Abstract
Purpose of review
Animal models published within the past 18 months on asthma, food allergy and anaphylaxis, all conditions of rising public health concern, were reviewed.
Recent findings
While domestic animals spontaneously develop asthma, food allergy and anaphylaxis, in animal models, divergent sensitization and challenge routes, dosages, intervals and antigens are used to induce asthmatic, food allergic or anaphylactic phenotypes. This must be considered in the interpretation of results. Instead of model antigens, gradually relevant allergens such as house dust mite in asthma, and food allergens like peanut, apple and peach in food allergy research were used. Novel engineered mouse models such as a mouse with a T-cell receptor for house dust mite allergen Der p 1, or with transgenic human hFcγR genes, facilitated the investigation of single molecules of interest. Whole-body plethysmography has become a state-of-the-art in-vivo readout in asthma research. In food allergy and anaphylaxis research, novel techniques were developed allowing real-time monitoring of in-vivo effects following allergen challenge. Networks to share tissues were established as an effort to reduce animal experiments in allergy which cannot be replaced by in-vitro measures.
Summary
Natural and artificial animal models were used to explore the pathophysiology of asthma, food allergy and anaphylaxis and to improve prophylactic and therapeutic measures. Especially the novel mouse models mimicking molecular aspects of the complex immune network in asthma, food allergy and anaphylaxis will facilitate proof-of-concept studies under controlled conditions.
Keywords: anaphylaxis, asthma, FcgammaR, food allergy, IgE, mouse model, rhinitis
INTRODUCTION
Animal models have significantly contributed to the understanding of the three allergy problems with the most destructive outcomes being asthma, food allergy and anaphylaxis. Although mice are the most commonly used species, the immunological limitations of this model have been increasingly realized, prompting for instance the generation of transgenic mice expressing IgE-receptors on cells that are relevant in human allergy, such as dendritic cells or eosinophils. Surprisingly, current studies with such models even suggested an anti-inflammatory role of IgE/FcεRI signals [1], reviewed in [2]. The situation in mice is also complicated by the fact that mouse FcγRIV acts as IgE-receptor similar to human FcεRI and contributes in IgE-induced lung inflammation [3]. More rarely, other allergy models than mice were used, for instance rats and guinea pigs [4,5], or piglets [6], especially for answering pharmacologic questions. In the investigated time period of this review (18 months back), no research articles involving monkey or nonhuman primates were published.
As companion animals may spontaneously develop skin (dogs, cats and horses), respiratory (cats and horses) and food allergies (dogs, cats, horses) like human patients, their inclusion as animal model ‘patients’ is increasingly encouraged [7]. Although in these animals little is known about the eliciting specific allergen molecules [8,9], the immune mechanisms relevant for allergies in dog, cat and horses are more similar to humans than any rodent models. These facts prompted experimental approaches including models for food sensitization [10] or for sublingual allergen-specific immunotherapy [11], up to clinical comparative investigations in animal patients, which are very similar to humans [12,13]. Technically, however, all investigations in animals are regulated by the animal experimentation directives [14]. This may prevent animal owners from participation in trials.
Therefore, and for practical reasons, most current work on animal models discussed below derives from murine studies and allows comparisons with previous mouse murine studies, but there are continuous efforts to advance these artificial models in terms of immune parameters and technical readouts.
Asthma models: using antigens or allergens?
Asthma in humans develops due to chronic inflammation of the airways, in contrast to murine asthma models that only develop acute airway inflammation [15]. Nevertheless, most of our knowledge about the pathogenesis relies heavily on available mouse models.
Ovalbumin from egg is often used as a model antigen, although it is not a respiratory allergen for humans. The rationale is that a great number of transgenic models have ovalbumin-specific immune cells, allowing elegant and precise studies of the immunological machinery. It should be noted, however, that also transgenic mice exist which specifically react to more relevant allergens, like the 1-DER mice expressing the T-cell receptor specific for the major house dust mite allergen Der p 1 from Dermatophagoides pteronyssinus[16]. Recent reports, all using ovalbumin have shown aggravation of asthma by pentraxin 3 [17], prostaglandin E2 [18], IL-15-deficiency [19] or suppression of asthma by anti-inflammatory protein 2 from hookworms [20▪▪], natural killer (NK) receptor 1 [21] and inhibition of hypoxia inducible factor-1α [22]. Sensitization with ovalbumin was usually performed by the intraperitoneal route, which, though valid, hardly represents the natural respiratory sensitization process in humans or animals. Allergen challenge to induce the pulmonary phenotype is conducted intranasally or by aerosolization in nebulization chambers (see below, Table 1).
Table 1.
Sensitization and challenge schemes used in murine asthma models
| Allergen | Sensitization | Challenge | AHR | Reference |
| Alternaria alternata extract | iTreg cells transferred i.v. into Rag2−/− mice followed by i.n. with IL-33 or 25 μg Alternaria alternata | n/a | i.t. | [35] |
| Amb a 11 | 2× 10 μg Amb an 11 i.p. | 4× 10 mg Amb a 1 + 100 μg LPS aerosol, 20 min | WBP | [40] |
| Birch allergen | 1× 5 Mio PBMCs + 20 μg birch allergen + 10 ng IL4 i.p. 1× 20 μg birch allergen + 10 ng IL4 | 3× 20 μg birch allergen | i.t. | [38] |
| CE | 3× 10 μg CE i.p. | 3× 10 μg CE i.n. | WBP | [36] |
| CE | 6× 120 μg CE + 3 μg CpG-ODN i.n. | n/a | WBP | [37] |
| HDM | 1× 200 μg, 2× 100 μg i.n. | 4 cycles: 2× 50 μg HDM i.n. | WBP | [41] |
| HDM | Severe asthma:3× 25 μg HDM + 7 μg c-di-GMP | 3 cycles with: 1× 25 μg HDM + 5 μg cyclic-di GMP and 2× 25 μg HDM i.n. | i.t. | [23] |
| HDM | 3× 25 μg HDM i.n. | 3× 5 μg HDM i.n. | i.t. | [24] |
| HDM | 3× 25 μg HDM i.n. | n/a | WBP | [25] |
| HDM | 1× 1 μg HDM i.n. | 5× 10 μg HDM i.n. or 1× 100 μg HDM i.n. | n/a | [26] |
| HDM | 2× 10 μg HDM i.p. | 100 μg i.t. on day 14 + 19 | i.t. | [27] |
| HDM | 1× 1 μg crude HDM i.t. | 2× 10 μg HDM i.n. | j.v., WBP | [28▪▪] |
| HDM | 5 cycles: 5× 25 μg HDM i.n. | n/a | WBP | [29] |
| HDM | 5 cycles: 3× 25 μg HDM i.n. 1× influenza virus ×31 i.n. | n/a | WBP | [30] |
| HDM | 1× 100 μg HDM i.n.; 4× 50 μg HDM i.n. | n/a | n/a | [31] |
| HDM | 4× 50 μg HDM i.n. | 3× 50 μg HDM i.n. | n/a | [32] |
| HDM | 5 cycles: 5× 25 μg HDM i.n. | n/a | n/a | [33] |
| HDM | 3 cycles: 5× 40 μg HDM i.n. and 1× 320 μg HDM i.p. | n/a | n/a | [42] |
| HDM + DEP | 3× 1 μg HDM + 25 μg DEP i.p. | n/a | WBP | [34] |
| KLH | 3 cycles: 2× 5 μg KLH ± 10 μg acrolein, 2 cycles: 2× 10 μg KLH ± 20 μg acrolein i.n. | 2× 10 μg KLH i.n. | WBP | [74] |
| OVA | 2× OVA + Alum i.p. | 2× OVA i.n. | i.t. | [17] |
| OVA | 2× 20 μg OVA + Alum i.p. | 5× 0.2% OVA aerosol, 20 min | i.t. | [20▪▪] |
| OVA | 3× 25 μg OVA + 2 mg Alum i.p. | 3 or 7× 1% OVA aerosol, 1 h | n/a | [19] |
| OVA | 2× OVA i.p. | 5× OVA i.n. | i.t. | [22] |
| OVA | 2× 0.1 μg OVA + 1 mg Alum i.p. | 2× 25 μg OVA i.n. | n/a | [21] |
| Ova | 3× 20 μg Ova with Alum i.p. | 3× 1% OVA aerosol, 30 min | i.t. | [18] |
AHR, airway hyperreactivity; CE, cockroach extract; DEP, diesel exhaust particles; HDM, house dust mite extract; i.n. intranasal; i.p. intraperitoneal; i.t. intratracheal; i.v.intravenous; j.v. jugular vein; KLH, keyhole limpet hemocyanin; LPS, lipopolysaccharide; n/a, not applicable.; OVA, ovalbumin; PBMC, peripheral blood mononuclear cells; WBP whole-body plethysmography.
Box 1.
no caption available
An increasing number of current studies tried to mimic the natural sensitization course, mainly using allergen extracts of house dust mites [23–27,28▪▪,29–34], but also from other relevant sources like from the fungus Alternaria alternata[35] and cockroaches [36,37].
Also transfer models were employed, in which human blood immune cells stimulated in-vitro with allergens are transferred to immune-deficient mice to mimic human asthma [38].
Considering the increasing number of molecular allergens available, more studies should be done with ‘true’ allergens which, however, will require the creation of new specific reagents and models.
Pathophysiology associated with immune changes in asthma models
As a readout for airway hyperreactivity and lung resistance, mice were exposed to increasing concentration of methacholine and monitored either by whole-body plethysmography (Fig. 1, from [39]) [29,34,40] or by surgically implanted means [23,24,27]. Studies investigating the mechanisms at the very start of allergic sensitization demonstrated that B-cells are dispensable for the primary CD4+ T-cell response, but crucial for expansion of primed Th2 cells and generation of central memory [28▪▪], ILC 2 were not an early source of Th2 cytokines, but contributed to allergic inflammation [26] together with IL-33 derived from monocytes [24], IL-23 secreted by bronchial epithelial cells [32], placenta growth factor [27] and lysophophatidylcholine [36]. Th2 and Th17 inflammatory pathways seemed to be reciprocally regulated in asthma [41]. On the other hand, CpG-oligodeoxynucleotides [37], STAT3 inhibition [42], CB2 receptors on NK cells [31] or secretoglobin superfamily protein SCGB3A2 [33] suppressed allergen-induced asthma in mice. Thus, the investigated factors illustrate that asthma can be counterregulated by reinforcing tolerance or skewing Th2-based asthma towards Th1 or Th17.
FIGURE 1.
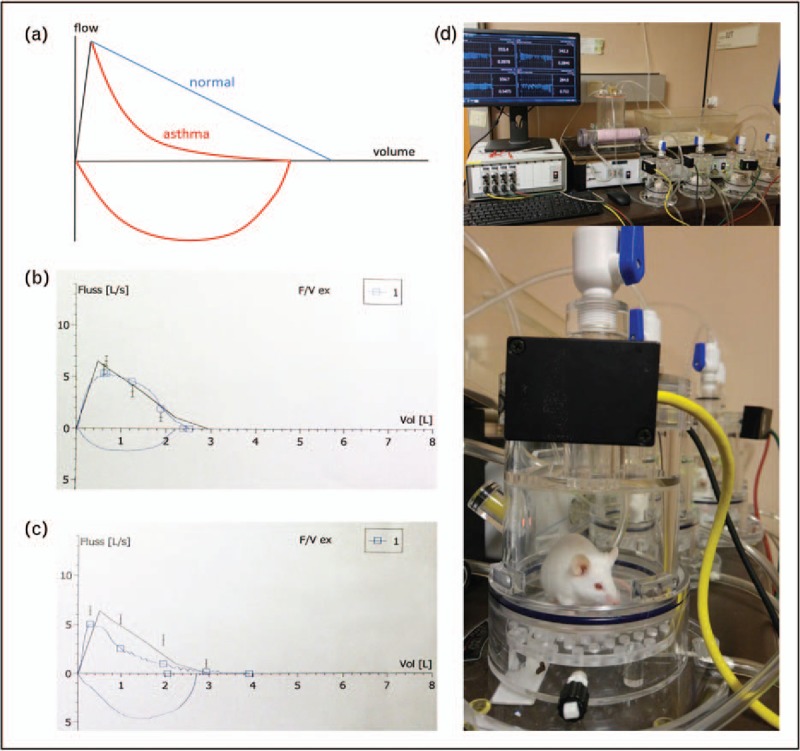
Spirometry: principle and typical test results. (a) Diagram showing the changes in airflow in healthy (blue) and asthmatic condition (red) (Adapted from [43]; (b) original spirometry test results of a human healthy patient; (c) of a patient suffering from bronchial asthma. Note the reduced airflow (upper blue line) due to bronchoconstriction above the x-axis in (c), as compared with normal lung function in (b); y-axis: forced expiratory flow (FEF) in l/s; x-axis: expired air volume in liters; (d) whole-body plethysmography in mouse studies for asthma research (d1, series of measurements in mice; d2, single tested mouse). The experimental principle is identical to the human diagnostic test. Reproduced with permission from Springer [39].
Models of severe asthma
Asthmatic patients immunologically cluster either in Th2-high, Th17-high or Th2/17 subgroups [41]. Severe asthma patients usually have high IFN-γ and low Th2 and IL-17 responses and do not respond to systemic corticosteroids [23]. Thus, models for severe asthma have been developed, in which cyclic-di-GMP (Guanosinmonophosphat), a second messenger produced by many pathogenic bacteria and being a strong Th1-Th17 adjuvant, was coapplied with the allergen extract during sensitization [23]. Similarly, severe asthma was also induced by infecting mice during the sensitization phase with influenza virus [30].
Food allergy models
Novel models: humanized and knockout
As a novel food allergy model, NOD-scidγc−/− mice with a human membrane-bound stem cell factor transgene were engrafted with human cord-blood derived CD34+ hematopoietic stem cells (HSCs) [44▪▪]. When peanut butter was given intragastrically, HSC-engrafted mice formed peanut-specific serum-IgE, systemic anaphylaxis (body temperature drop and human serum tryptase) and human IgE+ mast cells were observed in the small intestine, the spleen and the skin.
Using this model, CD4+CD25+ splenic T cells stimulated with or without allergen and/or recombinant glycoprotein A repetitions predominant (GARP) were transferred into the mice [45]. After allergen challenge, mini-endoscopy revealed that Tregs were responsible for inhibition of human allergen-specific IgE and allergen-specific gut inflammation. This inhibitory effect of Tregs was abolished when Treg-inducing protein GARP was blocked.
In another study, the phenomenon why Wiskott–Aldrich syndrome (WAS) patients develop higher rates of food allergy was investigated in a WAS protein-deficient mouse model. The results revealed that WAS protein expressed in Foxp3+ T cells has a critical function in the control of immune tolerance to chow antigens, such as soy and wheat, and thus maintenance of intestinal tolerance [46]. In summary, novel humanized mouse models confirmed the impact of Foxp3+ Tregs in tolerance induction to food.
Other animal models of food allergy
In addition to mouse and rat models, all relying on diverse sensitization methods, also pigs, dogs or sheep are used in food allergy research (reviewed by [47]). Epicutaneous sensitization was established in Beagles, where peanut paste was applied to normal and atopic dogs (each group n = 5), resulting in serum-IgE in all atopic vs. two control dogs [10]. After oral challenge, pruritic dermatitis, clinical symptoms scores, eosinophilic dermatitis and IgE-positive cells in skin were significantly higher in atopic dogs. This model is useful to mimic food-aggravated atopic dermatitis rather than gastrointestinal symptoms.
Microbial compounds, RNA and vitamins
To study the influence of microbial products, Li et al.[48] stimulated B cells with recombinant bacterial flagellin together with IL-4 as Th2-cytokine. In-vitro, flagellin suppressed expression of B-cell lymphoma-6 (Bcl6), a transcription factor repressing IgE-switch in B cells. Exposure to flagellin together with IL-4 induced IgE, while flagellin or IL-4 alone did not. In a wild-type mouse model, ovalbumin induced higher specific IgE, mast cells in intestinal mucosa and anaphylactic temperature drop when coapplied with flagellin. The same protocol in flagellin-receptor TLR-5−/− animals prevented these symptoms.
Micro-RNA can also regulate immune responses. B cells of ovalbumin-sensitized mice expressed high levels of the micro RNA miR-19a in the intestinal mucosa, in parallel with low levels of IL-10 [49]. Accordingly, addition of IL-4 increased the expression of miR-19a and suppressed IL-10 also in-vitro.
The physiologically observed neonatal inefficiency to induce oral tolerance to ovalbumin was studied in the context with vitamin A [50]. In neonatal animals, antigen transfer was markedly increased through the gut barrier, and only from the 3rd week of life on, animals were able to establish oral tolerance. The newborn mice showed a specific deficiency of retinaldehyde dehydrogenase expression in CD103+ dendritic cells (DC) of mesenteric lymph nodes associated with a reduced ability for antigen-specific T-cell activation, with retinol serum levels three times lower than in adults. When vitamin A was supplemented, oral tolerance could be induced right from birth. Therefore, bacterial compounds such as flagellin and micro-RNAs may potentiate food allergy, while vitamin A seems important for tolerance induction, at least in young age.
Novel approaches to treat food allergies
Oral treatment of peanut-allergic C3H/HeJ mice with CpG/peanut-poly(lactic-co-glycolic acid) nanoparticles as novel carrier system protected them from anaphylaxis, decreased peanut-specific IgE/IgG1 and Th2 cytokines [51], in accordance with previous own studies [52].
Antihuman IgE antibodies were used in a novel humanized murine model of peanut allergy: PBMC from human peanut-allergic donors were transferred into NOD-scid IL2Rgammanull mice, followed by an intraperitoneal challenge with peanut extract [53]. As therapy, an adeno-associated viral vector coding for a full-length, high-affinity, anti-human IgE antibody derived from the Fab-fragment of Omalizumab was applied once i.v. and significantly reduced specific and total serum IgE, free IgE, plasma histamine, clinical anaphylaxis and prolonged survival times.
Low-dose human recombinant IL-2 as survival factor for Tregs was injected in an ovalbumin- or peanut-allergic mouse model [54]. It prevented food allergy symptoms, anaphylactic body temperature drop, decreased serum mast cell protease-1 (MCP-1) and IL-5 in mesenteric lymph nodes and increased ovalbumin-specific IFN-γ production in mesenteric lymph nodes and Peyer's patches. Reduction of symptom scores was only effective after about 2 weeks. Anti-CD25 mAb treatment proved the protective role of Tregs in this model.
Mucosal mast cells (MMCs) are positively correlated with diarrhea and anaphylactic symptoms in murine food allergy. In ovalbumin-sensitized mice [55], pharmacologic inhibition of Notch-signaling by a γ-secretase-inhibitor suppressed food antigen-induced mast cell hyperplasia in the intestinal tract. Only 30% of treated vs. 89% of control animals showed allergic diarrhea.
A recombinant hypoallergenic mutant of the major carp allergen Cyp c 1 (mCyp c 1) was used to produce antisera in rabbits or mice [56]. When mice were sensitized to Cyp c 1 or carp-extract and received the antisera, allergic symptoms after oral challenge with Cyp c 1 and carp extract were markedly reduced. The induced specific IgG1 also reduced Cyp c 1-specific degranulation of IgE-loaded rat basophilic leukemia cells by 95%.
Plasmid DNA encoding the peanut-protein (p) Ara h 2 or pAra h 2 mixed with poly-l-lysine were injected intradermally either before or after sensitization with Ara h 2 [57]. The treatment containing poly-l-lysine was more effective in preventing specific serum antibodies and temperature drop upon challenge when applied before sensitization, inducing CD207+ DCs in draining lymph nodes of skin, CD25+Foxp3+ Treg cells in lymph nodes and spleen, as well as IFN-γ and IL-10 in splenocytes.
The natural molecule diosgenin from Chinese yam was administered daily to ovalbumin-sensitized BALB/c mice concomitantly with repeated ovalbumin challenges and increased the number of Th1-like Treg cells (Foxp3+, but coexpressing Th1 markers like CCR5, CXCR3, IFN-γ and T-bet) in the intestine, as well as in Peyer's patches [58].
Another natural molecule, baicalein (5,6,7-trihydroxyflavone) from Baikal skullcap (Scutellaria baicalensis) used in Traditional Chinese Medicine, supported integrity of intestinal tight junctions and prevented uptake of antigens and subsequent food allergy [59]. In an ovalbumin-food allergy mouse model, baicalein – an agonist of the aryl hydrocarbon receptor – reduced allergic symptoms (diarrhea, anaphylactic response, body temperature drop, serum IgE and effector T cells), whereas TGF-β was upregulated. As safety in food allergy therapy is a major concern, strategies to reduce the allergenicity of the therapeutic allergen by encapsulation or application of hypoallergens – besides inhibition of IgE pathways – may be most promising.
Anaphylaxis models
Allergens and routes investigated
Anaphylaxis models are mostly used in food allergy research with paradigmatic allergens like ovalbumin and intraperitoneal injections followed by oral challenges. A recent study used peach allergen Pru p 3 with lipopolysaccharide as adjuvant for intranasal sensitization, and anaphylaxis could be elicited by intraperitoneal challenge [60]. The authors suggested this model to be suitable for testing immunotherapeutic strategies. In another model hazelnut was applied orally and intraperitoneally. Usage of the Th2-adjuvant aluminium hydroxide (alum) led to stronger anaphylactic responses than using Staphylococcal enterotoxin B as adjuvant, which could in fact be therapeutically addressed by proteasome inhibitor bortezomib, reducing IgE and the B-cellular arm [61]. The question of the participation of IgG in the observed reactions was not addressed in this model.
PR-10 family members Bet v 1 from birch and Mal d 1 from apple induce clinically cross-reactive IgE in humans. To address whether allergen-specific immunotherapy (AIT) may affect cross-sensitization, mice were serially sensitized to both molecules with alum intraperitoneally up to a level when Mal d 1 elicited anaphylaxis. When mice then were subcutaneously treated with birch pollen extract adsorbed to alum, like in some human AIT formulations, this reduced the anaphylactic response [62]. Instead of causal treatment, histamine-1-receptor antagonist loratadine and histamine-4-receptor antagonist JNJ7777120 protected peanut-sensitized mice from intestinal anaphylaxis, potentially via preventing intestinal DC accumulation and antigen presentation to T cells [63]. Along this line, another study suggested that histamine-4-receptor antagonist JNJ28307474 should be applied already during the sensitization process to prevent allergy [64].
Taken together, only one of the above studies made efforts to truly mimic the oral sensitization route or used oral challenge with a food allergen, and all used Th2 adjuvants to induce anaphylaxis.
Addressing IgG mechanisms of anaphylaxis
To exclude any biases in terms of induced antibody isotypes in anaphylaxis models (see below), an adjuvant-free sensitization protocol was developed using the intraperitoneal route and ovalbumin [65▪]. Knockout models indicated that the anaphylactic drop of body temperature depended on mouse FcεRI, FcγRIII and γ-chain FcRγ, whereas FcγRIV related to inflammation only [65▪]. In this model, macrophages and neutrophils turned out to be less important in anaphylaxis than mast cells.
IgG-mediated anaphylaxis has recently become an emerging concern in humans too and can be mimicked by mouse models [66], in which besides IgE, also IgG (via FcγRIV) may act anaphylactogenic [3]. Different mouse IgG subclasses, however, have different effects depending on the used IgG receptor. The tested mouse IgG1 turned out to be exclusively activating and anaphylactogenic when triggered by the model allergen trinitrophenyl-bovine serum albumin, whereas IgG2a and IgG2b also bound to the inhibitory FcγRIIb receptor. This suggested that the sum of these reactions may decide on the anaphylaxis [67▪]. Efforts have been done to translate these findings into the human setting, in which four IgG subclasses complicate the events. A transgenic mouse was generated with human hFcγR genes (encompassing activating hFcγRIIA, hFcγRIIIA, hFcγRIIIB and inhibitory hFcγRIIB) being inserted into the corresponding mouse locus. Experiments with FcγR locus-switched mice injected with human heat-aggregated intravenous immunoglobulins suggested that despite the presence of inhibitory FcγRIIb, the activating receptors dominated the outcome of the anaphylaxis experiment [68▪▪].
Percutaneous sensitization and anaphylaxis
Also percutaneous exposure to food, especially in atopic dermatitis, may lead to sensitization, priming or boosting of existing allergy. The role of IL-33 binding to the IL-33 receptor ST2 (expressed in human and mouse skin) to trigger anaphylactogenic Th2 responses was investigated. Wild-type, ST2-deficient and mast cell-deficient KitW-sh/W-sh mice were percutaneously sensitized to ovalbumin and orally challenged [69]. Although food-allergic wild-type mice experienced anaphylaxis, it was attenuated in ST2-deficient and in ST2-blocked animals, and abrogated in mast cell-deficient KitW-sh/W-sh mice. The study highlighted the importance of IL-33 and mast cells to trigger anaphylaxis and might lead to novel anti-IL-33 therapeutics in humans [69]. Interestingly platelets, which participate in the anaphylactic reaction, have proven a significant source of IL-33 in humans as well as in an airway eosinophilia model in mice [70].
Novel readout methods
Following the 3R rules (replacement, reduction and refinement of animal experiments), efforts are done to minimize the number of animals used in the experiments, for instance by sharing tissues in networks as SEARCH [71]. However, in-vivo anaphylaxis models are needed in allergy research and for safety monitoring in vaccine development. Following single animals continuously over time during a reaction reduces the number of animals needed during an experiment.
The major anaphylaxis readouts in mouse models are drop of body temperature, reduction of physical mobility, eventually diarrhea and the elevation of MCP-1 levels released from anaphylactic mast cells. Telemedical devices can be surgically implanted into mice that allow the live reading of temperature, but this invasive method requires an experienced hand. Alternative methods based on imaging can only be done after isoflurane anesthesia and are usually not suitable for monitoring animals in motion [72]. A novel device and software has been developed that allows the noninvasive monitoring of surface body temperature and thereby also horizontal and vertical movements by imaging heat pictures (Fig. 2) [73]. Proof-of-concept studies were successful using casein, peanut and ovalbumin as food allergens for sensitization of BALB/c mice.
FIGURE 2.
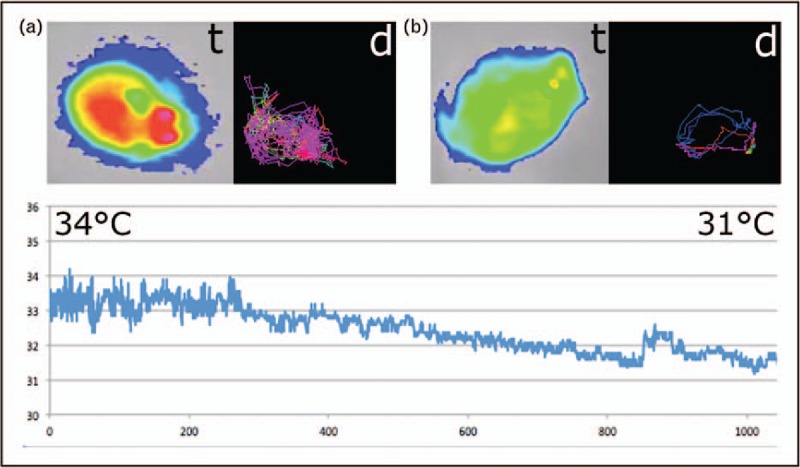
(a) Anaphylaxis imaging in mice with a heat-sensitive camera. The surface body temperature (t), in a healthy mouse around 34 °C, is shown in false colors: hottest point in red (usually the head) to > yellow > green > blue (coldest areas). Simultaneously, horizontal mouse movements can be electronically recorded and the running distance (d) calculated. (b) Anaphylaxis is associated with a drop of body temperature. Here, values around 31 °C are shown (t), associated with impairment in physical activity and shortest distances moved (d) [73]. The curve below shows the continuous evaluation of the surface body temperature drop (y-axis) recorded using 1000 frames per second, that is over approximately 4.1 min of an anaphylactic reaction (x-axis). The vertical movements evaluation is not depicted.
CONCLUSION
Summarized, a plethora of current murine asthma models helped us to an improved understanding of the pathophysiology of human asthma, food allergy and anaphylaxis. The routes, dosages, intervals and antigens used for sensitization, or used for challenges to induce an asthmatic (Table 1) or food allergic phenotype (Table 2), are, however, very diverse and should be considered in the interpretation. Increasingly, more relevant allergens such as house dust mite in asthma, and as peanut, apple or peach in food allergy research are used. In-vivo readouts are important to assess the ‘clinical’ relevance. This is done by whole-body plethysmography in asthma research (Fig. 1) [39]. In food allergy and anaphylaxis research, novel techniques were developed allowing the in-vivo monitoring of effects by allergen challenges, and, in accordance with the 3R principle, networks are formed to share tissues [70]. Future research directions may address the development of novel engineered mouse models facilitating the investigation of single-target molecules, whereas domestic animals represent natural animal models, spontaneously developing asthma, food allergy and anaphylaxis with immune mechanisms more similar to human allergy.
Table 2.
Sensitization, challenge and readout principles in food allergy models
| Allergen | Sensitization | Challenge | Readout | Ref. |
| Chaw components (wheat, soy bean, wheat middlings, yellow corn,fish meal) | Was−/−, Was−/− Il4−/−, Was−/− Rag2−/− mice: none (spontaneous sensitization) | None (spontaneous sensitization)orstarving for 3 h then fed 12.5 mg soy protein in 300 μl PBS | Surface LAMP-1,Intestinalmast cell expansion,Serum levels of mast cell protease 1 (MCPT1),body temperature by transponder | [46] |
| Cyp c 1;Hypoallergenic mCyp c 1;carp extract | C3H/HeJ mice: 5× i.g. weekly 100 mg rCyp c 1 + 20 mg cholera toxin in 200 ml 0.2-mol bicarbonate buffer (pH 9)20 mg hypoallergenic mCyp c 1 + Alum s.c. 6 times every month;Carp extract–sensitized C3H/HeJ, 600 ml of heat-inactivated mCyp c 1-specific mouse antisera i.p. | i.g. 100 mg rCyp c 1i.g. 10 mg carp extract | Symptom score | [56] |
| OVA | BALB/c mice: 2× i.p. 50 mg of OVA + Alum + magnesium hydroxide | 5× i.g. 50 mg of OVA every other day | Diarrhea,rectal temperature | [55] |
| OVA | BALB/c mice: gavaged daily with diosgenin | repeatedly i.g.OVA | Foxp3+ Treg cells coexpressing Th1-type transcription factors, cytokines, chemokines in intestine,mRNA expression of chemokines corresponding to Th1-like Treg cells | [58] |
| OVA | C57BL/6 mice or TLR5−/−:i.g. 0.1 mg OVA/mouse ± flagellin + cholera toxin (0.02 mg/mouse) weekly for 4 consecutive weeks | gavage-fed OVA (10 mg/mouse in 0.3 ml physiological saline) | Mast cell infiltration in the intestinal mucosa, intestinal CD4+ T-cell proliferation, core temperature, diarrhea | [48] |
| OVA | BALBc/c mice: 2× i.p. 20 μg OVA + Alum | 5× orally 50 mg OVA every 3 days;Baicalein (20 mg/kg) orally administered daily from day 28–40 | Diarrhea, anaphylaxis,rectal temperature | [59] |
| OVA | C57BL/6 mice or miR-17–92fl/fl: fed OVA 1 mg/mouse + cholera toxin (20 μg/mouse) weekly for 4 weeks;wild and miR-19a-deficient mice with recombinant IL-4 or/and LPS via i.p. injection daily for 5 days | Mast cell and eosinophil infiltration in intestinal mucosa, allergen-specific CD4+ T cells in the intestine;B cells isolated from intestine analyzed for IL-10 expression | [49] | |
| OVA | BALB/C (AnNR/J) mice: i.p. 2 × 10 mg OVA + Alum | OVA p.o. (20 mg/mouse), 5× within a 10-day period | Rectal body temperature,clinical score (diarrhea severity, appearance of hirsute pelage) | [54] |
| OVA | OVA (2 mg) i.g. 3×/week for lactating mothers 3 weeks after delivery or during lactation period ± VitA supplemention by maternal vitamin A-enriched diet from 2 days before delivery until the end of first week | 6–8-week-old adult mice: allergic airway inflammation to OVA by i.p. 10 mg OVA + Alum on days 0 and 7, day 17–21 mice exposed to OVA (0.5%) aerosols for 20 min in nebulizer | Serum OVA-specific IgE, IgA,eosinophils BAL;lung cell cytokinesecretion;ex vivo gut permeability;small intestine histology;serum retinol levels | [50] |
| Peanut extract | NOD-scid IL2Rgammanull mice: transferred i.p. 3 × 107 bloodmononuclear cells from peanut allergic donors + 100 mg crude peanut extract;i.p. 2× 100 mg of crude peanut extract;therapy: AAVrh.10 anti-hIgE (1011 genome copies)at week 3, reconstituted with human donor blood mononuclear cells at week 0 | i.g. 300 mg crude peanut extract at weeks 5–10 | Clinical score,anaphylaxis score,histamine in plasma,PCA | [53] |
| Peanut butter | huNSG mice: i.g. 22.5 mg (5 mg protein) skippy creamy peanut butter in 250 ml 0.2 mol sodium bicarbonate, pH 8.0, weekly for 8 weeks | Fed 350 mg peanut butter in 0.2 mol sodium bicarbonate | Temperature measurements using s.c. microchip transponders;PN-specific human IgE in serum;tissue mast cell expansion | [44▪▪] |
| Peanut extract,pAra h 2,PLL-pAra h 2 | BALB/c mice: i.d.PLL-pAra h 2 or pAra h 2 (25 μg pAra h 2 DNA) 3×, followed i.p. 2.5 μg purified Ara h 2 + Alum | i.p. 5 mg CPE | Rectal temperature | [57] |
| Peanut extract | i.p. 3× 500 mg peanut extract + Alum at 1 week interval | 7× orally peanut extract (15 mg) every 2 days | Body temperature, clinical symptoms:activity/lethargy, diarrhea, death | |
| Peanut | C3H/HeJ mice: oralpeanut + cholera toxin;therapy: 4× fed CpG/PN-nanoparticles | 5× monthly oral peanut | Visualsymptom scores, body temperature | [51] |
| Different allergens according to allergy of human donors | NOD.CB17-Prkdcscid/J γc−/−mice i.p. with 1 × 107 PBMCs from donors allergic to different allergens ± Treg cells (ratio of 1 : 10 or 1 : 20) ± respective allergen (20 mg) ± 4 mghuman recombinant GARP | i.p. allergen boost200 ml 0.9% NaCl | High-resolutionvideo endoscopicrectally | [45] |
| Peanut paste | Atopic beagles:e.c. 2× weekly for 8 weeks under occlusion on the axillae | Day 56: orally with roasted peanut (2 g/kg)day 66 e.c. peanut paste on one pinna under occlusion | Systemic signs (e.g. vomiting, diarrhea, collapse), pruritus, CADESI,skin biopsies | [10] |
AAVrh.10anti-hIgE, adeno-associated rh.10 serotype vector coding for a full-length, high-affinity, antihIgE antibody from Fab fragment of anti-hIgE mAb omalizumab; Alum, aluminum hydroxide; e.c., epicutaneous; GARP. glycoprotein A repetitions predominant; huNSG, nonobese diabetic severe combined immunodeficient common gamma chain-deficient stem cell factor; i.d., intradermal; i.g., intragastric; i.p., intraperitoneal; LPS, lipopolysaccharide; OVA, ovalbumin; p.o., per os; pAra h 2, plasmid encoding Ara h 2; PBMC, peripheral blood mononuclear cells; PCA, passive cutaneous anaphylaxis; PN, peanut; PLL-pAra h 2, pAra h 2 pretreated with poly-L-lysine; WASP, Wiskott–Aldrich syndrome protein.
Acknowledgements
No funding was obtained from National Institutes of Health (NIH), Wellcome Trust and Howard Hughes Medical Institute (HHMI).
Financial support and sponsorship
The work to this review was supported by grant SFB F4606-B28 of the Austrian Science Fund FWF to E.J.-J.
Conflicts of interest
E.J.-J is inventor of the Imaging method for non-invasive temperature and physical activity measurement in small animals EP 13158620.8-1657 and shareholder of Biomedical International R+D GmbH., Vienna, Austria. The other authors declare no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Platzer B, Baker K, Vera MP, et al. Dendritic cell-bound IgE functions to restrain allergic inflammation at mucosal sites. Mucosal Immunol 2015; 8:516–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Platzer B, Stout M, Fiebiger E. Functions of dendritic-cell-bound IgE in allergy. Mol Immunol 2015; 68:116–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mancardi DA, Iannascoli B, Hoos S, et al. FcgammaRIV is a mouse IgE receptor that resembles macrophage FcepsilonRI in humans and promotes IgE-induced lung inflammation. J Clin Invest 2008; 118:3738–3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cordoba-Rodriguez G, Vargas MH, Ruiz V, et al. Allergic sensitization modifies the pulmonary expression of 5-hydroxytryptamine receptors in guinea pigs. Respir Physiol Neurobiol 2016; 223:9–15. [DOI] [PubMed] [Google Scholar]
- 5.Xu Y, Kang T, Dou D, Kuang H. The evaluation and optimization of animal model for anaphylactoid reaction induced by injections. Asian Pac J Allergy Immunol 2015; 33:330–338. [DOI] [PubMed] [Google Scholar]
- 6.Kretzschmar M, Kozian A, Baumgardner JE, et al. Bronchoconstriction induced by inhaled methacholine delays desflurane uptake and elimination in a piglet model. Respir Physiol Neurobiol 2016; 220:88–94. [DOI] [PubMed] [Google Scholar]
- 7.Jensen-Jarolim E, Einhorn L, Herrmann I, et al. Pollen allergies in humans and their dogs, cats and horses: differences and similarities. Clin Transl Allergy 2015; 5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mueller RS, Janda J, Jensen-Jarolim E, et al. Allergens in veterinary medicine. Allergy 2016; 71:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pali-Schöll I, De Lucia M, Jackson H, Janda J, Mueller R, Jensen-Jarolim E. Comparing immediate type food allergy in humans and companion animals – definition of knowledge gaps: EAACI position paper. approved by ExCom, in submission. [DOI] [PubMed] [Google Scholar]
- 10.Marsella R. Experimental model for peanut allergy by epicutaneous sensitization in atopic beagle dogs. Exp Dermatol 2015; 24:711–712. [DOI] [PubMed] [Google Scholar]
- 11.Maina E, Pelst M, Hesta M, Cox E. Food-specific sublingual immunotherapy is well tolerated and safe in healthy dogs: a blind, randomized, placebo-controlled study. BMC Vet Res 2017; 13:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mueller RS, Olivry T, Prelaud P. Critically appraised topic on adverse food reactions of companion animals (2): common food allergen sources in dogs and cats. BMC Vet Res 2016; 12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olivry T, Mueller RS. Critically appraised topic on adverse food reactions of companion animals (3): prevalence of cutaneous adverse food reactions in dogs and cats. BMC Vet Res 2017; 13:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furdos I, Fazekas J, Singer J, Jensen-Jarolim E. Translating clinical trials from human to veterinary oncology and back. J Transl Med 2015; 13:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar RK, Herbert C, Foster PS. Mouse models of acute exacerbations of allergic asthma. Respirology 2016; 21:842–849. [DOI] [PubMed] [Google Scholar]
- 16.Jarman ER, Tan KA, Lamb JR. Transgenic mice expressing the T cell antigen receptor specific for an immunodominant epitope of a major allergen of house dust mite develop an asthmatic phenotype on exposure of the airways to allergen. Clin Exp Allergy 2005; 35:960–969. [DOI] [PubMed] [Google Scholar]
- 17.Balhara J, Shan L, Zhang J, et al. Pentraxin 3 deletion aggravates allergic inflammation through a TH17-dominant phenotype and enhanced CD4 T-cell survival. J Allergy Clin Immunol 2017; 139:950–963.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao Y, Zhao C, Wang W, et al. Prostaglandins E2 signal mediated by receptor subtype EP2 promotes IgE production in vivo and contributes to asthma development. Sci Rep 2016; 6:20505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathias CB, Schramm CM, Guernsey LA, et al. IL-15-deficient mice develop enhanced allergic responses to airway allergen exposure. Clin Exp Allergy 2017; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20▪▪.Navarro S, Pickering DA, Ferreira IB, et al. Hookworm recombinant protein promotes regulatory T cell responses that suppress experimental asthma. Sci Transl Med 2016; 8:362ra143. [DOI] [PubMed] [Google Scholar]; This outstanding article describes the use and efficacy of the anti-inflammatory protein 2 from hookworm as a novel agent for allergen immunotherapy in an asthma model.
- 21.Elhaik Goldman S, Moshkovits I, Shemesh A, et al. Natural killer receptor 1 dampens the development of allergic eosinophilic airway inflammation. PLoS One 2016; 11:e0160779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dewitz C, McEachern E, Shin S, et al. Hypoxia-inducible factor-1alpha inhibition modulates airway hyperresponsiveness and nitric oxide levels in a BALB/c mouse model of asthma. Clin Immunol 2017; 176:94–99. [DOI] [PubMed] [Google Scholar]
- 23.Raundhal M, Morse C, Khare A, et al. High IFN-gamma and low SLPI mark severe asthma in mice and humans. J Clin Invest 2015; 125:3037–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tashiro H, Takahashi K, Hayashi S, et al. Interleukin-33 from monocytes recruited to the lung contributes to house dust mite-induced airway inflammation in a mouse model. PLoS One 2016; 11:e0157571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan TK, Loh XY, Peh HY, et al. House dust mite-induced asthma causes oxidative damage and DNA double-strand breaks in the lungs. J Allergy Clin Immunol 2016; 138:84–96 e81. [DOI] [PubMed] [Google Scholar]
- 26.Li BW, de Bruijn MJ, Tindemans I, et al. T cells are necessary for ILC2 activation in house dust mite-induced allergic airway inflammation in mice. Eur J Immunol 2016; 46:1392–1403. [DOI] [PubMed] [Google Scholar]
- 27.Eiymo Mwa Mpollo MS, Brandt EB, Shanmukhappa SK, et al. Placenta growth factor augments airway hyperresponsiveness via leukotrienes and IL-13. J Clin Invest 2016; 126:571–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28▪▪.Dullaers M, Schuijs MJ, Willart M, et al. House dust mite-driven asthma and allergen-specific T cells depend on B cells when the amount of inhaled allergen is limiting. J Allergy Clin Immunol 2016; pii: S0091-6749(16)31131-9. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]; In elegantly conducted experiments, mechanistic insight at the very start of allergic sensitization are indicating that at the beginning B cells are dispensable, but crucial for expansion of primed Th2 cells and generation of central memory.
- 29.Vroman H, Bergen IM, Li BW, et al. Development of eosinophilic inflammation is independent of B-T cell interaction in a chronic house dust mite-driven asthma model. Clin Exp Allergy 2016; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 30.Ravanetti L, Dijkhuis A, Sabogal Pineros YS, et al. An early innate response underlies severe influenza-induced exacerbations of asthma in a novel steroid-insensitive and anti-IL-5-responsive mouse model. Allergy 2016; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 31.Ferrini ME, Hong S, Stierle A, et al. CB2 receptors regulate natural killer cells that limit allergic airway inflammation in a murine model of asthma. Allergy 2016; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee HS, Park DE, Lee JW, et al. IL-23 secreted by bronchial epithelial cells contributes to allergic sensitization in asthma model: role of IL-23 secreted by bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 2017; 312:L13–L21. [DOI] [PubMed] [Google Scholar]
- 33.Yoneda M, Xu L, Kajiyama H, et al. Secretoglobin superfamily protein SCGB3A2 alleviates house dust mite-induced allergic airway inflammation in mice. Int Arch Allergy Immunol 2016; 171:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Grove KC, Provoost S, Hendriks RW, et al. Dysregulation of type 2 innate lymphoid cells and TH2 cells impairs pollutant-induced allergic airway responses. J Allergy Clin Immunol 2017; 139:246–257 e244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rigas D, Lewis G, Aron JL, et al. Type 2 innate lymphoid cell suppression by regulatory T cells attenuates airway hyperreactivity and requires inducible T-cell costimulator-inducible T-cell costimulator ligand. J Allergy Clin Immunol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bansal P, Gaur SN, Arora N. Lysophosphatidylcholine plays critical role in allergic airway disease manifestation. Sci Rep 2016; 6:27430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim DH, Sohn JH, Park HJ, et al. CpG oligodeoxynucleotide inhibits cockroach-induced asthma via induction of IFN-gamma(+) Th1 cells or Foxp3(+) regulatory T cells in the lung. Allergy Asthma Immunol Res 2016; 8:264–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer-Martin H, Hahn SA, Beckert H, et al. GARP inhibits allergic airway inflammation in a humanized mouse model. Allergy 2016; 71:1274–1283. [DOI] [PubMed] [Google Scholar]
- 39.Hufnagl K, Hirt R, Robibaro B. Jensen-Jarolim E. Out of breath: asthma in humans and their animals. Comparative medicine: diseases linking humans with their animals. Switzerland:Springer International; 2017. 71–86. [Google Scholar]
- 40.Groeme R, Airouche S, Kopecny D, et al. Structural and functional characterization of the major allergen Amb a 11 from short ragweed pollen. J Biol Chem 2016; 291:13076–13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choy DF, Hart KM, Borthwick LA, et al. TH2 and TH17 inflammatory pathways are reciprocally regulated in asthma. Sci Transl Med 2015; 7:301ra129. [DOI] [PubMed] [Google Scholar]
- 42.Gavino AC, Nahmod K, Bharadwaj U, et al. STAT3 inhibition prevents lung inflammation, remodeling, and accumulation of Th2 and Th17 cells in a murine asthma model. Allergy 2016; 71:1684–1692. [DOI] [PubMed] [Google Scholar]
- 43.Reddel HK, Bateman ED, Becker A, et al. A summary of the new GINA strategy: a roadmap to asthma control. Eur Respir J 2015; 46:622–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44▪▪.Burton OT, Stranks AJ, Tamayo JM, et al. A humanized mouse model of anaphylactic peanut allergy. J Allergy Clin Immunol 2017; 139:314–322 e319. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article describes a mouse model with a newly established humanized adaptive immune system, including IgE induction and the effector cell engraftment in local tissues, and therefore allows for simulation of food-induced anaphylaxis upon ingestion.
- 45.Eschborn M, Weigmann B, Reissig S, et al. Activated glycoprotein A repetitions predominant (GARP)-expressing regulatory T cells inhibit allergen-induced intestinal inflammation in humanized mice. J Allergy Clin Immunol 2015; 136:159–168. [DOI] [PubMed] [Google Scholar]
- 46.Lexmond WS, Goettel JA, Lyons JJ, et al. FOXP3+ Tregs require WASP to restrain Th2-mediated food allergy. J Clin Invest 2016; 126:4030–4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bogh KL, van Bilsen J, Glogowski R, et al. Current challenges facing the assessment of the allergenic capacity of food allergens in animal models. Clin Transl Allergy 2016; 6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li LJ, Ma N, Zeng L, et al. Flagellin modulates IgE expression in B cells to initiate food allergy in mice. Am J Transl Res 2016; 8:2748–2757. [PMC free article] [PubMed] [Google Scholar]
- 49.Liu ZQ, Yang G, Geng XR, et al. Micro RNA-17-92 cluster mediates interleukin-4-suppressed IL-10 expression in B cells. Am J Transl Res 2016; 8:2317–2324. [PMC free article] [PubMed] [Google Scholar]
- 50.Turfkruyer M, Rekima A, Macchiaverni P, et al. Oral tolerance is inefficient in neonatal mice due to a physiological vitamin A deficiency. Mucosal Immunol 2016; 9:479–491. [DOI] [PubMed] [Google Scholar]
- 51.Srivastava KD, Siefert A, Fahmy TM, et al. Investigation of peanut oral immunotherapy with CpG/peanut nanoparticles in a murine model of peanut allergy. J Allergy Clin Immunol 2016; 138:536–543 e534. [DOI] [PubMed] [Google Scholar]
- 52.Pali-Scholl I, Szollosi H, Starkl P, et al. Protamine nanoparticles with CpG-oligodeoxynucleotide prevent an allergen-induced Th2-response in BALB/c mice. Eur J Pharm Biopharm 2013; 85:656–664. [DOI] [PubMed] [Google Scholar]
- 53.Pagovich OE, Wang B, Chiuchiolo MJ, et al. AntihIgE gene therapy of peanut-induced anaphylaxis in a humanized murine model of peanut allergy. J Allergy Clin Immunol 2016; 138:1652–1662 e1657. [DOI] [PubMed] [Google Scholar]
- 54.Bonnet B, Vigneron J, Levacher B, et al. Low-dose IL-2 induces regulatory T cell-mediated control of experimental food allergy. J Immunol 2016; 197:188–198. [DOI] [PubMed] [Google Scholar]
- 55.Honjo A, Nakano N, Yamazaki S, et al. Pharmacologic inhibition of Notch signaling suppresses food antigen-induced mucosal mast cell hyperplasia. J Allergy Clin Immunol 2017; 139:987–996.e10. [DOI] [PubMed] [Google Scholar]
- 56.Freidl R, Gstoettner A, Baranyi U, et al. Blocking antibodies induced by immunization with a hypoallergenic parvalbumin mutant reduce allergic symptoms in a mouse model of fish allergy. J Allergy Clin Immunol 2016; pii: S0091-6749(16)31348-3. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu Z, Yu J, Niu Y, et al. Enhanced prophylactic and therapeutic effects of polylysine-modified Ara h 2 DNA vaccine in a mouse model of peanut allergy. Int Arch Allergy Immunol 2016; 171:241–250. [DOI] [PubMed] [Google Scholar]
- 58.Huang CH, Wang CC, Lin YC, et al. Oral administration with diosgenin enhances the induction of intestinal T helper 1-like regulatory T cells in a murine model of food allergy. Int Immunopharmacol 2017; 42:59–66. [DOI] [PubMed] [Google Scholar]
- 59.Bae MJ, Shin HS, See HJ, et al. Baicalein induces CD4(+)Foxp3(+) T cells and enhances intestinal barrier function in a mouse model of food allergy. Sci Rep 2016; 6:32225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rodriguez MJ, Aranda A, Fernandez TD, et al. LPS promotes Th2 dependent sensitisation leading to anaphylaxis in a Pru p 3 mouse model. Sci Rep 2017; 7:40449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mudnakudu Nagaraju KK, Babina M, Weise C, et al. Bortezomib treatment diminishes hazelnut-induced intestinal anaphylaxis in mice. Eur J Immunol 2016; 46:1727–1736. [DOI] [PubMed] [Google Scholar]
- 62.Utsch L, Logiantara A, Wallner M, et al. Birch pollen immunotherapy inhibits anaphylaxis to the cross-reactive apple allergen Mal d 1 in mice. Clin Exp Allergy 2016; 46:1474–1483. [DOI] [PubMed] [Google Scholar]
- 63.Wang M, Han J, Domenico J, et al. Combined blockade of the histamine H1 and H4 receptor suppresses peanut-induced intestinal anaphylaxis by regulating dendritic cell function. Allergy 2016; 71:1561–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rossbach K, Schaper K, Kloth C, et al. Histamine H4 receptor knockout mice display reduced inflammation in a chronic model of atopic dermatitis. Allergy 2016; 71:189–197. [DOI] [PubMed] [Google Scholar]
- 65▪.Balbino B, Sibilano R, Starkl P, et al. Pathways of immediate hypothermia and leukocyte infiltration in an adjuvant-free mouse model of anaphylaxis. J Allergy Clin Immunol 2017; 139:584–596 e510. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article evidences that the IgG-receptor-related pathways differ between anaphylactic drop of body temperature and inflammation, with the subtypes of FcγR being decisive.
- 66.Khodoun MV, Strait R, Armstrong L, et al. Identification of markers that distinguish IgE- from IgG-mediated anaphylaxis. Proc Natl Acad Sci U S A 2011; 108:12413–12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67▪.Beutier H, Gillis CM, Iannascoli B, et al. IgG subclasses determine pathways of anaphylaxis in mice. J Allergy Clin Immunol 2017; 139:269–280 e267. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article evidences that the sum of all different IgG subclasses in a complex interplay with FcγRs decide on whether anaphylaxis occurs or not. Considering the novel findings that IgG is involved in human systemic hypersensitivity too, this mouse model is very useful and important.
- 68▪▪.Gillis CM, Jonsson F, Mancardi DA, et al. Mechanisms of anaphylaxis in human low-affinity IgG receptor locus knock-in mice. J Allergy Clin Immunol 2016; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]; This innovative transgenic mouse model expressing human activating and inhibitory hFcγR genes is a step forward to better understand the effects of human IgG subclasses in anaphylaxis – experiments suggest that in fact activating receptors dominates the outcome.
- 69.Galand C, Leyva-Castillo JM, Yoon J, et al. IL-33 promotes food anaphylaxis in epicutaneously sensitized mice by targeting mast cells. J Allergy Clin Immunol 2016; 138:1356–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takeda T, Unno H, Morita H, et al. Platelets constitutively express IL-33 protein and modulate eosinophilic airway inflammation. J Allergy Clin Immunol 2016; 138:1395–1403.e1396. [DOI] [PubMed] [Google Scholar]
- 71.Morrissey B, Blyth K, Carter P, et al. The sharing experimental animal resources, coordinating holdings (SEARCH) framework: encouraging reduction, replacement, and refinement in animal research. PLoS Biol 2017; 15:e2000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hayashi M, Takai J, Yu L, et al. Whole-body in vivo monitoring of inflammatory diseases exploiting human interleukin 6-luciferase transgenic mice. Mol Cell Biol 2015; 35:3590–3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Manzano-Szalai K, Pali-Scholl I, Krishnamurthy D, et al. Anaphylaxis imaging: non-invasive measurement of surface body temperature and physical activity in small animals. PLoS One 2016; 11:e0150819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roth-Walter F, Bergmayr C, Meitz S, et al. Janus-faced acrolein prevents allergy but accelerates tumor growth by promoting immunoregulatory Foxp3+ cells: mouse model for passive respiratory exposure. Sci Rep 2017; In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]


