Abstract
The majority of biomolecules are intrinsically atomically precise, an important characteristic that enables rational engineering of their recognition and binding properties. However, imparting similar precision to hybrid nanoparticles has been challenging due to inherent limitations of the existing chemical methods and availability of properly designed functional building blocks. Here we report a new approach to form atomically precise and highly tunable hybrid nanomolecules with well-defined three-dimensionality. Perfunctionalization of atomically precise clusters with pentafluoroaryl-terminated linkers produces size-tunable rigid cluster nanomolecules. These species are amenable to facile modification with a variety of thiol-containing molecules and macromolecules. Assembly proceeds at room temperature within hours under mild conditions, and the resulting nanomolecules exhibit high stabilities due to their full covalency. We further demonstrate how these nanomolecules grafted with saccharides can exhibit dramatically improved binding affinity toward a protein. Ultimately, the developed strategy allows the rapid generation of precise molecular assemblies for investigating multivalent interactions.
Natural systems feature very complex three-dimensional molecular architectures that can interact with a high degree of specificity and fidelity. Among the well-established interaction modes, multivalency has been known to enable myriad biological events by strengthening individually weak interactions between biomolecules that are either native or foreign to the organism1. Multivalent interactions can be found in such diverse processes as infection (viral/bacterial proteins–cell receptors), immune recognition (antibodies–cell receptors/antigens, cytokines–cell receptors), and gene expression regulation (transcription factors–DNA), due to the higher avidity and better recognition compared to the corresponding monovalent bindings1,2.
Inspired by nature’s design, chemists have taken an interest in developing synthetic multivalent ligands with the ability to bind specific target receptors with high affinity for the purposes of 1) elucidating the mechanistic details of multivalent interactions and 2) promoting or inhibiting biological interactions of interest3. Many examples of synthetic scaffolds have emerged over the past several decades4–18, including but not limited to polymeric nanoparticles4,5, dendrimers6,7, and hybrid nanoparticles8–16. Specifically, the advent of nanotechnology and the development of surface-functionalized metal nanoparticles has provided an extremely powerful class of multivalent scaffolds. For example, gold nanoparticles (AuNPs) capped with thiolated ligands can feature biocompatibility and stability, enabling them for applications requiring binding and recognition capabilities10,11. Hybrid AuNP systems are especially attractive due to the synthetic ease of producing systems that are well-defined and tunable in size. Nevertheless, it is well known that thiolated ligands often can desorb or undergo exchange processes with other surfactants in solution over short periods of time, and furthermore, various electrochemical conditions or elevated temperatures (>60 °C) dramatically accelerate these processes10,19–22. These events can be explained primarily by the relatively weak nature of the gold–thiolate bond (40–50 kcal/mol)19. Thiol ligand-exchange processes also readily occur on smaller Au-based cluster molecules (≤5 nm), potentially limiting the ability of researchers to create atomically precise hybrid nanomolecules that remain compositionally well-defined under biologically relevant conditions (e.g. in serum)21,23.
Here we report a new strategy for building robust atomically precise hybrid nanomolecules using air-stable inorganic clusters24–28 densely decorated with perfluoroaromatic functional groups. Using this organomimetic strategy29, we show that one can mimic the rigid surface of a Au-based nanoparticle core while producing assemblies that are fully covalent and thus stable under relatively harsh conditions (Fig. 1). Specifically, we demonstrate how dodecaborate clusters29,31–36 featuring a dense layer of rigid pentafluoroaryl functional groups can serve as excellent scaffolds for constructing atomically precise, multivalent organomimetic cluster nanomolecules (OCNs). The perfluoroaryls are able to undergo facile “click”-like nucleophilic aromatic substitution (SNAr) with a wide range of thiols at room temperature within hours, creating robust carbon–sulfur bonds (80–90 kcal/mol)37, thereby producing nanomolecules decorated with well-defined functional surfaces. This approach affords the functional advantages of using dendrimers while at the same time mimicking the synthetic ease with which thiol-capped AuNPs are normally constructed. Unlike the majority of dendritic scaffolds38,39, the resulting assemblies are highly rigid and can be synthesized and purified within hours. Furthermore, these OCNs are purely covalent and therefore feature improved stability in serum and various pH environments. Finally, we demonstrate the first example of using hybrid inorganic cluster scaffold as a highly competent multivalent recognition platform for binding a protein system.
Figure 1. Comparison of features between the thiol-capped gold nanoparticles (AuNPs) and the organomimetic cluster nanomolecules (OCNs).
a, Thiol-capped AuNPs between 5 and 100 nm can be easily prepared via self-assembly, giving rise to polydisperse hybrid particles comprising weak, non-covalent gold–thiolate bonds (40–50 kcal/mol). These AuNPs often undergo ligand-exchange processes that can compromise the structural integrity of the particles. b, Uniform and robust OCNs can be efficiently assembled with atomic precision and full covalency in the size range of 2 to 10 nm via perfluoroaryl-thiol SNAr chemistry under mild conditions. The formed carbon–sulfur bond (80–90 kcal/mol) is significantly stronger compared to the gold-sulfur interaction, resulting in nanomolecules that feature high structural stabilities.
Results and discussion
Given the high reactivity of the perfluoroarenes with thiol-based nucleophiles40–44, we hypothesized that perfluoroaryl-thiol SNAr chemistry could be utilized to conjugate various thiolated groups onto perfluoroaryl-containing clusters efficiently under mild conditions at room temperature. Our laboratory has recently reported a rapid perfunctionalization strategy of [B12(OH)12]2- cluster (1) originally discovered by Hawthorne31–34 featuring a wide scope of substituents35. Using this method, we synthesized perfunctionalized clusters grafted with rigid linkers containing peripheral pentafluoroaryl moieties (Fig. 2a). Specifically, perfunctionalized cluster scaffolds 2 and 3 can be synthesized in good yields under 30 minutes and isolated after purification by silica gel column chromatography in their neutral form as air-stable solids soluble in the majority of common polar organic solvents (Supplementary Information, pages 9–12). The single crystal X-ray structures of 2 and 3 reveal the highly rigid nature of these scaffolds (Fig. 2b and 2c, respectively). Importantly, by virtue of using size-tunable linker precursor in the synthesis of 2 and 3, the resulting rigid cluster species can be rationally controlled in size (2 is approximately 1.9 and 3 is about 2.7 nm lengthwise, as measured from the single crystal structures). These clusters represent a new class of atomically precise scaffolds that offer unique rigidity and structural covalency, making them topologically reminiscent of both dendrimers and small metal nanoparticles.
Figure 2. Synthesis and characterization of the perfluoroaryl-perfunctionalized dodecaborate clusters and the subsequent modification with thiols.
a, Perfunctionalization of 1 with rigid pentafluoroaryl-terminated linkers yields pure clusters 2 and 3, after isolation. b,c, Ball-and-stick and space-filling representations of the single crystal X-ray structures of 2 and 3, respectively. Size measurements of the crystal structures reveal that 2 is 1.9 nm and 3 is 2.7 nm (lengthwise). Salmon = B, red = O, grey = C, purple = F. d, “Click”-like modification of cluster 2 with the 1-hexanethiol reagent and the corresponding 19F and 11B NMR spectra associated with the transformation from the starting material 2 to the functionalized product 2a. Specifically, perfunctionalization of 2 with 1-hexanethiol results in a shift of the meta-F resonance and the complete disappearance of the para-F resonance as well as a characteristic upfield shift of the boron singlet resulting from the reduction of the cluster.
To test our hypothesis that 2 and 3 could be fully functionalized by thiols via SNAr reaction, we commenced conjugation studies between 2 and 1-hexanethiol (a). Cluster 2 was mixed with 12 eq. of thiol a in dimethylformamide (DMF) in the presence of base and left stirring under N2 atmosphere to mitigate the undesired oxidation of the thiol reagent. Notably, the initially dark red solution quickly turned colorless. 11B NMR spectroscopy of this colorless solution revealed a singlet resonance at δ −15 characteristic of a reduced cluster in a [2]2- oxidation state35,36, which is consistent with the reducing capacity of the thiolate species when exposed to [2]0 (Fig. 2d). Therefore, for all subsequent optimization studies we utilized an extra equivalent of the thiol reagent to account for this reduction (Supplementary Table 1). 19F NMR spectroscopy was utilized to monitor the conversion of 2 to 2a, given the diagnostic change associated with this transformation (Fig. 2d – disappearance of the para-F resonance and significant downfield shift of the meta-F resonances in 2a compared to the starting material 2). A base screen identified potassium carbonate (K2CO3) as the optimal reagent leading to the substantial conversion resulting in the formation of perfunctionalized cluster 2a (Supplementary Table 1, entry 4). After additional optimization using K2CO3, we found that using 13.3 eq. of a and 30 eq. of K2CO3 resulted in a nearly quantitative (>99%) substitution of 2, producing the 12-fold substituted OCN 2a (Table 1, entry 1, Fig. 2d). The crude product was dried and then purified via silica gel column chromatography and isolated as an oily substance in 70% yield (Supplementary Information, pages 13–14). Electrospray ionization-high resolution mass spectrometry (ESI-HRMS), 1H, 11B, and 19F NMR spectroscopy of purified 2a are consistent with its proposed structure and composition (Supplementary Information, pages 51–55). We further found that 2 could be fully conjugated with aromatic (b) and benzylic (c) thiols. Both reactions proceeded nearly quantitatively within 24 hours at room temperature using potassium phosphate (K3PO4), leading to pure OCNs 2b and 2c (Table 1, entries 2 and 3), respectively, after isolation in good yields (see Supplementary Information, pages 14–16 and 56–69, for details on reaction conditions, purification, and characterization). Importantly, results with 2a–c indicate that the developed chemistry can operate with a wide range of thiol-based species spanning a significant window of nucleophilicities (pKa of aliphatic thiols is approximately 17, aromatic – 10). Overall, these experiments suggest that using the developed method, it is possible to rapidly assemble OCNs via SNAr chemistry under very mild and operationally simple conditions mimicking the simplicity of the thiol-capped AuNPs assembly. Furthermore, unlike the previously developed Huisgen “click” cycloaddition and carbamate functionalization strategies of inherently non-rigid B12-based clusters, which require elevated temperatures, long reaction times (days) and a large excess of reagents (4–5 fold per vertex), the perfluoroaryl-thiol SNAr chemistry described here proceeds using significantly milder conditions33,34.
Table 1.
Conjugation Scope for 2 and 3.
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Symbol | L | R | Time (h) |
in situ yielda (%) |
Isolated yieldb (%) |
| 1 | 2a | none | 24 | 99 | 70 | |
| 2 | 2b | none | 24 | 99 | 90 | |
| 3 | 2c | none | 24 | 99 | 94 | |
| 4 | 2d | none | 24 | 99 | 40 | |
| 5 | 2e | none | 24 | 99 | 30 | |
| 6 | 2f | none | 24 | 99 | 49 | |
| 7 | 3a | 7c | 99 | 87 | ||
| 8 | 3b | 7c | 99 | 85 | ||
| 9 | 3c | 5 | 99 | 81 | ||
| 10 | 3d | 2 | 99 | 81 | ||
| 11 | 3e | 4 | 99 | 59 | ||
| 12 | 3f | 3 | 99 | 33 | ||
| 13 | 3g |  |
3 | 99 | 49 | |
| 14 | 3hd |  |
6 | 99 | 29 | |
Yield determined by 19F NMR spectroscopy.
Isolated yields after purification.
Small scale reactions show full conversion within 1 hour.
Additional 36 eq. isopropoxytrimethylsilane (iPrMe3Si) was employed to scavenge fluoride (F−) by-product.
Thiol-capped AuNP constructs are also extremely attractive given the chemical orthogonality of the gold-thiol interaction compared to other ligands, which provides an opportunity to use a wide variety of unprotected thiol reagents for facile and programmable self-assembly. Therefore, we decided to probe the degree to which SNAr chemistry on perfluorinated clusters can mimic this attractive feature. To evaluate the thiol-selectivity of our approach, we performed conjugation reactions between 2 and thiols featuring additional nucleophilic groups such as alcohols and amines. Consistent with the previous work by Pentelute and co-workers with unprotected peptides43,44, we found that the model thiol species (d–f) all reacted with 2 through the thiol site selectively to form the desired perfunctionalized OCNs 2d–f (Table 1, entries 4–6) within 24 hours as confirmed by 19F NMR spectroscopy (see Supplementary Information, pages 16–19 and 139–159, for details on reaction conditions, purification, and characterization). This finding is important as it suggests that this chemistry can be used to selectively conjugate thiol reagents containing multiple nucleophilic functional groups and fundamentally takes advantage of the mild conditions developed here, which allow one to guide the kinetic selectivity between the thiol and pentafluoroaryl fragment42–44. Most importantly, this chemoselectivity is reminiscent of that observed in the assembly of thiol-capped AuNPs.
With the successful perfunctionalization of 2 (vide supra), we hypothesized that the larger-sized cluster 3 could not only be perfunctionalized with the same thiols to create a new generation of OCNs that are modularly extended in size, but could also accommodate 12-fold conjugation with bulkier substrates. Indeed, under the same conditions as described above for functionalization of 2, cluster 3 undergoes clean and facile perfunctionalization chemistry with thiols a–f, yielding 3a–f (Table 1, entries 7–12 and Supplementary Information, pages 25–31 and 118–159). Importantly, when using 3 instead of 2, we observed a significantly faster conversion rate leading to perfunctionalized clusters (under six hours versus 24 hours), consistent with the surface of 3 being less sterically encumbered than 2. Therefore, using 3 allowed full substitution with a bulky cysteine derivative (g) as well as a small, unprotected peptide sequence C-A-G (h), yielding 3g and 3h, respectively (Table 1, entries 13 and 14; Supplementary Information, pages 31–33, 160–173, and 222).
Next, to test whether more complex molecular architectures could also be introduced onto the clusters, we turned our attention to poly(ethylene glycol) (PEG)4,22,45,46. Complete 12-fold conjugation between 2 and commercially available mPEG-thiol (MWavg = 356 Da) occurred within 24 hours at room temperature, yielding OCN 2i (Table 2, entry 1; Supplementary Information, pages 19–20 and 91–97). Subsequently, larger mPEG-thiols (MWavg = 766 Da and 2,000 Da) were tested and similarly afforded 2j and 2k, respectively, in quantitative conversions based on 19F NMR spectroscopy (Table 2, entries 2 and 3; Supplementary Information, pages 20–23 and 98–110). As expected, PEGylation conferred considerable hydrophilicity to these clusters: 2i–k are readily soluble in water. Due to the full covalency of PEGylated OCNs, we hypothesized that these species should be structurally stable under biological conditions. Using 2i as a model, we conducted stability studies in biologically relevant media (Supplementary Information, pages 204–221). A purified sample of 2i was exposed to cell culture media containing fetal bovine serum (FBS) for five days at room temperature, and no changes or degradation products were observed by monitoring this sample via 19F and 11B NMR spectroscopy. Similarly, no degradation occurred when this sample was incubated for an additional five days at 37 °C. Importantly, samples of 2i were dissolved in buffers of various pH (5, 7, and 9) for five days, and these were found to remain structurally intact as well. These results suggest that OCNs retain their structural integrity under the wide range of biologically relevant conditions. We then decided to investigate the stability of the conjugation linkage between the cluster core and the thiol. Given the full covalency of 2i, we expected that it should not undergo ligand-exchange, a process that commonly occurs with many ligand-capped AuNPs22. Significantly, no thiol-exchange occurred when 2i (0.8 mM) was exposed to 2-mercaptoethanol (20 mM) over a period of 11 days. Similar results were obtained with 2 mM glutathione (GSH). Overall, these experiments clearly demonstrate that the OCNs constructed via the SNAr approach feature superior robustness compared to many AuNP-based assemblies10,21.
Table 2.
PEGylation and glycosylation of 2 and 3.
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Symbol | L | R | Time (h) |
in situ yielda (%) |
Isolated yieldb (%) |
| 1 | 2i | none | 24 | 99 | 81 | |
| 2 | 2j | none | 24 | 99 | 19 | |
| 3 | 2k | none |  |
24 | 99 | 41 |
| 4 | 2lc | none |  |
24 | 99 | 17 |
| 5 | 3i | 5 | 99 | 78 | ||
| 6 | 3j | 4 | 99 | 21 | ||
| 7 | 3k |  |
20d | 99 | 54 | |
| 8 | 3lc |  |
5 | 99 | 32 | |
Yield determined by 19F NMR spectroscopy.
Isolated yields after purification.
2l and 3l have undergone partial K+/Na+ counterion exchange during the deprotection reaction with NaOMe.
Small scale reaction shows full conversion within 5 hours.
PEGylated OCNs were characterized by a number of techniques to ensure their proposed nearly monodisperse composition (while the OCN cores are monodisperse, the PEG chains used in this study feature some compositional variability due to the inherent limitations of PEG oligomer synthesis)47. First, we conducted 2D diffusion-ordered (DOSY) 1H NMR spectroscopy experiments with purified samples of 2i–k and the more extended OCNs 3i–k (Table 2, entries 5–7 and Supplementary Information, pages 33–36 and 174–190) in D2O (see 2D DOSY method and plots in the Supplementary Information, pages 6 and 198–203). Based on the diffusion constants obtained from these DOSY experiments, the respective hydrodynamic diameters were estimated (Fig. 3a). As expected, the results reveal a gradual increase in the sizes of the PEGylated clusters, both as a function of the cluster core size (from 2 to 3) and the length of the PEG chain used. We noted that the size of 3i measured by 2D DOSY 1H NMR spectroscopy was larger than expected, most likely due to aggregation under the conditions the measurement was performed – suggesting the small number of PEG units in 3i could not fully stabilize the hydrophobic core against self-aggregation. To determine the size of a single non-aggregated OCN 3i, we performed additional transmission electron microscopy (TEM) experiments on 3i (Fig. 3b and Supplementary Information, page 6). The TEM images reveal the presence of nearly monodisperse particles with an average size of 1.9 nm, which is in agreement with the expected value for a non-aggregated single particle. Consistent with these results, gel permeation chromatography (GPC) traces of 2k and 3k in water (Fig. 3c) also reveal nearly monodisperse samples (Ð = 1.003 ± 0.02, 1.081 ± 0.007, respectively; Supplementary Information, page 6). Furthermore, we performed molecular dynamics (MD) simulations of species 2i–k and 3i–k in water and calculated their hydrodynamic radii and radii of gyration (snapshots after 21 ns in Fig. 3d; Supplementary Information, pages 223–231 and movies). The results are in good agreement with the non-aggregated OCN sizes measured by TEM, and furthermore exhibit a trend similar to the one observed by 2D DOSY 1H NMR spectroscopy. A small discrepancy arises between the sizes estimated based on computational studies/TEM and 2D DOSY 1H NMR spectroscopy and is likely due to some aggregation of the particles under the conditions employed in 2D DOSY 1H NMR spectroscopy experiments. Overall, our measurements clearly show that using the developed SNAr assembly strategy, one can rationally prepare robust and nearly monodisperse samples of size-tunable PEGylated OCNs.
Figure 3. Characterization of the PEGylated OCNs 2i–k and 3i–k.
a, Plot of the particle sizes of the PEGylated OCNs 2i–k and 3i–k obtained via 2D diffusion-ordered (DOSY) 1H NMR spectroscopy experiments. The plot reveals a trend of a gradual increase in the sizes of the OCNs both as a function of the cluster precursor dimension and the chain-length of a PEG reagent. We note that the size of 3i is larger than expected, likely due to aggregation. b, Transmission electron microscopy (TEM) images of a negatively stained sample of 3i reveal the presence of nearly monodisperse particles with an average size of 1.9 nm, consistent with the expected size of 3i. c, Gel permeation chromatography (GPC) traces of 2k and 3k measured in water further confirm the monodispersity of the samples (Ð = 1.003 ± 0.02 and 1.081 ± 0.007, respectively). RI = refractive index. d, Molecular dynamics (MD) calculated structures of the PEGylated nanomolecules in pure water after 21 ns (see the Supplementary Information for the movies showcasing dynamics in 2i–k and 3i–k) of simulation indicate a trend in the sizes of the OCNs consistent with the one observed through the 2D DOSY 1H NMR spectroscopy experiments.
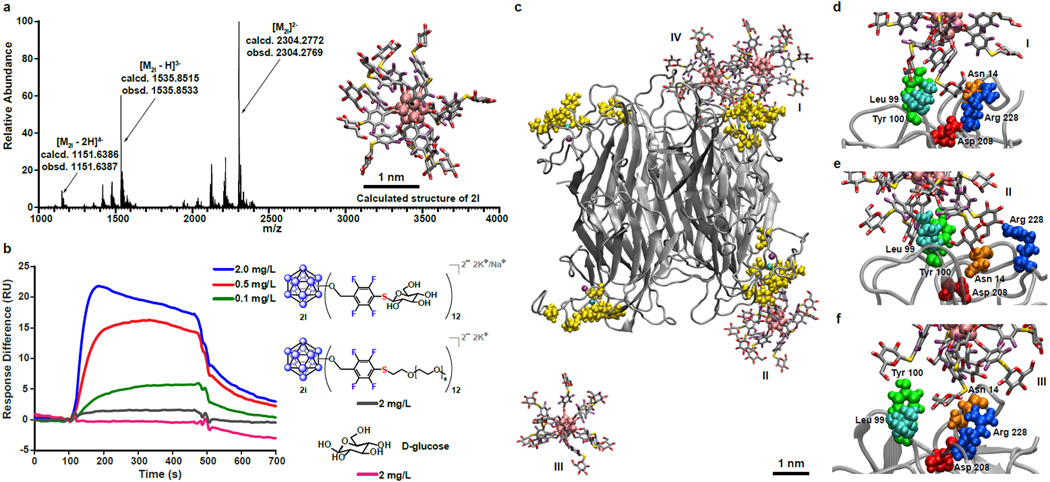
After demonstrating the scope of the developed chemistry with various classes of thiols, we next aimed to coat the scaffold clusters with recognition moieties in order to develop OCNs capable of multivalent binding interactions. In nature, multivalent glycoconjugates such as glycoproteins and glycolipids can bind lectins with relatively high avidity, thereby bypassing the fundamental limitation of weak monosaccharide binding (dissociation constants (KD) ranging between mM and µM)38,48–52. We hypothesized that cluster 2 and 3 can serve as a rigid, tunable scaffold for three-dimensional, precise display of carbohydrates. Using commercially available carbohydrate precursor 1-thio-β-D-glucose tetraacetate, we have synthesized functionalized OCNs 2l and 3l featuring 12 appended glucose molecules (Table 2, entries 4 and 8; Fig. 4a; Supplementary Information, pages 24–25, 36–37, 111–117, and 191–197). We then conducted surface plasmon resonance (SPR) experiments with a Biacore T100 instrument to monitor and quantify binding interactions between the glycosylated OCN 2l and a model lectin Concanavalin A (ConA) at pH 7.4 (Supplementary Information, pages 6–7). ConA was covalently attached to the Au-coated sensor chip’s dextran layer via conventional amide coupling53, and binding between ConA and the injected analyte was measured as a change in refractive index (RI) and expressed in response units (RU). From the binding sensorgrams (Fig. 4b), it is clear that the measured binding response was dependent on the concentration of 2l in the injected sample. Furthermore, when two controls (2i and D-glucose) were injected at the highest mass concentration of 2l shown (2.0 mg/L), minimal to no binding was observed. When the binding curves of 2l were fitted to the Langmuir 1:1 binding model, the KD value was estimated to be 54 nM, which corresponds to a 6,500-fold increase in affinity when compared to the KD between ConA and methyl D-glucopyranoside53. These results are consistent with the KD value previously reported by Munoz and co-workers between ConA and a 3rd generation D-glucose-functionalized glycodendrimer (15.8 nM, 27 saccharides). This result is significant since it demonstrates that a similar multivalent effect can be achieved by using a rigid OCN scaffold featuring significantly fewer (12 vs. 27) saccharides. Furthermore, compared to the glycodendrimer used in the work of Munoz and co-workers, which requires 8 synthetic steps, glycosylated OCNs can be rapidly constructed in 3 steps with an SNAr conjugation done under 24 hours (Huisgen click cycloaddition conjugations on large dendritic assemblies normally take several days for completion)54. Lastly, we note that the OCNs generated here are fully covalent and therefore feature enhanced stability properties compared to the species synthesized via coordination-based self-assembly55.
Figure 4. Multivalent binding of the glycosylated OCN 2l to the lectin Concanavalin A (ConA).
a, Electrospray ionization-high resolution mass spectrometry (ESI-HRMS) of 2l supports its proposed structure and composition (see inset for the MD simulated structure of 2l in aqueous environment). b, Surface plasmon resonance (SPR) sensorgram indicates that the measured binding response is dependent on the concentration of 2l. Furthermore, it suggests multivalent binding interactions between 2l and ConA as well as minimal binding of the PEGylated cluster 2i and D-glucose controls to ConA. c, A snapshot at 20 ns of a MD simulation showcases the interactions between four 2l particles (I, II, III, IV) and ConA. d–f, MD simulation close-up snapshots of three of the 2l particles (I, II, III) binding to ConA at the known monosaccharide-binding residues (colored and labeled). See the Supplementary Information for the movie illustrating this simulation and another movie illustrating the weakly binding interactions between D-glucose and ConA.
We hypothesized that the glycosylated OCN’s dramatically enhanced affinity over D-glucose toward ConA can be explained by the cluster glycoside effect38, and in order to better understand the mechanistic details of binding between 2l and ConA, we performed MD simulations of the interactions between 2l and ConA in water (see snapshots of the simulations in Fig. 4c–f; Supplementary Information, pages 231–237 and movies). Our calculation results are consistent with the experimental observations – that 2l exhibits much higher affinity than the monovalent D-glucose molecule toward ConA’s saccharide-binding sites. Furthermore, the higher affinity can be attributed to the multivalent statistical/rebinding effect provided by the densely functionalized surface filled with monosaccharide ligands positioned around the OCN cluster3,53.
Conclusions
We have developed a new strategy that allows rapid assembly of fully covalent nanoparticles with atomic precision. Specifically, we demonstrated that the rigid clusters densely decorated with perfluoroaryl-containing functional groups undergo efficient conjugation with various thiols via SNAr chemistry under very mild conditions at room temperature. Importantly, this chemistry is operationally reminiscent of the chemoselective assembly conditions associated with thiol-capped gold nanoparticles (AuNPs). Furthermore, similarly to thiol-capped AuNPs, these organomimetic cluster nanomolecules (OCNs) can be easily tuned in size and surface chemistry by choosing a specific thiol reagent. OCNs exhibit dramatically improved structural stability under a wide range of biologically relevant conditions due to the full covalency of all bonding interactions comprising these nanomolecules. Finally, using this assembly strategy we show how one can design and synthesize nanomolecules featuring a three-dimensional densely packed layer of saccharides that can participate in multivalent binding with a natural lectin leading to a dramatic increase in binding affinity. This work ultimately opens a new avenue for creating highly tailored synthetic mimics of ligand-capped AuNPs featuring rigid and fully covalent atomically precise assemblies.
Supplementary Material
Acknowledgments
A.M.S. acknowledges the UCLA Department of Chemistry and Biochemistry for start-up funds, 3M for a Non-Tenured Faculty Award and the American Chemical Society Petroleum Research Fund (56562-DNI3) for the Doctoral New Investigator Grant. E.A.Q. thanks the USPHS of the NIH for the Predoctoral Training Fellowship through the UCLA Chemistry-Biology Interface Training Program under the National Research Service Award (T32GM008496). A.S. is funded by the CARE Scholars Programs (NIH grant GM055052). M.S.M. is grateful to the NSF for the Bridge-to-Doctorate and the Predoctoral (GRFP) fellowships. P.K. acknowledges the NSF Division of Materials Research grants 1309765 and 1506886. H.D.M. thanks the NSF (CHE-1507735) for funding. We thank UCLA Molecular Instrumentation Center for mass spectrometry and NMR spectroscopy (NIH grant 1S10OD016387-01, NSF grant CHE-1048804). We also thank the Electron Imaging Center for Nanomachines at the UCLA CNSI for electron microscopy (NIH grant 1S10RR23057 to ZHZ) and the UCLA Biochemistry Instrumentation Facility for SPR measurements.
Footnotes
Author contributions
A.M.S. conceived the concept and supervised and guided the research. E.A.Q. and A.M.S. designed the experiments; E.A.Q. performed the majority of the experimental work. A.I.W., J.Y.W., J.C.A., and A.S. contributed to the synthesis of 2 and 3. A.T.R and A.L.R. performed the crystallographic characterization of compound 3. A.S., E.H.M., and D.M. contributed to the synthesis of 2j and 3j. S.C. assisted with optimizing the reaction conditions of 2/3 a–f, i, l. A.I.W. and E.H.M. contributed to the purification of the OCNs. D.J. conducted and interpreted the TEM experiments. M.S.M. and H.D.M. assisted with the GPC experiments. Y.H., P.R., and P.K. designed, conducted and interpreted the computational experiments. E.A.Q., A.I.W., and A.M.S. co-wrote the manuscript. All authors commented on the manuscript during its preparation.
Additional information
Supplementary information is available in the online version of this paper.
Competing financial interests
The authors declare no competing financial interests.
Data availability
The crystallographic data have been deposited at the Cambridge Crystallographic Data Centre (CCDC) as CCDC 1493022 (2 at 100 K) & 1511361 (3 at 100 K) and can be obtained free of charge from the CCDC via www.ccdc.cam.ac.uk/getstructures.
References
- 1.Mammen M, Choi S-K, Whitesides GM. Polyvalent interactions in biological systems: implications for design and use of multivalent ligands and inhibitors. Angew. Chem., Int. Ed. 1998;37:2754–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 2.Kiessling LL, Gestwicki JE, Strong LE. Synthetic multivalent ligands as probes of signal transduction. Angew. Chem., Int. Ed. 2006;45:2348–2368. doi: 10.1002/anie.200502794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones LH. Recent advances in the molecular design of synthetic vaccines. Nature Chem. 2015;7:952–960. doi: 10.1038/nchem.2396. [DOI] [PubMed] [Google Scholar]
- 4.Elsabahy M, Wooley KL. Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev. 2012;41:2545–2561. doi: 10.1039/c2cs15327k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao JP, Geckeler KE. Polymer nanoparticles: preparation techniques and size-control parameters. Prog. Polym. Sci. 2011;36:887–913. [Google Scholar]
- 6.Tomalia DA, et al. A new class of polymers: starburst-dendritic macromolecules. Polym. J. 1985;17:117–132. [Google Scholar]
- 7.Hawker CJ, Frechet JMJ. Preparation of polymers with controlled molecular architecture. A new convergent approach to dendritic macromolecules. J. Am. Chem. Soc. 1990;112:7638–7647. [Google Scholar]
- 8.Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3:16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 9.Peer D, et al. Nanocarriers as an emerging platform for cancer therapy. Nature Nanotech. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 10.Daniel M, Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004;104:293–346. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- 11.Dreaden EC, Alkilany AM, Huang X, Murphy CJ, El-Sayed MA. The golden age: gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012;41:2740–2779. doi: 10.1039/c1cs15237h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brust M, Walker M, Bethell D, Schiffrin DJ, Whyman R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid–liquid system. J. Chem. Soc., Chem. Commun. 1994;17:801–802. [Google Scholar]
- 13.Giljohann DA, et al. Oligonucleotide loading determines cellular uptake of DNA-modified gold nanoparticles. Nano Lett. 2007;7:3818–3821. doi: 10.1021/nl072471q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao H, et al. Reversible trapping and reaction acceleration within dynamically self-assembling nanoflasks. Nature Nanotech. 2015;11:82–88. doi: 10.1038/nnano.2015.256. [DOI] [PubMed] [Google Scholar]
- 15.Jones MR, Seeman NC, Mirkin CA. Programmable materials and the nature of the DNA bond. Science. 2015;347:1260901-1–1260901-11. doi: 10.1126/science.1260901. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki K, Sato S, Fujita M. Template synthesis of precisely monodisperse silica nanoparticles within self-assembled organometallic spheres. Nature Chem. 2010;2:25–29. doi: 10.1038/nchem.446. [DOI] [PubMed] [Google Scholar]
- 17.Heindl C, Peresypkina EV, Virovets AV, Kremer W, Scheer M. Giant rugby ball [{CpBnFe(η5-P5)}24Cu96Br96] derived from pentaphosphaferrocene and CuBr6 . J. Am. Chem. Soc. 2015;137:10938–10941. doi: 10.1021/jacs.5b06835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ambrogio MW, Thomas CR, Zhao Y-L, Zink JI, Stoddart JF. Mechanized silica nanoparticles: a new frontier in theranostic nanomedicine. Acc. Chem. Res. 2011;44:903–913. doi: 10.1021/ar200018x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Love JC, Estroff LA, Kriebel JK, Nuzzo RG, Whitesides GM. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 2005;105:1103–1170. doi: 10.1021/cr0300789. [DOI] [PubMed] [Google Scholar]
- 20.Hostetler MJ, Green SJ, Stokes JJ, Murray RW. Monolayers in three dimensions: synthesis and electrochemistry of ω-functionalized alkanethiolate-stabilized gold cluster compounds. J. Am. Chem. Soc. 1996;118:4212–4213. [Google Scholar]
- 21.Hostetler MJ, Templeton AC, Murray RW. Dynamics of place-exchange reactions on monolayer-protected gold cluster molecules. Langmuir. 1999;15:3782–3789. [Google Scholar]
- 22.MacLeod MJ, Johnson JA. PEGylated N-heterocyclic carbene anchors designed to stabilize gold nanoparticles in biologically relevant media. J. Am. Chem. Soc. 2015;137:7974–7977. doi: 10.1021/jacs.5b02452. [DOI] [PubMed] [Google Scholar]
- 23.Häkkinen H. The gold–sulfur interface at the nanoscale. Nature Chem. 2012;4:443–455. doi: 10.1038/nchem.1352. [DOI] [PubMed] [Google Scholar]
- 24.Yvon C, et al. Polyoxometalate clusters integrated into peptide chains and as inorganic amino acids: solution- and solid-phase approaches. Angew. Chem., Int. Ed. 2014;53:3336–3341. doi: 10.1002/anie.201311135. [DOI] [PubMed] [Google Scholar]
- 25.Lachkar D, Vilona D, Dumont E, Lelli M, Lacôte E. Grafting of secondary diolamides onto [P2W15V3O62]9- generates hybrid heteropoly acids. Angew. Chem., Int. Ed. 2016;55:5961–5965. doi: 10.1002/anie.201510954. [DOI] [PubMed] [Google Scholar]
- 26.Gouzerh P, Proust A. Main-group element, organic, and organometallic derivatives of polyoxometalates. Chem. Rev. 1998;98:77–112. doi: 10.1021/cr960393d. [DOI] [PubMed] [Google Scholar]
- 27.Müller A, Gouzerh P. From linking of metal-oxide building blocks in a dynamic library to giant clusters with unique properties and towards adaptive chemistry. Chem. Soc. Rev. 2012;41:7431–7463. doi: 10.1039/c2cs35169b. [DOI] [PubMed] [Google Scholar]
- 28.Li G, Wang L, Ni H, Pittman CU. Polyhedral oligomeric silsesquioxane (POSS) polymers, copolymers, and resin nanocomposites. J. Inorg. Organomet. Polym. 2002;11:123–154. [Google Scholar]
- 29.Spokoyny AM. New ligand platforms featuring boron-rich clusters as organomimetic substitutents. Pure & App. Chem. 2013;85:903–919. doi: 10.1351/PAC-CON-13-01-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee IS, Long JR, Prusiner SB, Safar JG. Selective Precipitation of prions by polyoxometalate complexes. J. Am. Chem. Soc. 2005;127:13802–13803. doi: 10.1021/ja055219y. [DOI] [PubMed] [Google Scholar]
- 31.Pitochelli AR, Hawthorne FM. The isolation of the icosahedral B12H12 -2 ion. J. Am. Chem. Soc. 1960;82:3228–3229. [Google Scholar]
- 32.Farha OK, et al. Synthesis of stable dodecaalkoxy derivatives of hypercloso-B12H12 . J. Am. Chem. Soc. 2005;127:18243–18251. doi: 10.1021/ja0556373. [DOI] [PubMed] [Google Scholar]
- 33.Jalisatgi SS, et al. A convenient route to diversely substituted icosahedral closomer nanoscaffolds. J. Am. Chem. Soc. 2011;133:12382–12385. doi: 10.1021/ja204488p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarma SJ, Khan AA, Goswami LN, Jalisatgi SS, Hawthorne MF. A trimodal closomer drug-delivery system tailored with tracing and targeting capabilities. Chem. Eur. J. 2016;22:12715–12723. doi: 10.1002/chem.201602413. [DOI] [PubMed] [Google Scholar]
- 35.Wixtrom AI, et al. Rapid synthesis of redox-active dodecaborane B12(OR)12 clusters under ambient conditions. Inorg. Chem. Front. 2016;3:711–717. doi: 10.1039/c5qi00263j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Messina MS, et al. Visible-light-induced olefin activation using 3D aromatic boron-rich cluster photooxidants. J. Am. Chem. Soc. 2016;138:6952–6955. doi: 10.1021/jacs.6b03568. [DOI] [PubMed] [Google Scholar]
- 37.Haynes WM. CRC Handbook of Chemistry and Physics. Taylor and Francis: CRC Press; 2016. [Google Scholar]
- 38.Lundquist JJ, Toone EJ. The cluster glycoside effect. Chem. Rev. 2002;102:555–578. doi: 10.1021/cr000418f. [DOI] [PubMed] [Google Scholar]
- 39.Moore JS, Xu Z. Synthesis of rigid dendritic macromolecules: enlarging the repeat unit size as a function of generation, permitting growth to continue. Macromolecules. 1991;24:5893–5894. [Google Scholar]
- 40.Birchall JM, Green M, Haszeldine RN, Pitts AD. The mechanism of the nucleophilic substitution reactions of polyfluoroarenes. Chem. Commun. 1967;7:338–339. [Google Scholar]
- 41.Becer CR, Hoogenboom R, Schubert US. Click chemistry beyond metal-catalyzed cycloaddition. Angew. Chem., Int. Ed. 2009;48:4900–4908. doi: 10.1002/anie.200900755. [DOI] [PubMed] [Google Scholar]
- 42.Becer CR, et al. Clicking pentafluorostyrene copolymers: synthesis, nanoprecipitation, and glycosylation. Macromolecules. 2009;42:2387–2394. [Google Scholar]
- 43.Spokoyny AM, et al. A perfluoroaryl-cysteine SNAr chemistry approach to unprotected peptide stapling. J. Am. Chem. Soc. 2013;135:5946–5949. doi: 10.1021/ja400119t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang C, et al. π-clamp-mediated cysteine conjugation. Nature Chem. 2015;8:120–128. doi: 10.1038/nchem.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoffman AS. The origins and evolution of ‘controlled’ drug delivery systems. J. Control. Release. 2008;132:153–163. doi: 10.1016/j.jconrel.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 46.Alconcel SNS, Baas AS, Maynard HD. FDA-approved poly(ethylene glycol)–protein conjugate drugs. Polym. Chem. 2011;2:1442–1448. [Google Scholar]
- 47.Veronese FM, Pasut G. PEGylation, successful approach to drug delivery. Drug Discov. Today. 2005;10:1451–1458. doi: 10.1016/S1359-6446(05)03575-0. [DOI] [PubMed] [Google Scholar]
- 48.Dam TK, Roy R, Das SK, Oscarson S, Brewer CF. Binding of multivalent carbohydrates to Concanavalin A and Dioclea grandiflora lectin. Thermodynamic analysis of the “multivalency effect”. J. Biol. Chem. 2000;275:14223–14230. doi: 10.1074/jbc.275.19.14223. [DOI] [PubMed] [Google Scholar]
- 49.Müller C, Despras G, Lindhorst TK. Organizing multivalency in carbohydrate recognition. Chem. Soc. Rev. 2016;45:3275–3302. doi: 10.1039/c6cs00165c. [DOI] [PubMed] [Google Scholar]
- 50.Muñoz A, et al. Synthesis of giant globular multivalent glycofullerenes as potent inhibitors in a model of Ebola virus infection. Nature Chem. 2016;8:50–57. doi: 10.1038/nchem.2387. [DOI] [PubMed] [Google Scholar]
- 51.Bhatia S, Camacho LC, Haag R. Pathogen inhibition by multivalent ligand architectures. J. Am. Chem. Soc. 2016;138:8654–8666. doi: 10.1021/jacs.5b12950. [DOI] [PubMed] [Google Scholar]
- 52.Bernardi A, et al. Multivalent glycoconjugates as anti-pathogenic agents. Chem. Soc. Rev. 2013;42:4709–4727. doi: 10.1039/c2cs35408j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Munoz EM, Correa J, Riguera R, Fernandez-Megia E. Real-time evaluation of binding mechanisms in multivalent interactions: a surface plasmon resonance kinetic approach. J. Am. Chem. Soc. 2013;135:5966–5969. doi: 10.1021/ja400951g. [DOI] [PubMed] [Google Scholar]
- 54.Fernandez-Megia E, Correa J, Rodríguez-Meizoso I, Riguera R. A click approach to unprotected glycodendrimers. Macromolecules. 2006;39:2113–2120. [Google Scholar]
- 55.Kamiya N, Tominaga M, Fujita M. Saccharide-coated M12L24 molecular spheres that form aggregates by multi-interaction with proteins. J. Am. Chem. Soc. 2007;129:3816–3817. doi: 10.1021/ja0693082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.