Abstract
Background:
Interleukin-6 (IL-6) is a multifunctional glycoprotein that regulates the growth of some tumors, including prostate carcinomas due to signal transducer and activator of transcription 3 (STAT3), extracellular signal-regulated kinases 1/2 (ERK1/2), and AKT signaling pathways. Hesperetin, as a flavanone, has several biological properties such as antitumor and anti-inflammatory.
Objective:
This study was carried out to evaluate the biological effects of hesperetin on the IL-6 gene expression and phosphorylated STAT3, AKT, and ERK1/2 signaling pathways in PC3 prostate cancer (PC) cells.
Materials and Methods:
In this study, we used real-time quantitative polymerase chain reaction (RT-qPCR) and ELISA to evaluate IL-6 gene expression and IL-6 protein secretion, respectively, in the treated PC3 cells with 0, 400, 450, and 500 μM of hesperetin. Cell survival studies were done by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay after 48 h treatment with hesperetin, and cell apoptosis was determined by flow cytometry. The protein levels of activated signaling molecules (pSTAT3, pAKT, and pERK1/2) analyzed by immunoprecipitation technique.
Results:
Hesperetin-treated PC3 cells resulted in reduction of cell viability. Hesperetin led to the elevation of phosphorylated STAT3, ERK1/2, and AKT signaling proteins after 48 h in a dose-dependent manner as compared to the control cells. IL-6 gene expression, as well as protein level, significantly increased (P < 0.05) in a dose-dependent pattern in treated PC3 with hesperetin compared to the control cells. Further, hesperetin exposure resulted in the induction of cell cycle arrest at G0/G1 phase.
Conclusion:
Hesperetin in PC3 cells led to elevation IL-6 gene expression, IL-6 protein secretion, pSTAT3, pERK1/2 and pAKT intracellular signaling proteins. Our results indicate that hesperetin treatment leads to the inhibition of cell proliferation and the induction of cell cycle arrest at the G1 phase. Hesperetin can be considered a potent agent which synchronizes and stops cell cycle at G0/G1 phase to apply suitable chemotherapeutic agents and radiotherapy in PC cells.
SUMMARY
This study evaluates biological effects of hesperetin on the cell cycle, interleukin-6 gene expression and some phosphorylated signaling pathways in PC3 prostate cancer cells. Hesperetin resulted in the inhibition of cell proliferation via inducing G0/G1 phase arrest in spite of the elevation of interleukin-6 gene expression and phosphorylated AKT, STAT3, and ERK1/2 intracellular signaling proteins. Therefore, hesperetin can be considered a potent agent which synchronizes and stop cell cycle at G0/G1 phase so that suitable chemotherapeutic agents can be applied in PC3 prostate cancer cells.
Abbreviations Used: PC: Prostate cancer, IL-6: Interleukin-6, STAT3: Signal transducer activator of transcription 3, ERK1/2: Extracellular signal–regulated kinases 1/2, IC50: Inhibitory concentration of 50%.
Key words: Hesperetin, interleukin-6, pAKT, phosphorylated extracellular signal-regulated kinases 1/2, phosphorylated signal transducer and activator of transcription 3, prostate cancer
INTRODUCTION
Prostate cancer (PC) is one of the most common malignancies and the second highest cause of cancer-related deaths among men in the United States.[1] In the past two decades, PC has increased strikingly in many countries.[2] Recent studies have exhibited the role of interleukin-6 (IL-6, a multifunctional glycoprotein including 212 amino) in the etiology and progression of PC.[3] In addition, IL-6 regulates some biological responses and in a cell type-dependent manner stimulates or inhibits cellular growth due to its activating of the signaling pathways, especially Janus kinase-signal transducer and activator of transcription 3 (STAT3) and extracellular signal-regulated kinases 1/2 (ERK1/2).[4,5] IL-6 is a suitable candidate for progression of targeted therapies for PC. Therefore, the suppressors of cytokine signaling lead to inhibiting phosphorylation of STAT3.[4,5]
Medicinal plants have long been used worldwide for treating a variety of diseases due to their flavonoids.[6,7,8] Flavonoids are polyphenolic compounds with various pharmacological properties which act as eliminator of free radicals by OH groups in their molecular structures. Hesperetin (3',5,7-trihydroxy-4-methoxyflavanone) is a member of the flavanone subclass of flavonoids which is found in large amounts in citrus fruits, including oranges and grapefruit. Hesperetin and its metabolites have several biological properties such as antitumor, antioxidant, anti-inflammatory, and lipid lowering effects.[9,10,11] Further, hesperetin possesses estrogenic properties and exerts an anti-atherogenic effect through estrogen receptor (ER)-mediated actions.[12,13] On the other hand, a previous study demonstrated that all ER-β isoforms, particularly ER-β2, are present in large amounts in PC3 cells.[14,15] Moreover, another study indicated that hesperetin can interact with estrogen-receptor on the cell surface.[16] In addition, many studies have shown that some antitumor effects of flavonoids, especially hesperetin, are exerted by modulating STAT3, AKT, and ERK1/2 cell signaling molecules.[17,18,19] There is accumulating evidence that flavonoids, especially hesperetin, regulate the activity of several protein kinases that control a number of various intracellular signaling proteins such as AKT/protein kinase B (AKT/PKB), protein kinase C, tyrosine kinases, phosphoinositide 3-kinase (PI3K), and mitogen-activated protein kinase (MAPK). This regulatory function is interfered due to the interaction of the flavonoids, with the ATP-binding sites on enzymes.[18,20,21] On the other hand, it is demonstrated that IL-6 is related to the activation of STAT3, AKT, and STAT3 cell signaling molecules in PC.[22,23] Therefore, considering the antioxidant properties of hesperetin, this study was carried out to evaluate the effects of hesperetin on the changes of the cellular phosphorylated STAT3, AKT, and ERK1/2 signaling pathways and the IL-6 gene expression in PC3 cells.
MATERIALS AND METHODS
The human PC3 cells were purchased from Pasteur Institute of Iran (Tehran, Iran). RPMI1640 medium, penicillin/streptomycin (PEN/STREP), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), trypan blue, and hesperetin were obtained from Sigma (St. Louis, MO). Fetal bovine serum (FBS) was prepared from Gibco (Rockville, MD, USA). Antibodies were purchased from Abcam Co. (San Francisco, CA, USA), and Biozol was prepared from BioFlux Kit Bioer Technology. All other chemicals used were of analytical grade.
Cell culture and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
The human PC3 cells were maintained in RPMI 1640 supplemented with 10% FBS, 100 U/mL PEN, and 0.1 mg/mL STREP. The cells were maintained at 37°C in a humidified incubator with 5% carbon dioxide. The cytotoxic effects of hesperetin on the human PC3 cells were determined by MTT assay. This technique depends on the capacity of living cells to reduce tetrazolium salt to a formazan crystal in their metabolizing mitochondria. Briefly, PC3 cells were seeded in 96-well culture plates at the density of 5 × 103 cells per well and were allowed to grow overnight. Then, the cells were treated with different concentrations of hesperetin (0–1000 μM in DMSO with final concentration of 0.1%) in their media. Each test was repeated 3 times. After treatment for 48 h, the medium was removed and 100 μL colorless RPMI and 10 μL MTT (5 mg/mL in phosphate-buffered saline [PBS]) were added to each well and incubated for 4 h.[24] The medium was removed and formazan was solubilized in 150 μL DMSO. Then, formazan absorbance was assessed at 490 nm using a microplate reader (Stat Fax 3200, Awareness Technology, USA).
Real-time quantitative polymerase chain reaction for interleukin-6 gene expression
Cells were harvested after treatment with different concentrations of hesperetin (0, 400, 450, and 500 μM) in a six-well plate for 48 h. Then, the media were removed for IL-6 protein levels determination. Then, the total RNA of cells was extracted using Biozol reagent according to the manufacturer's instructions. Total mRNA concentration and quality were analyzed by a NanoDrop spectrophotometer (Thermo-USA). cDNA was prepared from RNA using a synthesis Kit (Takara Bio, Japan) using 1 μg total mRNA according to the manufacturer's instructions. Then, cDNA was amplified by real-time polymerase chain reaction using (RT-PCR) SYBR ® Green PCR Master Mix (Qiagen). The cDNA was subjected to RT-quantitative PCR (RT-qPCR) using specific primers for IL-6 gene expression (Forward: 5'-AAGCCAGAGCTGTGCAGATGAGTA-3'; Reverse: 5' TGTCCTGCAGCCACTGGTTC-3') and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Forward: 5'-ACACCCACTCCTCCACCTTTG-3'; Reverse: 5'-CCACCACCCTGTTGCTGTAG-3'). The primers were designed using Oligo 6.0 software (Molecular Biology Insights, Cascade, CO), confirmed by blast (NCBI), and were purchased from Eurogentec (Seraing, Belgium). GAPDH, a housekeeping gene, was used as an internal control. IL-6 gene expression was detected using a Rotor-Gene 3000 (Corbett, Australia) for each experimental concentration. Amplification involved a first denaturation at 95°C for 5 min and RT-qPCR reaction was done for 40 cycles in a 3-step program (including 15 s at 95°C, 20 s at 62°C, and 25 s at 72°C).[18]
Western immunoblotting
The cells were cultured with different concentrations of hesperetin (0, 400, 450, and 500 μM) and they were harvested after 48 h. Then, the cells were lysed in RIPA buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1% Triton 100X, 0.5% sodium deoxycholate, 1 mM EDTA, 0.1% sodium azide, 50 mM NaF, and 0.1% sodium dodecyl sulfate [SDS], 1 mM PMSF). Protein concentrations were determined by the NanoDrop spectrophotometer. The protein lysates were mixed with an equal volume of Lameli buffer (0.125 M Tris-HCl pH 6.8, 4% SDS, 20% glycine, 10% 2-mercaptoethanol) and boiled for 5 min. The denatured proteins were separated on 10% SDS-polyacrylamide gel electrophoresis. Prestained blue protein markers (Bio-Rad) were used for molecular weight determination. The gels were blotted onto polyvinylidene difluoride membranes and were immediately placed in a blocking solution (5% w/v skim milk powder in tris-buffered saline (TBS)-Tween buffer containing 10 mM Tris pH 7.4, 100 mM NaCl, and 0.1 mM Tween-20) for overnight at 4°C. The membranes were washed in TBS-Tween buffer for 30 min and then incubated with primary antibodies against either pSTAT3, phosphorylated p44/42ERK1/2, and phosphorylated AKT or β-actin according to the manufacturer's instructions at room temperature for 3 h. Then, membranes were washed with TBS-Tween buffer 3 times for 10 min and incubated with an appropriate dilution of horseradish peroxidase-conjugated secondary antibody at room temperature for 2 h. After washing the membrane 3 times for 10 min in TBS-Tween buffer, the bands were revealed by adding BM blue POD substrate.
Cell cycle and apoptosis
PC3 cells were cultured in a 6-well plate and allowed to attach to plate for overnight. Then, the cells were treated with 0, 400, and 450 μM of hesperetin for 48 h. Apoptosis was analyzed by flow cytometry (Cyflow ® space flow cytometer, Partec, Münster, Germany) using a fluorescein isothiocyanate (FITC) Annexin V Apoptosis Detection Kit (BD Biosciences) according to the manufacturer's instructions. The excitation and emission wavelengths were 450 nm and 519 nm for FITC, respectively. The excitation and emission wavelengths were 540 nm and 620 nm for propidium iodide, respectively. Briefly, the treated PC3 cells with hesperetin were trypsinized, washed in PBS, diluted in 500 μL of binding buffer, and incubated in 10 μL of Annexin-binding buffer with 2 μL of Annexin V-FITC for 20 min in the dark.
Measurement of interleukin-6 protein level
Levels of the protein IL-6 in the different hesperetin-treated culture medium (0, 400, 450, and 500 μM) were determined by ELISA kit (AViBion Human IL-6 ELISA kit) according to the manufacturer's protocol.
Statistical analysis
The results were presented as the mean ± standard deviation. Statistical analysis was performed using SPSS version 20.0 software (SPSS, Chicago, IL, USA). For expression analysis, the relative quantitation of gene transcripts was estimated with the ΔΔCT method and was normalized by GAPDH expression in each sample and data were expressed as fold change. Melting curves for each PCR reaction were generated to ensure the purity of the amplification product. The experiments of western blot were repeated for 3 times. A value of P < 0.05 was considered to indicate a statistically significant result.
RESULTS
Effects of hesperetin on PC3 cells viability
Figure 1 shows that cell proliferation and viability in hesperetin-treated PC3 cells decreased after 48 h in a dose-dependent manner. Further, hesperetin-treated PC3 cells show fewer cells and more cell shrinkage as opposed to untreated cells [Figure 2]. PC3 cells that were exposed to hesperetin (0–700 μM) exhibited an inhibitory concentration of 50% (IC50) of about 450 μM.
Figure 1.
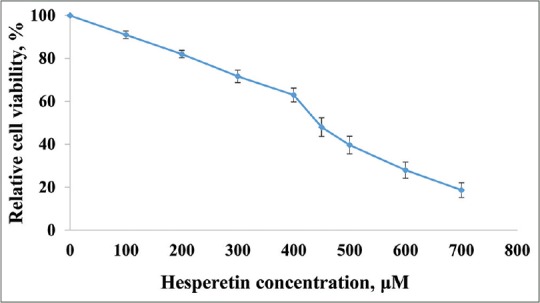
Inhibition of cell proliferation by hesperetin. PC3 cells were treated with different concentrations of hesperetin for 48 h. At the end of treatment times, cell viability was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay as described under materials and methods section. Each bar represents the mean ± standard deviation of three independent observations
Figure 2.
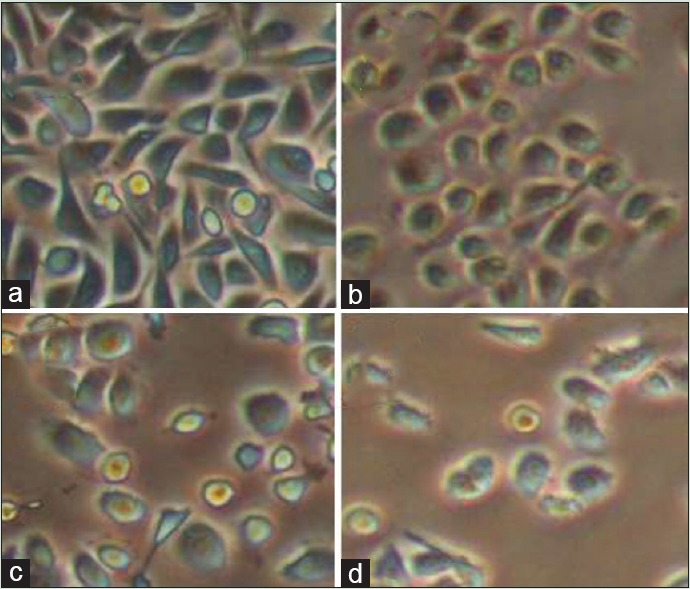
Morphological changes of cell by hesperetin in PC3 cells after treatment with various concentrations of hesperetin (a: 0; b: 400; c: 450; and d: 500 μM) after 48 h
The effect of hesperetin on interleukin-6 gene expression in PC3 cells line
Hesperetin reinforced IL-6 transcriptional activity in PC3 cells. Figure 3 shows the treated PC3 cells with/without hesperetin for IL-6 mRNA expression using RT-qPCR. mRNA expression of IL-6 with hesperetin treatment significantly upregulated (P < 0.05) in a dose-dependent pattern. As shown in Figure 3, there was a significant elevation (P < 0.05) in IL-6 gene expression by almost 6.2, 9.1, and 10.5 fold at 400, 450, and 500 μM of hesperetin when compared with control cells, respectively. Further, there was a significant increase (P < 0.05) in IL-6 gene expression in hesperetin-treated PC3 cells at 450 and 500 μM when compared with 400 μM of hesperetin.
Figure 3.
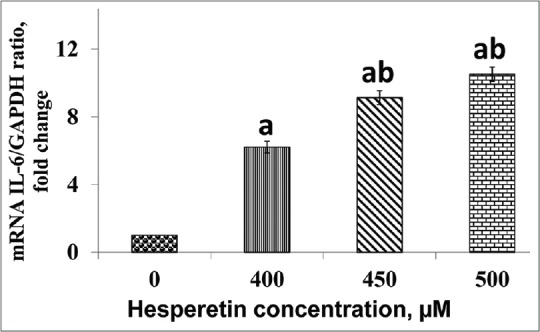
The effect of hesperetin on the interleukin-6 expression. Expression of interleukin-6 was significantly upregulated in PC3 being treated with hesperetin at the concentrations of 400, 450, and 500 μM after 48 h in a dose-dependent pattern. mRNA expression of interleukin-6 normalized with glyceraldehyde-3-phosphate dehydrogenase as an internal control. aP < 0.05 compared to the control cells. bP < 0.05 compared to 400 μM hesperetin-treated PC3 cells
Effect of hesperetin on the phosphorylated AKT, extracellular signal-regulated kinases, and signal transducer and activator of transcription 3 signaling pathways
Figure 4 shows the effect of hesperetin on the cellular levels of pSTAT3, pERK1/2 and pAKT signaling proteins. Our western blots data showed an increase in the expression of pSTAT3, pAKT, and pERK1/2, signaling pathway proteins in a dose-dependent manner compared with control cells after treatment with different doses of hesperetin for 48 h.
Figure 4.
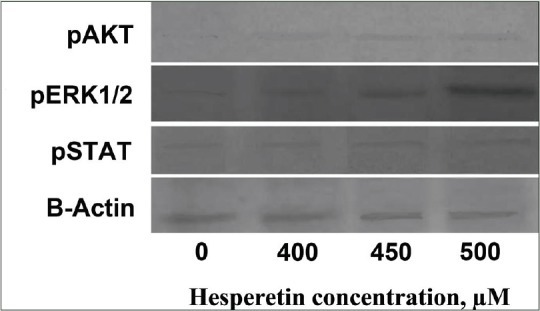
The effects of hesperetin on the level of signaling pathway proteins in PC3 cells which were treated with increasing doses of hesperetin for 48 h, and cell lysates were collected and subjected to Western blotting analysis. Equal amounts of lysate protein were subjected to gel electrophoresis. Hesperetin upregulated the expression of phosphorylated signal transducer and activator of transcription 3, pAKT, and phosphorylated extracellular signal-regulated kinases 1/2
Effect of hesperetin on cell cycle
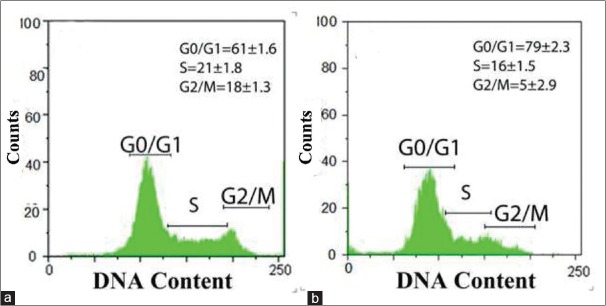
Hesperetin reduced the percentage of viable apoptotic cells in a flow cytometry analysis. Figure 5 shows the effect of hesperetin on the cell cycle of PC3 cells. Our data showed that hesperetin arrested PC3 cells at G0/G1 phase of the cell cycle. After exposure to 450 μM of hesperetin for 48 h, a significant increase (P < 0.05) in the number of G1-phase cells was seen from 61.6% to 79.4%. Further, the proportion of S-phase cells significantly decreased (P < 0.05) from 21.1% to 16.3%. In addition, Figure 6 shows the effect of hesperetin on the apoptosis of PC3 cells. Apoptosis in PC3 cells was slightly induced (not significantly) 5.4%, 7.8%, and 9.1% at 400, 450, and 500 μM of hesperetin, respectively. On the other hand, hesperetin significantly restrained the PC3 cell proliferation whereas the apoptosis of PC3 was not significant.
Figure 5.
Effects of hesperetin on cell cycle progression in PC3 cells. (a) Untreated PC3 cells with hesperetin after 48 h incubation. (b) Cells were treated with 450 μM of hesperetin for 48 h. The cell cycle distribution and proportions of the cells in G0/G1, S, and G2/M were determined using flow cytometric analysis. Data are presented as means ± standard deviation of the results from three independent experiments
Figure 6.
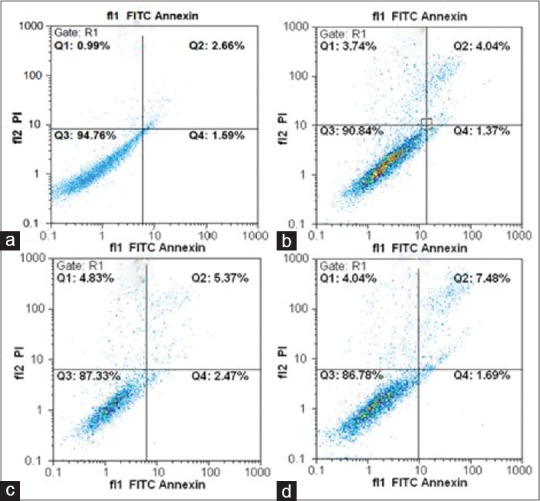
Apoptotic effects of hesperetin in PC3 cells. Apoptosis induced by hesperetin was analyzed by flow cytometry using dual staining with propidium iodide and fluorescein isothiocyanate-conjugated Annexin V in PC3 cells. (a) Control without treatment, (b-d) the cells were treated with 400, 450, and 500 μM of hesperetin, respectively
The effect of hesperetin on interleukin-6 protein secretion
IL-6 protein secretion by hesperetin-treated PC3 cells is shown in Figure 7. Hesperetin significantly resulted in IL-6 protein levels rising in culture supernatants PC3 cells media in a dose-dependent manner as compared to control cells. There was a significant increase (P < 0.05) in IL-6 protein levels by almost 35.91%, 64.80%, and 83.22% at 400, 450, and 500 μM in hesperetin-treated PC3 cells when compared with control cells, respectively.
Figure 7.
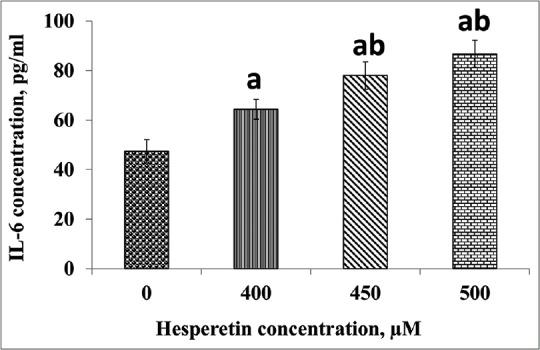
The levels of interleukin-6 after treatment with hesperetin in PC3 cells which were significantly elevated compared with the control cells. PC3 cells were treated with different concentrations of hesperetin for 48 h and secreted interleukin-6 was measured by ELISA. Data represent the mean ± standard deviation value of three consecutive experiments. aP < 0.05 compared to the control cells. bP < 0.05 compared to 400 μM treated cells with hesperetin
DISCUSSION
In the past decade, investigations on PC chemoprevention have increased considerably which makes PC a better target for chemoprevention. PC is initially androgen dependent, but it will progress to become a more aggressive and androgen-independent form.[25] IL-6 plays a key role in proliferation, apoptosis, and differentiation of PC3 cells.[3,4] Our results indicated that hesperetin treatment has anti-proliferative effects and results in the reduction of PC3 cells viability [Figures 1 and 2] as reported by other investigators.[9,10] Further, our findings indicated that IC50 of the potential cytotoxic effects of hesperetin was 450 μM, which is in accordance with other investigations.[26] On the other hand, IC50 in treated PC3 cells with hesperetin was 40 μM in the study of Sambantham et al.[18] The difference between our results and those of Sambantham et al. study may be, at least in part, due to using different media, experimental conditions, and the source preparation of hesperetin. In addition, apoptosis in PC3 cells treated with hesperetin by flow cytometry revealed that apoptosis increased slightly (not significant) in treated PC3 cells compared to the control group [Figure 6]. Apoptosis at 450 μM of hesperetin was about 8% in treated PC3 cells. It seems that the results of apoptosis is in line with elevated IL-6 protein secretion by PC3 cells. In our study, hesperetin led to the elevation of IL-6 protein level secretion by PC3 cells [Figure 7]. The elevation of IL-6 can be resulted from an internal reaction PC3 cells against hesperetin because IL-6 protein leads to an increase in cancer cell resistance against chemotherapeutic agent,[15,27] which leads to the induction of anti-apoptosis effects. On the other hand, previous studies have shown that all ER-β isoforms, particularly ER-β2, are present at high levels in PC3 cell lines.[14,28] In a study, it was demonstrated that hesperetin can interact with estrogen-receptor on the cell surface.[16] In addition, previous studies have depicted that hesperetin possesses estrogenic properties and it might act on receptors and modulate the activation of AKT/PKB, ERK1/2, and c-Jun N-terminal kinase (JNK) for pro-survival signaling responses.[17,19] Therefore, in our study, the reduction of apoptotic effects of hesperetin in PC3 cells, at least in part, may be resulted from cell cycle processing so that cell cycle balance between cell survival and cell death is regulated by both the G1 and G2/M portions of the cell cycle [Figure 5]. At present, radiotherapy is a major therapeutic approach in the treatment of cancer together with surgery and chemotherapy. The outcome of irradiation is affected by the cell cycle.[29,30,31] Mitotic cells are hypersensitive to irradiation. The cycle is minimal during the mitotic and late G1 or early DNA synthesis phases.[29,30,31] Therefore, using a cell sorter, which can be a flavonoid such as hesperetin, the cell-cycle phase in each cell can be selected precisely according to the duration of the G0/G1 phase for radiotherapy and chemotherapy. Previous studies have indicated that the effects of hesperetin on the cell cycle result in the inhibition of cell proliferation at G1 phase[32] and the arrest of most cells predominately at G0/G1 phase which in agreement with our study. As shown in Figure 5, in this study, hesperetin caused the elevation of G0/G1 phase in treated PC3 cells and a reduction at the S and G2 phases of the cell cycle which is in line with findings of other investigators.[32] It has been shown that flavanone exposure also leads to G1 and G2/M arrest in human cancer cells.[33] In addition, hesperetin results in cell cycle arrest in G1 phase in MCF-7 human breast cancer cells.[34] Therefore, in our study, hesperetin resulted in the inhibition of cell proliferation through inducing G0/G1 phase arrest in spite of the elevation of IL-6 protein secretion by PC3 cells. This may be, in part, an important molecular mechanism through which hesperetin inhibits the growth of cancer cells. On the other hand, in the study of Sambantham et al., they reported that hesperetin resulted in evoking apoptosis in PC3 cells at 40 μM.[18] The differences in apoptosis in our results with those of Sambantham et al. may be, in part, due to higher hesperetin concentration (450 μM) used in our experiments which led to the inhibition of cell proliferation via inducing G0/G1 phase arrest in PC3 cells.
Many published studies have reported that IL-6 protein can inhibit or stimulate several cancer cell lines due to its different interactions with cellular regulatory signaling pathways.[32] It is reported that in PC12 cells, estrogen activates membrane ER-mediated pro-survival AKT/PKB, Src/MEK/ERK, and MAPK/ERK pathways for pro-survival responses.[35] In addition, IL-6 protein is known to activate STAT3 signaling pathway which plays an essential role in the pathogenesis and prevention of apoptosis[36] as was observed in our study. In the present study, the hesperetin-treated PC3 cells induced the elevation of phosphorylated AKT, STAT3, and ERK1/2 intracellular signaling proteins [Figure 4], which is consistent with increased IL-6 protein secretion [Figure 7] by PC3 cells. Consistent with our observations, hesperetin has shown to lead to significant increases in the level of ERK1/2 phosphorylation when used at low concentrations.[16] Further, in another study, the protein expression of phospho-ERK, phospho-AKT, and phospho-CREB was induced by hesperetin which in agreement with our study.[37] Many studies reported that ER-α and β (ER-α/ER-β+) expression is increased in PC3 and PC3M, a highly metastatic variant of the PC cell line, and acts as oncogenes. On the other hand, it is now well established that cellular signals could be induced through signaling molecules, receptors, and proteins related to intracellular signal pathways.[28] There is a body of evidence which demonstrates that estrogens and its receptors are important regulators of the prostate function.[38] Therefore, the elevation of pSTAT3, pERK1/2 and pAKT in our study [Figure 4] can result, at least in part, in the interaction of hesperetin with ER on the PC3 cell surface. Previous studies demonstrated that hesperetin increased protein levels of PI3K isoforms and the phosphorylation of AKT through its action on receptors and its modulatory role in the activation of AKT/PKB, extracellular ERK, and JNK for pro-survival signaling responses,[12,39,40] which is in accordance with our study. AKT is a downstream target of PI3K which is activated through the phosphorylation by PI3K-dependent kinases PDK1 and PDK2.[41] Moreover, other published studies have shown that STAT and MAPK/ERK1/2 can be associated with cell survival and prevention of apoptosis,[42] which is in line with our study. Furthermore, in a study, it was shown that the activation of STAT3 by IL-6 increases gene expression of many survival proteins, such as Bcl-2, Bcl-X, Mcl-1, survivin, and X-linked inhibitor of apoptosis protein.[43] In addition, recent findings suggest that the MEK-1/MAPK (ERK1/2) pathway may play a role in suppressing apoptosis due to its interface with survival signaling at the level of Bcl2 to directly link these two critical growth pathways.[42] In our study, as shown in Figure 4, hesperetin resulted in the elevation of pERK1/2 which in turn suppresses apoptosis [Figure 6], which is in line with reported studies.[42,44] In another study, it has been demonstrated that human SH-SY5Y neuroblastoma cells or human primary dermal fibroblasts (PromoCells) as a result of exposure to hesperetin led to a siginficant elevation in the level of ERK1/2 phosphorylation,[15] which is in agreement with our results. The mechanism underlying these effects seems to be related to flavonoid antioxidant properties of hesperetin to alter signal transduction protein kinases. Therefore, hesperetin can be considered a potent agent which synchronizes and stops cell cycle at G0/G1 phase so that suitable chemotherapeutic agents and radiotherapy can be applied.
In our study, we did not evaluate the tumor suppressor gene p53 as an important key factor in a balance between cell survival and cell death through regulation of both the G1 and G2/M portions of the cell cycle. In addition, we did not evaluate the multiple caspases. Thus, we suggest that future studies focus on other possible mechanisms of hesperetin on the mentioned principle factors in PC3 cells.
CONCLUSION
Hesperetin in PC3 cells led to elevation IL-6 gene expression, IL-6 protein secretion, pSTAT3, pERK1/2 and pAKT intracellular signaling proteins. Furthermore, hesperetin inhibited cell proliferation due to the elevation of G0/G1 phase cell cycle. Our results indicate that hesperetin can be considered a potent agent which synchronizes and stop cell cycle at G0/G1 phase so that suitable chemotherapeutic agents and radiotherapy can be applied in PC3 PC cells.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We would like to express our gratitude to those who have helped us in Clinical Biochemistry Research Center of Shahrekord University of Medical Sciences. The results described in this paper were the MS dissertation of Mr. Moein Shirzad.
REFERENCES
- 1.Rasul A, Di J, Millimouno FM, Malhi M, Tsuji I, Ali M, et al. Reactive oxygen species mediate isoalantolactone-induced apoptosis in human prostate cancer cells. Molecules. 2013;18:9382–96. doi: 10.3390/molecules18089382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray NP, Reyes E, Fuentealba C, Orellana N, Jacob O. Sequential use of primary malignant circulating prostate cells to detect prostate cancer. Cancer Cell Microenviron. 2015;2:e714. [Google Scholar]
- 3.Nguyen DP, Li J, Tewari AK. Inflammation and prostate cancer: The role of interleukin 6 (IL-6) BJU Int. 2014;113:986–92. doi: 10.1111/bju.12452. [DOI] [PubMed] [Google Scholar]
- 4.Culig Z. Proinflammatory cytokine interleukin-6 in prostate carcinogenesis. Am J Clin Exp Urol. 2014;2:231–8. [PMC free article] [PubMed] [Google Scholar]
- 5.Heidarian E, Keloushadi M, Ghatreh-Samani K, Valipour P. The reduction of IL-6 gene expression, pAKT, pERK1/2, pSTAT3 signaling pathways and invasion activity by gallic acid in prostate cancer PC3 cells. Biomed Pharmacother. 2016;84:264–9. doi: 10.1016/j.biopha.2016.09.046. [DOI] [PubMed] [Google Scholar]
- 6.Heidarian E, Saffari J, Jafari-Dehkordi E. Hepatoprotective action of Echinophora platyloba DC leaves against acute toxicity of acetaminophen in rats. J Diet Suppl. 2014;11:53–63. doi: 10.3109/19390211.2013.859217. [DOI] [PubMed] [Google Scholar]
- 7.Valipour P, Heidarian E, Khoshdel A, Gholami-Arjenaki M. Protective effects of hydroalcoholic extract of ferulago angulata against gentamicin-induced nephrotoxicity in rats. Iran J Kidney Dis. 2016;10:189–96. [PubMed] [Google Scholar]
- 8.Rezaei A, Heidarian E. Co-administration of trientine and flaxseed oil on oxidative stress, serum lipids and heart structure in diabetic rats. Indian J Exp Biol. 2013;51:646–52. [PubMed] [Google Scholar]
- 9.Wang J, Zhu H, Yang Z, Liu Z. Antioxidative effects of hesperetin against lead acetate-induced oxidative stress in rats. Indian J Pharmacol. 2013;45:395–8. doi: 10.4103/0253-7613.115015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Song J, Wu D, Wang J, Dong W. Hesperetin induces the apoptosis of hepatocellular carcinoma cells via mitochondrial pathway mediated by the increased intracellular reactive oxygen species, ATP and calcium. Med Oncol. 2015;32:101. doi: 10.1007/s12032-015-0516-z. [DOI] [PubMed] [Google Scholar]
- 11.Chahar MK, Sharma N, Dobhal MP, Joshi YC. Flavonoids: A versatile source of anticancer drugs. Pharmacogn Rev. 2011;5:1–12. doi: 10.4103/0973-7847.79093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hwang SL, Yen GC. Effect of hesperetin against oxidative stress via ER- and TrkA-mediated actions in PC12 cells. J Agric Food Chem. 2011;59:5779–85. doi: 10.1021/jf104632a. [DOI] [PubMed] [Google Scholar]
- 13.Liu L, Xu DM, Cheng YY. Distinct effects of naringenin and hesperetin on nitric oxide production from endothelial cells. J Agric Food Chem. 2008;56:824–9. doi: 10.1021/jf0723007. [DOI] [PubMed] [Google Scholar]
- 14.Leung YK, Mak P, Hassan S, Ho SM. Estrogen receptor (ER)-beta isoforms: A key to understanding ER-beta signaling. Proc Natl Acad Sci U S A. 2006;103:13162–7. doi: 10.1073/pnas.0605676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rainey-Smith S, Schroetke LW, Bahia P, Fahmi A, Skilton R, Spencer JP, et al. Neuroprotective effects of hesperetin in mouse primary neurones are independent of CREB activation. Neurosci Lett. 2008;438:29–33. doi: 10.1016/j.neulet.2008.04.056. [DOI] [PubMed] [Google Scholar]
- 16.Hwang SL, Shih PH, Yen GC. Neuroprotective effects of citrus flavonoids. J Agric Food Chem. 2012;60:877–85. doi: 10.1021/jf204452y. [DOI] [PubMed] [Google Scholar]
- 17.Hwang SL, Yen GC. Modulation of Akt, JNK, and p38 activation is involved in citrus flavonoid-mediated cytoprotection of PC12 cells challenged by hydrogen peroxide. J Agric Food Chem. 2009;57:2576–82. doi: 10.1021/jf8033607. [DOI] [PubMed] [Google Scholar]
- 18.Sambantham S, Radha M, Paramasivam A, Anandan B, Malathi R, Chandra SR, et al. Molecular mechanism underlying hesperetin-induced apoptosis by in silico analysis and in prostate cancer PC-3 cells. Asian Pac J Cancer Prev. 2013;14:4347–52. doi: 10.7314/apjcp.2013.14.7.4347. [DOI] [PubMed] [Google Scholar]
- 19.Sak K. Cytotoxicity of dietary flavonoids on different human cancer types. Pharmacogn Rev. 2014;8:122–46. doi: 10.4103/0973-7847.134247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li S, Wang L, Hu Y, Sheng R. Autophagy regulators as potential cancer therapeutic agents: A review. Curr Top Med Chem. 2015;15:720–44. doi: 10.2174/1568026615666150302105343. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida H, Takamura N, Shuto T, Ogata K, Tokunaga J, Kawai K, et al. The citrus flavonoids hesperetin and naringenin block the lipolytic actions of TNF-alpha in mouse adipocytes. Biochem Biophys Res Commun. 2010;394:728–32. doi: 10.1016/j.bbrc.2010.03.060. [DOI] [PubMed] [Google Scholar]
- 22.Eskandari E, Heidarian E, Amini SA, Saffari- Chaleshtori J. Evaluating the effects of ellagic acid on pSTAT3, pAKT, and pERK1/2 signaling pathways in prostate cancer PC3 cells. J Can Res Ther. 2016;12:1266–71. doi: 10.4103/0973-1482.165873. [DOI] [PubMed] [Google Scholar]
- 23.Santoni M, Massari F, Del Re M, Ciccarese C, Piva F, Principato G, et al. Investigational therapies targeting signal transducer and activator of transcription 3 for the treatment of cancer. Expert Opin Investig Drugs. 2015;24:809–24. doi: 10.1517/13543784.2015.1020370. [DOI] [PubMed] [Google Scholar]
- 24.Gurushankar K, Gohulkumar M, Prasad NR, Krishnakumar N. Synthesis, characterization and in vitro anti-cancer evaluation of hesperetin-loaded nanoparticles in human oral carcinoma (KB) cells. Adv Nat Sci Nanosci Nanotechnol. 2014;5:015006 (10pp). doi:10.1088/2043-6262/5/1/015006. [Google Scholar]
- 25.Goldberg AA, Titorenko VI, Beach A, Sanderson JT. Bile acids induce apoptosis selectively in androgen-dependent and -independent prostate cancer cells. Peer J. 2013;1:e122. doi: 10.7717/peerj.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alshatwi AA, Ramesh E, Periasamy VS, Subash-Babu P. The apoptotic effect of hesperetin on human cervical cancer cells is mediated through cell cycle arrest, death receptor, and mitochondrial pathways. Fundam Clin Pharmacol. 2013;27:581–92. doi: 10.1111/j.1472-8206.2012.01061.x. [DOI] [PubMed] [Google Scholar]
- 27.Zarebczan B, Pinchot SN, Kunnimalaiyaan M, Chen H. Hesperetin, a potential therapy for carcinoid cancer. Am J Surg. 2011;201:329–32. doi: 10.1016/j.amjsurg.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hariri W, Sudha T, Bharali DJ, Cui H, Mousa SA. Nano-targeted delivery of toremifene, an estrogen receptor-a blocker in prostate cancer. Pharm Res. 2015;32:2764–74. doi: 10.1007/s11095-015-1662-x. [DOI] [PubMed] [Google Scholar]
- 29.Rae C, Mairs RJ. Evaluation of the radiosensitizing potency of chemotherapeutic agents in prostate cancer cells. Int J Radiat Biol. 2010:1–10. doi: 10.1080/09553002.2017.1231946. doi: 10.1080/09553002.2017.1231946. [DOI] [PubMed] [Google Scholar]
- 30.Otani K, Naito Y, Sakaguchi Y, Seo Y, Takahashi Y, Kikuta J, et al. Cell-cycle-controlled radiation therapy was effective for treating a murine malignant melanoma cell line in vitro and in vivo. Sci Rep. 2016;6:30689. doi: 10.1038/srep30689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao C, Wu X, Yan Y, Meng L, Shan D, Li Y, et al. Sensitization of radiation or gemcitabine-based chemoradiation therapeutic effect by nimotuzumab in pancreatic cancer cells. Technol Cancer Res Treat. 2016;15:446–52. doi: 10.1177/1533034615585209. [DOI] [PubMed] [Google Scholar]
- 32.Choi EJ, Lee JI, Kim GH. Anti-carcinogenic effect of a new analogue 4'-chloroflavanone from flavanone in human breast cancer cells. Int J Mol Med. 2010;25:293–8. [PubMed] [Google Scholar]
- 33.Park M, Chae HD, Yun J, Jung M, Kim YS, Kim SH, et al. Constitutive activation of cyclin B1-associated cdc2 kinase overrides p53-mediated G2-M arrest. Cancer Res. 2000;60:542–5. [PubMed] [Google Scholar]
- 34.Choi EJ. Hesperetin induced G1-phase cell cycle arrest in human breast cancer MCF-7 cells: Involvement of CDK4 and p21. Nutr Cancer. 2007;59:115–9. doi: 10.1080/01635580701419030. [DOI] [PubMed] [Google Scholar]
- 35.Alexaki VI, Charalampopoulos I, Kampa M, Nifli AP, Hatzoglou A, Gravanis A, et al. Activation of membrane estrogen receptors induce pro-survival kinases. J Steroid Biochem Mol Biol. 2006;98:97–110. doi: 10.1016/j.jsbmb.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 36.Mora LB, Buettner R, Seigne J, Diaz J, Ahmad N, Garcia R, et al. Constitutive activation of Stat3 in human prostate tumors and cell lines: Direct inhibition of Stat3 signaling induces apoptosis of prostate cancer cells. Cancer Res. 2002;62:6659–66. [PubMed] [Google Scholar]
- 37.Huang YC, Liu KC, Chiou YL. Melanogenesis of murine melanoma cells induced by hesperetin, a Citrus hydrolysate-derived flavonoid. Food Chem Toxicol. 2012;50:653–9. doi: 10.1016/j.fct.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 38.Dumbre RK, Kamble MB, Patil VR. Inhibitory effects by ayurvedic plants on prostate enlargement induced in rats. Pharmacognosy Res. 2014;6:127–32. doi: 10.4103/0974-8490.129031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim SY, Lee JY, Park YD, Kang KL, Lee JC, Heo JS. Hesperetin alleviates the inhibitory effects of high glucose on the osteoblastic differentiation of periodontal ligament stem cells. PLoS One. 2013;8:e67504. doi: 10.1371/journal.pone.0067504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vauzour D, Vafeiadou K, Rice-Evans C, Williams RJ, Spencer JP. Activation of pro-survival Akt and ERK1/2 signalling pathways underlie the anti-apoptotic effects of flavanones in cortical neurons. J Neurochem. 2007;103:1355–67. doi: 10.1111/j.1471-4159.2007.04841.x. [DOI] [PubMed] [Google Scholar]
- 41.Bader AG, Kang S, Zhao L, Vogt PK. Oncogenic PI3K deregulates transcription and translation. Nat Rev Cancer. 2005;5:921–9. doi: 10.1038/nrc1753. [DOI] [PubMed] [Google Scholar]
- 42.Deng X, Ruvolo P, Carr B, May WS., Jr Survival function of ERK1/2 as IL-3-activated, staurosporine-resistant Bcl2 kinases. Proc Natl Acad Sci U S A. 2000;97:1578–83. doi: 10.1073/pnas.97.4.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo Y, Xu F, Lu T, Duan Z, Zhang Z. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat Rev. 2012;38:904–10. doi: 10.1016/j.ctrv.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 44.Ramshankar V, Krishnamurthy A. Chemoprevention of oral cancer: Green tea experience. J Nat Sci Biol Med. 2014;5:3–7. doi: 10.4103/0976-9668.127272. [DOI] [PMC free article] [PubMed] [Google Scholar]