Abstract
Background: Anatomic pathology laboratory workflow consists of 3 major specimen handling processes. Among the workflow are preanalytic, analytic, and postanalytic phases that contain multistep subprocesses with great impact on patient care. A worldwide representation of experts came together to create a system of metrics, as a basis for laboratories worldwide, to help them evaluate and improve specimen handling to reduce patient safety risk.
Method: Members of the Initiative for Anatomic Pathology Laboratory Patient Safety (IAPLPS) pooled their extensive expertise to generate a list of metrics highlighting processes with high and low risk for adverse patient outcomes.
Results: Our group developed a universal, comprehensive list of 47 metrics for patient specimen handling in the anatomic pathology laboratory. Steps within the specimen workflow sequence are categorized as high or low risk. In general, steps associated with the potential for specimen misidentification correspond to the high-risk grouping and merit greater focus within quality management systems. Primarily workflow measures related to operational efficiency can be considered low risk.
Conclusion: Our group intends to advance the widespread use of these metrics in anatomic pathology laboratories to reduce patient safety risk and improve patient care with development of best practices and interlaboratory error reporting programs.
Keywords: anatomic pathology, patient safety, best practices, workflow metrics
Patient safety issues within the United States health care system were brought to wide public attention by a report from the Institute of Medicine (IOM) published in the year 2000.1 The report focused on adverse outcomes from surgical procedures or medication prescription errors and revealed an overwhelming number of undetected diagnostic errors, specifically in the outpatient setting.1 The IOM report also called for the formation of a federal agency to lead a unified effort in reducing errors that affect patient health. It also highlighted a need for anonymous and standardized reporting, to avoid frivolous malpractice lawsuits.1,2 One of the focus areas of the outpatient setting, detailed in the report, was the anatomic pathology (AP) laboratory.3
Self-monitoring and self-awareness among individual AP labs resulted in several publications from independent sites on diagnostic errors.2,4,5 In response to these initial autonomous efforts, the College of American Pathologists (CAP) established the Q-probes survey and unified the approach to collect AP laboratory workflow errors.6 A Q-probes survey conducted in the outpatient setting detected an average error incidence rate of 4.8% among a total of 660 institutions.6 Although accumulative, the Q-probes survey data continue to reflect errors associated with practices restricted within the United States and Clinical Laboratory Improvement Amendments (CLIA)–certified labs. Q-tracks is a voluntary program launched by CAP to capture individual laboratory performance for laboratory services, to track errors and variations in quality.6 The community based reporting allows individual laboratories to conduct comparisons and collectively improve best practices.
Beyond the CAP-officiated Q-probes survey and Q-tracks program, there were no collective data sets which reflect worldwide practices within the AP-laboratory workflow. Recognizing the value of community input, a few laboratories developed a shared error-reporting database. The Patient Safety Database was created at the Center of Excellence of Pathology Quality and Health Care Research, within the University of Pittsburgh, in Pennsylvania, to collect error data with 3 partner institutions (ie, Western Pennsylvania hospitals, the University of Iowa, and Henry Ford Medical Center).7 The error database was an attempt to provide measurable data to support resource allocation and decision making, as well as to provide robust evidence for implementation of best practices. However, no comprehensive report was derived from data mining, which likely happened due to the large collection of unstructured data and varied deficiencies in the AP laboratory workflow. The literature suggests that limitations in error reporting are fundamental to the current culture of health care institutions in which reluctance to release unfavorable diagnostics data is ingrained.8,9 To date, these combined challenges have hindered development of a comprehensive list on AP workflow process deficiencies for collection of error data.
After the IOM report was released, best estimates suggested that increased surveillance and accountability reduced the rate of misdiagnosis; however, issues remain with AP laboratory processes.10 The AP laboratory setting continues to be fundamentally challenged by a shrinking workforce. Essentially, there are fewer histotechnology training program graduates, and simultaneously, there has been an increase in retirement of experienced technologists.11 The strain on resources is offset somewhat as automation replaces a few manual specimen handling processes. However, to truly overcome potential challenges, AP laboratories have proactively adopted the root-cause analysis (RCA) system to identify the causes of common error, thus providing better training.12 However, data were needed to identify those processes most vulnerable to error that impact patient care, to improve training of the next generation of histotechnologists. Again, the need arises for a list of critical metrics for AP laboratory workflow, in the interest of improving patient care.
Previously, studies concluded that quality issues in the AP laboratory occurred due in part to the inherent diversity of specimens and procurement methods.4,12 For example, delivery or procurement of patient specimens into the AP laboratory occurs from various sites and often with no universally shared operating procedures for labeling or fixation. This and other challenges highlight the need to develop and implement new universal best practices. However, once more, there is a need to gather reliable data before implementation of new processes.
In this study, we identified 47 vulnerable workflow steps that can cause diagnostic errors within the AP laboratory. We report these results as a global initiative aimed at identifying the many potential deficiencies within the AP laboratory workflow, in the interest of improving worldwide public health.
Materials and Methods
In 2014, a group of AP laboratory management experts convened as an advisory board for industry. The group realized the expansive potential to improve AP laboratory management practices and rapidly assumed a new independent identity from that of an advocacy group; this new group called itself the Initiative for Anatomic Pathology Laboratory Patient Safety (IAPLPS). The group consisted of highly involved internationally recognized experts in AP laboratory organization, including pathologists, a laboratory administrator, and a pathology assistant. Their first position statement was published in March 2014, calling for an international effort to secure safe, reliable processing of all patient specimens for diagnosis.13
Members of this group pooled the experiences of their respective institutions to identify and rank a comprehensive list of steps that they perceived to represent patient risk within the AP laboratory workflow. All 47 metrics were subject to discussion before members reached a consensus on relative risk. Individual members were electronically surveyed with regard to grading each metric as too high, intermediate, or low risk for likelihood of adverse patient outcome. High and low risk groupings were separable, with significant agreement among the membership. However, a group of intermediate-risk measures was not distinguishable, thus leading to a final separation consisting of only high and low risk categories.
Globally harmonized practices and processes within the AP laboratory helped to generate this set of metrics. This list is different from Q-probes criteria or other checklists because it consists of workflow activities that influence risk to patient care and not only standard quality and efficiency. The list specifically focuses on safety threats in the AP laboratory and does not include potential patient safety threats from pathologist interpretative errors because professional training and remediation better address these risks. The scope also excludes preanalytic processes not directly controlled within the AP laboratory, such as specimen handling in the surgical or operating suite. Pathologist interpretation and AP laboratory specimen sourcing remain important issues for future investigation but are not within the scope of this research project.
Results
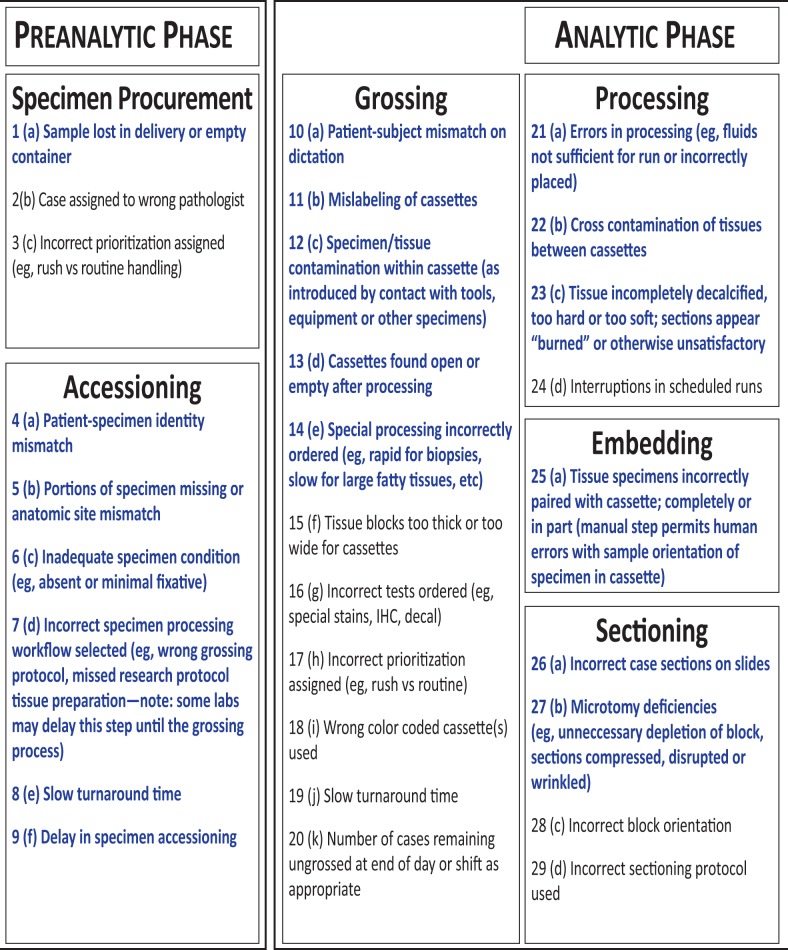
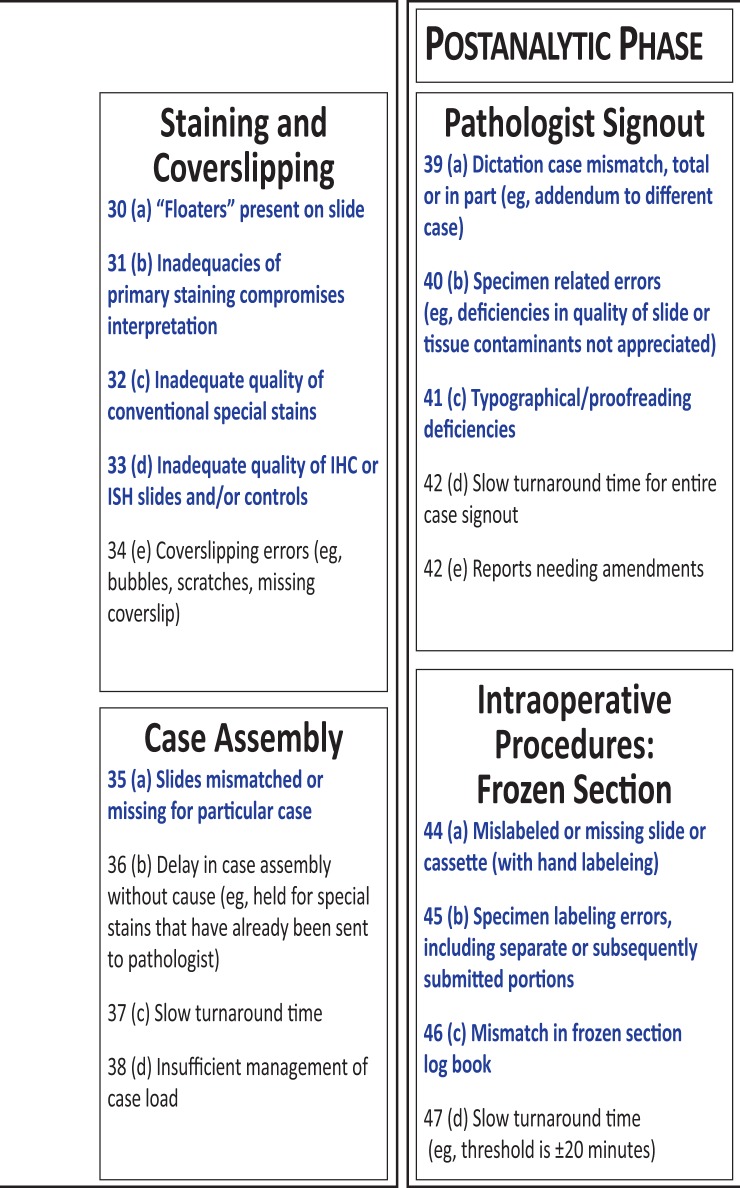
The list in Figure 1 consists of the final set of 47 metrics generated by members of the IAPLPS. Each item, within each phase, was a stand-alone metric. The workflow phases progressed from specimen procurement to completion of the pathologist report. The parenthetic-alphanumeric designations indicate the exact succession of steps within each phase. The 3 categories of AP laboratory processing are preanalytic, analytic, and postanalytic; together, these categories contain 10 independent subprocesses. Figure 1 identifies 26 of 47 metrics as high risk (shown in red) and 21 as low risk (in black).
Figure 1.
List of the anatomic pathology laboratory workflow metrics surveyed as being high risk and low risk for adverse patient outcomes. Within each category, the metrics are listed (alphabetically), and all 47 process metrics are listed numerically according to the entire workflow sequence.
Exhaustive discussion of this grading led us to realize that in extraordinarily rare circumstances, almost any step in the AP laboratory could lead to an adverse patient event. For example, although typographical discrepancies in the final report usually only pose a minor threat to misdiagnosis, the remote possibility exists that mislabeling can result in a serious medical misdiagnosis. However, although some metrics raise potential severe patient safety concerns, in most instances, even in riskier steps, laboratory professionals avoided actual adverse events by means of early detection.
Figure 1 indicates that we identified high and low risk processes in all 3 phases. Based on this list, the preanalytic phase had 9 possible deficiencies for safety in workflow, of which 4 were designated as high risk. Three metrics listed in the accessioning process were classified as high risk, and 3 others were considered to present low risk to patient care. An example of a high risk scenario is when an inadequate amount of fixative jeopardizes the specimen integrity and results in the need for another biopsy. Some low risk process deficiencies listed have mild consequences, requiring only remedial efforts for resolution, such as correct pathologist case assignment. Other deficiencies, such as mislabeling errors, may be resolved immediately further down the stepwise sequence with limited consequences. However, most of the metrics listed in this article are capable of generating an adverse patient outcome if not properly addressed.
The histotechnology committee affiliated with CAP and the National Society for Histotechnology (NSH) has recently updated a guidance document called the Practical Guide to Specimen Handling in Surgical Pathology.14 This committee reports to the Council on Scientific Affairs and currently contains 14 members, of which 6 are CAP fellows and 8 are certified histotechnologists and American Society for Clinical Pathology (ASCP) certified. The purpose of the committee is to provide technical information for the improvement of laboratory processes. The specimen handling guideline publication not only identifies process deficiencies but also provides literature to support the entire workflow. The publication also discusses patient safety issues, with the intention to bring about awareness of the Practical Guide to Specimen Handling. It is an extensive report of the types of errors in workflow that can occur in the AP laboratory, which could compromise the quality of the specimen. The report is full of details associated with various processes. Strict criteria for specimen procurement transfer are described in the guide, stating that all containers for specimen material should be rigid, impermeable, unbreakable, and nonreactive to fixative solutions.14 Also reported are the fixative programs for tissue processing, with references to peer-reviewed manuscripts and to proper waste-management documents.14 This report provides detailed insights toward optimal performance. However, its content is extensive and is not limited to processes with direct impact on patient care, unlike our metrics list, which focuses on high-risk issues.
Figure 1 suggests that the lengthy analytic phase contains multiple processes associated with the potential for high risk to patients. Features such as processing, staining, and coverslipping specifically contain high risk metrics. Similar to those in the preanalytic phase, most metrics in the analytic phase have the potential to lead to misdiagnosis, if not carefully designed and monitored. Possible solutions to reduce error include advanced automation using individual slide-staining technology and coverslipping, to avoid any cross-contamination among specimens. The potential for error associated with the processing subgroup can be readily addressed with implementation of best practices. Specifically, adhering to proper sequence timing (ie, incorporating tissue type specifications) and performing maintenance of fresh stock reagents will greatly minimize risk.
As highlighted in the final phase of the AP laboratory workflow, the postanalytic phase contains 2 distinct subprocesses. Within these, 6 of the 9 metrics are high risk, indicating that some are closely associated with patient safety. The first process was the pathologist sign-out and the second was grouping of intraoperative procedures associated with frozen sections. Similar to the previous phases, some metrics listed under pathologist sign-out can be corrected with use of automated equipment. For example, the metric of dictation case mismatch can be resolved if the pathologist report is electronically integrated with audio-file compatibility, thus synchronizing the report with the dictation. Some metrics listed in Figure 1 may have established, relatively simple solutions, but others continue to present challenges, which calls for a worldwide effort to compile error data before construction of best practices.
Conclusions
Management of the AP laboratory requires a dedicated effort from health care staff and pathologists, to avoid diagnostic error and patient mismanagement. The challenge is to obtain measureable quality improvements in specimen workflow, reducing risk of adverse outcomes in patient care. The metrics list generated in this study is a comprehensive tool for standardizing collection of quality metrics, derived from pooled experience and expertise. The metrics list, in combination with collection of error data, allows a comprehensive approach to initiate an engaged collaborative dialogue and to reduce risk of adverse outcomes from the AP laboratory workflow. Ultimately, doing this will require support and participation from patient care areas outside the laboratory, particularly those submitting tissue specimens. Surgical suites and other sites, such as gastrointestinal endoscopic and gynecologic clinics, would need to be involved. Implicit in such a scenario is the direct support of health care facility officers, patient safety officers, and other stakeholders, possibly even including patients.
The key objective of the IAPLPS in publishing this list of 47 metrics is to increase data collection regarding workflow deficiencies. In the past few decades since the release of the IOM Report, several individual laboratories in the academic and private sectors have published their collection of error data.15,16 However, to our knowledge, only our report contains a list of error metrics composed by a team of global experts from 6 different countries. The metrics listed herein are universal and more comprehensive than those represented in the Q-probes survey, as well as being inclusive of intraoperative-procedure components.7
This list of metrics also requires a proactive style of management for improvement of the AP laboratory workflow systems. Because specimen procurement is the beginning of the workflow, it is essential for harmonious cooperation to take place during that step among nonlaboratory health care professionals and institutional administrators. Also, the metrics include risks with pathologist interpretation that continue to remind pathologists to integrate with the AP laboratory workflow before receipt of diagnostics slides. In the past, individual laboratories have attempted to borrow management practices from other fields, such as the Lean principles.17,18 Some have utilized the concept of Lean production methods to streamline and quantitate error data into measurable metrics within the AP laboratory with positive results.15
However, not all laboratories generate the same volume of specimens. Also, not all laboratories have the resources to approach all 47 metrics at once; more data would be beneficial in selecting solutions that are high in priority but low in cost. Grading of the metrics into higher and lower risk allows prioritization for lower initial cost expenditures. The set of metrics that we present herein will provide a framework from which management can collect data and justify the cost of implementing quality improvement systems.
Many of the metrics listed herein are more readily resolved with the installation of advanced technology, such as barcode readers, specimen-tracking instruments, computerized organizational systems, or advanced automated staining systems. However, in some instances, simple solutions are just as effective. For example in a recent report from the Department of Dermatology at Duke University Medical Center, misplacement of patient biopsy specimens was identified as a major problem within the workflow.19 Requiring attachment of a sticker to each specimen container, along with the initials of the person who had performed the specimen-retrieval procedure, reduced the error rate from 5.79 to 3.53 per 1000 cases.19 Thus, by increasing traceable individual accountability, the laboratory achieved dramatic improvement in an area of critical risk to patient safety.
The IAPLPS group hopes that the list of metrics provided in this article will streamline attempts to create effective interinstitutional best practices for AP laboratories. Our goal is to stimulate interest in widespread use of these metrics to encourage standardized, revolutionary safety-management approaches in the laboratory.
Acknowledgments
We thank Denise Smith and Sherry Dean for their assistance in coordinating the survey and data collection. We also thank Laya Bhavaraju, PhD, for review of the manuscript and assistance with final manuscript preparation.
Glossary
Abbreviations
- IOM
Institute of Medicine
- AP
anatomic pathology
- CAP
College of American Pathologists
- CLIA
Clinical Laboratory Improvement Amendments
- RCA
root cause analysis
- IAPLPS
Initiative for Anatomic Pathology Laboratory Patient Safety
Personal and Professional Conflicts of Interest
The initial collaboration of authors began as a corporate advisory board, in which Ventana Medical Systems Inc, a member of the Roche Group, compensated the members for their attendance. Subsequently, the authors formed the Initiative for Anatomic Pathology Laboratory Patient (IAPLPS) and creation of these metrics, and any other continued autonomous effort is voluntary and uncompensated. Authors PB and ED are currently employed by Ventana Medical Systems. All other authors have disclosed all employment and affiliations.
References
- 1. Sirota LR. The Institute of Medicine’s report on medical error. Arch Pathol Lab Med 2000;12411:1674-1678. [DOI] [PubMed] [Google Scholar]
- 2. Kohn LT, Corrigan JM, Donaldson MS.. To Err Is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000. [PubMed] [Google Scholar]
- 3. James JT. A new, evidence-based estimate of patient harms associated with hospital care. J Patient Saf. 2013;93:122-128. [DOI] [PubMed] [Google Scholar]
- 4. Nakhleh RE, Zarbo RJ.. Surgical pathology specimen identification and accessioning: a college of American pathologists Q-Probes study of 1 004 115 cases from 417 institutions. Arch Pathol Lab Med. 1996;1203:227-233. [PubMed] [Google Scholar]
- 5. Valenstein PN, Sirota RL.. Identification errors in pathology and laboratory medicine. Clin Lab Med. 2004;244:979-996, vii. [DOI] [PubMed] [Google Scholar]
- 6. Novis DA. Detecting and preventing the occurrence of errors in the practices of laboratory medicine and anatomic pathology: 15 years’ experience with the College of American Pathologists’ Q-PROBES and Q-TRACKS programs. Clin Lab Med. 2004;244:965-978. [DOI] [PubMed] [Google Scholar]
- 7. Becich MJ, Gilbertson JR, Gupta D, Patel A, Grzybicki DM, Raab SS.. Pathology and patient safety: the critical role of pathology informatics in error reduction and quality initiatives. Clin Lab Med. 2004;244:913-943. [DOI] [PubMed] [Google Scholar]
- 8. Sheikh A, Hurwitz B.. A national database of medical error. J R Soc Med. 1999;9211:554-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sheikh A, Hurwitz B.. Setting up a database of medical error in general practice: conceptual and methodological considerations. Br J Gen Pract. 2001;51462:57-60. [PMC free article] [PubMed] [Google Scholar]
- 10. Institute of Medicine. Chapter 3. Overview of Diagnostic Error in Health Care. In: Balogh EP, Miller BT, Ball JR, eds. Improving Diagnosis in Health Care. Washington, DC: The National Academies Press; 2015. p. 84–88. [PubMed] [Google Scholar]
- 11. Buesa RJ. Histology aging workforce and what to do about it. Ann Diagn Pathol. 2009;133:176-184. [DOI] [PubMed] [Google Scholar]
- 12. Schmidt RL, Messinger BL, Layfield LJ.. Internal labeling errors in a surgical pathology department: a root cause analysis. Lab Med. 2013;442:176-185. [Google Scholar]
- 13.McGinley P. International Panel of Pathology Leaders Develop Principles for Patient Safety, Current News. In: Patient Safety and Quality Healthcare. Middleton, MA: Business and Legal Resources Media; 2014. [Google Scholar]
- 14. Lott R, Tunnicliffe J, Sheppard E, et al. practical guide to specimen handling in surgical pathology In: Online Histotechnology Resources. Northfield, IL: College of American Pathologists; 2015. [Google Scholar]
- 15. Tworek JA. Safety practices in surgical pathology: practical steps to reduce error in the pre-analytic, analytic, and post-analytic phases of surgical pathology. Diagn Histopathol. 2008;147:292-298. [Google Scholar]
- 16. Zarbo RJ, Tuthill JM, D’Angelo R, et al. The Henry Ford production system reduction of surgical pathology in-process misidentification defects by bar code–specified work process standardization. Am J Clin Pathol. 2009;1314:468-477. [DOI] [PubMed] [Google Scholar]
- 17. Serrano L, Hegge P, Sato B, Richmond B, Stahnke L.. Using LEAN principles to improve quality, patient safety, and workflow in histology and anatomic pathology. Adv Anat Pathol. 2010;173:215-221. [DOI] [PubMed] [Google Scholar]
- 18. Zarbo RJ, Tuthill JM, D’Angelo R.. Creating and sustaining a lean culture of continuous process improvement. Am J Clin Pathol. 2012;1383:321-326. [DOI] [PubMed] [Google Scholar]
- 19. Kim JK, Dotson B, Thomas S, Nelson KC.. Standardized patient identification and specimen labeling: a retrospective analysis on improving patient safety. J Am Acad Dermatol. 2013;681:53-56. [DOI] [PubMed] [Google Scholar]