Abstract
Red blood cells (RBCs) constitute a unique drug delivery system as a biologic or hybrid carrier capable of greatly enhancing pharmacokinetics, altering pharmacodynamics (for example, by changing margination within the intravascular space), and modulating immune responses to appended cargoes. Strategies for RBC drug delivery systems include internal and surface loading, and the latter can be performed both ex vivo and in vivo. A relatively new avenue for RBC drug delivery is their application as a carrier for nanoparticles. Efforts are also being made to incorporate features of RBCs in nanocarriers to mimic their most useful aspects, such as long circulation and stealth features. RBCs have also recently been explored as carriers for the delivery of antigens for modulation of immune response. Therefore, RBC-based drug delivery systems represent supercarriers for a diverse array of biomedical interventions, and this is reflected by several industrial and academic efforts that are poised to enter the clinical realm.
Keywords: Red blood cells, Erythrocytes, Drug delivery, Diagnostics, Nanoparticles
1. Introduction: artificial, natural, and hybrid drug delivery systems
Drug delivery systems (DDSs) can be divided into three types: artificial, natural (biological) and hybrids. Artificial DDSs, including nanocarriers made of synthetic or natural components, offer advantages of rational design, diversity, and control of materials. Biological DDSs including cells and their fragments offer biocompatibility and utilization of natural mechanisms for transport, localization, and responsiveness to factors of microenvironment in the body. Hybrid systems offer the theoretical possibility of combining these advantages while mitigating shortcomings.
This article reviews a class of DDSs that largely falls within the second and third categories, namely, erythrocytes or red blood cells (RBCs). RBCs represent natural transport agents that can be used to improve vascular and systemic delivery of drugs and probes, either encapsulated in the cell’s inner volume, or coupled to the surface of RBCs. This review focuses on the second approach for loading drugs to RBCs and explores the role of RBCs in other drug delivery strategies, including: (i) their effect on other DDSs; (ii) DDSs using RBC as a “supercarrier”; (iii) artificial DDSs imitating features of RBCs; and (iv) hybrid DDSs that combine artificial elements and RBC components.
2. RBC as carriers in drug delivery: brief overview
RBCs naturally deliver “cargoes” including oxygen throughout the body via prolonged circulation in the vascular system. RBCs circulate in humans for about 3 months and in mice for about 40 days. RBCs lack organelles and have a biconcave shape; hence their entire inner volume and extended surface can be used for carriage of diverse compounds. From a practical standpoint, RBCs represent the most abundant, biocompatible, affordable, and easy to handle biological carrier for DDSs [1–5].
These natural carriers can be employed for drug delivery, for example, via loading drugs into donor or autologous RBCs prior to transfusion to a patient. For more than forty years, RBCs have been explored for delivery of many drugs in models ranging from in vitro, to small animal models and primates, as well as in clinical studies in human patients. This section provides a background pertaining to key aspects of drug delivery by RBCs. Detailed reviews of this subject can be found in this volume (see Magnani) and elsewhere [2,4–7].
2.1. Pharmacokinetics, biodistribution, and targets of RBC-loaded drugs
RBC carriage offers unique features advantageous for delivery of many agents for improved management of some pathological conditions. First, carriage by RBCs cardinally changes the pharmacokinetics (PK) of drugs by prolonging their circulation time in the bloodstream, facilitating redistribution in blood from plasma to cellular elements and redistribution in tissues. This change in PK is especially desirable for drugs that: (i) require a long-lasting depot in blood; (ii) have intra-vascular therapeutic targets accessible to RBCs; or (iii) are supposed to act in the extravascular compartments accessible to RBCs. In many cases, these changes to PK effectively enhance drug bioavailability, allowing reduced dosages and therefore alleviating adverse effects.
Carriage by RBCs shifts the pathway for drug clearance from renal filtration to bile excretion, typical of products of hemoglobin degradation. This switch in clearance mechanism results from: (i) RBC size, which, by many orders of magnitude, exceeds the limit of glomerular filtration; and (ii) macrophages in the reticuloendothelial system (RES), which have direct access to blood cells (particularly, in the hepatic sinuses and splenic follicles) and normally eliminate senescent and damaged RBCs. As such, these host defense cells represent natural targets for drug delivery using RBC carriage.
With the exception of these compartments of the RES, “normal areas” for RBCs in the body are limited to areas connected by the vascular system. However, RBCs often exit this space in pathological sites including: (i) the CNS (for example, in stroke or traumatic brain injury); (ii) penumbra of the myocardial infarction zone in immediate post-ischemic period; (iii) alveolar and airway compartments in acute lung injury, pulmonary hypertension, and other conditions which lead to damaged endothelium; and (iv) other numerous cases of pathological vascular permeability and vessel damage. In theory, RBCs may provide “passive” drug delivery to these sites.
In the absence of these pathological conditions, the list of main targets accessible to RBCs includes components of blood, endothelium, and a few constituents of the RES. RBC-coupled drugs do not act upon targets inaccessible to their carrier, which limits non-specific, systemic, and other undesirable off-target effects. Furthermore, coupling to RBCs impedes a drug’s freedom to interact with some targets even within the vascular compartment. For example, fibrinolytic plasminogen activators bound to RBC surfaces can act upon soluble therapeutic targets in blood, but not with endothelial receptors, which circumvents a main side effect of free plasminogen activators [8–10].
RBCs are attractive carriers for drugs that: (i) metabolize or capture agents circulating in blood; (ii) regulate blood clotting and thrombi transformations; (iii) require delivery to the endothelium; (iv) replace deficient lysosomal enzymes; and (v) regulate immune system (antigens, anti-inflammatory agents, stimulators, and inhibitors of phagocytes).
2.2. Loading drugs in RBC inner volume and to RBC surface
Drugs and probes can either be encapsulated into the RBC inner volume or coupled to the RBC surface (Fig. 1). Drugs encapsulated in RBC may interact with molecules diffusing from blood through the RBC membrane, but not with molecules outside the RBC membrane including components of blood and vascular walls. Thus, encapsulation in RBCs may reduce immune reaction to biotherapeutic agents or protect from pathways of inactivation.
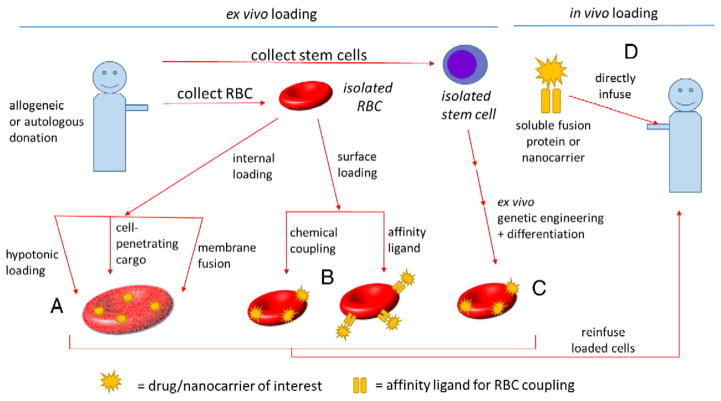
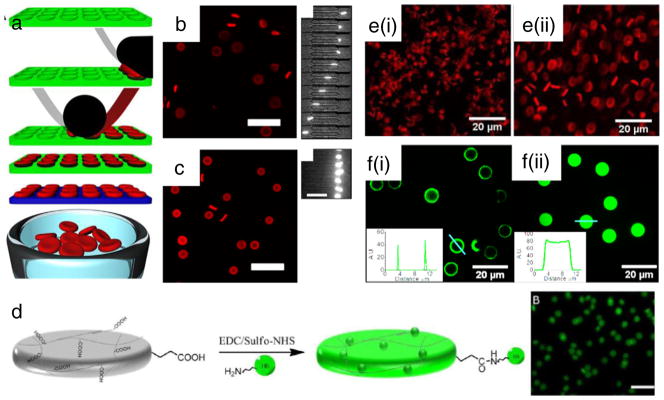
Fig. 1.
Strategies for RBC-based DDSs. Approaches can be broadly categorized as ex vivo versus in vivo RBC loading. Among ex vivo approaches are included: (A) internal loading of drugs/nanocarriers by penetration through the RBC membrane, for example, by hypotonic loading, cell-penetrating cargo, or fusion with liposomes; (B) surface loading by chemical coupling and binding of RBC-targeted affinity ligands; (C) differentiation of engineered, enucleated RBCs derived from stem cells (peripheral blood CD34+ cells, iPSCs, etc.). All ex vivo approaches would ultimately require reinfusion of the manipulated cellular RBC product after quality control testing. In vivo loading would be comprised of (D) drugs or nanocarriers fused or coupled to RBC-targeted affinity ligands such that they would bind rapidly to RBC in circulation.
In most protocols, loading drugs into the carrier RBC inner volume necessitates ex vivo manipulation. Hypotonic loading is achieved by incubating washed RBCs or blood with the cargo at hypotonic solutions which induce the formation of transient pores in the plasma membrane of swelling cells. This approach is currently used for several RBC carriers in clinical trials (Table 1) for the treatment of oncologic, inflammatory, and neurological diseases and commercial development is underway (Table 2). Newer developments include attempts to use cell-penetrating peptides to import therapeutic proteins in the carrier RBCs [11] and fusion of RBCs with drug-loaded liposomes [12].
Table 1.
List of clinical trials for RBC DDS which are currently active or recruiting (retrieved from www.clinicaltrials.gov).
| Sponsor (product) | Principal investigator | Drug and loading method | Application/indication | ClinicalTrials.gov Identifier |
|---|---|---|---|---|
| Erydel: EryDex | Larry J. Dumont | Dexamethasone sodium phosphate loaded into RBCs (A) | Determining in vivo recovery and survival of dexamethasone sodium phosphate loaded into RBCs | NCT02380924 |
| ERYtech Pharma: | • Richard A. Larson | L-asparaginase loaded in RBCs | • Acute lymphoblastic leukemia (H) | • NCT01910428 |
| • ERY-ASP | • Xavier Thomas | • Acute myeloblastic leukemia | • NCT01810705 | |
| • GRASPA | • Yves Bertand | • Relapse of acute lymphoblastic leukemia | • NCT01518517 | |
| • GRASPA | • Pascal | • Progressive metastatic pancreatic carcinoma (in combination with gemcitabine or FOLFOX) | • NCT02195180 | |
| • ERY001 | • Hammel | • Acute lymphoblastic leukemia (combination with polychemotherapy) | • NCT02197650 | |
| • GRASPA | • Yves Bertand | |||
| • University of Iowa | • John A. Widnes) | Biotin labeled RBCs (A) | • Investigating biotin labeled RBC survival in patients that previously developed antibody response against biotin labeled RBCs | • NCT02077751 |
| • VA Office of Research and Development | • Robert Cohen | • Investigating RBC survival in diabetics and pre-diabetics | • NCT01204216 | |
| North Shore Long Island Jewish Health System | Lawrence A. Yannuzzi | Indocyanine green loaded RBCs (A) | Diagnosing retinal pathologies via quantification of capillary blood flow | NCT02445001 |
Table 2.
Commercialization of RBC DDS.
| Company | Technology | Target disease/application | Current status and results | Refs |
|---|---|---|---|---|
| Erydel | Hypotonic swelling and resealing for loading of dexamethasone sodium phosphate | Ataxia telangiectasia | Beginning phase III studies Received ODD in both US and Europe |
[202,203] |
| Erytech | RBC encapsulated:
|
|
1. Acute lymphoblastic leukemia: Phase I/II and Phase II trials completed Currently being investigated for Phase IbODD in Europe and USA Acute myeloblastic leukemia: Phase IIb initiated ODD in Europe Pancreatic cancer: Phase I study completed Phase II study started |
[204,205] |
| Orphan Technologies | Encapsulation of thymidine phosphorylase | Mitochondrial neurogastrointestinal encephalomyopathy | ODD by both FDA and EMA If approved, this will be the only treatment available for any mitochondrial disease |
[206] |
| Rubius | Genetically modified hematopoietic stem cells are turned into RBCs in culture that eventually express therapeutic proteins | Treat metabolic diseases, autoimmune diseases, and cancer | Company recently launched (December 2015) | [13] |
| Anokion | Engineered proteins are attached to circulating RBCs following IV injection, and via natural cell death the RBCs and the attached engineering proteins are processed by immune cells for enhanced antigen processing | Inducing protein specific immune tolerance without immunosuppression | Expanding preclinical applications | [42,43,207] |
| NanoBlood | A 7 nm particle containing a hemoglobin core, a hydrated PEG outer shell, and an inner superoxide dismutase mimetic gold shell | Alternative to RBC transfusions with applications in stroke treatment, trauma situations, traumatic brain injuries, and sickle cell disease | Patent granted | [208,209] |
| Arytha Biosciences | Developing nanoparticles that mimic key properties of RBCs by using natural RBC membranes to | Improving nanoparticle therapies (e.g. extended circulation, vaccine applications, toxin removal applications) | Expanding preclinical applications | [170,184,188,210] |
| RxMP Therapeutics | RBC derived microparticles synthesized from packed RBCs | Hemostatic agents to prevent bleeding in trauma situations | Currently in preclinical development | [211,212] |
The technologies farthest along the commercialization path are focused on the encapsulation of therapeutics into RBCs, as these RBC delivery strategies were developed earlier than the other RBC delivery systems highlighted here (e.g. cellular hitchhiking, RBC-inspired carriers, or genetically engineered RBCs). For example, both Erytech and Erydel have entered clinical trials (see Table 1). The technologies that have received Orphan Drug Designation from either the FDA or EMA are also RBC-encapsulation based. Other RBC-based delivery strategies are being commercialized that make use of either engineered RBCs (Rubius) or engineered proteins (Anokion) attached to the surface of RBCs. These specific technologies will require a commercialization strategy that deviates from RBC encapsulation technologies, since they rely on genetic modifications or protein engineering, which will likely necessitate additional considerations during the approval process. While more recently described in preclinical settings, a few particle-based technologies are being commercialized to enhance current approaches toward blood transfusions, hemostatic agents, and nanoparticle biotechnologies (e.g. drug delivery, vaccination, and toxin removal). The majority of RBC delivery systems being commercialized have academic roots, highlighting the central role of academia in the development of RBC DDS.
Surface loading to RBCs can be achieved ex vivo by incubating either intact or modified RBCs with drugs or drug carriers. In such an approach, the drug of interest may react chemically with the RBC surface to form a covalent linkage or be non-specifically adsorbed. Most recently, biotechnological methods to culture genetically engineered RBCs ex vivo that bear recognition sequences have allowed for site-specific, covalent coupling of therapeutic proteins by way of transpeptidases [13]. Alternatively, drugs and carriers can be conjugated or fused with antibodies, antibody fragments, peptides, or other ligands that bind to RBC’s surface. In the latter approach, RBC-targeted drugs can be used in two main protocols. First, this can be employed for ex vivo surface loading on donor or autologous RBCs, to avoid multi-step and inevitable damaging loading by encapsulation. Second, a simple intravascular injection of these RBC-targeted agents leads to rapid binding to RBC circulating in the bloodstream [14].
2.3. RBC surface loading: effects on cargo
RBC’s surface can be used for the coupling of drugs, their carriers, and targeting agents. The prototype for the latter approach was designed in the early 1980s for targeting RBC-encapsulated drugs to the sites of vascular injury [15–18]. Antibody-coated RBCs have been studied in vitro and in vivo for targeting drugs to diverse cells—endothelium, smooth muscle cells, leukocytes, etc. [19–21]. More recently, RBCs painted with affinity ligands have been tested for targeting to circulating leukocytes [22,23].
Anchoring of drugs to RBC surface changes not only their PK and distribution but in some cases also modulates their functional features. For example, as discussed above, coupling of plasminogen activators including tPA and urokinase to RBC’s surface restricts their ability to interact with receptors on other cells [9]. This mitigates adverse effects mediated by such interaction of soluble plasminogen activators, which is especially dangerous in the CNS [24]. As a result, RBC-bound tPA demonstrates an unparalleled safety profile in very challenging animal models including stroke [25], TBI [26], hypoxia [27], and thrombosis [28] in the cerebral vasculature [29].
On the other hand, coupling of drugs and carriers to RBC’s surface, as well as the features of RBC-associated agents is influenced by the glycocalyx [30]. For example, RBC-coupled tPA is protected against plasma inhibitors by RBC glycocalyx [31]. Furthermore, coupling to RBC carrier creates high local concentrations of tPA, allowing expedient focal dissolution of blood clot matter in the close vicinity of RBC–tPA complexes, which in models of thrombotic occlusion of blood vessels results in the formation of patent channels spanning the plug and permitting reperfusion prior to dissolution of the thrombus [32,33]. Therefore, drug loading to carrier RBCs profoundly alters their pharmacological profile, in some cases, enabling effects simply unavailable to free drugs. It is tempting to postulate that RBCs may also significantly and favorably modulate delivery and therapeutic effects of nanocarriers, among other artificial drug delivery systems, and these interactions with nanocarriers are detailed below.
2.4. Immunological and biotechnological aspects of surface-loading of RBCs
When considering surface-coupling as a strategy for RBC-based DDSs, one must also consider the potential for immunomodulatory effects, which may be favorable or unfavorable depending on the desired application. In transfusion medicine, immune responses to allogeneic RBCs represent a significant obstacle to multiple transfusions in clinical practice. Despite decades of experience in the transfusion of RBCs and the observation of a multitude of allo-antibodies thereafter, the precise mechanisms by which transfused RBCs induce alloantibody formation in certain individuals and in certain clinical scenarios remain to be fully understood and they are the subject of extensive investigation and are reviewed elsewhere [34,35].
A key observation is that despite the high degree of polymorphism of RBC antigens, and the fact that most transfusions are not matched for antigens other than that of ABO and RhD systems, only a subset of RBC recipients ultimately develop allo-antibodies [36]. Contributory factors likely include recipient immune status and degree of mismatch between donor and recipient populations [37]. Recently developed models of RBC alloimmunization to the human Kell antigen have revealed several interesting features of immunologic responses to red blood cells [38,39], including the observation that C3 is involved in “antigen modulation” on the RBC surface [40], and that a subset of allo-geneic RBCs may be resistant to clearance by antibodies in mouse models [41].
Of particular interest are recent studies that coupling of antigens to RBCs may induce tolerance. Hubbell demonstrated that injection of albumin conjugated with peptides binding to RBCs leads to T-cell deletion and immune tolerance to the antigen [42]. The authors also demonstrated efficacy in a model of autoimmune type 1 diabetes. It was speculated that such a strategy exploits natural regulatory mechanisms of eryptosis (programmed RBC death) whereby the body’s own pathways are designed to clear RBCs via mechanisms that avoid auto-reactivity and provide tolerogenic signals. This same group recently furthered this approach by coupling of E. coli L-asparaginase (ASNase) [43], a biomolecular therapeutic used for treatment of acute lymphoblastic leukemia and non-Hodgkin lymphoma that was previously explored in RBC DDSs using internal loading approaches [44–46]. This therapeutic is limited by the high frequency of development of anti-ASNase antibodies, many of which are drug-neutralizing [47]. The authors found that in vivo loading of ASNase onto RBCs resulted in >1000-fold reduction of anti-ASNase antibodies, >10-fold improvement in pharmacodynamic effect, and tolerized mice to subsequent challenges with the wild-type enzyme.
These findings carry major implications for RBCs as DDS. Immunogenicity of protein therapeutics continues to complicate their repeated administration in clinical settings, including drug-neutralizing antibodies and infusion reactions, which can be severe [48]. Although strategies for immune tolerance induction have been developed [49], many include potent immunosuppressive agents such as rituximab and corticosteroids. RBC and platelet internal loading [44,50] and particle-based delivery systems [51] have demonstrated promise in overcoming these limitations, but the efficacy of the RBC surface loading for induction of tolerance is remarkable given its relative simplicity, and opens the possibility of RBCs as carriers of virtually all protein therapeutics, including enzyme replacement therapies (for example, FVIII replacement in hemophilia) where development of inhibitors remains a major clinical hurdle [52]. Overall, these important studies, together with decades of experience in transfusion medicine, have revealed that the immune responses to red blood cells may represent a complex balance between immunogenicity and tolerogenicity, dependent on a multitude of factors, including the inflammatory or disease state of the host.
Further expanding the RBC DDS platform, the recent progress in ex vivo differentiation of stem cells [53] (including peripheral blood CD34+ stem cells, umbilical cord stem cells, human embryonic stem cells, and inducible pluripotent stem cells (iPSC)) into mature, enucleated RBCs has opened the possibility of ex vivo production of molecularly engineered RBCs as drug carriers. The early focus of ex vivo production of RBCs has largely been placed on the production and feasibility of transfusable packed red cell units, and has been reviewed elsewhere [54]. Such an approach offers the promise of overcoming limitations in donor supply, alloimmunization, and infectious disease safety in human blood products. However, production of multiple units of transfusable red blood cells faces major hurdles with respect to the ability to produce sufficient transfusable RBCs and the scalability of these approaches, although significant advances have been made [55]. In 2011, the group of Giarratana et al. demonstrated successful ex vivo GMP-grade production and transfusion of 1010 RBCs into a healthy donor [56]. Using 51Cr labeling, they demonstrated survival of the cultured RBCs in circulation was comparable to what has been reported for native RBCs. In drug delivery applications, far fewer numbers of carrier RBCs might be expected to be required, which lessens concerns over scalability.
Indeed, building upon these successes in ex vivo RBC production, the group of Lodish and Ploegh demonstrated the successful production of engineered RBCs expressing modified proteins on the surface of in vitro-differentiated reticulocytes [13]. The modified RBCs were labeled with various probes, demonstrated long-circulation, and were able to be targeted by attachment of single domain antibodies. Modified RBCs produced with these technologies have enormous potential as drug carriers, potentially with cytoplasmic as well as surface cargoes, and have already prompted industrial development (see Table 2). Further complementing this approach is the significant recent advancement in genome editing strategies, such as CRISPR-Cas9 and zinc finger nucleases [57–59]. The optimal source of stem cells, and whether an autologous or allogeneic source should be used, remains an open question. The enucleated nature of red cell carriers, also lessens concerns over undesired effects of genetic manipulation such as the potential for neoplastic transformation.
2.5. Surface loading of RBC using targeting ligands
The surface of RBCs offers myriad membrane proteins representing potential sites for carriage of therapeutics. The diversity of RBC membrane proteins has been largely informed by the decades of experience in transfusion medicine, where phenotypic diversity across individuals [60], attributed in part to evolutionary pressure for protection against infections, can result in allo-antibody formation after transfusion. Although, in transfusion medicine, the focus is largely on epitopes present only in fractions of the population (and its subsequent potential for alloimmunization), drug delivery using RBCs is likely to focus on epitopes with very high frequency across populations. Several such targets have been described and are reviewed elsewhere [1,61] and a summary is provided in Table 3.
Table 3.
Summary of RBC surface targets and estimated copy numbers.
| RBC membrane protein | Blood group designation | Approximate copy number per RBC |
|---|---|---|
| Band 3 (anion exchanger 1, AE1) | Diego | 1,000,000 |
| Glycophorin A (GPA) | MNS | 1,000,000 |
| GLUT1 | None identified | 500,000 |
| Glycophorin B (GPB) | MNS | 250,000 |
| RHCE and RHD | Rhesus | 100,000–200,000 |
| CD59 | Undefined | 10,000–20,000 |
| Kell | Kell | 10,000 |
| Decay accelerating factor (DAF, CD55) | Cromer | 3000–5000 |
| ICAM-4 | LW | 3000–5000 |
| Complement receptor 1 (CR1) | Knops | 1000 |
| ADP-ribosyltransferase 4 (ART4) | Dombrock | Uncertain |
Despite the multitude of potential sites for drug coupling, few of these epitopes have been assessed for their suitability in drug targeting. Clinical experience in RBC membrane disorders, such as hereditary spherocytosis, demonstrate the critical importance of preserving (or allowing control of) RBC integrity, deformability, membrane-fluidity, shape, rheology, and resistance to phagocytosis and clearance [62–64]. Indeed, it has been demonstrated that extracellular ligands, including monovalent Fabs, can significantly alter membrane rigidity, with studies suggesting that extracellular ligands can promote strong or transient interactions between membrane proteins, thereby impeding local distortion [65]. Such alteration of surface proteins can significantly alter RBC physiology, for example, by impeding malarial parasite entry of RBCs [66], altering oxidant susceptibility [67], RBC margination and hemodynamics [68], and interaction with platelets and vessel walls [69]. Considering these potential effects, the surface ligand chosen for RBC coupling should be chosen as to maximize biocompatibility, and will likely also depend on the number of ligands that may need to be coupled for therapeutic effect.
Among the earliest targets for surface coupling of therapeutics to RBCS was complement receptor type 1 (CR1). In normal RBC physiology, CR1 serves as a ligand for complement C3b, wherein immune complexes containing the activated component of complement C3b bind to CR1 on RBC, resulting in transfer to macrophages and clearance without untoward damage to other tissues [70]. In primates and humans, the large majority of CR1 (>90%) is found on RBCs (although at relatively low numbers per cell, ~500–1500 copies per cell, relative to other RBC proteins) [71,72]. Several decades ago, the group of Ron Taylor proposed emulating this natural mechanism using bispecific antibodies to clear pathogens or toxins by coupling them to RBC CR1 [73]. Importantly, the binding of anti-CR1 conjugates to RBCs apparently facilitates pathogen clearance without overt RBC phagocytosis [74] or damage to cells [75,76]. This approach has demonstrated efficacy for clearance of bacterial and viral pathogens and pathogenic antibodies, including in primate models [77–82], as well as coupling of therapeutics [83]. CR1 constitutes the Knops blood group, of which many high-frequency epitopes have been described, although their levels of expression are variable [84]. Interestingly, alloantibodies to the Knops antigens are not associated with hemolysis of transfused RBCs, suggesting that this target can tolerate drug loading with little adverse effects.
Among the most abundant proteins on the RBC surface are the band 3 and glycophorin A proteins. Band 3, an anion exchange protein, represents approximately 20% of the protein mass on the RBC surface, and exists both in isolation and in multiprotein complexes that are critical for maintaining linkages to the spectrin skeletal network below [85,86]. Band 3 carries a large portion of the ABO blood group sugars and is central to the maintenance of RBC membrane integrity and structure, as evidenced by the various membrane disorders associated with band 3 mutations. It may represent an attractive target for surface coupling due to the very large number of available sites such that only a relatively small fraction would need to be occupied. Band 3 is classified as the Diego blood group and high-frequency epitopes (such as Wr(b)) have been described [87]. Glycophorin A (GPA) is a type I membrane-bound sialoglycoprotein that functions as a receptor for complement, a chaperone for band 3 transport, and contributes to RBC structural integrity and the negative charge of the RBC glycocalyx [88]. It is similarly present in very high numbers and, like band 3, is also expressed on portions of the kidney. This protein has been successfully used to couple therapeutics to RBCs in animal models [14,89,90], and is among the most commonly used markers for RBC lineage. Glycophorin A is classified as the MNS blood group and high-frequency epitopes, such as En(a) have been described. Additional glycophorins on the red cell surface, although fewer in number, include glycophorins B, C, and D which are part of the MNS (glycophorin B) and Gerbich (glycophorins C and D) blood groups.
Other potential targets for surface coupling include the Kell, ART4, ICAM-4, CD59, DAF, GLUT1, and Rh family proteins, among many others. These proteins have a range of expressions from low to high copy numbers, facilitating design of therapeutics according to the number of moieties that should be coupled to RBCs. The Kell family, although relatively low in expression (1e4/cell) [91], has been successfully modified in genetic engineering of ex vivo derived RBCs [13], although when considering in vivo loading, it should be noted that Kell is also expressed on many other tissues including parts of the brain, and lack of Kell is associated with a severe clinical phenotype [92]. ART4 constitutes the Dombrock blood group, and although it is known to encode an ADP-ribosyl-transferase, the enzymatic function in RBC physiology is unknown [93,94]. ICAM-4 constitutes the LW (Landsteiner Weiner) blood group and is involved in RBC adhesion and erythropoiesis [95, 96]. CD59 and DAF are involved in complement regulation, and although CD59 has yet to be designated as a specific blood group [97], DAF constitutes the Cromer system [98]. GLUT1 is a highly expressed, minimally polymorphic RBC antigen involved in glucose transport that is also expressed in many other tissues [99]. The Rh family is among the most well-described in transfusion medicine, is thought to be involved in ammonia transport, has moderate expression levels, and is relatively erythroid-specific [100–102]. High-frequency epitopes for all the above blood groups have been characterized.
Binding of antibodies and other multivalent ligands to some high-density determinants such as GPA (e.g., TER119 antibody) causes RBC aggregation, and therefore monovalent scFv fragments are likely to be preferable and, in addition, support modular recombinant engineering of diverse fusion proteins. Indeed, TER119 antibody-derived scFv-fusions have been used by John Atkinson’s and other labs for coupling to RBC of therapeutic fusions including those controlling RBC damage by complement [14,90,103]. Interestingly, Rh-antigen D targeted ligands using remodeled monovalent scFv in multivalent format renders agglutinating ability without activation of complement [104], and multivalent anti-Rh(D) scFv/CR1 fusion restores the function of this natural RBCs glycoprotein involved in host defense [105]. Selection of ligands using phage display libraries provides a means to discover novel peptide and antibody ligands [106] that facilitate binding of diverse cargoes and improve their pharmacokinetics [107].
3. RBC and nanocarriers
Artificial carriers—liposomes, polymeric carriers, dendrimers, filomicelles, and other nanoparticles—are capable performing diversified biomedical applications [108]. Their features can be controlled via addition of surface modifications (inclusion of hydrophilic polymers, self-peptides, targeting moieties, etc.) [109], inclusion of stimuli-responsive functions (e.g. enzyme, pH, near-infrared light, or magnetic responsiveness) [110], or modifications to their physical parameters (e.g. size, shape, elasticity) [111]. It is tempting to speculate that hybridization of artificial DDS with RBC may help overcome some of the problematic issues that limit the utility of nanocarriers, such as inadequate behavior in the bloodstream. On the other hand, RBCs represent an important and influential, yet underappreciated in the past, counterpart of DDSs.
Indeed, RBCs normally occupy ~50% of blood volume and, due to such high concentration and peculiar hemodynamics, greatly influence circulation of other particles and their interaction with vascular walls [112]. In arteries, where blood perfusion is associated with high levels of fluid shear stress, RBCs tend to occupy the mainstream and avoid the micron-scale marginal layer of blood that directly contacts endothelial surface, thereby pushing other circulating particles to the cell-free layer (Fig. 2a) [113]. This hydrodynamic phenomenon intensifying interactions of particles with endothelial cells, known as “margination” [114], is of extreme importance in designing vascular targeted nanocarriers [115]. Margination, or margination potential, can also be described as a particle’s ability to either travel from bulk flow to vessel walls or, more generally, the ability to travel close to vessel walls. In general, this has been studied using perfused model vessels [116] and computations simulating interactions between nanocarriers and endothelium in differently sized vessels, both with and without RBC presence [113,117].
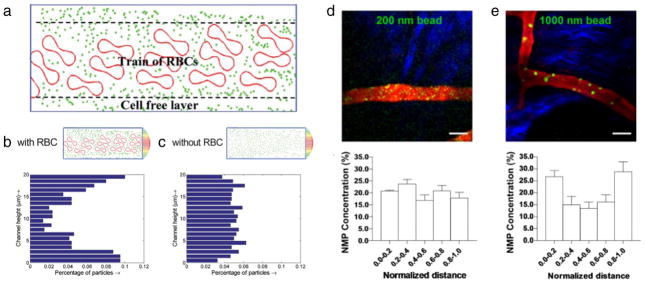
Fig. 2.
Interactions between red blood cells and nanocarriers in microcirculation. (a) Schematic of the cell-free layer with flowing nanocarriers and RBCs. Distribution of particles flowing in a straight channel in a particle cell computational model (b) with and (c) without RBCs. Intravital microscopy and quantitative analysis of the radial distribution of nanocarriers of (d) 200 nm and (e) 1000 nm diameter. (a, b, and c) Adapted from [113] with permission from The Royal Society of Chemistry. (d and e) Adapted by permission from Macmillan Publishers Ltd.: Scientific Reports [125], copyright (2013).
The amplitude of margination effect depends on a variety of factors including hydrodynamic conditions, vessel diameter and shape, and geometry of particles [112,118–120]. Here, we will focus on the role that circulating RBCs, either alone or interplayed with the above factors, have on influencing particle margination. In one of the earliest reports, a particle-cell computational model was leveraged to show how nanocarriers more effectively marginate to vessel walls in the presence of RBCs (Fig. 2b), as compared to a RBC-free vessel (Fig. 2c) [113]. These effects were attributed as the cell-free layer effect (Fig. 2a), which captures interactions between particles and RBCs. RBC-mediated margination effects are also dependent on particle shape, as shown by a recent dissipative dynamics model where both spherical and ellipsoidal particles increase as hematocrit levels increase, with spheres marginating more efficiently [120]. Other computational studies have reported mixed results regarding margination advantages of higher aspect ratio particles in the presence of RBCs, where in some cases higher aspect ratio particles marginate better [121], and in other cases worse [120], than spherical particles. However, it should be noted that a recent in vitro microfluidic study, in contrast to the above computational studies, has reported enhanced margination of high aspect ratios particles in the presence of RBCs [122]. Other shapes, notably discoidal particles, have exhibited enhanced margination in the presence of RBCs, both in vitro [123] and in vivo [123,124].
Particle size has also been shown to greatly impact margination in the presence of RBCs. Overall, the majority of computational [120,125] (exception here: [113]), in vitro [126,127], and in vivo reports [123, 125] have reported enhanced margination of larger particles, independent of shape, as compared to their smaller counterparts in the presence of RBCs. A recent in vivo study highlighted how small 200 nm particles (Fig. 2d) are distributed randomly and non-specifically throughout a vessel section, whereas larger 1000 nm particles (Fig. 2e) show enhanced accumulation at the vessel’s outer layers or the vessel walls [125]. Indeed, quantitative analysis revealed that the 1000 nm particles were 3× more concentrated at the vessel walls than their smaller 200 nm counterparts, highlighting their enhanced margination abilities [125].
More advanced in vitro models are combining RBCs, flow, and vessel geometry effects in devices containing inflamed endothelial cells, so as to better simulate binding to pathological tissues [128]. While it is widely known that functionalization of nanocarriers occurs with affinity ligands that bind to endothelial surface determinants such as intracellular cell adhesion molecule 1 (ICAM-1, which is used in several examples given below in this article) [129,130], it has only recently been shown that affinity-mediated targeting is further enhanced due to the cell-free layer and RBC-mediated nanocarrier-margination effects [126].
The interaction of nanocarriers with endothelial luminal surface is greatly influenced by the cellular glycocalyx [131]. One of the postulated functions of this brush-like structure formed on the surface of endothelial and other cells by anionic carbohydrate chains of glycoproteins is to control vascular adhesion of blood cells [132]. The interplay between cellular interactions of the glycocalyx and circulating nanocarriers awaits more definitive characterization. For example, the glycocalyx spatial barrier may inhibit binding of neutral and anionic particles, facilitating the binding of cationic counterparts; however, the effects of circulating RBCs on glycocalyx–nanocarrier interactions are not well known. Treatment with glycocalyx-degrading enzymes in animals markedly augments endothelial binding of polymeric spheres (diameter ~100 nm) targeted to ICAM-1 [133], so it seems logical that blood cells and other circulatory molecules can further influence interactions between nanocarriers and the glycocalyx. Additionally, the endothelial glycocalyx modulates endocytic uptake and thus is involved in the intracellular delivery of nanocarriers [134]. The glycocalyx also modulates the interaction of endogenous regulatory entities (e.g. plasma cofactors and inhibitors) with biotherapeutic agents bound to endothelium, similarly as RBC’s glycocalyx protects bound serine proteases from plasma inhibitors [31,135].
4. Particles/carriers designed for targeting to or carriage by RBC
In the 1980s, Daan Crommelin and coworkers showed that injection of liposomes carrying targeting Fab fragments to RBCs results in the prolongation of the attached liposomes at the cost of enhanced clearance and hepatic uptake of RBC themselves [136]. In other words, RBC-adhered particles circulate for longer times, but the modified cells exhibit more rapid clearance than unmodified cells. In this study, RBCs were just a model target cell to test an approach for the elimination of tumor cells from the circulation. In a modern application, RBC-targeted liposomes are being studied for delivery of anti-malarial agents. In these studies, antibodies and semi-specific agents such as heparin have been used to target liposomes to Plasmodium-infected RBCs [137,138].
An important corollary of targeting DDSs to RBCs is that, theoretically, similar to RBCs being utilized as a carrier for drugs, RBCs can also be used for carriage of nanocarriers. Encapsulation of drug-loaded hydrogel nanoparticles in RBCs and other nano-scale drug formulations has been studied in vitro [139,140]. Loading in RBCs and laser-triggered release of gold nanoparticles has also been reported [141]. Encapsulation of iron oxide nanoparticles into RBCs renders them responsive the magnetic field in vitro [142]. These ideas might evolve in mechanisms for controlled release and magnetic guiding of RBC-carried nanocarriers for therapeutic and imaging purposes [143]. The main challenges of these approaches are the loss of RBC’s compatibility and the need for extracorporeal loading. As such, surface loading of nanocarriers to RBCs seems preferable [144].
Recent iterations of binding carriers to RBC supercarrier, called “cellular hitchhiking” may combine the advantages of synthetic nano-particles and the natural delivery abilities of circulatory cells [145, 146]. RBCs represent arguably the simplest biological carrier for cellular hitchhiking.
4.1. Loading nanoparticles on/in RBCs
There are a variety of methods to attach nanoparticles of various compositions, surface chemistries, sizes, and shapes to the RBC surface (Fig. 3a, b and c), or encapsulate nanoparticles in RBCs (Fig. 3d). In either case, it is essential to consider both the properties of the particles and the end application of the RBC delivery system so that the chosen method offers both optimal attachment/inclusion while limiting damage or impaired function of the RBC. Here, we will review the three main strategies used to combine nanoparticles and RBCs: (i) non-specific adsorption of nanoparticles to RBCs, (ii) specific binding, either ligand-receptor mediated or through chemical conjugation, and (iii) encapsulation of nanoparticles within RBCs.
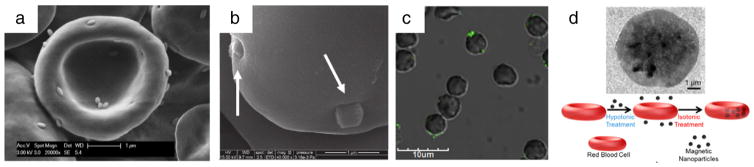
Fig. 3.
Attachment/inclusion of nanocarriers to/in red blood cells. (a) Polystyrene rod-shaped, (b) mesoporous silica, and (c) antibody-functionalized polystyrene spheres adsorbed to the surface of RBCs. (d) Hypotonic swelling and subsequent sealing of RBCs to include nanocarriers inside RBCs. (a) Reprinted from [159], Copyright (2015), with permission from Elsevier. (b) Adapted with permission from [149]. Copyright (2011) American Chemical Society. (c) Adapted with permission from [158]. Copyright (2013) American Chemical Society. (d) Adapted with permission from [142]. Copyright (2014) American Chemical Society.
The simplest method involves non-specific physical adsorption of the particle to the RBC surface. Similarly to other cells, RBC’s plasma membrane contains relatively hydrophobic domains, which present an area for binding hydrophobic particles. These interactions have been shown to be independent of charge, as both positive and negatively charged hydrophobic particles bind to RBC surfaces (Fig. 3a) [147]. In other examples, cationic particles bind to the anionic RBC’s glycocalyx [148], while mesoporous silica nanoparticles with silanol functional groups have been shown to adsorb to RBCs via interacting with the phosphatidyl choline present on RBC membranes (Fig. 3b) [149].
Covalent and non-covalent loading protocols with variable degrees of molecular specificity have also been reported. Proteins and carbohydrates on RBC’s surface contain an abundance of amine and thiol groups [150] available for conjugation via a variety of chemistries involving modification of the RBC surface. For example, chemical conjugation of biotin to RBC surface allows avidin functionalized particles to readily bind and attach to the biotinylated RBC [151–154]. Coating particles with antibodies or other ligands of RBC proteins offers most specific loading [1]. Recently, phage display has been exploited to discover novel peptides that bind to RBCs [42,107] where some of these peptides have been shown to facilitate nanoparticle binding to RBCs [106].
Generally, particle encapsulation in RBCs is based on the same principle of hypotonic swelling that opens up pores in the membrane, allowing nanoparticles to diffuse into the cell before returning the cell to isotonic conditions to seal the pores (Fig. 3d) [142]. This method requires that the nanoparticles be small enough to enter the pores (<100 nm [141]) during hypotonic treatment. Typically, inorganic nanoparticles (e.g. iron oxide or gold) have been utilized for encapsulation inside RBCs for imaging applications [155,156].
4.2. RBC-bound carriers
Coupling to RBC supercarrier prolongs blood circulation and alters tissue distribution of nanoparticles in animals. Hydrophobic polystyrene particles bound to RBC circulated in animals for markedly longer time than free counterparts with particle sizes ranging from 100 to 1100 nm, with 220 nm and 450 nm nanoparticles exhibiting the longest circulation times when hitchhiked onto RBCs [157]. This outcome was verified using adsorption onto RBCs of anionic and other surface chemistries nanoparticles, showing prolongation of circulation of hitchhiked particles of 220 nm for all surface chemistries examined [147].
Hitchhiked nanoparticles detach from circulating carrier RBCs in a time-dependent manner likely via shear stress-based forces and physical contact with vascular walls [147]. This manifests as transfer from RBC and pulmonary accumulation of RBC-hitchhiked nanoparticles, while simultaneously lowering uptake in the liver and spleen (i.e. clearance organs) [158]. Lung accumulation of RBC-hitchhiked nanoparticles was as high as 7-fold as compared to free nanoparticles. Transfer of nanoparticles from RBC surfaces to lung endothelium was confirmed via independent tracking of RBCs and nanoparticles.
Pulmonary accumulation of RBC-carried nanocarriers was further enhanced via attachment of lung-specific antibodies to the surface of RBC-adsorbed nanoparticles (Fig. 3c) [158]. In a follow-up study [159], lung accumulation and clearance organ avoidance of RBC-hitchhiked nanoparticles were improved further by leveraging the use of rod-shaped particles, which have recently been used for lung targeting [160]. Spherical and rod-shaped particles, RBC-hitchhiked and non-hitchhiked, were functionalized either with clearance organ targeting moiety, IgG, or with ICAM antibodies providing affinity to endothelium and directly compared for their liver, spleen, and lung uptake (Fig. 4a). The formulation which provided the best lung targeting, while decreasing uptake in the liver and spleen, leveraged synergistic contributions from RBC-hitchhiking, lung-targeting antibodies, and rod-shaped nanoparticles [159].
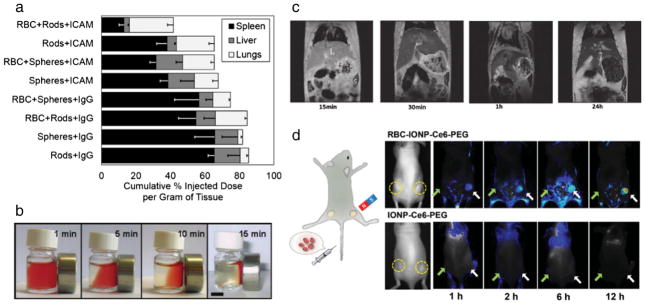
Fig. 4.
Applications of RBC-bound or loaded nanocarriers. (a) The adsorption of spherical or rod-shaped polystyrene particles functionalized with endothelium targeting antibody or immune system targeting antibody exhibit distinct distribution in liver, spleen, and lungs. (b) Magnetic responsiveness of RBC-adsorbed iron oxide nanoparticles, used for (c) magnetic resonance imaging contrast. (d) Biotinylated RBCs with avidin-conjugated iron oxide nanoparticles used for enhanced magnetic targeting in a tumor model in mice. (a) Reprinted from [159], Copyright (2015), with permission from Elsevier. (b and c).
In other RBC-hitchhiking work, focus has been placed on transferring the long-circulation abilities of RBCs to hitchhiked inorganic nano-particles for diagnostic and imaging purposes. For example, 35 nm iron oxide nanoparticles with a silica shell were non-specifically adsorbed to the surface of human RBCs, and hitchhiked RBCs were shown to respond to a constant magnetic field (Fig. 4b) [161]. In this study, human RBCs that were injected into mice showed negative contrast enhancement in the liver and spleen, which increased over a period of 24 h (Fig. 4c), likely due to an increased clearance of RBC-hitchhiked iron oxide particles [161].
Alternative approaches used RBC-encapsulated nanoparticles as a means to increase their circulation time [162]. These studies have also shown that iron oxide-loaded RBC can be propelled and controlled using both magnetization and ultrasound stimulation, while preserving the integrity of the cell during stimulation [142]. In one of the first examples of using RBC-hitchhiking for a therapeutic benefit, RBCs were loaded with both iron oxide nanoparticles and aspirin for the magnetic delivery and treatment of arterial thrombosis in dogs [163]. Given the large number of clinically approved iron oxide-based imaging agents [164], RBC-hitchhiking could potentially be integrated with a number of these clinically relevant imaging agents given non-traumatic conditions for particle loading into RBCs [155]. RBC encapsulation of gold nanoparticles can potentially be used in tandem with X-ray absorption to track RBC flow in vessels [156].
The RBC-hitchhiking approach offers then combination of drug delivery and imaging. For example, coupling to biotinylated RBCs of avidin-conjugated iron oxide nanoparticles (14–18 nm) prolonged their blood circulation and provided magnetic targeting in a tumor model in mice, which improved tumor visualization using fluorescent and MR imaging (Fig. 4d) and tumor shrinkage by doxorubicin encapsulated into the RBC interior by diffusion and a photodynamic agent loaded onto the RBC-bound nanoparticles [151].
These examples illustrate diverse and powerful potential biomedical utility of RBC-coupled nanocarriers and provide a strong impetus to both extend the versatility of this DDS platform and rigorous analysis of its translational and clinical aspects, including adverse effects (see below in Section 5).
5. RBC-inspired and RBC-hybrid nanocarriers
The aim of RBC mimicry is to yield carriers capable of navigating the vasculature and remaining in circulation similarly to RBCs. To date, no synthetic carrier has proven capable of circulating for up to a month, and in fact, most are cleared from the circulation in minutes [165]. Carriers capable of long-circulation will shift the paradigm of nanoparticle drug delivery and diagnostics, and as such, synthetic versions of RBCs are of high interest for applications in drug delivery and imaging. There are two main modern approaches to combining the advantages of synthetic materials and RBCs [166]. The first is to design carriers mimicking desirable physical features of RBCs—size, shape, elasticity, and lack of adhesion [167,168]. The second is to design synthetic carriers that contain proteins [169] or cell membranes [170] or other components of natural RBCs.
5.1. Synthetic RBCs
Synthetic RBCs (sRBCs) have long been the Holy Grail of bioengineering [171], given the demand for blood substitutes in transfusion medicine [172,173]. New technological developments offer hope that this task will be ultimately achieved.
A technique of particle replication in non-wetting templates (PRINT®) has been devised (Fig. 5a) for the high-throughput automated synthesis of hydrogel sRBCs. Injection in mice of iterations of sRBCs produced using this technique showed that highly elastic PEG-hydrogel sRBCs (Fig. 5b) with diameters similar to RBC (6μm) were capable of circulating for longer times than rigid sRBCs, which were unable to deform through identical channels (Fig. 5c) [168]. Further, sRBCs with a diameter most closely matching that of RBCs circulate for the longest times [174]. Moreover, PRINT® sRBCs were designed to harbor hemoglobin and were shown to possess oxygen binding capacity (Fig. 5d) [175]. PRINT® sRBCs were also engineered to possess functions that natural RBCs do not have, namely, pH-modulated swelling (Fig. 5e–i and e–ii)) and the ability to control the spatial conjugation (e.g. exterior versus interior) of small molecules (Fig. 5f–i and f–ii) [176]. These studies highlight the role of size, shape, and elasticity in designing drug carriers, whereas the versatility of the PRINT® approach gives hope that it may yield medically useful sRBCs with the potential of functionalities that RBCs themselves do not possess.
Fig. 5.
PRINT® for synthesis of sRBCs. (a) Schematic of the PRINT® process. (b) Deformable PEG-hydrogel sRBCs squeeze through small diameter channels, whereas (c) stiffer counterparts cannot. (d) PRINT® sRBCs functionalized with hemoglobin exhibit oxygen binding capacity. Additional functions of PRINT® sRBCs include (e) pH modulated swelling and (f) control over spatial (e.g. exterior versus interior) conjugation of small molecules. (a–c) Reprinted from [168]. (d) Adapted with permission from [175]. Copyright (2012) American Chemical Society. (e and f) Adapted with permission from [176]. Copyright (2014) American Chemical Society.
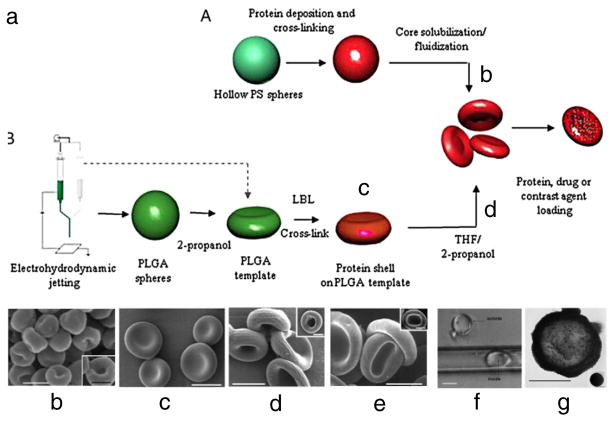
Two other recently devised approaches, layer-by-layer (LbL) particle templating of polyelectrolytes, and electrohydrodynamic jetting were used independently and in combination to create a variety of sRBC formulations (Fig. 6a), including sRBCs made of: (i) natural RBC proteins and synthetic polyelectrolytes created via LbL on hollow polystyrene spheres subjected to core dissolution (Fig. 6b), (ii) PLGA prepared from electrohydrodynamic jetting (Fig. 6c), and (iii) a combination of protein/polyelectrolyte LbL templating on sRBCs prepared from electrohydrodynamic jetting with core dissolution (Fig. 6d). These particles were shown to possess RBC-like features, including geometry (Fig. 6e), elasticity allowing transport through capillaries smaller in diameter than the sRBC (Fig. 6f), and oxygen binding capabilities. Further, sRBCs have been tested for loading and release of heparin, and loading of iron oxide nanoparticles (Fig. 6g) [167]. Other methods for synthesis of LbL [177] and hydrogel [178] sRBCs are also being devised and may evolve to yield DDSs formulations that might find medical utility.
Fig. 6.
Layer-by-layer (LbL) and electrohydrodynamic jetting for sRBC synthesis. (a) Schematic illustrating the synthesis mechanism for both sRBCs consisting of (b) natural RBC proteins and synthetic polyelectrolytes made via LbL, (c) PLGA made via electrohydrodynamic jetting, and (d) a protein/polyelectrolyte mixture leveraging both methods. (e) Reference image of mouse RBCs. (f) sRBCs were able to travel though capillaries smaller in diameter than the sRBCs themselves. (g) sRBCs loaded with iron oxide. (a–g) Reprinted from [167].
5.2. Hybrid nanoparticles containing RBC components
An alternative approach is to employ RBC components as elements of synthetic DDSs to improve biocompatibility. Historically, exosome-like fragments of RBC, i.e. vesicles called “nanoerythrosomes”, which could theoretically reach target sites including tumors that are typically inaccessible to RBCs, were proposed two decades ago [179,180]. Initial in vitro studies showed some promise of anti-tumor effects of prototype derivatives of RBC membrane ghosts loaded with cytotoxic agents. Notwithstanding, this DDS apparently had not been pursued to the level of systematic studies in vivo, perhaps due to very rapid clearance from blood, as this formulation lost biocompatibility [181]. In one recent study, nanoerythrosomes filled with anti-malarial agents were cleared in rats within a few hours (although slightly slower than free drug) [182].
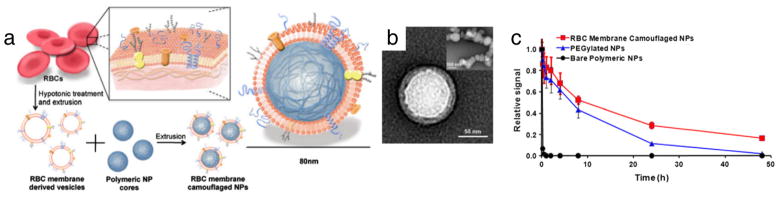
Recently, alternative strategies emerged. In one strategy, RBC membranes were used to form a shell that shields the synthetic nanoparticles from direct contact with the immune system (so-called “RBC-cloaked” nanoparticles). In this approach, RBC membranes isolated after hypotonic lysis (RBC ghosts) are extruded alongside carboxylated PLGA nanoparticles (~70 nm) to form the RBC-cloaked particles (Fig. 7a and b) [170]. These particles have prolonged circulation versus PLGA-based and, more impressively, PEGylated particles (Fig. 7c) [170]. These hybrid formulations, which can also be synthesized with an inorganic core [183], offer additional functions and possibilities to conjugate targeting moieties [183], controlled release of chemotherapeutics (e.g. doxorubicin) [184], acoustical propulsion [185] and photothermal effect [186]. In a therapeutic application of this, RBC-cloaked gold nanocages were shown to provide enhanced photothermal tumor ablation versus non-cloaked counterparts [186].
Fig. 7.
RBC-cloaked nanoparticles. (a) Schematic highlighting the extrusion approach for synthesis of RBC-cloaked particles. (b) TEM-image of final RBC-cloaked PLGA particle. (c) RBC-cloaked particles exhibit enhanced circulation as compared to both the PLGA template particle and its PEGylated counterpart. Reprinted from [170].
RBC-cloaked carriers, in theory, can provide a platform for targeted drug delivery [187], vaccination [188], and toxin absorption [189], among other applications. For example, toxoid (a pore-forming staphylococcal α-hemolysin, Hla) damages cell membranes and can lead to skin damage if used subcutaneously. Mice immunized with toxoid incorporated in RBC-cloaked versus control particles showed dramatic resistance to Hla subcutaneous infection as compared to unvaccinated and heat-treated Hla vaccinated groups.
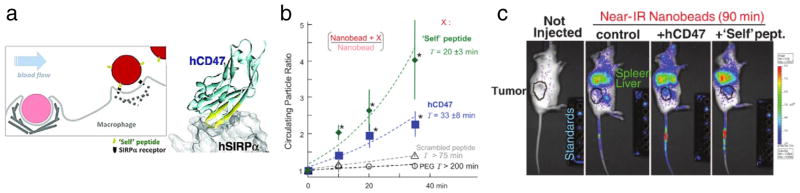
In another strategy, RBC components such as RBC proteins that inhibit RBC’s clearance by phagocytes, or peptides derived from these proteins, are conjugated to the surface of particles to provide more specific mechanisms to avoid immune system clearance as compared to widely used “stealth” methods of PEG conjugation, which persists with unresolved problematic issues [190]. For example, a glycoprotein CD47, which regulates the RBCs ability to be recognized as “self” and thus avoid clearance by macrophages (Fig. 8a), has recently been exploited as a functional molecule that can be incorporated in nanocarriers [191]. In prototype studies, coating of polystyrene particles by human CD47 inhibited their uptake by human phagocytes even when particles were opsonized with IgG [191]. In the follow-up study [169], small “self” peptides derived computationally from human CD47 were shown to increase the circulation time of 160 nm polystyrene nanoparticles (Fig. 7b) by inhibiting uptake by macrophages. As a result of improved circulation, CD47 and CD47 “self” peptide particles loaded with paclitaxel showed increased tumor accumulation (Fig. 7c) and tumor shrinkage, compared to “non-self” control formulations [169]. CD47 and “self” peptides present one of the only examples of a molecule that give off an active “self” signals, which, unlike non-active hydrophilic coatings that delay opsonization and clearance, addresses the clearance issue directly to succeed even in cases where opsonization has occurred.
Fig. 8.
“Self” peptide particles inspired by RBC stealth protein, CD47. (a) Mechanism by which phagocytes actively avoid CD47-based particles. (b) Polystyrene particles functionalized with either a CD47-derived “self” peptide or human CD47 exhibit enhanced circulation as compared to scrambled peptide or PEG-coated particles. (c) By virtue of CD47-mediated extended circulation, stealth particles exhibit enhanced tumor accumulation. (a–c) From [169]. Reprinted with permission from AAAS.
6. Current challenges and opportunities
In this decade, a few RBC-based DDSs have entered (Table 1), are entering, or are approaching the clinic.
For RBC-based DDSs, the dependence on blood typing and storage conditions on their circulation and therapeutic effect should be defined. Concerning RBC-specific cellular therapies, questions concerning the source (e.g. autologous versus allogeneic), storage, and extent of chemical modification of RBCs need to be addressed. Drug loading might affect RBC’s biocompatibility, and strict control of this aspect is prescribed to ensure the safety of the medical use of RBC drug carriers. The key issue in this approach is to avoid RBC damage by cross-linking cell membrane or other mechanisms associated with an excessive coupling of therapeutic molecules to RBC surface. For example, drugs endowed with an affinity to RBC surface determinants may inhibit molecules in RBC membrane that normally protect against phagocytosis or damage by complement system, alter plasticity of RBC membrane, or interact in an undesirable or unintended fashion with components of blood and other accessible vascular targets. The therapeutically effective and potentially toxic levels of drugs and nanocarriers remain to be determined for each formulation [158]. Loading schemas that affect RBC biocompatibility, for example, by reducing their elasticity and deformability [149], are likely to be problematic [192]. While many studies have focused on the positive aspects of RBC drug delivery, few have investigated the potential negative effects.
Non-specific physical adsorption of nanoparticles onto RBCs has been shown to have minimal hemolysis effect on the modified RBCs [149,158] and also have minimal effects on RBC structure following desorption from the RBC surface [158]. Specific methods for RBC loading by nanoparticles have not been widely explored for particle binding, and as such, it is not clear if specific binding or chemical modifications will damage or impair the hitchhiked RBCs; however, it has been shown that some methods conjugating drugs directly to RBCs can lead to damaged membranes and subsequently rapid clearance in vivo [5]. In vitro studies characterized how particle size, genesis, and surface chemistry of super-paramagnetic iron oxide nanoparticles (SPION) influence the cell integrity of the RBCs following encapsulation and implicated agents used for particle dispersion [155], particle loads, and duration of the loading [156]. Loading conditions producing the least RBC damage in vitro must be recapitulated in vivo [162].
In general, other limitations and advantages for RBC delivery systems designed to deliver drugs or nanoparticles exist. For example, RBCs are typically restricted to the vascular space, and as such are naturally limited in their abilities to penetrate and cross tissue barriers. This can affect the delivery of both drugs and nanoparticles for RBC delivery systems, and consideration of the best application for RBC delivery systems should be taken. Alongside this, tissue penetration issues for RBC delivery systems through microscopic barriers (e.g. the blood–brain barrier, endothelial barriers) also translate down to nanoscale barriers (e.g. cellular internalization). For these barriers, contact and interactions between nanoparticles/drugs loaded onto/into RBCs may be difficult depending on the location of the target cell.
Although RBC-hitchhiking brings together cellular therapies and nanotechnology, it may compete with both approaches. Future key areas of interest include: (i) investigating highly perfused tissues other than the lungs as potential targets for RBC-hitchhiking therapies, (ii) developing methods that can allow for on-demand detachment of the particle from the RBC so as to facilitate nanoparticle accumulation at any site in the body, and (iii) examining whether or not covalent attachment of particles to RBCs could improve nanoparticle circulation times even further. Hybrid RBC–nanocarrier approaches are intriguing and offer a chance to avoid immunological issues of PEG coating [190]. Exciting studies of RBC-cloaking and “self-signaling” DDSs warrant rigorous analysis of biocompatibility and toxicity.
Overall, RBC drug carriers present complex regulatory challenges. The regulatory challenges will be dictated by whether the materials are entirely synthetic, hybrid biologic/synthetic, or entirely cell-based. For entirely synthetic nanomaterials, these challenges have been reviewed elsewhere [164,193] and although they remain difficult and often costly, successful examples of clinically approved nanomaterials include liposomal formulations, protein-based nanoparticles, imaging agents, and device components [194]. For methods incorporating RBC elements into hybrid materials, the same standards can be expected to apply, with the additional consideration that they now contain biopharmaceuticals and, potentially, blood-derived elements that are subject to additional regulatory procedures. As part of the FDA, the Center for Biologics Evaluation and Research (CBER) regulates blood products and biological therapies [195] and provides guidance to investigators and manufacturers regarding these additional regulatory procedures.
Although the Center for Devices and Radiological Health (CDRH) regulates most medical devices, CBER continues to regulate those used with blood and cellular products, for example, the equipment expected to be used for the collection and processing of RBC products and other cellular therapies. Alteration of donor red blood cells (for example, leukoreduction, irradiation, and washing) is already practiced in the clinical setting through hospital blood banks. Such modifications lead to the designation of blood banks as “manufacturers” of drugs, and makes them subject to FDA licensure, registration, and regulation [196], including FDA inspection every two years. If ex vivo loading of red cells is to become widely used in the clinic, the resulting complex regulatory and compliance issues should be addressed early in the translational process for successful RBC-based carriers. Important practical considerations include the logistics, technique, and timing of cell collection, processing, and reinfusion. For example, peripheral blood stem cells for hematopoietic transplantation are usually harvested by apheresis and subsequently frozen before later reinfusion, while other cellular therapies such as chimeric antigen receptor T cells are generated on demand and reinfused upon successful manufacture. The techniques by which RBC-based delivery systems would be collected and manufactured will likely depend on their application and source. As one example, the EryDex system for RBCs loaded with dexamethasone utilizes a simple 50 mL peripheral blood draw that is then loaded using a proprietary RBC loading device [202,203].
Although red cell transfusion has long been successfully practiced clinically, cellular therapy using engineered cells, both autologous and allogeneic, represents a field still in its earliest clinical stages and with regulatory issues that remain to be fully defined and addressed [197–199]. Nonetheless, successful examples of cellular therapies that involve autologous cells manipulated ex vivo are growing in number [200,201]. As a potential alternative, methods permitting the in vivo loading of RBCs [14,42] are advantageous over those requiring ex vivo cellular loading and reinfusion in this respect, as the manufactured product (such as a recombinant protein) can be separately subjected to well-established quality control and manufacturing practices and likely faces better-defined regulatory hurdles.
7. Conclusion
Since the first exploratory studies four decades ago, red blood cell-based drug delivery systems have evolved into a diverse series of DDS platforms for drugs and nanocarriers loaded either inside or on the surface of this supercarrier, which profoundly changes the PK, biodistribution, clearance, and metabolism of the cargoes. In many (if not all) instances, RBC carriage also changes the activity, regulation, and pharmacodynamic characteristics of the cargoes, including their immunological and therapeutic features. Furthermore, naive RBCs circulating in the bloodstream influence behavior in vivo and interaction with target and non-target cells of carriers that do not (or at least are not supposed to) interact with RBCs. Using RBCs as “mother ship supercarriers” enables previously unrecognized options for transfer of nanocarriers to the vascular cells, in some cases, augmenting the efficacy of targeting of nanocarriers directed to cells of interest by affinity ligands. Both coupling to RBC and hybridization of RBC with synthetic components are being explored to combine affinity, geometry, plasticity, and features of nanocarriers with unique biocompatibility and transporting features of RBCs. New biotechnological techniques to produce modified RBCs, RBC carrier hybrids, and RBC carriers are currently undergoing transition from academic to industrial and clinical domains.
Footnotes
This review is part of the Advanced Drug Delivery Reviews theme issue on “Biologically-inspired drug delivery systems”.
Funding: National Institutes of Health R01 HL121134 and R01 HL125462.
References
- 1.Villa CH, et al. Delivery of drugs bound to erythrocytes: new avenues for an old intravascular carrier. Ther Deliv. 2015;6:795–826. doi: 10.4155/tde.15.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Godfrin Y, et al. International seminar on the red blood cells as vehicles for drugs. Expert Opin Biol Ther. 2012;12:127–133. doi: 10.1517/14712598.2012.631909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krantz A. Red cell-mediated therapy: opportunities and challenges. Blood Cells Mol Dis. 1997;23:58–68. doi: 10.1006/bcmd.1997.0119. [DOI] [PubMed] [Google Scholar]
- 4.Magnani M, et al. Erythrocyte engineering for drug delivery and targeting. Biotechnol Appl Biochem. 1998;28(Pt 1):1–6. [PubMed] [Google Scholar]
- 5.Muzykantov VR. Drug delivery by red blood cells: vascular carriers designed by mother nature. Expert Opin Drug Deliv. 2010;7:403–427. doi: 10.1517/17425241003610633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel PD, et al. Drug loaded erythrocytes: as novel drug delivery system. Curr Pharm Des. 2008;14:63–70. doi: 10.2174/138161208783330772. [DOI] [PubMed] [Google Scholar]
- 7.Hamidi M, Tajerzadeh H. Carrier erythrocytes: an overview. Drug Deliv. 2003;10:9–20. doi: 10.1080/713840329. [DOI] [PubMed] [Google Scholar]
- 8.Ganguly K, et al. Fibrin affinity of erythrocyte-coupled tissue-type plasminogen activators endures hemodynamic forces and enhances fibrinolysis in vivo. J Pharmacol Exp Ther. 2006;316(3):1130–1136. doi: 10.1124/jpet.105.093450. [DOI] [PubMed] [Google Scholar]
- 9.Murciano JC, et al. Soluble urokinase receptor conjugated to carrier red blood cells binds latent pro-urokinase and alters its functional profile. J Control Release. 2009;139(3):190–196. doi: 10.1016/j.jconrel.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murciano J-C, et al. Prophylactic fibrinolysis through selective dissolution of nascent clots by tPA-carrying erythrocytes. Nat Biotechnol. 2003;21(8):891–896. doi: 10.1038/nbt846. An early example of how RBC-coupled therapeutics (in this case, a tissue-type plasminogen activator) dramatically alters their pharmacokinetics and pharmacodynamics. [DOI] [PubMed] [Google Scholar]
- 11.He H, et al. Cell-penetrating peptides meditated encapsulation of protein therapeutics into intact red blood cells and its application. J Control Release. 2014;176:123–132. doi: 10.1016/j.jconrel.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Favretto ME, et al. Human erythrocytes as drug carriers: loading efficiency, side effects of hypotonic dialysis, hlorpromazine treatment and fusion with liposomes. J Control Release. 2013;170(3):343–351. doi: 10.1016/j.jconrel.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 13.Shi J, et al. Engineered red blood cells as carriers for systemic delivery of a wide array of functional probes. Proc Natl Acad Sci. 2014;111:10131–10136. doi: 10.1073/pnas.1409861111. This study demonstrated successful ex vivo production of genetically engineered red blood cells from hematopoietic progenitors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaitsev S, et al. Targeting recombinant thrombomodulin fusion protein to red blood cells provides multifaceted thromboprophylaxis. Blood. 2012;119(20):4779–4785. doi: 10.1182/blood-2011-12-398149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samokhin GP, et al. Red blood cell targeting to collagen-coated surfaces. FEBS Lett. 1983;154(2):257–261. doi: 10.1016/0014-5793(83)80160-4. [DOI] [PubMed] [Google Scholar]
- 16.Muzykantov VR, et al. Targeting of enzyme immobilized on erythrocyte membrane to collagen-coated surface. EBS Lett. 1985;182(1):62–66. doi: 10.1016/0014-5793(85)81154-6. [DOI] [PubMed] [Google Scholar]
- 17.Muzykantov VR, et al. Immunotargeting of erythrocyte-bound streptokinase provides local lysis of a fibrin clot. iochim Biophys Acta. 1986;884(2):355–362. doi: 10.1016/0304-4165(86)90184-4. [DOI] [PubMed] [Google Scholar]
- 18.Smirnov VN, et al. Carrier-directed targeting of liposomes and erythrocytes to denuded areas of vessel wall. Proc Natl Acad Sci U S A. 1986;83(17):6603–6607. doi: 10.1073/pnas.83.17.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muzykantov VR, et al. Directed targeting of immunoerythrocytes provides local protection of endothelial cells from damage by hydrogen peroxide. Am J Pathol. 1987;128(2):276–285. [PMC free article] [PubMed] [Google Scholar]
- 20.Glukhova MA, et al. Red blood cell targeting to smooth muscle cells. FEBS Lett. 1986;198(1):155–158. doi: 10.1016/0014-5793(86)81203-0. [DOI] [PubMed] [Google Scholar]
- 21.Magnani M, Rossi L. Approaches to erythrocyte-mediated drug delivery. Expert Opin Drug Deliv. 2014;11(5):677–687. doi: 10.1517/17425247.2014.889679. [DOI] [PubMed] [Google Scholar]
- 22.Mukthavaram R, et al. Targeting and depletion of circulating leukocytes and cancer cells by lipophilic antibody-modified erythrocytes. J Control Release. 2014;183:146–153. doi: 10.1016/j.jconrel.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi G, et al. Distearoyl anchor-painted erythrocytes with prolonged ligand retention and circulation properties in vivo. Adv Healthcare Mater. 2014;3(1):142–148. doi: 10.1002/adhm.201300084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armstead WM, et al. Signaling, delivery and age as emerging issues in the benefit/risk ratio outcome of tPA for treatment of CNS ischemic disorders. J Neurochem. 2010;113(2):303–312. doi: 10.1111/j.1471-4159.2010.06613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Danielyan K, et al. Cerebrovascular thromboprophylaxis in mice by erythrocyte-coupled tissue-type plasminogen activator. Circulation. 2008;118(14):1442–1449. doi: 10.1161/CIRCULATIONAHA.107.750257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stein SC, et al. Erythrocyte-bound tissue plasminogen activator is neuroprotective in experimental traumatic brain injury. J Neurotrauma. 2009;26(9):1585–1592. doi: 10.1089/neu.2008.0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armstead WM, et al. Red blood cell-coupled tissue plasminogen activator prevents impairment of cerebral vasodilatory responses through inhibition of c-Jun-N-terminal kinase and potentiation of p38 mitogen-activated protein kinase after cerebral photothrombosis in the newborn pig. Pediatr Crit Care Med. 2011;12(6):e369–e375. doi: 10.1097/PCC.0b013e3181fe40a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pisapia JM, et al. Microthrombosis after experimental subarachnoid hemorrhage: time course and effect of red blood cell-bound thrombin-activated pro-urokinase and clazosentan. Exp Neurol. 2012;233(1):357–363. doi: 10.1016/j.expneurol.2011.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Armstead WM, et al. Red blood cells-coupled tPA prevents impairment of cerebral vasodilatory responses and tissue injury in pediatric cerebral hypoxia/ischemia through inhibition of ERK MAPK activation. J Cereb Blood Flow Metab. 2009;29(8):1463–1474. doi: 10.1038/jcbfm.2009.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atukorale PU, et al. Influence of the glycocalyx and plasma membrane composition on amphiphilic gold nanoparticle association with erythrocytes. Nanoscale. 2015;7(26):11420–11432. doi: 10.1039/c5nr01355k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ganguly K, et al. The glycocalyx protects erythrocyte-bound tissue-type plasmin-ogen activator from enzymatic inhibition. J Pharmacol Exp Ther. 2007;321(1):158–164. doi: 10.1124/jpet.106.114405. [DOI] [PubMed] [Google Scholar]
- 32.Gersh KC, et al. The spatial dynamics of fibrin clot dissolution catalyzed by erythrocyte-bound vs. free fibrinolytics. J Thromb Haemost. 2010;8(5):1066–1074. doi: 10.1111/j.1538-7836.2010.03802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gersh KC, et al. Flow-dependent channel formation in clots by an erythrocyte-bound fibrinolytic agent. Blood. 2011;117(18):4964–4967. doi: 10.1182/blood-2010-10-310409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryder AB, Zimring JC, Hendrickson JE. Factors influencing RBC alloimmunization: lessons learned from murine models. Transfus Med Hemother. 2014;41:406–419. doi: 10.1159/000368995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hendrickson JE, Tormey CA, Shaz BH. Red blood cell alloimmunization mitigation strategies. Transfus Med Rev. 2014;28:137–144. doi: 10.1016/j.tmrv.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Heddle NM, et al. A prospective study to determine the frequency and clinical significance of alloimmunization post-transfusion. Br J Haematol. 1995;91:1000–1005. doi: 10.1111/j.1365-2141.1995.tb05425.x. [DOI] [PubMed] [Google Scholar]
- 37.Chou ST, et al. High prevalence of red blood cell alloimmunization in sickle cell disease despite transfusion from Rh-matched minority donors. Blood. 2013;122:1062–1071. doi: 10.1182/blood-2013-03-490623. [DOI] [PubMed] [Google Scholar]
- 38.Stowell SR, et al. Transfusion of murine red blood cells expressing the human KEL glycoprotein induces clinically significant alloantibodies. Transfusion. 2014;54:179–189. doi: 10.1111/trf.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith NH, et al. Generation of transgenic mice with antithetical KEL1 and KEL2 human blood group antigens on red blood cells. Transfusion. 2012;52:2620–2630. doi: 10.1111/j.1537-2995.2012.03641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Girard-Pierce KR, et al. A novel role for C3 in antibody-induced red blood cell clearance and antigen modulation. lood. 2013;122:1793–1801. doi: 10.1182/blood-2013-06-508952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liepkalns JS, et al. Resistance of a subset of red blood cells to clearance by antibodies in a mouse model of incompatible transfusion. Transfusion. 2013;53:1319–1327. doi: 10.1111/j.1537-2995.2012.03910.x. [DOI] [PubMed] [Google Scholar]
- 42.Kontos S, et al. Engineering antigens for in situ erythrocyte binding induces T-cell deletion. Proc Natl Acad Sci. 2013;110:17–18. doi: 10.1073/pnas.1216353110. This study demonstrated a tolerogenic effect of RBC coupling that has significant implications on their use as delivery vehicles for biologic agents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lorentz KM, et al. Engineered binding to erythrocytes induces immunological tolerance to E. coli asparaginase. Sci Adv. 2015;1:e1500112. doi: 10.1126/sciadv.1500112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kravtzoff R, et al. Tolerance evaluation of L-asparaginase loaded in red blood cells. Eur J Clin Pharmacol. 1996;51:221–225. doi: 10.1007/s002280050187. [DOI] [PubMed] [Google Scholar]
- 45.Kravtzoff R, et al. Improved pharmacodynamics of L-asparaginase-loaded in human red blood cells. Eur J Clin Pharmacol. 1996;49:465–470. doi: 10.1007/BF00195932. [DOI] [PubMed] [Google Scholar]
- 46.Kravtzoff R, et al. Erythrocytes as carriers for L-asparaginase. Methodological and mouse in-vivo studies. J Pharm Pharmacol. 1990;42:473–476. doi: 10.1111/j.2042-7158.1990.tb06598.x. [DOI] [PubMed] [Google Scholar]
- 47.Panosyan EH, et al. Asparaginase antibody and asparaginase activity in children with higher-risk acute lymphoblastic leukemia: Children’s Cancer Group Study CCG-1961. J Pediatr Hematol Oncol. 2004;26:217–226. doi: 10.1097/00043426-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 48.Nechansky A, Kircheis R. Immunogenicity of therapeutics: a matter of efficacy and safety. Expert Opin Drug Discovery. 2010;5:1067–1079. doi: 10.1517/17460441.2010.514326. [DOI] [PubMed] [Google Scholar]
- 49.Kishnani PS, et al. Immune response to enzyme replacement therapies in lyso-somal storage diseases and the role of immune tolerance induction. Mol Genet Metab. 2016;117(2):68–83. doi: 10.1016/j.ymgme.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 50.Pickens B, et al. Platelet-delivered ADAMTS13 inhibits arterial thrombosis and prevents thrombotic thrombocytopenic purpura in murine models. Blood. 2015;125:3326–3334. doi: 10.1182/blood-2014-07-587139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maldonado RA, et al. Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc Natl Acad Sci. 2015;112:E156–E165. doi: 10.1073/pnas.1408686111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Georgescu MT, et al. War and peace: factor VIII and the adaptive immune response. Cell Immunol. 2016;301:2–7. doi: 10.1016/j.cellimm.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 53.Giarratana M-C, et al. Ex vivo generation of fully mature human red blood cells from hematopoietic stem cells. Nat Biotechnol. 2005;23:69–74. doi: 10.1038/nbt1047. [DOI] [PubMed] [Google Scholar]
- 54.Anstee DJ, Gampel A, Toye AM. Ex-vivo generation of human red cells for transfusion. Curr Opin Hematol. 2012;19:163–169. doi: 10.1097/MOH.0b013e328352240a. [DOI] [PubMed] [Google Scholar]
- 55.Huang X, et al. Extensive ex vivo expansion of functional human erythroid precursors established from umbilical cord blood cells by defined factors. ol Ther. 2014;22:451–463. doi: 10.1038/mt.2013.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Giarratana M-C, et al. Proof of principle for transfusion of in vitro-generated red blood cells. Blood. 2011;118:5071–5079. doi: 10.1182/blood-2011-06-362038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maruyama T, et al. Increasing the efficiency of precise genome editing with CRISPR-Cas9 by inhibition of nonhomologous end joining. Nat Biotechnol. 2015;33:538–542. doi: 10.1038/nbt.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sander JD, Joung JK. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol. 2014;32:347–355. doi: 10.1038/nbt.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Urnov FD, et al. Genome editing with engineered zinc finger nucleases. Nat Rev Genet. 2010;11:636–646. doi: 10.1038/nrg2842. [DOI] [PubMed] [Google Scholar]
- 60.Reid ME, Mohandas N. Red blood cell blood group antigens: structure and function. Semin Hematol. 2004;41:93–117. doi: 10.1053/j.seminhematol.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 61.Reid ME, Lomas-Francis C, Olsson ML. The Blood Group Antigen FactsBook. Elsevier/Academic Press; Amsterdam: 2012. [Google Scholar]
- 62.Mohandas N, Gallagher PG. Red cell membrane: past, present, and future. Blood. 2008;112:3939–3948. doi: 10.1182/blood-2008-07-161166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sosa JM, et al. The relationship between red blood cell deformability metrics and perfusion of an artificial microvascular network. Clin Hemorheol Microcirc. 2013;57(3):275–289. doi: 10.3233/CH-131719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.An X, Mohandas N. Disorders of red cell membrane. Br J Haematol. 2008;141:367–375. doi: 10.1111/j.1365-2141.2008.07091.x. [DOI] [PubMed] [Google Scholar]
- 65.Paulitschke M, et al. Perturbation of red blood cell membrane rigidity by extracellular ligands. Blood. 1995;86:342–348. [PubMed] [Google Scholar]
- 66.Pasvol G, et al. Inhibition of malarial parasite invasion by monoclonal antibodies against glycophorin A correlates with reduction in red cell membrane deformability. Blood. 1989;74:1836–1843. [PubMed] [Google Scholar]
- 67.Simmonds MJ, et al. Assessment of oxidant susceptibility of red blood cells in various species based on cell deformability. Biorheology. 2011;48:293–304. doi: 10.3233/BIR-2012-0599. [DOI] [PubMed] [Google Scholar]
- 68.Kaul DK, et al. Additive effect of red blood cell rigidity and adherence to endothelial cells in inducing vascular resistance. Am J Physiol Heart Circ Physiol. 2008;295:H1788–H1793. doi: 10.1152/ajpheart.253.2008. [DOI] [PubMed] [Google Scholar]
- 69.Aarts PA, Heethaar RM, Sixma JJ. Red blood cell deformability influences platelets–vessel wall interaction in flowing blood. Blood. 1984;64:1228–1233. [PubMed] [Google Scholar]
- 70.Hess C, Schifferli JA. Immune adherence revisited: novel players in an old game. News Physiol Sci. 2003;18:104–108. doi: 10.1152/nips.01425.2002. [DOI] [PubMed] [Google Scholar]
- 71.Fearon DT. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leukocyte, B lymphocyte and monocyte. J Exp Med. 1980;152:20–30. doi: 10.1084/jem.152.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lindorfer MA, et al. Heteropolymer-mediated clearance of immune complexes via erythrocyte CR1: mechanisms and applications. Immunol Rev. 2001;183:10–24. doi: 10.1034/j.1600-065x.2001.1830102.x. [DOI] [PubMed] [Google Scholar]
- 73.Taylor RP, et al. Use of heteropolymeric monoclonal antibodies to attach antigens to the C3b receptor of human erythrocytes: a potential therapeutic treatment. Proc Natl Acad Sci U S A. 1991;88:3305–3309. doi: 10.1073/pnas.88.8.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reinagel ML, et al. The primate erythrocyte complement receptor (CR1) as a privileged site: binding of immunoglobulin G to erythrocyte CR1 does not target erythrocytes for phagocytosis. Blood. 1997;89:1068–1077. [PubMed] [Google Scholar]
- 75.Craig ML, Bankovich AJ, Taylor RP. Visualization of the transfer reaction: tracking immune complexes from erythrocyte complement receptor 1 to macrophages. Clin Immunol. 2002;105:36–47. doi: 10.1006/clim.2002.5266. [DOI] [PubMed] [Google Scholar]
- 76.Craig ML, Waitumbi JN, Taylor RP. Processing of C3b-opsonized immune complexes bound to non-complement receptor 1 (CR1) sites on red cells: phagocytosis, transfer and associations with CR1. J Immunol. 2005;174:3059–3066. doi: 10.4049/jimmunol.174.5.3059. [DOI] [PubMed] [Google Scholar]
- 77.Ferguson PJ, et al. Antigen-based heteropolymers. A potential therapy for binding and clearing autoantibodies via erythrocyte CR1. Arthritis Rheum. 1995;38:190–200. doi: 10.1002/art.1780380207. [DOI] [PubMed] [Google Scholar]
- 78.Kuhn SE, et al. Escherichia coli bound to the primate erythrocyte complement receptor via bispecific monoclonal antibodies are transferred to and phagocytosed by human monocytes in an in vitro model. J Immunol. 1998;160:5088–5097. [PubMed] [Google Scholar]
- 79.Lindorfer MA, et al. Targeting of Pseudomonas aeruginosa in the bloodstream with bispecific monoclonal antibodies. J Immunol. 2001;167:2240–2249. doi: 10.4049/jimmunol.167.4.2240. [DOI] [PubMed] [Google Scholar]
- 80.Reist CJ, et al. Cross-linked bispecific monoclonal antibody heteropolymers facilitate the clearance of human IgM from the circulation of squirrel monkeys. Eur J Immunol. 1994;24:2018–2025. doi: 10.1002/eji.1830240913. [DOI] [PubMed] [Google Scholar]
- 81.Asher DR, Cerny AM, Finberg RW. The erythrocyte viral trap: transgenic expression of viral receptor on erythrocytes attenuates coxsackievirus B infection. Proc Natl Acad Sci U S A. 2005;102:12897–12902. doi: 10.1073/pnas.0506211102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hahn CS, et al. Bispecific monoclonal antibodies mediate binding of dengue virus to erythrocytes in a monkey model of passive viremia. J Immunol. 2001;166:1057–1065. doi: 10.4049/jimmunol.166.2.1057. [DOI] [PubMed] [Google Scholar]
- 83.Zaitsev S, et al. Human complement receptor type 1-directed loading of tissue plasminogen activator on circulating erythrocytes for prophylactic fibrinolysis. Blood. 2006;108:1895–1902. doi: 10.1182/blood-2005-11-012336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moulds JM. The Knops blood-group system: a review. Immunohematology. 2010;26(1):2–7. [PubMed] [Google Scholar]
- 85.Tanner MJ. The structure and function of band 3 (AE1): recent developments (review) Mol Membr Biol. 1997;14:155–165. doi: 10.3109/09687689709048178. [DOI] [PubMed] [Google Scholar]
- 86.Peters LL, et al. Anion exchanger 1 (band 3) is required to prevent erythrocyte membrane surface loss but not to form the membrane skeleton. Cell. 1996;86:917–927. doi: 10.1016/s0092-8674(00)80167-1. [DOI] [PubMed] [Google Scholar]
- 87.Figueroa D. The Diego blood group system: a review. Immunohematology. 2013;29:73–81. [PubMed] [Google Scholar]
- 88.Reid ME. MNS blood group system: a review. Immunohematology. 2009;25:95–101. [PubMed] [Google Scholar]
- 89.Zaitsev S, et al. Sustained thromboprophylaxis mediated by an RBC-targeted pro-urokinase zymogen activated at the site of clot formation. Blood. 2010;115:5241–5248. doi: 10.1182/blood-2010-01-261610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zaitsev S, et al. Targeting of a mutant plasminogen activator to circulating red blood cells for prophylactic fibrinolysis. J Pharmacol Exp Ther. 2010;332:1022–1031. doi: 10.1124/jpet.109.159194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Parsons SF, Gardner B, Anstee DJ. Monoclonal antibodies against Kell glycoprotein: serology, immunochemistry and quantification of antigen sites. Transfus Med. 1993;3:137–142. doi: 10.1111/j.1365-3148.1993.tb00051.x. [DOI] [PubMed] [Google Scholar]
- 92.Jung HH, Danek A, Frey BM. McLeod syndrome: a neurohaematological disorder: McLeod syndrome. Vox Sang. 2007;93:112–121. doi: 10.1111/j.1423-0410.2007.00949.x. [DOI] [PubMed] [Google Scholar]
- 93.Gubin AN, et al. Identification of the Dombrock blood group glycoprotein as a polymorphic member of the ADP-ribosyltransferase gene family. Blood. 2000;96(7):2621–2627. [PubMed] [Google Scholar]
- 94.Lomas-Francis C, Reid ME. The Dombrock blood group system: a review. Immunohematology. 2010;26:71–78. [PubMed] [Google Scholar]
- 95.Grandstaff Moulds MK. The LW blood group system: a review. Immunohematology. 2011;27(4):136–142. [PubMed] [Google Scholar]
- 96.Zennadi R, et al. Epinephrine-induced activation of LW-mediated sickle cell adhesion and vaso-occlusion in vivo. Blood. 2007;110:2708–2717. doi: 10.1182/blood-2006-11-056101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Anliker M, et al. A new blood group antigen is defined by anti-CD59, detected in a CD59-deficient patient. ransfusion. 2014;54(7):1817–1822. doi: 10.1111/trf.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hill A, et al. Protection of erythrocytes from human complement-mediated lysis by membrane-targeted recombinant soluble CD59: a new approach to PNH therapy. Blood. 2006;107:2131–2137. doi: 10.1182/blood-2005-02-0782. [DOI] [PubMed] [Google Scholar]
- 99.Montel-Hagen A, et al. The Glut1 and Glut4 glucose transporters are differentially expressed during perinatal and postnatal erythropoiesis. Blood. 2008;112:4729–4738. doi: 10.1182/blood-2008-05-159269. [DOI] [PubMed] [Google Scholar]
- 100.Avent ND, Reid ME. The Rh blood group system: a review. Blood. 2000;95:375–387. [PubMed] [Google Scholar]
- 101.Chou ST, Westhoff CM. The Rh and RhAG blood group systems. Immunohematology. 2010;26:178–186. [PubMed] [Google Scholar]
- 102.Gruswitz F, et al. Function of human Rh based on structure of RhCG at 2.1 A. Proc Natl Acad Sci U S A. 2010;107:9638–9643. doi: 10.1073/pnas.1003587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Spitzer D, et al. ScFv-mediated in vivo targeting of DAF to erythrocytes inhibits lysis by complement. Mol Immunol. 2004;40(13):911–919. doi: 10.1016/j.molimm.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 104.Libyh MT, et al. A recombinant human scFv anti-Rh(D) antibody with multiple valences using a C-terminal fragment of C4-binding protein. Blood. 1997;90(10):3978–3983. [PubMed] [Google Scholar]
- 105.Oudin S, et al. A soluble recombinant multimeric anti-Rh(D) single-chain Fv/CR1 molecule restores the immune complex binding ability of CR1-deficient erythrocytes. J Immunol. 2000;164(3):1505–1513. doi: 10.4049/jimmunol.164.3.1505. [DOI] [PubMed] [Google Scholar]
- 106.Hall SS, Mitragotri S, Daugherty PS. Identification of peptide ligands facilitating nanoparticle attachment to erythrocytes. Biotechnol Prog. 2007;23(3):749–754. doi: 10.1021/bp060333l. [DOI] [PubMed] [Google Scholar]
- 107.Kontos S, Hubbell JA. Improving protein pharmacokinetics by engineering erythrocyte affinity. Mol Pharm. 2010;7(6):2141–2147. doi: 10.1021/mp1001697. [DOI] [PubMed] [Google Scholar]
- 108.Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9(8):615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 109.Byrne JD, Betancourt T, Brannon-Peppas L. Active targeting schemes for nano-particle systems in cancer therapeutics. Adv Drug Deliv Rev. 2008;60(15):1615–1626. doi: 10.1016/j.addr.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 110.Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12(11):991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 111.Albanese A, Tang PS, Chan WC. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Eng. 2012;14:1–16. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- 112.Myerson JW, et al. Non-affinity factors modulating vascular targeting of nano-and microcarriers. Adv Drug Deliv Rev. 2016;99:97–112. doi: 10.1016/j.addr.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tan J, Thomas A, Liu Y. Influence of red blood cells on nanoparticle targeted delivery in microcirculation. Soft Matter. 2012;8(6):1934–1946. doi: 10.1039/C2SM06391C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kumar A, Graham MD. Mechanism of margination in confined flows of blood and other multicomponent suspensions. hys Rev Lett. 2012;109(10):108102. doi: 10.1103/PhysRevLett.109.108102. [DOI] [PubMed] [Google Scholar]
- 115.Gentile F, et al. The margination propensity of spherical particles for vascular targeting in the microcirculation. J Nanobiotechnol. 2008;6(1):9. doi: 10.1186/1477-3155-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Samokhin G, et al. Effect of flow rate and blood cellular elements on the efficiency of red blood cell targeting to collagen-coated surfaces. J Appl Biochem. 1983;6(1–2):70–75. [PubMed] [Google Scholar]
- 117.Zhang J, Johnson PC, Popel AS. Effects of erythrocyte deformability and aggregation on the cell free layer and apparent viscosity of microscopic blood flows. Microvasc Res. 2009;77(3):265–272. doi: 10.1016/j.mvr.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Toy R, et al. The effects of particle size, density and shape on margination of nanoparticles in microcirculation. anotechnology. 2011;22(11):115101. doi: 10.1088/0957-4484/22/11/115101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tan J, et al. The influence of size, shape and vessel geometry on nanoparticle distribution. Microfluid Nanofluid. 2013;14(1–2):77–87. doi: 10.1007/s10404-012-1024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Muller K, Fedosov DA, Gompper G. Margination of micro- and nano-particles in blood flow and its effect on drug delivery. Sci Rep. 2014;4:4871. doi: 10.1038/srep04871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vahidkhah K, Bagchi P. Microparticle shape effects on margination, near-wall dynamics and adhesion in a three-dimensional simulation of red blood cell suspension. Soft Matter. 2015;11(11):2097–2109. doi: 10.1039/c4sm02686a. [DOI] [PubMed] [Google Scholar]
- 122.Thompson AJ, Mastria EM, Eniola-Adefeso O. The margination propensity of ellipsoidal micro/nanoparticles to the endothelium in human blood flow. Biomaterials. 2013;34(23):5863–5871. doi: 10.1016/j.biomaterials.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 123.D’Apolito R, et al. Red blood cells affect the margination of microparticles in synthetic microcapillaries and intravital microcirculation as a function of their size and shape. J Control Release. 2015;217:263–272. doi: 10.1016/j.jconrel.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 124.Anselmo AC, et al. Platelet-like nanoparticles: mimicking shape, flexibility and surface biology of platelets to target vascular injuries. ACS Nano. 2014;8(11):11243–11253. doi: 10.1021/nn503732m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee T-R, et al. On the near-wall accumulation of injectable particles in the microcirculation: smaller is not better. ci Rep. 2013;3 doi: 10.1038/srep02079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Thomas A, Tan J, Liu Y. Characterization of nanoparticle delivery in microcirculation using a microfluidic device. icrovasc Res. 2014;94:17–27. doi: 10.1016/j.mvr.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Charoenphol P, Huang RB, Eniola-Adefeso O. Potential role of size and hemodynamics in the efficacy of vascular-targeted spherical drug carriers. Biomaterials. 2010;31(6):1392–1402. doi: 10.1016/j.biomaterials.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 128.Namdee K, et al. Margination propensity of vascular-targeted spheres from blood flow in a microfluidic model of human microvessels. Langmuir. 2013;29(8):2530–2535. doi: 10.1021/la304746p. [DOI] [PubMed] [Google Scholar]
- 129.Calderon AJ, et al. Optimizing endothelial targeting by modulating the antibody density and particle concentration of anti-ICAM coated carriers. J Control Release. 2011;150(1):37–44. doi: 10.1016/j.jconrel.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Calderon AJ, et al. Flow dynamics, binding and detachment of spherical carriers targeted to ICAM-1 on endothelial cells. Biorheology. 2009;46(4):323–341. doi: 10.3233/BIR-2009-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Calderon AJ, et al. Effect of glycocalyx on drug delivery carriers targeted to endothelial cells. Int J Transp Phenom. 2011;12(1–2):63. [PMC free article] [PubMed] [Google Scholar]
- 132.Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng. 2007;9:121–167. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- 133.Mulivor AW, Lipowsky HH. Role of glycocalyx in leukocyte-endothelial cell adhesion. Am J Physiol Heart Circ Physiol. 2002;283(4):H1282–H1291. doi: 10.1152/ajpheart.00117.2002. [DOI] [PubMed] [Google Scholar]
- 134.Favretto ME, et al. Glycosaminoglycans in the cellular uptake of drug delivery vectors—bystanders or active players? J Control Release. 2014;180:81–90. doi: 10.1016/j.jconrel.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 135.Greineder CF, et al. Vascular Immunotargeting to Endothelial Determinant ICAM-1 Enables Optimal Partnering of Recombinant scFv-Thrombomodulin Fusion with Endogenous Cofactor. 2013 doi: 10.1371/journal.pone.0080110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Peeters PA, et al. Immunospecific targeting of immunoliposomes, F(ab′)2 and IgG to red blood cells in vivo. Biochim Biophys Acta. 1988;943(2):137–147. doi: 10.1016/0005-2736(88)90545-7. [DOI] [PubMed] [Google Scholar]
- 137.Moles E, et al. Immunoliposome-mediated drug delivery to Plasmodium-infected and non-infected red blood cells as a dual therapeutic/prophylactic antimalarial strategy. J Control Release. 2015;210:217–229. doi: 10.1016/j.jconrel.2015.05.284. [DOI] [PubMed] [Google Scholar]
- 138.Marques J, et al. Application of heparin as a dual agent with antimalarial and liposome targeting activities toward plasmodium-infected red blood cells. Nanomedicine. 2014;10(8):1719–1728. doi: 10.1016/j.nano.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 139.Hamidi M, et al. Encapsulation of valproate-loaded hydrogel nanoparticles in intact human erythrocytes: a novel nano-cell composite for drug delivery. J Pharm Sci. 2011;100(5):1702–1711. doi: 10.1002/jps.22395. [DOI] [PubMed] [Google Scholar]
- 140.Staedtke V, et al. In vitro inhibition of fungal activity by macrophage-mediated sequestration and release of encapsulated amphotericin B nanosupension in red blood cells. Small. 2010;6(1):96–103. doi: 10.1002/smll.200900919. [DOI] [PubMed] [Google Scholar]
- 141.Delcea M, et al. Nanoplasmonics for dual-molecule release through nanopores in the membrane of red blood cells. CS Nano. 2012;6(5):4169–4180. doi: 10.1021/nn3006619. [DOI] [PubMed] [Google Scholar]
- 142.Wu Z, et al. Turning erythrocytes into functional micromotors. ACS Nano. 2014;8(12):12041–12048. doi: 10.1021/nn506200x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Antonelli A, Magnani M. Red blood cells as carriers of iron oxide-based contrast agents for diagnostic applications. Biomed Nanotechnol. 2014;10(9):1732–1750. doi: 10.1166/jbn.2014.1916. [DOI] [PubMed] [Google Scholar]
- 144.Sternberg N, et al. Surface-modified loaded human red blood cells for targeting and delivery of drugs. J Microencapsul. 2012;29(1):9–20. doi: 10.3109/02652048.2011.629741. [DOI] [PubMed] [Google Scholar]
- 145.Anselmo AC, Mitragotri S. Cell-mediated delivery of nanoparticles: taking advantage of circulatory cells to target nanoparticles. J Control Release. 2014;190:531–541. doi: 10.1016/j.jconrel.2014.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Batrakova EV, Gendelman HE, Kabanov AV. Cell-mediated drug delivery. Expert Opin Drug Deliv. 2011;8(4):415–433. doi: 10.1517/17425247.2011.559457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chambers E, Mitragotri S. Long circulating nanoparticles via adhesion on red blood cells: mechanism and extended circulation. Exp Biol Med (Maywood) 2007;232(7):958–966. [PubMed] [Google Scholar]
- 148.Mai TD, et al. Red blood cells decorated with functionalized core-shell magnetic nanoparticles: elucidation of the adsorption mechanism. Chem Commun (Camb) 2013;49(47):5393–5395. doi: 10.1039/c3cc41513a. [DOI] [PubMed] [Google Scholar]
- 149.Zhao Y, et al. Interaction of mesoporous silica nanoparticles with human red blood cell membranes: size and surface effects. ACS Nano. 2011;5(2):1366–1375. doi: 10.1021/nn103077k. [DOI] [PubMed] [Google Scholar]
- 150.Stephan MT, Irvine DJ. Enhancing cell therapies from the outside in: cell surface engineering using synthetic nanomaterials. Nano Today. 2011;6(3):309–325. doi: 10.1016/j.nantod.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang C, et al. Multifunctional theranostic red blood cells for magnetic-field-enhanced in vivo combination therapy of cancer. Adv Mater. 2014;26(28):4794–4802. doi: 10.1002/adma.201400158. [DOI] [PubMed] [Google Scholar]
- 152.Muzykantov VR, et al. Regulation of the complement-mediated elimination of red blood cells modified with biotin and streptavidin. Anal Biochem. 1996;241(1):109–119. doi: 10.1006/abio.1996.0384. [DOI] [PubMed] [Google Scholar]
- 153.Zaltzman AB, et al. Enhanced complement susceptibility of avidin-biotin-treated human erythrocytes is a consequence of neutralization of the complement regulators CD59 and decay accelerating factor. Biochem J. 1995;307(Pt 3):651–656. doi: 10.1042/bj3070651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Muzykantov VR, Taylor RP. Attachment of biotinylated antibody to red blood cells: antigen-binding capacity of immunoerythrocytes and their susceptibility to lysis by complement. Anal Biochem. 1994;223(1):142–148. doi: 10.1006/abio.1994.1559. [DOI] [PubMed] [Google Scholar]
- 155.Antonelli A, et al. Encapsulation of superparamagnetic nanoparticles into red blood cells as new carriers of MRI contrast agents. Nanomedicine. 2011;6(2):211–223. doi: 10.2217/nnm.10.163. [DOI] [PubMed] [Google Scholar]
- 156.Ahn S, et al. Gold nanoparticle-incorporated human red blood cells (RBCs) for X-ray dynamic imaging. Biomaterials. 2011;32(29):7191–7199. doi: 10.1016/j.biomaterials.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 157.Chambers E, Mitragotri S. Prolonged circulation of large polymeric nanoparticles by non-covalent adsorption on erythrocytes. J Control Release. 2004;100(1):111–119. doi: 10.1016/j.jconrel.2004.08.005. This paper showed how nanoparticles attached, or hitchhiked, to the surface of RBCs that are capable of circulating significantly longer than non-attached nanoparticles. [DOI] [PubMed] [Google Scholar]
- 158.Anselmo AC, et al. Delivering nanoparticles to lungs while avoiding liver and spleen through adsorption on red blood cells. ACS Nano. 2013;7(12):11129–11137. doi: 10.1021/nn404853z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Anselmo AC, et al. Exploiting shape, cellular-hitchhiking, antibodies to target nanoparticles to lung endothelium: synergy between physical, chemical and biological approaches. Biomaterials. 2015;68:1–8. doi: 10.1016/j.biomaterials.2015.07.043. [DOI] [PubMed] [Google Scholar]
- 160.Kolhar P, et al. Using shape effects to target antibody-coated nanoparticles to lung and brain endothelium. Proc Natl Acad Sci. 2013;110(26):10753–10758. doi: 10.1073/pnas.1308345110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Laurencin M, et al. Human erythrocytes covered with magnetic core-shell nano-particles for multimodal imaging. dv Healthcare Mater. 2013;2(9):1209–1212. doi: 10.1002/adhm.201200384. [DOI] [PubMed] [Google Scholar]
- 162.Antonelli A, et al. New biomimetic constructs for improved in vivo circulation of superparamagnetic nanoparticles. J Nanosci Nanotechnol. 2008;8(5):2270–2278. doi: 10.1166/jnn.2008.190. [DOI] [PubMed] [Google Scholar]
- 163.Orekhova N, et al. Local prevention of trombosis in animal arteries by means of magnetic targeting of aspirin-loaded red cells. Thromb Res. 1990;57(4):611–616. doi: 10.1016/0049-3848(90)90078-q. [DOI] [PubMed] [Google Scholar]
- 164.Anselmo AC, Mitragotri S. A review of clinical translation of inorganic nanoparticles. AAPS J. 2015;17(5):1041–1054. doi: 10.1208/s12248-015-9780-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yoo J-W, Chambers E, Mitragotri S. Factors that control the circulation time of nanoparticles in blood: challenges, solutions and future prospects. Curr Pharm Des. 2010;16(21):2298–2307. doi: 10.2174/138161210791920496. [DOI] [PubMed] [Google Scholar]
- 166.Yoo JW, et al. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat Rev Drug Discov. 2011;10(7):521–535. doi: 10.1038/nrd3499. [DOI] [PubMed] [Google Scholar]
- 167.Doshi N, et al. Red blood cell-mimicking synthetic biomaterial particles. Proc Natl Acad Sci. 2009;106(51):21495–21499. doi: 10.1073/pnas.0907127106. This paper provided a method to synthesize synthetic RBCs from relevant biomolecules (e.g. natural RBC proteins) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Merkel TJ, et al. Using mechanobiological mimicry of red blood cells to extend circulation times of hydrogel microparticles. Proc Natl Acad Sci. 2011;108(2):586–591. doi: 10.1073/pnas.1010013108. This paper highlighted how RBC-shaped particles exhibiting elasticities comparable to natural RBCs circulate longer than RBC-shaped particles of non-natural elasticities. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rodriguez PL, et al. Minimal “self” peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science. 2013;339(6122):971–975. doi: 10.1126/science.1229568. This paper highlights how particles decorated with “self” peptides from RBCs are capable of decreasing immune system uptake, extending circulation times, and increasing tumor accumulation, even when pre-opsonized, as compared to non-decorated particles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hu C-MJ, et al. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. roc Natl Acad Sci. 2011;108(27):10980–10985. doi: 10.1073/pnas.1106634108. This paper is a recent example of utilizing RBC membranes coated onto nanoparticles to transfer natural RBC properties to nanoparticles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Modery-Pawlowski CL, et al. Synthetic approaches to RBC mimicry and oxygen carrier systems. Biomacromolecules. 2013;14(4):939–948. doi: 10.1021/bm400074t. [DOI] [PubMed] [Google Scholar]
- 172.Myhre BA. The first recorded blood transfusions: 1656 to 1668. Transfusion. 1990;30(4):358–362. doi: 10.1046/j.1537-2995.1990.30490273445.x. [DOI] [PubMed] [Google Scholar]
- 173.Wilson K, Code C, Ricketts MN. Risk of acquiring Creutzfeldt–Jakob disease from blood transfusions: systematic review of case–control studies. BMJ. 2000;321(7252):17–19. doi: 10.1136/bmj.321.7252.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Merkel TJ, et al. The effect of particle size on the biodistribution of low-modulus hydrogel PRINT particles. J Control Release. 2012;162(1):37–44. doi: 10.1016/j.jconrel.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chen K, et al. Low modulus biomimetic microgel particles with high loading of hemoglobin. Biomacromolecules. 2012;13(9):2748–2759. doi: 10.1021/bm3007242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chen K, et al. Design of asymmetric particles containing a charged interior and a neutral surface charge: comparative study on in vivo circulation of polyelectrolyte microgels. J Am Chem Soc. 2014;136(28):9947–9952. doi: 10.1021/ja503939n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Luo R, et al. Engineering of erythrocyte-based drug carriers: control of protein release and bioactivity. J Mater Sci Mater Med. 2012;23(1):63–71. doi: 10.1007/s10856-011-4485-2. [DOI] [PubMed] [Google Scholar]
- 178.Haghgooie R, Toner M, Doyle PS. Squishy non-spherical hydrogel microparticles. Macromol Rapid Commun. 2010;31(2):128–134. doi: 10.1002/marc.200900302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Moorjani M, et al. Nanoerythrosomes, a new derivative of erythrocyte ghost II: identification of the mechanism of action. Anticancer Res. 1996;16(5 A):2831–2836. [PubMed] [Google Scholar]
- 180.Lejeune A, et al. Nanoerythrosome, a new derivative of erythrocyte ghost: preparation and antineoplastic potential as drug carrier for daunorubicin. Anticancer Res. 1994;14(3 A):915–919. [PubMed] [Google Scholar]
- 181.Desilets J, et al. Nanoerythrosomes, a new derivative of erythrocyte ghost: IV. Fate of reinjected nanoerythrosomes. Anticancer Res. 2001;21(3B):1741–1747. [PubMed] [Google Scholar]
- 182.Agnihotri J, et al. Development and evaluation of anti-malarial bio-conjugates: artesunate-loaded nanoerythrosomes. Drug Deliv Transl Res. 2015;5(5):489–497. doi: 10.1007/s13346-015-0246-y. [DOI] [PubMed] [Google Scholar]
- 183.Fang RH, et al. Lipid-insertion enables targeting functionalization of erythrocyte membrane-cloaked nanoparticles. anoscale. 2013;5(19):8884–8888. doi: 10.1039/c3nr03064d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Aryal S, et al. Erythrocyte membrane-cloaked polymeric nanoparticles for controlled drug loading and release. anomedicine. 2013;8(8):1271–1280. doi: 10.2217/nnm.12.153. [DOI] [PubMed] [Google Scholar]
- 185.Wu ZG, et al. Cell-membrane-coated synthetic nanomotors for effective biodetoxification. Adv Funct Mater. 2015;25(25):3881–3887. [Google Scholar]
- 186.Piao JG, et al. Erythrocyte membrane is an alternative coating to polyethylene glycol for prolonging the circulation lifetime of gold nanocages for photothermal therapy. ACS Nano. 2014;8(10):10414–10425. doi: 10.1021/nn503779d. [DOI] [PubMed] [Google Scholar]
- 187.Luk BT, Zhang L. Cell membrane-camouflaged nanoparticles for drug delivery. J Control Release. 2015;220:600–607. doi: 10.1016/j.jconrel.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hu CMJ, et al. A biomimetic nanosponge that absorbs pore-forming toxins. Nat Nanotechnol. 2013;8(5):336–340. doi: 10.1038/nnano.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Fang RH, et al. Engineered nanoparticles mimicking cell membranes for toxin neutralization. Adv Drug Deliv Rev. 2015;90:69–80. doi: 10.1016/j.addr.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Abu Lila AS, Kiwada H, Ishida T. The accelerated blood clearance (ABC) phenomenon: clinical challenge and approaches to manage. J Control Release. 2013;172(1):38–47. doi: 10.1016/j.jconrel.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 191.Tsai RK, Discher DE. Inhibition of “self” engulfment through deactivation of myosin-II at the phagocytic synapse between human cells. J Cell Biol. 2008;180(5):989–1003. doi: 10.1083/jcb.200708043. This paper first described the use of “self” RBC peptides and their implications in mediating nanoparticle–cell interactions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sosale NG, et al. Cell rigidity and shape override CD47’s ‘self’ signaling in phagocytosis by hyperactivating myosin-II. Blood. 2015;125(3):542–552. doi: 10.1182/blood-2014-06-585299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Anselmo AC, Mitragotri S. An overview of clinical and commercial impact of drug delivery systems. J Control Release. 2014;190:15–28. doi: 10.1016/j.jconrel.2014.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Etheridge ML, et al. The big picture on nanomedicine: the state of investigational and approved nanomedicine products. Nanomed Nanotechnol Biol Med. 2013;9(1):1–14. doi: 10.1016/j.nano.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.U.S. Food and Drug Administration. [Accessed February 14th, 2016];Vaccines, Blood & Biologics. Available from: http://www.fda.gov/BiologicsBloodVaccines/default.htm.
- 196.Fung MK, et al. Technical Manual. American Association of Blood Banks; 2014. [Google Scholar]
- 197.Heathman TR, et al. The translation of cell-based therapies: clinical landscape and manufacturing challenges. egen Med. 2015;10(1):49–64. doi: 10.2217/rme.14.73. [DOI] [PubMed] [Google Scholar]
- 198.McAllister TN, Audley D, L’Heureux N. Autologous cell therapies: challenges in US FDA regulation. egen Med. 2012;7(6 Suppl):94–97. doi: 10.2217/rme.12.83. [DOI] [PubMed] [Google Scholar]
- 199.Werner M, Mayleben T, Bokkelen GV. Autologous cell therapies: the importance of regulatory oversight. Regen Med. 2012;7(6 Suppl):100–103. doi: 10.2217/rme.12.90. [DOI] [PubMed] [Google Scholar]
- 200.Maude SL, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Davila ML, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6(224):224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Chessa L, et al. Intra-erythrocyte infusion of dexamethasone reduces neurological symptoms in ataxia teleangiectasia patients: results of a phase 2 trial. Orphanet J Rare Dis. 2014;9:5. doi: 10.1186/1750-1172-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Leuzzi V, et al. Positive effect of erythrocyte-delivered dexamethasone in ataxia-telangiectasia. Neurol Neuroimmunol Neuroinflamm. 2015;2(3):e98. doi: 10.1212/NXI.0000000000000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hunault-Berger M, et al. A phase 2 study of L-asparaginase encapsulated in erythrocytes in elderly patients with Philadelphia chromosome negative acute lymphoblastic leukemia: the GRASPALL/GRAALL-SA2-2008 study. Am J Hematol. 2015;90(9):811–818. doi: 10.1002/ajh.24093. [DOI] [PubMed] [Google Scholar]
- 205.Bachet JB, et al. Asparagine synthetase expression and phase I study with L-asparaginase encapsulated in red blood cells in patients with pancreatic adenocarcinoma. Pancreas. 2015;44(7):1141–1147. doi: 10.1097/MPA.0000000000000394. [DOI] [PubMed] [Google Scholar]
- 206.Levene M, et al. Preclinical toxicity evaluation of erythrocyte-encapsulated thymidine phosphorylase in BALB/c mice and beagle dogs: an enzyme-replacement therapy for mitochondrial neurogastrointestinal encephalomyopathy. oxicol Sci. 2013;131(1):311–324. doi: 10.1093/toxsci/kfs278. [DOI] [PubMed] [Google Scholar]
- 207.Grimm AJ, et al. Memory of tolerance and induction of regulatory T cells by erythrocyte-targeted antigens. Sci Rep. 2015;5 doi: 10.1038/srep15907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Shellington DK, et al. Polynitroxylated pegylated hemoglobin: a novel neuroprotective hemoglobin for acute volume-limited fluid resuscitation after combined traumatic brain injury and hemorrhagic hypotension in mice. Crit Care Med. 2011;39(3):494–505. doi: 10.1097/CCM.0b013e318206b1fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Brockman EC, et al. Polynitroxylated-pegylated hemoglobin attenuates fluid requirements and brain edema in combined traumatic brain injury plus hemorrhagic shock in mice. J Cereb Blood Flow Metab. 2013;33(9):1457–1464. doi: 10.1038/jcbfm.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hu C-MJ, et al. Nanoparticle-detained toxins for safe and effective vaccination. Nat Nanotechnol. 2013;8(12):933–938. doi: 10.1038/nnano.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Jy W, et al. Comparison of pharmacokinetics and hemostatic efficacy of red cell microparticles (RMP) in rabbits using different infusion regimens. Blood. 2014;124(21):2811–2811. [Google Scholar]
- 212.Jy W, et al. Red cell-derived microparticles (RMP) as haemostatic agent. Thromb Haemost. 2013;110(4):751–760. doi: 10.1160/TH12-12-0941. [DOI] [PubMed] [Google Scholar]
- 213.Klein HG, Anstee DJ. Mollison’s Blood Transfusion in Clinical Medicine. 12. John Wiley and Sons, Inc; Chichester, West Sussex, UK: 2014. [Google Scholar]