Abstract
Background:
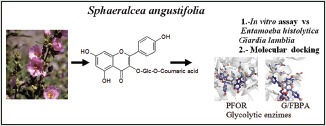
Sphaeralcea angustifolia (Malvaceae) is extensively used in Mexican traditional medicine for the treatment of gastrointestinal disorders such as diarrhea and dysentery.
Objective:
The current study was to validate the traditional use of S. angustifolia for the treatment of diarrhea and dysentery on biological grounds using in vitro antiprotozoal activity and computational experiments.
Materials and Methods:
The ethanol extract, subsequent fractions, flavonoids, phenolic acids, and a sterol were evaluated on Entamoeba histolytica and Giardia lamblia trophozoites. Moreover, molecular docking studies on tiliroside were performed; it was tested for its affinity against pyruvate:ferredoxin oxidoreductase (PFOR) and fructose-1,6-bisphosphate aldolase (G/FBPA), two glycolytic enzymes of anaerobic protozoa.
Results:
Bioassay-guided fractionation of extract of the aerial parts of S. angustifolia gives tiliroside and apigenin, caffeic acid, protocatechuic acid, and β-sitosterol. The in vitro antiprotozoal assay showed that tiliroside was the most potent antiprotozoal compound on both protozoa with 50% inhibitory concentration values of 17.5 μg/mL for E. histolytica and 17.4 μg/mL for G. lamblia. Molecular docking studies using tiliroside showed its probable antiprotozoal mechanism with PFOR and G/FBPA. In both cases, tiliroside showed high affinity and inhibition constant theoretic for PFOR (lowest free binding energy from −9.92 kcal/mol and 53.57 μM, respectively) and G/FBPA (free binding energy from −7.17 kcal/mol and 55.5 μM, respectively), like to metronidazole, revealing its potential binding mode at molecular level.
Conclusion:
The results suggest that tiliroside seems to be a potential antiprotozoal compound responsible for antiamoebic and antigiardial activities of S. angustifolia. Its in vitro antiprotozoal activities are in good agreement with the traditional medicinal use of S. angustifolia in gastrointestinal disorders such as diarrhea and dysentery.
SUMMARY
Bioassay-guided fractionation of extract of the aerial parts of S. angustifolia gives: tiliroside and apigenin, caffeic acid, protocatechuic acid) and β-sitosterol. The in vitro antiprotozoal assay showed that tiliroside was the most potent antiprotozoal compound on both protozoa with IC50 values of 17.5 mg/mL for E. histolytica and 17.4 μg/mL for G. lamblia. Molecular docking studies using tiliroside showed its probable antiprotozoal mechanism with PFOR and G/FBPA. In both cases tiliroside showed high affinity and inhibition constant theoretic for PFOR (lowest free binding energy from -9.92 kcal/mol and 53.57 mM, respectively) and G/FBPA (free binding energy from -7.17 kcal/mol, respectively and 55.5 μM), like to metronidazole, revealing its potential binding mode at molecular level. The results suggest that tiliroside seems to be a potential antiprotozoal compound responsible for antiamoebic and antigiardial activities of Sphaeralcea angustifolia.
Abbreviations Used: PFOR: Pyruvate:ferredoxin oxidoreductase; G/FBPA: Fructose 1,6 bisphosphate aldolase.
Key words: Antiprotozoal activity, gastrointestinal disorders, fructose-1, 6-bisphosphate aldolase, molecular docking, pyruvate:ferredoxin oxidoreductase, Sphaeralcea angustifolia, tiliroside
INTRODUCTION
The anaerobic protozoa Entamoeba histolytica and Giardia lamblia collectively infect over one billion people each year. Both are pathogens of the intestinal tract of humans; the first causes were dysentery, liver abscess, and invade other organs; the second causes were intestinal malabsorption, bloating, nausea, loss of appetite, vomiting, and severe diarrhea. These enteric parasitic infections are a health problem in the world, particularly in developing countries where there are poor sanitary conditions.[1,2,3,4] In Mexico, amoebiasis is an endemic disease, with incidence rates that vary among the geographic regions of the country. In the last 7 years, it has been a serious health problem, and it was the 10th cause of morbidity among all age groups. In the case of giardiasis, it currently accounts for an estimated nine millions of sick each year, and it is the leading cause of intestinal parasitosis of medical importance in children.[5,6,7,8]
Treatments with antiprotozoal drugs as metronidazole, tinidazole, iodoquinol, diloxanide furoate, and paromomycin fail at a rate of ~20%. In the case of metronidazole, it is the drug of choice currently used in Mexico for the treatment of infections caused by G. lamblia and E. histolytica. It is effective; however, it is mutagenic in bacteria and carcinogenic in rodents. In addition, this drug has several other side effects including gastrointestinal disturbance, especially nausea and vomiting. Furthermore, infrequent adverse effects include headache and stomatitis. In long-term systemic treatment, patients experience dry mouth, metallic taste, headache, and vertigo. Moreover, it is associated with the development of leukopenia, neutropenia, and central nervous system toxicity. In addition, clinical and laboratory-generated resistant strains of both protozoa have been reported.[4,9,10,11] Clearly, there are needs for alternative antiamoebic and antigiardial agents. In this sense, many plants species are used in Mexican traditional medicine for the treatment of diarrhea and dysentery; these plants should be analyzed to determine their efficacy and thus their potential as sources of new antiprotozoal drugs.
Sphaeralcea angustifolia (Cav.) G. Don (Malvaceae) is an erect perennial plant of 0.5–2 m of height, densely stellate-pubescent throughout with grayish or slightly yellowish hairs; the gray-green leaf blades are lanceolate to lance-oblong, cuneate at base, margin usually finely and regularly crenate. The leafy inflorescence bears have several flowers each one with five wedge-shaped mauve or lavender petals. It is widely distributed in the USA and Mexico; it grows in desert and plateau habitat. S. angustifolia is commonly known as “Copper Globemallow” in the USA, while in Mexico, it is locally called “Hierba del negro and Vara de San José.” In Mexican traditional medicine, the leaves from S. angustifolia are used for the treatment of inflammatory disorders such as rheumatism, inflamed skin, and arthritis. In addition, they are used for the treatment of diseases of the gastrointestinal tract as abdominal pain, diarrhea, and dysentery.[12,13]
Previous pharmacological investigations reported the utility for the treatment in hand osteoarthritis and anti-inflammatory properties of this plant. Scopoletin, tomentin, and sphaeralcic acid were thought to be responsible for anti-inflammatory properties of S. angustifolia. In addition, β-sitosterol, stigmasterol, α- and β-amyrins, and transcinnamic acid have been isolated of this plant.[14,15,16,17,18] On the other hand, intraperitoneal administration of the dichloromethane extract in rodents did not indicate severe systematic toxicity, adverse effects, hematological or biochemical alterations.[16]
Despite the traditional medicinal use of S. angustifolia, no data are available with respect to its usefulness in gastrointestinal disorders caused by protozoa such as diarrhea and dysentery. Therefore, the aim of this study was to examine the effects of the ethanol extract, fractions, and pure compounds obtained from the aerial parts of S. angustifolia using in vitro antiprotozoal activity and computational experiments, so as to assess some of the possible mechanisms involved in the traditional use.
MATERIALS AND METHODS
Plant material
The aerial parts of S. angustifolia were collected from La Conchita-Zapotitlan, Tláhuac, Ciudad de México (Mexico), in March 2012 by Dr. Fernando Calzada. Sample was authenticated by MS Abigail Aguilar-Contreras of Instituto Mexicano del Seguro Social (IMSS), Mexico. Specimen having voucher no. 15794 was deposited in the Herbarium IMSSM of IMSS.
Extraction from Sphaeralcea angustifolia
The air-dried and finely powdered aerial parts (600 g) were extracted by maceration at room temperature with EtOH (5 times × 20 L). After filtration, the extract was combined and evaporated in vacuo to yield 80 g of green residue.
Isolation of antiprotozoal compounds from the ethanol extract of Sphaeralcea angustifolia
A portion of crude ethanol extract (30 g) was suspended in methanol-water (50 mL; 5:45) and fractionated into chloroform fraction (11.8 g, 100 mL × 2 times), ethyl acetate fraction (0.80 g, 100 mL × 2 times), and the aqueous residual fraction (AR, 17.4 g). 10 g of chloroform fraction was subjected to a silica gel column chromatography (CC, 167 g 70–230 mesh, E. Merck, Germany) and eluted with chloroform (100%), chloroform-methanol (90:10; v/v), chloroform-methanol (80:20; v/v), and chloroform-methanol (70:30; v/v) to give three fractions: Fr1 (1.5 L), Fr2 (0.5 L), and Fr3 (1. 3 L). From Fraction 1, β-sitosterol (1, 1.58 g) crystallized spontaneously. From Fraction 2, tiliroside (2, 400 mg) crystallized spontaneously. Fraction 3 (115 mg) was purified by preparative thin-layer chromatography (TLC) (25 mg each plate × 5; silica gel Merck; EtOAc-MeOH-water, 100: 16.5: 13.5) to give caffeic acid (3, 15.5 mg), protocatechuic acid (4, 12 mg), apigenin (5, 11.7 mg), and additional amounts of tiliroside (27.5 mg). The structure of pure compounds 1–5 was ascertained by physical and spectroscopic properties (MP, TLC, HPLC, and RMN) compared with those reported in the literature and authentic samples (Sigma) available in our laboratory.
Antiprotozoal assays
E. histolytica strain HM1-IMSS used in all experiments was grown axenically at 37°C in TYI-S-33 medium supplemented with 10% heat-inactivated bovine serum. In the case of G. lamblia, strain IMSS: 8909:1 was grown in TYI-S-33 modified medium supplemented with 10% calf serum and bovine bile. The trophozoites were axenically maintained and for assays were employed in the log phase of growth. In vitro susceptibility tests were performed using a subculture method previously described.[19] Briefly, E. histolytica (6 × 103) or G. lamblia (5 × 104) trophozoites were incubated for 48 h at 37°C in the presence of different concentrations (2.5–200 μg/mL) of the crude extract or pure compounds in dimethyl sulfoxide (DMSO). Each test included metronidazole (Sigma) as standard amoebicidal and giardicidal drugs, a control (culture medium plus trophozoites and DMSO), and a blank (culture medium). After incubation, the trophozoites were detached by chilling and 50 μL samples of each tube were subcultured in fresh medium for another 48 h, without antiprotozoal samples. The final number of parasites was determined with an hemocytometer, and the percentages of trophozoites growth inhibition were calculated by comparison with the control culture. The results were confirmed by a colorimetric method: The trophozoites were washed and incubated for 45 min at 37°C in phosphate buffer saline with 3-45-dimethylhiazol-2-il]-2,5-diphenyl tetrazolium bromide and phenazine methosulfate. The dye produced (formazan) was extracted and the absorbance was determined at 570 nm. The experiments were performed in duplicate for each protozoan and repeated at least three times. Data were analyzed using probit analysis.[20] The in vitro results were classified as follows: If the samples displayed a 50% inhibitory concentration (IC50) <20.0 μg/mL, the antiprotozoal activity was considered good; from 21.0 to 60 μg/mL, the antiprotozoal activity was considered moderate; from 61 to 200 μg/mL, the antiprotozoal activity was considered weak; and over 200 μg/mL, the samples were considered inactive.
Statistical analysis
Data were analyzed using probit analysis. The percentage of trophozoites surviving was calculated by comparison with the growth in the control group. The plot of probit against log concentration was made, the best straight line was determined by regression analysis, and the IC50 values were calculated. The regression coefficient, its level of significance (P < 0.05 indicates significant difference between group), and correlation coefficient were calculated and 95% confidence interval (CI) values determined.[20,21,22,23]
Computational study
Considering that enzymes pyruvate:ferredoxin oxidoreductase (PFOR) and G/FBPA are two potential targets for drug design in anaerobic pathogens as E. histolytica and G. lamblia and that both are target of inhibitor metronidazole, we decide test tiliroside in silico.[24,25] Furthermore, to known the potential binding mode at molecular level of tiliroside 2 as antiprotozoal agent, it was compared versus metronidazole in molecular docking experiments. We carried out docking studies employing two glycolytic enzymes as potential targets, PFOR and G/FBPA from Desulfovibrio africanus and G. lamblia, respectively.[26,27,28] Briefly, the crystal structures were retrieved from RCSB (PDB IDs: 1KEK and 3GAY, respectively); total molecules of water and ions no needed to catalytic activity were stripped to preserve the entire protein. Subsequently, ligands structures (tiliroside and metronidazole) were drawn with ChemBioDraw Ultra 11.0 software. Then, the complete optimization of geometry and energy before docking simulations was done with Gaussian 09 package; the output minimized structures were converted into input files to docking with GaussView 5.0. Docking simulations were conducted with AutoDock 4.2 applying the follow search parameters: Total Kollman charges were computed after polar hydrogen atoms were added in such atoms from the residues with capability to establish H bonds; a blind docking procedure was conducted in all the simulations with a grid box of 126 Å ×126 Å × 126 Å in each space coordinates establishing a spacing in each grid point of 0.375 Å; the scoring function selected was the Lamarckian genetic algorithm with a randomized initial population of 100 individuals delimiting a total of 1 × 107 cycles such as maximum number of energy evaluations. Resulting docking orientations within 1.0 Å in the root-mean-square deviation tolerance of each other were clustered together and represented by the result with the most favorable free energy of binding. Nonbonded interactions were analyzed with PyMOL graphical viewer. AutoDock 4.2 is currently being distributed free of charge as open source under a GPL license at the WWW site: http://autodock.scripps.edu. ADT is being distributed free of charge as part of the MGLTools package, at the WWW site: http://mgltools.scripps.edu/downloads. AutoDock & this web site are Copyright © 1989-2017 The Scripps Research Institute. All Rights Reserved.
RESULTS AND DISCUSSION
S. angustifolia is used in Mexican traditional medicine for the treatment of gastrointestinal diseases, including diarrhea and dysentery, as well inflammatory disorders.[12,13] In the present work as part our research to obtain potential antiprotozoal agents by medicinal plants and to rationalize their traditional use in Mexican traditional medicine the bioassay-guided fractionation of the active ethanol extract of the aerial parts of S. angustifolia against E. histolytica and G. lamblia was performed. Bioassay-guided fractionation of the active ethanol extract of the aerial parts of S. angustifolia against E. histolytica and G. lambli a was performed [Table 1]. The ethanol extract was fractionated into organic and aqueous soluble fractions by organic solvent extractions with CHCl3 and EtOAc. All fractions (CHCl3, EtOAc, and AR) were tested for antiprotozoal activity using E. histolytica and G. lamblia trophozoites. As a result of this process, chloroform fraction showed the best inhibitory activity on both protozoa; it was purified by CC to give two flavonoids (tiliroside 2 and apigenin 5), two phenolic acids (caffeic acid 3 and protocatechuic acid 4), and a sterol (β-sitosterol 1). In addition, the ethyl acetate and the aqueous residual fractions were discarded because they showed weak activity against both protozoa.
Table 1.
Antiprotozoal activity of the ethanol extract, fractions, and pure compounds obtained of the aerial parts of Sphaeralcea angustifoliaa
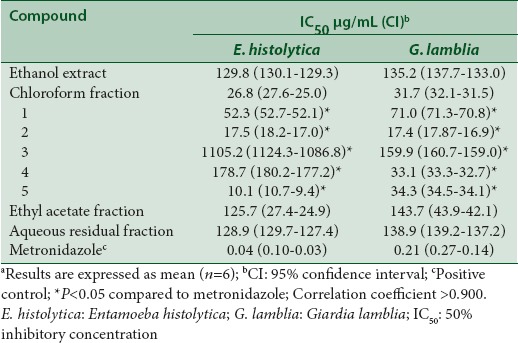
The antiprotozoal activities of all isolated compounds in this work were tested on E. histolytica and G. lamblia trophozoites [Table 1]. Among these, tiliroside 2 was the most potent compound on both protozoa with IC50 values of 17.5 μg/mL for E. histolytica and 17.4 μg/mL for G. lamblia. Apigenin showed selectivity against G. lamblia (IC50 10.1 μg/mL). The remaining compounds tested showed moderate or weak activity. All compounds were less active than metronidazole, drug used as positive control. To the best of our knowledge, this is the first report of antiamoebic and antigiardial properties of compounds 3 and 4. Further, this is the first bioassay-guided work to obtain the antiprotozoal compounds of the aerial parts of S. angustifolia. Tiliroside 2 is a flavonoid isolated from several medicinal plants with important in vivo and in vitro antiprotozoal activity. In this sense, it has been speculated that antidiarrheic properties of medicinal plants are a consequence of their inhibitory actions on protozoa.[29,30] In addition, tiliroside which was obtained in a large amount may be a prodrug of kaempferol considering that flavonoid glycosides are hydrolyzed by intestinal bacteria, cell-free extract from the small intestine, or gastrointestinal fluid.[31,32,33,34] In this context, kaempferol is a flavonoid with significant in vitro and in vivo antiprotozoal activity; then, it may contribute to the observed antiprotozoal activity of the aerial parts of S. angustifolia. The antiprotozoal activity of tiliroside is in agreement with the results published by the author's group and confirms that it flavonoid glycoside may be a leading compound in the development of novel antiamoebic and antigiardial drugs.[29,30]
In relation with the molecular docking experiments, they are based on the fact that genome studies have recently contributed to the discovery of a new array of virulence determinants in pathogenic protozoa as E. histolytica, as well as identification of novel drug candidates.[24,25,35] However, no data are available that explain its potential binding mode at molecular level. In this regard, in silico approaches has been applied to search the possible interaction between the potential targets and molecules obtained from natural products as flavonoids yielding new pharmacological horizons.[36,37,38] In the present study, results obtained from in vitro and in silico experiments yield important findings of the possible inhibition activity of tiliroside over two enzymes involved in the glycolytic pathway of anaerobic protozoa. In specific, tiliroside [Figure 1] docked over PFOR, it showed interactions with 14 amino acids residues (Ala 117 [at 2.99 Å], Ala 118 [at 2.16 Å], Leu 121 [at 3.10 Å], Phe 665 [at 2.74 Å], Leu 667 [at 3.55 Å], Gly 845 [at 2.10 Å], Ala 845 [at 4.10 Å], Met 851 [at 4.17 Å], Pro 852 [at 2.89 Å], Tyr 853 [at 3.94 Å], Trp 864 [at 3.28 Å], Gly 865 [at 3.29 Å], Asn 866 [at 3.91 Å], and Tyr 875 [at 3.90 Å]) in a region different that metronidazole; the interactions were established downstream of the iron-sulfur cluster forming mainly H-bond interactions according to its chemical nature [Figure 2, lower right panel]. In contrast, metronidazole (Phe 174 [at 3.25 Å], His 178 [at 3.33 Å], Lys 435 [at 2.83 Å], Phe 453 [at 3.24 Å], and Tyr 455 [at 2.90 Å]) showed a binding posed most closed to iron-sulfur cluster [Figure 2, upper right panel]. In the case of interaction of tiliroside over G/FBPA, it showed a similar behavior in which tiliroside interacted with ten amino acids residues (Pro 2 [at 3.10 Å], Leu 3 [at 2.54 Å], Lys 63 [at 4.27 Å], Cys 66 [at 3.15 Å], Glu 67 [at 3.43 Å], Leu 70 [at 3.86 Å], Glu 71 [at 1.83 Å], Ile 80 [at 4.55 Å], and Leu 97 [at 4.25 Å]) in a place different [Figure 3, upper right panel] that the inhibitor metronidazole [Figure 3, lower right panel]; its interactions were with seven amino acids residues (His 178 [at 2.92 Å], Gly 211 [at 2.91 Å], Ser 213 [at 2.06 Å], Asn 253 [at 3.22 Å], Val 254 [at 5.37 Å], Asp 255 [at 3.25 Å], and Ser 256 [at 4.36 Å]), and Zn 2+ (at 2.23 Å) atom. Our results showed that tiliroside exhibited high affinities on both glycolytic enzymes with lowest free binding energy from −9.92 kcal/mol for PFOR and from −7.17 for G/FBPA kcal/mol like to metronidazole (−5.09 and −10.04 kcal/mol, respectively). In addition, inhibition constant theoretic for PFOR (53.57 μM) and G/FBPA (55.5 μM) was like to metronidazole (47.64 μM and 44.01 μM). In both cases, the results explain the possible mode of interaction of tiliroside with the amino acid environment of the binding site in the PFOR and G/FBPA at molecular level.
Figure 1.
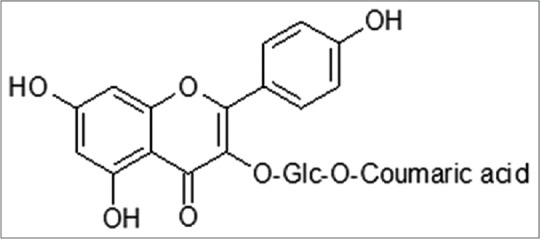
Chemical structure of tiliroside 2
Figure 2.
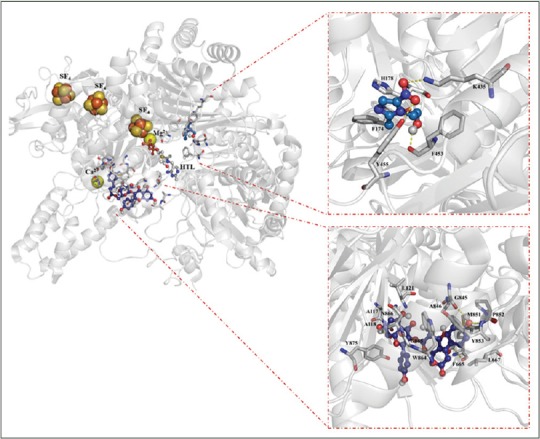
Inhibition sites over pyruvate:ferredoxin oxidoreductase to tiliroside and metronidazole (left panel). Close contact of the binding modes of metronidazole (upper right panel) and tiliroside (lower right panel). H bonds are showed in red dashes. Y = Tyr; W = Trp, G = Gly, N = Asn, L = Leu, M = Met, A = Ala, P = Pro, F = Phe, H = His, K = Lys. SF4= [4Fe-4S] cluster
Figure 3.
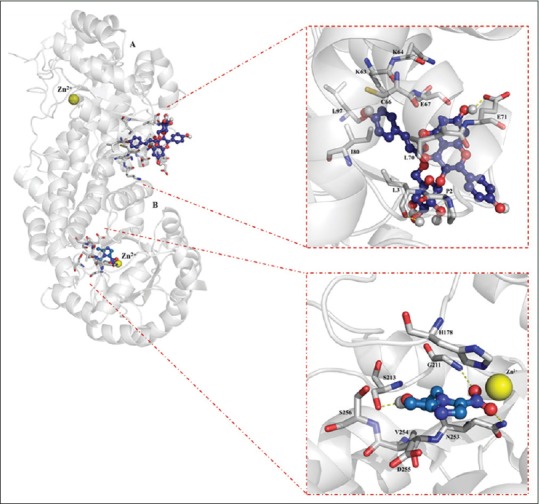
Inhibition sites over G/FBPA to metronidazole and tiliroside (left panel). Close contact of the binding modes of metronidazole (lower right panel) in short distance to Zn2+ atom and tiliroside (upper right panel). H bonds are showed in red dashes. G = Gly, N = Asn; L = Leu, P = Pro, H = His, V = Val, S = Ser, E = Glu, C = Cys, D = Asp, I = Ile, and K = Lys
Glycolysis is an important metabolic pathway for most organisms, including protozoan parasites such as E. histolytica and G. lamblia. Many of these primitive eukaryotes have streamlined their metabolism, favoring glycolysis for generating ATP in the glucose-rich environments in which they reside. Therefore, the enzymes involved in glucose metabolisms such as PFOR and G/FBPA could prove to be attractive targets for therapeutic development.[26,27,28,29,39,40]
CONCLUSION
Results of the present work along with the in vivo and in vitro antiprotozoal properties previously describes to tiliroside[29,30] could suggest that part of the mechanism by which S. angustifolia inhibits diarrhea and dysentery involves antigiardial and antiamoebic effects. In this context, the antidiarrheic properties reputed for S. angustifolia in Mexican traditional medicine may be due to the presence of flavonoids: tiliroside and apigenin. In addition, in agreement with docking analysis, the antiprotozoal activity of tiliroside on anaerobic protozoa as E. histolytica and G. lamblia may be through of inhibition of glycolytic enzymes PFOR and G/FBPA. In addition, although tiliroside was less active than metronidazole, it may be of interest to medicinal chemists who are interested in developing new antiprotozoal agents, considering that tiliroside interacted in a region different that metronidazole in the enzymes PFOR and G/FBPA. To confirm the results obtained in computational experiments on the interaction between tiliroside and glycolytic enzymes, proteomic approach based on two-dimensional gel electrophoresis and mass spectrometry (ESI-MS/MS) analysis is in process.[41]
Financial support and sponsorship
We are grateful the research scholarship of IMSS Foundation A.C. given to Dr. Normand Garcia Hernandez.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We thank MS Abigail Aguilar, IMSS Herbarium, for authentication of plant material.
REFERENCES
- 1.Baldursson S, Karanis P. Waterborne transmission of protozoan parasites: Review of worldwide outbreaks – An update 2004-2010. Water Res. 2011;45:6603–14. doi: 10.1016/j.watres.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Casburn-Jones AC, Farthing MJ. Management of infectious diarrhoea. Gut. 2004;53:296–305. doi: 10.1136/gut.2003.022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan MA, Khan NA, Qasmi IA, Ahmad G, Zafar S. Protective effect of Arque-Ajeeb on acute experimental diarrhoea in rats. BMC Complement Altern Med. 2004;4:8. doi: 10.1186/1472-6882-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Upcroft P, Upcroft JA. Drug targets and mechanisms of resistance in the anaerobic protozoa. Clin Microbiol Rev. 2001;14:150–64. doi: 10.1128/CMR.14.1.150-164.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.SS (Secretaria de Salud). Sistema Nacional de Vigilancia Epidemiologica; Epidemiologia 34, semana 34. 2008 ISSN 1405-2636. [Google Scholar]
- 6.Dirección General de Epidemiología (DGE) Anuarios de Morbilidad 2008-2013. 2013. [Last accesed on 2017 Dec 12]. Available from: http://www.epidemiologia.salud.gob.mx/anuario/html/anuarios.html .
- 7.Hernández EG, Granados J, Partida-Rodríguez O, Valenzuela O, Rascón E, Magaña U, et al. Prevalent HLA class II alleles in Mexico City appear to confer resistance to the development of amebic liver abscess. PLoS One. 2015;10:e0126195. doi: 10.1371/journal.pone.0126195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guía Práctica Clínica (GPC) Prevención, diagnóstico y tratamiento farmacológico de la giardiasis en niños y adolescentes de 1 a 18 años en el primer y segundo nivel de atención. Evidencias y recomendaciones. Publicado por CENETEC. 2012. [Last accesed on 2017 Dec 12]. Available from: http://www.cenetec.salud.gob.mx/interior/gpc.html .
- 9.Peraza-Sánchez SR, Poot-Kantân S, Torres-Tapia LW, May-Pat F, Simá-Polanco P, Cedillo-Rivera R. Screening of native plants from Yucatán for anti-Giardia lamblia activity. Pharm Biol. 2005;43:594–8. [Google Scholar]
- 10.Müller J, Rühle G, Müller N, Rossignol JF, Hemphill A. In vitro effects of thiazolides on Giardia lamblia WB clone C6 cultured axenically and in coculture with Caco2 cells. Antimicrob Agents Chemother. 2006;50:162–70. doi: 10.1128/AAC.50.1.162-170.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behnia M, Haghighi A, Komeylizadeh H, Tabaei SJ, Abadi A. Inhibitory effects of Iranian Thymus vulgaris extracts on in vitro growth of Entamoeba histolytica. Korean J Parasitol. 2008;46:153–6. doi: 10.3347/kjp.2008.46.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aguilar A, Camacho J, Chino S, Jáquez P, López ME. Información Etnobotánica. 1st ed. México: IMSS; 1994. Herbário medicinal del Instituto Mexicano del Seguro Social; p. 140. [Google Scholar]
- 13.Argueta A, Cano L, Rodarte M. Atlas de las Plantas de la Medicina Tradicional Mexicana. Vol. I-III. Mexico City: Instituto Nacional Indigenista; 1994. [Google Scholar]
- 14.Meckes M, David-Rivera AD, Nava-Aguilar V, Jimenez A. Activity of some Mexican medicinal plant extracts on carrageenan-induced rat paw edema. Phytomedicine. 2004;11:446–51. doi: 10.1016/j.phymed.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Juárez-Ciriaco M, Román-Ramos R, González-Márquez H, Meckes-Fischer M. Efecto de Sphaeralcea angustifoli a sobre la expresión de citocinas pro y antiinflamatorias. Lab Cienc. 2008;2:21–4. [Google Scholar]
- 16.García-Rodríguez RV, Chamorro-Cevallos G, Siordia G, Jiménez-Arellanes MA, Chavez-Soto MA, Meckes-Fischer M. Sphaeralcea angustifolia (Cav.) G. Don extract, potential phytomedicine to treat chronic inflammation. Bol Latinoam Caribe Plant Med Aromat. 2012;11:468–77. [Google Scholar]
- 17.Pérez-Hernández J, González-Cortazar M, Marquina S, Herrera-Ruiz M, Meckes-Fischer M, Tortoriello J, et al. Sphaeralcic acid and tomentin, anti-inflammatory compounds produced in cell suspension cultures of Sphaeralcea angustifolia. Planta Med. 2014;80:209–14. doi: 10.1055/s-0033-1360302. [DOI] [PubMed] [Google Scholar]
- 18.Romero-Cerecero O, Meckes-Fischer M, Zamilpa A, Enrique Jiménez-Ferrer J, Nicasio-Torres P, Pérez-García D, et al. Clinical trial for evaluating the effectiveness and tolerability of topical Sphaeralcea angustifolia treatment in hand osteoarthritis. J Ethnopharmacol. 2013;147:467–73. doi: 10.1016/j.jep.2013.03.040. [DOI] [PubMed] [Google Scholar]
- 19.Calzada F, Meckes M, Cedillo-Rivera R, Tapia-Contreras A, Mata R. Screening of Mexican medicinal plants for antiprotozoal activity. Pharm Biol. 1998;36:305–30. [Google Scholar]
- 20.Finney DL. Probit Analysis. New York: Cambridge University Press; 1977. p. 20. [Google Scholar]
- 21.Keene AT, Harris A, Phillipson JD, Warhurst DC. In vitro amoebicidal testing of natural products: Part I. Methodology. Planta Med. 1986;4:278–85. [PubMed] [Google Scholar]
- 22.Calzada F, Yepez-Mulia L, Tapia-Contreras A, Ortega A. Antiprotozoal and antibacterial properties of Decachaeta incompta. Rev Latinoam Quím. 2009;37:97–103. [Google Scholar]
- 23.Calzada F, Yepez-Mulia L, Tapia-Contreras A, Bautista E, Maldonado E, Ortega A. Evaluation of the antiprotozoal activity of neo-clerodane type diterpenes from Salvia polystachya against Entamoeba histolytica and Giardia lamblia. Phytother Res. 2010;24:662–5. doi: 10.1002/ptr.2938. [DOI] [PubMed] [Google Scholar]
- 24.Leitsch D, Burgess AG, Dunn LA, Krauer KG, Tan K, Duchêne M, et al. Pyruvate:ferredoxin oxidoreductase and thioredoxin reductase are involved in 5-nitroimidazole activation while flavin metabolism is linked to 5-nitroimidazole resistance in Giardia lamblia. J Antimicrob Chemother. 2011;66:1756–65. doi: 10.1093/jac/dkr192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlosser S, Leitsch D, Duchêne M. Entamoeba histolytica: Identification of thioredoxin-targeted proteins and analysis of serine acetyltransferase-1 as a prototype example. Biochem J. 2013;451:277–88. doi: 10.1042/BJ20121798. [DOI] [PubMed] [Google Scholar]
- 26.Saavedra E, Encalada R, Pineda E, Jasso-Chávez R, Moreno-Sánchez R. Glycolysis in Entamoeba histolytica. Biochemical characterization of recombinant glycolytic enzymes and flux control analysis. FEBS J. 2005;272:1767–83. doi: 10.1111/j.1742-4658.2005.04610.x. [DOI] [PubMed] [Google Scholar]
- 27.Chabrière E, Charon MH, Volbeda A, Pieulle L, Hatchikian EC, Fontecilla-Camps JC. Crystal structures of the key anaerobic enzyme pyruvate:ferredoxin oxidoreductase, free and in complex with pyruvate. Nat Struct Biol. 1999;6:182–90. doi: 10.1038/5870. [DOI] [PubMed] [Google Scholar]
- 28.Chabrière E, Vernède X, Guigliarelli B, Charon MH, Hatchikian EC, Fontecilla-Camps JC. Crystal structure of the free radical intermediate of pyruvate:ferredoxin oxidoreductase. Science. 2001;294:2559–63. doi: 10.1126/science.1066198. [DOI] [PubMed] [Google Scholar]
- 29.Calzada F, Meckes M, Cedillo-Rivera R. Antiamoebic and antigiardial activity of plant flavonoids. Planta Med. 1999;65:78–80. doi: 10.1055/s-2006-960445. [DOI] [PubMed] [Google Scholar]
- 30.Barbosa E, Calzada F, Campos R. In vivo antigiardial activity of three flavonoids isolated of some medicinal plants used in Mexican traditional medicine for the treatment of diarrhea. J Ethnopharmacol. 2007;109:552–4. doi: 10.1016/j.jep.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 31.Lozoya X, Meckes M, Abou-Zaid M, Tortoriello J, Nozzolillo C, Arnason JT. Quercetin glycosides in Psidium guajava L. leaves and determination of a spasmolytic principle. Arch Med Res. 1994;25:11–5. [PubMed] [Google Scholar]
- 32.Day AJ, Dupont MS, Ridley S, Rhodes M, Rhodes MJ, Morgan MR, et al. Deglycosylation of flavonoid and isoflavonoid glycosides by human small intestine and liver beta-glucosidase activity. FEBS Lett. 1998;436:71–5. doi: 10.1016/s0014-5793(98)01101-6. [DOI] [PubMed] [Google Scholar]
- 33.Kim DH, Kim SY, Park SY, Han MJ. Metabolism of quercitrin by human intestinal bacteria and its relation to some biological activities. Biol Pharm Bull. 1999;22:749–51. doi: 10.1248/bpb.22.749. [DOI] [PubMed] [Google Scholar]
- 34.Shin NR, Moon JS, Shin SY, Li L, Lee YB, Kim TJ, et al. Isolation and characterization of human intestinal Enterococcus avium EFEL009 converting rutin to quercetin. Lett Appl Microbiol. 2016;62:68–74. doi: 10.1111/lam.12512. [DOI] [PubMed] [Google Scholar]
- 35.Bolaños V, Díaz-Martínez A, Soto J, Rodríguez MA, López-Camarillo C, Marchat LA, et al. The flavonoid (-)-epicatechin affects cytoskeleton proteins and functions in Entamoeba histolytica. J Proteomics. 2014;111:74–85. doi: 10.1016/j.jprot.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 36.Kapetanovic IM. Computer-aided drug discovery and development (CADDD): In silico-chemico-biological approach. Chem Biol Interact. 2008;171:165–76. doi: 10.1016/j.cbi.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alonso H, Bliznyuk AA, Gready JE. Combining docking and molecular dynamic simulations in drug design. Med Res Rev. 2006;26:531–68. doi: 10.1002/med.20067. [DOI] [PubMed] [Google Scholar]
- 38.Ha CH, Fatima A, Gaurav A. In Silico investigation of flavonoids as potential trypanosomal nucleoside hydrolase inhibitors. Adv Bioinformatics. 2015;2015:826047. doi: 10.1155/2015/826047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galkin A, Kulakova I, Melamud E, Li L, Wu C, Mariano P, et al. Characterization, kinetics, and crystal structure of fructose-1, 6-bisphosphate aldolase from the human parasite, Giardia lamblia. J Biol Chem. 2007;58:7072–82. doi: 10.1074/jbc.M609534200. [DOI] [PubMed] [Google Scholar]
- 40.Singh S, Malik BK, Sharma DK. Molecular modeling and docking analysis of Entamoeba histolytica glyceraldehyde-3 phosphate dehydrogenase, a potential target enzyme for anti-protozoal drug development. Chem Biol Drug Des. 2008;71:554–62. doi: 10.1111/j.1747-0285.2008.00666.x. [DOI] [PubMed] [Google Scholar]
- 41.Bolaños V, Díaz-Martínez A, Soto J, Marchat LA, Sanchez-Monroy V, Ramírez-Moreno E. Kaempferol inhibits Entamoeba histolytica growth by altering cytoskeletal functions. Mol Biochem Parasitol. 2015;204:16–25. doi: 10.1016/j.molbiopara.2015.11.004. [DOI] [PubMed] [Google Scholar]


