Abstract
Thirty years ago Robert F. Furchgott concluded that nitric oxide, a compound traditionally known to be a toxic component of fuel exhaust, is in fact released from the endothelium, and in a paracrine fashion, induces relaxation of underlying vascular smooth muscle resulting in vasodilation. This discovery has helped pave the way for a more thorough understanding of vascular inter- and intracellular communication that supports the process of regulating regional perfusion to match local tissue oxygen demand. Vasoregulation is not only controlled by endothelial release of a diverse class of vasoactive compounds such as nitric oxide, arachidonic acid metabolites, and reactive oxygen species, but also via physical forces on the vascular wall and through electrotonic conduction through gap junctions. Although the endothelium is a critical source of vasoactive compounds, paracrine mediators can also be released from surrounding parenchyma such as perivascular fat, myocardium, and cells in the arterial adventitia to exert either local or remote vasomotor effects. The focus of this review will highlight the various means by which intercellular communication contributes to mechanisms of vasodilation. Paracrine signaling and parenchymal influences will be reviewed as well as regional vessel communication via gap junctions, connexons, and myoendothelial feedback. More recent modes of communication such as vesicular and microRNA signaling will also be discussed.
Introduction
The key function of the microcirculation is to supply oxygen (O2) and nutrients to parenchymal tissue while removing metabolic waste. A vascular network comprised of microvessels, including small arterioles (30-150μm in diameter), capillaries, and venules is primarily responsible for these tasks. The microcirculation is ubiquitous, required to be in contact with each living cell in the human body.(1) The majority of flow regulation in the heart and other tissues is provided by adjustments in arteriolar vascular smooth muscle tone with only a small contribution from larger conduit arteries. Due to its high basal level of oxygen extraction, the heart is particularly sensitive to adjustments in perfusion, thus it is not surprising that multiple mechanisms exist for fine-tuning arteriolar resistance to tightly control coronary flow. To achieve this degree of control, vessels are capable of sensing signals within the vessel lumen, as well as external environment cues (e.g. neural, metabolic, and mechanical activity) to adjust vascular tone.(2) The overall control of vascular tone relies heavily on proper synchronization via an organized network of communication within the vascular wall.(3)
Furchgott is credited with defining the endothelium as a critical regulator of vascular tone. Being the innermost layer of the vessel wall, the endothelium can quickly sense and respond to changes in circulating humoral factors as well as physical effects of blood flow itself, resulting in signal transmission to the underlying smooth muscle cells to adjust vascular tone.(4) While the list of endothelial-derived diffusible vasoactive agents continues to expand, the paradigm is shifting from one where autocoids released from the endothelium act on vascular smooth muscle cells, to a more globally coordinated model involving electrotonic and mechanical communication along and through the vascular wall. Control of vascular tone operates in a larger arena with multiple inputs from adjacent vascular segments. An example is “ascending dilation,” which electrically propagates longitudinally up and downstream from a local dilator response via gap junctions.(5) Likewise, electrical communication occurs in the radial direction between endothelial and smooth muscle cells via myoendothelial projections, extensions of endothelial cells which protrude through holes in the internal elastic lamina and abut smooth muscle cell membranes.(6)
All layers of the cell wall participate in regulating arteriolar vasomotion. Recent evidence suggests that paracrine mediators released from neighboring adipose tissue, myocardium, and skeletal muscle also contribute to the control of vascular tone. This review will highlight the various means of vascular intercellular messaging and discuss how each mechanism specifically contributes to the regulation of microcirculatory tone and thereby tissue perfusion.
Endothelial-Derived Paracrine Mediators
The endothelium is the largest continuous layer of cells in the body and serves as a critical interface between flowing blood and the static vascular wall. The endothelial lining is responsive to shear stress, and through mechanotransduction releases vasomotor factors or hyperpolarizes sarcolemmal membranes to actively modulate contractile activity of underlying smooth muscle in regulating flow.(7) This phenomenon, now referred to as endothelial-dependent vasodilation, was first identified by Robert F. Furchgott through a now classic series of elegant experiments demonstrating that acetylcholine (ACh) elicits vasodilation in an endothelium-dependent manner in rabbit aortic rings.(8) That study launched a massive search for and identification by innumerable laboratories of many other endothelium-dependent dilator stimuli. In many cases the endothelial-dependent smooth muscle relaxation was due to a transferrable substance released from the endothelium acting on the underlying smooth muscle, however in some cases direct hyperpolarization (via potassium channel opening) was responsible. The identification of a diffusible dilator factor was established using a bioassay or “sandwich” model where ACh was applied to a vessel with endothelium intact in close proximity to one denuded of endothelium.(8) Dilation of the denuded vessel ring in this situation but not when tested in isolation is indicative of a transferrable mediator of vasodilation.(8) These experiments confirmed the presence of an endothelium-derived relaxing factor, or EDRF which is now known to be nitric oxide (NO), a mediator that diffuses out of the endothelial cell and communicates with underlying smooth muscle in a paracrine manner to regulate vascular tone and ultimately organ perfusion.
Nitric Oxide
Thirty years later we now understand that multiple endothelial-derived vasodilatory compounds exist with the most prototypical substance being nitric oxide (NO) formed from the endothelial isoform of nitric oxide synthase (eNOS) by a variety of pathways resulting in phosphorylation (S1799) or an elevation in endothelial cell calcium.(9),(10) This gaseous mediator diffuses to the smooth muscle cell where it activates soluble guanylyl cyclase (sGC) to produce cyclic guanosine monophosphate (cGMP).(11) Increases in cGMP activate specific cGMP-dependent protein kinases (PKGs)(12), and initiates smooth muscle cell relaxation via multiple proposed mechanisms including alteration of membrane potential and intracellular calcium levels, activation of myosin light chain phosphatases, and even regulation of smooth muscle cell contraction through thin filament regulation.(13) The development of nitric oxide synthase (NOS) inhibitors such as NG-monomethyl l-arginine (L-NMMA) not only helped to verify the contribution of NO in endothelial-dependent vasodilation(14), but also laid the groundwork for experimental observations that hyperpolarization and dilation can occur with mediators other than NO.(15)
In addition to eNOS, other forms of NOS contribute to smooth muscle relaxation and subsequent vasodilation. Neuronal nitric oxide synthase (nNOS) initially was thought to only play a role in the cerebral vasculature. S-methyl-L-thiocitrulline (SMTC), a selective inhibitor of nNOS has been shown to decrease blood flow in the human forearm as well as the coronary circulation.(16) It is now known that vascular smooth muscle cells express nNOS and interestingly can contribute to vasodilation when eNOS is dysfunctional. (17) Also located in the vascular wall, inducible nitric oxide synthase (iNOS) contributes to the significant vasodilation observed during inflammatory processes such as septic shock which is primarily due to the overproduction of NO.(18)
Prostacyclin
In 1976, four years before Furchgott's pioneering report, Moncada et al. isolated an enzyme from rabbit and pig aortas that catalyzed the conversion of endoperoxides to an unstable substance they referred to as PGX that was capable of inhibiting platelet aggregation and relaxing mesenteric arteries.(19) PGX, known today as prostacyclin (PGI2), is an eicosanoid derivative of arachidonic acid (AA), and like NO has vasodilator properties which are mediated by activation of adenylyl cyclase leading to formation of cAMP and activation of PKA.(20),(21) Even though two Nobel prizes were awarded for the discovery of prostaglandin- and NO-mediated vasodilation (1982 and 1998, respectively), the critical role of the endothelium was first reported with NO and only later recognized for PGI2 by Moncada.(19) Through microscopic observations of the rabbit aorta, Moncada et al concluded that the endothelial surface of the arterial wall generates the majority of PGI2.(22) Following release from cell membranes via phospholipase A2 (PLA2), AA is metabolized by cyclooxygenase (COX) enzymes to generate PGI2 along with other eicosanoids.(23) Initial studies demonstrated that PGI2 is capable of activating the adenylyl cyclase/cyclic-AMP transduction system. It was then later discovered that PGI2 can also induce (19) hyperpolarization and relaxation of surrounding smooth muscle cells.(24) Although the most abundant prostaglandin in the vasculature is PGI2, additional vasodilator prostaglandins are derived from endothelial cells including PGD2 and PGE2,(25) which may compensate for loss of endothelial-dependent dilation. For instance it has been shown that the mediator of ACh-induced vasodilation transitions from NO to PGD2 in patients with inflammatory bowel disease. (26)
Endothelial derived hyperpolarizing factors (EDHF)
AA can also be metabolized by cytochrome P450 (CYP450) to form a mixture of 4 regioisomers of epoxyeicosatrienoic acid (EET; 5,6-, 8,9-, 11,12-, and 14,15-), EETs are one of the most widely studied endothelial derived hyperpolarizing factors.(27), (28) Responsible for dilation to bradykinin in arterioles from the human heart,(29) EETs contribute significantly to bovine conduit coronary dilation.(30) Importantly EETs can serve as a compensatory dilator mechanism when other endothelium-dependent dilator mediators such as NO, are compromised.(31) Through activation of smooth muscle large conductance Ca+2-activated potassium (BKCa) channels, EETs have been shown to vasodilate rat renal arteries(32) as well as cat cerebral arteries.(33) Activation of BKCa channels is achieved through increases in intracellular calcium sparks via opening of transient receptor potential (TRP) channels.(34) Interestingly, in human coronary endothelial cells AA but not EETs participate in activating TRP channels.(35)
Hydrogen Peroxide
In 2000, Matoba and colleagues observed that ACh-induced vasodilation and hyperpolarization were well preserved in eNOS-KO mice, however in the presence of catalase, an enzyme that converts hydrogen peroxide (H2O2) to oxygen and water, dilation was inhibited.(36) Further studies indicated that H2O2 was indeed an EDHF capable of inducing smooth muscle hyperpolarization, relaxation and vessel dilation in human, as well as in pig mesenteric arteries and canine coronary vessels.(37) Our lab has demonstrated that H2O2 contributes to FMD in coronary arterioles collected from patients diagnosed with CAD,(38) and further that the mitochondria is the primary source of H2O2 as evidenced by the fact that flow-mediated dilation (FMD) is greatly impaired in CAD arterioles in the presence of rotenone, a mitochondrial complex I inhibitor.(39) It is now accepted that H2O2, traditionally thought to be a destructive reactive oxygen species (ROS), is also an important endothelial-derived signaling molecule that is prominently active in the human microcirculation and acts as a compensatory mediator in patients with cardiovascular disease.(38) H2O2 is crucial in mediating dilation during shear stress, and contributes to the dilation evoked by agonists such as bradykinin (29). H2O2 importantly links myocardial metabolism to blood flow.(40) Thus the coronary arteriolar medial layer of smooth muscle is stimulated by H2O2 derived both from the endothelium during flow and from the underlying metabolic signal from the myocardium. While the exact mechanism of H2O2-induced dilation remains unclear, data supports numerous pathways as capable of mediating H2O2-induced dilation including actions on cGMP(41), cAMP(42), as well as various potassium channels (e.g. BKCa) channels(43), ATP-sensitive potassium (KATP) channels, and voltage-sensitive potassium (Kv) channels.(44)
Hydrogen Sulfide
More recently, hydrogen sulfide (H2S), a gaseous molecule similar to NO, has been identified as a EDHF.(45) Endothelial and smooth muscle H2S formation is catalyzed by either cystathionine β-synthase (CBS) and/or cystathionine γ-lyase (CSE) by converting l-cysteine to l-serine, and H2S.(46) Similar to NO, formation of H2S is dependent on the calcium-calmodulin system for activation of CSE.(47) Despite the controversial evidence that H2S can act as both an effective vasodilator as well as vasoconstrictor, studies show that the endothelial-derived version hyperpolarizes smooth muscle in a dose-dependent manner via activation of ATP-sensitive potassium (KATP) channels.(48) Other work has suggested a role for BKCa channels(49) as well as Kv channels, specifically Kv7.4. (50) Additional studies suggest that H2S-induced vasoconstriction versus dilation is dependent on physiological oxygen (O2) levels, where rapid contraction has been observed in the rat aorta with high O2 levels as opposed to vasorelaxation in low O2 environments. It is further believed that the vasoconstriction is primarily due to oxidation products formed between H2S and O2 rather than a direct effect of H2S alone. (51)
As can be appreciated, substantial variation and redundancy exists among mediators of endothelium-dependent dilation across species and vascular beds. Beyond their ability to modulate vascular tone, these released substances have broader paracrine effects, modulating other physiological processes including inflammation, proliferation, fibrosis, apoptosis, and thrombosis. (1) To achieve such broad effects, these mediators are capable of moving beyond the vascular wall to influence surrounding parenchymal cells. It is likely that the diversity in mediator compounds coordinates in paracrine fashion, both tissue perfusion and tissue function. The nature of compensatory dilator substances may contribute to such diversity in physiology and pathology. (52)
The K+ ion itself is considered an EDHF. Edwards et al in 1999 concluded that K+ transferred from endothelial to smooth muscle cells was in fact an EDHF.(53) The authors went on to show that gap junction inhibitors (carbenoxolone and gap 27) eliminated ACh-induced smooth muscle hyperpolarization in carotid arteries indicating that gap junctions play a role in the radial propagation of electronic signals between the endothelium and medial layer of the vascular wall.(54) This work was expanded upon by Dora and colleagues who demonstrated that activation of SKCa channels in endothelial cells in response to ACh results in hyperpolarization of smooth muscle cells even in the presence of the NOS inhibitor, L-NG-Nitroarginine methyl ester (L-NAME).(55) Further, this group showed that carbenoxolone inhibits endothelial-dependent smooth muscle hyperpolarization and subsequent relaxation in mouse mesenteric arteries.(56)
Non-Endothelial-Derived Paracrine Communication
Parenchymal Cell Influences
Since Furchgott's findings in 1980, the intimal layer of the vessel wall has been viewed as a paracrine tissue, capable of forming and releasing various factors to influence the medial layer and thus overall tone (see above section). During this period of discovery the outermost layer, the adventitial layer, comprised of fibroblasts, collagen, nerve endings, and the vasa vasorum, was primarily considered structural support for the blood vessel.(57) This view was changed by innovative studies demonstrating in vivo transfer and expression of recombinant eNOS in the adventitia of canine cerebral arteries.(58) Later studies would show improved vessel relaxation in both normal and atherosclerotic cerebral arteries indicating that adventitial cells can possibly compensate for loss of endothelium-derived NO.(59, 60) What has emerged from these and other studies is that adventitial fibroblasts are able to regulate vascular function through release of vasoactive mediators and may compensate for decreased endothelial delivery of NO during times of stress or disease.(61) On the contrary, the adventitia can also impair NO-mediated vasorelaxation. This was demonstrated by Pagano and colleagues who showed that NADPH oxidase activity in the rabbit aorta adventitial layer was capable of generating superoxide, (O2· -) thus decreasing NO bioavailability.(62) Interestingly, eNOS gene transfer to the adventitial layer of rabbit carotid arteries decreases the contractile response to phenylephrine and may serve as a novel therapeutic strategy for cardiovascular disease where NO bioavailability is diminished.(63)
Vasoactive compounds also arise from plasma cells which have a critical role in the inflammatory process and can trigger significant dilation throughout the vasculature. (64) For instance histamine and serotonin are both considered vasoactive amines that cause profound dilation once released from mast cells and/or platelets. Substance P, a vasoactive peptide, is released from secretory vesicles found in sensory neurons and is also capable of vasodilation as well as triggering mast cell degranulation. (64)
Perivascular Fat
Perivascular adipose tissue (PVAT) was considered primarily a structural feature of arteries until Soltis and Cassis showed that the presence of PVAT decreased noradrenaline-induced constriction in rat aortic rings.(65) However interest in this novel paracrine peri-vascular region did not gain traction until ten years later when perivascular inflammation was observed following angioplasty in porcine coronary arteries.(66) Broad support exists for the idea that PVAT contributes to endothelial dysfunction and reduced vascular tone in subjects with cardiovascular disease.(67-69) Similar to the endothelium, multiple PVAT-derived relaxing factors (PVRF) have emerged as being responsible for vasodilation. In 2007 Fesus et al demonstrated using an adiponectin knock-out (Apn 1-/-) rat model that adiponectin, a protein secreted by adipocytes (“adipokine)” is a vasodilator of rat aorta and mesenteric arteries.(70) The cholesterol-lowering lipophilic HMG-coA reductase inhibitor Atorvastatin activates release of H2S from PVAT causing subsequent relaxation in denuded rat aortic vessels.(71) Angiotensin 1-7 (Ang 1-7) was identified immunohistochemically in rat aortic PVAT where it stimulates an endothelium-dependent vasodilation inhibitable by A779, an antagonist of the Ang1-7 endothelial cell receptor (Mas receptor).(72) Using rat aortic rings, Gao et al demonstrated that anti-contractile effects of PVAT involve the release of NO and H2O2 (73). PGI2 and palmitic acid methyl esters have also emerged as potential PVRFs.(74, 75)
Mechanisms through which PVRFs induce smooth muscle hyperpolarization and relaxation remain unclear. PVAT-derived H2O2 may directly activate sGC in smooth muscle cells to initiate relaxation however evidence also suggests that PVRF activates endothelial cells to release NO with downstream K+ channel activation. Scavenging of NO, NOS inhibition, high extracellular K+, and inhibition of KCa channels block PVAT-dependent vasodilation.(76) As with EDRFs, the effect of PVRFs on vascular tone vary among species and may contribute to the heterogeneity observed between vascular beds. For example, peripheral vessel PVAT primarily promotes vasorelaxation whereas coronary PVAT impairs endothelial-dependent vasodilation and induces constriction.(77, 78) The nature of the fat depot may account for some of this variation since pericoronary adipose is much more pro-inflammatory than subcutaneous fat stores.(79) Although virtually all arteries are in contact with adipose tissue, the amount of PVAT at each specific artery can vary and may also account for variation. For instance coronary PVAT increases with age and is more abundant in men versus women. (80) As the majority of data has been derived from animal studies, additional studies in human tissue are required to fully understand the effect of PVAT on vascular tone.(81)
Metabolic Vasodilation
The most important and powerful regulator of coronary microvascular tone is cardiac metabolism. Coronary arterioles are designed to provide optimal second-to-second regulation of flow to meet the heart's metabolic demand.(82) This requires close communication between cardiac myocytes and the vasculature. In response to a stressor that increases local oxygen demand, vasoactive metabolites released from the myocardium act directly on coronary arterioles to increase local blood flow. In addition, the mechanism by which myocardial metabolism evokes an increase in local blood flow also involves remote signaling through shear stress. Myocardial metabolite-induced local arteriolar relaxation increases blood flow and dynamically shifts a larger percentage of overall vascular resistance upstream to larger arterioles and small arteries. The local increase in flow is also experienced by the upstream vessels serving the same myocardial area. In response to flow-induced shear stress the endothelium releases NO which promotes dilation. This further reduces overall vascular resistance specifically in the region of myocardium where metabolism is increased. In this way the metabolic vasodilation in the microcirculation recruits proximal vascular segments to further facilitate an increase in flow.
Regional Vessel Communication
Ascending Vasodilation and Gap Junctions
Following the first observation of remote vasodilation in the frog hindlimb by Krogh in the 1922,(83) a wealth of information has surfaced regarding the mechanisms involved in this tightly regulated process by which a local vasodilator stimulus is transmitted up- and down-stream. Elegant experiments performed by Segal and Duling in the late 1980's demonstrated that microiontophoretic application of ACh in the hamster cheek pouch resulted in local as well as propagated vasodilation. The observed dilations were bidirectional and blocked by inhibitors of gap junctions including hypertonic sucrose solution, CO2, octanol,(84) and carbenoxolone (54). The ensuing thirty years of experimentation have strengthened the hypothesis that gap junctions, bidirectional channels that connect the cytoplasm between adjacent cells, allow for a rapid means of communicating electrotonic impulses within the arteriole wall.(85)
Gap junctions permit direct transfer of molecules and ions between adjacent cells allowing for precise control and coordination of regional vascular tone.(86) For instance binding of ACh to the endothelium initiates production of inositol 1,4,5-trisphosphate (IP3) which subsequently releases Ca+2 from the endoplasmic reticulum to then stimulate Ca+2-activated potassium channels (KCa) in the plasma membrane. This induces a hyperpolarization that is conducted from endothelial cell to endothelial cells via gap junctions or radially into the medial layer through myoendothelial gap junctions. Vasodilation is ultimately achieved by the closure of voltage-gated Ca+2 channels.(87) Cell-to-cell contact is accomplished by the physical interaction between hemichannels or connexons, with each connexon containing six connexins (Cx).(88) Interestingly, connexin isoforms exhibit different cellular localization as well as substrate specificity, implying that these connections mediate specific signaling events.(89) Of the twenty known connexin isoforms, five are expressed in the vasculature, including Cx 32, 37, 40, 43, and 45.(90),(91) Using immunohistochemistry techniques, Van Kempen and colleagues have shown that these connexins are differentially expressed throughout the pig, rat, and bovine aortic wall and coronary arteries, with endothelial cell connxeons primarily composed of Cx37, while the majority of connexons in smooth muscle cells are comprised of Cx43 (92) which allow for the movement of Ca+2 between each cell. (93) The complexity of these channels in the vasculature has been confirmed with knock-out studies of specific connexins. Liao and colleagues have shown that deletion of endothelial Cx43 in mice results in hypotension,(94) whereas smooth muscle knockout of Cx43 in the same species initiates accelerated growth of the neointima and adventitia.(95) Interestingly, neointimal formation is reduced in mice following Cx43 deletion in all cell types that express this specific connexin.(96) Substantial evidence indicates that vasoactive compounds modulate gap junctions, and vice versa, both in vitro and in vivo.(97, 98) Prior studies suggest that NO can either enhance or inhibit gap junction communication based on Cx type and cellular location. In microvascular endothelial cell culture, NO inhibits Cx37 in a non-cGMP-dependent manner(99) that likely involves S-nitrosylation.(100) Studies in human umbilical vein endothelial cells (HUVECs) indicate that NO-induced protein kinase A activation increases formation of Cx40, however inhibition of NOS has no effect on Cx expression. In vivo studies support these findings and indicate that NO impairs Cx37-mediated communication.(97) Conversely, connexins can regulate eNOS activity with Cx37 and Cx40 decreasing eNOS activity, with Cx40 being required for proper enzyme expression.(101)
The effect of EETs on gap junctions remains unclear. That EETs increase cAMP levels supports the notion that EETs can enhance gap junction Cx activity via cAMP-dependent phosphorylation.(98) Conversely, H2O2 inhibits gap junction-mediated communication in rat epithelial cells through phosphorylation of Cx43. Effects of other vasoactive substances (e.g. PGI2, H2S, adenosine) on gap junction activity are largely unexplored but may play a role in fine vasomotor control.
Myoendothelial Projections
Myoendothelial projections (MEPs), endothelial cell protrusions that extend through the elastic lamina allowing contact with the underlying smooth muscle cell, act as an anatomical hub for gap junctions and intercellular communication across the basement membrane.(102) Under 100nm in diameter, their distribution is heterogeneous throughout the vasculature, with a higher concentration located in smaller caliber arteries as opposed to the larger proximal arteries.(103) To date, only Cx37, Cx40, and Cx43 have been identified within MEPs, with Cx40 shown to be involved in endothelial-derived hyperpolarization (EDH)-mediated vasodilation.(104) Variation in MEP distribution not only occurs within the vascular bed but between microvascular territories, species, and can act as a compensatory pathway for vasodilation with aging or during disease where NO bioavailability is decreased.(105) Also noteworthy, in addition to the traditional endothelium-to-smooth muscle cell signaling, MEPs may contribute to reverse signaling via myoendothelial feedback. In this situation, stimulated elevations in smooth muscle calcium are transmitted to the endothelium via MEPs resulting in endothelial hyperpolarization, which in turn acts as a brake by relaxing smooth muscle.(106)
Vesicular Communication
Microvesicles
In addition to the more classical forms of cell-to-cell communication (e.g. soluble mediators, gap junctions), microvesicles (MV), small particles (1 micron in diameter and lower) released by practically every cell type, have also emerged as a means for remote intercellular communication within the vasculature.(107) The concept of vesicles as tools contributing to cellular crosstalk was introduced decades ago.(108) Originally believed to be the result of cellular necrosis, more recent evidence suggests that vesicle release is a highly regulated active cellular process.(109) Different pathways lead to formation of vesicles of different sizes, and with varying content and membrane composition. Those between 500nm and 1μm in diameter are commonly referred to as ‘microparticles’ (MPs) whereas smaller vesicles, ranging approximately 50nm to 100nm are termed ‘exosomes.’ Collectively, these two populations are referred to as ‘microvesicles.’
Information regarding the formation of microvesicles has primarily been derived from experiments involving cultured cells. Over the last decade the list of stimuli identified to induce MV release has expanded greatly and includes shear stress,(110) plasminogen-activator inhibitor 1,(111) and tumor necrosis factor alpha.(112) Once in the extracellular space microvesicles can be broken up within minutes, or, circulate and travel to distant sites. These vesicles can circulate throughout the vasculature, activate transmembrane receptors on neighboring cells in an autocrine or paracrine manner, and bind to and release their contents into other cells.(113) The content is varied and can consist of proteins, lipids, and genetic material. Within the spheroid, proteomic studies performed on MPs from various cell types indicate the presence of proteins involved in cytoskeletal arrangement, adhesion, fusion, protein folding, and metabolism,(114, 115) suggesting a selective composition. Valadi et al showing that exosomes contain functional mRNA as well as non-coding microRNAs.(116) Circulating microRNA has recently entered the spotlight as a potential biomarker for numerous diseases, however questions have been raised pertaining to the stability of secreted microRNA. Microvesicles may provide a protected microenvironment for microRNA allowing them to travel long distances within the vasculature to target sites.(117)
Despite the fact that microvesicles can interact with other circulating cells, the majority of information exchange occurs at the endothelial cell level, thus these particles can have a tremendous impact on vascular homeostasis and overall tone utilizing the vasculature as a “tube system” for remote communication.(118) In fact a strong correlation exists between impaired FMD in patients with chronic disease and the elevation of MPs. Amabile and colleagues reported increased levels of endothelial-derived MPs in patients with chronic renal disease who also exhibited reduced FMD.(119) Likewise, microvesicles collected from patients during acute myocardial infarction abolished ACh-induced relaxation in aortic rings.(120)
Although many studies have shown that MPs can evoke endothelial dysfunction, data regarding the impact of exosomes on vascular function is lacking. Interestingly, Wu and colleagues demonstrated beneficial effects of exosomal transfer of microRNA in cardiac progenitor cells within ischemic myocardium.(121) Although the majority of studies to date have supported the role of microvesicles having a detrimental effect on the vasculature, emerging data indicates that vesicles are capable of eliciting a positive or negative paracrine functional effect depending on their content or origin. Microvesicles as a novel means of remote vascular cell communication is an exciting new area for investigation.
Mechanistic Cross-Talk and Vasodilation
The various means of intercellular communication described in this review allow for redundancy in mechanisms of vasodilation to ensure adequate delivery of blood in order to match the demand of the organ. This redundancy not only allows for compensatory pathways to emerge during times of stress or disease, but also allows for cross talk between local regulatory factors and regional vascular control, providing precise control over tissue perfusion. For instance increased tissue metabolism triggers dilation locally however blood flow to the region is limited without the concurrent upstream vessel dilation initiated by the release of vasoactive substances in response to an increase in flow. Although the initial dilation may be due to metabolic factors, FMD fine-tunes support of flow to that specific vascular bed. Likewise following an initial local hyperpolarization stimulus, gap junctions allow for the propagation of ions resulting in smooth muscle relaxation, or ascending dilation along the vasculature wall. And finally remote communication throughout the vasculature can be achieved via release and downstream engulfment of microvesicles. Together these mechanisms may create a hierarchy of dilator control working in sync to efficiently provide proper flow to the tissue bed.
Summary and Conclusions
As a result of Furchgott's original discovery of an EDRF, a new scientific domain has emerged describing a highly coordinated chemical-based system of communication within and along the vasculature, extending to adjacent tissues. This review highlights the various mechanisms by which vascular intercellular communication can impact vasomotor responses and tissue perfusion. This complex yet coordinated physiological process relies on multiple modalities including paracrine mediators, gap junctions, and myoendothelial feedback. Novel regulators of vascular tone continue to emerge including remote delivery of vasoactive compounds in discretely secreted vesicular packets. The vasculature not only responds to meet tissue metabolic demands, but also directly influences parenchymal cell processes, establishing blood vessels as dynamic components of an organ's health and functional capacity. It is increasingly important to consider vascular function when assessing or treating any disease process.
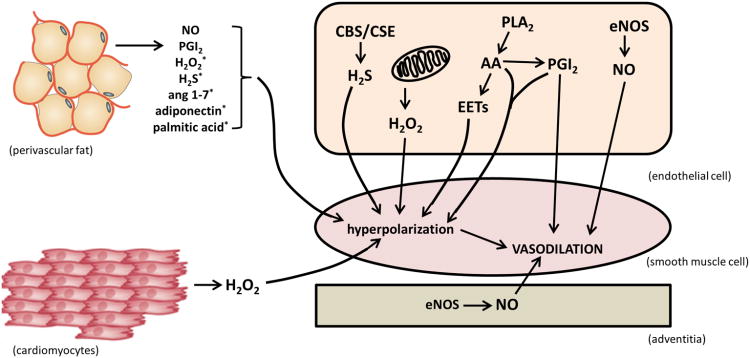
Fig. 1. Paracrine Signaling Resulting in Vasodilation.
Endothelial activation may lead to formation of NO, PGI2, AA, EETs, H2S, or H2O2 which subsequently cause smooth muscle cell relaxation through various signaling pathways or via membrane hyperpolarization. The adventitial layer also contributes through activation of eNOS and generation of NO. Cardiomyocytes generate H2O2 during metabolism that results in vessel relaxation. Perivascular fat is capable of producing multiple vasoactive compounds including NO, PGI2, H2S, H2O2, ang 1-7, adiponectin, and palmitic acid. *These compounds elicit vasodilation via membrane hyperpolarization.
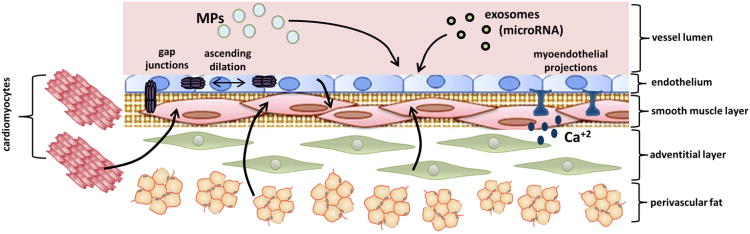
Fig. 2. Mechanisms of Intercellular Signaling Leading to Vasodilation.
Cell-to-cell communication can occur via endothelial and non-endothelial-derived (e.g. cardiomyocytes, perivascular fat, adventitial cells) paracrine mediators, gap junctions, myoendothelial projections, as well as via vesicular communication (e.g. microparticles, exosomes, circulating microRNA).
References
- 1.Gutterman DD, Chabowski DS, Kadlec AO, Durand MJ, Freed JK, Ait-Aissa K, Beyer AM. The Human Microcirculation: Regulation of Flow and Beyond. Circulation research. 2016 Jan 8;118(1):157–72. doi: 10.1161/CIRCRESAHA.115.305364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Welsh DG, Taylor MS. Cell-cell communication in the resistance vasculature: the past, present, and future. Microcirculation. 2012 Jul;19(5):377–8. doi: 10.1111/j.1549-8719.2012.00195.x. [DOI] [PubMed] [Google Scholar]
- 3.Segal SS, Duling BR. Flow control among microvessels coordinated by intercellular conduction. Science. 1986 Nov 14;234(4778):868–70. doi: 10.1126/science.3775368. [DOI] [PubMed] [Google Scholar]
- 4.Ellinsworth DC, Sandow SL, Shukla N, Liu Y, Jeremy JY, Gutterman DD. Endothelium-Derived Hyperpolarization and Coronary Vasodilation: Diverse and Integrated Roles of Epoxyeicosatrienoic Acids, Hydrogen Peroxide, and Gap Junctions. Microcirculation. 2016 Jan;23(1):15–32. doi: 10.1111/micc.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Segal SS. Integration of blood flow control to skeletal muscle: key role of feed arteries. Acta physiologica Scandinavica. 2000 Apr;168(4):511–8. doi: 10.1046/j.1365-201x.2000.00703.x. [DOI] [PubMed] [Google Scholar]
- 6.Ledoux J, Taylor MS, Bonev AD, Hannah RM, Solodushko V, Shui B, Tallini Y, Kotlikoff MI, Nelson MT. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proceedings of the National Academy of Sciences of the United States of America. 2008 Jul 15;105(28):9627–32. doi: 10.1073/pnas.0801963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vanhoutte PM. Endothelial dysfunction and vascular disease. Verhandelingen - Koninklijke Academie voor Geneeskunde van Belgie. 1998;60(3):251–66. [PubMed] [Google Scholar]
- 8.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–6. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 9.Ignarro LJ, Byrns RE, Buga GM, Wood KS, Chaudhuri G. Pharmacological evidence that endothelium-derived relaxing factor is nitric oxide: use of pyrogallol and superoxide dismutase to study endothelium-dependent and nitric oxide-elicited vascular smooth muscle relaxation. The Journal of pharmacology and experimental therapeutics. 1988 Jan;244(1):181–9. [PubMed] [Google Scholar]
- 10.Marletta MA. Nitric oxide: biosynthesis and biological significance. Trends in biochemical sciences. 1989 Dec;14(12):488–92. doi: 10.1016/0968-0004(89)90181-3. [DOI] [PubMed] [Google Scholar]
- 11.Arnold WP, Mittal CK, Katsuki S, Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′-cyclic monophosphate levels in various tissue preparations. Proceedings of the National Academy of Sciences of the United States of America. 1977 Aug;74(8):3203–7. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lohmann SM, Vaandrager AB, Smolenski A, Walter U, De Jonge HR. Distinct and specific functions of cGMP-dependent protein kinases. Trends in biochemical sciences. 1997 Aug;22(8):307–12. doi: 10.1016/s0968-0004(97)01086-4. [DOI] [PubMed] [Google Scholar]
- 13.Lincoln TM, Dey N, Sellak H. Invited review: cGMP-dependent protein kinase signaling mechanisms in smooth muscle: from the regulation of tone to gene expression. J Appl Physiol (1985) 2001 Sep;91(3):1421–30. doi: 10.1152/jappl.2001.91.3.1421. [DOI] [PubMed] [Google Scholar]
- 14.Rees DD, Palmer RM, Hodson HF, Moncada S. A specific inhibitor of nitric oxide formation from L-arginine attenuates endothelium-dependent relaxation. British journal of pharmacology. 1989 Feb;96(2):418–24. doi: 10.1111/j.1476-5381.1989.tb11833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen RA, Vanhoutte PM. Endothelium-dependent hyperpolarization. Beyond nitric oxide and cyclic GMP. Circulation. 1995 Dec 1;92(11):3337–49. doi: 10.1161/01.cir.92.11.3337. [DOI] [PubMed] [Google Scholar]
- 16.Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012 Apr;33(7):829–37. 37a–37d. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwarz PM, Kleinert H, Forstermann U. Potential functional significance of brain-type and muscle-type nitric oxide synthase I expressed in adventitia and media of rat aorta. Arteriosclerosis, thrombosis, and vascular biology. 1999 Nov;19(11):2584–90. doi: 10.1161/01.atv.19.11.2584. [DOI] [PubMed] [Google Scholar]
- 18.Lange M, Enkhbaatar P, Nakano Y, Traber DL. Role of nitric oxide in shock: the large animal perspective. Front Biosci (Landmark Ed) 2009 Jan 01;14:1979–89. doi: 10.2741/3357. [DOI] [PubMed] [Google Scholar]
- 19.Moncada S, Gryglewski R, Bunting S, Vane JR. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976 Oct 21;263(5579):663–5. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- 20.Parkington HC, Coleman HA, Tare M. Prostacyclin and endothelium-dependent hyperpolarization. Pharmacological research. 2004 Jun;49(6):509–14. doi: 10.1016/j.phrs.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 21.Kukovetz WR, Holzmann S, Wurm A, Poch G. Prostacyclin increases cAMP in coronary arteries. Journal of cyclic nucleotide research. 1979 Dec;5(6):469–76. [PubMed] [Google Scholar]
- 22.Moncada S. Prostaglandin endoperoxides and thromboxanes: formation and effects. Naunyn-Schmiedeberg's archives of pharmacology. 1977;297(1):S81–4. doi: 10.1007/BF00587788. [DOI] [PubMed] [Google Scholar]
- 23.Larsen BT, Gutterman DD, Hatoum OA. Emerging role of epoxyeicosatrienoic acids in coronary vascular function. European journal of clinical investigation. 2006 May;36(5):293–300. doi: 10.1111/j.1365-2362.2006.01634.x. [DOI] [PubMed] [Google Scholar]
- 24.Parkington HC, Tonta MA, Coleman HA, Tare M. Role of membrane potential in endothelium-dependent relaxation of guinea-pig coronary arterial smooth muscle. The Journal of physiology. 1995 Apr 15;484(Pt 2):469–80. doi: 10.1113/jphysiol.1995.sp020679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaborska KE, Wareing M, Austin C. Comparisons between perivascular adipose tissue and the endothelium in their modulation of vascular tone. British journal of pharmacology. 2016 Oct 16; doi: 10.1111/bph.13648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hatoum OA, Gauthier KM, Binion DG, Miura H, Telford G, Otterson MF, Campbell WB, Gutterman DD. Novel mechanism of vasodilation in inflammatory bowel disease. Arteriosclerosis, thrombosis, and vascular biology. 2005 Nov;25(11):2355–61. doi: 10.1161/01.ATV.0000184757.50141.8d. [DOI] [PubMed] [Google Scholar]
- 27.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circulation research. 1996 Mar;78(3):415–23. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- 28.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiological reviews. 2002 Jan;82(1):131–85. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 29.Larsen BT, Bubolz AH, Mendoza SA, Pritchard KA, Jr, Gutterman DD. Bradykinin-induced dilation of human coronary arterioles requires NADPH oxidase-derived reactive oxygen species. Arteriosclerosis, thrombosis, and vascular biology. 2009 May;29(5):739–45. doi: 10.1161/ATVBAHA.108.169367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosolowsky M, Campbell WB. Synthesis of hydroxyeicosatetraenoic (HETEs) and epoxyeicosatrienoic acids (EETs) by cultured bovine coronary artery endothelial cells. Biochimica et biophysica acta. 1996 Jan 19;1299(2):267–77. doi: 10.1016/0005-2760(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 31.Larsen BT, Gutterman DD, Sato A, Toyama K, Campbell WB, Zeldin DC, Manthati VL, Falck JR, Miura H. Hydrogen peroxide inhibits cytochrome p450 epoxygenases: interaction between two endothelium-derived hyperpolarizing factors. Circulation research. 2008 Jan 4;102(1):59–67. doi: 10.1161/CIRCRESAHA.107.159129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zou AP, Fleming JT, Falck JR, Jacobs ER, Gebremedhin D, Harder DR, Roman RJ. Stereospecific effects of epoxyeicosatrienoic acids on renal vascular tone and K(+)-channel activity. The American journal of physiology. 1996 May;270(5 Pt 2):F822–32. doi: 10.1152/ajprenal.1996.270.5.F822. [DOI] [PubMed] [Google Scholar]
- 33.Gebremedhin D, Ma YH, Falck JR, Roman RJ, VanRollins M, Harder DR. Mechanism of action of cerebral epoxyeicosatrienoic acids on cerebral arterial smooth muscle. The American journal of physiology. 1992 Aug;263(2 Pt 2):H519–25. doi: 10.1152/ajpheart.1992.263.2.H519. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003 Jul 24;424(6947):434–8. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- 35.Bubolz AH, Mendoza SA, Zheng X, Zinkevich NS, Li R, Gutterman DD, Zhang DX. Activation of endothelial TRPV4 channels mediates flow-induced dilation in human coronary arterioles: role of Ca2+ entry and mitochondrial ROS signaling. American journal of physiology Heart and circulatory physiology. 2012 Feb 1;302(3):H634–42. doi: 10.1152/ajpheart.00717.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kanaide H, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. The Journal of clinical investigation. 2000 Dec;106(12):1521–30. doi: 10.1172/JCI10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimokawa H. Hydrogen peroxide as an endothelium-derived hyperpolarizing factor. Pflugers Archiv : European journal of physiology. 2010 May;459(6):915–22. doi: 10.1007/s00424-010-0790-8. [DOI] [PubMed] [Google Scholar]
- 38.Miura H, Bosnjak JJ, Ning G, Saito T, Miura M, Gutterman DD. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circulation research. 2003 Feb 7;92(2):e31–40. doi: 10.1161/01.res.0000054200.44505.ab. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC, Gutterman DD. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circulation research. 2003 Sep 19;93(6):573–80. doi: 10.1161/01.RES.0000091261.19387.AE. [DOI] [PubMed] [Google Scholar]
- 40.Saitoh S, Zhang C, Tune JD, Potter B, Kiyooka T, Rogers PA, Knudson JD, Dick GM, Swafford A, Chilian WM. Hydrogen peroxide: a feed-forward dilator that couples myocardial metabolism to coronary blood flow. Arteriosclerosis, thrombosis, and vascular biology. 2006 Dec;26(12):2614–21. doi: 10.1161/01.ATV.0000249408.55796.da. [DOI] [PubMed] [Google Scholar]
- 41.Burke TM, Wolin MS. Hydrogen peroxide elicits pulmonary arterial relaxation and guanylate cyclase activation. The American journal of physiology. 1987 Apr;252(4 Pt 2):H721–32. doi: 10.1152/ajpheart.1987.252.4.H721. [DOI] [PubMed] [Google Scholar]
- 42.Iida Y, Katusic ZS. Mechanisms of cerebral arterial relaxations to hydrogen peroxide. Stroke; a journal of cerebral circulation. 2000 Sep;31(9):2224–30. doi: 10.1161/01.str.31.9.2224. [DOI] [PubMed] [Google Scholar]
- 43.Zhang DX, Borbouse L, Gebremedhin D, Mendoza SA, Zinkevich NS, Li R, Gutterman DD. H2O2-induced dilation in human coronary arterioles: role of protein kinase G dimerization and large-conductance Ca2+-activated K+ channel activation. Circulation research. 2012 Feb 3;110(3):471–80. doi: 10.1161/CIRCRESAHA.111.258871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park SW, Noh HJ, Sung DJ, Kim JG, Kim JM, Ryu SY, Kang K, Kim B, Bae YM, Cho H. Hydrogen peroxide induces vasorelaxation by enhancing 4-aminopyridine-sensitive Kv currents through S-glutathionylation. Pflugers Archiv : European journal of physiology. 2015 Feb;467(2):285–97. doi: 10.1007/s00424-014-1513-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang R. Hydrogen sulfide: a new EDRF. Kidney international. 2009 Oct;76(7):700–4. doi: 10.1038/ki.2009.221. [DOI] [PubMed] [Google Scholar]
- 46.Kamoun P. Endogenous production of hydrogen sulfide in mammals. Amino acids. 2004 Jun;26(3):243–54. doi: 10.1007/s00726-004-0072-x. [DOI] [PubMed] [Google Scholar]
- 47.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008 Oct 24;322(5901):587–90. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, Bhunia AK, Barodka VM, Gazi FK, Barrow RK, Wang R, Amzel LM, Berkowitz DE, Snyder SH. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circulation research. 2011 Nov 11;109(11):1259–68. doi: 10.1161/CIRCRESAHA.111.240242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jackson-Weaver O, Paredes DA, Gonzalez Bosc LV, Walker BR, Kanagy NL. Intermittent hypoxia in rats increases myogenic tone through loss of hydrogen sulfide activation of large-conductance Ca(2+)-activated potassium channels. Circulation research. 2011 Jun 10;108(12):1439–47. doi: 10.1161/CIRCRESAHA.110.228999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martelli A, Testai L, Breschi MC, Lawson K, McKay NG, Miceli F, Taglialatela M, Calderone V. Vasorelaxation by hydrogen sulphide involves activation of Kv7 potassium channels. Pharmacological research. 2013 Apr;70(1):27–34. doi: 10.1016/j.phrs.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 51.Koenitzer JR, Isbell TS, Patel HD, Benavides GA, Dickinson DA, Patel RP, Darley-Usmar VM, Lancaster JR, Jr, Doeller JE, Kraus DW. Hydrogen sulfide mediates vasoactivity in an O2-dependent manner. American journal of physiology Heart and circulatory physiology. 2007 Apr;292(4):H1953–60. doi: 10.1152/ajpheart.01193.2006. [DOI] [PubMed] [Google Scholar]
- 52.Durand MJ, Gutterman DD. Diversity in mechanisms of endothelium-dependent vasodilation in health and disease. Microcirculation. 2013 Apr;20(3):239–47. doi: 10.1111/micc.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998 Nov 19;396(6708):269–72. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- 54.Edwards G, Feletou M, Gardener MJ, Thollon C, Vanhoutte PM, Weston AH. Role of gap junctions in the responses to EDHF in rat and guinea-pig small arteries. British journal of pharmacology. 1999 Dec;128(8):1788–94. doi: 10.1038/sj.bjp.0703009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crane GJ, Gallagher N, Dora KA, Garland CJ. Small- and intermediate-conductance calcium-activated K+ channels provide different facets of endothelium-dependent hyperpolarization in rat mesenteric artery. The Journal of physiology. 2003 Nov 15;553(Pt 1):183–9. doi: 10.1113/jphysiol.2003.051896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dora KA, Sandow SL, Gallagher NT, Takano H, Rummery NM, Hill CE, Garland CJ. Myoendothelial gap junctions may provide the pathway for EDHF in mouse mesenteric artery. Journal of vascular research. 2003 Sep-Oct;40(5):480–90. doi: 10.1159/000074549. [DOI] [PubMed] [Google Scholar]
- 57.Fernandez-Alfonso MS. Regulation of vascular tone: the fat connection. Hypertension. 2004 Sep;44(3):255–6. doi: 10.1161/01.HYP.0000140056.64321.f9. [DOI] [PubMed] [Google Scholar]
- 58.Chen AF, Jiang SW, Crotty TB, Tsutsui M, Smith LA, O'Brien T, Katusic ZS. Effects of in vivo adventitial expression of recombinant endothelial nitric oxide synthase gene in cerebral arteries. Proceedings of the National Academy of Sciences of the United States of America. 1997 Nov 11;94(23):12568–73. doi: 10.1073/pnas.94.23.12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ooboshi H, Chu Y, Rios CD, Faraci FM, Davidson BL, Heistad DD. Altered vascular function after adenovirus-mediated overexpression of endothelial nitric oxide synthase. The American journal of physiology. 1997 Jul;273(1 Pt 2):H265–70. doi: 10.1152/ajpheart.1997.273.1.H265. [DOI] [PubMed] [Google Scholar]
- 60.Ooboshi H, Rios CD, Chu Y, Christenson SD, Faraci FM, Davidson BL, Heistad DD. Augmented adenovirus-mediated gene transfer to atherosclerotic vessels. Arteriosclerosis, thrombosis, and vascular biology. 1997 Sep;17(9):1786–92. doi: 10.1161/01.atv.17.9.1786. [DOI] [PubMed] [Google Scholar]
- 61.Gutterman DD. Adventitia-dependent influences on vascular function. The American journal of physiology. 1999 Oct;277(4 Pt 2):H1265–72. doi: 10.1152/ajpheart.1999.277.4.H1265. [DOI] [PubMed] [Google Scholar]
- 62.Pagano PJ, Clark JK, Cifuentes-Pagano ME, Clark SM, Callis GM, Quinn MT. Localization of a constitutively active, phagocyte-like NADPH oxidase in rabbit aortic adventitia: enhancement by angiotensin II. Proceedings of the National Academy of Sciences of the United States of America. 1997 Dec 23;94(26):14483–8. doi: 10.1073/pnas.94.26.14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kullo IJ, Mozes G, Schwartz RS, Gloviczki P, Crotty TB, Barber DA, Katusic ZS, O'Brien T. Adventitial gene transfer of recombinant endothelial nitric oxide synthase to rabbit carotid arteries alters vascular reactivity. Circulation. 1997 Oct 7;96(7):2254–61. doi: 10.1161/01.cir.96.7.2254. [DOI] [PubMed] [Google Scholar]
- 64.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008 Jul 24;454(7203):428–35. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 65.Soltis EE, Cassis LA. Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clinical and experimental hypertension Part A, Theory and practice. 1991;13(2):277–96. doi: 10.3109/10641969109042063. [DOI] [PubMed] [Google Scholar]
- 66.Okamoto E, Couse T, De Leon H, Vinten-Johansen J, Goodman RB, Scott NA, Wilcox JN. Perivascular inflammation after balloon angioplasty of porcine coronary arteries. Circulation. 2001 Oct 30;104(18):2228–35. doi: 10.1161/hc4301.097195. [DOI] [PubMed] [Google Scholar]
- 67.Takemori K, Gao YJ, Ding L, Lu C, Su LY, An WS, Vinson C, Lee RM. Elevated blood pressure in transgenic lipoatrophic mice and altered vascular function. Hypertension. 2007 Feb;49(2):365–72. doi: 10.1161/01.HYP.0000255576.16089.b9. [DOI] [PubMed] [Google Scholar]
- 68.Lu C, Su LY, Lee RM, Gao YJ. Alterations in perivascular adipose tissue structure and function in hypertension. European journal of pharmacology. 2011 Apr 10;656(1-3):68–73. doi: 10.1016/j.ejphar.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 69.Marchesi C, Ebrahimian T, Angulo O, Paradis P, Schiffrin EL. Endothelial nitric oxide synthase uncoupling and perivascular adipose oxidative stress and inflammation contribute to vascular dysfunction in a rodent model of metabolic syndrome. Hypertension. 2009 Dec;54(6):1384–92. doi: 10.1161/HYPERTENSIONAHA.109.138305. [DOI] [PubMed] [Google Scholar]
- 70.Fesus G, Dubrovska G, Gorzelniak K, Kluge R, Huang Y, Luft FC, Gollasch M. Adiponectin is a novel humoral vasodilator. Cardiovascular research. 2007 Sep 1;75(4):719–27. doi: 10.1016/j.cardiores.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 71.Wojcicka G, Jamroz-Wisniewska A, Atanasova P, Chaldakov GN, Chylinska-Kula B, Beltowski J. Differential effects of statins on endogenous H2S formation in perivascular adipose tissue. Pharmacological research. 2011 Jan;63(1):68–76. doi: 10.1016/j.phrs.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 72.Lee RM, Lu C, Su LY, Gao YJ. Endothelium-dependent relaxation factor released by perivascular adipose tissue. Journal of hypertension. 2009 Apr;27(4):782–90. doi: 10.1097/HJH.0b013e328324ed86. [DOI] [PubMed] [Google Scholar]
- 73.Gao YJ, Lu C, Su LY, Sharma AM, Lee RM. Modulation of vascular function by perivascular adipose tissue: the role of endothelium and hydrogen peroxide. British journal of pharmacology. 2007 Jun;151(3):323–31. doi: 10.1038/sj.bjp.0707228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee YC, Chang HH, Chiang CL, Liu CH, Yeh JI, Chen MF, Chen PY, Kuo JS, Lee TJ. Role of perivascular adipose tissue-derived methyl palmitate in vascular tone regulation and pathogenesis of hypertension. Circulation. 2011 Sep 6;124(10):1160–71. doi: 10.1161/CIRCULATIONAHA.111.027375. [DOI] [PubMed] [Google Scholar]
- 75.Chang L, Villacorta L, Li R, Hamblin M, Xu W, Dou C, Zhang J, Wu J, Zeng R, Chen YE. Loss of perivascular adipose tissue on peroxisome proliferator-activated receptor-gamma deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation. 2012 Aug 28;126(9):1067–78. doi: 10.1161/CIRCULATIONAHA.112.104489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brown NK, Zhou Z, Zhang J, Zeng R, Wu J, Eitzman DT, Chen YE, Chang L. Perivascular adipose tissue in vascular function and disease: a review of current research and animal models. Arteriosclerosis, thrombosis, and vascular biology. 2014 Aug;34(8):1621–30. doi: 10.1161/ATVBAHA.114.303029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Owen MK, Noblet JN, Sassoon DJ, Conteh AM, Goodwill AG, Tune JD. Perivascular adipose tissue and coronary vascular disease. Arteriosclerosis, thrombosis, and vascular biology. 2014 Aug;34(8):1643–9. doi: 10.1161/ATVBAHA.114.303033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Owen MK, Witzmann FA, McKenney ML, Lai X, Berwick ZC, Moberly SP, Alloosh M, Sturek M, Tune JD. Perivascular adipose tissue potentiates contraction of coronary vascular smooth muscle: influence of obesity. Circulation. 2013 Jul 2;128(1):9–18. doi: 10.1161/CIRCULATIONAHA.112.001238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee HY, Despres JP, Koh KK. Perivascular adipose tissue in the pathogenesis of cardiovascular disease. Atherosclerosis. 2013 Oct;230(2):177–84. doi: 10.1016/j.atherosclerosis.2013.07.037. [DOI] [PubMed] [Google Scholar]
- 80.Iozzo P. Myocardial, perivascular, and epicardial fat. Diabetes care. 2011 May;34(2):S371–9. doi: 10.2337/dc11-s250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ozen G, Daci A, Norel X, Topal G. Human perivascular adipose tissue dysfunction as a cause of vascular disease: Focus on vascular tone and wall remodeling. European journal of pharmacology. 2015 Nov 5;766:16–24. doi: 10.1016/j.ejphar.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 82.Duncker DJ, Koller A, Merkus D, Canty JM., Jr Regulation of coronary blood flow in health and ischemic heart disease. Progress in cardiovascular diseases. 2015 Mar-Apr;57(5):409–22. doi: 10.1016/j.pcad.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Krogh A, Harrop GA, Rehberg PB. Studies on the physiology of capillaries: III. The innervation of the blood vessels in the hind legs of the frog. The Journal of physiology. 1922 May 16;56(3-4):179–89. doi: 10.1113/jphysiol.1922.sp002000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Segal SS, Duling BR. Conduction of vasomotor responses in arterioles: a role for cell-to-cell coupling? The American journal of physiology. 1989 Mar;256(3 Pt 2):H838–45. doi: 10.1152/ajpheart.1989.256.3.H838. [DOI] [PubMed] [Google Scholar]
- 85.Evans WH, Martin PE. Gap junctions: structure and function (Review) Molecular membrane biology. 2002 Apr-Jun;19(2):121–36. doi: 10.1080/09687680210139839. [DOI] [PubMed] [Google Scholar]
- 86.Figueroa XF, Duling BR. Gap junctions in the control of vascular function. Antioxidants & redox signaling. 2009 Feb;11(2):251–66. doi: 10.1089/ars.2008.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bagher P, Segal SS. Regulation of blood flow in the microcirculation: role of conducted vasodilation. Acta Physiol (Oxf) 2011 Jul;202(3):271–84. doi: 10.1111/j.1748-1716.2010.02244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiological reviews. 2003 Oct;83(4):1359–400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- 89.White TW. Nonredundant gap junction functions. News in physiological sciences : an international journal of physiology produced jointly by the International Union of Physiological Sciences and the American Physiological Society. 2003 Jun;18:95–9. doi: 10.1152/nips.01430.2002. [DOI] [PubMed] [Google Scholar]
- 90.Haefliger JA, Nicod P, Meda P. Contribution of connexins to the function of the vascular wall. Cardiovascular research. 2004 May 1;62(2):345–56. doi: 10.1016/j.cardiores.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 91.Okamoto T, Akiyama M, Takeda M, Gabazza EC, Hayashi T, Suzuki K. Connexin32 is expressed in vascular endothelial cells and participates in gap-junction intercellular communication. Biochemical and biophysical research communications. 2009 May 1;382(2):264–8. doi: 10.1016/j.bbrc.2009.02.148. [DOI] [PubMed] [Google Scholar]
- 92.van Kempen MJ, Jongsma HJ. Distribution of connexin37, connexin40 and connexin43 in the aorta and coronary artery of several mammals. Histochemistry and cell biology. 1999 Dec;112(6):479–86. doi: 10.1007/s004180050432. [DOI] [PubMed] [Google Scholar]
- 93.Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Hori M, Tada M. Intercellular calcium signaling via gap junction in connexin-43-transfected cells. The Journal of biological chemistry. 1998 Jan 16;273(3):1519–28. doi: 10.1074/jbc.273.3.1519. [DOI] [PubMed] [Google Scholar]
- 94.Liao Y, Day KH, Damon DN, Duling BR. Endothelial cell-specific knockout of connexin 43 causes hypotension and bradycardia in mice. Proceedings of the National Academy of Sciences of the United States of America. 2001 Aug 14;98(17):9989–94. doi: 10.1073/pnas.171305298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liao Y, Regan CP, Manabe I, Owens GK, Day KH, Damon DN, Duling BR. Smooth muscle-targeted knockout of connexin43 enhances neointimal formation in response to vascular injury. Arteriosclerosis, thrombosis, and vascular biology. 2007 May;27(5):1037–42. doi: 10.1161/ATVBAHA.106.137182. [DOI] [PubMed] [Google Scholar]
- 96.Chadjichristos CE, Matter CM, Roth I, Sutter E, Pelli G, Luscher TF, Chanson M, Kwak BR. Reduced connexin43 expression limits neointima formation after balloon distension injury in hypercholesterolemic mice. Circulation. 2006 Jun 20;113(24):2835–43. doi: 10.1161/CIRCULATIONAHA.106.627703. [DOI] [PubMed] [Google Scholar]
- 97.Looft-Wilson RC, Billaud M, Johnstone SR, Straub AC, Isakson BE. Interaction between nitric oxide signaling and gap junctions: effects on vascular function. Biochimica et biophysica acta. 2012 Aug;1818(8):1895–902. doi: 10.1016/j.bbamem.2011.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ellinsworth DC, Earley S, Murphy TV, Sandow SL. Endothelial control of vasodilation: integration of myoendothelial microdomain signalling and modulation by epoxyeicosatrienoic acids. Pflugers Archiv : European journal of physiology. 2014 Mar;466(3):389–405. doi: 10.1007/s00424-013-1303-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McKinnon RL, Bolon ML, Wang HX, Swarbreck S, Kidder GM, Simon AM, Tyml K. Reduction of electrical coupling between microvascular endothelial cells by NO depends on connexin37. American journal of physiology Heart and circulatory physiology. 2009 Jul;297(1):H93–H101. doi: 10.1152/ajpheart.01148.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Straub AC, Billaud M, Johnstone SR, Best AK, Yemen S, Dwyer ST, Looft-Wilson R, Lysiak JJ, Gaston B, Palmer L, Isakson BE. Compartmentalized connexin 43 s-nitrosylation/denitrosylation regulates heterocellular communication in the vessel wall. Arteriosclerosis, thrombosis, and vascular biology. 2011 Feb;31(2):399–407. doi: 10.1161/ATVBAHA.110.215939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alonso F, Boittin FX, Beny JL, Haefliger JA. Loss of connexin40 is associated with decreased endothelium-dependent relaxations and eNOS levels in the mouse aorta. American journal of physiology Heart and circulatory physiology. 2010 Nov;299(5):H1365–73. doi: 10.1152/ajpheart.00029.2010. [DOI] [PubMed] [Google Scholar]
- 102.Straub AC, Zeigler AC, Isakson BE. The myoendothelial junction: connections that deliver the message. Physiology (Bethesda) 2014 Jul;29(4):242–9. doi: 10.1152/physiol.00042.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sandow SL, Hill CE. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circulation research. 2000 Feb 18;86(3):341–6. doi: 10.1161/01.res.86.3.341. [DOI] [PubMed] [Google Scholar]
- 104.Mather S, Dora KA, Sandow SL, Winter P, Garland CJ. Rapid endothelial cell-selective loading of connexin 40 antibody blocks endothelium-derived hyperpolarizing factor dilation in rat small mesenteric arteries. Circulation research. 2005 Aug 19;97(4):399–407. doi: 10.1161/01.RES.0000178008.46759.d0. [DOI] [PubMed] [Google Scholar]
- 105.Sandow SL, Haddock RE, Hill CE, Chadha PS, Kerr PM, Welsh DG, Plane F. What's where and why at a vascular myoendothelial microdomain signalling complex. Clinical and experimental pharmacology & physiology. 2009 Jan;36(1):67–76. doi: 10.1111/j.1440-1681.2008.05076.x. [DOI] [PubMed] [Google Scholar]
- 106.Nagaraja S, Kapela A, Tran CH, Welsh DG, Tsoukias NM. Role of microprojections in myoendothelial feedback--a theoretical study. The Journal of physiology. 2013 Jun 1;591(Pt 11):2795–812. doi: 10.1113/jphysiol.2012.248948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney international. 2010 Nov;78(9):838–48. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- 108.Bastida E, Ordinas A, Escolar G, Jamieson GA. Tissue factor in microvesicles shed from U87MG human glioblastoma cells induces coagulation, platelet aggregation, and thrombogenesis. Blood. 1984 Jul;64(1):177–84. [PubMed] [Google Scholar]
- 109.Beaudoin AR, Grondin G. Shedding of vesicular material from the cell surface of eukaryotic cells: different cellular phenomena. Biochimica et biophysica acta. 1991 Nov 13;1071(3):203–19. doi: 10.1016/0304-4157(91)90014-n. [DOI] [PubMed] [Google Scholar]
- 110.Vion AC, Ramkhelawon B, Loyer X, Chironi G, Devue C, Loirand G, Tedgui A, Lehoux S, Boulanger CM. Shear stress regulates endothelial microparticle release. Circulation research. 2013 May 10;112(10):1323–33. doi: 10.1161/CIRCRESAHA.112.300818. [DOI] [PubMed] [Google Scholar]
- 111.Brodsky SV, Malinowski K, Golightly M, Jesty J, Goligorsky MS. Plasminogen activator inhibitor-1 promotes formation of endothelial microparticles with procoagulant potential. Circulation. 2002 Oct 29;106(18):2372–8. doi: 10.1161/01.cir.0000033972.90653.af. [DOI] [PubMed] [Google Scholar]
- 112.Peterson DB, Sander T, Kaul S, Wakim BT, Halligan B, Twigger S, Pritchard KA, Jr, Oldham KT, Ou JS. Comparative proteomic analysis of PAI-1 and TNF-alpha-derived endothelial microparticles. Proteomics. 2008 Jun;8(12):2430–46. doi: 10.1002/pmic.200701029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends in cell biology. 2009 Feb;19(2):43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 114.Banfi C, Brioschi M, Wait R, Begum S, Gianazza E, Pirillo A, Mussoni L, Tremoli E. Proteome of endothelial cell-derived procoagulant microparticles. Proteomics. 2005 Nov;5(17):4443–55. doi: 10.1002/pmic.200402017. [DOI] [PubMed] [Google Scholar]
- 115.Sander TL, Ou JS, Densmore JC, Kaul S, Matus I, Twigger S, Halligan B, Greene AS, Pritchard KA, Jr, Oldham KT. Protein composition of plasminogen activator inhibitor type 1-derived endothelial microparticles. Shock. 2008 Apr;29(4):504–11. doi: 10.1097/shk.0b013e3181454898. [DOI] [PubMed] [Google Scholar]
- 116.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature cell biology. 2007 Jun;9(6):654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 117.Chen X, Liang H, Zhang J, Zen K, Zhang CY. Secreted microRNAs: a new form of intercellular communication. Trends in cell biology. 2012 Mar;22(3):125–32. doi: 10.1016/j.tcb.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 118.Martinez MC, Tesse A, Zobairi F, Andriantsitohaina R. Shed membrane microparticles from circulating and vascular cells in regulating vascular function. American journal of physiology Heart and circulatory physiology. 2005 Mar;288(3):H1004–9. doi: 10.1152/ajpheart.00842.2004. [DOI] [PubMed] [Google Scholar]
- 119.Amabile N, Guerin AP, Leroyer A, Mallat Z, Nguyen C, Boddaert J, London GM, Tedgui A, Boulanger CM. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. Journal of the American Society of Nephrology : JASN. 2005 Nov;16(11):3381–8. doi: 10.1681/ASN.2005050535. [DOI] [PubMed] [Google Scholar]
- 120.Boulanger CM, Scoazec A, Ebrahimian T, Henry P, Mathieu E, Tedgui A, Mallat Z. Circulating microparticles from patients with myocardial infarction cause endothelial dysfunction. Circulation. 2001 Nov 27;104(22):2649–52. doi: 10.1161/hc4701.100516. [DOI] [PubMed] [Google Scholar]
- 121.Ong SG, Lee WH, Huang M, Dey D, Kodo K, Sanchez-Freire V, Gold JD, Wu JC. Cross talk of combined gene and cell therapy in ischemic heart disease: role of exosomal microRNA transfer. Circulation. 2014 Sep 9;130(11 Suppl 1):S60–9. doi: 10.1161/CIRCULATIONAHA.113.007917. [DOI] [PMC free article] [PubMed] [Google Scholar]