Abstract
Background:
Inflammation is a normal and necessary prerequisite to healing of the injured tissues. Inflammation contributes to all disease process including immunity, vascular pathology, trauma, sepsis, chemical, and metabolic injuries. The secretory phospholipase A2 (sPLA2) is a key enzyme in the production of pro-inflammatory mediators in chronic inflammatory disorders such as rheumatoid arthritis, coronary heart disease, diabetes, and asthma. The sPLA2 also contribute to neuroinflammatory disorders such as Parkinson's, Alzheimer's, and Crohn's disease.
Aims:
The present study aims to investigate the inhibition of human sPLA2 by a popular medicinal herb Boerhaavia diffusa Linn. as a function of anti-inflammatory activity.
Materials and Methods:
The aqueous and different organic solvents extracts of B. diffusa were prepared and evaluated for human synovial fluid, human pleural fluid, as well as Vipera russelli and Naja naja venom sPLA2 enzyme inhibition.
Results:
Among the extracts, the ethanol extract of B. diffusa (EEBD) showed the highest sPLA2 inhibition and IC50 values ranging from 17.8 to 27.5 μg. Further, antioxidant and lipid peroxidation activities of B. diffusa extract were checked using 2,2-diphenyl-1-picrylhydrazyl radical, thiobarbituric acid, and rat liver homogenate. The antioxidant activity of EEBD was more or less directly proportional to in vitro sPLA2 inhibition. Eventually, the extract was subjected to neutralize sPLA2-induced mouse paw edema and indirect hemolytic activity. The EEBD showed similar potency in both the cases.
Conclusions:
The findings suggest that the bioactive molecule/s from the EEBD is/are potentially responsible for the observed in vitro and in vivo sPLA2 inhibition and antioxidant activity.
SUMMARY
The present study aims to investigate the inhibition of human sPLA2 by a popular medicinal herb Boerhaavia diffusa Linn. as a function of anti inflammatory activity.
Abbreviation Used: EEBD: Ethanolic extract of boerhaavia diffusa, sPLA2: Secretory phospholipase A2, HSF: Human synovial fluid, HPF: Human pleural fluid, VRV-PLA2-V: Vipera russelli phospholipase A2, NN-PLA2-I: Naja naja phospholipase A2.
Key words: Ethanolic extract of Boerhaavia diffusa L., human pleural fluid, human synovial fluid, Naja naja phospholipase A2, Vipera russelli phospholipase A2
INTRODUCTION
Inflammation is a normal and necessary prerequisite to heal the injured tissues.[1] Inflammation contributes to all disease processes including immunity, vascular pathology, trauma, and sepsis. External force or internally disruptive chemical or biological agents also cause tissue injury, where inflammatory reactions are initiated with the results that blood vessels and other tissues in or near the injured area will exhibit dynamic complex cytological and histological reactions. These reactions are local in nature and are designed to cause the destruction or removal of injurious materials, such as bacteria, foreign matter, or chemicals, injured tissue and to promote morphological responses that lead to repair and healing.[1]
However, inflammation is a double-edged sword, with undesirable consequences such as systemic shock and circulatory collapse, if prolonged leads to local tissue damage in many organs.[2,3] Inflammatory response can destroy healthy tissue and cause more damage than the original problem; as a result, uncontrolled inflammatory reactions is the biochemical basis for the origin of chronic inflammatory disease such as rheumatoid arthritis, coronary heart disease, diabetes, and asthma. Hence, inflammatory diseases contribute the greatest clinical suffering and provide major targets for improved treatment.
Secretory phospholipase A2
Inflammation is mainly mediated by secretory phospholipase A2 (sPLA2), which catalyzes the hydrolysis of membrane phospholipids at the second position. Number of studies has reported dramatic increase of sPLA2 levels in biological fluids of patients with various inflammatory diseases. Initially, Vadas et al. showed that septic shock is associated with high levels of sPLA2 in the plasma of patients.[4] Later, it was shown that high level of sPLA2 is present in the inflammatory exudates and plasma of patients with diseases such as rheumatoid arthritis, septic shock, asthma, Crohn's disease, and bronchoalveolar lavage fluids.[5,6,7] The other functions of sPLA2s are cell migration, apoptosis, reactive oxygen species (ROS) generation and cytotoxicity, coagulation,[8,9,10,11] and several snake venom PLA2 enzymes are responsible for local tissue damage such as edema and hemorrhage.[12]
Pro-inflammatory mediators and reactive oxygen species
Elevated level of sPLA2 enzymes in many inflammatory disorders plays a key role by releasing free arachidonic acid and lysophospholipid which are precursors for the production of pro-inflammatory lipid mediators and ROS. Cyclooxygenase-1/2 and lipoxygenase catalyze the synthesis of pro-inflammatory mediators such as prostaglandins, thromboxanes, and leukotrienes. Lysophospholipid is further converted into platelet activation factor (PAF) by acetyltransferase that continued to cause inflammation.[13]
In addition, the arachidonic acid cascade is considered to be a significant source of ROS such as O2 − and OH˙,[11,14] which serves as a part of defensive mechanism by harming the invading pathogens. The generation of free radicals, if persisted after the defensive role, results in a more deleterious complication. In such conditions, regulation of these free radicals is equally important to that of the regulation of pro-inflammatory eicosanoids. However, there is a continuous production of pro inflammatory lipid mediators along with existing free radicals cause augmentation of inflammatory disease by several folds. Thus, PLA2 catalyzes the rate-limiting step in the eicosanoid pathway by generating pro-inflammatory lipid mediators and free radicals and is therefore regarded as a crucial enzyme in various inflammatory diseases.
Boerhaavia diffusa Linn. (Punarnava)
A perennial creeping weed belongs to the 4 o'clock family or Nyctaginaceae. Boerhaavia diffusa L. is commonly called hogweed, encountered in different terrestrial habitat, ranging from managed grasslands, wastelands, agroecosystems to large forest gaps.[15] The plant B. diffusa has a long history of uses by indigenous and tribal people and in ayurvedic and leaves of B. diffusa are often used as a green vegetable in many parts of India. It is taken in herbal medicines for pain relief and other uses.[16]
The B. diffusa roots have been widely used for the treatment of dyspepsia, inflammations, heart diseases, jaundice, abdominal pain, and enlargement of spleen.[17] Pharmacological studies have demonstrated that extracts of B. diffusa possesses diuretic,[15] anti-inflammatory,[16] antifibrinolytic,[18] anticonvulsant, and antibacterial properties.[19] The B. diffusa contains secondary metabolites such as hypoxanthine 9-L-arabinofuranoside, boeravinone A to F,[20,21,22] serratagenic acid, liriodendron,[23] oleanolic acid,[24] ursolic acid, and punarnavoside.[25] All these properties have made this plant very interesting and the plant has played an important role in the treatment of human diseases. Hence, in the present study, the ethanolic extract of Boerhaavia diffusa (EEBD) was subjected for PLA2 inhibition as an anti-inflammation.
MATERIALS AND METHODS
Materials
14C-oleic acid was obtained from PerkinElmer Life Sciences Inc., Boston, MA, USA. Fatty acid-free bovine serum albumin (BSA) fraction V was purchased from PAA Laboratories, GmbH Haidmannweg, Austria. Agar, beef extract, peptone, and lactose were obtained from HiMedia Laboratories Private Limited, Mumbai, India. Scintillation cocktail (Ultima Gold) was obtained from Packard Bioscience, USA. Escherichia coli (lyophilized cells of strain W [ATCC 9637]), Sephadex (G-25, 50, and 75), CM-Sephadex C-25, 2,2-diphenyl-1-picrylhydrazyl radical (DPPH˙), thiobarbituric acid (TBA), gallic acid (GA), dimethyl sulfoxide (DMSO), oleanolic acid were purchased from Sigma-Aldrich Chemical Laboratories, St. Louis, MO, USA. Human pleural fluid (HPF) and synovial fluid were obtained from Princes Krishnajammanni Tuberculosis and Chest Disease Hospital, Mysore, and Dr. Hegde Orthopaedic Clinic, Mysore, India. Blood samples for indirect hemolytic activity were obtained from the healthy volunteers, Department of Studies in Biochemistry, University of Mysore. All other chemicals and reagents used in this study were of analytical grade or better and solvents were redistilled before use.
Plant materials
Aegle marmelos, Ailanthus excelsa, B. diffusa, Clerodendrum indicum, Clerodendrum phlomidis, Clerodendrum serratum, Gmelina arborea, and Oroxylum indicum plants were collected from “Chandravana,” the garden of medicinal plants maintained by Ayurvedic College, Mysore, India. These plants were authenticated with the help of botanist Prof. T. C. Shivashankarmurthy. The voucher specimens were deposited in the Herbarium, Department of Studies in Botany, University of Mysore, India.
Venom
Lyophilized powder of Naja naja and Vipera russelli snake venoms were purchased from Hindustan Park, Kolkata, India, and Irula Co-operative Society Ltd., Chennai, India, respectively.
Animal
Swiss albino mice and rats weighing 20–25 g and 150–200 g were obtained from Central Animal House Facility, Department of Studies in Zoology, University of Mysore, Mysore, India. The animal care and handling were conducted in compliance with the National Regulations for Animal Research. The animal experiments were carried out after reviewing the protocols by the Animal Ethical Committee of the University of Mysore, Mysore, India.
Methods
Preparation of plant extracts
The plants were shade dried and powdered using conventional mixer. The pulverized powder of each plant was extracted hexane, benzene, chloroform, acetone, ethanol, methanol, and water using Soxhlet apparatus (100/750; w/v). The entire sample obtained was centrifuged at 5000 ×g for 15 min, and solvents in the supernatant were removed by vacuum rotary evaporator (Heidolph, HB Digit, Q-01). Aqueous extract was reduced to powder by lyophilization. The solid powder obtained was weighed to calculate the percentage yield from the initial weight. For the further studies, the extracts were dissolved in ethanol.
Preparation of 14C-oleate-labeled Escherichia coli substrate
14C-oleate-labeled E. coli substrate was prepared according to Patriarca et al.[26] A single colony of E. coli was picked to prepare a miniculture using 10 ml of Luria-Bertani (LB) medium. The culture was grown overnight in rotating water bath at rpm of 90 at 37°C. This miniculture was diluted 100 times with LB medium and continued the incubation. An absorbance of 0.6–0.9 at 520 nm indicates that the bacteria are in log phase of their growth. These cells were harvested by centrifugation at 1500 ×g for 5 min. The cell pellet was resuspended in 100 ml of LB medium. In a separate conical flask, 125 μCi of 14 C-oleate was evaporated and was resuspended in 5 ml of 20% fatty acid-free BSA. The prepared E. coli culture was mixed with 14 C-oleate and continued incubation at 37°C in rotating shaking water bath for 4 h at an rpm of 90. The labeled E. coli were washed 3–4 times with phosphate buffer saline (PBS) and autoclaved to inactivate endogenous PLA2 activity. The labeled E. coli cells were diluted with PBS and similarly prepared autoclaved cold E. coli cells to get 10,000 cpm in 30 μl of diluted sample. This preparation was used further as substrate for PLA2 enzyme activity.
Purification of secretory phospholipase A2 from Vipera russelli venom
The sPLA2 from V. russelli venom was purified to homogeneity according to modified method of Kasturi and Gowda.[27] Briefly, V. russelli venom (110 mg) was fractionated on Sephadex G-75 column (1.5 cm × 110 cm) using 0.05 M phosphate buffer pH 7.0. The venom was resolved into three fractions. The second fraction, which constitutes about 30% of the total protein loaded, showed PLA2 activity. This PLA2 fraction was lyophilized and further subjected to CM Sephadex-C-25 column (2.5 cm × 20 cm) chromatography. The fractions were eluted stepwise using phosphate buffers of varying molarities and pH (0.05–0.2 M, pH 7.0–8.0). They were resolved into two fractions labeled as V and VIII, respectively. The above eluted two fractions are similar to V and VIII of Kasturi and Gowda protein profile. The lyophilized fractions V and VIII were next subjected to Sephadex G-50 column (1.0 cm × 40 cm) chromatography and eluted using 0.05 M phosphate buffer pH 7.0. Both the peaks were homogenous and showed PLA2 activity. Homogeneity was checked by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)[28] and reversed-phase high-performance liquid chromatography.
Purification of secretory phospholipase A2 from human inflammatory fluids
The sPLA2 from these inflammatory fluids, HPF, and human synovial fluid (HSF) is purified by the modification method previously described by Stefanski et al.[28] Briefly, fluids were centrifuged at 1000 ×g for 15 min to sediment cells and debris. The supernatant was mixed with equal volume of ice-cold 0.36 N H2 SO4 constant stirring at 4°C overnight. After centrifugation at 7500 ×g, the supernatant was dialyzed against 10 mM sodium acetate buffer, pH 4.5, and the dialysate was centrifuged at 7500 ×g for 5 min. The supernatant was incubated at 80°C for 5 min and centrifuged to yield a supernatant enriched in sPLA2 activity. The above procedure was repeated several times to obtain considerable amount of sPLA2 enriched source, which was lyophilized and stored. This lyophilized powder was dissolved in 0.5 M sodium acetate buffer, pH 5.0 and applied to CM-Sephadex C-50, which had been preequilibrated with the same buffer. The column was discontinuously eluted with 0.5 M sodium acetate buffer, pH 5.0; 0.3 M NaCl in 0.2 M Tris-HCl, pH 8.5; and 3 M NaCl in 0.2 M Tris-HCl buffer pH 8.5. The PLA2 was eluted in the latter buffer. Fractions with PLA2 activity were pooled, dialyzed against 0.05 M Tris-HCl buffer, pH 8.0, and lyophilized. The lyophilized residue was dissolved in 0.05 M Tris-HCl, pH 7.5. This PLA2 fraction was passed through Sephadex G-75 column using 10 mM Tris-HCl buffer, pH 7.5. The protein peak with PLA2 activity from Sephadex G-75 column was further checked for purity on SDS-PAGE. This peak was further resolved into two protein bands in 12.5% SDS-PAGE. Both bands showed PLA2 activity after electroelution. The fractions with PLA2 activity were pooled, lyophilized, and further used as purified pleural fluid sPLA2 (Group-IIA).
Inhibition of phospholipase A2 activity
Inhibition of PLA2 activity was studied by previous method.[29] Solutions of B. diffusa extracts were prepared by dissolving 1 mg in very small volume of DMSO (30 μl) and made up to 1 ml with 100 mM Tris-HCl buffer (pH 7.4). Inhibition was carried out with indicated concentrations of inhibitors for HSF, HPF, Vipera russelli phospholipase A2 (VRV-PLA2-V), and Naja naja phospholipase A2 (NN-PLA2-I) enzymes. The amount of enzyme was chosen such that a 60%–70% hydrolysis of substrate by concentration dependent assay at 37°C for 60 min. IC50 concentration was calculated using software GraphPad version Prism 5.0 (GraphPad Software, San Diego California USA). Ursolic acid is well-known inhibitor of sPLA2 and used as positive control. Control experiments were performed with DMSO (the highest concentration of DMSO used is 0.022%).
Effect of substrate and calcium concentration on secretory phospholipase A2 inhibition
The reaction mixture 350 μl containing VRV-PL-V alone or with IC50 concentration of inhibitors in 100 mM Tri-HCl buffer, pH 7.4 and 5 mM calcium was used for PLA2 assay. Addition of various concentration of substrate (20–120 nM) started the reaction. Calcium concentration ranging from 2.5 to 15 mM was added to PLA2 assay mixture. The assay was carried out as described above.
Neutralization of indirect hemolytic activity
Indirect hemolytic activity was assayed as described by Boman and Kaletta.[30] The substrate for the indirect hemolytic assay was prepared by suspending 1 ml of packed fresh human RBC and 1 ml fresh hen's egg yolk in 8 ml of PBS. Extracts and EEBD were preincubated with 30 μg of VRV-PL-V for 30 min at 37°C. To this preincubated sample, 1 ml of substrate was added and allowed to react for 45 min at 37°C. The reaction was stopped by adding 9 ml of ice-cold PBS. The suspension was mixed and centrifuged at 1500 ×g for 20 min. The released hemoglobin was read at 530 nm. Sample with venom alone served as positive control.
Neutralization of edema-inducing activity
The modified procedure of Vishwanath and Gowda was followed.[31] Mice weighing 20–25 g were injected with 5 μg of VRV-PL-V enzyme alone or mixed with different concentrations of EEBD in a total volume of 20 μl saline into their intraplantar surface of right hind footpads. Respective left footpads received 20 μl of saline or vehicle and served as controls. After 45 min, the mice were sacrificed after giving anesthesia (pentobarbital, 30 mg/kg, i.p.) and both hind limbs were removed at the ankle joint and weighed individually. The increase in weight due to edema is expressed as the ratio of the weight of edematous limb to the weight of normal (sham injected) limb × 100. Percentage increase of sham-injected control compared to the uninjected limb is 117 ± 4.
Determination of total phenolics by Folin–Ciocalteu assay
The concentration of total phenolics in the extracts of medicinal plants was determined by the method of Singleton et al., with slight modification.[32] Extracts were taken at 100 μg concentration by their dry weight and mixed with 500 μl of 5% sodium carbonate; 250 μl of Folin–Ciocalteu reagent (1:1 diluted with water) was added, mixed thoroughly, and incubated at room temperature for 1 h. After the incubation, the absorbance was measured at 725 nm using a spectrophotometer (Shimadzu 1601A). The phenolic contents of the extracts were determined from calibration curve generated using GA and the concentration was expressed as equivalents of GA (GAE/100 g).
Antioxidant activity by 2,2-diphenyl-1-picrylhydrazyl radical method
Antioxidant activity of plant extracts was determined using DPPH as described by Blios.[33] The medicinal plants extracts were taken at 100 μg concentration by their dry weight and mixed with 5 ml of 0.1 mM methanolic solution of DPPH˙ and incubated at 20°C for 20 min in darkness. The control was prepared as above without any extract and methanol was used for the baseline correction. Changes in the absorbance of the samples were measured at 517 nm. Radical scavenging activity was expressed as percentage activity using the following formula. Butylated hydroxyanisole and GA were used for competition.
Percentage radical scavenging activity = ([control absorbance − sample absorbance]/[control absorbance]) × 100.
Antioxidant activity by thiobarbituric acid method
Antioxidant activity of the extracts was performed using TBA according to the protocol of Halliwell and Gutteridge.[34] Lipid peroxidation induced by ferric chloride resulted in the production of malondialdehyde (MDA), lipid peroxide. TBA reacts with MDA to form a di-adduct, a pink chromogen, which can be detected spectrophotometrically at 532 nm.
Preparation of rat liver homogenate for lipid peroxidation
The animal used was normal albino rats of Swiss Wistar strain and rats perfused liver were isolated, and 10% (w/v) homogenate was prepared using a Potter-Elvehjem homogenizer under ice-cold (0°C–4°C) condition. The homogenate was centrifuged at 1500 ×g for 5 min, and clear supernatant was used for lipid peroxidation analysis.[35]
Plant extracts were taken at 100 μg concentration by their dry weight and mixed with 1.0 ml of 0.15 M KCl and 0.5 ml of rat liver homogenate. Peroxidation was initiated by adding 100 μl of 0.2 mM ferric chloride. After incubation at 37°C for 30 min, the reaction was stopped by adding 2 ml of ice-cold HCl (0.25 N) containing 4% trichloroacetic acid (TCA) and 0.38% TBA. The reaction mixtures were heated at 80°C for 60 min. The samples were cooled and centrifuged, and the absorbance of the supernatants was measured at 532 nm. An identical experiment was performed in the absence of the extracts to determine the amount of lipid peroxidation obtained in the absence of extract. The anti-lipid peroxidative (ALP) activity is expressed as percentage ALP using the following formula:
Percentage ALP = 1 − sample absorbance/control absorbance × 100.
Determination of reducing power
The reducing powers of extracts were determined according to the method of Yen Duh.[36] The extracts were taken at 60 μg concentration of their dry weight and mixed with 2.5 ml of phosphate buffer (0.2 M, pH 6.6) and 2.5 ml of 1% potassium ferricyanide and incubated at 50°C for 20 min. The reaction was terminated by adding 2.5 ml of 10% TCA and the mixture was centrifuged at 3500 × g for 10 min. An aliquot of supernatant (2.5 ml) was mixed with 2.5 ml of distilled water and 0.5 ml of 0.1% ferric chloride to form a colored complex which is measured at 700 nm.
Acute toxicity
Acute oral toxicity study was performed as per the OECD-423 guidelines (acute toxic class method). Albino mice (n = 6) of either sex selected by random sampling technique were used for the study. The animals were kept fasting for overnight providing only water after which the extracts were administered orally at the dose level of 5 mg/kg body weight by oral feeding needle and observed for 14 days. Mortality was not observed; the procedure was repeated for further higher dose such as 50, 300, and 2000 mg/kg body weight.[37]
Statistical analysis
Inhibition percentages were calculated from the difference between inhibitor treated group and control animals, which received the vehicle. Student's t-test for comparisons of unpaired data was used for statistical evaluation. The experimental results were presented as mean ± standard deviation of three determinations. The IC50 concentration was calculated by Boltzmann dose–response analysis using originpro 6.1 OriginLab, Northampton, MA. The statistical significance between different sPLA2s at a given concentration was analyzed by Duncan's multiple range tests at P < 0.05 using ANOVA of SPSS software (SPSS Inc. Released 2007. SPSS for Windows, Version 16.0. Chicago, SPSS Inc.).
RESULTS
Inhibition of sPLA2 is the intense pharmacological interest because the release of arachidonic acid from the sn-2 position of phospholipids is the rate-limiting step in the production of eicosanoid mediators of inflammation.[38,39] The oxidative metabolism of arachidonic acid also produces ROS, which are implicated in many degenerative neurological conditions.[40] Some medicinal plants such as Andrographis paniculata and Tamarindus indica show inhibition of sPLA2 at less IC50.[41,42] The present study report the potency of ethanolic leaf extract of B. diffusa against enzymatic and pharmacological activities of secretary PLA2 enzymes.
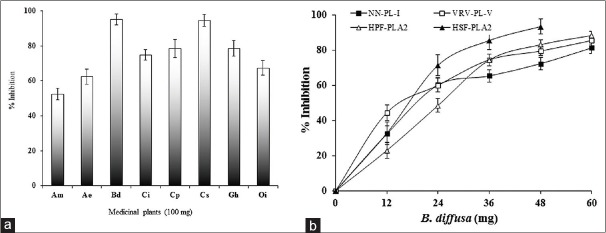
Initially, inhibition of sPLA2 (VRV-PL-V) was carried out using the extracts of all the medicinal plants at 100 μg/350 μl concentration. The extracts of plants inhibit to the various extents. Out of the eight ethanol extracts, B. diffusa and C. serratum plants inhibited VRV-PL-V activity to the maximum extent (>90%). The extracts of Aegie mermelosa, A. excelsa, C. indicum, Clerodendrum phlomides, G. arborea, and O. indicum were also showed more than 50% inhibition [Figure 1a]. The EEBD showed highest inhibition VRV-PL-V [Table 1].
Figure 1.
Inhibition of sPLA2 (VRV-PL-V) enzyme by ethanol extracts of medicinal plants (a) and inhibition of sPLA2 from different sources by ethanol extracts of Boerhaavia diffusa leaf (b). The reaction mixture, 350 ml contained VRV-PL-V (4μg), 5 mM calcium, 100 μM Tris-HCl buffer, and 100μg ethanolic extract of Boerhaavia diffusa. The reaction was initiated by adding 30μl of substrate and incubated for 1 h at 37°C. Data represents mean ± standard deviation (n = 3). Plants: (Am: Aegle marmelos; Ae: Ailanthus excelsa; Bd: Boerhaavia diffusa; Ci: Clerodendrum indicum; Cp: Clerodendrum phlomidis; Cs: Clerodendrum serratum; Ga: Gmelina arborea; and Oi: Oroxylum indicum). sPLA2: Secretory phospholipase A2; VRV-PLA2-V: Vipera russelli phospholipase A2
Table 1.
Percentage inhibition of Vipera russelli venom phospholipase-V by different solvent extracts of medicinal plants. 75 g powder of each source was extracted sequentially using Soxhlet apparatus with increasing polarity of organic solvents at 50°C-70°C for 48 h each and inhibition of secretory phospholipase A2 (Vipera russelli venom phospholipase-V) enzyme was studied by extracts at 100 μg concentration.

Inhibition of secretory phospholipase A2 enzymes
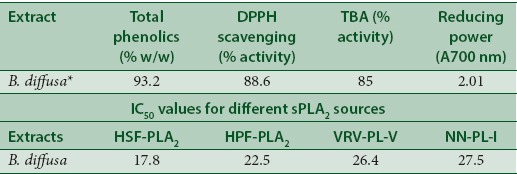
Further, inhibition of sPLA2 of different source by the EEBD was carried out. The sPLA2s from HPF, HSF PLA2, VRV-PL-V (belongs to Group-IIA), and NN-PL-I (belongs to Group-I) inhibition was carried out for the comparison. The extract which was in the range of 12–60 μg was incubated with sPLA2 enzymes in standard set of conditions. The EEBD inhibited sPLA2 enzymes in concentration-dependent manner to the various extents. The extent of inhibition of HSF-PLA2 was highest (96%) at the extract concentration of 48 μg, followed by HPF-PLA2 (92.1%), VRV-PL-V (85.6%), and NN-PL-I (78.0%) at 60 μg concentration [Figure 1b]. The oleanolic acid is a well-known inhibitor of the Group-IA and Group-IIA PLA2 enzymes taken as standard (data not shown). The linear regression analysis of linear portion of concentration-dependent inhibition curves used to calculate IC50 values. IC50 values of HSF-PLA2 and HPF-PLA2 enzymes are 17.8 and 22.5 μg, respectively, whereas VRV-PL-V and NN-PL-I are 26.4 μg and 27.5 μg, respectively [Table 2].
Table 2.
Total phenolics, antioxidant, reducing power, and activity of Boerhaavia diffusa and IC50 values for different secretory phospholipase A2 enzymes. The linear regression analysis of the linear portion of the dose-dependent inhibition curve of each enzyme was used for the calculation of IC50
Effect of substrate and calcium concentration on inhibition
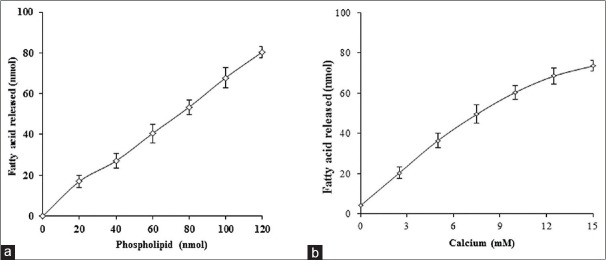
The mechanism of inhibition of PLA2 enzymes by the EEBD was examined as a function of substrate concentration. The sPLA2 (VRV-PL-V) activity was measured in the presence and absence of IC50 concentration of EEBD. The substrate concentration was increased up to 120 nM. Activity of VRV-PL-V enzyme increases almost linearly with increased substrate concentration. The percentage of inhibition is in the range of 49%–52% and almost remained constant over the entire range of substrate concentration used [Figure 2a]. Over all the results, the inhibition of sPLA2 by EEBD is independent of substrate concentration.
Figure 2.
Effect of substrate (a) and calcium (b) concentration on inhibition of VRV-PL-V by the extracts at IC50 concentration: The reaction mixture contains VRV-PL-V alone or with IC50 concentrations of extracts in 100 mM Tri-HCl buffer pH 7.4 and 5 mM CaCl2. The reaction was initiated by adding indicated concentration of substrate and incubated for 1 h. The data were represented as mean ± standard deviation (n < 4). VRV-PL-V: Vipera russelli phospholipase A2
The sPLA2 enzymes are calcium dependent and require calcium for optimum activity. The possibility of inhibition by metal ion chelation was determined by VRV-PL-V inhibition of with IC50 concentrations of extracts EEBD. The range of calcium concentration was 0–15 mM. Increasing calcium concentration from 0–15 mM increases sPLA2 activity linearly but did not change the percentage of inhibition at IC50 concentration of extracts [Figure 2b]. The inhibition sPLA2 ranges 48%–54%. The inhibition of sPLA2 is independent of calcium concentration and does not inhibit the activity by chelating calcium ions required for the enzyme activity.
Determination of total phenolics, antioxidant activity, and anti-lipid peroxidation
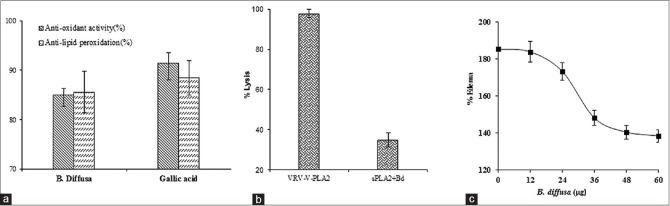
The extracts of B. diffusa inhibited sPLA2s in the in vitro condition and the inhibition was independent of substrate and calcium concentrations. Further, the total phenolic content, antioxidant activity, and reducing power were estimated at 100 μg concentration. The result showed that extracts of B. diffusa contain 93% phenolics [Table 2]. The antioxidant activity was determined by DPPH method. The extract of B. diffusa showed the considerable antioxidant activity if compared to standard GA. ALP activity of the extracts was performed using TBA. The extract of B. diffusa showed 87.5% ALP activity [Figure 3a]. These data suggested that the percentage of PLA2 inhibition by these sources is almost accordance of the amount of phenolic content, antioxidant, reducing power and anti-lipid peroxidative activity [Table 2].
Figure 3.
Estimation of antioxidant activity and anti-lipid peroxidation (a), neutralization of indirect hemolytic activity (b), and edema-inducing activity (c) of Boerhaavia diffusa. Ethanol extracts of Boerhaavia diffusa were assayed for antioxidant activity by 2,2-diphenyl-1-picrylhydrazyl radical method and anti-lipid peroxidation activity by thiobarbituric acid method at 60 mg concentration. Neutralization of indirect hemolytic activity and neutralization of edema-inducing activity were assayed according to the established protocol. Data were represented as mean ± standard deviation (n = 3)
In situ and in vivo studies
Since the extract inhibited sPLA2 activity in the in vitro, the effect of these extracts on in situ hemolytic activity of sPLA2 enzymes was examined. In situ hemolytic activity is an indirect way of measuring PLA2 activity using egg yolk and washed erythrocytes as substrate. VRV-PL-V was subjected to inhibition by the extracts (60 μg) of B. diffusa. The reaction mixture without enzyme served as control. The extract of B. diffusa inhibited indirect hemolytic activity of VRV-PL-V. The maximum inhibition was achieved at 48 μg [Figure 3b].
The effect of extracts on edema-inducing activity of VRV-PL-V was determined. The sPLA2 (VRV-PL-V) was injected into the mouse footpad; the edema ratio was >180% compared with controlled experiments. The enzyme was preincubated for 30 min at 37°C with the extracts of medicinal plants and legumes before the co-injection into mice footpads. The extracts inhibited edema formation in dose-dependent manner when co-injected with VRV-PL-V [Figure 3c]. The edema ratio was decreased to 137% by B. diffusa at 60 μg concentration. The apparent IC50 for inhibition of edema was about 30 μg.
Thus, EEBDinhibited the activity of all sPLA2 enzymes from both human and snake venom sources as well as neutralized the indirect hemolytic and edema-inducing activities of PLA2 enzymes.
DISCUSSION
The extracellular Group-IIA sPLA2 activity could be implicated in the control of wide range of physiological states, such as acute inflammation, pancreatitis, and rheumatoid arthritis.[41] Thus, the relationship of sPLA2 activation to the inflammatory response has been assumed an important manifestation. Inhibition of increased PLA2 activity has tremendous scope in the development of pharmacologically potent molecules to control inflammation. Further, the PLA2 inhibitors could minimize the tissue damage and other deleterious effects that take place secondary to inflammation.
In many countries, extracts of medicinal plants have been traditionally used for treatment of inflammation and related diseases. These plant extracts constitute rich source of novel compounds with a variety of pharmacological activities.[43] Although biologically active phytochemicals have been reported to be present in a wide range of plants source, their efficacy in a wide range of plants, their efficacy in disease prevention has been clearly established only in case of some. The potency of a plant in preventing a disease is the sum of bioactivities of all phytochemicals present in that source with a common property. These phytochemicals are reported to prevent disease mainly through their functions as antioxidants detoxifiers, neuropharmacological and immunopotentiating agents, and source of dietary fiber.
The preliminary screening data with several folk medicinal plants that are extensively used against inflammation clearly inhibited sPLA2 activity. In the present study, crude extracts of medicinal plants showed sPLA2 inhibition, which inhibits enzymatic and toxic activities exerted by a variety of purified sPLA2 enzymes from snake venoms and human inflammatory exudates. The extracts from Soxhlet method of extraction, the polar solvents, showed good inhibition compared to extracts from nonpolar solvents. The highest inhibition was achieved with ethanol extracts, followed by water and methanol extracts. The data indicate that B. diffusa containing more of hydrophilic inhibitors than hydrophobic inhibitors.
To check whether the extract inhibits other PLA2 enzymes, extract of B. diffusa, was subjected to inhibit sPLA2 enzymes from different sources. It inhibited sPLA2 enzymes from human inflammatory fluids (HSF and HPF) and V. russelli snake venom (VRV-PL-V) which all belongs to Group-IIA sPLA2. The result showed that human inflammatory PLA2s are more sensitive to inhibition by these extracts than snake venom PLA2 (VRV-PL-V). The extracts were further checked for their ability to inhibit other group sPLA2 enzyme, NN-PLA2-I, which belongs to Group-IA, but percentage of inhibition is slightly less compared to Group-IIA sPLA2s, indicating that these extracts are more competent to Group-IIA sPLA2s either from snake venom or human inflammatory fluids. The extent of inhibition is differing from Group-I to Group-II PLA2 enzymes. Although they exhibit more than 70% homology, many inhibitors inhibit these enzymes differentially.[7] The variable extent to which these PLA2 enzymes are inhibited may be due to the differential binding affinities at the active site.
Inhibition of sPLA2 by binding of inhibitors to the phospholipid substrate and affecting the quality of interface that renders phospholipids inaccessible to the enzyme is doubt against sPLA2 inhibitors potency. The steroid-inducible inhibitors of sPLA2 such as lipocortin I and II are shown to inhibit sPLA2s by nonspecific binding and by affecting the “quality of interface” of the membrane phospholipids. However, the inhibition by these agents was relieved when the substrate concentration was increased in the reaction mixture.[43] Contrast to this, the extracts of B. diffusa retained the sPLA2 inhibition potency even when the substrate concentration was increased by twofold. This observation suggests that the crude extracts of these plants do not compete for the substrate-binding site and may interact at a separate site strongly, and the inhibition cannot be affected by increased substrate concentration.
Some of the sPLA2 inhibitors are shown to mediate displacement of catalytically essential calcium from the enzyme, and thus, inhibition by these agents appears to be dependent on calcium. Our investigation shows that inhibition of sPLA2 by the extracts B. diffusa is independent of calcium concentration and not by chelation. Since the enzyme was inhibited to the extent of about 50% even when the calcium concentration was four times more than that of standard calcium concentration used in regular assay system. The involvement of molecules in extracts in binding to calcium ion hence leading to inhibition is ruled out. The above results indicated that the compounds in the crude extract directly interact with the enzyme and bring about irreversible inhibition.
The phenolic concentration, antioxidant activity, anti-lipid peroxidation activity, and reducing power of B. diffusa were not proportional to the phenolic concentration. The reducing power of the extract in terms of antioxidant activity was determined. The inhibition of sPLA2 activity in the in vitro and in vivo condition by B. diffusa was more or less directly proportional to their antioxidant activity and anti-lipid peroxidation activity.
Probably, molecules of extract bind at the substrate-binding region of the enzyme and enzyme activity was inhibited irrespective of concentration of substrate provided for its activity. This is reflected in the inhibition of both in vitro sPLA2 inhibition and indirect hemolytic activity where the substrate nature is totally different. The intact E. coli membrane was used as substrate in the in vitro assay and crude egg phospholipids mixture dispersed in the buffer as micelles in the indirect hemolytic assay. Because of this nature, these can be readily administered into in vivo model.
Injection of venom sPLA2 or inflammatory fluid into joint of animal causes acute inflammatory response such as edema, swelling of synovial cell, and hyperplasia.[44] The inhibitors on co-injection with sPLA2 enzymes resulted in neutralization of edema-inducing activity in animal model.[45,46] The extract of B. diffusa on co-injection with VRV-PL-V decreased the edema-inducing activity in dose-dependent manner. Since extracts inhibit sPLA2 in vivo method, they are effective in preventing edema.
Conclusively, the juice of fresh leaves of B. diffusa reported to markedly reduces pain in mice.[16] Ethanolic and aqueous extracts of B. diffusa effectively reduced the sPLA2-induced paw edema. These effects established some pharmacological evidence to support the folklore claim that it is used as anti-inflammatory agent. The EEBD has shown the higher potency in inhibiting sPLA2 enzymes. This kind of herbs is reported to have higher concentration of compounds such as D-mannitol, oleanolic acid, ursolic acid, apigenin, stigmosterols, alpha-spinasterol, luteolin, baicalin, quercetin, and genistein. Since the extracts used in the assay were crude, there is always a possibility of single molecule or cumulative effect, which may be the other reason for sPLA2 inhibition. However, several important questions remain open and further investigations are necessary to confirm which compound/s is/are responsible for the anti-inflammatory activity.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We are grateful to Prof. B. S. Vishwanath, Department of Studies in Biochemistry, University of Mysore, Mysore, and Department of Studies in Biochemistry, Mangalore University, Post Graduate Centre, Chikka Aluvara, for providing necessary facilities and continuous support.
REFERENCES
- 1.Kay AB. Mediators and inflammatory cells in allergic disease. Ann Allergy. 1987;59(6 Pt 2):35–42. [PubMed] [Google Scholar]
- 2.Granger DN, Kubes P. The microcirculation and inflammation: Modulation of leukocyte-endothelial cell adhesion. J Leukoc Biol. 1994;55:662–75. [PubMed] [Google Scholar]
- 3.DiNapoli M, Arakelyan A, Boyajyan A, Godoy A, Papa F. The acute phase inflammatory response in stroke: Systemic inflammation and neuroinflammation. In: Pitzer JA, editor. Progress in Inflammation Research. USA: Nova Science Publishers Inc.; 2006. pp. 95–145. [Google Scholar]
- 4.Vadas P, Stefanski E, Wloch M, Grouix B, Van Den Bosch H, Kennedy B. Secretory non-pancreatic phospholipase A2 and cyclooxygenase-2 expression by tracheobronchial smooth muscle cells. Eur J Biochem. 1996;235:557–63. doi: 10.1111/j.1432-1033.1996.t01-1-00557.x. [DOI] [PubMed] [Google Scholar]
- 5.Hallstrand TS, Lai Y, Altemeier WA, Appel CL, Johnson B, Frevert CW, et al. Regulation and function of epithelial secreted phospholipase A2 group X in asthma. Am J Respir Crit Care Med. 2013;188:42–50. doi: 10.1164/rccm.201301-0084OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghosh S. Estimating benefits of therapy in Crohn's disease in terms of indirect costs. Can J Gastroenterol. 2011;25:412. doi: 10.1155/2011/310417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim HP, Son KH, Chang HW, Kang SS. Anti-inflammatory plant flavonoids and cellular action mechanisms. J Pharmacol Sci. 2004;96:229–45. doi: 10.1254/jphs.crj04003x. [DOI] [PubMed] [Google Scholar]
- 8.Minami T, Shinomura Y, Miyagawa J, Tojo H, Okamoto M, Matsuzawa Y. Immunohistochemical localization of group II phospholipase A2 in colonic mucosa of patients with inflammatory bowel disease. Am J Gastroenterol. 1997;92:289–92. [PubMed] [Google Scholar]
- 9.Gambero A, Landucci EC, Toyama MH, Marangoni S, Giglio JR, Nader HB, et al. Human neutrophil migration in vitro induced by secretory phospholipases A2: A role for cell surface glycosaminoglycans. Biochem Pharmacol. 2002;63:65–72. doi: 10.1016/s0006-2952(01)00841-3. [DOI] [PubMed] [Google Scholar]
- 10.Costa-Junior HM, Hamaty FC, da Silva Farias R, Einicker-Lamas M, da Silva MH, Persechini PM. Apoptosis-inducing factor of a cytotoxic T cell line: Involvement of a secretory phospholipase A2. Cell Tissue Res. 2006;324:255–66. doi: 10.1007/s00441-005-0095-y. [DOI] [PubMed] [Google Scholar]
- 11.Sakuma S, Kitamura T, Kuroda C, Takeda K, Nakano S, Hamashima T, et al. All-trans Arachidonic acid generates reactive oxygen species via xanthine dehydrogenase/xanthine oxidase interconversion in the rat liver cytosol in vitro. J Clin Biochem Nutr. 2012;51:55–60. doi: 10.3164/jcbn.11-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mounier CM, Bon C, Kini RM. Anticoagulant venom and mammalian secreted phospholipases A2: Protein versus phospholipid-dependent mechanism of action. Haemostasis. 2001;31:279–87. doi: 10.1159/000048074. [DOI] [PubMed] [Google Scholar]
- 13.Serhan CN, Haeggstrom JH. Lipid mediators in acute inflammation and resolution: Eicosanoids, PAF, resolvins and protectins. In: Serhan CN, Ward PA, Gilroy DW, editors. Fundamentals of Inflammation. New York: Cambridge University Press; 2011. pp. 153–75. [Google Scholar]
- 14.Chan PH, Chen SF, Yu AC. Induction of intracellular superoxide radical formation by arachidonic acid and by polyunsaturated fatty acids in primary astrocytic cultures. J Neurochem. 1988;50:1185–93. doi: 10.1111/j.1471-4159.1988.tb10591.x. [DOI] [PubMed] [Google Scholar]
- 15.Anand RK. Biodiversity and tribal association of Boerhaavia diffusa in India-Nepal Himalayan Terai region. Flora Fauna. 1995;4:167–70. [Google Scholar]
- 16.Hiruma-Lima CA, Gracioso JS, Bighetti EJ, Germonsén Robineou L, Souza Brito AR. The juice of fresh leaves of Boerhaavia diffusa L. (Nyctaginaceae) markedly reduces pain in mice. J Ethnopharmacol. 2000;71:267–74. doi: 10.1016/s0378-8741(00)00178-1. [DOI] [PubMed] [Google Scholar]
- 17.Awasthi LP, Menzel G. Effect of root extract from Boerhaavia diffusa L. containing an antiviral principle upon plaque formation of RNA bacteriophages. Zentralbl Mikrobiol. 1986;141:415–9. [PubMed] [Google Scholar]
- 18.Jain GK, Khanna NM. Punarnavoside: A new antifibrinolytic agent from Boerhaavia diffusa Linn. Indian J Chem. 1989;28:163–6. [Google Scholar]
- 19.Olukoya DK, Idika N, Odugbemi T. Antibacterial activity of some medicinal plants from Nigeria. J Ethnopharmacol. 1993;39:69–72. doi: 10.1016/0378-8741(93)90051-6. [DOI] [PubMed] [Google Scholar]
- 20.Kadota S, Lami N, Tezuka Y, Kikuchi T. Constituents of the roots of Boerhaavia diffusa Linn. I. Examination of sterols and structures of new rotenoids (boeravinones A and B) Chem Pharm Bull. 1989;37:3214–20. [Google Scholar]
- 21.Lami N, Kadota S, Tezuka Y, Kikuchi T. Constituents of the roots of Boerhaavia diffusa Linn. II. Structure and stereochemistry of a new rotenoid boeravinone C2. Chem Pharm J. 1990;38:1558–62. [Google Scholar]
- 22.Lami N, Kadota S, Kikuchi T. Constituents of the roots of Boerhaavia diffusa Linn. IV. Isolation and structure determination of boeravinones D, E and F. Chem Pharm Bull. 1992;39:1863–5. doi: 10.1248/cpb.39.1551. [DOI] [PubMed] [Google Scholar]
- 23.Aftab K, Usmani SB, Ahmad SI, Usmanghani K. Naturally occurring calcium channel blockers-II. Hamdard Med. 1996;39:44–54. [Google Scholar]
- 24.Pereira DM, Faria J, Gaspar L, Valentão P, de Pinho PG, Andrade PB. Boerhaavia diffusa: Metabolite profiling of a medicinal plant from Nyctaginaceae. Food Chem Toxicol. 2009;47:2142–9. doi: 10.1016/j.fct.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 25.Mishra AN, Tiwari HP. Constituents of the roots of Boerhaavia diffusa. Phytochemistry. 1971;10:3318. [Google Scholar]
- 26.Patriarca P, Beckerdite S, Elsbach P. Phospholipases and phospholipid turnover in Escherichia coli spheroplasts. Biochim Biophys Acta. 1972;260:593–600. doi: 10.1016/0005-2760(72)90008-2. [DOI] [PubMed] [Google Scholar]
- 27.Kasturi S, Gowda TV. Purification and characterization of a major phospholipase A2 from Russell's viper (Vipera russelli) venom. Toxicon. 1989;27:229–37. doi: 10.1016/0041-0101(89)90136-0. [DOI] [PubMed] [Google Scholar]
- 28.Stefanski E, Pruzanski W, Sternby B, Vadas P. Purification of a soluble phospholipase A2 from synovial fluid in rheumatoid arthritis. J Biochem. 1986;100:1297–303. doi: 10.1093/oxfordjournals.jbchem.a121836. [DOI] [PubMed] [Google Scholar]
- 29.Dharmappa KK, Kumar RV, Nataraju A, Mohamed R, Shivaprasad HV, Vishwanath BS. Anti-inflammatory activity of oleanolic acid by inhibition of secretory phospholipase A2. Planta Med. 2009;75:211–5. doi: 10.1055/s-0028-1088374. [DOI] [PubMed] [Google Scholar]
- 30.Boman HG, Kaletta U. Chromatography of rattlesnake venom; a separation of three phosphodiesterases. Biochim Biophys Acta. 1957;24:619–31. doi: 10.1016/0006-3002(57)90256-1. [DOI] [PubMed] [Google Scholar]
- 31.Vishwanath BS, Gowda TV. Interaction of aristolochic acid with Vipera russelli phospholipase A2: Its effect on enzymatic and pathological activities. Toxicon. 1987;25:929–37. doi: 10.1016/0041-0101(87)90155-3. [DOI] [PubMed] [Google Scholar]
- 32.Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidative substrates by means of Folin-Ciocalteau reagent. Methods Enzymol. 1999;299:152. [Google Scholar]
- 33.Blios MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199. [Google Scholar]
- 34.Halliwell B, Gutteridge JM. Free Radicals in Biology and Medicine. 2nd ed. Oxford: Clarendon Press; 1989. [DOI] [PubMed] [Google Scholar]
- 35.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 36.Yen Duh PD. Antioxidative properties of methanolic extracts from peanut hulls. J Am Oil Chem Soci. 1993;70:383–6. [Google Scholar]
- 37.Brahmane RI, Pathak SS, Wanmali VV, Salwe KJ, Premendran SJ, Shinde BB. Partial in vitro and in vivo red scorpion venom neutralization activity of Andrographis paniculata. Pharmacognosy Res. 2011;3:44–8. doi: 10.4103/0974-8490.79115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Touqui L, Alaoui-El-Azher M. Mammalian secreted phospholipases A2 and their pathophysiological significance in inflammatory diseases. Curr Mol Med. 2001;1:739–54. doi: 10.2174/1566524013363258. [DOI] [PubMed] [Google Scholar]
- 39.Murakami M, Kudo I. Secretory phospholipase A2. Biol Pharm Bull. 2004;27:1158–64. doi: 10.1248/bpb.27.1158. [DOI] [PubMed] [Google Scholar]
- 40.Lewén A, Matz P, Chan PH. Free radical pathways in CNS injury. J Neurotrauma. 2000;17:871–90. doi: 10.1089/neu.2000.17.871. [DOI] [PubMed] [Google Scholar]
- 41.Kishore V, Yarla NS, Zameer F, Nagendra Prasad MN, Santosh MS, More SS, et al. Inhibition of group IIA secretory phospholipase A2 and its inflammatory reactions in mice by ethanolic extract of Andrographis paniculata, a well-known medicinal food. Pharmacognosy Res. 2016;8:213–6. doi: 10.4103/0974-8490.182916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhadoriya SS, Ganeshpurkar A, Narwaria J, Rai G, Jain AP. Tamarindus indica: Extent of explored potential. Pharmacogn Rev. 2011;5:73–81. doi: 10.4103/0973-7847.79102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petrovska BB. Historical review of medicinal plants' usage. Pharmacogn Rev. 2012;6:1–5. doi: 10.4103/0973-7847.95849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davidson FF, Dennis EA, Powell M, Glenney JR., Jr Inhibition of phospholipase A2 by “lipocortins” and calpactins. An effect of binding to substrate phospholipids. J Biol Chem. 1987;262:1698–705. [PubMed] [Google Scholar]
- 45.Vishwanath BS, Appu Rao AG, Gowda TV. Interaction of phospholipase A2 from Vipera russelli venom with aristolochic acid: A circular dichroism study. Toxicon. 1987;25:939–46. doi: 10.1016/0041-0101(87)90156-5. [DOI] [PubMed] [Google Scholar]
- 46.Marshall LA, Blazek E, Chang J. Characterization of an in-vivo model for evaluation of phospholipase A2 inhibitors. Fed Proc. 1987;46:854–62. [Google Scholar]