Synopsis
Short stature is a common and heterogeneous condition which is often genetic in etiology. For most children with genetic short stature, the specific molecular causes remain unknown, but with advances in exome/genome sequencing and bioinformatics approaches, new genetic causes of growth disorders have been identified, contributing to the understanding of the underlying molecular mechanisms of longitudinal bone growth and growth failure. These genetic causes can involve not only hormonal deficiencies, including the growth hormone-IGF-1 axis, thyroid hormone or glucocorticoid and defects in hormonal receptors or subsequent signaling, but also defects in fundamental cellular processes (intracellular signaling pathways, transcriptional regulation, and DNA repair), extracellular matrix, or paracrine signaling. Especially, heterozygous and/or mild mutations in SHOX, NPR2, ACAN, IGF1, IGF1R, or FGFR3 have been associated with isolated short stature without other prominent or noticeable phenotype while homozygous and/or severe mutations in these genes cause severe short stature with bone malformation, that is, a chondrodysplasia. Identifying new genetic causes of growth disorders has the potential to improve diagnosis, prognostic accuracy, individualized management, and help avoid unnecessary testing for endocrine and other disorders.
Key Terms: Short stature, genetic causes, growth plate, genome wide association study, exome sequencing
Introduction
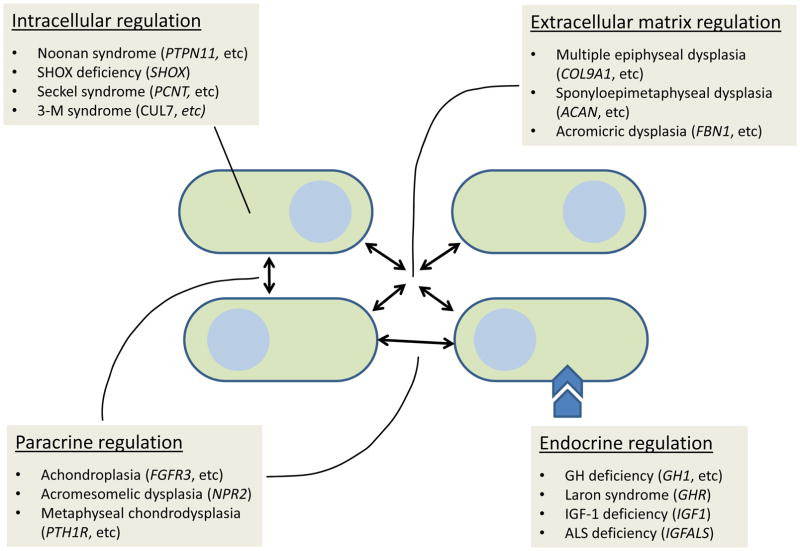
Short stature is a common medical concern that pediatricians and pediatric endocrinologists often evaluate in their daily practice since poor growth may be a symptom of an underlying, treatable medical condition [1]. Linear growth is the result of chondrogenesis at the growth plate and all forms of short stature are therefore due to decreased chondrogenesis at the growth plates [2]. Growth plate chondrogenesis and therefore linear growth are regulated by multiple systemic factors including nutritional intake, hormones, and inflammatory cytokines [3]. Consequently, systemic diseases, such as hypothyroidism, celiac disease and other chronic disorders impair childhood growth. In addition, growth plate chondrogenesis is regulated by multiple local factors, including intracellular regulatory mechanisms in the growth plate chondrocytes, cartilage extracellular matrix components, and paracrine factors in the growth plate. As a result, genetic defects in these local growth plate systems can also result in short stature (figure 1).
Figure 1.
Molecular mechanisms of short stature. Short stature is caused by multiple molecular defects including intracellular signaling, extracellular matrix, paracrine and endocrine regulation. Ovoid shapes represent growth plate chondrocytes. Arrows indicate mechanisms regulating chondrocytes. Examples of clinical syndrome and the genetic cause under different molecular mechanisms are shown in each box.
Height variation within the normal range involves similar mechanisms. In 2010, a genome wide association (GWA) study revealed 180 loci that explain approximately 10% of height variation [4] and a more recent GWA study identified about 400 loci that are associated with adult height in the general population [5]. It is likely that many children have mild short stature because they have inherited multiple polymorphisms each of which tends to slightly inhibit growth plate chondrogenesis and in fact, the loci implicated by GWA study are shown enriched in genes that are expressed and important for growth plate function [4–6]. Taken together, these findings suggest that normal growth is modulated by several hundred or maybe even thousands of genes that affect growth plate function. Therefore, polymorphism and mild mutations in these identified genes may modulate height within the normal range and perhaps cause mild polygenic short stature, whereas mutations with a stronger effect on protein function and/or biallelic mutations may cause significant monogenic short stature or skeletal dysplasias.
The high-throughput sequencing and bioinformatics approaches have enabled identification of genetic causes for many human disorders [7–8]. In particular, exome sequencing has been successfully applied to reveal genetic variants responsible for unknown causes of rare diseases [7–8]. Consequently, exome sequencing has become a powerful research tool to identify the etiology of disorders with a monogenic pattern of inheritance. Using this approach, an increasing number of monogenic causes of growth disorders are being identified, thereby gradually diminishing the number of children who receive the unhelpful diagnosis of “idiopathic” short stature. The GWA studies on height variation as well as the expanding genetic diagnoses of growth disorders indicate that childhood growth disorders are highly genetically heterogeneous [1–2] and that a large fraction of the genes important for growth are involved in cellular processes previously not implicated in regulation of growth. These findings will likely affect the way we diagnose childhood growth disorders. Previously, the approach to short stature was primarily focused on clinical manifestations, for example primordial dwarfism, syndromic short stature, or skeletal dysplasia to categorize them by similar clinical features. Currently, the combination of the clinical approach and improved genetic diagnosis is advancing our understanding of genetic growth disorders and has helped to further widen the understanding of the clinical variability and genetic heterogeneity of short stature syndromes.
Identifying and understanding the genetic basis of short stature will have significant impact on the care of children seeking medical attention for severe short stature. An accurate genetic diagnosis will guide management and help limit unnecessary testing, recognize associated health risks, enable proper genetic counseling, and will improve basic understanding of skeletal development and growth and may eventually lead to the development of new treatment approaches [1, 2]. Therefore, we here review genetic causes of growth disorders by the molecular mechanisms (Table1 and figure 1), emphasizing recently discovered causes of childhood growth disorders.
Table 1.
Overview of Genetics of Short Stature
| Genes | Function | Disorder | Key Clinical features | * GWA list |
|---|---|---|---|---|
| 1) Defects in intracellular pathways | ||||
| Intracellular signaling pathways | ||||
| FGD1 | Guanine nucleotide exchange factor | Aarskog-Scott (Faciogenital dysplasia) | IUGR, hypertelorism, ptosis, everted lower lip vermilion, joint hyper-extension, finger abnormalities, shawl scrotum [21–23] | No |
| PRKAR1A | Cyclic AMP (cAMP)-dependent regulatory subunit of protein kinase A | Acrodysostosis, type 1 | IUGR, skeletal dysplasia, severe brachydactyly, facial dysostosis, nasal hypoplasia, advanced bone age, obesity, hormone resistance [24] | No |
| PDE4D | Cyclic AMP-specific 3’, 5’-cyclic phospho-diesterase 4D | Acrodysostosis, type 2 | IUGR (variable), skeletal dysplasia, accelerated bone age progression, variable hormone resistance [25] | No |
| GNAS | G protein alpha subunit | Albright hereditary osteodystrophy | IUGR, obesity, round-shaped face, subcutaneous ossifications and brachymetacarpal bone (4th and 5th) [26–28] | Yes |
| RPS6KA3 | Serine/threonine kinase in RAS-MAPK pathway | Coffin-Lowry syndrome | No IUGR, microcephaly, facial dysmorphism, skeletal abnormalities, intellectual disability, hypotonia, X-linked disorder [9–10] | No |
| HRAS | Signal transduction with GTPase activity in RAS-MAPK pathway | Costello (faciocutaneoskeletal syndrome) | No IUGR, delayed development, intellectual disability, distinctive facial features, loose folds of extra skin (especially, hands and feet), flexible joints [11] | No |
| PTPN11, RAF1, BRAF | Protein-tyrosine phosphatase/RAS-MAP kinase regulation | Multiple lentigines syndrome (LEOPARD syndrome) | No IUGR, lentigines, hypertrophic myopathy, electro-cardiographic conduction abnormalities, ocular hypertelorism, pulmonic stenosis, abnormalities of genitalia, sensorineural deafness [12–16] | Yes (RAF1) |
| NF1 | RAS signal transduction | Neurofibromatosis type 1 | No IUGR, cafe-au-lait spot, malignancy (pheochromocytoma and gastrointestinal stromal tumor), Lisch nodules, osteoporosis [17–18] | No |
| PTPN11, BRAF, SOS1, KRAS, RAF1, NRAS, RASA2, SHOC2, CBL, RIT1 (activating) | Protein-tyrosine phosphatase/RAS-MAP kinase regulation | Noonan Syndrome or Noonan-like syndrome | No IUGR, distinctive facial appearance, a broad or webbed neck, congenital heart defects, coagulopathy, skeletal malformations, developmental delay [11, 19–20] | Yes (RAF1) |
| ROR2, WNT5A, DVL1 | Cell surface receptor, secreted signaling protein | Robinow syndrome (acral dysostosis with facial and genital abnormalities) | IUGR (variable), short-limb dwarfism, costovertebral segmentation defects, abnormalities of head, face and external genitalia, chest deformities, rib fusions, scoliosis, brachydactyly, aplasia/hypoplasia of the phalanges and metacarpal/metatarsal bones [29–32] | Yes (WNT5A) |
| Transcriptional regulation | ||||
| LARP7 | Transcriptional regulator of polymerase II genes | Alazami syndrome | IUGR (variable), facial dysmorphism (triangular face), intellectual disability, tendon or skeletal abnormalities [48–49] | No |
| SOX9 | Chondrocyte differentiation factor | Campomelic dysplasia | IUGR, born with bowing of the long bones, short legs, dislocated hips, ambiguous genitalia, distinctive facial features [39–41] | Yes |
| BRF1 | RNA polymerase III transcription initiation factor | Cerebello-facio-dental syndrome | IUGR, facial dysmorphism, hypoplastic cerebellum, markedly delayed bone age [50] | No |
| SOX11 | Transcriptional regulation of GDF5 | Coffin-Siris syndrome | IUGR (variable), mental retardation, facial dysmorphism, hearing or vision impairment, severe scoliosis [51] | No |
| MLL2 (KMT2D), KDM6A | Histone methyltransferase/3 Histone demethylase | Kabuki syndrome | IUGR (variable), facial features that resemble the make-up worn by actors of kabuki (long eye openings slanting upwards, arched eyebrows, prominent ears, and corners of the mouth turning downwards), mild to moderate intellectual disability, problems involving heart, skeleton, teeth, and immune system [45–47] | No |
| ANKRD11 | Transcription regulator | KBG syndrome | IUGR (variable), facial dysmorphism, hearing loss, congenital heart defect, skeletal anomalies, global developmental delay, seizures, intellectual disability [52–53] | No |
| SHOX | Transcription factor | Leri-Weill dyschondrosteosis, mesomelic dysplasia (Langer type) | No IUGR, skeletal dysplasia, Madelung deformity [42–44] | No |
| CREBBP, EP300 | Transcriptional coactivator | Rubinstein-Taybi syndrome | IUGR (variable), facial dysmorphism, moderate to severe intellectual disability, broad thumbs and first toes [54–55] | Yes (CREBBP) |
| DNA repair | ||||
| BLM | DNA repair enzyme | Bloom syndrome | IUGR (as case report), increased risk of cancer, sun-sensitive skin changes on face, hands and/or arms, a high-pitched voice, distinctive facial features including a long, narrow face, small lower jaw, large nose and prominent ears [72–73] | No |
| ERCC6, ERCC8 | DNA repair | Cockayne syndrome | IUGR (variable), microcephaly, photosensitivity progeroid appearance, progressive pigmentary retinopathy, sensorineural deafness [74–75] | No |
| FANCA, FANCC, FANCG | DNA repair | Fanconi anemia | IUGR, absence of thumb, hyperpigmentation, early onset bone marrow failure, predisposition to cancers [56] | Yes (FANCA) |
| LIG4 | DNA repair | LIG 4 syndrome | No IUGR, distinctive facial features, microcephaly, pancytopenia, various skin abnormalities, immune deficiency [69] | No |
| NSMCE2 | DNA repair | Microcephalic primordial dwarfism-insulin resistance syndrome | No IUGR reported, microcephaly, insulin resistance [76] | No |
| NBN (NBS1) | DNA repair | Nijmegan breakage syndrome | No IUGR, microcephaly, distinctive facial features, immunodeficiency, and cancer predisposition [70–71] | No |
| SMARCAL1 | DNA repair | Schimke immunoosseous dysplasia | IUGR, kidney disease, immune deficiency, stroke, bone marrow failure, kidney failure [77] | No |
| ATR, ATRIP, CENPJ, CEP152, CEP63, DNA2, PCNT, PLK4, RBBP8, XRCC4 | DNA repair, centrosome maintenance, DNA stability | Seckel syndrome | IUGR, microcephaly, beak-like protrusion of nose, facial dysmorphism [56–66] | Yes (DNA2) |
| Other fundamental cellular processes | ||||
| CUL7, OBSL1, CCDC8 | Microtubule stabilization and genome stability | 3-M syndrome | IUGR, facial dysmorphism (triangular face), relatively large head circumference, prominent fleshy heels [78–80] | No |
| ALMS1 | Microtubule organization | Alström syndrome | No IUGR, vision and hearing abnormalities, childhood obesity, diabetes mellitus, heart disease and slowly progressive kidney dysfunction [96] | No |
| SMARCB1, SMARCE1, SMARCA4, ARID1A, ARID1B | Chromatin remodeling | Coffin-Siris syndrome | IUGR (variable), mental retardation, facial dysmorphism, hearing or vision impairment, severe scoliosis [89] | Yes (ARD1B) |
| NIPBL (50%), SMC1A, HDAC8, RAD21, SMC3, | Cohesin pathway (sister chromatid cohesion) | Cornelia de Lange syndrome | IUGR, dysmorphic facial features (facial hirsutism), microcephaly, limb reduction defects, cardiac defect, and intellectual disability [81] | Yes (NIPBL) |
| SRCAP | Chromatin remodeling | Floating-Harbor syndrome | IUGR (variable), facial dysmorphism, abnormal thumb, delayed bone age, early puberty, delay in expressive language [86–88] | No |
| LMNA | Nuclear stability | Hutchinson-Gilford Progeria | No IUGR, failure to thrive, distinctive facial features (aged-looking skin), alopecia, loss of subcutaneous fat, joint abnormalities [82–83] | No |
| RNU4ATAC | Minor intron splicing | MOPD I | IUGR, microcephaly, dysmorphic face, skin and skeletal abnormalities, developmental delay [90–91] | No |
| PCNT | Mitotic spindle/chromosome segregation | MOPD II | IUGR, facial dysmorphism, microcephaly, near normal intelligence, cancer susceptibility [67–68] | No |
| TRIM37 | Persoxisomal protein, possibly ubiquitin-dependent degradation | Mulibrey nanism | IUGR, dysmorphic craniofacial features, heart disease (constrictive pericardium), hepatomegaly, Wilms tumor [92–94] | No |
| CRIPT | Interaction with cytoskeleton | Primordial dwarfism | IUGR (not established), facial dysmorphism, microcephaly, ophthalmological abnormalities, intellectual disabilities, skeletal abnormalities, pigmentation abnormalities [95] | No |
| POC1A | Centriole assembly/ciliogenesis | SOFT syndrome | IUGR, disproportionate short stature, onychodysplasia, facial dysmorphism, and hypotrichosis [84–85] | No |
| DHCR7 | Steroid biosynthesis | Smith-Lemli-Opitz syndrome | IUGR, distinctive facial features, microcephaly, intellectual disability or learning problems, behavioral problems, malformations of heart, lungs, kidneys, gastrointestinal tract, and genitalia [97] | No |
| 2) Defects in cartilage extracellular matrix | ||||
| COL2A1 | Extracellular matrix, collagen | Achondrogenesis (Type II), hypochondrogenesis, Kniest dysplasia, Spondylo-epiphyseal dysplasia congenita, Stickler syndrome type 1 | IUGR, skeletal abnormalities and problems with vision and hearing [101–102] | No |
| FBN1 | Extracellular matrix, fibrillin 1 | Acromicric dysplasia, Geleophysic dysplasia 2 | No IUGR, short hands and feet, thickened skin and joint contractures, limited range of motion in fingers, toes, wrists, and elbows, cardiac issue [109] | Yes |
| COL11A1 | Extracellular matrix, collagen 11 | Fibrochondrogenesis | IUGR (variable), skeletal dysplasia, broad long bone metaphyses, pear-shaped vertebral bodies, flat midface with a small nose and anteverted nares, significant shortening of all limb segments [103] | Yes |
| COL10A1 | Extracellular matrix, collagen 10 | Metaphyseal dysplasia, Schmid type | No IUGR, coxa vara, relatively short limbs, bow legs, waddling gait [104] | No |
| MATN3, COL9A1, COL9A2, COL9A3 | Extracellular matrix, cartilage oligomeric matrix protein, collagen, matrillin-3 | Multiple epiphyseal dysplasia | No IUGR, skeletal dysplasia, Joint pain, joint deformity, waddling gait [105–108, 112] | Yes (COL9A2) |
| COMP | Extracellular matrix, cartilage oligomeric matrix protein | Multiple epiphyseal dysplasia, Pseudoachondroplasia | No IUGR, short arms and legs, a waddling walk, early-onset joint pain (osteoarthritis), limited range of motion at elbows and hips [110–111] | No |
| HSPG2 | Extracellular matrix, perlecan | Schwartz-Jampel syndrome | IUGR (not established), permanent myotonia (prolonged failure of muscle relaxation), skeletal dysplasia, kyphoscoliosis, bowing of diaphyses and irregular epiphyses [115] | No |
| ACAN | Extracellular matrix, aggrecan | Sponyloepimetaphyseal dysplasia, aggrecan/Kimberly type | IUGR, macrocephaly, severe midface hypoplasia, short neck, barrel chest, brachydactyly, advanced bone age [113–114] | Yes |
| 3) Defects in paracrine signaling | ||||
| FGFR3 (activating) | Fibroblast growth factor receptor | Achondroplasia, hypochondroplasia | IUGR, short upper arms and thighs, limited range of motion at elbows, relative macrocephaly with a prominent forehead, trident hand [119–121] | No |
| IHH | Secreted signaling molecule, Indian hedgehog | Acrocapitofemoral dysplasia, Brachydactyly, type A1 | No IUGR, brachydactyly [129] | No |
| NPR2 (inactivating) | CNP receptor | Acromesomelic dysplasia, Maroteaux type | IUGR (variable), short limbs and hand/foot malformations [126] | Yes |
| BMPR1B, GDF5 | BMP receptor/interacting protein (ligand) | Brachydactyly, type A1 and A2 | No IUGR, brachydactyly [135–136] | Yes (GDF5) |
| PTHLH | Secreted signaling molecules (PTH-related protein) | Brachydactyly, type E2 | No IUGR, shortening of fingers mainly in metacarpals and metatarsals [130–131] | No |
| IGF2 | Secreted signaling molecule (insulin-like growth factor-II) | IGF2 deficiency | IUGR, Silver-Russel like facies [137] | No |
| PTH1R | PTH and PTHrP receptor | Metaphyseal chondrodysplasia (Jansen type), Eikan Dysplasia, Chondrodysplasia (Blomstrand type) | No IUGR, skeletal dysplasia, micrognathia, failure of tooth eruption, low-set/posteriorly rotated ears, proptosis [132–133] | No |
| 4) Defects in endocrine ligands, receptors, and signaling pathways | ||||
| IGFALS | Acid labile subunit | ALS deficiency | IUGR (variable), low IGF-1 and IGF-BP3 [148–149] | No |
| GH1, GHRHR, SOX3, BTK | Growth hormone production | GH deficiency | No IUGR, GH deficiency [138] | No |
| IGF1 | IGF-1 | IGF1 deficiency | IUGR, microcephaly, mental retardation, low IGF-1 level [143–144] | No |
| IGF1R | Insulin-like growth factor receptor | IGF-1 insensitivity | IUGR, normal to high IGF-1 level [145–147] | Yes |
| STAT5B | Growth hormone signaling | Immune deficiency and GH resistance | No IUGR, elevated random GH but low IGF-1 or IGFBP-3, immunodeficiency [142] | No |
| GHR | Growth hormone receptor | Laron syndrome | IUGR, elevated growth hormone and low IGF-1[139–141] | No |
670 height–associated SNPs according to GWA study by Wood et al [5]
Genetics of short stature
1) Defects in intracellular pathways
Mutations causing loss- or gain-of-function of proteins important for fundamental cellular processes often result in severe short stature with or without an obvious skeletal dysplasia. The mutations may also be associated with microcephaly, intellectual disability, distinctive facial features or other clinical abnormalities (Table 1).
Intracellular signaling pathways
Depending on the pathway affected, defects in intracellular signaling cause a wide spectrum in the degree of growth failure as well as in the conditions associated with each defect. The intracellular defects are highly heterogeneous, such as RAS (rat sarcoma)-MAPK (mitogen-activated protein kinase) pathway [9–20], guanine nucleotide exchange factor [21–23], cyclic AMP (cAMP) dependent regulatory subunit of protein kinase A [24], and other signaling proteins [25–32]. Especially, altered RAS-MAPK signaling has been identified as a key pathway in regulation of growth plate chondrogenesis and is affected in several disorders, referred to as RASopathies [33]. These conditions include Coffin-Lowry syndrome [9–10], Costello (faciocutaneoskeletal syndrome) [11], multiple lentigines syndrome (LEOPARD syndrome) [12–16], neurofibromatosis type 1 [17–18], and Noonan Syndrome or Noonan-like syndrome [11, 19–20]. Patients with these disorders have overlapping phenotypes of short stature, skin manifestation, cardiovascular abnormalities, and variable degree of learning disability/cognitive dysfunction and/or predisposition to cancers. RAS cycles between a GDP-bound form (inactive) and GTP-bound form (active) [34], and RAS-GTP activates a large number of effector pathways facilitating downstream signaling taking an important role in cell proliferation and differentiation [35–36]. Moreover, RAS-MAPK is downstream of fibroblast growth factor (FGF) signaling which is another major pathway involved in skeletal disease [37]. Some of the conditions caused by defects in RAS-MAPK pathway carry higher risks of malignancies, including mutations in neurofibromin (NF1), RAS, or RAFs [38]. Mutations in FGD1, which encodes a protein similar to small GTP-binding proteins, cause Aarskog-Scott syndrome presenting with disproportionate short stature, skeletal and urogenital anomalies [21–23].
Interestingly, impairment of signaling through the cAMP-protein kinase A also cause skeletal dysplasias with growth failure associated with accelerated bone age progression and/or poor pubertal growth spurts which include Acrodysostosis, type 1 (caused by mutations in PRKAR1A that encodes cAMP-dependent protein kinase type I-alpha regulatory subunit [24]), Acrodysostosis, type 2 (caused by mutations in PDE4D that encodes cAMP-specific 3’, 5’-cyclic phosphodiesterase 4D [25]) and Albright’s hereditary osteodystrophy (caused by mutations in GNAS that encodes guanine nucleotide-binding protein stimulatory G subunit alpha protein [26–28]). Mutations in these genes may not only result in skeletal dysplasias but also variable hormone resistance, thus demonstrating the important role of this pathway both in the regulation of growth plate chondrogenesis and in signaling of the G-protein coupled hormone receptors [25]. In addition, defects in the WNT5A/JNK signaling pathway including mutations in ROR2, DVL1 as well as Wnt5a, the ligand of ROR2, cause skeletal dysplasia, Robinow syndrome characterized by dysmorphic facial features, frontal bossing, hypertelorism, broad nose, short-limbed dwarfism, vertebral segmentation, and genital hypoplasia [29–32].
Transcriptional regulation
Mutations in transcriptional factors or genes that are involved in transcription repression can cause short stature. Mutations in SOX9, an important transcription factor for sex development and chondrocyte differentiation, cause Campomelic dysplasia which can cause sex reversal, ambiguous genitalia, chondrodysplasia and bent bones [39–41]. SHOX is another transcription factor that is important in growth plate chondrocytes [42]. Homozygous mutations cause severe short stature in Langer mesomelic dysplasia whereas heterozygous mutations, can present either as a milder skeletal dysplasia, Leri-Weill dyschondrosteosis or as isolated short stature [43–44]. Mutations in MLL2 (KMT2D) and KDM6A cause abnormal histone methylation and demethylation, respectively, resulting in short stature and unique facial features of Kabuki syndrome [45–47]. Mutations in transcriptional regulator of polymerase II genes (LARP7) cause Alazami syndrome [48–49] whereas mutations in the transcription initiator factor for RNA polymerase III (BRF1) cause the newly identified cerebello-facio-dental syndrome [50]. Mutations in SOX11 can cause an autosomal dominant mental retardation 27, a form of Coffin-Siris syndrome presenting with relatively mild mental retardation, microcephaly, short stature and hypoplastic fifth toe nails [51]. Similarly, the ankyrin repeat domain-containing protein 11 (ANKRD11) interacts with nuclear receptor complexes to modify transcriptional activation and mutations in this gene cause short stature, developmental delay, seizures as well as other features of the KBG syndrome, including macrodontia of the upper central incisors, and skeletal anomalies [52–53]. In addition, heterozygous mutations in transcription coactivators (CREBBP and EP300) cause Rubinstein-Taybi syndrome, characterized by severe short stature, intellectual disability, microcephaly, hearing loss, broad thumbs/halluces, and distinct facial features [54–55].
DNA repair
Impairment of DNA repair can result in severe short stature (often primordial dwarfism), microcephaly, photosensitivity and/or predisposition to leukemia/other cancers. Skeletal abnormalities may not be prominent (Table 1). Especially, Seckel syndrome has many subtypes which are caused by mutations in several genes but mostly involved in DNA repair processes [56–66]. Pericentrin (PCNT) encodes a centrosomal protein in the microtubule network and the mutations cause MOPD II but also Seckel syndrome [66]. Moreover, clinical overlaps may occur between Seckel syndrome [56, 66], MOPD II [56, 66–68], Fanconi anemia (FANC) [56], LIG4 syndrome (LIG4) [56, 69], and Nijimegen breakage syndrome (NBS1) [56, 70–71]. Mutations in BLM cause Bloom syndrome which manifests as sun-sensitive skin and increased risk of malignancies, including leukemia, lymphoma, adeno-, and squamous cell carcinoma [72–73]. Due to the increased risk of malignancy, growth hormone treatment in children with Bloom syndrome is not recommended [73]. Impaired DNA repair mechanisms may also cause short stature and microcephaly without an increased risk of malignancies. For example, no cancer predisposition has been demonstrated for Cockayne syndrome [74–75]. In addition, recent reports show that heterozygous mutation in NSMCE2 causes microcephalic primordial dwarfism with severe insulin resistance [76] and homozygous mutation in SMARCAL1 causes Schimke immunoosseous dysplasia characterized by severe growth restriction, immune deficiency, as well as renal and bone marrow failure [77], but no known increased risk of malignancies.
Other fundamental cellular processes
This group of syndromes can be caused by mutations in genes important for genome or nuclear stability (3M syndrome [78–80], Cornelia de Lange syndrome [81], Hutchinson-Gilford Progeria [82–83], microcephalic osteodysplastic primordial dwarfism type 2, MOPD II [67–68], SOFT syndrome [84–85]), chromatin remodeling (Floating-Harbor syndrome [86–88], Coffin-Siris syndrome [89]), RNA processing (microcephalic osteodysplastic primordial dwarfism type 1, MOPD I [90–91]), ubiquitination (Mulibrey nanism [92–94]), cytoskeletal interaction (primordial dwarfism due to CRIPT mutation [95]), microtubule organization (Alström syndrome [96]) or cholesterol biosynthesis (Smith-Lemli-Opitz syndrome [97], (Table 1). The patients are often born small for gestational age indicating that growth is affected during intrauterine life. Interestingly, although the molecular mechanisms are different, the clinical phenotype may have significant overlap. For example, Cornelia de Lange syndrome caused by mutation in the NIPBL has similar features with Rubinstein-Taybi syndrome caused by EP300 [98] although their molecular mechanisms are different.
2) Defects in cartilage extracellular matrix defect
Growth plate chondrocytes also secrete a characteristic extracellular matrix rich in collagens and proteoglycans that are critical to maintain structure and function of the growth plate [99]. The extracellular matrix does not only provide support, but also interacts with paracrine signaling molecules regulating chondrocyte proliferation and differentiation [99–100]. Therefore, mutations in genes that encode matrix collagens, proteoglycans, non-collagenous proteins and their processing enzymes affect growth plate chondrogenesis by several mechanisms and causes growth failure with a wide phenotypic spectrum (Table 1). For example, mutations in collagens [101–108], fibrillin 1 [109], cartilage oligomeric matrix protein [110–111], matrillin-3 [112], aggrecan [113–114] and perlecan [115] have been reported to cause short stature of variable severity. Mutations in genes encoding extracellular matrix proteins may also affect connective tissue beyond the growth plate, causing various degrees of skeletal and joint problems, as well as some conditions with low bone mineral density (Table 1).
3) Abnormal paracrine signaling
In the growth plate, paracrine factors coordinate sequential changes in chondrocyte morphology, proliferation, differentiation and matrix assembly [116]. The understanding of the paracrine mechanisms acting in the growth plate has advanced substantially during the last decade. Some of the identified factors include fibroblast growth factors (FGFs), C-type natriuretic peptide (CNP), Indian hedgehog (IHH), parathyroid hormone-related protein (PTHrP) encoded by PTHLH, and bone morphogenetic factors (BMPs). FGF receptor-3 (FGFR3) signals through several pathways including the MAPK and JAK/STAT pathways and acts as a negative regulator of growth plate chondrogenesis [117–118]. Consequently, activating mutations in FGFR3 result in inhibition of growth [119], which can result in skeletal dysplasias ranging from moderate disproportionate short stature (hypochondroplasia) to severe short stature and bone malformation (achondroplasia), and to the most severe form (thanatophoric dysplasia), a perinatal lethal skeletal dysplasia with very short limbs and underdeveloped ribs [120]. In addition, several families with autosomal dominant proportionate short stature were recently reported to have mild activating mutations of FGFR3 further expanding the phenotype [121]. Interestingly, both heterozygous and homozygous inactivating mutations in FGFR3 have been reported in patients with a tall stature syndrome including scoliosis, camptodactyly, and hearing loss [122–123].
Despite its name, C-natriuretic peptide, CNP act as an important paracrine factor in the growth plate. Homozygous knockout of CNP causes dwarfism in mice [124]. In humans, overexpression of CNP results in overgrowth [125], whereas homozygous inactivating mutations of its receptor, NPR2 causes a skeletal dysplasia with short stature and heterozygous inactivating mutations present as isolated short stature [126]. Conversely, activating mutation in NPR2 can cause tall stature [127]. CNP inhibits MAPK signaling and thus antagonizes FGFR3 signaling, and has therefore been proposed as a potential treatment for achondroplasia [128]. PTHrP and IHH form a negative feedback loop critical to the spatial coordination of proliferation and differentiation of growth plate chondrocytes [116]. Consequently, mutations in the genes for IHH [129], PTHrP [130–131], and PTH1R (the receptor for both PTH and PTHrP) [132–133] as well as mutations in their signaling pathway cause specific skeletal dysplasias. Disorders of linear growth can also result from genetic defects in other paracrine signaling systems. For example, BMP signaling is important for endochondrial ossification and growth plate regulation [134] and defects in BMP family members or the receptors cause skeletal dysplasia [135–136], characterized by brachydactyly. Mutation in IGF2, which encodes insulin-like growth factor-II, a paracrine factor that is important for intrauterine growth, was recently reported to cause pre- and postnatal growth failure [137].
4) Abnormal hormone, receptor, or signaling pathway
Growth hormone (GH) plays a major role in childhood growth and has, especially since the development of recombinant GH, been the main therapeutic approach available to treat patients with short stature. It is therefore common that children presenting with suboptimal growth are subjected to clinical testing for growth hormone deficiency or resistance even though true growth hormone deficiency or resistance is a rare cause of short stature [2]. Patients with mutations in GH1, GHRHR, SOX3, and BTK present with isolated growth hormone deficiency [138]. On the other hand, mutations in GHR [139–141] and STAT5B [142] cause GH resistance. GHR encodes the growth hormone receptor. Because an extracellular cleavage fragment of the GH receptor circulates as a growth hormone binding protein (GHBP), mutations in the extracellular domain of GHR tend to cause low growth hormone binding protein (GHBP) while mutations in intracellular (signaling) domain cause normal or high GHBP [139–141]. STAT5 proteins contribute to the common pathway of growth hormone and interleukin-2 cytokine family signaling, therefore mutations in STAT5B cause growth failure and immune deficiency [142]. Mutations in IGF1 or IGF1R cause intrauterine growth restriction (because IGF1 signaling is important for intrauterine growth while GH is not required), postnatal growth failure, microcephaly and other various anomalies including developmental delay [143–147]. Mutations in a gene that stabilizes IGF-1, IGFALS, forming an IGF-1-IGFBP3-ALS complex, cause growth deficiency, insulin resistance and osteoporosis [148–149].
Mutations in the same gene may cause a wide phenotypic spectrum
For many genes that support growth plate chondrogenesis, homozygous and/or severe mutations cause bones to be markedly short and malformed, presenting clinically as a chondrodysplasia, whereas heterozygous and/or milder mutations may cause “isolated” short stature, with no or only subtle signs of a skeletal dysplasia. For example, SHOX [43], NPR2 [125], ACAN [114], IGF1 [143], IGF1R [146], or FGFR3 [120] have been associated with “isolated” short stature without other prominent phenotypic features. There are likely numerous genetic causes still remaining to be discovered to fully explain the molecular mechanism of “isolated” growth disorders. It is likely that there soon will be a large number of genetically characterized monogenic short stature syndromes with no or only minor associated abnormalities. A suitable term for this group of patients would be “isolated” short stature.
Summary and future considerations
Over the last decades, advances in clinical genetics, including exome sequencing, have accelerated the identification of new genetic growth disorders and thereby greatly contributed to the understanding of the underlying molecular mechanisms of longitudinal bone growth and growth failure. This new knowledge will help the individual patient seeking medical attention due to severe short stature, as it will improve the chances of an exact mechanistic diagnosis, which in turn enables individualized management, improved prognosis, better genetic counseling and may also help avoid unnecessary testing for endocrine and other disorders. As more genetic causes become identified, better classifications of growth disorders become possible. Fewer children will receive the unhelpful diagnosis of “idiopathic” short stature, and instead will be categorized clinically as having a skeletal dysplasia, syndromic short stature, or isolated short stature, will be categorized genetically as having polygenic or monogenic short stature and will be categorized mechanistically depending on how their specific genetic defects diminish growth plate chondrogenesis and therefore linear growth.
Key Points.
Over the last decades, advances in clinical genetics, including exome sequencing, have accelerated the identification of new genetic growth disorders and thereby greatly contributed to the understanding of the underlying molecular mechanisms of longitudinal bone growth and growth failure.
This new knowledge will help the individual patient seeking medical attention due to severe short stature, as it will improve the chances of an exact mechanistic diagnosis, which in turn enables individualized management, improved prognosis, and better genetic counseling and may also help avoid unnecessary testing for endocrine and other disorders.
As more genetic causes become identified, better classifications of growth disorders become possible.
Footnotes
Disclosure: This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH. Dr. Nilsson was supported by grants from the Swedish Research Council (Grant no. 521-2014-3063 & 2015-02227), the Swedish Governmental Agency for Innovation Systems (Vinnova) (2014-01438), Marianne and Marcus Wallenberg Foundation, the Stockholm County Council, Byggmästare Olle Engkvist’s Foundation, Stiftelsen Frimurare Barnhuset i Stockholm, and Karolinska Institutet. Dr. Andrade was supported by grants from Sällskapet Barnavård.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jee YH, Baron J. The Biology of Stature. J Pediatr. 2016;173:32–8. doi: 10.1016/j.jpeds.2016.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baron J, Sävendahl L, De Luca F, Dauber A, Phillip M, Wit JM, Nilsson O. Short and tall stature: a new paradigm emerges. Nat Rev Endocrinol. 2015;11(12):735–46. doi: 10.1038/nrendo.2015.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nilsson O, Marino R, De Luca F, Phillip M, Baron J. Endocrine regulation of the growth plate. Horm Res. 2005;64(4):157–65. doi: 10.1159/000088791. [DOI] [PubMed] [Google Scholar]
- 4.Lango A, et al. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature. 2010;467(7317):832–8. doi: 10.1038/nature09410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood AR, et al. Defining the role of common variation in the genomic and biological architecture of adult human height. Nat Genet. 2014;46(11):1173–86. doi: 10.1038/ng.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lui JC, Nilsson O, Chan Y, Palmer CD, Andrade AC, Hirschhorn JN, Baron J. Synthesizing genome-wide association studies and expression microarray reveals novel genes that act in the human growth plate to modulate height. Hum Mol Genet. 2012;21(23):5193–201. doi: 10.1093/hmg/dds347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gahl WA, Markello TC, Toro C, Fajardo KF, Sincan M, Gill F, Carlson-Donohoe H, Gropman A, Pierson TM, Golas G, Wolfe L, Groden C, Godfrey R, Nehrebecky M, Wahl C, Landis DM, Yang S, Madeo A, Mullikin JC, Boerkoel CF, Tifft CJ, Adams D. The National Institutes of Health Undiagnosed Diseases Program: insights into rare diseases. Genet Med. 2012;14(1):51–9. doi: 10.1038/gim.0b013e318232a005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gahl WA, Adams DR, Markello TC, Boerkoel NF, Tifft CJ. In: Genetic Approaches to Rare and Undiagnosed Diseases, chapter 83, Nelson's Textbook of Pediatrics, Twentieht Edition. Kliegman, Stanton, St Gene, Shore, Behrman, editors. Elsevier; Philadelphia, PA: pp. 629–633. [Google Scholar]
- 9.Delaunoy J, Abidi F, Zeniou M, Jacquot S, Merienne K, Pannetier S, Schmitt M, Schwartz C, Hanauer A. Mutations in the X-linked RSK2 gene (RPS6KA3) in patients with Coffin-Lowry syndrome. Hum Mutat. 2001;17(2):103–16. doi: 10.1002/1098-1004(200102)17:2<103::AID-HUMU2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 10.Rogers RC, Abidi FE. Coffin-Lowry Syndrome. Seattle (WA): University of Washington, Seattle; 1993–2016. [Google Scholar]
- 11.Noonan JA. Noonan syndrome and related disorders: alterations in growth and puberty. Rev Endocr Metab Disord. 2006;7(4):251–5. doi: 10.1007/s11154-006-9021-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalev I, Muru K, Teek R, Zordania R, Reimand T, Köbas K, Ounap K. LEOPARD syndrome with recurrent PTPN11 mutation Y279C and different cutaneous manifestations: two case reports and a review of the literature. Eur J Pediatr. 2010;169(4):469–73. doi: 10.1007/s00431-009-1058-1. [DOI] [PubMed] [Google Scholar]
- 13.Sarkozy A, Digilio MC, Dallapiccola B. Leopard syndrome. Orphanet J Rare Dis. 2008;3:13. doi: 10.1186/1750-1172-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martínez-Quintana E, Rodríguez-González F. LEOPARD Syndrome: Clinical Features and Gene Mutations. Mol Syndromol. 2012;3(4):145–57. doi: 10.1159/000342251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pandit B, Sarkozy A, Pennacchio LA, Carta C, Oishi K, Martinelli S, Pogna EA, Schackwitz W, Ustaszewska A, Landstrom A, Bos JM, Ommen SR, Esposito G, Lepri F, Faul C, Mundel P, López Siguero JP, Tenconi R, Selicorni A, Rossi C, Mazzanti L, Torrente I, Marino B, Digilio MC, Zampino G, Ackerman MJ, Dallapiccola B, Tartaglia M, Gelb BD. Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nat Genet. 2007;39(8):1007–12. doi: 10.1038/ng2073. [DOI] [PubMed] [Google Scholar]
- 16.Sarkozy A, Carta C, Moretti S, Zampino G, Digilio MC, Pantaleoni F, Scioletti AP, Esposito G, Cordeddu V, Lepri F, Petrangeli V, Dentici ML, Mancini GM, Selicorni A, Rossi C, Mazzanti L, Marino B, Ferrero GB, Silengo MC, Memo L, Stanzial F, Faravelli F, Stuppia L, Puxeddu E, Gelb BD, Dallapiccola B, Tartaglia M. Germline BRAF mutations in Noonan, LEOPARD, and cardiofaciocutaneous syndromes: molecular diversity and associated phenotypic spectrum. Hum Mutat. 2009;30(4):695–702. doi: 10.1002/humu.20955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunning-Davies BM, Parker AP. Annual review of children with neurofibromatosis type 1. Arch Dis Child Educ Pract Ed. 2016;101(2):102–11. doi: 10.1136/archdischild-2014-308084. [DOI] [PubMed] [Google Scholar]
- 18.Bizzarri C, Bottaro G. Endocrine implications of neurofibromatosis 1 in childhood. Horm Res Paediatr. 2015;83(4):232–41. doi: 10.1159/000369802. [DOI] [PubMed] [Google Scholar]
- 19.Roberts AE, Allanson JE, Tartaglia M, Gelb BD. Noonan syndrome. Lancet. 2013;381(9863):333–42. doi: 10.1016/S0140-6736(12)61023-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aoki Y, Niihori T, Banjo T, Okamoto N, Mizuno S, Kurosawa K, Ogata T, Takada F, Yano M, Ando T, Hoshika T, Barnett C, Ohashi H, Kawame H, Hasegawa T, Okutani T, Nagashima T, Hasegawa S, Funayama R, Nagashima T, Nakayama K, Inoue S, Watanabe Y, Ogura T, Matsubara Y. Gain-of-function mutations in RIT1 cause Noonan syndrome, a RAS/MAPK pathway syndrome. Am J Hum Genet. 2013;93(1):173–80. doi: 10.1016/j.ajhg.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasteris NG, Cadle A, Logie LJ, Porteous ME, Schwartz CE, Stevenson RE, Glover TW, Wilroy RS, Gorski JL. Isolation and characterization of the faciogenital dysplasia (Aarskog-Scott syndrome) gene: a putative Rho/Rac guanine nucleotide exchange factor. Cell. 1994;79(4):669–78. doi: 10.1016/0092-8674(94)90552-5. [DOI] [PubMed] [Google Scholar]
- 22.Shalev SA, Chervinski E, Weiner E, Mazor G, Friez MJ, Schwartz CE. Clinical variation of Aarskog syndrome in a large family with 2189delA in the FGD1 gene. Am J Med Genet A. 2006;140(2):162–5. doi: 10.1002/ajmg.a.31033. [DOI] [PubMed] [Google Scholar]
- 23.Orrico A, Galli L, Cavaliere ML, Garavelli L, Fryns JP, Crushell E, Rinaldi MM, Medeira A, Sorrentino V. Phenotypic and molecular characterisation of the Aarskog-Scott syndrome: a survey of the clinical variability in light of FGD1 mutation analysis in 46 patients. Eur J Hum Genet. 2004;12(1):16–23. doi: 10.1038/sj.ejhg.5201081. [DOI] [PubMed] [Google Scholar]
- 24.Linglart A, Menguy C, Couvineau A, Auzan C, Gunes Y, Cancel M, Motte E, Pinto G, Chanson P, Bougnères P, Clauser E, Silve C. Recurrent PRKAR1A mutation in acrodysostosis with hormone resistance. N Engl J Med. 2011;364(23):2218–26. doi: 10.1056/NEJMoa1012717. [DOI] [PubMed] [Google Scholar]
- 25.Lindstrand A, Grigelioniene G, Nilsson D, Pettersson M, Hofmeister W, Anderlid BM, Kant SG, Ruivenkamp CA, Gustavsson P, Valta H, Geiberger S, Topa A, Lagerstedt-Robinson K, Taylan F, Wincent J, Laurell T, Pekkinen M, Nordenskjöld M, Mäkitie O, Nordgren A. Different mutations in PDE4D associated with developmental disorders with mirror phenotypes. J Med Genet. 2014;51(1):45–54. doi: 10.1136/jmedgenet-2013-101937. [DOI] [PubMed] [Google Scholar]
- 26.Ahrens W, Hiort O, Staedt P, Kirschner T, Marschke C, Kruse K. Analysis of the GNAS1 gene in Albright's hereditary osteodystrophy. J Clin Endocrinol Metab. 2001;86(10):4630–4. doi: 10.1210/jcem.86.10.7946. [DOI] [PubMed] [Google Scholar]
- 27.Lemos MC, Thakker RV. GNAS mutations in Pseudohypoparathyroidism type 1a and related disorders. Hum Mutat. 2015;36(1):11–9. doi: 10.1002/humu.22696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mantovani G1, Ferrante E, Giavoli C, Linglart A, Cappa M, Cisternino M, Maghnie M, Ghizzoni L, de Sanctis L, Lania AG, Beck-Peccoz P, Spada A. Recombinant human GH replacement therapy in children with pseudohypoparathyroidism type Ia: first study on the effect on growth. J Clin Endocrinol Metab. 2010;95(11):5011–7. doi: 10.1210/jc.2010-1649. [DOI] [PubMed] [Google Scholar]
- 29.Afzal AR, Rajab A, Fenske CD, Oldridge M, Elanko N, Ternes-Pereira E, Tüysüz B, Murday VA, Patton MA, Wilkie AO, Jeffery S. Recessive Robinow syndrome, allelic to dominant brachydactyly type B, is caused by mutation of ROR2. Nat Genet. 2000;25(4):419–22. doi: 10.1038/78107. [DOI] [PubMed] [Google Scholar]
- 30.Person AD, Beiraghi S, Sieben CM, Hermanson S, Neumann AN, Robu ME, Schleiffarth JR, Billington CJ, Jr, van Bokhoven H, Hoogeboom JM, Mazzeu JF, Petryk A, Schimmenti LA, Brunner HG, Ekker SC, Lohr JL. WNT5A mutations in patients with autosomal dominant Robinow syndrome. Dev Dyn. 2010;239(1):327–37. doi: 10.1002/dvdy.22156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roifman M, Marcelis CL, Paton T, Marshall C, Silver R, Lohr JL, Yntema HG, Venselaar H, Kayserili H, van Bon B, Seaward G, Brunner HG, Chitayat D FORGE Canada Consortium. De novo WNT5A-associated autosomal dominant Robinow syndrome suggests specificity of genotype and phenotype. Clin Genet. 2015;87(1):34–41. doi: 10.1111/cge.12401. [DOI] [PubMed] [Google Scholar]
- 32.Bunn KJ, Daniel P, Rösken HS, O'Neill AC, Cameron-Christie SR, Morgan T, Brunner HG, Lai A, Kunst HP, Markie DM, Robertson SP. Mutations in DVL1 cause an osteosclerotic form of Robinow syndrome. Am J Hum Genet. 2015;96(4):623–30. doi: 10.1016/j.ajhg.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aoki Y, Niihori T, Inoue S, Matsubara Y. Recent advances in RASopathies. J Hum Genet. 2016;61(1):33–9. doi: 10.1038/jhg.2015.114. [DOI] [PubMed] [Google Scholar]
- 34.Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81(1):153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- 35.Giehl K. Oncogenic Ras in tumour progression and metastasis. Biol Chem. 2005;386(3):193–205. doi: 10.1515/BC.2005.025. [DOI] [PubMed] [Google Scholar]
- 36.Santarpia L, Lippman SM, El-Naggar AK. Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16(1):103–19. doi: 10.1517/14728222.2011.645805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teven CM, Farina EM, Rivas J, Reid RR. Fibroblast growth factor (FGF) signaling in development and skeletal diseases. Genes Dis. 2014;1(2):199–213. doi: 10.1016/j.gendis.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cizmarova M, Kostalova L, Pribilincova Z, Lasabova Z, Hlavata A, Kovacs L, Ilencikova D. Rasopathies - dysmorphic syndromes with short stature and risk of malignancy. Endocr Regul. 2013;47(4):217–22. doi: 10.4149/endo_2013_04_217. [DOI] [PubMed] [Google Scholar]
- 39.Foster JW, Dominguez-Steglich MA, Guioli S, Kwok C, Weller PA, Stevanović M, Weissenbach J, Mansour S, Young ID, Goodfellow PN, et al. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature. 1994;372(6506):525–30. doi: 10.1038/372525a0. [DOI] [PubMed] [Google Scholar]
- 40.Meyer J, Südbeck P, Held M, Wagner T, Schmitz ML, Bricarelli FD, Eggermont E, Friedrich U, Haas OA, Kobelt A, Leroy JG, Van Maldergem L, Michel E, Mitulla B, Pfeiffer RA, Schinzel A, Schmidt H, Scherer G. Mutational analysis of the SOX9 gene in campomelic dysplasia and autosomal sex reversal: lack of genotype/phenotype correlations. Hum Mol Genet. 1997;6(1):91–8. doi: 10.1093/hmg/6.1.91. [DOI] [PubMed] [Google Scholar]
- 41.Gimovsky M1, Rosa E, Tolbert T, Guzman G, Nazir M, Koscica K. Campomelic dysplasia: case report and review. J Perinatol. 2008;28(1):71–3. doi: 10.1038/sj.jp.7211875. [DOI] [PubMed] [Google Scholar]
- 42.Beiser KU, Glaser A, Kleinschmidt K, Scholl I, Röth R, Li L, Gretz N, Mechtersheimer G, Karperien M, Marchini A, Richter W, Rappold GA. Identification of novel SHOX target genes in the developing limb using a transgenic mouse model. PLoS One. 2014;9(6):e98543. doi: 10.1371/journal.pone.0098543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Binder G. Short stature due to SHOX deficiency: genotype, phenotype, and therapy. Horm Res Paediatr. 2011;75(2):81–9. doi: 10.1159/000324105. [DOI] [PubMed] [Google Scholar]
- 44.Ambrosetti F, Palicelli A, Bulfamante G, Rivasi F. Langer mesomelic dysplasia in early fetuses: two cases and a literature review. Fetal Pediatr Pathol. 2014;33(2):71–83. doi: 10.3109/15513815.2013.807322. [DOI] [PubMed] [Google Scholar]
- 45.Ng SB, Bigham AW, Buckingham KJ, Hannibal MC, McMillin MJ, Gildersleeve HI, Beck AE, Tabor HK, Cooper GM, Mefford HC, Lee C, Turner EH, Smith JD, Rieder MJ, Yoshiura K, Matsumoto N, Ohta T, Niikawa N, Nickerson DA, Bamshad MJ, Shendure J. Exome sequencing identifies MLL2 mutations as a cause of Kabuki syndrome. Nat Genet. 2010;42(9):790–3. doi: 10.1038/ng.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dentici ML, Di Pede A, Lepri FR, Gnazzo M, Lombardi MH, Auriti C, Petrocchi S, Pisaneschi E, Bellacchio E, Capolino R, Braguglia A, Angioni A, Dotta A, Digilio MC, Dallapiccola B. Kabuki syndrome: clinical and molecular diagnosis in the first year of life. Arch Dis Child. 2015;100(2):158–64. doi: 10.1136/archdischild-2013-305858. [DOI] [PubMed] [Google Scholar]
- 47.Lederer D, Grisart B, Digilio MC, Benoit V, Crespin M, Ghariani SC, Maystadt I, Dallapiccola B, Verellen-Dumoulin C. Deletion of KDM6A, a histone demethylase interacting with MLL2, in three patients with Kabuki syndrome. Am J Hum Genet. 2012;90(1):119–24. doi: 10.1016/j.ajhg.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alazami AM, Al-Owain M, Alzahrani F, Shuaib T, Al-Shamrani H, Al-Falki YH, Al-Qahtani SM, Alsheddi T, Colak D, Alkuraya FS. Loss of function mutation in LARP7, chaperone of 7SK ncRNA, causes a syndrome of facial dysmorphism, intellectual disability, and primordial dwarfism. Hum Mutat. 2012;33(10):1429–34. doi: 10.1002/humu.22175. [DOI] [PubMed] [Google Scholar]
- 49.Hollink IH, Alfadhel M, Al-Wakeel AS, Ababneh F, Pfundt R, de Man SA, Jamra RA, Rolfs A, Bertoli-Avella AM, van de Laar IM. Broadening the phenotypic spectrum of pathogenic LARP7 variants: two cases with intellectual disability, variable growth retardation and distinct facial features. J Hum Genet. 2016;61(3):229–33. doi: 10.1038/jhg.2015.134. [DOI] [PubMed] [Google Scholar]
- 50.Borck G, Hög F2, Dentici ML, Tan PL, Sowada N, Medeira A, Gueneau L, Thiele H, Kousi M, Lepri F, Wenzeck L, Blumenthal I, Radicioni A, Schwarzenberg TL, Mandriani B, Fischetto R, Morris-Rosendahl DJ, Altmüller J, Reymond A, Nürnberg P, Merla G, Dallapiccola B, Katsanis N, Cramer P, Kubisch C. BRF1 mutations alter RNA polymerase III-dependent transcription and cause neurodevelopmental anomalies. Genome Res. 2015;25(2):155–66. doi: 10.1101/gr.176925.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsurusaki Y, Koshimizu E, Ohashi H, Phadke S, Kou I, Shiina M, Suzuki T, Okamoto N, Imamura S, Yamashita M, Watanabe S, Yoshiura K, Kodera H, Miyatake S, Nakashima M, Saitsu H, Ogata K, Ikegawa S, Miyake N, Matsumoto N. De novo SOX11 mutations cause Coffin-Siris syndrome. Nat Commun. 2014;5:4011. doi: 10.1038/ncomms5011. [DOI] [PubMed] [Google Scholar]
- 52.Sirmaci A, Spiliopoulos M, Brancati F, Powell E, Duman D, Abrams A, Bademci G, Agolini E, Guo S, Konuk B, Kavaz A, Blanton S, Digilio MC, Dallapiccola B, Young J, Zuchner S, Tekin M. Mutations in ANKRD11 cause KBG syndrome, characterized by intellectual disability, skeletal malformations, and macrodontia. Am J Hum Genet. 2011;89(2):289–94. doi: 10.1016/j.ajhg.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ockeloen CW, Willemsen MH, de Munnik S, van Bon BW, de Leeuw N, Verrips A, Kant SG, Jones EA, Brunner HG, van Loon RL, Smeets EE, van Haelst MM, van Haaften G, Nordgren A, Malmgren H, Grigelioniene G, Vermeer S, Louro P, Ramos L, Maal TJ, van Heumen CC, Yntema HG, Carels CE, Kleefstra T. Further delineation of the KBG syndrome phenotype caused by ANKRD11 aberrations. Eur J Hum Genet. 2015;23(9):1176–85. doi: 10.1038/ejhg.2014.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Menke LA, van Belzen MJ, Alders M, Cristofoli F, Ehmke N, Fergelot P, Foster A, Gerkes EH, Hoffer MJ, Horn D, Kant SG, Lacombe D, Leon E, Maas SM, Melis D, Muto V, Park SM, Peeters H, Peters DJ, Pfundt R, van Ravenswaaij-Arts CM, Tartaglia M, Hennekam RC DDD Study. CREBBP mutations in individuals without Rubinstein-Taybi syndrome phenotype. Am J Med Genet A. 2016 doi: 10.1002/ajmg.a.37800. In print. [DOI] [PubMed] [Google Scholar]
- 55.Negri G, Magini P, Milani D, Colapietro P, Rusconi D, Scarano E, Bonati MT, Priolo M, Crippa M, Mazzanti L, Wischmeijer A, Tamburrino F, Pippucci T, Finelli P, Larizza L, Gervasini C. From Whole Gene Deletion to Point Mutations of EP300-Positive Rubinstein-Taybi Patients: New Insights into the Mutational Spectrum and Peculiar Clinical Hallmarks. Hum Mutat. 2016;37(2):175–83. doi: 10.1002/humu.22922. [DOI] [PubMed] [Google Scholar]
- 56.Khetarpal P, Das S, Panigrahi I, Munshi A. Primordial dwarfism: overview of clinical and genetic aspects. Mol Genet Genomics. 2016;291(1):1–15. doi: 10.1007/s00438-015-1110-y. [DOI] [PubMed] [Google Scholar]
- 57.O'Driscoll M1, Ruiz-Perez VL, Woods CG, Jeggo PA, Goodship JA. A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nat Genet. 2003;33(4):497–501. doi: 10.1038/ng1129. [DOI] [PubMed] [Google Scholar]
- 58.Ogi T, Walker S, Stiff T, Hobson E, Limsirichaikul S, Carpenter G, Prescott K, Suri M, Byrd PJ, Matsuse M, Mitsutake N, Nakazawa Y, Vasudevan P, Barrow M, Stewart GS, Taylor AM, O'Driscoll M, Jeggo PA. Identification of the first ATRIP-deficient patient and novel mutations in ATR define a clinical spectrum for ATR-ATRIP Seckel Syndrome. PLoS Genet. 2012;8(11):e1002945. doi: 10.1371/journal.pgen.1002945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Al-Dosari MS, Shaheen R, Colak D, Alkuraya FS. Novel CENPJ mutation causes Seckel syndrome. J Med Genet. 2010;47(6):411–4. doi: 10.1136/jmg.2009.076646. [DOI] [PubMed] [Google Scholar]
- 60.Kalay E, Yigit G, Aslan Y, Brown KE, Pohl E, Bicknell LS, Kayserili H, Li Y, Tüysüz B, Nürnberg G, Kiess W, Koegl M, Baessmann I, Buruk K, Toraman B, Kayipmaz S, Kul S, Ikbal M, Turner DJ, Taylor MS, Aerts J, Scott C, Milstein K, Dollfus H, Wieczorek D, Brunner HG, Hurles M, Jackson AP, Rauch A, Nürnberg P, Karagüzel A, Wollnik B. CEP152 is a genome maintenance protein disrupted in Seckel syndrome. Nat Genet. 2011;43(1):23–6. doi: 10.1038/ng.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marjanović M, Sánchez-Huertas C, Terré B, Gómez R, Scheel JF, Pacheco S, Knobel PA, Martínez-Marchal A, Aivio S, Palenzuela L, Wolfrum U, McKinnon PJ, Suja JA, Roig I, Costanzo V, Lüders J, Stracker TH. CEP63 deficiency promotes p53-dependent microcephaly and reveals a role for the centrosome in meiotic recombination. Nat Commun. 2015;6:7676. doi: 10.1038/ncomms8676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shaheen R, Faqeih E, Ansari S, Abdel-Salam G, Al-Hassnan ZN, Al-Shidi T, Alomar R, Sogaty S, Alkuraya FS. Genomic analysis of primordial dwarfism reveals novel disease genes. Genome Res. 2014;24(2):291–9. doi: 10.1101/gr.160572.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Griffith E1, Walker S, Martin CA, Vagnarelli P, Stiff T, Vernay B, Al Sanna N, Saggar A, Hamel B, Earnshaw WC, Jeggo PA, Jackson AP, O'Driscoll M. Mutations in pericentrin cause Seckel syndrome with defective ATR-dependent DNA damage signaling. Nat Genet. 2008;40(2):232–6. doi: 10.1038/ng.2007.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martin CA, Ahmad I, Klingseisen A, Hussain MS, Bicknell LS, Leitch A, Nürnberg G, Toliat MR, Murray JE, Hunt D, Khan F, Ali Z, Tinschert S, Ding J, Keith C, Harley ME, Heyn P, Müller R, Hoffmann I, Daire VC, Dollfus H, Dupuis L, Bashamboo A, McElreavey K, Kariminejad A, Mendoza-Londono R, Moore AT, Saggar A, Schlechter C, Weleber R, Thiele H, Altmüller J, Höhne W, Hurles ME, Noegel AA, Baig SM, Nürnberg P, Jackson AP. Mutations in PLK4, encoding a master regulator of centriole biogenesis, cause microcephaly, growth failure and retinopathy. Nat Genet. 2014;46(12):1283–92. doi: 10.1038/ng.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qvist P, Huertas P, Jimeno S, Nyegaard M, Hassan MJ, Jackson SP, Børglum AD. CtIP Mutations Cause Seckel and Jawad Syndromes. PLoS Genet. 2011;7(10):e1002310. doi: 10.1371/journal.pgen.1002310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Willems M, Geneviève D, Borck G, Baumann C, Baujat G, Bieth E, Edery P, Farra C, Gerard M, Héron D, Leheup B, Le Merrer M, Lyonnet S, Martin-Coignard D, Mathieu M, Thauvin-Robinet C, Verloes A, Colleaux L, Munnich A, Cormier-Daire V. Molecular analysis of pericentrin gene (PCNT) in a series of 24 Seckel/microcephalic osteodysplastic primordial dwarfism type II (MOPD II) families. J Med Genet. 2010;47(12):797–802. doi: 10.1136/jmg.2009.067298. [DOI] [PubMed] [Google Scholar]
- 67.Rauch A, Thiel CT, Schindler D, Wick U, Crow YJ, Ekici AB, van Essen AJ, Goecke TO, Al-Gazali L, Chrzanowska KH, Zweier C, Brunner HG, Becker K, Curry CJ, Dallapiccola B, Devriendt K, Dörfler A, Kinning E, Megarbane A, Meinecke P, Semple RK, Spranger S, Toutain A, Trembath RC, Voss E, Wilson L, Hennekam R, de Zegher F, Dörr HG, Reis A. Mutations in the pericentrin (PCNT) gene cause primordial dwarfism. Science. 2008;319(5864):816–9. doi: 10.1126/science.1151174. [DOI] [PubMed] [Google Scholar]
- 68.Bober MB, Niiler T, Duker AL, Murray JE, Ketterer T, Harley ME, Alvi S, Flora C, Rustad C, Bongers EM, Bicknell LS, Wise C, Jackson AP. Growth in individuals with Majewski osteodysplastic primordial dwarfism type II caused by pericentrin mutations. Am J Med Genet A. 2012;158A(11):2719–25. doi: 10.1002/ajmg.a.35447. [DOI] [PubMed] [Google Scholar]
- 69.Chistiakov DA, Voronova NV, Chistiakov AP. Ligase IV syndrome. Eur J Med Genet. 2009;52(6):373–8. doi: 10.1016/j.ejmg.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 70.Pastorczak A, Szczepanski T, Mlynarski W International Berlin-Frankfurt-Munster (I-BFM) ALL host genetic variation working group. Clinical course and therapeutic implications for lymphoid malignancies in Nijmegen breakage syndrome. Eur J Med Genet. 2016;59(3):126–32. doi: 10.1016/j.ejmg.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 71.Berardinelli F, di Masi A, Antoccia A. NBN Gene Polymorphisms and Cancer Susceptibility: A Systemic Review. Curr Genomics. 2013;14(7):425–40. doi: 10.2174/13892029113146660012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ellis NA, Lennon DJ, Proytcheva M, Alhadeff B, Henderson EE, German J. Somatic intragenic recombination within the mutated locus BLM can correct the high sister-chromatid exchange phenotype of Bloom syndrome cells. Am J Hum Genet. 1995;57(5):1019–27. [PMC free article] [PubMed] [Google Scholar]
- 73.Renes JS, Willemsen RH, Wagner A, Finken MJ, Hokken-Koelega AC. Bloom syndrome in short children born small for gestational age: a challenging diagnosis. J Clin Endocrinol Metab. 2013;98(10):3932–8. doi: 10.1210/jc.2013-2491. [DOI] [PubMed] [Google Scholar]
- 74.Troelstra C, van Gool A, de Wit J, Vermeulen W, Bootsma D, Hoeijmakers JH. ERCC6, a member of a subfamily of putative helicases, is involved in Cockayne's syndrome and preferential repair of active genes. Cell. 1992;71(6):939–53. doi: 10.1016/0092-8674(92)90390-x. [DOI] [PubMed] [Google Scholar]
- 75.Bregman DB1, Halaban R, van Gool AJ, Henning KA, Friedberg EC, Warren SL. UV-induced ubiquitination of RNA polymerase II: a novel modification deficient in Cockayne syndrome cells. Proc Natl Acad Sci U S A. 1996;93(21):11586–90. doi: 10.1073/pnas.93.21.11586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Payne F, Colnaghi R, Rocha N, Seth A, Harris J, Carpenter G, Bottomley WE, Wheeler E, Wong S, Saudek V, Savage D, O'Rahilly S, Carel JC, Barroso I, O'Driscoll M, Semple R. Hypomorphism in human NSMCE2 linked to primordial dwarfism and insulin resistance. J Clin Invest. 2014;124(9):4028–38. doi: 10.1172/JCI73264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boerkoel CF, Takashima H, John J, Yan J, Stankiewicz P, Rosenbarker L, André JL, Bogdanovic R, Burguet A, Cockfield S, Cordeiro I, Fründ S, Illies F, Joseph M, Kaitila I, Lama G, Loirat C, McLeod DR, Milford DV, Petty EM, Rodrigo F, Saraiva JM, Schmidt B, Smith GC, Spranger J, Stein A, Thiele H, Tizard J, Weksberg R, Lupski JR, Stockton DW. Mutant chromatin remodeling protein SMARCAL1 causes Schimke immuno-osseous dysplasia. Nat Genet. 2002;30(2):215–20. doi: 10.1038/ng821. [DOI] [PubMed] [Google Scholar]
- 78.Huber C, Dias-Santagata D, Glaser A, O'Sullivan J, Brauner R, Wu K, Xu X, Pearce K, Wang R, Uzielli ML, Dagoneau N, Chemaitilly W, Superti-Furga A, Dos Santos H, Mégarbané A, Morin G, Gillessen-Kaesbach G, Hennekam R, Van der Burgt I, Black GC, Clayton PE, Read A, Le Merrer M, Scambler PJ, Munnich A, Pan ZQ, Winter R, Cormier-Daire V. Identification of mutations in CUL7 in 3-M syndrome. Nat Genet. 2005;37(10):1119–24. doi: 10.1038/ng1628. [DOI] [PubMed] [Google Scholar]
- 79.Hanson D1, Murray PG, Sud A, Temtamy SA, Aglan M, Superti-Furga A, Holder SE, Urquhart J, Hilton E, Manson FD, Scambler P, Black GC, Clayton PE. The primordial growth disorder 3-M syndrome connects ubiquitination to the cytoskeletal adaptor OBSL1. Am J Hum Genet. 2009;84(6):801–6. doi: 10.1016/j.ajhg.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hanson D1, Murray PG, O'Sullivan J, Urquhart J, Daly S, Bhaskar SS, Biesecker LG, Skae M, Smith C, Cole T, Kirk J, Chandler K, Kingston H, Donnai D, Clayton PE, Black GC. Exome sequencing identifies CCDC8 mutations in 3-M syndrome, suggesting that CCDC8 contributes in a pathway with CUL7 and OBSL1 to control human growth. Am J Hum Genet. 2011;89(1):148–53. doi: 10.1016/j.ajhg.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boyle MI, Jespersgaard C, Brøndum-Nielsen K, Bisgaard AM, Tümer Z. Cornelia de Lange syndrome. Clin Genet. 2015;88(1):1–12. doi: 10.1111/cge.12499. [DOI] [PubMed] [Google Scholar]
- 82.Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L, Erdos MR, Robbins CM, Moses TY, Berglund P, Dutra A, Pak E, Durkin S, Csoka AB, Boehnke M, Glover TW, Collins FS. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423(6937):293–8. doi: 10.1038/nature01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gonzalo S, Kreienkamp R, Askjaer P. Hutchinson-Gilford Progeria Syndrome: A premature aging disease caused by LMNA gene mutations. Ageing Res Rev. 2016 doi: 10.1016/j.arr.2016.06.007. pii: S1568–1637(16)30134–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sarig O, Nahum S, Rapaport D, Ishida-Yamamoto A, Fuchs-Telem D, Qiaoli L, Cohen-Katsenelson K, Spiegel R, Nousbeck J, Israeli S, Borochowitz ZU, Padalon-Brauch G, Uitto J, Horowitz M, Shalev S, Sprecher E. Short stature, onychodysplasia, facial dysmorphism, and hypotrichosis syndrome is caused by a POC1A mutation. Am J Hum Genet. 2012;91(2):337–42. doi: 10.1016/j.ajhg.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shaheen R, Faqeih E, Shamseldin HE, Noche RR, Sunker A, Alshammari MJ, Al-Sheddi T, Adly N, Al-Dosari MS, Megason SG, Al-Husain M, Al-Mohanna F, Alkuraya FS. POC1A truncation mutation causes a ciliopathy in humans characterized by primordial dwarfism. Am J Hum Genet. 2012;91(2):330–6. doi: 10.1016/j.ajhg.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hood RL, Lines MA, Nikkel SM, Schwartzentruber J, Beaulieu C, Nowaczyk MJ, Allanson J, Kim CA, Wieczorek D, Moilanen JS, Lacombe D, Gillessen-Kaesbach G, Whiteford ML, Quaio CR, Gomy I, Bertola DR, Albrecht B, Platzer K, McGillivray G, Zou R, McLeod DR, Chudley AE, Chodirker BN, Marcadier J, Majewski J, Bulman DE, White SM, Boycott KM FORGE Canada Consortium. Mutations in SRCAP, encoding SNF2-related CREBBP activator protein, cause Floating-Harbor syndrome. Am J Hum Genet. 2012;90(2):308–13. doi: 10.1016/j.ajhg.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nikkel SM, Dauber A, de Munnik S, Connolly M, Hood RL, Caluseriu O, Hurst J, Kini U, Nowaczyk MJ, Afenjar A, Albrecht B, Allanson JE, Balestri P, Ben-Omran T, Brancati F, Cordeiro I, da Cunha BS, Delaney LA, Destrée A, Fitzpatrick D, Forzano F, Ghali N, Gillies G, Harwood K, Hendriks YM, Héron D, Hoischen A, Honey EM, Hoefsloot LH, Ibrahim J, Jacob CM, Kant SG, Kim CA, Kirk EP, Knoers NV, Lacombe D, Lee C, Lo IF, Lucas LS, Mari F, Mericq V, Moilanen JS, Møller ST, Moortgat S, Pilz DT, Pope K, Price S, Renieri A, Sá J, Schoots J, Silveira EL, Simon ME, Slavotinek A, Temple IK, van der Burgt I, de Vries BB, Weisfeld-Adams JD, Whiteford ML, Wierczorek D, Wit JM, Yee CF, Beaulieu CL, White SM, Bulman DE, Bongers E, Brunner H, Feingold M, Boycott KM FORGE Canada Consortium. The phenotype of Floating-Harbor syndrome: clinical characterization of 52 individuals with mutations in exon 34 of SRCAP. Orphanet J Rare Dis. 2013;8:63. doi: 10.1186/1750-1172-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Messina G, Atterrato MT, Dimitri P. When chromatin organisation floats astray: the Srcap gene and Floating-Harbor syndrome. J Med Genet. 2016 doi: 10.1136/jmedgenet-2016-103842. pii: jmedgenet-2016–103842. [DOI] [PubMed] [Google Scholar]
- 89.Kosho T, Okamoto N Coffin-Siris Syndrome International Collaborators. Genotype-phenotype correlation of Coffin-Siris syndrome caused by mutations in SMARCB1, SMARCA4, SMARCE1, and ARID1A. Am J Med Genet C Semin Med Genet. 2014;166C(3):262–75. doi: 10.1002/ajmg.c.31407. [DOI] [PubMed] [Google Scholar]
- 90.He H, Liyanarachchi S, Akagi K, Nagy R, Li J, Dietrich RC, Li W, Sebastian N, Wen B, Xin B, Singh J, Yan P, Alder H, Haan E, Wieczorek D, Albrecht B, Puffenberger E, Wang H, Westman JA, Padgett RA, Symer DE, de la Chapelle A. Mutations in U4atac snRNA, a component of the minor spliceosome, in the developmental disorder MOPD I. Science. 2011;332(6026):238–40. doi: 10.1126/science.1200587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nagy R, Wang H, Albrecht B, Wieczorek D, Gillessen-Kaesbach G, Haan E, Meinecke P, de la Chapelle A, Westman JA. Microcephalic osteodysplastic primordial dwarfism type I with biallelic mutations in the RNU4ATAC gene. Clin Genet. 2012;82(2):140–6. doi: 10.1111/j.1399-0004.2011.01756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hämäläinen RH, Avela K, Lambert JA, Kallijärvi J, Eyaid W, Gronau J, Ignaszewski AP, McFadden D, Sorge G, Lipsanen-Nyman M, Lehesjoki AE. Novel mutations in the TRIM37 gene in Mulibrey Nanism. Hum Mutat. 2004;23(5):522. doi: 10.1002/humu.9233. [DOI] [PubMed] [Google Scholar]
- 93.Avela K1, Lipsanen-Nyman M, Idänheimo N, Seemanová E, Rosengren S, Mäkelä TP, Perheentupa J, Chapelle AD, Lehesjoki AE. Gene encoding a new RING-B-box-Coiled-coil protein is mutated in mulibrey nanism. Nat Genet. 2000;25(3):298–301. doi: 10.1038/77053. [DOI] [PubMed] [Google Scholar]
- 94.Kallijärvi J, Lahtinen U, Hämäläinen R, Lipsanen-Nyman M, Palvimo JJ, Lehesjoki AE. TRIM37 defective in mulibrey nanism is a novel RING finger ubiquitin E3 ligase. Exp Cell Res. 2005;308(1):146–55. doi: 10.1016/j.yexcr.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 95.Leduc MS, Niu Z, Bi W, Zhu W, Miloslavskaya I, Chiang T, Streff H, Seavitt JR, Murray SA, Eng C, Chan A, Yang Y, Lalani SR. CRIPT exonic deletion and a novel missense mutation in a female with short stature, dysmorphic features, microcephaly, and pigmentary abnormalities. Am J Med Genet A. 2016;170(8):2206–11. doi: 10.1002/ajmg.a.37780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Collin GB, Marshall JD, Ikeda A, So WV, Russell-Eggitt I, Maffei P, Beck S, Boerkoel CF, Sicolo N, Martin M, Nishina PM, Naggert JK. Mutations in ALMS1 cause obesity, type 2 diabetes and neurosensory degeneration in Alström syndrome. Nat Genet. 2002;31(1):74–8. doi: 10.1038/ng867. Epub 2002 Apr 8. [DOI] [PubMed] [Google Scholar]
- 97.Nowaczyk MJ, Irons MB. Smith-Lemli-Opitz syndrome: phenotype, natural history, and epidemiology. Am J Med Genet C Semin Med Genet. 2012;160C(4):250–62. doi: 10.1002/ajmg.c.31343. [DOI] [PubMed] [Google Scholar]
- 98.Woods SA, Robinson HB, Kohler LJ, Agamanolis D, Sterbenz G, Khalifa M. Exome sequencing identifies a novel EP300 frame shift mutation in a patient with features that overlap Cornelia de Lange syndrome. Am J Med Genet A. 2014;164A(1):251–8. doi: 10.1002/ajmg.a.36237. [DOI] [PubMed] [Google Scholar]
- 99.Dierker T, Bachvarova V, Krause Y, Li JP, Kjellén L, Seidler DG, Vortkamp A. Altered heparan sulfate structure in Glce(−/−) mice leads to increased Hedgehog signaling in endochondral bones. Matrix Biol. 2016;49:82–92. doi: 10.1016/j.matbio.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 100.Melrose J, Shu C, Whitelock JM, Lord MS. The cartilage extracellular matrix as a transient developmental scaffold for growth plate maturation. Matrix Biol. 2016;52–54:363–83. doi: 10.1016/j.matbio.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 101.Terhal PA, Nievelstein RJ, Verver EJ, Topsakal V, van Dommelen P, Hoornaert K, Le Merrer M, Zankl A, Simon ME, Smithson SF, Marcelis C, Kerr B, Clayton-Smith J, Kinning E, Mansour S, Elmslie F, Goodwin L, van der Hout AH, Veenstra-Knol HE, Herkert JC, Lund AM, Hennekam RC, Mégarbané A, Lees MM, Wilson LC, Male A, Hurst J, Alanay Y, Annerén G, Betz RC, Bongers EM, Cormier-Daire V, Dieux A, David A, Elting MW, van den Ende J, Green A, van Hagen JM, Hertel NT, Holder-Espinasse M, den Hollander N, Homfray T, Hove HD, Price S, Raas-Rothschild A, Rohrbach M, Schroeter B, Suri M, Thompson EM, Tobias ES, Toutain A, Vreeburg M, Wakeling E, Knoers NV, Coucke P, Mortier GR. A study of the clinical and radiological features in a cohort of 93 patients with a COL2A1 mutation causing spondyloepiphyseal dysplasia congenita or a related phenotype. Am J Med Genet A. 2015;167A(3):461–75. doi: 10.1002/ajmg.a.36922. [DOI] [PubMed] [Google Scholar]
- 102.Terhal PA, van Dommelen P, Le Merrer M, Zankl A, Simon ME, Smithson SF, Marcelis C, Kerr B, Kinning E, Mansour S, Hennekam RC, van der Hout AH, Cormier-Daire V, Lund AM, Goodwin L, Mégarbané A, Lees M, Betz RC, Tobias ES, Coucke P, Mortier GR. Mutation-based growth charts for SEDC and other COL2A1 related dysplasias. Am J Med Genet C Semin Med Genet. 2012;160C(3):205–16. doi: 10.1002/ajmg.c.31332. [DOI] [PubMed] [Google Scholar]
- 103.Tompson SW, Bacino CA, Safina NP, Bober MB, Proud VK, Funari T, Wangler MF, Nevarez L, Ala-Kokko L, Wilcox WR, Eyre DR, Krakow D, Cohn DH. Fibrochondrogenesis results from mutations in the COL11A1 type XI collagen gene. Am J Hum Genet. 2010;87(5):708–12. doi: 10.1016/j.ajhg.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bonaventure J, Chaminade F, Maroteaux P. Mutations in three subdomains of the carboxy-terminal region of collagen type X account for most of the Schmid metaphyseal dysplasias. Hum Genet. 1995;96(1):58–64. doi: 10.1007/BF00214187. [DOI] [PubMed] [Google Scholar]
- 105.Czarny-Ratajczak M1, Lohiniva J, Rogala P, Kozlowski K, Perälä M, Carter L, Spector TD, Kolodziej L, Seppänen U, Glazar R, Królewski J, Latos-Bielenska A, Ala-Kokko L. Am J A mutation in COL9A1 causes multiple epiphyseal dysplasia: further evidence for locus heterogeneity. Hum Genet. 2001;69(5):969–80. doi: 10.1086/324023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Spayde EC, Joshi AP, Wilcox WR, Briggs M, Cohn DH, Olsen BR. Exon skipping mutation in the COL9A2 gene in a family with multiple epiphyseal dysplasia. Matrix Biol. 2000;19(2):121–8. doi: 10.1016/s0945-053x(00)00055-x. [DOI] [PubMed] [Google Scholar]
- 107.Paassilta P, Lohiniva J, Annunen S, Bonaventure J, Le Merrer M, Pai L, Ala-Kokko L. COL9A3: A third locus for multiple epiphyseal dysplasia. Am J Hum Genet. 1999;64(4):1036–44. doi: 10.1086/302328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jackson GC, Mittaz-Crettol L, Taylor JA, Mortier GR, Spranger J, Zabel B, Le Merrer M, Cormier-Daire V, Hall CM, Offiah A, Wright MJ, Savarirayan R, Nishimura G, Ramsden SC, Elles R, Bonafe L, Superti-Furga A, Unger S, Zankl A, Briggs MD. Pseudoachondroplasia and multiple epiphyseal dysplasia: a 7-year comprehensive analysis of the known disease genes identify novel and recurrent mutations and provides an accurate assessment of their relative contribution. Hum Mutat. 2012;33(1):144–57. doi: 10.1002/humu.21611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Le Goff C, Mahaut C, Wang LW, Allali S, Abhyankar A, Jensen S, Zylberberg L, Collod-Beroud G, Bonnet D, Alanay Y, Brady AF, Cordier MP, Devriendt K, Genevieve D, Kiper PÖ, Kitoh H, Krakow D, Lynch SA, Le Merrer M, Mégarbane A, Mortier G, Odent S, Polak M, Rohrbach M, Sillence D, Stolte-Dijkstra I, Superti-Furga A, Rimoin DL, Topouchian V, Unger S, Zabel B, Bole-Feysot C, Nitschke P, Handford P, Casanova JL, Boileau C, Apte SS, Munnich A, Cormier-Daire V. Mutations in the TGFβ binding-protein-like domain 5 of FBN1 are responsible for acromicric and geleophysic dysplasias. Am J Hum Genet. 2011;89(1):7–14. doi: 10.1016/j.ajhg.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Briggs MD, Hoffman SM, King LM, Olsen AS, Mohrenweiser H, Leroy JG, Mortier GR, Rimoin DL, Lachman RS, Gaines ES, et al. Pseudoachondroplasia and multiple epiphyseal dysplasia due to mutations in the cartilage oligomeric matrix protein gene. Nat Genet. 1995;10(3):330–6. doi: 10.1038/ng0795-330. [DOI] [PubMed] [Google Scholar]
- 111.Hecht JT, Nelson LD, Crowder E, Wang Y, Elder FF, Harrison WR, Francomano CA, Prange CK, Lennon GG, Deere M, et al. Mutations in exon 17B of cartilage oligomeric matrix protein (COMP) cause pseudoachondroplasia. Nat Genet. 1995;10(3):325–9. doi: 10.1038/ng0795-325. [DOI] [PubMed] [Google Scholar]
- 112.Chapman KL, Mortier GR, Chapman K, Loughlin J, Grant ME, Briggs MD. Mutations in the region encoding the von Willebrand factor A domain of matrilin-3 are associated with multiple epiphyseal dysplasia. Nat Genet. 2001;28(4):393–6. doi: 10.1038/ng573. [DOI] [PubMed] [Google Scholar]
- 113.Gleghorn L, Ramesar R, Beighton P, Wallis G. A mutation in the variable repeat region of the aggrecan gene (AGC1) causes a form of spondyloepiphyseal dysplasia associated with severe, premature osteoarthritis. Am J Hum Genet. 2005;77(3):484–90. doi: 10.1086/444401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nilsson O, Guo MH, Dunbar N, Popovic J, Flynn D, Jacobsen C, Lui JC, Hirschhorn JN, Baron J, Dauber A. Short stature, accelerated bone maturation, and early growth cessation due to heterozygous aggrecan mutations. J Clin Endocrinol Metab. 2014;99(8):E1510–8. doi: 10.1210/jc.2014-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nicole S, Davoine CS, Topaloglu H, Cattolico L, Barral D, Beighton P, Hamida CB, Hammouda H, Cruaud C, White PS, Samson D, Urtizberea JA, Lehmann-Horn F, Weissenbach J, Hentati F, Fontaine B. Perlecan, the major proteoglycan of basement membranes, is altered in patients with Schwartz-Jampel syndrome (chondrodystrophic myotonia) Nat Genet. 2000;26(4):480–3. doi: 10.1038/82638. [DOI] [PubMed] [Google Scholar]
- 116.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423(6937):332–6. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 117.Matsushita T, Wilcox WR, Chan YY, Kawanami A, Bükülmez H, Balmes G, Krejci P, Mekikian PB, Otani K, Yamaura I, Warman ML, Givol D, Murakami S. FGFR3 promotes synchondrosis closure and fusion of ossification centers through the MAPK pathway. Hum Mol Genet. 2009;18(2):227–40. doi: 10.1093/hmg/ddn339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li M, Seki Y, Freitas PH, Nagata M, Kojima T, Sultana S, Ubaidus S, Maeda T, Shimomura J, Henderson JE, Tamura M, Oda K, Liu Z, Guo Y, Suzuki R, Yamamoto T, Takagi R, Amizuka N. FGFR3 down-regulates PTH/PTHrP receptor gene expression by mediating JAK/STAT signaling in chondrocytic cell line. J Electron Microsc (Tokyo) 2010;59(3):227–36. doi: 10.1093/jmicro/dfq002. [DOI] [PubMed] [Google Scholar]
- 119.Webster MK, Donoghue DJ. Constitutive activation of fibroblast growth factor receptor 3 by the transmembrane domain point mutation found in achondroplasia. EMBO J. 1996;15(3):520–7. [PMC free article] [PubMed] [Google Scholar]
- 120.Foldynova-Trantirkova S, Wilcox WR, Krejci P. Sixteen years and counting: the current understanding of fibroblast growth factor receptor 3 (FGFR3) signaling in skeletal dysplasias. Hum Mutat. 2012;33(1):29–41. doi: 10.1002/humu.21636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kant SG, Cervenkova I, Balek L, Trantirek L, Santen GW, de Vries MC, van Duyvenvoorde HA, van der Wielen MJ, Verkerk AJ, Uitterlinden AG, Hannema SE, Wit JM, Oostdijk W, Krejci P, Losekoot M. A novel variant of FGFR3 causes proportionate short stature. Eur J Endocrinol. 2015;172(6):763–70. doi: 10.1530/EJE-14-0945. [DOI] [PubMed] [Google Scholar]
- 122.Toydemir RM, Brassington AE, Bayrak-Toydemir P, Krakowiak PA, Jorde LB, Whitby FG, Longo N, Viskochil DH, Carey JC, Bamshad MJ. A novel mutation in FGFR3 causes camptodactyly, tall stature, and hearing loss (CATSHL) syndrome. Am J Hum Genet. 2006;79(5):935–41. doi: 10.1086/508433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Makrythanasis P, Temtamy S, Aglan MS, Otaify GA, Hamamy H, Antonarakis SE. A novel homozygous mutation in FGFR3 causes tall stature, severe lateral tibial deviation, scoliosis, hearing impairment, camptodactyly, and arachnodactyly. Hum Mutat. 2014;35(8):959–63. doi: 10.1002/humu.22597. [DOI] [PubMed] [Google Scholar]
- 124.Chusho H, Tamura N, Ogawa Y, Yasoda A, Suda M, Miyazawa T, Nakamura K, Nakao K, Kurihara T, Komatsu Y, Itoh H, Tanaka K, Saito Y, Katsuki M, Nakao K. Dwarfism and early death in mice lacking C-type natriuretic peptide. Proc Natl Acad Sci U S A. 2001;98(7):4016–21. doi: 10.1073/pnas.071389098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Moncla A, Missirian C, Cacciagli P, Balzamo E, Legeai-Mallet L, Jouve JL, Chabrol B, Le Merrer M, Plessis G, Villard L, Philip N. A cluster of translocation breakpoints in 2q37 is associated with overexpression of NPPC in patients with a similar overgrowth phenotype. Hum Mutat. 2007;28(12):1183–8. doi: 10.1002/humu.20611. [DOI] [PubMed] [Google Scholar]
- 126.Olney RC, Bükülmez H, Bartels CF, Prickett TC, Espiner EA, Potter LR, Warman ML. Heterozygous mutations in natriuretic peptide receptor-B (NPR2) are associated with short stature. J Clin Endocrinol Metab. 2006;91(4):1229–32. doi: 10.1210/jc.2005-1949. [DOI] [PubMed] [Google Scholar]
- 127.Miura K, Namba N, Fujiwara M, Ohata Y, Ishida H, Kitaoka T, Kubota T, Hirai H, Higuchi C, Tsumaki N, Yoshikawa H, Sakai N, Michigami T, Ozono K. An overgrowth disorder associated with excessive production of cGMP due to a gain-of-function mutation of the natriuretic peptide receptor 2 gene. PLoS One. 2012;7(8):e42180. doi: 10.1371/journal.pone.0042180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lorget F, Kaci N, Peng J, Benoist-Lasselin C, Mugniery E, Oppeneer T, Wendt DJ, Bell SM, Bullens S, Bunting S, Tsuruda LS, O'Neill CA, Di Rocco F, Munnich A, Legeai-Mallet L. Evaluation of the therapeutic potential of a CNP analog in a Fgfr3 mouse model recapitulating achondroplasia. Am J Hum Genet. 2012;91(6):1108–14. doi: 10.1016/j.ajhg.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hellemans J, Coucke PJ, Giedion A, De Paepe A, Kramer P, Beemer F, Mortier GR. Homozygous mutations in IHH cause acrocapitofemoral dysplasia, an autosomal recessive disorder with cone-shaped epiphyses in hands and hips. Am J Hum Genet. 2003;72(4):1040–6. doi: 10.1086/374318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Klopocki E, Hennig BP, Dathe K, Koll R, de Ravel T, Baten E, Blom E, Gillerot Y, Weigel JF, Krüger G, Hiort O, Seemann P, Mundlos S. Deletion and point mutations of PTHLH cause brachydactyly type E. Am J Hum Genet. 2010;86(3):434–9. doi: 10.1016/j.ajhg.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pereda A, Garin I, Garcia-Barcina M, Gener B, Beristain E, Ibañez AM, Perez de Nanclares G. Brachydactyly E: isolated or as a feature of a syndrome. Orphanet J Rare Dis. 2013;8:141. doi: 10.1186/1750-1172-8-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Savoldi G1, Izzi C, Signorelli M, Bondioni MP, Romani C, Lanzi G, Moratto D, Verdoni L, Pinotti M, Prefumo F, Superti-Furga A, Pilotta A. Prenatal presentation and postnatal evolution of a patient with Jansen metaphyseal dysplasia with a novel missense mutation in PTH1R. Am J Med Genet A. 2013;161A(10):2614–9. doi: 10.1002/ajmg.a.36115. [DOI] [PubMed] [Google Scholar]
- 133.Jobert AS, Zhang P, Couvineau A, Bonaventure J, Roume J, Le Merrer M, Silve C. Absence of functional receptors for parathyroid hormone and parathyroid hormone-related peptide in Blomstrand chondrodysplasia. J Clin Invest. 1998;102(1):34–40. doi: 10.1172/JCI2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nilsson O, Parker EA, Hegde A, Chau M, Barnes KM, Baron J. Gradients in bone morphogenetic protein-related gene expression across the growth plate. J Endocrinol. 2007;193(1):75–84. doi: 10.1677/joe.1.07099. [DOI] [PubMed] [Google Scholar]
- 135.Lehmann K, Seemann P, Stricker S, Sammar M, Meyer B, Süring K, Majewski F, Tinschert S, Grzeschik KH, Müller D, Knaus P, Nürnberg P, Mundlos S. Mutations in bone morphogenetic protein receptor 1B cause brachydactyly type A2. Proc Natl Acad Sci U S A. 2003;100(21):12277–82. doi: 10.1073/pnas.2133476100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Seemann P, Schwappacher R, Kjaer KW, Krakow D, Lehmann K, Dawson K, Stricker S, Pohl J, Plöger F, Staub E, Nickel J, Sebald W, Knaus P, Mundlos S. Activating and deactivating mutations in the receptor interaction site of GDF5 cause symphalangism or brachydactyly type A2. J Clin Invest. 2005;115(9):2373–81. doi: 10.1172/JCI25118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Begemann M1, Zirn B, Santen G, Wirthgen E, Soellner L, Büttel HM, Schweizer R, van Workum W, Binder G, Eggermann T. Paternally Inherited IGF2 Mutation and Growth Restriction. N Engl J Med. 2015;373(4):349–56. doi: 10.1056/NEJMoa1415227. [DOI] [PubMed] [Google Scholar]
- 138.Alatzoglou KS, Webb EA, Le Tissier P, Dattani MT. Isolated growth hormone deficiency (GHD) in childhood and adolescence: recent advances. Endocr Rev. 2014;35(3):376–432. doi: 10.1210/er.2013-1067. [DOI] [PubMed] [Google Scholar]
- 139.Laron Z. LESSONS FROM 50 YEARS OF STUDY OF LARON SYNDROME. Endocr Pract. 2015;21(12):1395–402. doi: 10.4158/EP15939.RA. [DOI] [PubMed] [Google Scholar]
- 140.Ross RJ. The GH receptor and GH insensitivity. Growth Horm IGF Res. 1999;(Suppl B):42–5. doi: 10.1016/s1096-6374(99)80080-x. [DOI] [PubMed] [Google Scholar]
- 141.Iida K, Takahashi Y, Kaji H, Nose O, Okimura Y, Abe H, Chihara K. Growth hormone (GH) insensitivity syndrome with high serum GH-binding protein levels caused by a heterozygous splice site mutation of the GH receptor gene producing a lack of intracellular domain. J Clin Endocrinol Metab. 1998;83(2):531–7. doi: 10.1210/jcem.83.2.4601. [DOI] [PubMed] [Google Scholar]
- 142.Bernasconi A, Marino R, Ribas A, Rossi J, Ciaccio M, Oleastro M, Ornani A, Paz R, Rivarola MA, Zelazko M, Belgorosky A. Characterization of immunodeficiency in a patient with growth hormone insensitivity secondary to a novel STAT5b gene mutation. Pediatrics. 2006;118(5):e1584–92. doi: 10.1542/peds.2005-2882. [DOI] [PubMed] [Google Scholar]
- 143.Fuqua JS, Derr M, Rosenfeld RG, Hwa V. Identification of a novel heterozygous IGF1 splicing mutation in a large kindred with familial short stature. Horm Res Paediatr. 2012;78(1):59–66. doi: 10.1159/000337249. [DOI] [PubMed] [Google Scholar]
- 144.van Duyvenvoorde HA1, van Setten PA, Walenkamp MJ, van Doorn J, Koenig J, Gauguin L, Oostdijk W, Ruivenkamp CA, Losekoot M, Wade JD, De Meyts P, Karperien M, Noordam C, Wit JM. Short stature associated with a novel heterozygous mutation in the insulin-like growth factor 1 gene. J Clin Endocrinol Metab. 2010;95(11):E363–7. doi: 10.1210/jc.2010-0511. [DOI] [PubMed] [Google Scholar]
- 145.Gannagé-Yared MH, Klammt J, Chouery E, Corbani S, Mégarbané H, Abou Ghoch J, Choucair N, Pfäffle R, Mégarbané A. Homozygous mutation of the IGF1 receptor gene in a patient with severe pre- and postnatal growth failure and congenital malformations. Eur J Endocrinol. 2012;168(1):K1–7. doi: 10.1530/EJE-12-0701. [DOI] [PubMed] [Google Scholar]
- 146.Kansra AR, Dolan LM, Martin LJ, Deka R, Chernausek SD. IGF receptor gene variants in normal adolescents: effect on stature. Eur J Endocrinol. 2012;167(6):777–81. doi: 10.1530/EJE-12-0565. [DOI] [PubMed] [Google Scholar]
- 147.Juanes M, Guercio G, Marino R, Berensztein E, Warman DM, Ciaccio M, Gil S, Bailez M, Rivarola MA, Belgorosky A. Three novel IGF1R mutations in microcephalic patients with prenatal and postnatal growth impairment. Clin Endocrinol (Oxf) 2015;82(5):704–11. doi: 10.1111/cen.12555. [DOI] [PubMed] [Google Scholar]
- 148.Domené HM, Scaglia PA, Martínez AS, Keselman AC, Karabatas LM, Pipman VR, Bengolea SV, Guida MC, Ropelato MG, Ballerini MG, Lescano EM, Blanco MA, Heinrich JJ, Rey RA, Jasper HG. Heterozygous IGFALS gene variants in idiopathic short stature and normal children: impact on height and the IGF system. Horm Res Paediatr. 2013;80(6):413–23. doi: 10.1159/000355412. [DOI] [PubMed] [Google Scholar]
- 149.Högler W, Martin DD, Crabtree N, Nightingale P, Tomlinson J, Metherell L, Rosenfeld R, Hwa V, Rose S, Walker J, Shaw N, Barrett T, Frystyk J. IGFALS gene dosage effects on serum IGF-I and glucose metabolism, body composition, bone growth in length and width, and the pharmacokinetics of recombinant human IGF-I administration. J Clin Endocrinol Metab. 2014;99(4):E703–12. doi: 10.1210/jc.2013-3718. [DOI] [PubMed] [Google Scholar]