Abstract
Background:
The morphological identification is an effective and simple quality evaluation method in Chinese drugs, and the traits of mealiness and color were widely used in the commercial market of Chinese drugs.
Objective:
The objective of this study was to explore the correlation between mealiness of herbal drugs and its quality; licorice was selected as an example.
Materials and Methods:
The mealiness of licorice was graded by its weight; meanwhile, the content of glycyrrhizic acid and liquiritin was determined by high-performance liquid chromatography-diode-array detection method; the content of polysaccharides, soluble sugars, pectin, total starch, amylose, and amylopectin was measured by colorimetric method; and the number and diameter of starch granule were observed by microscope.
Results:
The results showed that the mealiness of licorice which collected from wild and cultivated plants is positively correlated with the content of glycyrrhizic acid, liquiritin, the ratio of amylose to total starch, and the number of starch granules whose diameter was over 5 μm. However, the mealiness is negatively correlated with the total starch. Further, the formation mechanism of starch granule was discussed.
Conclusion:
It is for the first time to report the positive correlation between the mealiness and the starch granule size, the ratio of amylose to total starch, which can provide rationality for the quality evaluation using the character of mealiness in herbal medicine.
SUMMARY
It is a convenient method to justify the quality of herbal medicine. To explore the correlation between mealiness of herbal drugs and its quality, licorice was selected as an example. The result indicated that the effective constituent is correlated with mealiness of licorice.

Abbreviations Used: TCM: Traditional Chinese Medicine.
Key words: Liquorice, mealiness, quality
INTRODUCTION
The traditional Chinese medicines (TCMs) have been used in China for thousands of years, and several traditional standards based on the appearance traits of drugs have been attained by observation of correlations between the drug traits and its clinical efficacy, such as mealiness and color. They are generally used for the quality evaluation of root drugs. Nowadays, the quality of drugs is mainly evaluated by their chemical components; but in some instances, only one single or several components could not truly reflect the efficacy of the Chinese drugs. The quality of a drug, by its nature, should be determined by its clinical efficacy. Take licorice for example, it mainly contains saponins and flavonoids, only glycyrrhizic acid or flavonoids could not reflect its quality, even both of them could not stand for its efficacy for there is polysaccharides in licorice which possess the immunomodulating functions and polysaccharides may enhance the efficacy of licorices.[1] There are still many traditional standards used in TCM nowadays, but its real connotation is absence of research; in addition, the present medicinal materials are different from that of ancient medicinal materials in that most drug materials are made by cultivation now, but almost all the drug materials are derived from wild resources in the past.
It is well known that plants are the production factory of natural chemical components which are regulated by the factors of genes and environments, and the plant-growing status is an effective way to affect its appearance and internal chemical components, so the appearance of a drug is associated with its internal quality. The practitioners of TCM took a longer time to establish a set of unique and simple methods to evaluate the quality of Chinese medicine in the absence of modern analytical instruments. Mealiness is an important morphological index to judge the quality of root drugs. However, its rationality still needs to be elucidated. Previously, the correlation between the appearance, color, and glycyrrhizic acid, liquiritin of licorice, was studied.
MATERIALS AND METHODS
Plant materials
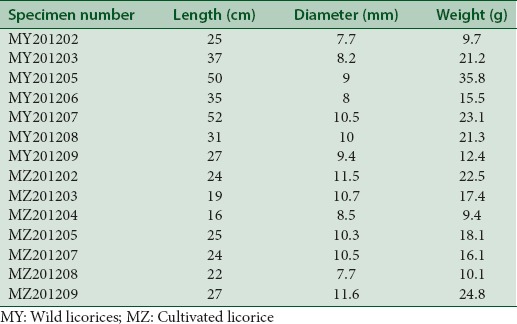
Seven wild and seven cultivated specimens of the medicinally used licorice (Glycyrrhiza uralensis Fisch.) which were both 5 years old were collected from inner Mongolia in the fall of 2012 [Table 1]. Moreover, all the samples were authenticated by Professor Wang Bing (College of Pharmacy, Liaoning University of TCM), and the voucher specimens (No. 20121204) were deposited in the Herbarium of College of Pharmacy, Liaoning University of TCM. Each root of licorice was cut off with a section of 7–11 mm in diameter for the subsequent analysis.
Table 1.
Samples of licorice
Standard compounds and reagents
Ammonium glycyrrhetate (ID: AK6D-KMWT) and liquiritin (ID: 9UJD-1ATS) were both purchased from National Institute for the Control of Pharmaceutical and Biological Products. Amylopectin and amylose were isolated from starch, and they were exactly identified by comparison of the spectral data (ultraviolet [UV] spectrum) with those reported in previous literature. High-performance liquid chromatography (HPLC)-grade acetonitrile and methanol were purchased from Oceanpak Alexative Chemical, Ltd. Analytical grade anthrone, concentrated sulfuric acid, glucose, phenol, carbazole, pectinase, galacturonic acid, ethanol, and glycerol were all purchased from Sinopharm Chemical Reagent Co., Ltd., China.
Apparatus
The Agilent 1100 HPLC system (Agilent Technologies, USA) comprised G1311C quat pump, G1329B autosampler, and G1316A Column thermostat. G1315D diode-array detection (DAD) was used to determinate the contents of glycyrrhetinic acid and liquiritin. UV-2100 spectrophotometer (UNICO Ltd., USA) was used to determinate the contents of polysaccharides, pectin, soluble sugars, total sugars, total starch, amylose, and amylopectin. MOTIC digital microscope system (Motic Ltd., China) and BX51 biological microscope (Olympus Co., Japan) were used to determinate the diameter and the amount of starch granules.
Measurement of glycyrrhizic acid and liquiritin
The contents of glycyrrhizic acid and liquiritin were determined.[2] The chromatographic conditions were as follows. All samples were performed on an Agilent 1100 HPLC equipped with DAD detector over Phenomenex HyperClone ODS-C18 (4.6 mm × 250 mm, 5 μm). The mobile phase composed of 0.1% phosphoric acid–water (A) and acetonitrile (B) with gradient elution (15%–20% B in 0–10 min, 20%–24.8% B in 10–17 min, 24.8%–26.9% B in 17–20 min, 26.9%–30% A in 20–24.5 min, 30%–80% A in 24.5–65 min, and 80%–15% A in 65–70 min). The flow rate of the mobile phase was 0.5 mL/min in 0–10 min and 0.8 mL/min in 10–70 min. Detector wavelengths were 276 nm in 0–17 min, 360 nm in 17–24.5 min, and 248 nm in 24.5–248 min. And, the temperature was maintained at 25°C.
The preparation of sample solution is as follows. First, the licorice was pulverized to pass through a 60 mesh sieve, accurately weighed 100 mg licorice powder was transfered to 25 mL volumetric flask and soaked for 24 h with 67% methanol, and then extracted with ultrasonic cleanser (250 W, 80 Hz) for 30 min. And then, after cooling, 67% methanol was added to the constant volume and filtered with 0.45 μm millipore membranes before HPLC analysis.
Measurement of the mealiness
Mealiness can be indicated by the amount of the powder falling from the fracture of licorice. As usual, the more falling powders when the liquorice is snapped, the stronger mealiness of licorice is, so the mealiness was ranked as low grade (<1.5 mg), moderate grade (1.5–3.0 mg), or high grade (>3 mg), depending on the weight of the falling powders and they are scored as 1, 2, and 3, respectively.
Measurement of the amount and the size of starch granules
About 20 mg of the sample was dried for 12 h at 60°C and then passed to 60 mesh fineness, transferred into 25 mL volumetric flask, 25 mL glycerinum-50% acetic acid–water (1:1:1) was added, and then fixed. Moderate sample solution was added into cell-count boards, and then four areas were random selected, composed of 64 compartments. In the view of a polariscope, the starch granules were identified, and in the view of normal field of vision, its amounts and areas with MOTIC digital microscope system were measured. The radius was converted by its areas.
Measurement of soluble sugar content
The method of anthrone colorimetry was used,[3] accurately weighed 50 mg of licorice powder was passed through a 60 mesh sieve, into a 10 mL glass tube, and then 8 mL 80% ethanol was added and sealed. The mixture was heated in a water bath at 80°C to extract it for 30 min, centrifuged (4000 rev/min) for 5 min, and then the supernatant layer was collected. The process was repeated twice more and the supernatant layers were collected of 3 times into 25 mL volumetric flask and 80% ethanol was added to the volume. 1 mL of the extract was pipetted, diluted with 10 mL of water, and mixed. 2 mL of the mixed extract was added to 0.5 mL of 2% anthrone ethyl acetate solution, and then 5 mL of concentrated sulfuric acid was slowly added along the wall of the tube, mixed well, placed on the tube rack in water bath at 100°C for 1 min, and then cooled it with ice water. The absorbance was measured under 620 nm of wavelength.
Measurement of total polysaccharide content
The method of phenol colorimetry was used.[4] In brief, the dried residue extracted with 80% ethanol was ultrasonically extracted (250 W, 80 Hz) with water for 30 min and centrifuged (4000 rev/min) for 5 min to give the supernatant solution. The process was repeated twice more and the supernatant solution was collected of 3 times into 25 mL volumetric flask and water was added to the volume.
To 1 mL of the solution, 1 mL of 5% phenol was added, 5 mL of concentrated sulfuric acid was added dropwise along the wall of the tube, mixed well, and standstill for 25 min at room temperature. And then, the absorbance was measured at 490 nm.
Measurement of pectin
Carbazole colorimetry was used for the measurement.[5,6] In brief, accurately weighed 300 mg of licorice fine powder was passed through 60 mesh sieve into a 50 mL centrifuge tube and extracted with 25 mL 80% ethanol in water bath at 80°C for 30 min, centrifuged (4000 rev/min) for 5 min, and then the supernatant solution was discarded. Then, the process was repeated once and the residue was dried. 200 U pectinase and 25 mL phosphate-buffered saline (pH 4) were added to hydrolyze the residue at 50°C for 24 h, cooled with ice water, and 5 mL of concentrated sulfuric acid was added slowly. After heating in boiling water bath for 10 min, it was cooled to room temperature, 0.5 mL of 5% carbazole diluted with ethanol was added, heated in boiling water bath for 15 min, and cooled to room temperature. The absorbance was measured at 530 nm.
Measurement of amylose, amylopectin, and total starch
Iodine colorimetry was used.[7,8] In brief, accurately weighed 100 mg of licorice powder was passed through 60 mesh sieve into a 50 mL beaker, 1 mL anhydrous ethanol and 9 mL of 1 mol/L NaOH were added, stirred in water bath at 85°C ± 1°C for 20 min, transferred to 25 mL volumetric flask, and water was added to the volume. A volume of 10 mL of the solution was taken and centrifuged (4000 rev/min) for 10 min. Then, 5 mL of the supernatant was taken in 50 mL volumetric flask and 25 mL water was added, the pH value was adjusted to 3.0 with 0.1 mol/L HC1 solution, and then 0.5 mL iodine-potassium iodide reagent and water were added to the volume. Standstill at 25°C ± 2°C for 25 min, the difference between the absorbance value at 492 nm and 634 nm was measured to calculate the content of amylose, difference between the absorbance value at 560 and 735 nm was measured to calculate the content of amylopectin, and the total starch is the summation of amylose and amylopectin.
Statistical analysis
Statistical analysis was performed to analyze the correlation between each pair of diameter, the content of glycyrrhizic acid and liquiritin, polysaccharides, soluble sugars, pectin, total polysaccharides, total starch, amylose and amylopectin, the amount and diameter of starch granule; P < 0.05 or 0.01 was deemed a statistically significant difference. Correlation analysis was performed using IBM SPSS Statistic software (version 19.0).
RESULTS
Determination of the constituents related to mealiness
Several primary and secondary metabolites of licorice which may reflect the physical characteristics of its plant or had been deemed to be active components were chosen to analyze their correlations with mealiness and their results were summarized in Table 2. Further, the correlation analysis results of wild and cultivated liquorice were, respectively, shown in Tables 3 and 4.
Table 2a.
Results of different indexes of licorice
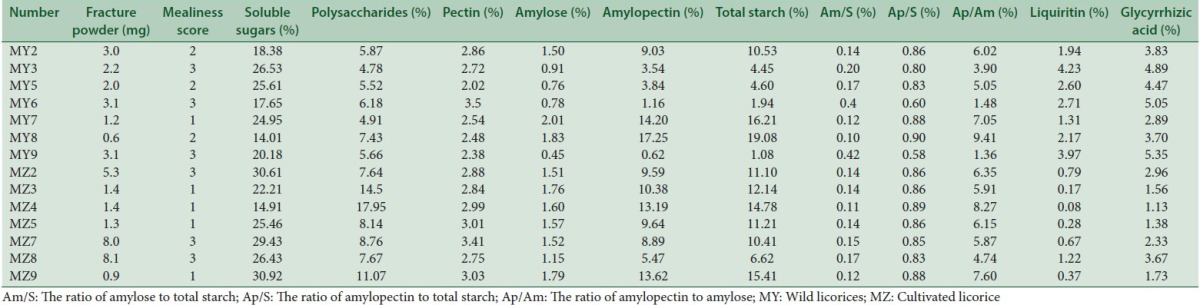
Table 3.
Pearson's correlation coefficients in the correlation analysis of wild liquorice
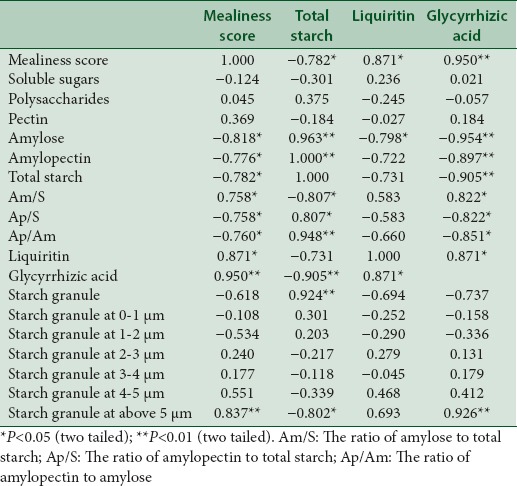
Table 4.
Pearson's correlation coefficients in the correlation analysis of cultivated liquorice
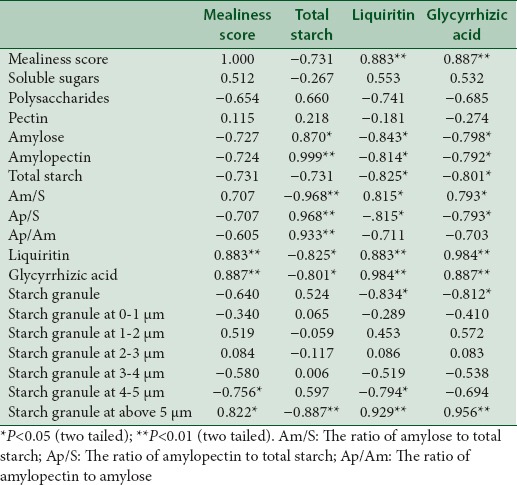
Table 2b.
Results of different sizes of starch granules
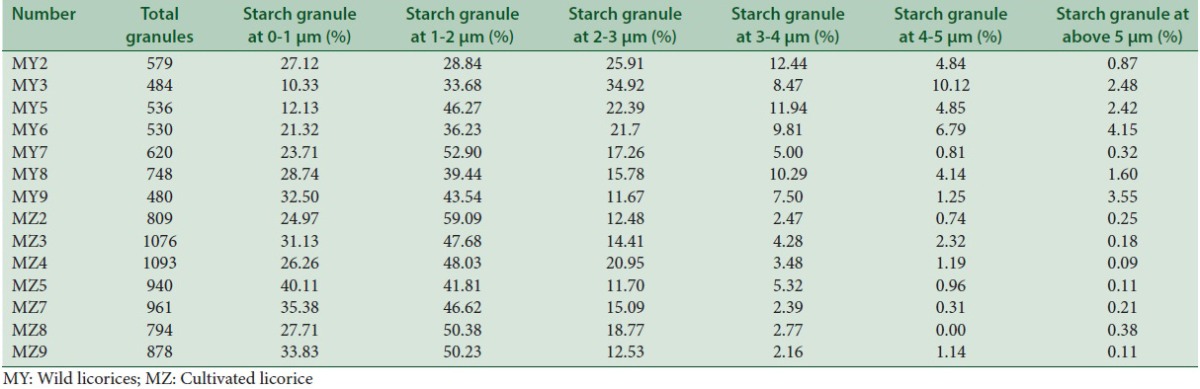
In the correlation analysis of wild liquorice, the mealiness had significant positive correlation with the contents of glycyrrhizic acid, liquiritin, the content ratios of amylose to total starch, and the amount of starch granule with the radius over 5 μm. However, it had a negative correlation with the contents of amylose, amylopectin, total starch, the content ratios of amylopectin to total starch and amylopectin to amylose.
In the correlation analysis of cultivated liquorice, mealiness had significant positive correlation with the contents of glycyrrhizic acid, liquiritin, the ratio of starch granule with the radius over 5 μm, and also the content ratios of amylose to total starch (Pearson's coefficient: 0.707, P = 0.075). The mealiness had negative correlation with the contents of polysaccharides (Pearson's coefficient: −0.654, P = 0.111), amylose (Pearson's coefficient: −0.727, P = 0.064), amylopectin (Pearson's coefficient: −0.724, P = 0.066), total starch (Pearson's coefficient: −0.731, P = 0.062), content ratios of amylopectin to total starch (Pearson's coefficient: −0.707, P = 0.075), amylopectin to amylose (Pearson's coefficient: −0.605, P = 0.15), and also the ratio of starch granule with the radius at 4–5 μm, which was different from the wild liquorice.
The chromatogram of liquiritin, glycyrrhizic acid, and liquorice is shown in Figure 1.
Figure 1.
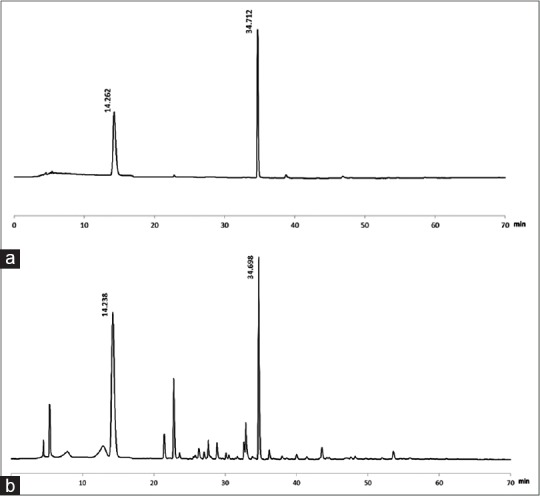
(a) The chromatogram of liquiritin and glycyrrhizic acid. Liquiritin tR = 14.263 min, glycyrrhizic acid tR = 34.712 min. (b) Chromatogram of liquorice. Liquiritin tR = 14.238 min, glycyrrhizic acid tR = 34.698 min
DISCUSSION
The mealiness, the external visual index for the quality evaluation of licorice, has still been used nowadays for the preliminary examination by the sophisticated apothecaries and it is an experience discovered by the ancient practitioners of TCM. This would have given researchers an obvious inspiration to analyze the falling power, which was the direct factor to the mealiness phenomenon. By microscopic identify the collecting powders from fracture of licorice, we found that the field under microscope was full of starch granules and other substances were hardly found. This indicated that the starch had a significant impact on the mealiness formation.
The traditional practitioners think that mealiness is related to the contents of starch, even in college textbooks.[9] However, the recent research indicated that the mealiness is positively correlated with glycyrrhizic acid and liquiritin, but negatively correlated with total starch,[10,11] enlightening that the traditional recognition is in question. Traditionally, only wild licorice was used, but now, most of our materials of licorice are obtained from cultivation resources. Thus, the wild and cultivated licorices were collected and only the similar radius section of licorice was selected so as to give a parallel comparison. In addition, the weight of powder from fracture was first used to rank the level of the mealiness of licorice to diminish the deviation from observation assay. Our result indicated that the mealiness is positively correlated with glycyrrhizic acid and liquiritin and negatively correlated with starch in both wild and cultivated licorice. Further analysis indicated that mealiness is positively correlated with the amount of bigger size starch granule, revealing that mealiness is only positively correlated with bigger size starch granule instead of total starch. As usual, glycyrrhizic acid and liquiritin are regarded as the active constituents of licorice. Thus, mealiness, as a traditional index, is reasonable in quality evaluation of licorice.
The mechanism of mealiness formation
As we know, the mealiness is the main sensory evaluation of Chinese drugs possessing higher starch. As usual, in the process of drying, starch would gelatinize to be a kind of adhesive, which helps to bind the cells and substances together, enlightening us that maybe the size of starch particles can affect its mealiness.
Starch consists of amylose and amylopectin. The starch granules usually exist in the plants. The process of starch gelatinization took place on the reaction between water and starch granule, and its sizes could affect the reaction, the smaller size of starch granule has larger surface area to interact easily with water. However, we can only observe the ambiguous correlation between the mealiness and the total amount of starch granule and it may be caused by the composition of different sizes of starch granule. However, the starch granule with size over 5 μm in both wild and cultivated licorice had significant positive correlation with mealiness. It can be known that the starch granules grow larger in the plants as the growing years increase; simultaneously, the glycyrrhizic acid and liquiritin increase. Thus, the formation of mealiness may have a relationship with the larger starch granules.
Meanwhile, the ratio of amylose to total starch in both wild and cultivated liquorice had significant positive correlation with mealiness. Researches indicated that the amylopectin in plant synthesizes first, and then the chain in the periphery of amylopectin broke and falls off to further form amylose.[12,13] As usual, the glycyrrhizic acid and liquiritin were regarded as the active components accumulated as the plants grow and their contents in licorice with above 4 years could reach the standard of Chinese Pharmacopeia.
CONCLUSION
Mealiness is an important marker to evaluate the quality of root and rhizome of herbal drugs. Our results found that in both cultivated and wild liquorice, the mealiness had a significant positive correlation with glycyrrhizic acid and liquiritin, and ratios of amylose to total starch and the amount of starch granule at over 5 μm, revealing the bioaccumulation trends of different chemical constituents in plants. In addition, it was interesting that in both cultivated and wild liquorice, the mealiness had significant negative correlation with the content of amylose, indicating that the amylose or content ratios of amylose to total starch could affect the size of starch granule formation. It is for the first time that the mealiness of licorice is correlated with the starch granule size and the rationality of mealiness in the quality control was further elucidated.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Liu H, Wang J, Zhou W, Wang Y, Yang L. Systems approaches and polypharmacology for drug discovery from herbal medicines: An example using licorice. J Ethnopharmacol. 2013;146:773–93. doi: 10.1016/j.jep.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Tian-Xuan D, Mi-Mi YU, Chun-sheng L, Chang-Hua M, Wen-Quan W, Sheng-Li W. Simultaneous determination of glycyrrhizic acid, liquiritin and fingerprint of licorice by RP-HPLC. Chin Tradit Pat Med. 2006;28:161–5. [Google Scholar]
- 3.He-Sheng L. Plant Physiology Experiment Instruction. Beijing: China Agriculture Press; 1999. pp. 194–6. [Google Scholar]
- 4.Villanueva-Suárez MJ, Redondo-Cuenca A, Rodríguez-Sevilla MD, de las Heras Martínez M. Characterization of nonstarch polysaccharides content from different edible organs of some vegetables, determined by GC and HPLC: Comparative study. J Agric Food Chem. 2003;51:5950–5. doi: 10.1021/jf021010h. [DOI] [PubMed] [Google Scholar]
- 5.Taylor KA. A colorimetric method for the quantitation of galacturonic acid. Appl Biochem Biotechnol. 1993;43:51–4. [Google Scholar]
- 6.Bitter T, Muir HM. A modified uronic acid carbazole reaction. Anal Biochem. 1962;4:330–4. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- 7.Ming-Shun F, Chong-Gu Z, Qin Z, Xiao-Fei L. Determination of amylose and amylopectin content in sorghum by dual wavelength method. China Brewing. 2008;15:21–7. [Google Scholar]
- 8.Mei-Juan L, Jiang-Feng S, Da-Jing L, Chun-Quan L. Determination of amylose and amylopectin content in fresh corn by dual-wavelength spectrophotometry. Acta Agric Jiangxi. 2010;15:22:117–9. [Google Scholar]
- 9.Ting-Guo K. Chinese medicine identification. Beijing: Chinese Press of Traditional Chinese Medicine; 2011. [Google Scholar]
- 10.Chun-Sheng L, Xue-Yong W, Ren-Quan L, Bo N, Yan-Jie Z. Research on the correlation between the content of starch and glycyrrhizic acid of licorice. China J Chin Mater Med. 2009;21:2831–2. [Google Scholar]
- 11.Jun-Bo X, Wen-Quan W. Comparative Study on the Chemical Characters in Different Authentic Traits of Glycyrrhiza uralensis. The Ninth Session of Chinese Medicine Identification of Academic Conference Proceedings of China Chinese Medicine Institute.2008. [Google Scholar]
- 12.van de Wal M, D'Hulst C, Vincken JP, Buléon A, Visser R, Ball S. Amylose is synthesized in vitro by extension of and cleavage from amylopectin. J Biol Chem. 1998;273:22232–40. doi: 10.1074/jbc.273.35.22232. [DOI] [PubMed] [Google Scholar]
- 13.Maddelein ML, Libessart N, Bellanger F, Delrue B, D'Hulst C, Van den Koornhuyse N, et al. Toward an understanding of the biogenesis of the starch granule. Determination of granule-bound and soluble starch synthase functions in amylopectin synthesis. J Biol Chem. 1994;269:25150–7. [PubMed] [Google Scholar]


