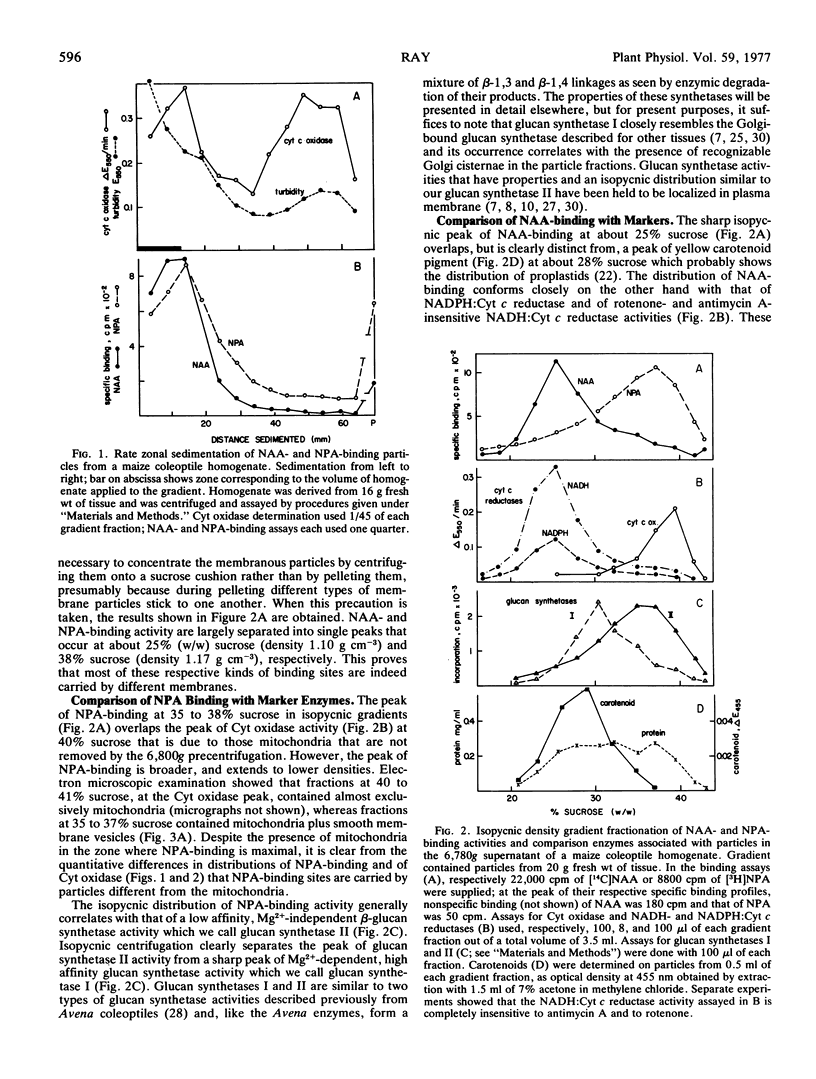
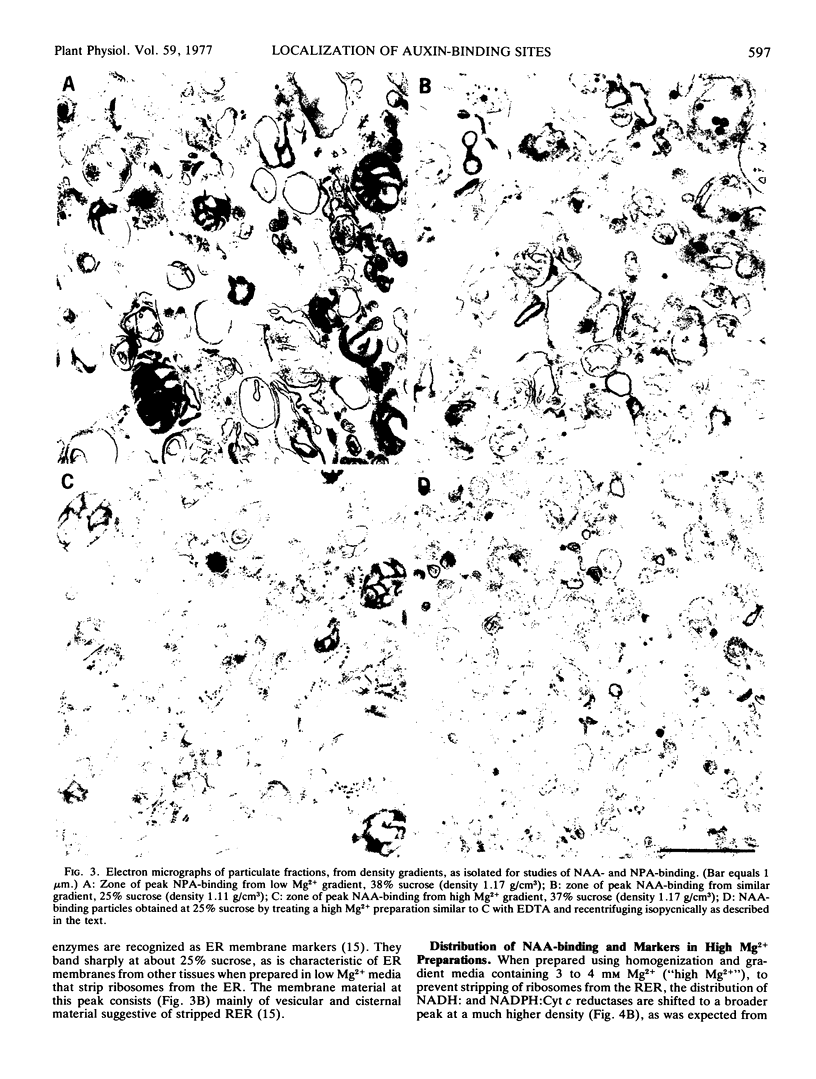
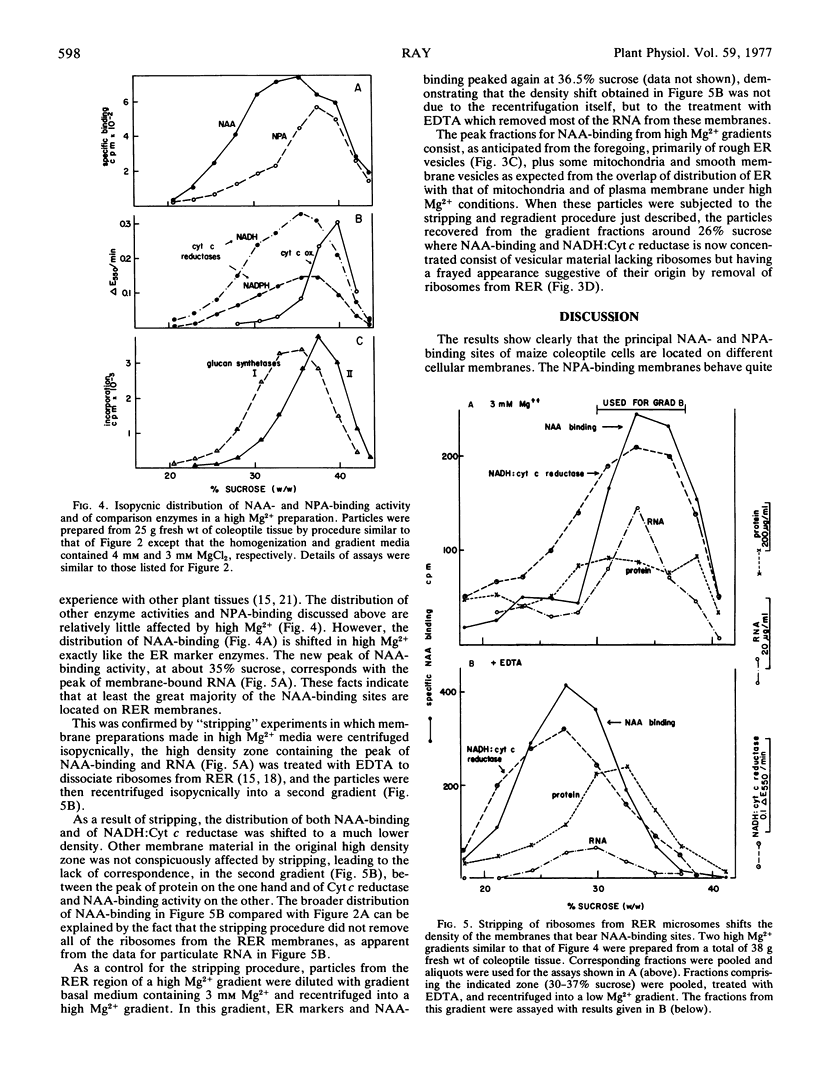
Abstract
Sites in maize (Zea mays L.) coleoptile homogenates that reversibly bind naphthalene-1-acetic acid with high affinity and may represent receptor sites for auxins are located primarily on cellular membranes that show the enzymic and buoyant density characteristics of membranes of the rough endoplasmic reticulum. The sites remain attached to the endoplasmic reticulum (ER) membranes after the ribosomes have been stripped off them. Binding sites for naphthylphthalamic acid, an inhibitor of auxin transport, are located on membranes different from those that carry the naphthalene-1-acetic-acid (NAA)-binding sites, and which are probably plasma membrane. The two kinds of binding sites can be largely separated by appropriate density gradient centrifugation. The results raise the possibility that primary auxin action occurs at ER membranes and could represent facilitation of the transfer of hydrogen ions and nascent secretory protein into the ER lumen followed by secretory transport of these products to the cell exterior via the Golgi system.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowles D. J., Northcote D. H. The amounts and rates of export of polysaccharides found within the membrane system of maize root cells. Biochem J. 1974 Jul;142(1):139–144. doi: 10.1042/bj1420139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner M., Chrispeels M. J. Involvement of the Golgi Apparatus in the Synthesis and Secretion of Hydroxyproline-rich Cell Wall Glycoproteins. Plant Physiol. 1975 Mar;55(3):536–541. doi: 10.1104/pp.55.3.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin J. W., Cherry J. H., Morré D. J., Lembi C. A. Enhancement of RNA polymerase activity by a factor released by auxin from plasma membrane. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3146–3150. doi: 10.1073/pnas.69.11.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Jacobs M., Ray P. M. Rapid Auxin-induced Decrease in Free Space pH and Its Relationship to Auxin-induced Growth in Maize and Pea. Plant Physiol. 1976 Aug;58(2):203–209. doi: 10.1104/pp.58.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leonard R. T., Vanderwoude W. J. Isolation of plasma membranes from corn roots by sucrose density gradient centrifugation: an anomalous effect of ficoll. Plant Physiol. 1976 Jan;57(1):105–114. doi: 10.1104/pp.57.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Moore T. S., Beevers H. Endoplasmic reticulum as the site of lecithin formation in castor bean endosperm. J Cell Biol. 1973 Jun;57(3):659–667. doi: 10.1083/jcb.57.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechler B., Vassalli P. Membrane-bound ribosomes of myeloma cells. I. Preparation of free and membrane-bound ribosomal fractions. Assessment of the methods and properties of the ribosomes. J Cell Biol. 1975 Oct;67(1):1–15. doi: 10.1083/jcb.67.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M., Dohrmann U. Characterization of naphthaleneacetic Acid binding to receptor sites on cellular membranes of maize coleoptile tissue. Plant Physiol. 1977 Mar;59(3):357–364. doi: 10.1104/pp.59.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M., Shininger T. L., Ray M. M. ISOLATION OF beta-GLUCAN SYNTHETASE PARTICLES FROM PLANT CELLS AND IDENTIFICATION WITH GOLGI MEMBRANES. Proc Natl Acad Sci U S A. 1969 Oct;64(2):605–612. doi: 10.1073/pnas.64.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Hassid W. Z. Substrate Activation of beta-(1 --> 3) Glucan Synthetase and Its Effect on the Structure of beta-Glucan Obtained from UDP-d-glucose and Particulate Enzyme of Oat Coleoptiles. Plant Physiol. 1973 Jun;51(6):998–1001. doi: 10.1104/pp.51.6.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Woude W. J., Lembi C. A., Morré D. J. beta-Glucan Synthetases of Plasma Membrane and Golgi Apparatus from Onion Stem. Plant Physiol. 1974 Sep;54(3):333–340. doi: 10.1104/pp.54.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhoef L. N., Stahl C. A., Williams C. A., Brinkmann K. A. Additional evidence for separable responses to auxin in soybean hypocotyl. Plant Physiol. 1976 May;57(5):817–819. doi: 10.1104/pp.57.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]