Abstract
Context
Xerostomia and hyposalivation are associated with diabetes. Research is sparse regarding electrostimulation as a mainstream therapy for salivary gland hypofunction.
Objective
To clinically evaluate the effectiveness of transcutaneous electric nerve stimulation (TENS) therapy in stimulating whole salivary flow among patients with xerostomia and hyposalivation caused by diabetes mellitus.
Design
Forty patients between age 30 to 75 years with diabetes mellitus categorized as controlled or uncontrolled who had subjective symptoms of xerostomia and an objective sign of hyposalivation were included in a prospective study.
Main Outcome Measures
Unstimulated saliva through the “low forced spitting” method and stimulated saliva collection using TENS were assessed and compared. Longer-term effects of TENS application were evaluated by recalling the patient 24 hours later.
Results
A statistically significant increase in stimulated whole saliva after TENS application in continuous mode (p < 0.001) was demonstrated compared with unstimulated saliva, especially in xerostomic patients with diabetes. Burst mode inferred a statistically significant decrease in salivary flow (p < 0.001).
Conclusion
In patients with diabetes with xerostomia and hyposalivation, TENS was highly effective in stimulating whole salivary flow.
INTRODUCTION
Saliva is a precious oral fluid that often is taken for granted. It is critical to the preservation and maintenance of oral health, yet it receives little attention until quantity or quality is diminished. Decreased salivary production or altered salivary composition may result in various clinical conditions affecting oral health, comfort, and quality of life. Clinical assessment of oral dryness is a vital component of care. A person may have clinically identified oral desiccation with or without hyposalivation.1
Xerostomia and hyposalivation are diverse words that should not be used interchangeably. Xerostomia is defined as a subjective complaint of dry mouth that may result from a decrease in the production of saliva, and hyposalivation is an objective reduction in salivary secretion and is defined as unstimulated whole saliva below 0.12 mL/min and 0.16 mL/min and stimulated whole salivary flow rate below 0.5 mL/min.2 Xerostomia often develops when the quantity of saliva that bathes the oral mucous membranes is reduced. However, symptoms may occur without a considerable reduction in salivary gland output.3 Both xerostomia and hyposalivation have been associated with diabetes mellitus (DM).4–6 Dryness of the mouth as a facet of uncontrolled DM was first described in 1942. Xerostomia in Type 1 DM could be dependent on glucose control, whereas in Type 2 DM, salivary secretion seems to be predisposed by xerogenic drugs and autonomic neuropathy.7
A large number of treatment options are available for patients with xerostomia depending on the cause. Palliative treatment includes frequent sipping of water and application of various types of sprays and gels, which appear to be helpful for reducing the morbidity related to this condition; however, many of the current treatment options are merely transient and as such are not considered to be satisfactory treatment options. Novel approaches for treating xerostomia are being investigated, which include acupuncture and transcutaneous electric nerve stimulation (TENS).2,8–10
Electrostimulation of neural and muscular structures is of therapeutic potential in several areas of medicine (pacemakers, phrenic stimulators, etc), and because of the known autonomic control of salivary secretion, a similar approach could potentially be applied to the management of salivary gland hypofunction. Application of electric impulses to one or more of the three components of the salivary reflex arch should, in theory, improve salivary secretion and ultimately lessen the various long-term effects of hyposalivation.11
Research is sparse regarding electrostimulation as a mainstream therapy for salivary gland hypofunction. As recommended by Hargitai et al,12 future studies on the TENS unit for salivary stimulation in a cohort of patients with dry mouth was warranted. Thus, the purpose of the present study was to clinically evaluate the effectiveness of TENS in stimulating salivary flow among patients with xerostomia and hyposalivation caused by DM.
METHODS
Participant Selection
Forty patients with DM with xerostomia and hyposalivation between age 45 and 70 years were recruited from the outpatient Department of Oral Medicine and Radiology, Oxford Dental College and Hospital, Rajiv Gandhi University of Health Sciences, Karnataka, India. The study protocol was approved by the university’s ethical committee. All subjects completed a written, institutional review board-approved informed consent document. Patients having DM associated with other systemic disorders, the presence of pacemakers and cochlear implants, or a history of salivary gland disease were excluded, as were patients who refused oral examination, did not provide informed consent, or did not have sufficient clinical data.
Diabetic Status
Fasting blood glucose test and glycosylated hemoglobin (HbA1c) test results were evaluated by the ion exchange method to categorize patients with DM into controlled and poorly controlled status of DM. The American Diabetes Association recommends that patients with DM attempt to achieve a target HbA1c value of less than 7%, whereas an HbA1c value of more than 8% suggests that a change in patient management may be needed to improve glycemic control.13,14 Accordingly, in the present study, HbA1c values of more than 7% were defined as poorly controlled DM, and HbA1c values of 7% and less were considered controlled DM. All subjects with diabetes were receiving treatment by dietary modifications, oral hypoglycemic agents, insulin, or a combination of these treatments.
Each subject’s demographic data and dental and medical history were obtained. Xerostomia was assessed by using four questions modified from Fox et al.15 Oral mucosa was checked for moistness on the basis of visible saliva in the oral cavity. The questions asked were
Does the amount of saliva in your mouth seem to be too little, too much, or you do not notice it?
Do you have any difficulty swallowing?
Does your mouth feel dry when eating a meal?
Do you sip liquids to aid in swallowing dry food?
Saliva Collection
Collection of Unstimulated Whole Saliva
All the participants were asked to refrain from eating, drinking, chewing gum, smoking, and oral hygiene procedures such as brushing and mouth rinsing for at least one hour before the appointment. The subjects were made to sit in an upright, comfortable, and relaxed position, with the head inclined forward and minimal body and orofacial movements. Subjects then were asked to swallow their saliva first and to stay motionless so that the saliva could collect passively in the anterior region of the floor of the mouth. With use of the “low forced spitting” method,16 unstimulated saliva was collected for ten minutes in a graduated measuring cylinder fitted with a funnel. After ten minutes of the whole saliva collection, TENS electrodes were placed.
Collection of Stimulated Whole Saliva by Transcutaneous Electric Nerve Stimulation
Surface electrode pads were placed externally on the skin overlying the parotid glands (anteroposteriorly between the tragus of the ear and the midmasseter region and superoinferiorly between the region of the head of the mandible and above the lower border of the mandible) with the TENS unit in the off position. Tabletop TENS unit (INDOTENS 10, HMS Medical Systems, Chennai, India) was then activated, the pulse rate was fixed at 50 Hz, and the intensity was gradually increased to a maximum tolerable level for each patient. At optimal intensity (the maximum intensity that the subject still perceived to be comfortable),12 stimulated saliva was collected in continuous mode for 10 minutes into a separate graduated measuring cylinder; after a gap of 1 minute this procedure was repeated in burst mode. The whole saliva collected was allowed to settle down for 10 minutes so that the bubbles would not interfere with the measured volume of the saliva.17 The amount of unstimulated and TENS-stimulated whole salivary flow was assessed and compared. A log of adverse effects was kept during and after the experiment. All the subjects were recalled after 1 day to evaluate the 24-hour effects of TENS therapy. The level of significance was 0.05, and a paired t test was used for analysis.
RESULTS
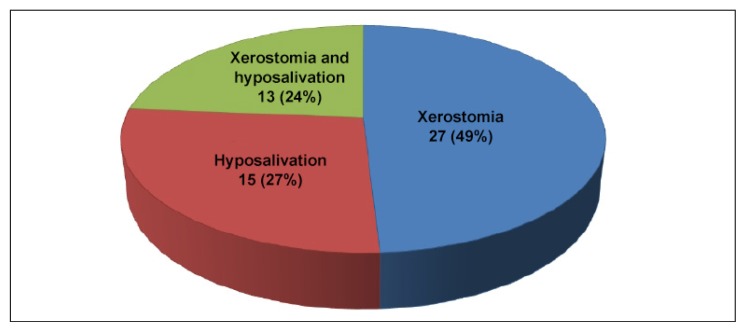
The distribution of study participants with respect to sex, duration of diabetes, and their type of diabetes is demonstrated in Table 1. Thirty men and 10 women participated in the study. Their mean age was 48.15 years. Thirteen patients had controlled diabetes and 27 had uncontrolled diabetes. Of 40 patients with DM, xerostomia was present in 27 (68%) of the patients, hyposalivation was present in 15 (27%), and 13 (24%) had both xerostomia and hyposalivation.
Table 1.
Demographic characteristics of study sample
| Demographic characteristics | Women | Men | Overall |
|---|---|---|---|
| Age, years | |||
| Mean | 48.40 | 48.07 | 48.15 |
| Median | 49.5 | 48.0 | 48.0 |
| Range | 32–57 | 32–63 | 32–63 |
| Duration of diabetes mellitus, years | |||
| Mean | 4.50 | 4.47 | 4.48 |
| Median | 5.0 | 4.0 | 4.0 |
| Range | 2–7 | 1–10 | 1–10 |
| Type of diabetes mellitus,a no. | |||
| Controlled | 0 | 13 | 13 |
| Uncontrolled | 10 | 17 | 27 |
| Total | 10 | 30 | 40 |
Hemoglobin A1c concentration above 7% was defined as uncontrolled diabetes, and 7% or less as controlled diabetes.
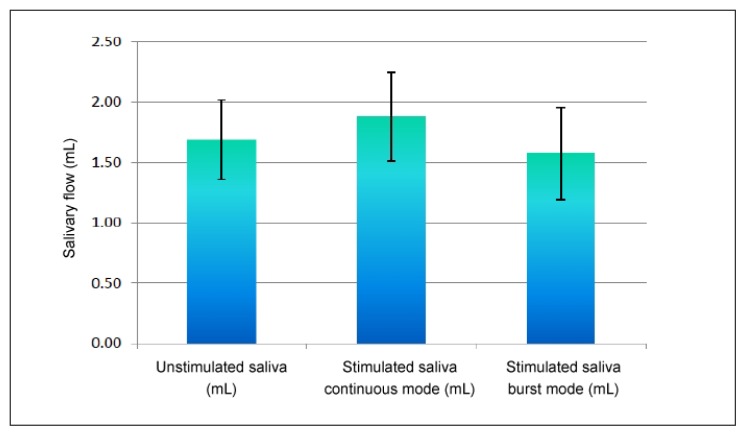
Table 2 and Figure 1 show the comparison of whole salivary flow (in milliliters per 10 minutes) among all patients with DM before and after TENS application in continuous and burst modes. The mean ± standard deviation (SD) increase in stimulated saliva after TENS application in continuous mode (1.88 ± 0.36 mL) compared with unstimulated saliva (1.69 ± 0.33 mL) was found to be statistically significant (p < 0.001). The mean ± SD decrease in stimulated saliva in burst mode (1.58 ± 0.38 mL) compared with unstimulated saliva (1.69 ± 0.33 mL) also was found to be statistically significant (p < 0.001).
Table 2.
Comparison of whole salivary flow among all patients with diabetes before and after transcutaneous electric nerve stimulation (N = 40)
| Whole salivary flow | Mean (mL/10 min)a | SD | SEM | Mean difference | t | p value |
|---|---|---|---|---|---|---|
| Unstimulated saliva | 1.69 | 0.33 | 0.05 | Referent | ||
| Stimulated saliva, continuous mode | 1.88 | 0.36 | 0.06 | −0.190 | −5.479 | < 0.001b |
| Stimulated saliva, burst mode | 1.58 | 0.38 | 0.06 | 0.113 | 4.869 | < 0.001b |
Normal range of unstimulated whole salivary flow = 0.1–0.5 mL/min.
Denotes significant difference.
SD = standard deviation; SEM =standard error of the mean.
Figure 1.
Comparison of whole salivary flow (mL/10 min) among all patients with diabetes mellitus before and after application of transcutaneous electric nerve stimulation in continuous and burst modes.
There were no major side effects with the use of TENS noticed in the study subjects. One subject had slight twitching of the facial musculature during application of TENS, and two subjects experienced a tingling sensation, which was transient and ceased immediately once the TENS unit was turned off. No significant correlation was observed between sex, age, or duration of DM and stimulated whole salivary flow after TENS application.
Table 3 shows the correlation of sex with xerostomia and hyposalivation in patients with DM. Among 30 men, xerostomia was present in 21 (70%); among 10 women, 6 (60%) had xerostomia. Hyposalivation was present in 10 male patients with DM (33%) and 5 female patients with DM (50%). This correlation of sex with xerostomia (p = 0.559) and with hyposalivation (p = 0.346) was not found to be statistically significant.
Table 3.
Correlation of sex of patients with diabetes with xerostomia and hyposalivation
| Condition | Men No. (%) | Women No. (%) | Total No. (%) | χ2 | p value |
|---|---|---|---|---|---|
| Xerostomia | |||||
| Present | 21 (70) | 6 (60) | 27 (67.5) | 0.342 | 0.559 |
| Absent | 9 (30) | 4 (40) | 13 (32.5) | ||
| Total | 30 (100) | 10 (100) | 40 (100) | ||
| Hyposalivation | |||||
| Present | 10 (33) | 5 (50) | 15 (37.5) | 0.889 | 0.346 |
| Absent | 20 (67) | 5 (50) | 25 (62.5) | ||
| Total | 30 (100) | 10 (100) | 40 (100) | ||
Table 4 shows the comparison of whole salivary flow (mL/10 min) among patients with DM having hyposalivation before and after TENS application in continuous and burst modes. The mean ± SD stimulated saliva in continuous mode (1.55 ± 0.31 mL) after TENS application was increased (0.21 mL) compared with unstimulated saliva (1.34 ± 0.23 mL), and this difference was found to be statistically significant (p < 0.014). The mean stimulated saliva in burst mode (1.21 ± 0.31 mL) was decreased compared with unstimulated saliva (1.34 ± 0.23 mL), which was found to be statistically significant (p < 0.001).
Table 4.
Comparison of whole salivary flow among patients with diabetes with hyposalivation before and after transcutaneous electric nerve stimulation (N = 15)
| Whole salivary flow | Mean (mL/10 min) | SD | SEM | Mean difference | t | p value |
|---|---|---|---|---|---|---|
| Unstimulated saliva | 1.34 | 0.23 | 0.06 | Referent | ||
| Stimulated saliva, continuous mode | 1.55 | 0.31 | 0.08 | −0.213 | −2.802 | −0.014a |
| Stimulated saliva, burst mode | 1.21 | 0.31 | 0.08 | 0.127 | 2.679 | 0.018a |
Denotes significant difference.
SD = standard deviation; SEM =standard error of the mean.
The correlation between duration of DM and unstimulated saliva or stimulated saliva in continuous mode and burst mode demonstrated no statistically significant (p > 0.05) association. However, an objective increase in stimulated whole salivary flow was found in 36 patients (8 women and 28 men), and an objective decrease in salivary flow after TENS application was observed in 4 patients (2 men and 2 women).
Among 40 patients with DM, only 6 patients (15%) returned for follow-up after 24 hours of TENS application. Among them, 2 patients had a remarkable increase in the unstimulated whole salivary flow (1.2 mL to 4.3 mL and 2.0 mL to 6.0 mL), and the 4 others had almost the same unstimulated whole salivary flow as the previous day.
DISCUSSION
Saliva plays a crucial role in maintaining a healthy oral environment. A diminution in saliva output, or hyposalivation, has been linked with changes in oral microflora and various oral pathologies.4,18 Several studies were conducted in the past to see the efficiency of electrostimulation in increasing salivary flow, but only a couple of studies have been conducted so far to demonstrate TENS as a means of stimulating salivary production in healthy adult subjects.12 In this study, TENS was used to evaluate its effectiveness as a therapeutic modality in patients with DM having xerostomia and hyposalivation.
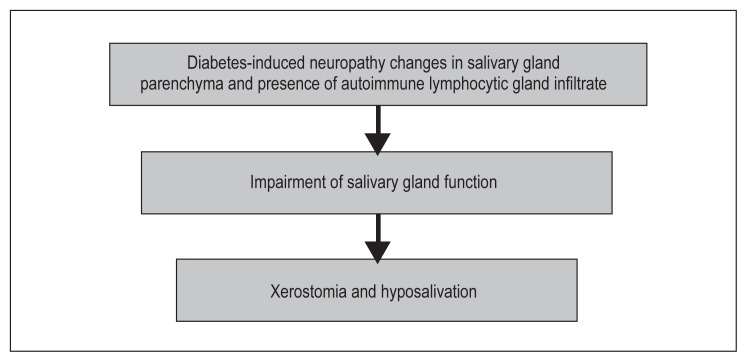
Salivary flow rates have often been found to be considerably reduced in patients with DM, although these findings have not always been established. Because the secretion of saliva is controlled by both sympathetic and parasympathetic autonomic nervous systems, it is possible that diabetic neuropathies could have differential effects on salivary secretion with either fluid or protein phases altered, depending on the nervous supply affected (Figure 2).19 Adverse hormonal, microvascular, and neuronal changes in poorly controlled diabetes could contribute to salivary gland hypofunction in older individuals. It has also been suggested that focal sensory neuropathies and microvascular changes may play a part in a patient with DM’s inability to detect xerostomia, perhaps through a mechanism similar to painless diabetic neuropathy associated with foot ulcers.20
Figure 2.
Pathophysiology in diabetes mellitus of xerostomia and hyposalivation.
The present study reported 27 complaints of xerostomia and 15 complaints of hyposalivation among 40 patients with DM (Figure 3). These complaints can be explained by the microvascular and autonomic neuropathic complications of diabetes, both of which may affect salivary secretion. Sreebny et al6 have pointed out that patients with diabetes, who often complain of oral dryness because of disturbances in glycemic control and poor salivary flow were associated with direct metabolic effects on salivary glands.
Figure 3.
Distribution of patients with diabetes mellitus with xerostomia and hyposalivation.
In the present study, 15 patients with DM had hyposalivation without a complaint of xerostomia. Chavez et al20 made a similar observation wherein patients with DM did not report significantly more xerostomic complaints, despite the fact that they experienced significantly lower stimulated parotid flow rates. A diminished stimulated saliva secretion could reflect dysfunction of the neuronal parasympathetic saliva secretion pathway and the simultaneous involvement of the neuronal sympathetic saliva secretion pathway in Type 2 DM.19 Another explanation for this finding could be that subjects may have found ways to compensate for salivary hypofunction (eg, taking liquids with meals) when diminished stimulated salivary flow might have been noticed. Also, there may be multiple physiologic factors, such as alterations in oral mucosal tissues or changes in baroreceptors, which contribute to decreased perception of xerostomia.12
Much of the variability in the literature regarding xerostomia can be attributed to different protocols for its measurement. A questionnaire for xerostomia is a good screening tool; however, the results may not correlate well with the salivary flow.21,22 Longman et al22 found that 34% of patients who had hyposalivation did not have symptoms of xerostomia. Similarly, 37% of patients who reported all 3 symptoms of xerostomia did not have hyposalivation.4 Dodds and Dodds19 reported that subjective responses to questions about salivary hypofunction indicated that salivation was not altered in well-controlled DM. Conversely, in the present study, among 13 patients with controlled DM, 9 complained of xerostomia and 4 had hyposalivation. However, Chavez et al5 reported that persons with poorly controlled DM had lower stimulated parotid flow rates than did persons with well-controlled diabetes and nondiabetic control subjects. There were no significant differences in xerostomic complaints on the basis of diabetic or glycemic control status or salivary flow rates.5 Thus, xerostomia does not equate consistently with hyposalivation.
There was a vast inconsistency in the amount of unstimulated whole saliva produced in our study. Initially some subjects demonstrated no salivary flow. This was not astonishing, as previous literature revealed that 21% to 22% of the healthy population demonstrates no parotid flow even when measured over 5 minutes.12,23
No significant (p > 0.05) association was observed between age and the saliva in unstimulated and stimulated conditions (ie, continuous and burst modes). The previous literature has also shown that salivary flow does not diminish with age and that TENS-stimulated salivary flow is not dependent on age.12,24 Thus, our results are in agreement with this prior observation.
A positive finding in this study was an objective increase in whole salivary flow among 36 patients (8 women and 28 men) after TENS application. The mean increase in stimulated saliva after TENS application in continuous mode compared with unstimulated saliva was found to be statistically significant (p < 0.001). The mean increase in stimulated saliva in continuous mode in xerostomic patients was 0.24 mL and 0.21 mL in patients with hyposalivation. Thus, xerostomic patients had a greater benefit from TENS application than did patients with hyposalivation in the study.
Previous studies have used the TENS unit in normal mode. Thus, an additional parameter was added in the study to compare continuous and burst modes of the TENS unit so that the most beneficial mode could be selected for saliva stimulation. Another important finding in this study was a statistically significant (p < 0.001) decrease in stimulated saliva in burst mode compared with unstimulated saliva. This may provide evidence to avoid burst mode of TENS application in xerostomic patients with DM in the future and that the continuous mode would be a better option for giving prompt relief from xerostomia and hyposalivation.
The mechanism by which the TENS unit works on the parotid gland is still unclear. Previous literature has mentioned that the auriculotemporal nerve that supplies secretomotor drive to the parotid gland may be directly stimulated and that it is also less clear if peripheral stimulation of the gland results in a reflex facilitation of central output from the salivatory nucleus of the medulla. To electrically stimulate sympathetic salivation, higher frequencies and longer pulse duration are required. Alternatively, electric stimulation of parasympathetic nerves of the salivary glands produces copious amounts of watery saliva at lower frequencies. It has been suggested that it is this voluminous, serous saliva of the parotid gland that would be clinically most practical for managing xerostomia.12,25
Sex-based differences were noticed in this study. In men, the increase in stimulated saliva after TENS application in continuous mode (1.88 mL) compared with unstimulated saliva (1.69 mL) was found to be statistically significant (p < 0.001). As mentioned in previous studies, men produced more saliva than women did and responded better to TENS, yet these differences probably have no clinical significance.12,24 Women were a small number in our study. On the basis of this finding, inclusion of more women in future studies in this area seems to be indispensable.
On its own, TENS is less likely to be effective in cases where there is no baseline salivary flow, such as in long-standing Sjögren syndrome or high-dose radiation therapy where complete destruction of the salivary gland unit has occurred. This is a limitation it would share with the other current treatment modalities. However, TENS appears to have potential in cases where there is residual salivary function. Based on these findings, it is suggestive that TENS may work best or even synergistically with other sialogogues.12,26
Whole salivary flow was decreased among four patients with DM after TENS application. The mechanism for this may entail the frequency and intensity settings and whether the brain professed the stimulus as being painful. Classically, the salivary reflex is improved when nociceptive input reaches the brain via the trigeminal sensory nuclei. However, it is known that not all preganglionic parasympathetic fibers are necessarily facilitated; some may be inhibited.5 This study did not observe which intensity and frequency produced the most saliva. We tried to minimize the adverse effects by keeping the stimulus at a tolerable level.
Of 40 patients with DM, only 6 patients returned for follow-up 24 hours after TENS application. Among them, 2 patients had remarkable increases in the unstimulated whole salivary flow, and the others had almost the same unstimulated whole salivary flow as the previous day. These findings suggest that TENS may stimulate the glands temporarily and may not have a significantly enduring effect after turning off the TENS unit, and that this technique may not work in every individual; however, in those who have dramatic results, relief from dry mouth would be most welcome.
The main benefit of the TENS unit over other nonpharmacologic measures such as chewing gum and citric lozenges is that it may be used while eating. Some chewing gum bases with sugar components need to be avoided by patients with DM and in those with temporomandibular disorders. Thus, xerostomic patients with DM may derive more benefit from TENS application. However, the great difficulty in recalling the subjects for follow-up was a drawback that affected the overall standardization of results.
CONCLUSION
The present study has been one of the few studies to show TENS having a potential for increasing salivary flow in a cohort of patients with dry mouth, such as patients with Sjögren syndrome and radiation-induced xerostomia (patients with residual saliva). To our knowledge, this study is the first of its kind to use TENS as a therapeutic modality in patients with DM with xerostomia and hyposalivation.
Because the initial results are encouraging, further studies are required to evaluate the long-term clinical effectiveness of TENS in Sjögren syndrome and xerostomia secondary to head and neck radiation therapy. Possibly, TENS acts more efficiently as an accelerator of salivary flow rather than an initiator. Therefore, it is likely to be more useful in cases of decreased salivary gland function rather than absolute absence of function. Future aspects of research should be encouraged, including the lasting effects in increasing salivary flow after turning off the TENS unit, the ability of TENS to stimulate parotid salivary flow specifically when there is none at baseline, patient acceptance, subjective measures, and usefulness of TENS alone vs in combination with other sialogogues, which is lacking in the present study.
Acknowledgment
Kathleen Louden, ELS, of Louden Health Communications provided editorial assistance.
Footnotes
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
References
- 1.Wiener RC, Wu B, Crout R, et al. Hyposalivation and xerostomia in dentate older adults. J Am Dent Assoc. 2010 Mar;141(3):279–84. doi: 10.14219/jada.archive.2010.0161. DOI: https://doi.org/10.14219/jada.archive.2010.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Napeñas JJ, Brennan MT, Fox PC. Diagnosis and treatment of xerostomia (dry mouth) Odontology. 2009 Jul;97(2):76–83. doi: 10.1007/s10266-008-0099-7. DOI: https://doi.org/10.1007/s10266-008-0099-7. [DOI] [PubMed] [Google Scholar]
- 3.Guggenheimer J, Moore PA. Xerostomia: Etiology, recognition and treatment. J Am Dent Assoc. 2003 Jan;134(1):61–9. doi: 10.14219/jada.archive.2003.0018. DOI: https://doi.org/10.14219/jada.archive.2003.0018. [DOI] [PubMed] [Google Scholar]
- 4.Khovidhunkit SO, Suwantuntula T, Thaweboon S, Mitrirattanakul S, Chomkhakhai U, Khovidhunkit W. Xerostomia, hyposalivation, and oral microbiota in type 2 diabetic patients: A preliminary study. J Med Assoc Thai. 2009 Sep;92(9):1220–8. [PubMed] [Google Scholar]
- 5.Chavez EM, Taylor GW, Borrell LN, Ship JA. Salivary function and glycemic control in older persons with diabetes. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000 Mar;89(3):305–11. doi: 10.1016/s1079-2104(00)70093-x. DOI: https://doi.org/10.1016/s1079-2104(00)70093-x. [DOI] [PubMed] [Google Scholar]
- 6.Sreebny LM, Yu A, Green A, Valdini A. Xerostomia in diabetes mellitus. Diabetes Care. 1992 Jul;15(7):900–4. doi: 10.2337/diacare.15.7.900. DOI: https://doi.org/10.2337/diacare.15.7.900. [DOI] [PubMed] [Google Scholar]
- 7.Vivek V. Xerostomia: A review. Journal of the Indian Academy of Oral Medicine and Radiology. 2007 Apr-Jun;19(2):319–28. [Google Scholar]
- 8.Lafaurie G, Fedele S, López RM, et al. Biotechnological advances in neuro-electro-stimulation for the treatment of hyposalivation and xerostomia. Med Oral Patol Oral Cir Bucal. 2009 Feb 1;14(2):E76–80. [PubMed] [Google Scholar]
- 9.Porter SR, Scully C, Hegarty AM. An update of the etiology and management of xerostomia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004 Jan;97(1):28–46. doi: 10.1016/j.tripleo.2003.07.010. DOI: https://doi.org/10.1016/j.tripleo.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Givens E., Jr Update on xerostomia: Current treatment modalities and future trends. Gen Dent. 2006 Mar-Apr;:99–101. [PubMed] [Google Scholar]
- 11.Fedele S, Wolff A, Strietzel F, López RM, Porter SR, Konttinen YT. Neuroelectrostimulation in treatment of hyposalivation and xerostomia in Sjögren’s syndrome: A salivary pacemaker. J Rheumatol. 2008 Aug;35(8):1489–94. [PubMed] [Google Scholar]
- 12.Hargitai IA, Sherman RG, Strother JM. The effect of electrostimulation on parotid saliva flow: A pilot study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005 Mar;99(3):316–20. doi: 10.1016/j.tripleo.2004.06.080. DOI: https://doi.org/10.1016/j.tripleo.2004.06.080. [DOI] [PubMed] [Google Scholar]
- 13.Greenberg MS, Glick M, Ship JA. Burket’s oral medicine. 11th ed. Hamilton, Ontario, Canada: BC Decker Inc; 2008. pp. 509–14. [Google Scholar]
- 14.Skamagas M, Breen TL, LeRoith D. Update on diabetes mellitus: Prevention, treatment, and association with oral diseases. Oral Dis. 2008 Mar;14(2):105–14. doi: 10.1111/j.1601-0825.2007.01425.x. DOI: https://doi.org/10.1111/j.1601-0825.2007.01425.x. [DOI] [PubMed] [Google Scholar]
- 15.Fox PC, Busch KA, Baum BJ. Subjective reports of xerostomia and objective measures of salivary gland performance. J Am Dent Assoc. 1987 Oct;115(4):581–4. doi: 10.1016/s0002-8177(87)54012-0. DOI: https://doi.org/10.1016/s0002-8177(87)54012-0. [DOI] [PubMed] [Google Scholar]
- 16.Navazesh M, Kumar SK University of Southern California School of Dentistry. Measuring salivary flow: Challenges and opportunities. J Am Dent Assoc. 2008 May;139( Suppl):35S–40S. doi: 10.14219/jada.archive.2008.0353. DOI: https://doi.org/10.14219/jada.archive.2008.0353. [DOI] [PubMed] [Google Scholar]
- 17.Kaufman E, Lamster IB. The diagnostic applications of saliva—a review. Crit Rev Oral Biol Med. 2002;13(2):197–212. doi: 10.1177/154411130201300209. DOI: https://doi.org/10.1177/154411130201300209. [DOI] [PubMed] [Google Scholar]
- 18.Humphrey SP, Williamson RT. A review of saliva: Normal composition, flow, and function. J Prosthet Dent. 2001 Feb;85(2):162–9. doi: 10.1067/mpr.2001.113778. DOI: https://doi.org/10.1067/mpr.2001.113778. [DOI] [PubMed] [Google Scholar]
- 19.Dodds MW, Dodds AP. Effects of glycemic control on saliva flow rates and protein composition in non-insulin-dependent diabetes mellitus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997 Apr;83(4):465–70. doi: 10.1016/s1079-2104(97)90147-5. DOI: https://doi.org/10.1016/s1079-2104(97)90147-5. [DOI] [PubMed] [Google Scholar]
- 20.Chávez EM, Borrell LN, Taylor GW, Ship JA. A longitudinal analysis of salivary flow in control subjects and older adults with type 2 diabetes. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001 Feb;91(2):166–73. doi: 10.1067/moe.2001.112054. DOI: https://doi.org/10.1067/moe.2001.112054. [DOI] [PubMed] [Google Scholar]
- 21.Nederfors T. Xerostomia and hyposalivation. Adv Dent Res. 2000 Dec;14:48–56. doi: 10.1177/08959374000140010701. DOI: https://doi.org/10.1177/08959374000140010701. [DOI] [PubMed] [Google Scholar]
- 22.Longman LP, McCracken CF, Higham SM, Field EA. The clinical assessment of oral dryness is a significant predictor of salivary gland hypofunction. Oral Dis. 2000 Nov;6(6):366–70. doi: 10.1111/j.1601-0825.2000.tb00128.x. DOI: https://doi.org/10.1111/j.1601-0825.2000.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 23.Ship JA, Fox PC, Baum BJ. How much saliva is enough? ‘Normal’ function defined. J Am Dent Assoc. 1991 Mar;122(3):63–9. doi: 10.14219/jada.archive.1991.0098. DOI: https://doi.org/10.14219/jada.archive.1991.0098. [DOI] [PubMed] [Google Scholar]
- 24.Percival RS, Challacombe SJ, Marsh PD. Flow rates of resting whole and stimulated parotid saliva in relation to age and gender. J Dent Res. 1994 Aug;73(8):1416–20. doi: 10.1177/00220345940730080401. DOI: https://doi.org/10.1177/00220345940730080401. [DOI] [PubMed] [Google Scholar]
- 25.Erlichman M. Patient selection criteria for electrostimulation of salivary production in the treatment of xerostomia secondary to Sjogren’s syndrome. Health Technol Assess Rep. 1990;(8):1–7. [PubMed] [Google Scholar]
- 26.Steller M, Chou L, Daniels TE. Electrical stimulation of salivary flow in patients with Sjögren’s syndrome. J Dent Res. 1988 Oct;67(10):1334–7. doi: 10.1177/00220345880670101701. DOI: https://doi.org/10.1177/00220345880670101701. [DOI] [PubMed] [Google Scholar]