Abstract
Background:
Atherosclerosis is one of the major causes of morbidity and mortality in the world. Antioxidants play a major role in prophylaxis and prevention of progression and complications of atherosclerosis.
Objective:
In this study, we are evaluating the antiatherosclerotic effect of two antioxidants such as astaxanthin and lycopene.
Materials and Methods:
After acclimatization, 24 male SD rats, 8–10 weeks old, 150 ± 10 g, maintained as per CPCSEA guidelines were divided into four groups of six rats each. Baseline values of weight lipid profile and 2-Thiobarbituric Acid Reactive Substances (TBARS) assay were taken. All the rats were fed with high cholesterol diet (HCD). HCD only, HCD + atorvastatin (50 mg/kg), HCD + lycopene (50 mg/kg), and HCD + astaxanthin (50 mg/kg) were given to control, standard, lycopene, and astaxanthin groups, respectively, through oral gavage for 45 days. The rats were sacrificed at the end of the study, blood sample collected from aorta, and then aorta was dissected for histopathology.
Results:
The lipid profile showed lycopene and astaxanthin decreased total cholesterol, low-density lipoprotein-cholesterol (LDL-C), very LDL-C, and triglycerides and increased high-density lipoprotein-cholesterol level significantly (P < 0.05) compared to the control but less than atorvastatin. The TBARS value of lycopene was significantly lower compared to HCD and atorvastatin groups, whereas astaxanthin was significantly less than HCD group only. The histopathology showed only Type I lesions, no naked fatty streaks, few foam cells in lycopene, and astaxanthin groups compared to control where we observed Type II and III lesions, visible fatty streaks and many foam cells with intimal thickening in HCD group.
Conclusion:
In this study, lycopene and astaxanthin showed antioxidant, antihyperlipidemic, and antiatherosclerotic property. This warrants further study for including them in the treatment of atherosclerosis.
SUMMARY
Antioxidants play a major role in prophylaxis and prevention of progression and complications of atherosclerosis.
Lycopene and Astaxanthin are suitable candidates for further research in cardiovascular disease and there is paucity of studies evaluating their role in prevention of atherosclerosis.
The effect of lycopene and Astaxanthin for anti-atherosclerotic property was evaluated in high cholesterol diet fed rats.
The lipid profile showed lycopene and Astaxanthin decreased TC, LDL-C, VLDL-C and triglycerides and increased HDL-C level significantly (P < 0.05) compared to the control but less than atorvastatin.
The TBARS value of Lycopene was significantly lower compared to HCD and atorvastatin groups while Astaxanthin was significantly less than HCD group only.
The histopathology showed only type I lesions, no naked fatty streaks, few foam cells in lycopene and Astaxanthin groups compared to control.
The study proves Lycopene and Astaxanthin have hypolipideamic and anti-atherogenic potential can be included in the treatment of hypercholesterolemia and atherosclerosis.

Abbreviations Used: TC: total cholesterol, HDL-C: high density lipoprotein cholesterol, LDL-C: low density lipoprotein cholesterol, VLDL-C: very Low density lipoprotein cholesterol, TG: triglycerides, and TBARS: thiobarbituric acid reactive substances, HCD: high cholesterol diet.
Key words: Antioxidant, astaxanthin, atherosclerosis, hypercholesterolemia, lycopene
INTRODUCTION
Atherosclerosis is one of the major modifiable risk factors in cardiovascular disease, and stroke responsible for most of the casualty admission all over the world. The global burden of noncommunicable diseases is on the rise not only in the developed countries but also in the developing and underdeveloped countries are contributing to the burden. According to the World Health Organization, Global burden of diseases (2014), ischemic heart disease and stroke together are responsible for approximately 13.2% of deaths worldwide.[1] The thought that the noncommunicable diseases were only the burden of developed countries is changing.
Atherosclerosis develops slowly, and its progress can be modified by various factors. Reviews of scientific and epidemiological studies reveal that the onset and progression of atherosclerosis is delayed or reversed in some demographic population due to differences in lifestyle, food habits, smoking, and family history. Oxidative stress and inflammation are the important processes in the development of atherosclerosis.[2,3]
Apart from the conventional medical treatment, the need for new, effective, and less toxic substances for the prevention of atherosclerosis turned the scientific community toward the search for antioxidants. Vitamin A served as the prototype for most of the studies, followed by Vitamin C and Vitamin E. These studies established the role of antioxidants in scavenging the free radicals produced during oxidative stress and paved the way for the discovery of a new group of compounds present in nature called carotenoids. The first of these was β-carotene, the precursor of Vitamin A.
Carotenoids are classified into carotene and xanthophyll groups, both of which are potent antioxidants, evaluated for antiatherosclerotic property.[4]
Lycopene is an antioxidant belonging to carotene group abundantly found in processed red tomatoes (tomato sauce) and red fruits. It is one of the most potent singlet oxygen quenchers among the natural carotenoids. Its quenching ability is twice as high as that of ß-carotene, 100 times as that of α-tocopherol.[5]
Astaxanthin is a strong antioxidant and anti-inflammatory carotenoid, predominantly present in marine food products.[6,7] The unique structure of the terminal ring moiety is probably the cause behind the efficient antioxidant activity of the naturally occurring carotenoid pigment.[8]
Thus, lycopene and astaxanthin are suitable candidates for further research in cardiovascular disease, and there is a paucity of studies evaluating their role in the prevention of atherosclerosis. We planned to evaluate the effect of lycopene and astaxanthin for antiatherosclerotic property in high cholesterol diet (HCD)-fed rats.
MATERIALS AND METHODS
Animals
Healthy Male Sprague-Dawley rats of 8–10 weeks old weighing 150 ± 10 g procured from registered breeder and maintained in institutional central animal house. The rats were sheltered in the animal house in clean polypropylene cage, under the temperature range of 18–29°C, humidity range of 30%–70%, with well-ventilated, air-conditioned room. The rats were provided with dry paddy husk bedding changed every alternate day, standard pellet feeding, water ad libitum, and 12:12 h light dark cycle in the animal house and taken care according to the CPCSEA guidelines.[9] The study protocol was approved by the Institutional Animal Ethics Committee, Registration No. 686/02/a/CPCSEA.
Chemicals and drugs
Lycopene was procured from Biorigine Life Science Pvt Ltd., Puducherry, India as 10% formulation. Astaxanthin was also procured from Biorigine Life Science Pvt Ltd., Puducherry, India as 20% formulation. Atorvastatin manufactured by Abbott Laboratories, Mumbai, Maharashtra, India was purchased from the local chemist. Propylthiouracil manufactured by Macleods Pharmaceuticals, Mumbai, Maharashtra, India was purchased from the local chemist. Chemicals and reagents used for the study were of analytical grade. Total cholesterol (TC), high-density lipoprotein-cholesterol (HDL-C), triglycerides, and Thiobarbituric Acid Reactive Substances (TBARS) kit manufactured by Sigma-Aldrich Corporation, Bengaluru, Karnataka, India was purchased from the local chemist.
High cholesterol diet
An oil-based suspension of 100 g cholesterol, 30 g propylthiouracil, and 100 g cholic acid in 1 L of peanut oil prepared fresh and fed to the rats at a dose of 1 ml/100 g body weight daily.[10,11]
Methods
After acclimatizing the rats for 1 week, the animals were weighed and divided into four groups of six rats each. Before commencing the experiment, baseline values of weight, lipid profile (TC, HDL, low-density lipoprotein [LDL], very LDL [VLDL], and TG) and TBARS were taken for all the four groups. Group 1 received HCD (1 ml/kg BW) orally with gavage and served as control. Group 2 received HCD and atorvastatin (50 mg/kg BW/day) orally with gavage and served as standard. Group 3 received HCD and lycopene (50 mg/kg BW/day) orally with gavage and served as test group for lycopene. Group 4 received HCD and astaxanthin (50 mg/kg BW/day) orally with gavage and served as test group for astaxanthin. All the four groups were fed with their respective drugs and chemicals for 45 days once daily with a steel gavage tube. Apart from the gavage feed, the rats were provided with standard pellet diet, water ad libitam associated with care as mentioned above.
The rats were weighed every week and observed for any change in the behavior throughout the study period. The dose of lycopene was taken from the European Food Safety Authority,[12] and the dose of astaxanthin was taken from the study done by Hussein et al.[13]
After 45 days of gavage feeding, the rats were sacrificed by cervical dislocation with minimum pain. As per the method of Filios et al., immediately, the rat's thorax was cut open the aorta was identified and cannulated with a needle. Blood sample was collected and labeled for TBARS assay, then flushed with a normal physiological salt solution with an outlet in the jugular vein. After flushing, the aorta is dissected at the root of the arch of aorta and at the root of the iliac bifurcation. The aorta is slit open vertically in physiological solution, and the gross features of atherosclerosis were noted and photographed and then preserved for histopathology in 10% formaldehyde. Each specimen was labeled with the rat number in specimen bottles and despatched to central laboratory.
The histopathological study of the gross specimen of the aorta for fatty streaks
The specimen was injected with Sudan IV and observed for lipid deposition (red color) with ×40 magnification, and photomicrograph was taken for analysis. Grittiness while sectioning the specimen was observed for identifying calcification of atheromatous plaque.
The slices which had the streaks and/or grittiness and those suspected of having the plaques were taken for preparing the slides for microscopic confirmation. The slices were made with microtome and fixed on the slides, followed by staining procedure with hematoxylin and eosin. The prepared slides were visualized under light microscope with various magnification, and the percentage of intimal thickening and atherosclerosis was calculated with image analyzer as per the classification in Stary et al. from Type I to Type VI as below:[14,15]
Type I (initial) lesion: Isolated macrophage, foam cells
Type II (fatty streak) lesion: Mainly intracellular, lipid accumulation
Type III (intermediate) lesion: Type II changes + small extracellular lipid pool
Type IV (atheroma) lesion: Type II changes + core of extracellular lipid
Type V (fibroatheroma) lesion: Lipid core + fibrotic layer or multiple lipid cores + fibrotic layers or mainly calcific or mainly fibrotic
Type VI (complicated) lesion: Surface defect, hematoma – hemorrhage, thrombus.
The estimation of TC was done by the method described by Wybenga et al.,[16] Triglycerides by Bucolo and David,[17] HDL-C was estimated by the method described by Burstein et al.,[18] all using the kit already described, LDL-cholesterol (LDL-C) and VLDL-cholesterol (VLDL-C) were calculated as described by Friedewald et al.[19] Serum peroxidation level was measured with Lipid Peroxidation (TBARS) Assay Kit of Sigma-Aldrich, Inc. In this assay, metabolites of lipid peroxidation (malondialdehyde) combine with the thiobarbituric acid to form thiobarbituric acid reactive species which produces a reddish pink color. This is read spectrometrically at 532 nm and expressed in nmol/ml.[20]
Statistics
All the variables are presented as mean ± SD. Data were analyzed using a one-way ANOVA followed by post hoc Least Significant Difference test. Calculations were performed using SPSS (Statistical Package for Social Sciences) Statistics for Windows, Version 17.0. Chicago: SPSS Inc. Released 2008. In all cases, P < 0.05 was considered statistically significant.
RESULTS
Effect of lycopene and astaxanthin on body weight
The weight of the rats in the HCD, atorvastatin, and lycopene group at the beginning of the study was 149.16 ± 4.83 g, 152.33 ± 7.06 g, and 149.00 ± 7.64 g, respectively. All the groups showed increase in weight at the end of the study. The increase in weight was significantly more in HCD group (292.83 ± 5.45 g) than atorvastatin group (196.16 ± 4.26 g), lycopene group (208.16 ± 7.38 g), and astaxanthin group (174.50 ± 4.84 g) with P < 0.05. Increase in weight in astaxanthin group was less marked than atorvastatin group and lycopene group [Table 1].
Table 1.
Comparison of weights of the rats at the end of the study to that of baseline

Effect of lycopene and astaxanthin on lipid profile
The total serum cholesterol has increased significantly in all groups except Group 2 compared to the baseline. The total serum cholesterol in Group 3 (125.16 ± 03.86 mg/dl) and Group 4 (119.83 ± 03.92 mg/dl) is decreased significantly compared to the Group 1, but Group 2 was found better than both test groups. The serum HDL-C is increased significantly in Group 3 (51 ± 6.29 mg/dl) and Group 4 (49.5 ± 2.34 mg/dl) compared to the baseline and Group 1 (31.83 ± 2.78 mg/dl). The increase in serum HDL-C in between Group 2, Group 3, and Group 4 is insignificant, but the lycopene group has shown a maximum rise in serum HDL-C level compared to other groups.
The serum LDL-C is reduced significantly in Group 2, Group 3, and Group 4 compared to the baseline and the Group 1. The reduction of serum LDL-C levels by Group 2 is significantly more than the other three groups, even though there is slight rise compared to the baseline.
The serum VLDL-C was reduced significantly by Group 2, Group 3, and Group 4 compared to the baseline and Group 1. The reduction of serum VLDL-C levels in Group 2 is significantly more compared to the Group 3 and Group 4. The reduction in serum VLDL-C is significantly greater in Group 4 compared to Group 3.
The serum triglycerides are reduced significantly by Group 2, Group 3, and Group 4 compared to the baseline and Group 1. The reduction of serum triglycerides by Group 2 is significantly greater compared to Group 3 and Group 4. The reduction in serum triglycerides is significantly greater by Group 4 compared to Group 3.
The result shows Group 3 (lycopene) and Group 4 (astaxanthin) reduced the TC, LDL-C, VLDL-C, and TG levels significantly compared to control, but reduction is not as significant as standard atorvastatin group. Lycopene and astaxanthin both increased HDL-C without significant difference compared to atorvastatin, but significant increase is present compared to the control [Table 2].
Table 2.
Serum total cholesterol, high.density lipoprotein.cholesterol, low.density lipoprotein.cholesterol, very low-density lipoprotein-cholesterol, and triglyceride levels of control, standard (atorvastatin), lycopene, and astaxanthin groups
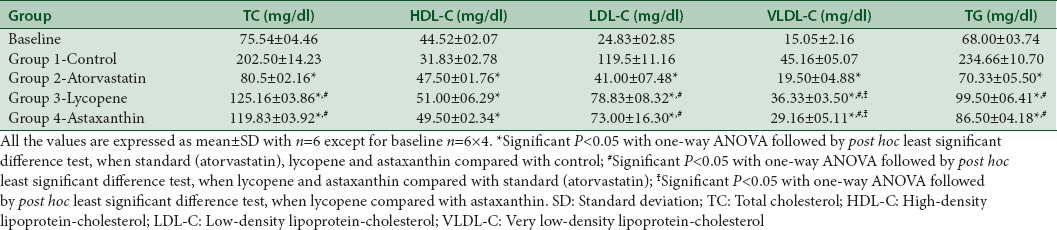
Effect of lycopene and astaxanthin on 2-Thiobarbituric Acid Reactive Substances assay
Serum TBARS levels in group 1 (6.4 ± 0.59 nmol/ml) were significantly higher than the baseline and the other three groups. Group 3 (lycopene) showed significantly lower values of TBARS levels compared to Group 1 (HCD) and Group 2 (atorvastatin) and approximately equals to baseline values. The reduction of TBARS levels of Group 4 (astaxanthin) was significantly lower compared to Group 1, but the difference is insignificant when compared to the Group 2 and Group 3 [Graph 1].
Graph 1.
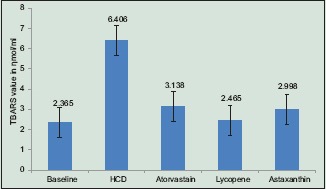
Bar diagram showing the levels of Thiobarbituric Acid Reactive Substances of control, standard (Atorvastatin), lycopene, and astaxanthin groups
Effect of lycopene and astaxanthin on histopathological changes in aorta
Gross
The fatty streaks in the aorta were visible to the naked eye only in two rats of the HCD group, and none were observed in the atorvastatin, lycopene, and astaxanthin groups. After Sudan IV injection into the aorta and observation under ×40 magnification showed multiple red dots in all the six rats of the HCD group, few tiny dots in 1 rat of atorvastatin and few tiny dots in all the six rats of the lycopene and astaxanthin group were seen.
The areas of these red dots were measured using the microphotograph taken by image analyzer. The results of the analysis are depicted in the bar diagram [Graph 2] in square micrometer in mean ± SD. There was a significant difference in the atherosclerosis area formed between HCD group and the other three groups. The difference in the value between atorvastatin group and lycopene seems to be present (P = 0.29) and to that of astaxanthin group (P = 0.652). The difference between lycopene group and astaxanthin group was statistically insignificant [Graph 2].
Graph 2.
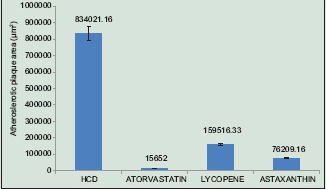
Effect of high cholesterol diet, atorvastatin, lycopene, and astaxanthin in atherosclerotic rats (Atherosclerotic plaque area measured in μm2)
Histopathology
Effect of high cholesterol diet on aorta of control group rats
The H- and E-stained aorta on × 400 magnification observation showed intimal thickening (double headed arrow) of Type III in only one rat of HCD group, and the other five showed Type I and II grade intimal thickening with numerous foam cells (single-headed arrow) in the intima and media [Figure 1].
Figure 1.
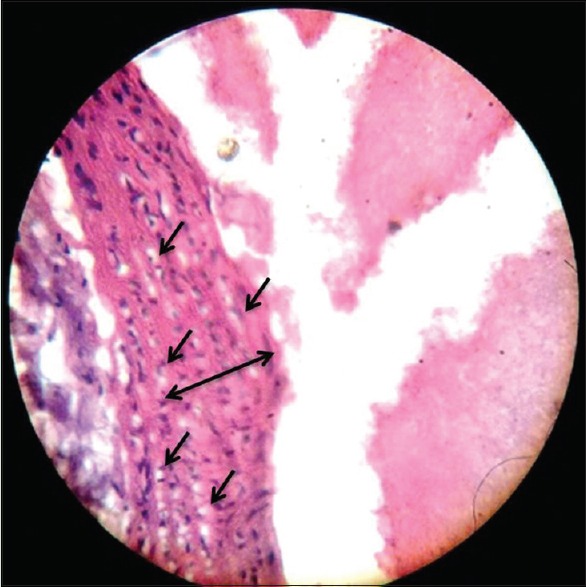
Atheromatous lesion showing intimal thickening, multiple foam cells in high cholesterol diet-fed rat's aorta (×400)
Effect of atorvastatin on aorta of high cholesterol-fed rats
Atorvastatin group had only one rat with intimal thickening of Type I with few foam cells. Atorvastatin-fed rats showed minimal or no sign of atherosclerosis [Figure 2].
Figure 2.
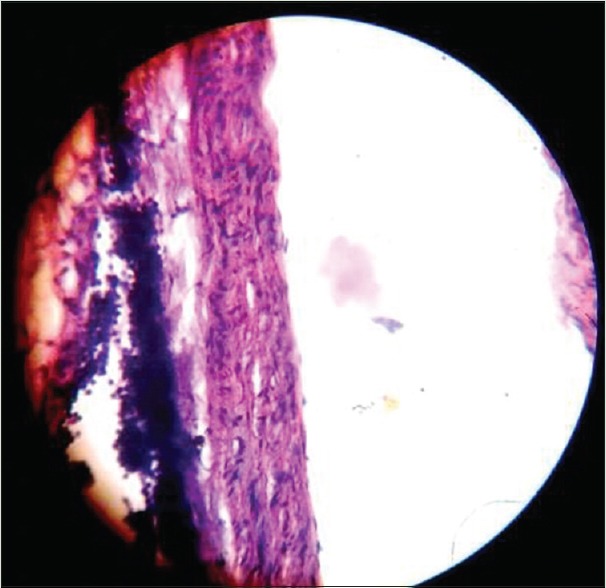
Aorta showing normal histology in aorta of atorvastatin-fed rats (×400)
Effect of lycopene on aorta of high cholesterol-fed rats
Lycopene group showed intimal thickening (double headed arrow) of Type I and II with few foam (single-headed arrow) cells in two rats out of six in the groups, which is significantly low compared to the control [Figure 3].
Figure 3.
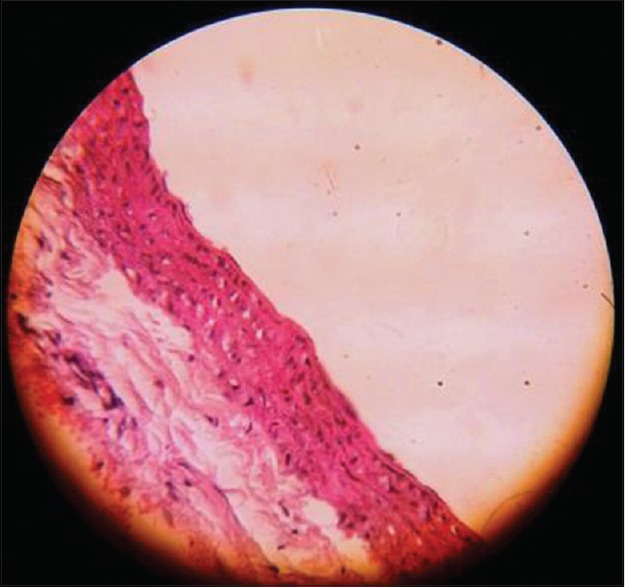
Atheromatous lesion showing minimal intimal thickening and few foam cells in lycopene-fed rats (×400)
Effect of astaxanthin on aorta of high cholesterol-fed rats
Astaxanthin group showed intimal thickening (double headed arrow) of Type I and II with few foam cells (single-headed arrow) in two rats out of six in the group [Figure 4].
Figure 4.
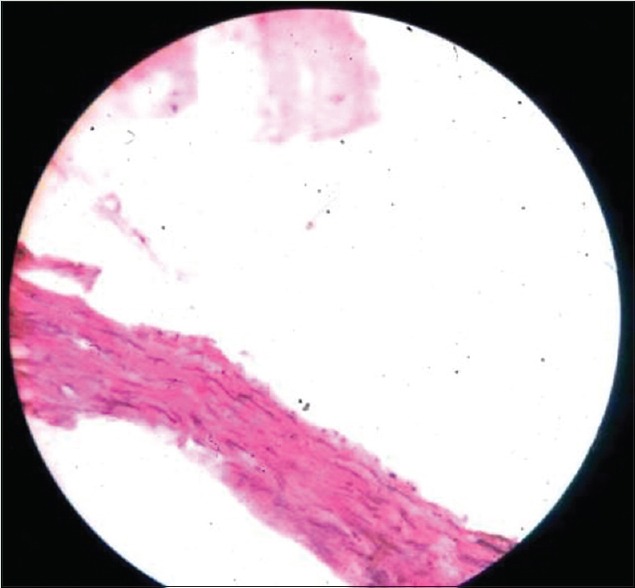
Aorta of astaxanthin fed rat with almost normal histology of aorta (×400)
The histopathology of control has shown a significant intimal thickness of Type I, Type II, and Type III atherosclerotic lesions, with multiple foam cells compared to only Type I and Type II lesion in only two rats with few foam cells in lycopene group. There is a significant reduction in atherosclerotic lesions when the comparison is done by types of lesions with the control group P < 0.05. However, the reduction in intimal thickening and foam cells produced by atorvastatin is significantly greater compared to the other two groups [Figures 1–4].
DISCUSSION
Our aim was to evaluate lycopene and astaxanthin for beneficial effect in the prevention of atherosclerosis. We assessed the antiatherosclerotic property of lycopene and astaxanthin in atherosclerosis-induced rats by assessing the following parameters – weight, lipid profile, TBARS assay, gross, and histopathological features of aorta.
In our study, weight of the control group steadily increased from the beginning significantly compared to the other groups due to the effect of HCD. In the atorvastatin group, the weight gain was significantly less compared to that of control. Lycopene produced a decrease in weight comparable to that of atorvastatin. Astaxanthin produced a significant reduction in weight compared to all the other three groups in the study. Similar result was obtained in the study by Ikeuchi et al. where astaxanthin produced a considerable reduction in weight gain with an increase in dose of astaxanthin.[21]
High lipid content in diet is known to cause hypercholesterolemia in humans and animals. Hypercholesterolemia is a major risk factor for atherosclerosis, and this has produced atherosclerosis in HCD-fed rats.
In our study, the HCD has induced hypercholesterolemia in rats. The lipid profile in control rats has proved this association by producing higher values of serum TC, LDL-C, VLDL-C, triglycerides, and lower values of HDL-C. In contrast, the standard group fed with atorvastatin has maintained the lipid profile with only 5% change compared to the baseline. Lycopene has decreased the serum TC, LDL-C, VLDL-C, and triglycerides and increased the values of HDL-C significantly when compared to the control group. This shows that lycopene has a beneficial effect in hyperlipidemia.
This property may be due to the prevention of oxidation of lipids by lycopene. In our study, astaxanthin has not only reduced the oxidation of LDL-C but also reduced serum TC, triglyceride, LDL-C, and VLDL-C and increased the value of HDL-C compared to the control. This showed that astaxanthin can be beneficial in hypercholesterolemia though atorvastatin was found better than astaxanthin. The difference was statistically insignificant when lycopene was compared with astaxanthin.
Antioxidant property was measured by TBARS assay and by assessing the prevention of atherosclerosis in aorta of the rats. Control group showed significantly higher level of TBARS values compared to all the other groups. Atorvastatin showed antioxidant properties with a significant difference compared to the control, but lycopene showed a greater antioxidant property than the standard control atorvastatin.
Lycopene showed a lower TBARS value compared to all the other three groups which may be attributed to its antioxidant property and nonoxidative mechanisms such as inhibition of 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase, suppression of endothelial cell tissue factor, and inhibition of expression of cell adhesion molecules.[22]
Astaxanthin showed a significant difference in lowering the TBARS value compared to control group. TBARS value for astaxanthin did not differ significantly compared to the standard atorvastatin or lycopene fed groups.
LDL plays a critical role in atherogenesis, by passing through the arterial wall and accumulating locally in the intima of arterial walls where they are prone to undergo oxidation. With continued high level of oxidized lipids, blood vessel damage to the reaction process continues and can lead to generation of foam cells and plaque, the symptoms of atherosclerosis. Oxidized LDL is atherogenic and is thought to be important in the formation of atherosclerosis plaques. Furthermore, oxidized LDL is cytotoxic and can directly damage endothelial cells. Antioxidants such as β-carotene play a vital role in the prevention of various cardiovascular diseases.[23,24,25]
The atherosclerotic plaque was seen in all the control group rats with larger mean area compared to other groups. The plaques were not visible to naked eye as in Andrus et al.[10] This may be due to smaller duration of our study period. Atorvastatin group had shown minimum atheromatous area, probably due to its hypolipidemic and antioxidant property.
Lycopene showed significantly lower plaque area compared to control. This showed that lycopene has significant antiatherogenic property but less than atorvastatin.
In the Rotterdam study, the serum concentration of lycopene was evaluated in atherosclerotic patients and was found to be inversely related to atherosclerosis.[26]
In our study, the control rats presented with higher aortic intima-media thickening than the lycopene-fed rats. This is in accordance with the Kuopio Ischaemic Heart Disease Risk Factor Study, where in low serum lycopene level showed association with higher common carotid artery intima-media thickening in the study population.[27]
Astaxanthin and lycopene showed stronger antiatherogenic property compared to control but less compared to atorvastatin. The antiatherogenic effect of lycopene was less compared to astaxanthin.
In the study conducted by Miki, on rats, he has shown that carotenoids showed stronger antioxidant property compared to Vitamin E, and among the carotenoids, astaxanthin was a stronger antioxidant than others determined by TBARS, and this agrees with our study.[28]
Similarly, astaxanthin has shown strong antioxidant property in high fructose fat diet in a study by Bhuvaneswari et al., where hepatoprotection is conferred by antioxidant property of astaxanthin.[29]
Astaxanthin lowered the lipid peroxidation of LDL-C by the lower TBARS value. This is in agreement with the study by Iwamoto et al., who showed that astaxanthin consumption in humans inhibits oxidation of LDL.[30]
Implication of the study: Our study implies that lycopene and astaxanthin are potent antioxidants with beneficial activity in the prevention of atherosclerosis. However, further research is needed both in animals and in humans before we can recommend their use as an antiatherosclerotic agent.
Further research is required to study the weight reduction property of astaxanthin for its clinical application in the obesity management. Even though astaxanthin is proved to have hypolipidemic, antiatherosclerotic, and anti-obesity property, the mechanism of action is not clearly known.
The limitations of our study include our inability to assess the serum levels of lycopene, astaxanthin, and atorvastatin. The antioxidant property was aimed only at lipid peroxidation, and TBARS alone was used. The duration of the study was short to assess the full blown and long-term benefit of lycopene and astaxanthin.
In our study, the results show that lycopene and astaxanthin decreased the oxidative stress (antioxidant property), LDL-C (hypolipidemic property), and decreased the atheromatous plaque area (anti-atherosclerotic property). Astaxanthin decreased the weight gain compared to all the other groups. All these properties demand further study for establishing safety and efficacy to be used in humans.
CONCLUSION
This study shows that the antioxidants – lycopene and astaxanthin have antiatherogenic property which has been proved by decreased LDL-C cholesterol, increased HDL-C levels and by preventing the formation of atherosclerotic plaques in lycopene and astaxanthin-fed rats compared to the control rats. The study also confirms the antioxidant property of lycopene and astaxanthin by decreased TBARS level. Therefore, lycopene and astaxanthin have hypolipidemic and antiatherogenic potential to be included in the treatment of hypercholesterolemia and atherosclerosis. This warrants further study in this field before human use.
Financial support and sponsorship
This study was financially supported by Sri Balaji Vidyapeeth, Puducherry, India.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
Authors offer their sincere thanks to Dr. Archana, MD postgraduate pathology for her help. We are also thankful for the help of technicians and attenders of Pharmacology and Pathology Departments.
REFERENCES
- 1.World Health Organization. The Top 10 Causes of Death. [Last cited on 2015 Sep 25]. Available from: http://www.who.int/mediacentre/factsheets/fs310/en/
- 2.Robbins SL, Kumar V, Cotran RS. Robbins and Cotran Pathologic Basis of Disease. 8th ed. Philadelphia, PA: Saunders/Elsevier; 2010. pp. 496–506. [Google Scholar]
- 3.Ramchoun M, Harnafi H, Alem C, Benlyas M, Elrhaffari L, Amrani S. Study on antioxidant and hypolipidemic effects of polyphenol-rich extracts from Thymus vulgaris and Lavandula multifida. Pharmacognosy Res. 2009;1:106–12. [Google Scholar]
- 4.IUPAC Commission on the Nomenclature of Organic Chemistry and the IUPAC-IUB Commission on Biochemical Nomenclature. Nomenclature of carotenoids (rules approved 1974) Pure Appl Chem. 1975;41:405–31. [Google Scholar]
- 5.Di Mascio P, Kaiser S, Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch Biochem Biophys. 1989;274:532–8. doi: 10.1016/0003-9861(89)90467-0. [DOI] [PubMed] [Google Scholar]
- 6.Fassett RG, Coombes JS. Astaxanthin: A potential therapeutic agent in cardiovascular disease. Mar Drugs. 2011;9:447–65. doi: 10.3390/md9030447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozen T, Türkekul I. Antioxidant activities of Sarcodon imbricatum wildly grown in the Black Sea Region of Turkey. Pharmacogn Mag. 2010;6:89–97. doi: 10.4103/0973-1296.62892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goto S, Kogure K, Abe K, Kimata Y, Kitahama K, Yamashita E, et al. Efficient radical trapping at the surface and inside the phospholipid membrane is responsible for highly potent antiperoxidative activity of the carotenoid astaxanthin. Biochim Biophys Acta. 2001;1512:251–8. doi: 10.1016/s0005-2736(01)00326-1. [DOI] [PubMed] [Google Scholar]
- 9.CPCSEA. CPCSEA guidelines for laboratory animal facility. Indian J Pharmacol. 2003;35:257. [Google Scholar]
- 10.Andrus SB, Fillios LC, Mann GV, Stare FJ. Experimental production of gross atherosclerosis in the rat. J Exp Med. 1956;104:539–54. doi: 10.1084/jem.104.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vogel GH. Anti atherosclerotic activity. Drug Discovery and Evaluation: Pharmacological Assays. 2nd ed. Heidelberg: Springer; 2008. pp. 1661–717. [Google Scholar]
- 12.EFSA – Opinion of the Scientific Committee/Scientific Panel: Use of Lycopene as a Food colour [1] – Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food. [Last cited on 2013 Oct 03]. Available from: http://www.efsa.europa.eu/en/efsajournal/pub/674.htm .
- 13.Hussein G, Nakamura M, Zhao Q, Iguchi T, Goto H, Sankawa U, et al. Antihypertensive and neuroprotective effects of astaxanthin in experimental animals. Biol Pharm Bull. 2005;28:47–52. doi: 10.1248/bpb.28.47. [DOI] [PubMed] [Google Scholar]
- 14.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92:1355–74. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 15.Stary HC, Chandler AB, Glagov S, Guyton JR, Insull W, Jr, Rosenfeld ME, et al. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1994;89:2462–78. doi: 10.1161/01.cir.89.5.2462. [DOI] [PubMed] [Google Scholar]
- 16.Wybenga DR, Pileggi VJ, Dirstine PH, Di Giorgio J. Direct manual determination of serum total cholesterol with a single stable reagent. Clin Chem. 1970;16:980–4. [PubMed] [Google Scholar]
- 17.Bucolo G, David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem. 1973;19:476–82. [PubMed] [Google Scholar]
- 18.Burstein M, Scholnick HR, Morfin R. Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. J Lipid Res. 1970;11:583–95. [PubMed] [Google Scholar]
- 19.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 20.Lipid Peroxidation (MDA) Assay Kit sufficient for 100 colorimetric or fluorometric tests | Sigma-Aldrich. [Last cited on 2013 Dec 06]. Available from: http://www.sigmaaldrich.com/catalog/product/sigma/mak085?lang=en®ion=IN .
- 21.Ikeuchi M, Koyama T, Takahashi J, Yazawa K. Effects of astaxanthin in obese mice fed a high-fat diet. Biosci Biotechnol Biochem. 2007;71:893–9. doi: 10.1271/bbb.60521. [DOI] [PubMed] [Google Scholar]
- 22.Palozza P, Colangelo M, Simone R, Catalano A, Boninsegna A, Lanza P, et al. Lycopene induces cell growth inhibition by altering mevalonate pathway and Ras signaling in cancer cell lines. Carcinogenesis. 2010;31:1813–21. doi: 10.1093/carcin/bgq157. [DOI] [PubMed] [Google Scholar]
- 23.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–25. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 24.Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev. 2010;4:118–26. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nambiar SS, Shetty NP, Bhatt P, Neelwarne B. Inhibition of LDL oxidation and oxidized LDL-induced foam cell formation in RAW 264.7 cells show anti-atherogenic properties of a foliar methanol extract of Scoparia dulcis. Pharmacogn Mag. 2014;10(Suppl 2):S240–8. doi: 10.4103/0973-1296.133241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klipstein-Grobusch K, Launer LJ, Geleijnse JM, Boeing H, Hofman A, Witteman JC. Serum carotenoids and atherosclerosis. The Rotterdam Study. Atherosclerosis. 2000;148:49–56. doi: 10.1016/s0021-9150(99)00221-x. [DOI] [PubMed] [Google Scholar]
- 27.Rissanen TH, Voutilainen S, Nyyssönen K, Salonen R, Kaplan GA, Salonen JT. Serum lycopene concentrations and carotid atherosclerosis: The Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr. 2003;77:133–8. doi: 10.1093/ajcn/77.1.133. [DOI] [PubMed] [Google Scholar]
- 28.Miki W. Biological functions and activities of animal carotenoids. Pure Appl Chem. 1991;63:141–6. [Google Scholar]
- 29.Bhuvaneswari S, Yogalakshmi B, Sreeja S, Anuradha CV. Astaxanthin reduces hepatic endoplasmic reticulum stress and nuclear factor-κB-mediated inflammation in high fructose and high fat diet-fed mice. Cell Stress Chaperones. 2014;19:183–91. doi: 10.1007/s12192-013-0443-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwamoto T, Hosoda K, Hirano R, Kurata H, Matsumoto A, Miki W, et al. Inhibition of low-density lipoprotein oxidation by astaxanthin. J Atheroscler Thromb. 2000;7:216–22. doi: 10.5551/jat1994.7.216. [DOI] [PubMed] [Google Scholar]


