Abstract
Background:
The chemical composition of plants used in traditional medicine exhibits biologically active compounds, such as tannins, flavonoids, and alkaloids and becomes a promising approach to treat microbial infections, mainly with drug-resistant bacteria.
Objective:
The aim of the present study was to evaluate the hydroethanolic leaf extracts of Tamarindus indica (tamarind) and Manihot esculenta (cassava) as antimicrobial potential against Pseudomonas aeruginosa clinical isolated and Methicillin-resistant Staphylococcus aureus.
Materials and Methods:
Hydroethanolic leaf extracts were prepared and characterized by high-performance liquid chromatography/diode array detection, Fourier transform infrared, 1,1-diphenyl-2-picrylhydrazyl, and ultraviolet-visible methods. The antimicrobial activity against four strains of clinical relevance was evaluated by the microdilution method at minimum inhibitory concentrations.
Results:
Phenolic compounds such as flavonoids were detected in the plant extracts. T. indica extract at 500 μg/mL showed antimicrobial activity against S. aureus and P. aeruginosa; however, M. esculenta showed only activity against P. aeruginosa in this concentration.
Conclusions:
Our results suggested that polyphenols and flavonoids present in T. indica leaf extracts are a potential source of antimicrobial compound. The T. indica extract showed antibacterial activity against S. aureus and P. aeruginosa while M. esculenta had effect only on P. aeruginosa meropenem resistant.
SUMMARY
Antibacterial effect of T. indica and M. esculenta leaf extract was evaluated.
T. indica extract displayed activity against S. aureus and P. aeruginosa strains.
M. esculenta showed effect on P. aeruginosa meropenem resistant.
Abbreviations Used: BHI: Agar brain heart infusion, CAPES: Coordination for the improvement of higher education personnel, DPPH: 1,1-diphenyl-2-picrylhydrazyl, FAPITEC/SE: Foundation for support to research and technological innovation of the state of sergipe, FTIR: Fourier transform infrared spectroscopy, HPLC: High-performance liquid chromatography, KBr: Potassium bromide, MIC: Minimum inhibitory concentration, MRSA: Methicillin-resistant Staphylococcus aureus, RSC: Radical scavenging capacity, UV-vis: Ultraviolet-visible.
Key words: Antibiotic-resistant bacteria, antimicrobial, plant extract
INTRODUCTION
Ethnobotanical and experimental evidence supports the use of plants due to their wide variety of secondary metabolites, such as tannins, terpenoids, alkaloids, and flavonoids, which have been found to have antimicrobial activity,[1,2,3,4] displaying even synergistic effects with existing antimicrobial drugs.[5]
Thus, due to the growing problem of antibiotic resistance of Pseudomonas aeruginosa and Staphylococcus aureus, there is a great interest in plants considering their phytochemicals as potential therapeutic agents.[6,7] In this context, Tamarindus indica L. and Manihot esculenta have significant importance in traditional medicine for different medicinal purposes.[8,9,10,11,12]
The T. indica L. and M. esculenta phytochemical studies have correlated their antimicrobial activities to several metabolites. T. indica leaf extract revealed the presence of many compounds such as ascorbic acid, β-carotene, polyphenols, and flavonoids (e.g., apigenin, catechin, epicatechin, and naringenin).[13]
Regarding M. esculenta leaf phytocomposition, it indicated the flavonoids, tannins, ascorbic acid, alkaloids, and saponins presence,[14] which display antimicrobial, antioxidant, and anti-inflammatory effects.[15]
Overall, phenolic compounds display a structural diversity which could be used for therapeutic intervention due to their biological broad spectrum.[16,17] These bioactivities are attributed to polyphenol interactions with proteins, lipids, and carbohydrates. These interaction induced cell permeability, causing membrane disruption.[18]
Therefore, the chemical composition of T. indica L. and M. esculenta leaf extracts was evaluated, as well as their antimicrobial activity against P. aeruginosa clinical isolated and Methicillin-resistant S. aureus (MRSA).
MATERIALS AND METHODS
Plant material and leaf extract preparation
T. indica L. and M. esculenta were collected in Porto da Folha, Sergipe, Brazil. The plants species were identified by Prof. Marla Ibrahim Uehbe de Oliveira and deposited in the Tiradentes University herbarium as AJU154 and AJU155, respectively. The leaves were previously oven-dried at 65°C for 2 days and powdered before extract preparation according to Sultana et al.[19] In brief, 10 g of each dried leaves was mixed with 100 mL of hydro-alcohol (20:80) and ultra-sounded for 1 h. Then, the obtained aqueous phases were concentrated and lyophilized. The dried crude concentrated extracts were stored at −4°C, until used for analyses.
Determination of total phenolic compounds
Total phenolic extract content was determined by Folin-Ciocalteu method.[20] The absorbance was measured in triplicate at 745 nm (DR 5000™ ultraviolet-visible [UV-vis] Spectrophotometer) and results expressed as mg of gallic acid equivalents per 100 mg of extract.
Scavenging activity of 1,1-diphenyl-2-picrylhydrazyl radicals
The extract for antioxidant activity was evaluated by 1,1-diphenyl-2-picrylhydrazyl (DPPH) method. Experiments were carried out according to Shaker et al.[21] with some modification. Briefly, 3 mL of each hydroethanolic extract (50–250 μg/mL) was mixed with 750 μL of ethanolic solution of DPPH 400 mM. The reaction mixture was shaken thoroughly and incubated in the dark at room temperature for 15 min. The absorbance was measured in triplicate at 517 nm (DR 5000™ UV-vis Spectrophotometer). The free radicals percentage inhibition was calculated using the following formula: % RSC = (A0 − A)/A × 100, where RSC = radical scavenging capacity (%), A0 = absorbance of control sample (t = 0 h), and A = absorbance of a tested sample at the end of the reaction (t = 1 h).
Qualitative phytochemical characterization
High-performance liquid chromatography/diode array detection
The hydroethanolic leaf extract composition was performed by reversed phase high performance liquid chromatography according to Aznar et al.[22] Dry leaves extract samples (5 mg/mL) were dissolved in methanol and 9 μL was injected into the HPLC (Varian ProStar HPLC system, Walnut Creek, Au). The samples were eluted using 0.1% formic acid and acetonitrile as mobile phase for gradient elution (flow rate = 1 mL/min) through the column Phenomenex C18 (4.6 mm × 100 mm, 2.6 μ particle size, Torrance, CA, USA). The peaks were detected at 280 nm. The compounds identification was done by comparison of their retention's time and UV absorption spectrum with those of the standards.
Ultraviolet-visible and fourier transform infrared
To detect T. indica and M. esculenta phenolic compounds, the plant extracts were scanned by UV-vis in the wavelength ranging from 200 to 800 nm using a LAMBDA™ 45 UV-vis Perkin Elmer spectrophotometer. Fourier transform infrared (FTIR) analysis was performed using 10 mg of dried powder of each plant extracts, encapsulated in 100 mg of KBr, and loaded in FTIR spectroscope (Spectrum BX, Perkin Elmer), with a scan range from 400 to 4000 cm −1 with a resolution of 4 cm −1.
Microorganisms
Microorganism strains (P. aeruginosa ATCC 27853, S. aureus [MRSA] ATCC 25923, S. aureus [MRSA] ATCC 33591, and S. aureus [MRSA] ATCC 43300) were supplied by the National Institute for Quality Control in Health (INCQS) from the Oswaldo Cruz Foundation (FIOCRUZ). The bacteria maintenance was carried out on Agar Brain Heart Infusion (BHI) (stationary culture) for 37°C at 24 h.
Minimum inhibitory concentration Determination
The microdilution method was used to estimate the minimum inhibitory concentration (MIC).[23] Briefly, 96-well microplate was coated with 100 μL BHI, 100 μL leaves extract (0, 1 g/mL), and 20 μL of microorganisms suspensions previously diluted at 0.5 McFarland turbidity standard. The microplates were incubated at 37°C for 24 h and microbial growth inhibition was determined at 595 nm (Microplate Reader Model 550, Bio Rad, USA). Vancomycin and meropenem were used as standard positive drug against S. aureus and P. aeruginosa, respectively. Experiments were done in triplicate.
Statistical analysis
All experimental results were centered using three parallel measurements of the mean ± standard deviation. Analysis of variance was performed followed by Tukey's test as applicable, using the statistical software R (R Development Core Team, 2008). P < 0.05 was considered statistically significant.
RESULTS
Phenolic content and antioxidant activity of the extracts
The total phenolic content of T. indica and M. esculenta extracts showed 132.85 ± 1.43 μg/100 mg and 121.64 ± 1.71 μg/100 mg of phenolic compounds, respectively, expressed as gallic acid equivalents.
The RSC evaluated by DPPH method is presented in Figure 1. Both extracts were able to reduce the DPPH in a concentration-dependent manner; however, T. indica showed better antioxidant capacity than M. esculenta compared to silymarin control, ranged from 18% to 75% of RSC.
Figure 1.
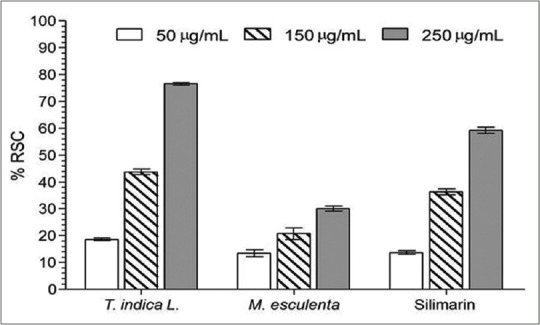
1,1-diphenyl-2-picrylhydrazyl radical scavenging activity of the Tamarindus indica and Manihot esculenta extracts. Values are means ± standard deviation (n = 3). RSC: Radical scavenging capacity
Qualitative phytochemical composition
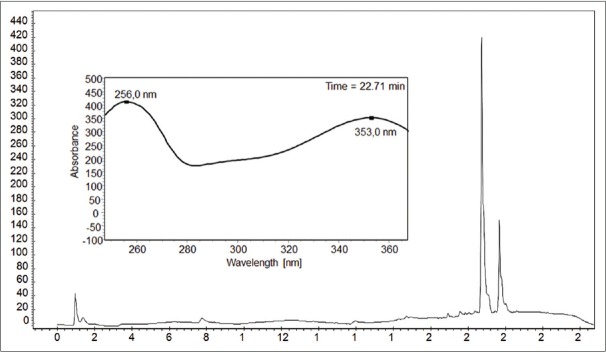
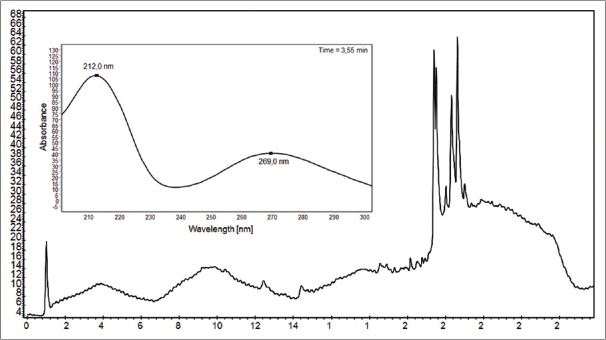
The chromatographic profiles of M. esculenta leaf extract showed two major peaks whose absorption spectrum matching to 353 nm (band I) and 256 nm (band II) and for T. indica 212 and 269 nm [Figures 2 and 3].
Figure 2.
Chromatographic profile of Manihot esculenta leaf extract used to investigate the qualitative content of polyphenols
Figure 3.
Chromatogram of the extract of leaves of Tamarindus indica used to investigate the presence of flavonoids
Ultraviolet-visible and fourier transform infrared analysis
With respect to UV-vis analyses, the extracts showed bands at 283 and 327–328 nm for T. indica, whereas bands at 286 and 309–321 nm were observed for M. esculenta extract [Figure 4].
Figure 4.
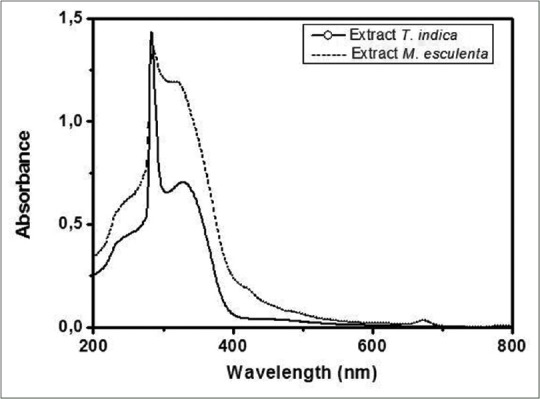
Ultraviolet-visible spectrum of Tamarindus indica and Manihot esculenta leaf extract
On the other hand, the FTIR spectra obtained from T. indica and M. esculenta extracts showed bands between 3.500 and 3.000 cm −1 assigned to OH group stretching vibration (alcohol and water), and also bands ranged from 2.950 to 2800 cm −1 indicating CH groups vibrations (methyl and methylene) were observed. The peak obtained at 1.680–1.630 cm −1 showed stretching vibration of the C=O (carbonyl) groups while bands between 1.150–1.050 cm −1 and 900–1.300 cm −1 corresponding to C-OH and CH groups, respectively [Figure 5].
Figure 5.
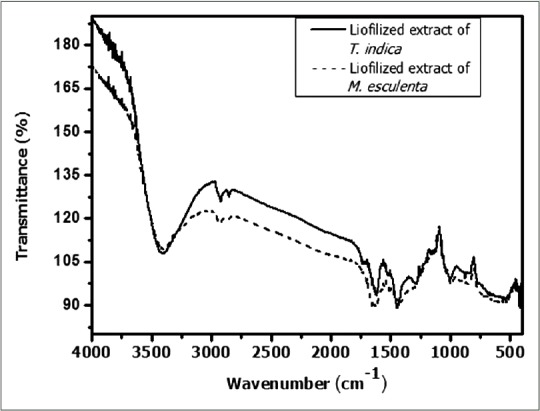
Fourier transform infrared of Tamarindus indica and Manihot esculenta extract
Antimicrobial activity
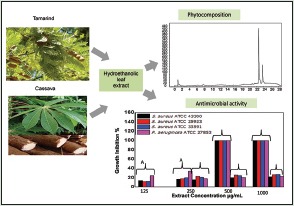
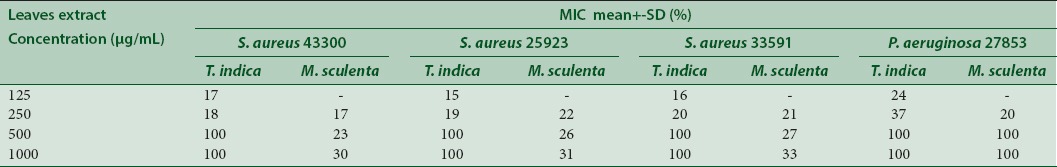
The antibacterial activity of the plant extracts is shown in Table 1. The results indicated that extracts showed antibacterial activities at variable degrees against clinical isolates (P. aeruginosa) and MRSA bacteria, with MICs values varying from 125 to 1000 μg/mL. T. indica extracts displayed the most important spectrum of activity at 500 and 1000 μg/mL, by inhibiting 100% growth of all microorganisms, whereas M. esculenta at 500 μg/mL had satisfactory antibacterial activity only against P. aeruginosa. Vancomycin at 1.95 μg/mL inhibited the S. aureus growth while meropenem had no activity against P. aeruginosa at 500 μg/mL (data not shown).
Table 1.
Minimal inhibitory concentration of Tamarindus indica and Manihot esculenta extract against Pseudomonas aeruginosa and Staphylococcus aureus. (-) No inhibition
DISCUSSION
Total phenolic content in plant extracts and their antioxidant activity are strongly related. Phenols have ability of eliminating free radicals due to its hydroxyl groups; therefore, the presence of these compounds in plants may directly contribute to their antioxidant and antimicrobial actions.[24,25,26,27] In this study, the phenolic content of T. indica leaf extract was high than in M. esculenta. Although the presence of polyphenols in M. esculenta was observed, its antioxidant activity is decreased due to glycosides substitutions in their molecular structure, interfering in this activity.[28] This may explain the lower value of the DPPH with M. esculenta extract despite its phenols content. Similar results were obtained by Rahiman et al.[29] by analyzing the antioxidant activity and total phenolic content of plants used as home remedies.
Thus, the better antioxidant capacity of T. indica extract can be explained by a variety of flavonoids and phenolic compounds presence, which act as reducing agents, stabilizing the free radical DPPH.[21]
The extract UV-vis spectra revealed two bands at 310–350 and 250–290 nm, indicating the presence of flavones and flavonols, respectively.[30,31] This result is relevant considering the antimicrobial activity of these compounds.
UV-vis and FTIR data are supported by chromatographic profiles of M. esculenta and T. indica leaf extracts that indicated the phenolic compounds and flavonoids presence, suggested by two typical bands of flavonoids structure and functional groups present (e.g., acids, carbonyl, alcohols, aldehydes, alkanes, and ethers) in these compounds; this is consistent with several data described in scientific literature.[15,32]
According to Jakobek,[18] the detected flavonoids and phenolic compounds in plant extracts act as antimicrobial agents against various human pathogens. The polyphenols mechanism of action on microorganisms are still poorly understood, and some authors suggest that polyphenol acts by reducing the iron availability, inhibiting protein expression, changing the cell membrane permeability, and fluidity.[33,34,35]
Although antimicrobial compounds present in T. indica leaves are not well elucidated, our results are in line with Cuban and Puerto Rican plant extract studies from the same species, which showed antimicrobial potential probably due to the presence of phenolic compounds.[36,37]
T. indica extract inhibited the S. aureus and P. aeruginosa growth at 500 and 1000 μg/mL. MIC values between 50 and 500 μg/mL indicate strong activity while 600–1500 μg/mL values denote a moderate antimicrobial action,[38] showing that T. indica extract has activity against the bacteria studied.
Although literature reports that Gram-negative bacteria are more resistant than Gram-positive to polyphenols due to the cell wall chemical composition,[39] this resistance was not observed in our results, since the T. indica extract showed an inhibitory capacity against P. aeruginosa.
On the other hand, M. esculenta extract has inhibition at 500 μg/mL only against P. aeruginosa meropenem resistant, evidencing the antimicrobial potential of the extract.. The low inhibitory effect of M. esculenta on S. aureus is probably due to attachment of carbohydrates in polyphenols and flavonoids compounds that decreased their antioxidant and antimicrobial activities.[18,33,40]
CONCLUSIONS
In the present study, two plant extracts (T. indica L. and M. esculenta) were studied and their antioxidant and antimicrobial capacities were evaluated. Biomolecules such as polyphenols and flavonoids present in leaf extracts exhibited antimicrobial activity on drug-resistant bacteria. The T. indica extract showed antibacterial effect against Gram-positive and Gram-negative bacteria, while M. esculenta had activity only on P. aureginosa meropenem resistant. Therefore, results suggest that both extracts are an affordable antioxidants source and have antimicrobial effects.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
Authors are thankful to CAPES and Fapitec/SE for the financial support and fellowship. We also would like to thank Embrapa Coastal Tablelands, the National Laboratory Síncronton Light and Sergipe Federal University for technical supports.
REFERENCES
- 1.Upadhyay A, Upadhyaya I, Kollanoor-Johny A, Venkitanarayanan K. Combating pathogenic microorganisms using plant-derived antimicrobials: A minireview of the mechanistic basis. Biomed Res Int. 2014;2014:761741. doi: 10.1155/2014/761741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh M, Khatoon S, Singh S, Kumar V, Rawat AK, Mehrotra S. Antimicrobial screening of ethnobotanically important stem bark of medicinal plants. Pharmacognosy Res. 2010;2:254–7. doi: 10.4103/0974-8490.69127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roumy V, Gutierrez-Choquevilca AL, Lopez Mesia JP, Ruiz L, Ruiz Macedo JC, Abedini A, et al. In vitro antimicrobial activity of traditional plant used in mestizo shamanism from the Peruvian amazon in case of infectious diseases. Pharmacogn Mag. 2015;11(Suppl 4):S625–33. doi: 10.4103/0973-1296.172975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kali A. Antibiotics and bioactive natural products in treatment of methicillin resistant Staphylococcus aureus: A brief review. Pharmacogn Rev. 2015;9:29–34. doi: 10.4103/0973-7847.156329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brahim MA, Fadli M, Hassani L, Boulay B, Markouk M, Bekkouche K, et al. Chenopodium ambrosioides var. ambrosioides used in Moroccan traditional medicine can enhance the antimicrobial activity of conventional antibiotics. Ind Crops Prod. 2015;71:37–43. [Google Scholar]
- 6.Dharmaprakash A, Thandavarayan R, Joseph I, Thomas S. Development of broad-spectrum antibiofilm drugs: strategies and challenges. Future Microbiol. 2015;10:1035–48. doi: 10.2217/fmb.15.14. [DOI] [PubMed] [Google Scholar]
- 7.Al-Azzawi A, Alguboori A, Hachim MY, Najat M, Al Shaimaa A, Sad M. Preliminary phytochemical and antibacterial screening of Sesuvium portulacastrum in the United Arab Emirates. Pharmacognosy Res. 2012;4:219–24. doi: 10.4103/0974-8490.102269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dewprashad B, Zakia S, Katayama S, Hendrix R. Antibacterial effects of the sauce from cassava. J Med Plants Res. 2009;3:880–2. [Google Scholar]
- 9.Goyal B, Alok S, Jain SK, Verma A. Evaluation of analgesic activity of ethanolic extract of Tamarindus indica leaves on experimental animal model. Int J Pharm Sci Res. 2013;4:1994–7. [Google Scholar]
- 10.Kuru P. Tamarindus indica and its health related effects. Asian Pac J Trop Biomed. 2014;4:676–81. [Google Scholar]
- 11.Bhadoriya SS, Ganeshpurkar A, Narwaria J, Rai G, Jain AP. Tamarindus indica: Extent of explored potential. Pharmacogn Rev. 2011;5:73–81. doi: 10.4103/0973-7847.79102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nwodo UU, Obiiyeke GE, Chigor VN, Okoh AI. Assessment of Tamarindus indica extracts for antibacterial activity. Int J Mol Sci. 2011;12:6385–96. doi: 10.3390/ijms12106385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samina KK, Shaikh W, Shahzadi S, Kazi TG, Usmanghani K, Kabir A, et al. Chemical constituents of Tamarindus indica L. medicinal plant in Sindh. Pak J Bot. 2008;40:2553–9. [Google Scholar]
- 14.Blagbrough IS, Bayoumi SA, Rowan MG, Beeching JR. Cassava: An appraisal of its phytochemistry and its biotechnological prospects. Phytochemistry. 2010;71:1940–51. doi: 10.1016/j.phytochem.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Raimi MM, Oyekanmi AM, Farombi AG. Proximate and phytochemical composition of leaves of Ceiba pentandra, Manihot esculentus and Abelmoschus esculentus in Southwestern Nigeria. Sci Res J. 2014;2:30–4. [Google Scholar]
- 16.Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients. 2010;2:1231–46. doi: 10.3390/nu2121231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asowata-Ayodele AM, Otunola GA, Afolayan AJ. Assessment of the polyphenolic content, free radical scavenging, anti-inflammatory, and antimicrobial activities of acetone and aqueous extracts of Lippia javanica (Burm. F.) spreng. Pharmacogn Mag. 2016;12:353–62. doi: 10.4103/0973-1296.185770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jakobek L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015;175:556–67. doi: 10.1016/j.foodchem.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Sultana B, Anwar F, Ashraf M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules. 2009;14:2167–80. doi: 10.3390/molecules14062167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slinkard K, Singleton VL. Total phenol analyses: Automation and comparison with manual methods. Am J Enol Vitic. 1977;28:49–55. [Google Scholar]
- 21.Shaker E, Mahmoud H, Mnaa S. Silymarin, the antioxidant component and Silybum marianum extracts prevent liver damage. Food Chem Toxicol. 2010;48:803–6. doi: 10.1016/j.fct.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 22.Aznar O, Checa A, Oliver R, Hernández-Cassou S, Saurina J. Determination of polyphenols in wines by liquid chromatography with UV spectrophotometric detection. J Sep Sci. 2011;34:527–35. doi: 10.1002/jssc.201000816. [DOI] [PubMed] [Google Scholar]
- 23.Manual Clinical and Laboratory Standards Institute (CLSI) Reference Method for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard-Ninth Edition: CLSI document M07-A9. Wayne, PA: Clinical and Laboratory Standards Institute; 2015. National Committee for Clinical Laboratory Standards. [Google Scholar]
- 24.Mamta, Mehrotra S, Amitabh, Kirar V, Vats P, Nandi SP, et al. Phytochemical and antimicrobial activities of Himalayan Cordyceps sinensis (Berk.) Sacc. Indian J Exp Biol. 2015;53:36–43. [PubMed] [Google Scholar]
- 25.Rekha K, Sivasubramanian C, Thiruvengadam M. Evaluation of polyphenol composition and biological activities of two samples from summer and winter seasons of Ligularia fischeri var. Spiciformis Nakai. Acta Biol Hung. 2015;66:179–91. doi: 10.1556/018.66.2015.2.5. [DOI] [PubMed] [Google Scholar]
- 26.D'Sousa' Costa CO, Ribeiro PR, Loureiro MB, SimÁμes RC, de Castro RD, Fernandez LG. Phytochemical screening, antioxidant and antibacterial activities of extracts prepared from different tissues of Schinus terebinthifolius Raddi that occurs in the coast of Bahia, Brazil. Pharmacogn Mag. 2015;11:607–14. doi: 10.4103/0973-1296.160459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pochapski MT, Fosquiera EC, Esmerino LA, Dos Santos EB, Farago PV, Santos FA, et al. Phytochemical screening, antioxidant, and antimicrobial activities of the crude leaves' extract from Ipomoea batatas (L.) Lam. Pharmacogn Mag. 2011;7:165–70. doi: 10.4103/0973-1296.80682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prawat H, Mahidol C, Ruchirawat S, Prawat U, Tuntiwachwut-tikul P, Tooptakong U, et al. Cyanogenic and non-cyanogenic glycosides from Manihot esculenta. Phytochemistry. 1995;40:1167–73. doi: 10.1016/0031-9422(95)00398-q. [DOI] [PubMed] [Google Scholar]
- 29.Rahiman S, Tantry BA, Kumar A. Variation of antioxidant activity and phenolic content of some common home remedies with storage time. Afr J Tradit Complement Altern Med. 2012;10:124–7. doi: 10.4314/ajtcam.v10i1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Markham RK, Mitcheel KA, Wilkins AL, Daldy JA, Lu Y. HPLC and GC-MS identification of the major organic constituents in New Zealand propolis. Photochemistry. 1996;42:205–11. [Google Scholar]
- 31.Tsimogiannis D, Samiotaki M, Panayotou G, Oreopoulou V. Characterization of flavonoid subgroups and hydroxy substitution by HPLC-MS/MS. Molecules. 2007;12:593–606. doi: 10.3390/12030593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naseem B, Shah SW, Hasan A, Sakhawat Shah S. Interaction of flavonoids, the naturally occurring antioxidants with different media: A UV-visible spectroscopic study. Spectrochim Acta A Mol Biomol Spectrosc. 2010;75:1341–6. doi: 10.1016/j.saa.2009.12.083. [DOI] [PubMed] [Google Scholar]
- 33.Daglia M. Polyphenols as antimicrobial agents. Curr Opin Biotechnol. 2012;23:174–81. doi: 10.1016/j.copbio.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Mila I, Scalbert A, Expert D. Iron withholding by plant polyphenols and resistance to pathogens and rots. Phytochemistry. 1996;42:1551–5. [Google Scholar]
- 35.Petti S, Scully C. Polyphenols, oral health and disease: A review. J Dent. 2009;37:413–23. doi: 10.1016/j.jdent.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Escalona-Arranz JC, Péres-Roses R, Urdaneta-Laffita I, Camacho-Pozo MI, Rodríguez-Amado J, Licea-Jiménez I. Antimicrobial activity of extracts from Tamarindus indica L. leaves. Pharmacogn Mag. 2010;6:242–7. doi: 10.4103/0973-1296.66944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meléndez PA, Capriles VA. Antibacterial properties of tropical plants from Puerto Rico. Phytomedicine. 2006;13:272–6. doi: 10.1016/j.phymed.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 38.Aligiannis N, Kalpoutzakis E, Mitaku S, Chinou IB. Composition and antimicrobial activity of the essential oils of two Origanum species. J Agric Food Chem. 2001;49:4168–70. doi: 10.1021/jf001494m. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura K, Ishiyama K, Sheng H, Ikai H, Kanno T, Niwano Y. Bactericidal activity and mechanism of photoirradiated polyphenols against Gram-positive and -negative Bacteria. J Agric Food Chem. 2015;63:7707–13. doi: 10.1021/jf5058588. [DOI] [PubMed] [Google Scholar]
- 40.Abd Aziz SM, Low CN, Chai LC, Abd Razak SS, Selamat J, Son R, et al. Screening of selected Malaysian plants against several food borne pathogen bacteria. Int Food Res J. 2011;18:1195–201. [Google Scholar]