cerda P., Cervello E., Cocca A., Viciana J. Effect of an aerobic training program as complementary therapy in patients with moderate depression. Perceptual and Motor Skills. 2011;112(3):761–769. doi: 10.2466/02.15.PMS.112.3.761-769. [DOI] [PubMed] [Google Scholar]
- 468.Doyne E. J., Ossip-Klein D. J., Bowman E. D., Osborn K. M., McDougall-Wilson I. B., Neimeyer R. A. Running versus weight lifting in the treatment of depression. Journal of Consulting and Clinical Psychology. 1987;55(5):748–754. doi: 10.1037//0022-006X.55.5.748. [DOI] [PubMed] [Google Scholar]
- 469.Dunn A. L., Trivedi M. H., Kampert J. B., Clark C. G., Chambliss H. O. Exercise treatment for depression: efficacy and dose response. American Journal of Preventive Medicine. 2005;28(1):1–8. doi: 10.1016/j.amepre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 470.Euteneuer F., Schwarz M. J., Schmidmaier R., et al. Blunted exercise-induced mobilization of monocytes in somatization syndromes and major depression. Journal of Affective Disorders. 2014;166:156–164. doi: 10.1016/j.jad.2014.04.060. [DOI] [PubMed] [Google Scholar]
- 471.Greer T. L., Grannemann B. D., Chansard M., Karim A. I., Trivedi M. H. Dose-dependent changes in cognitive function with exercise augmentation for major depression: results from the TREAD study. European Neuropsychopharmacology. 2015;25(2):248–256. doi: 10.1016/j.euroneuro.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 472.Gusi N., Reyes M. C., Gonzalez-Guerrero J. L., Herrera E., Garcia J. M. Cost-utility of a walking programme for moderately depressed, obese, or overweight elderly women in primary care: a randomised controlled trial. BMC Public Health. 2008;8(1):p. 231. doi: 10.1186/1471-2458-8-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Hoffman B. M., Blumenthal J. A., Babyak M. A., et al. Exercise fails to improve neurocognition in depressed middle-aged and older adults. Medicine and Science in Sports and Exercise. 2008;40(7):1344–1352. doi: 10.1249/MSS.0b013e31816b877c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Kerling A., Tegtbur U., Gutzlaff E., et al. Effects of adjunctive exercise on physiological and psychological parameters in depression: a randomized pilot trial. Journal of Affective Disorders. 2015;177:1–6. doi: 10.1016/j.jad.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 475.Kerse N., Hayman K. J., Moyes S. A., et al. Home-based activity program for older people with depressive symptoms: DeLLITE—a randomized controlled trial. Annals of Family Medicine. 2010;8(3):214–223. doi: 10.1370/afm.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Legrand F., Heuze J. P. Antidepressant effects associated with different exercise conditions in participants with depression: a pilot study. Journal of Sport & Exercise Psychology. 2007;29(3):348–364. doi: 10.1123/jsep.29.3.348. [DOI] [PubMed] [Google Scholar]
- 477.Leppamaki S., Partonen T., Lonnqvist J. Bright-light exposure combined with physical exercise elevates mood. Journal of Affective Disorders. 2002;72(2):139–144. doi: 10.1016/S0165-0327(01)00417-7. [DOI] [PubMed] [Google Scholar]
- 478.Leppamaki S. J., Partonen T. T., Hurme J., Haukka J. K., Lonnqvist J. K. Randomized trial of the efficacy of bright-light exposure and aerobic exercise on depressive symptoms and serum lipids. The Journal of Clinical Psychiatry. 2002;63(4):316–321. doi: 10.4088/JCP.v63n0408. [DOI] [PubMed] [Google Scholar]
- 479.Martiny K., Refsgaard E., Lund V., et al. Maintained superiority of chronotherapeutics vs. exercise in a 20-week randomized follow-up trial in major depression. Acta Psychiatrica Scandinavica. 2015;131(6):446–457. doi: 10.1111/acps.12402. [DOI] [PubMed] [Google Scholar]
- 480.Mather A. S., Rodriguez C., Guthrie M. F., McHarg A. M., Reid I. C., McMurdo M. E. Effects of exercise on depressive symptoms in older adults with poorly responsive depressive disorder: randomised controlled trial. The British Journal of Psychiatry. 2002;180(5):411–415. doi: 10.1192/bjp.180.5.411. [DOI] [PubMed] [Google Scholar]
- 481.Penninx B. W., Rejeski W. J., Pandya J., et al. Exercise and depressive symptoms: a comparison of aerobic and resistance exercise effects on emotional and physical function in older persons with high and low depressive symptomatology. The Journals of Gerontology Series B, Psychological Sciences and Social Sciences. 2002;57(2):P124–P132. doi: 10.1093/geronb/57.2.P124. [DOI] [PubMed] [Google Scholar]
- 482.Schuch F. B., Vasconcelos-Moreno M. P., Borowsky C., Fleck M. P. Exercise and severe depression: preliminary results of an add-on study. Journal of Affective Disorders. 2011;133(3):615–618. doi: 10.1016/j.jad.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 483.Schuch F. B., Vasconcelos-Moreno M. P., Borowsky C., Zimmermann A. B., Rocha N. S., Fleck M. P. Exercise and severe major depression: effect on symptom severity and quality of life at discharge in an inpatient cohort. Journal of Psychiatric Research. 2015;61:25–32. doi: 10.1016/j.jpsychires.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 484.Singh N. A., Clements K. M., Singh M. A. The efficacy of exercise as a long-term antidepressant in elderly subjects: a randomized, controlled trial. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2001;56(8):M497–M504. doi: 10.1093/gerona/56.8.M497. [DOI] [PubMed] [Google Scholar]
- 485.Martinsen E. W., Hoffart A., Solberg O. Comparing aerobic with nonaerobic forms of exercise in the treatment of clinical depression: a randomized trial. Comprehensive Psychiatry. 1989;30(4):324–331. doi: 10.1016/0010-440X(89)90057-6. [DOI] [PubMed] [Google Scholar]
- 486.Pfaff J. J., Alfonso H., Newton R. U., Sim M., Flicker L., Almeida O. P. ACTIVEDEP: a randomised, controlled trial of a home-based exercise intervention to alleviate depression in middle-aged and older adults. British Journal of Sports Medicine. 2014;48(3):226–232. doi: 10.1136/bjsports-2013-092510. [DOI] [PubMed] [Google Scholar]
- 487.Pereira D. S., de Queiroz B. Z., Miranda A. S., et al. Effects of physical exercise on plasma levels of brain-derived neurotrophic factor and depressive symptoms in elderly women—a randomized clinical trial. Archives of Physical Medicine and Rehabilitation. 2013;94(8):1443–1450. doi: 10.1016/j.apmr.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 488.Siqueira C. C., Valiengo L. L., Carvalho A. F., et al. Antidepressant efficacy of adjunctive aerobic activity and associated biomarkers in major depression: a 4-week, randomized, single-blind, controlled clinical trial. PLoS One. 2016;11(5, article e0154195) doi: 10.1371/journal.pone.0154195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Sarubin N., Nothdurfter C., Schule C., et al. The influence of hatha yoga as an add-on treatment in major depression on hypothalamic-pituitary-adrenal-axis activity: a randomized trial. Journal of Psychiatric Research. 2014;53:76–83. doi: 10.1016/j.jpsychires.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 490.Krogh J., Rostrup E., Thomsen C., Elfving B., Videbech P., Nordentoft M. The effect of exercise on hippocampal volume and neurotrophines in patients with major depression—a randomized clinical trial. Journal of Affective Disorders. 2014;165:24–30. doi: 10.1016/j.jad.2014.04.041. [DOI] [PubMed] [Google Scholar]
- 491.Krogh J., Gotze J. P., Jorgensen M. B., Kristensen L. O., Kistorp C., Nordentoft M. Copeptin during rest and exercise in major depression. Journal of Affective Disorders. 2013;151(1):284–290. doi: 10.1016/j.jad.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 492.Krogh J., Saltin B., Gluud C., Nordentoft M. The DEMO trial: a randomized, parallel-group, observer-blinded clinical trial of strength versus aerobic versus relaxation training for patients with mild to moderate depression. The Journal of Clinical Psychiatry. 2009;70(6):790–800. doi: 10.4088/JCP.08m04241. [DOI] [PubMed] [Google Scholar]
- 493.Duman C. H., Schlesinger L., Russell D. S., Duman R. S. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Research. 2008;1199:148–158. doi: 10.1016/j.brainres.2007.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Malisch J. L., Breuner C. W., Kolb E. M., et al. Behavioral despair and home-cage activity in mice with chronically elevated baseline corticosterone concentrations. Behavior Genetics. 2009;39(2):192–201. doi: 10.1007/s10519-008-9246-8. [DOI] [PubMed] [Google Scholar]
- 495.Otsuka A., Shiuchi T., Chikahisa S., Shimizu N., Sei H. Voluntary exercise and increased food intake after mild chronic stress improve social avoidance behavior in mice. Physiology & Behavior. 2015;151:264–271. doi: 10.1016/j.physbeh.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 496.Lapmanee S., Charoenphandhu J., Charoenphandhu N. Beneficial effects of fluoxetine, reboxetine, venlafaxine, and voluntary running exercise in stressed male rats with anxiety- and depression-like behaviors. Behavioural Brain Research. 2013;250:316–325. doi: 10.1016/j.bbr.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 497.Epps S. A., Kahn A. B., Holmes P. V., Boss-Williams K. A., Weiss J. M., Weinshenker D. Antidepressant and anticonvulsant effects of exercise in a rat model of epilepsy and depression comorbidity. Epilepsy & Behavior. 2013;29(1):47–52. doi: 10.1016/j.yebeh.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Cunha M. P., Oliveira A., Pazini F. L., et al. The antidepressant-like effect of physical activity on a voluntary running wheel. Medicine and Science in Sports and Exercise. 2013;45(5):851–859. doi: 10.1249/MSS.0b013e31827b23e6. [DOI] [PubMed] [Google Scholar]
- 499.Sartori C. R., Vieira A. S., Ferrari E. M., Langone F., Tongiorgi E., Parada C. A. The antidepressive effect of the physical exercise correlates with increased levels of mature BDNF, and proBDNF proteolytic cleavage-related genes, p11 and tPA. Neuroscience. 2011;180:9–18. doi: 10.1016/j.neuroscience.2011.02.055. [DOI] [PubMed] [Google Scholar]
- 500.Zheng H., Liu Y., Li W., et al. Beneficial effects of exercise and its molecular mechanisms on depression in rats. Behavioural Brain Research. 2006;168(1):47–55. doi: 10.1016/j.bbr.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Moraska A., Fleshner M. Voluntary physical activity prevents stress-induced behavioral depression and anti-KLH antibody suppression. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2001;281(2):R484–R489. doi: 10.1152/ajpregu.2001.281.2.R484. [DOI] [PubMed] [Google Scholar]
- 502.Bjornebekk A., Mathe A. A., Brene S. The antidepressant effects of running and escitalopram are associated with levels of hippocampal NPY and Y1 receptor but not cell proliferation in a rat model of depression. Hippocampus. 2010;20(7):820–828. doi: 10.1002/hipo.20683. [DOI] [PubMed] [Google Scholar]
- 503.Greenwood B. N., Strong P. V., Dorey A. A., Fleshner M. Therapeutic effects of exercise: wheel running reverses stress-induced interference with shuttle box escape. Behavioral Neuroscience. 2007;121(5):992–1000. doi: 10.1037/0735-7044.121.5.992. [DOI] [PubMed] [Google Scholar]
- 504.Hunsberger J. G., Newton S. S., Bennett A. H., et al. Antidepressant actions of the exercise-regulated gene VGF. Nature Medicine. 2007;13(12):1476–1482. doi: 10.1038/nm1669. [DOI] [PubMed] [Google Scholar]
- 505.Hutton C. P., Dery N., Rosa E., et al. Synergistic effects of diet and exercise on hippocampal function in chronically stressed mice. Neuroscience. 2015;308:180–193. doi: 10.1016/j.neuroscience.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 506.Kiuchi T., Lee H., Mikami T. Regular exercise cures depression-like behavior via VEGF-Flk-1 signaling in chronically stressed mice. Neuroscience. 2012;207:208–217. doi: 10.1016/j.neuroscience.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 507.Tal-Krivisky K., Kronfeld-Schor N., Einat H. Voluntary exercise enhances activity rhythms and ameliorates anxiety- and depression-like behaviors in the sand rat model of circadian rhythm-related mood changes. Physiology & Behavior. 2015;151:441–447. doi: 10.1016/j.physbeh.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 508.Yau S. Y., Li A., Zhang E. D., et al. Sustained running in rats administered corticosterone prevents the development of depressive behaviors and enhances hippocampal neurogenesis and synaptic plasticity without increasing neurotrophic factor levels. Cell Transplantation. 2014;23(4-5):481–492. doi: 10.3727/096368914X678490. [DOI] [PubMed] [Google Scholar]
- 509.Greenwood B. N., Loughridge A. B., Sadaoui N., Christianson J. P., Fleshner M. The protective effects of voluntary exercise against the behavioral consequences of uncontrollable stress persist despite an increase in anxiety following forced cessation of exercise. Behavioural Brain Research. 2012;233(2):314–321. doi: 10.1016/j.bbr.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Hare B. D., Beierle J. A., Toufexis D. J., Hammack S. E., Falls W. A. Exercise-associated changes in the corticosterone response to acute restraint stress: evidence for increased adrenal sensitivity and reduced corticosterone response duration. Neuropsychopharmacology. 2014;39(5):1262–1269. doi: 10.1038/npp.2013.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Clark P. J., Amat J., McConnell S. O., et al. Running reduces uncontrollable stress-evoked serotonin and potentiates stress-evoked dopamine concentrations in the rat dorsal striatum. PLoS One. 2015;10(11, article e0141898) doi: 10.1371/journal.pone.0141898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Greenwood B. N., Strong P. V., Foley T. E., Thompson R. S., Fleshner M. Learned helplessness is independent of levels of brain-derived neurotrophic factor in the hippocampus. Neuroscience. 2007;144(4):1193–1208. doi: 10.1016/j.neuroscience.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Fuss J., Ben Abdallah N. M., Vogt M. A., et al. Voluntary exercise induces anxiety-like behavior in adult C57BL/6J mice correlating with hippocampal neurogenesis. Hippocampus. 2010;20(3):364–376. doi: 10.1002/hipo.20634. [DOI] [PubMed] [Google Scholar]
- 514.Thompson R. S., Roller R., Greenwood B. N., Fleshner M. Wheel running improves REM sleep and attenuates stress-induced flattening of diurnal rhythms in F344 rats. Stress. 2016;19(3):312–324. doi: 10.1080/10253890.2016.1174852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Greenwood B. N., Strong P. V., Loughridge A. B., et al. 5-HT2C receptors in the basolateral amygdala and dorsal striatum are a novel target for the anxiolytic and antidepressant effects of exercise. PLoS One. 2012;7(9, article e46118) doi: 10.1371/journal.pone.0046118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Martin S. A., Dantzer R., Kelley K. W., Woods J. A. Voluntary wheel running does not affect lipopolysaccharide-induced depressive-like behavior in young adult and aged mice. Neuroimmunomodulation. 2014;21(1):52–63. doi: 10.1159/000356144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Li Y., Luikart B. W., Birnbaum S., et al. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59(3):399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]