Abstract
Purpose of review
Animal studies published within the past 18 months were assessed, focusing on innate and specific immunomodulation, providing knowledge of high translational relevance for human atopic and allergic diseases.
Recent findings
Allergic companion animals represent alternative models, but most studies were done in mice. Atopic dermatitis mouse models were refined by the utilization of cytokines like IL-23 and relevant skin allergens or enzymes. A novel IL-6 reporter mouse allows biomonitoring of inflammation. Both skin pH and the (transferable) microflora have a pivotal role in modulating the skin barrier. The microflora of the gastrointestinal mucosa maintains tolerance to dietary compounds and can be disturbed by antiacid drugs. A key mouse study evidenced that dust from Amish households, but not from Hutterites protected mice against asthma. In studies on subcutaneous and sublingual allergen-specific immunotherapy, much focus was given on delivery and adjuvants, using poly-lacto-co-glycolic particles, CpGs, probiotics or Vitamin D3. The epicutaneous and intralymphatic routes showed promising results in mice and horses in terms of prophylactic and therapeutic allergy treatment.
Summary
In atopic dermatitis, food allergies and asthma, environmental factors, together with the resident microflora and barrier status, decide on sensitization versus tolerance. Also allergen-specific immunotherapy operates with immunomodulatory principles.
Keywords: allergen-specific immunotherapy, allergy, atopic dermatitis, barrier, diet, microbiome, mouse model
INTRODUCTION
Failure in innate defense mechanisms (inborn or acquired barrier leakage of skin and mucosa and misbalanced microbiome) often is the first step toward atopic and allergic diseases. Animal models should help us mimicking these pathophysiological conditions and should allow proof-of-concept studies for the efficacy of novel therapies with a certain predictive value for humans. Many aspects of mouse models are suitable to picture the human condition in its extreme and were very successfully used also in current animal models of allergy and immunomodulation. Several immune parameters can be directly compared between animal models and humans; for instance, mice harbor natural killer T cells, dendritic cells (although lacking FcεRI in wild type mice) and regulatory T-cells (Tregs). However, mouse models are limited, e.g. because of the absence of the immunoglobulin G4 (IgG4) immunoglobulin class, which is a prominent hallmark of successful allergen immunotherapy and was recently suggested as a reliable marker for the compliance and adherence of patients to allergen-specific immunotharepy [1].
In comparison, other mammals, for instance canines, express four immunoglobulin subclasses and a similar immunoglobulin E (IgE)-receptor repertoire on the same cells like humans, including eosinophils and dendritic cells. Therefore, to overcome the limitations of murine models, atopic and allergic domestic animals could be investigated as natural models of disease with a potentially higher predictive value [2,3] (Fig. 1).
FIGURE 1.
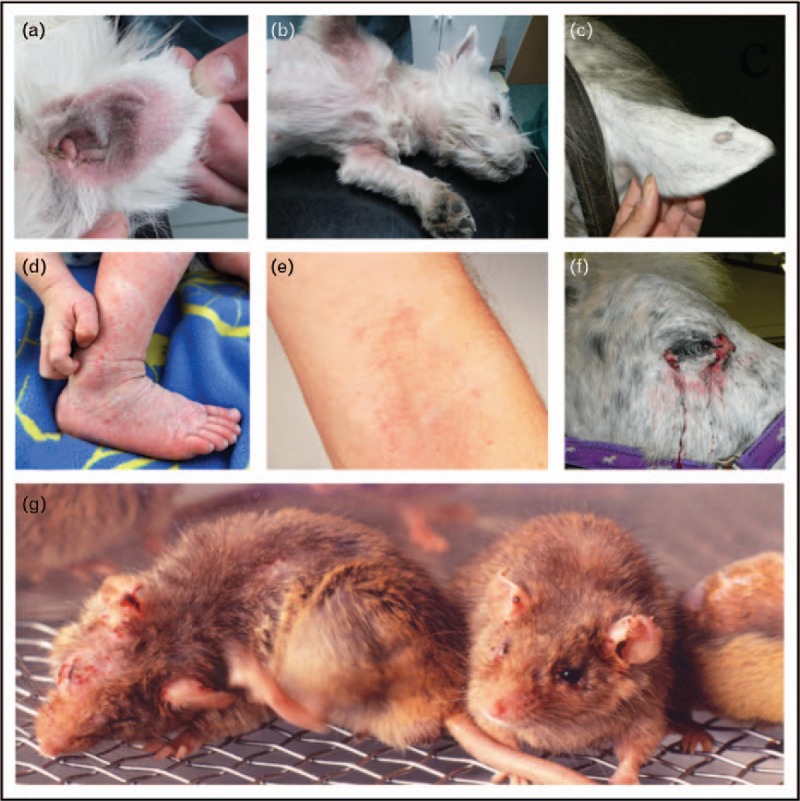
Typical atopic dermatitis lesions in domestic animals and humans versus NC/Tnd mice. Atopic lesions in a Maltese dog's ear (a) and subaxillary (b); itchy atopic dermatitis (neurodermitis) in a child (d) and in flexural site of a human adult (e); on ear and around the eye of a horse (c, f) (a–f from [3], reproduced with permission of Springer); (g) in an NC/Tnd mouse an inbred strain originating from NC/Nga, modeling all features of natural atopic dermatitis including barrier leakage and itchiness (by courtesy of Professor Hiroshi Matsuda, Tokyo University of Agriculture and Technology, Japan).
Box 1.

no caption available
It has to be emphasized that most of the generated knowledge discussed below derives from murine studies, but prompted key observations with respect to the power of prophylactic or therapeutic treatments, innate or specific immunomodulation, being translatable for humans.
ATOPIC DERMATITIS MODELS
The barrier function of a healthy skin is characterized by intact intercellular junctions between keratinocytes fixed by a ceramide glue, being supported by acidic skin pH and an intact microflora that via epithelia is in constant dialogue with the cutaneous innate immune defense. The tolerance to skin commensals is established very early in neonatal life by a wave of Tregs, which invade the skin [4]. This was shown by engineered Staphylococcus epidermidis expressing a model T-cell antigen (model peptide antigen 2W linked to a fluorescent protein) applied to disrupted skin: only in 7 days old (but not in adult) C57Bl/6 mice, Foxp3+ Tregs increased within the antigen-specific CD4+ population in the skin-draining lymph nodes and skin.
In atopic dermatitis on the contrary, innate and specific immune defense arms accelerate acute toward chronic inflammation linked with a mixed Th1/Th17/Th2 immune response [5] (Fig. 1d, e). Disturbances in the skin and mucosal barrier are typically associated with a misbalanced microflora, which may be the ‘hen or the egg’ in this disease. As a result, atopic patients have a higher risk for developing allergies. Several animal models, mostly in mice, have been created or applied recently to mimic atopic dermatitis and to understand these interconnected problems.
Immune Effects of the Microflora in Atopic Dermatitis
Although pathogenic skin bacteria via interleukin (IL)-23 strongly boost Th1/Th17 inflammation, other stimuli such as enzymes via protease-activated receptor (PAR)-2 and thymic stromal lymphopoietin (TSLP) trigger Th2 responses, involving basophil-derived IL-4 [6]. Both counteracting axes contribute to the mixed phenotype in human atopic dermatitis patients and could in fact be mimicked in mouse and canine models [7]. In a transcriptomic profiling study, however, IL-23-injected, NC/NgaTnd [8] (Fig. 1 g) and oxazolone-challenged mice had the highest homology to human disease, each reflecting different immune or barrier aspects [9▪▪].
Dogs’ spontaneous atopic dermatitis not only has inflammatory and prurigenic pathways like humans [10] (Fig. 1 a,b), but also a reduced microbiome diversity with a dominance of Staphylococcus aureus and Corynebacterium species, which correlate with barrier leakage [11]. Only gram-negative bacteria collected from healthy controls, but not from atopic dermatitis patients, were able to maintain barrier integrity and control of Staphylococcus when inoculated in MC903 atopic dermatitis mice [12▪▪], suggesting a ‘live-biotherapeutic approach’ for human patients.
The chronic aspects in atopic eczema are accompanied by IL-6 induction. This can now be monitored in vivo by bioluminescence imaging in the novel AhR-CA transgenic mouse model [13▪], allowing also transverse section image. In this lipopolysaccharid-induced luciferase reporter system, ‘WIM-6’ luminescence could be detected in each tissue, allowing the monitoring of progression of atopic dermatitis as well as treatment efficacy with dexamethason.
Environmental factors may also disturb the robustness of the skin. Alkalization of the skin of NC/Tnd mice induced epidermis kallikrein-5 mRNA and protein, an endogenous serine protease, resulting in PAR-2 activation, TSLP secretion and a cutaneous Th2 response, but could be blocked by PAR-2 antagonist ENMD-1068 [14]. As their phenotype could be counterbalanced by acidification, the study emphasizes the pivotal role of the physiological, slightly acidic cutaneous pH and translates into care recommendations for atopic dermatitis skin.
The exogenous enzyme papain, a prototypic cysteine protease, was used as surrogate for the house dust mite allergen Der p 1 for several studies. Enzymatically active papain induced immediate inflammation via intact skin in vivo, and it retained the sensitization potential when its enzymatic activity was inhibited [15]. On tape stripped skin, papain acted as adjuvant for another model allergen, ovalbumin [16], and primed specific respiratory hypersensitivity, mirroring the atopic march.
Interestingly, silver particles adjuvanted house dust mite proteins to become percutaneous allergens, thereby aggravating atopic dermatitis and activating mast cells [17].
Intrinsic barrier defects play a major role in the development of atopic dermatitis. A filaggrin mutant mouse on BALB/c background [18], like previously NC/Nga mice [19], developed spontaneous atopic dermatitis and compromised pulmonary function, thereby also mimicking the atopic march. Intriguingly, it could be healed by application of acidic skin cream [19].
Reduced expression of tight junction compound claudin-1 correlated with more severe atopic dermatitis in mice [20]. Being pronounced in infancy, symptoms improved with age, imitating the course of human atopic eczema.
Also, adherens junctions contribute to the regulation of the extracellular matrix: in a cadherin-11 knockout mouse model, cutaneous levels of elastin and collagen as well as mechanical properties were significantly reduced [21].
By concentrating on keratinocyte turnover, another useful atopic dermatitis model was developed: epidermis-restricted ablation of the proto-oncogene BRAF/RAF1 led to barrier defects and local and systemic IgE and Th2 inflammation; Janus kinase inhibition prevented disease onset [22].
In terms of potential novel therapies in atopic dermatitis, a proof-of-concept study in NC/Nga mice sensitized by papain revealed that Bacillus-derived poly-γ-glutamic acid-treatment induced invariant natural killer T cells driving basophils into apoptosis, thereby correcting the pronounced Th2 inflammation [6].
Astaxanthin, a xanthophyll carotenoid [23], and AM1030, a serotonin antagonist [24], showed promising anti-inflammatory effects, and targeted the itch in NC/Nga mice. Allergen-specific oral tolerance could be achieved in an atopic dermatitis mouse model epicutaneously sensitized to ovalbumin, with practical implications in human atopic dermatitis patients suffering from exacerbations due to the intake of dietary allergens [25]. A study in atopic dogs, which represent a relevant model for human atopic dermatitis [3], demonstrated that a chemokine receptor-4 antagonist prevented homing of lymphocytes into the lesional skin [26].
THE MICROBIOME: INNATE IMMUNOMODULATION IN FOOD ALLERGY AND ASTHMA MODELS
The influence of diet on the microbiome and subsequent food allergy development is today well accepted [27]. For instance, in a house dust mite-mouse model, a high-fiber diet increased Bacteroidetes, especially high-acetate-producing Bacteroides acidifaciens, which produce high levels of short-chain fatty acids like acetate in serum and feces [28]. These short-chain fatty acids inhibited histone deacetylase-C9 and prevented asthma development via Tregs. These findings were even true in offspring when mothers were on high-fiber diet during pregnancy. Asthma-preventive effects were mediated in utero predominantly by acetate (not other short-chain fatty acids), and independent of transfer of specific microbiota.
Medications and Microbiota
There is also a link between the gastric digestion capability, the establishment of a specific microbiota and the development of food allergy: mice orally treated with ovalbumin concomitantly with antiacid drugs which impair the gastric peptic digestion differed in the fecal microbiome and subsequently developed allergic and even anaphylaxis symptoms [29].
Environment and Housing
For the microbiological composition, environmental aspects also play an important role. Laboratory mice live under abnormal specific pathogen free (SPF) conditions and lack differentiated memory CD8+ T-cell subsets and mucosal memory T cells. When SPF animals were cohoused with pet store mice [30▪], their resistance to infection and T-cell differentiation upon de-novo viral infection increased, making ‘dirty’ mice valuable models for investigating, immune function and treatments in transplantation, allergy, autoimmunity and vaccination in context with the hygiene hypothesis [31].
Allergy-preventive effects are not the same on every farm: Amish folks were better protected against asthma than Hutterite people, implicating that the different living conditions are decisive in the two populations with almost identical genetic background [32▪▪]. Higher endotoxin levels in the house dust of Amish were made responsible for the observed protective effects. In accordance, aqueous house dust samples given intranasally concomitant with allergen sensitization protected mice during allergen challenge in an ovalbumin-asthma mouse model, whereas MyD88/Trif (innate immunity signaling molecules)-deficient mice were not protected.
Probiotics
Lactobacillus gasseri OLL2809 (LG2809) fed in a DO11.10-mouse model (carrying a T cell receptor transgene, Tg (DO11.10) reactive to ovalbumin peptide) together with ovalbumin resulted in induction of oral tolerance, shown as decreased T-cell proliferation and IL-2 levels but increased IL-10 from spleen cells and more plasmacytoid dendritic cells in the lamina propria [33]. In another ovalbumin-food allergy BALB/c mouse model [34], the administration of probiotic Bifidobacterium longum KACC 91563, but not Enterococcus faecalis KACC 91532, via the intragastric route or in the food, applied concomitantly with sensitization, alleviated food allergy symptoms (diarrhea). This was rather due to apoptosis induction in mast cells but not in T cells, B cells or eosinophils, than by production of acetate.
To study immunomodulatory effects, human microbiota-associated mice are widely used, wherein a human fecal microbiome was established in germ-free mice through microbiota transplantation [35,36].
IMMUNOMODULATION BY ALLERGEN-SPECIFIC IMMUNOTHERAPY
Barrier integrity and immune maintenance described above relate to allergen-independent mechanisms. In contrast, allergen-specific immunotherapy so far is the only causative, allergen-specific treatment of IgE-mediated allergic disorders. The mechanisms involve the induction of Tregs and blocking IgG4 antibodies, altogether resulting in immunomodulation combating the allergic Th2 bias. In general, repeated administration of increasing amounts of allergens results in a state of clinical hypo-responsiveness to the respective allergen. Conventional treatment usually employs the subcutaneous or the sublingual route. Mouse models of allergen immunotherapy are challenging for the following reasons: allergy mouse models rely on allergen application in context with Th2 adjuvants (often by nonphysiological routes) and thereby induce robust disease that is difficult to modulate; symptomatic allergy in mice is, unlike in humans, associated with IgE and IgG1; mice express Tregs, but do not harbor IgG4 antibodies which is an important biomarker in human allergen immunotherapy [1]. This makes comparative trials in domestic animal patients with spontaneous allergies and 4 IgG subclasses an attractive alternative approach.
Immune Regulation by Sublingual Immunotherapy
In humans, the efficacy of sublingual immunotherapy depends on the sensitization level of the individual and the dose administered during therapy. Indeed, highly sensitized mice required higher sublingual immunotherapy (SLIT) dose than less sensitized mice to improve airway hyperresponsiveness [37]. Moreover, upon SLIT treatment, a lower proportion of CD4-CD8 γδ cells and a higher frequency of CD8+CD25+IFNγ+ T cells accompanied by reduced inflammatory responses in the lung were observed [38]. The efficacy of SLIT may also depend on Vitamin D3 administration that potentiated subcutaneous allergen immunotherapy in mice by accumulating Tregs in the lung in an allergen-driven manner [39]. Interestingly, the smoke compound acrolein, in a completely antigen-independent manner, had a similar effect as it prevented allergic sensitization by promoting suppressive T cells via aryl hydrocarbon receptor activation [40].
Encapsulation for Safety in Subcutaneous Application
Focus was given on encapsulation procedures of allergy vaccines to enhance not only the immunogenicity, but also to confer safety. Strontium-doped hydroxyapatite porous spheres have been used in subcutaneous immunotherapy trials in mice to reduce side-effects, resulting in reduced symptoms after nasal challenge despite the higher percentage of eosinophils found in the bronchial lavage samples [41].
Alternative route: oral
Although subcutaneous allergen immunotherapy is extensively used for some respiratory and venom allergies, it is unsuitable for food allergens because of the high risk of adverse reactions. In a study using the oral route as a safer alternative, ovalbumin was applied in conjunction with the probiotic Clostridium butyricum to reduce symptoms in a food allergic murine model [42▪]. The in-vitro data suggested that by addition of C. butyricum, ovalbumin-specific regulatory B cells were generated and the plasmacytic differentiation of B cells was inhibited. However, oral immunotherapy usually does not confer a sustained protection.
Alternative routes: epicutaneous
In the pioneering experiments of epicutaneous immunotherapy without the use of adjuvant or tape stripping, sustained protection against anaphylaxis was induced by de-novo generation of latency-associated peptide+ Tregs, which was not achieved by the oral route in allergic mice. Tregs did not function by suppressing IgE antibodies, but instead directly suppressed mast cell activation, leading to sustained clinical protection against food-induced anaphylaxis [43▪▪]. Another study aimed to improve epidermal delivery of allergen using a microfractional laser to generate an array of self-renewable microchannels in the skin [44]. Epidermal application of allergens containing CpG and VitaminD3 turned out to be superior to the same treatment by the subcutaneous route, and resulted in a significant reduction of airway wall thickness, requiring a lower number of treatments. Similarly, in a murine atopic dermatitis model, epicutanous application of ovalbumin in conjunction with CpG was effective for treatment by promoting a strong Th1-mediated immune response leading to a decrease in antigen-specific IgE and increase in IgG2a antibodies [45].
Alternative routes: intravenous
Allergen-specific immunotherapy by the intravenous route was considered safe and effective when using allergen entrapped into biodegrable poly-lacto-glycolic (PLG) particles, whereas surface-conjugation of the allergen to PLG particles showed less efficacy [46▪▪].
Alternative routes: intralymphatic
In studies using the novel hCD2-DsRedxProx1-green fluorescent protein mice, featuring red T cells and green lymphatics, intravital microscopy depicted that lymphocytes crawl intralymphatically from inflamed tissue to lymphnodes [47▪▪].
Also the antigen itself was cargoed to lymph nodes by plasmocytoic dendritic cells, which resulted in tolerogenic effects [48].
In a proof-of-concept study for allergen-specific immunotherapy, intralymphatic application with ovalbumin adjuvanted with bacterial flagellin, a Toll-like receptor 5 agonist, was advantageous over the intranasal and sublingual applications in reducing symptoms in ovalbumin-sensitized mice [49▪].
Also for the intralymphatic allergy vaccines, comparative studies in veterinary patients are useful and were proposed for the treatment of canine atopic dermatitis [50]. Apart from atopy (Fig. 1c, f), insect bite hypersensitivity is a common problem in horses, which could be prevented by intralymphatic injections with recombinant insect allergens, adjuvanted with monophosphoryl lipid A (MPL) or aluminium hydroxide [51].
CONCLUSION
Innovative efforts were undertaken in models for atopic dermatitis by exploiting mediators previously correlated with atopic dermatitis, such as IL-23 [8], or using IL-6 for biomonitoring atopic dermatitis [12▪▪]. Notably, it was demonstrated that the atopic dermatitis microbiota was transferable and, vice versa, inoculation of mouse skin with healthy microbiota corrected the disease [11]. Novel mouse models helped to understand the significance of the skin barrier, enzymatic allergens [14] and physicochemical factors like skin pH. Also in atopic dermatitis models, the great influence of the microbiota was apparent. Animal studies provided evidence that the diet and gastric digestive functions affect colonization of microbiota [29] and correlate with food allergy and asthma, or protect against it. The induction or maintenance of tolerance to allergens could be supported by probiotics [42▪], but even more by immunostimulatory environmental compounds [32▪▪]. Germ-free mice transplanted with human microbiome [35] are valid models, but have limitations, such as the incorrect host specificity for human microbes, the greater microbial colonization of the small intestine in mice and disregard of host-specific coevolutionary factors such as resistance and nutrient utilization [52].
A plethora of animal models for specific allergen immunotherapy tried optimizing the application mode and route of allergen, by entrapment in tolerogenic PLG particles [46▪▪], or application concomitantly with CpG [45], probiotics or Vitamin D3 (Table 1) [37–39,41,42▪,43▪▪,44,45,46▪▪,49▪,51]. In particular, epicutaneous application has achieved promising results without adjuvants [43▪▪]. On the basis of therapeutic mouse studies [49▪] and preventive approaches in horses [51], the frequency of usage of the intralymphatic route is expected to increase in the near future.
Table 1.
Allergen immunotherapy models
| Allergen | Sensitization | Therapy | Challenge | References |
| Der p 2 | 2 × 1 μg rDer p 2 + 2 mg Alum i.p. | Chemically modified monomeric allergoid of Derp2, d2-OID or d2-OID + VitD3 | 1 × 1 μg rDer p 2 i.p. and 5 × 1% HDM | (Petrarca C et al.) [39] |
| HDM | 3, 5 or 7 weeks: 5 × 25 μg Der f extract i.n. | SLIT 2 weeks Der f extract 0.5 or 5 mg | 5 × 25 μg Der f extract i.n. | (Shima K et al.) [37] |
| HDM | 2 × 0.5 DU Der f extract + Alhydrogel i.p. or 4 × 1.5 DU Der f extract i.n. | SLIT 2 cycles 5 × 3 or 12 DU of Der f extract or 4 cycles: 3 × SLIT 12.6 DU extract | 4 × 0.5 DU Der f extract i.n. | (Hagner S et al.) [38] |
| OVA | OVA+ Alum i.p. | 2 × 0.2 mg OVA or SHAS-OVA s.c. | n/a | (Garbani M et al.) [41] |
| OVA | 2 × 10 μg OVA + 1 mg Alum i.p. | 3 × OVA+VD3 and CpG powder for 2 h on laser-treated skin, μEPIT or 8 × s.c.-treatments with 0.5, 1, 2, 4, 8, 16, 32, 50 μg OVA | 3 × 0.500 μg OVA i.n. | (Kumar MN et al.) [44] |
| OVA | 2 × 10 μg OVA + 3 mg Alum i.p. | Prophylactic or therapeutical 2 × PLG-OVA i.v. | 3 × 20 min with 10 mg/ml OVA aerosol | (Smarr CB et al.) [46▪▪] |
| OVA | 4 × 1 mg OVA + 20 μg cholera toxin o.g. | Oral 2 × 1 mg OVA, 2 × 5 mg OVA, 3 × 10 mg OVA, 2 × 25 mg OVA, 5 × 50 mg OVA +/– 10^9C. butyricum o.g. | n/a | (Shi Y et al.) [42▪] |
| OVA | 4 × 100 μg OVA +/– 100 μg CpG e.c. left total of 14 days | 2 × OVA or OVA+CpG e.c. left total of 8 days | 2 × OVA e.c. | (Majewska-Szczepanik et al.) [45] |
| OVA | 3 × 25 μg OVA + 1 mg Alum i.p. | 6 × 10 μg OVA and 10 μg FlaB i.n. or sublingual or 2 × 10 μg OVA and 1 μg FlaB i.l. | 6 × OVA 100 μg i.n. | (Kim EH et al.) [49▪] |
| OVA or Peanut | 100 μg OVA or 1 mg peanut extract on shaved skin | 8 × 100 μg OVA e.c. or 8 weeks 1 mg OVA orally | Oral challenge with 10, 20 and 50 mg OVA every 30 min | (Tordesillas L et al.) [43▪▪] |
| rCul n 3, rCul n4, rCul n8 rCul n10 | n/a | 3 × 10 μg of rCul n 3, rCul n4, rCul n8 rCul n10 + 500 μg Alum or Alum+50 μg MPLA (Avantilipids) s.l. in iceland horses | n/a | (Jonsdottier S et al.) [51] |
e.c., epicutaneous application; HDM, house dust mite; i.n., intranasal; i.p., intraperitoneal; i.v., intravenous; n/a, not applicable; o.g., oral gavage; OVA, ovalbumin; rCul, recombinant Culicoides nubeculosus allergens; s.c., subcutaneous; s.l., submandibular lymph node; t.a., topical application.
Acknowledgements
No funding was obtained from National Institutes of Health (NIH), Wellcome Trust and Howard Hughes Medical Institute (HHMI).
Financial support and sponsorship
The work to this article was supported by grant SFB F4606-B28 of the Austrian Science Fund FWF to EJJ.
Conflicts of interest
The other authors declare no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Shamji M, Kappen J, Akdis M, et al. Biomarkers for monitoring clinical efficacy of allergen immunotherapy for allergic rhinoconjunctivitis and allergic asthma: an EAACI Position Paper. Allergy 2017; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 2.Grimm H, Huth M. Jensen-Jarolim E. One health: many patients? A short theory on what makes an animal a patient. In Comparative medicine: diseases linking humans with their animals. Switzerland:Springer International Publishing; 2017. 219–230. [Google Scholar]
- 3.Jensen-Jarolim E, Herrmann I, Panakova L, Janda J. Jensen-Jarolim E. Allergic and atopic eczema in humans and their animals. In Comparative medicine: diseases linking humans with their animals. Switzerland:Springer International Publishing; 2017. 131–150. [Google Scholar]
- 4.Scharschmidt TC, Vasquez KS, Truong HA, et al. A wave of regulatory T cells into neonatal skin mediates tolerance to commensal microbes. Immunity 2015; 43:1011–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berker M, Frank LJ, Gessner AL, et al. Allergies: a T cells perspective in the era beyond the TH1/TH2 paradigm. Clin Immunol 2017; 174:73–83. [DOI] [PubMed] [Google Scholar]
- 6.Park HJ, Lee SW, Park SH, Hong S. iNKT cells are responsible for the apoptotic reduction of basophils that mediate Th2 immune responses elicited by papain in mice following gamma PGA stimulation. PLoS One 2016; 11:e0152189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J, Leyva-Castillo JM, Hener P, et al. Counterregulation between thymic stromal lymphopoietin- and IL-23-driven immune axes shapes skin inflammation in mice with epidermal barrier defects. J Allergy Clin Immunol 2016; 138:150–161. e113. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka A, Matsuda H. Evaluation of itch by using NC/NgaTnd mice: a model of human atopic dermatitis. J Biomed Biotechnol 2011; 2011:790436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9▪▪.Ewald DA, Noda S, Oliva M, et al. Major differences between human atopic dermatitis and murine models, as determined by using global transcriptomic profiling. J Allergy Clin Immunol 2017; 139:562–571. [DOI] [PubMed] [Google Scholar]; In search for the ideal mouse model of atopic dermatitis, the authors identify the IL-23-injected, NC/Nga and oxazolone-challenged mouse model to possess the largest homology to a human atopic dermatitis transcriptome from a meta-analysis.
- 10.Olivry T, Mayhew D, Paps JS, et al. Early activation of Th2/Th22 inflammatory and pruritogenic pathways in acute canine atopic dermatitis skin lesions. J Invest Dermatol 2016; 136:1961–1969. [DOI] [PubMed] [Google Scholar]
- 11.Bradley CW, Morris DO, Rankin SC, et al. Longitudinal evaluation of the skin microbiome and association with microenvironment and treatment in canine atopic dermatitis. J Invest Dermatol 2016; 136:1182–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12▪▪.Myles IA, Williams KW, Reckhow JD, et al. Transplantation of human skin microbiota in models of atopic dermatitis. JCI Insight 2016; 1:e86955. [DOI] [PMC free article] [PubMed] [Google Scholar]; When microbiota from healthy controls or from atopic dermatitis patients were inoculated in MC903 atopic dermatitis mice, only the microbiome from the healthy were able to control S. aureus growth and even corrected the atopic dermatitis. This result should be interesting to be transferred to humans.
- 13▪.Hayashi M, Takai J, Yu L, et al. Whole-body in vivo monitoring of inflammatory diseases exploiting human interleukin 6-luciferase transgenic mice. Mol Cell Biol 2015; 35:3590–3601. [DOI] [PMC free article] [PubMed] [Google Scholar]; A novel mouse model (hIL6-BAC-Luc mice) was used for demonstrating the development of chronic inflammation in atopic dermatitis by in-vivo bioluminescence imaging. This method will significantly contribute to our understanding in this disease.
- 14.Jang H, Matsuda A, Jung K, et al. Skin pH is the master switch of kallikrein 5-mediated skin barrier destruction in a murine atopic dermatitis model. J Invest Dermatol 2016; 136:127–135. [DOI] [PubMed] [Google Scholar]
- 15.Stremnitzer C, Manzano-Szalai K, Willensdorfer A, et al. Papain degrades tight junction proteins of human keratinocytes in vitro and sensitizes C57BL/6 mice via the skin independent of its enzymatic activity or TLR4 activation. J Invest Dermatol 2015; 135:1790–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimura S, Takai T, Iida H, et al. Epicutaneous allergic sensitization by cooperation between allergen protease activity and mechanical skin barrier damage in mice. J Invest Dermatol 2016; 136:1408–1417. [DOI] [PubMed] [Google Scholar]
- 17.Kang H, Kim S, Lee KH, et al. 5 nm silver nanoparticles amplify clinical features of atopic dermatitis in mice by activating mast cells. Small 2016; 13: doi: 10.1002/smll.201602363. [DOI] [PubMed] [Google Scholar]
- 18.Saunders SP, Moran T, Floudas A, et al. Spontaneous atopic dermatitis is mediated by innate immunity, with the secondary lung inflammation of the atopic march requiring adaptive immunity. J Allergy Clin Immunol 2016; 137:482–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee HJ, Lee NR, Jung M, et al. Atopic march from atopic dermatitis to asthma-like lesions in NC/Nga mice is accelerated or aggravated by neutralization of stratum corneum but partially inhibited by acidification. J Invest Dermatol 2015; 135:3025–3033. [DOI] [PubMed] [Google Scholar]
- 20.Tokumasu R, Yamaga K, Yamazaki Y, et al. Dose-dependent role of claudin-1 in vivo in orchestrating features of atopic dermatitis. Proc Natl Acad Sci U S A 2016; 113:E4061–E4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Row S, Liu Y, Alimperti S, et al. Cadherin-11 is a novel regulator of extracellular matrix synthesis and tissue mechanics. J Cell Sci 2016; 129:2950–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raguz J, Jeric I, Niault T, et al. Epidermal RAF prevents allergic skin disease. Elife 2016; 5:e14012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshihisa Y, Andoh T, Matsunaga K, et al. Efficacy of astaxanthin for the treatment of atopic dermatitis in a murine model. PLoS One 2016; 11:e0152288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palmqvist N, Siller M, Klint C, Sjodin A. A human and animal model-based approach to investigating the anti-inflammatory profile and potential of the 5-HT2B receptor antagonist AM1030. J Inflamm (Lond) 2016; 13:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baek JO, Roh JY, Jung Y. Oral tolerance inhibits atopic dermatitis-like type 2 inflammation in mice by modulating immune microenvironments. Allergy 2016; 72:397–406. [DOI] [PubMed] [Google Scholar]
- 26.Murray C, Ahrens K, Devalaraja M, et al. Use of a canine model of atopic dermatitis to investigate the efficacy of a CCR4 antagonist in allergen-induced skin inflammation in a randomized study. J Invest Dermatol 2016; 136:665–671. [DOI] [PubMed] [Google Scholar]
- 27.Barcik W, Untersmayr E, Pali-Schöll I, et al. Influence of microbiome and diet on immune responses in food allergy models. Drug Discovery Today: Disease Models 2017; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thorburn AN, McKenzie CI, Shen S, et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat Commun 2015; 6:7320. [DOI] [PubMed] [Google Scholar]
- 29.Diesner SC, Bergmayr C, Pfitzner B, et al. A distinct microbiota composition is associated with protection from food allergy in an oral mouse immunization model. Clin Immunol 2016; 173:10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30▪.Beura LK, Hamilton SE, Bi K, et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 2016; 532:512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]; This outstanding article describes that laboratory mice, living under abnormal SPF conditions, lack differentiated memory CD8+ T-cell subsets and mucosally distributed memory T cells. Cohousing SPF animals with pet store mice resulted in an immune system more similar to those of adult humans with subsequent resistance to infection and T-cell differentiation at de-novo viral infection.
- 31.Reardon S. Dirty room-mates make lab mice more useful. Nature 2016; 532:294–295. [DOI] [PubMed] [Google Scholar]
- 32▪▪.Stein MM, Hrusch CL, Gozdz J, et al. Innate immunity and asthma risk in Amish and Hutterite farm children. N Engl J Med 2016; 375:411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]; House dust samples from two genetically homologous populations, but with different allergy and asthma risk, were extracted and used for preventive treatment in an asthma mouse model. Only the dust from the healthier cohort protected from disease. This emphasizes the great impact of environmental compounds for immune modulation.
- 33.Aoki-Yoshida A, Yamada K, Hachimura S, et al. Enhancement of oral tolerance induction in DO11.10 mice by Lactobacillus gasseri OLL2809 via increase of effector regulatory T cells. PLoS One 2016; 11:e0158643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JH, Jeun EJ, Hong CP, et al. Extracellular vesicle-derived protein from Bifidobacterium longum alleviates food allergy through mast cell suppression. J Allergy Clin Immunol 2016; 137:507–516. e508. [DOI] [PubMed] [Google Scholar]
- 35.Arrieta MC, Sadarangani M, Brown EM, et al. A humanized microbiota mouse model of ovalbumin-induced lung inflammation. Gut Microbes 2016; 7:342–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arrieta MC, Walter J, Finlay BB. Human microbiota-associated mice: a model with challenges. Cell Host Microbe 2016; 19:575–578. [DOI] [PubMed] [Google Scholar]
- 37.Shima K, Koya T, Tsukioka K, et al. Effects of sublingual immunotherapy in a murine asthma model sensitized by intranasal administration of house dust mite extracts. Allergol Int 2017; 66:89–96. [DOI] [PubMed] [Google Scholar]
- 38.Hagner S, Rask C, Brimnes J, et al. House dust mite-specific sublingual immunotherapy prevents the development of allergic inflammation in a mouse model of experimental asthma. Int Arch Allergy Immunol 2016; 170:22–34. [DOI] [PubMed] [Google Scholar]
- 39.Petrarca C, Clemente E, Amato V, et al. Vitamin D3 improves the effects of low dose Der p 2 allergoid treatment in Der p 2 sensitized BALB/c mice. Clin Mol Allergy 2016; 14:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roth-Walter F, Bergmayr C, Meitz S, et al. Janus-faced Acrolein prevents allergy but accelerates tumor growth by promoting immunoregulatory Foxp3+ cells: mouse model for passive respiratory exposure. Sci Rep 2017; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garbani M, Xia W, Rhyner C, et al. Allergen-loaded strontium-doped hydroxyapatite spheres improve allergen-specific immunotherapy in mice. Allergy 2017; 72:570–578. [DOI] [PubMed] [Google Scholar]
- 42▪.Shi Y, Xu LZ, Peng K, et al. Specific immunotherapy in combination with Clostridium butyricum inhibits allergic inflammation in the mouse intestine. Sci Rep 2015; 5:17651. [DOI] [PMC free article] [PubMed] [Google Scholar]; The probiotic C. butyricum was applied orally in conjunction with a food allergen and this approach could reduce symptoms in a food allergy mouse model. The study is thus supportive for probiotics.
- 43▪▪.Tordesillas L, Mondoulet L, Blazquez AB, et al. Epicutaneous immunotherapy induces gastrointestinal LAP+ regulatory T cells and prevents food-induced anaphylaxis. J Allergy Clin Immunol 2017; 139:189–201. e184. [DOI] [PMC free article] [PubMed] [Google Scholar]; This pioneering article shows long-term efficacy by allergen immunotherapy without the use of adjuvants via the epicutaneous route, inducing allergen-specific regulatory T cells that directly suppress mast cell activation.
- 44.Kumar MN, Zhou C, Wu MX. Laser-facilitated epicutaneous immunotherapy to IgE-mediated allergy. J Control Release 2016; 235:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Majewska-Szczepanik M, Askenase PW, Lobo FM, et al. Epicutaneous immunization with ovalbumin and CpG induces TH1/TH17 cytokines, which regulate IgE and IgG2a production. J Allergy Clin Immunol 2016; 138:262–273. e266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46▪▪.Smarr CB, Yap WT, Neef TP, et al. Biodegradable antigen-associated PLG nanoparticles tolerize Th2-mediated allergic airway inflammation pre and postsensitization. Proc Natl Acad Sci U S A 2016; 113:5059–5064. [DOI] [PMC free article] [PubMed] [Google Scholar]; Although it is known that encapsulation in PLG particles are interesting biodegradable vectors for allergen immunotherapy models, the authors show here that they are able to induce tolerization of Th2 cells in an asthma model both in presensitization and postsensitization set-up.
- 47▪▪.Teijeira A, Hunter MC, Russo E, et al. T cell migration from inflamed skin to draining lymph nodes requires intralymphatic crawling supported by ICAM-1/LFA-1 interactions. Cell Rep 2017; 18:857–865. [DOI] [PubMed] [Google Scholar]; In the context of intralymphatic traveling of immune cells, this work provides for the first time a method allowing intravital microscopic observations in mice.
- 48.Lexmond WS, Goettel JA, Lyons JJ, et al. FOXP3+ Tregs require WASP to restrain Th2-mediated food allergy. J Clin Invest 2016; 126:4030–4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49▪.Kim EH, Kim JH, Samivel R, et al. Intralymphatic treatment of flagellin-ovalbumin mixture reduced allergic inflammation in murine model of allergic rhinitis. Allergy 2016; 71:629–639. [DOI] [PubMed] [Google Scholar]; Approaching the intralymphatic route for allergen immunotherapy, the TLR-5 ligand flagellin in context with model allergen ovalbumin effectively reduced allergic inflammation in this allergic rhinitis mouse model.
- 50.DeBoer DJ. The future of immunotherapy for canine atopic dermatitis: a review. Vet Dermatol 2017; 28:e25–e26. [DOI] [PubMed] [Google Scholar]
- 51.Jonsdottir S, Svansson V, Stefansdottir SB, et al. A preventive immunization approach against insect bite hypersensitivity: intralymphatic injection with recombinant allergens in Alum or Alum and monophosphoryl lipid A. Vet Immunol Immunopathol 2016; 172:14–20. [DOI] [PubMed] [Google Scholar]
- 52.Roth-Walter F, Berger S, Luckschander-Zeller N. Jensen-Jarolim E. Inflammatory bowel disease in humans, pets, and horses. In Comparative medicine: disorders linking humans with their animals. Switzerland:Springer International Publishing; 2017. 47–69. [Google Scholar]


